Abstract
Background
Comprehensive description of ketamine’s molecular binding profile becomes increasingly pressing as use in real-life patient cohorts widens. Animal studies attribute a significant role in the substance’s antidepressant effects to the serotonergic system. The serotonin transporter is a highly relevant target in this context, because it is central to depressive pathophysiology and treatment. This is, to our knowledge, the first study investigating ketamine’s serotonin transporter binding in vivo in humans.
Methods
Twelve healthy subjects were assessed twice using [11C]DASB positron emission tomography. A total of 0.50 mg/kg bodyweight ketamine was administered once i.v. prior to the second positron emission tomography scan. Ketamine plasma levels were determined during positron emission tomography. Serotonin transporter nondisplaceable binding potential was computed using a reference region model, and occupancy was calculated for 4 serotonin transporter-rich regions (caudate, putamen, thalamus, midbrain) and a whole-brain region of interest.
Results
After administration of the routine antidepressant dose, ketamine showed <10% occupancy of the serotonin transporter, which is within the test-retest variability of [11C]DASB. A positive correlation between ketamine plasma levels and occupancy was shown.
Conclusions
Measurable occupancy of the serotonin transporter was not detectable after administration of an antidepressant dose of ketamine. This might suggest that ketamine binding of the serotonin transporter is unlikely to be a primary antidepressant mechanism at routine antidepressant doses, as substances that facilitate antidepressant effects via serotonin transporter binding (e.g., selective serotonin reuptake inhibitors) show 70% to 80% occupancy. Administration of high-dose ketamine is widening. Based on the positive relationship we find between ketamine plasma levels and occupancy, there is a need for investigation of ketamine’s serotonin transporter binding at higher doses.
Keywords: ketamine, antidepressant, serotonin transporter, positron emission tomography
Significance Statement
This is, to our knowledge, the first study investigating ketamine’s binding of the serotonin transporter in humans in vivo. The serotonin transporter is integral to depressive pathophysiology and treatment and is therefore a relevant target in elucidating the role of the serotonergic system in ketamine’s antidepressant effects. In this study, administration of an antidepressant dose of ketamine was not associated with measurable occupancy of the serotonin transporter. These results are in contrast with the 70% to 80% occupancy shown in previous studies with antidepressant doses of selective serotonin reuptake inhibitors. However, our study revealed a positive association between ketamine plasma levels and occupancy, which may be relevant considering the trend towards administration of higher doses of ketamine and inter-individual variability in ketamine metabolism. Our results further differentiate the role of the serotonergic system in ketamine’s antidepressant effects while garnering pharmacodynamic information that benefits safe and effective implementation in clinical routine.
Introduction
Ketamine has rapidly gained clinical significance and wide application as a swift and effective treatment option for patients with depression. In this indication, ketamine is applied in subanesthetic dosages, with most studies utilizing i.v. routes of application (Zarate et al., 2006). Early trials performed in patients with treatment-resistant depression reported response rates of approximately 50% to 70% within the timeframe of 24 to 72 hours after a single ketamine infusion (Berman et al., 2000; Zarate et al., 2006). Though numerous studies have been performed in the meantime, meta-analytic evidence generally confirms these promising original findings (Romeo et al., 2015).
Despite its increasing use as an antidepressant (Kraus et al., 2017), our understanding of the neurobiological mechanisms driving ketamine’s antidepressant effects remains incomplete. Ketamine exerts influence on a variety of neurotransmitters. As an NMDA receptor antagonist (Lener et al., 2016), ketamine modulates the glutamatergic system, though it has also been shown to effect dopaminergic (Li et al., 2015) and GABAergic (Hevers et al., 2008) changes.
In addition, the relevance of the serotonergic system to ketamine’s antidepressant effects has recently come to light (du Jardin et al., 2016b). Studies have shown that ketamine’s antidepressant properties in mice and rats were inhibited by serotonin (5-HT) depletion (Gigliucci et al., 2013; Fukumoto et al., 2014, 2016; du Jardin et al., 2016a). These findings suggest that the serotonergic system is essential for ketamine’s role as an antidepressant (du Jardin et al., 2016b). The serotonin transporter (SERT) is a significant target when aiming to elucidate the complex mechanisms mediating ketamine’s antidepressant properties. A potential role for the SERT is based on the antidepressant efficacy of selective serotonin reuptake inhibitors (SSRIs) and numerous studies showing changes to the SERT in depression (Gryglewski et al., 2014; Spies et al., 2015).
In fact, ketamine has been shown in studies using positron emission tomography (PET) to bind the SERT in vitro (Martin et al., 1990) and in vivo in monkeys (Yamamoto et al., 2013). Furthermore, ketamine inhibits SERT-dependent reuptake of 5-HT (Zhao and Sun, 2008; Barann et al., 2015). On an in vitro level, ketamine therefore mirrors the effects of SSRIs (Bel and Artigas, 1992). Microdialysis studies in monkeys have demonstrated that i.v. ketamine administration is associated with an increase in cortical 5-HT (Yamamoto et al., 2013). An increase in cortical 5-HT induced through intracerebral injection of ketamine was even shown to correlate with antidepressant effects (Pham et al., 2017). However, these animal studies demonstrate ketamine’s occupancy of the SERT at doses higher than those routinely implemented when ketamine is applied as an antidepressant in humans. Furthermore, it is generally unclear whether the pharmacodynamic and pharmacokinetic properties ketamine shows in animals carry over to humans. Therefore, in vivo human studies on ketamine’s binding characteristics are of utmost importance.
Furthermore, ketamine is increasingly being administered to real-life clinical cohorts, which comes with additional challenges, including psychiatrically and somatically multi-morbid patients receiving polypharmaceutical treatment (Moller et al., 2014). In addition, application of ketamine in clinical settings has brought to light the importance of incorporating ketamine into a treatment concept or scheme (Zhang et al., 2015) and raised questions as to how often the substance should be applied (Murrough et al., 2013) and in combination with what other potentiating or effect-stabilizing treatments (Chiu et al., 2014; Xu et al., 2015; Anderson et al., 2017). Many medications taken by depressed patients (Meyer et al., 2004; Spindelegger et al., 2009), as well as treatments performed in this patient group (i.e., electroconvulsive therapy), affect the serotonergic system (Lanzenberger et al., 2013). Investigation of ketamine’s occupancy of the SERT in humans is necessary for comprehensive description of ketamine’s complex molecular binding profile. This in turn is a prerequisite for safe and effective implementation in clinical practice.
Therefore, we aimed to investigate the extent and pattern of ketamine’s binding on the SERT in humans. We utilized PET imaging with [11C]-N,N-dimethyl-2-(2-amino-4-cyanophenylthio)-benzylamine ([11C]DASB), a highly selective radioligand for imaging of the SERT (Wilson et al., 2001).
Materials and Methods
Subjects
Twelve healthy male subjects (mean age±SD = 26.92±3.45) were included in this study. Subjects were free from all severe internal, neurological, and psychiatric disorders, assessed via an extensive medical history, physical examination, electrocardiography, and blood draw. A structured clinical interview for DSM-IV (SCID) was performed by an experienced physician to exclude all previous or current psychiatric disorders. Subjects had no history of drug abuse, and current drug abuse was excluded using a urine drug test performed at the screening and both PET visits. Subjects were also screened for any magnetic resonance imaging (MRI) contraindications, including implants, pacemakers, and claustrophobia. All subjects provided written informed consent and received financial reimbursement for participation. This study was approved by the Ethics Committee of the Medical University of Vienna and was performed according to the Declaration of Helsinki.
PET
All subjects underwent 2 [11C]DASB PET investigations performed using a GE Advance full-ring scanner (General Electric Medical Systems) at the Department of Biomedical Imaging and Image-Guided Therapy, Department of Nuclear Medicine, Medical University of Vienna. The first PET scan (PET 1) functioned as a baseline scan, and no pharmacologic challenge was administered. Prior to the second PET scan (PET 2), all subjects received ketamine. [11C]DASB is a highly selective radioligand for assessment of SERT distribution in the human brain (Wilson et al., 2001). A transmission scan, which is necessary for tissue attenuation correction, was performed prior to i.v. injection of [11C]DASB during 5 minutes using retractable 68GE rod sources. Then 4.7 MBq/kg bodyweight [11C]DASB was diluted in phosphate buffered saline and applied as a bolus simultaneously with the start of PET scanning. [11C]DASB was synthesized at the PET Center at the Medical University of Vienna according to (Haeusler et al., 2009; Ungersboeck et al., 2012). To minimize head motion artifacts during PET measurement, each subject’s head was fixed within the padded PET scanner headpiece using a head-strap. Data were acquired over the course of 90 minutes using 50 successive time frames. Subsequently, data were reconstructed in volumes consisting of 35 transaxial sections (128 x 128 matrix) using Fourier rebinning iterative filter back-projection algorithm with a spatial resolution of 4.36 mm full-width half maximum 1 cm next to the center of the field of view.
Ketamine Administration
A total of 0.50 mg/kg bodyweight racemic ketamine (Ketamin-hameln, 50 mg/mL ampoules; Hameln Pharma Plus GmbH) was applied once i.v. via a cubital vein over the course of 40 minutes, with the infusion ending 5 minutes before start of PET and simultaneous [11C]DASB administration. The dose of 0.50 mg/kg bodyweight racemic ketamine was chosen as this is the generally accepted and thoroughly investigated subanesthetic, antidepressant dose (Perry et al., 2007; Romeo et al., 2015). Blood pressure was measured at regular intervals during ketamine administration while heart rate and blood oxygenation were measured over the course of ketamine infusion and subsequent PET 2 measurement. A physician was present at all times during ketamine infusion and PET 2 measurement to ensure subject safety.
MRI
One structural MRI scan was performed in each subject using a 3 Tesla PRISMA MR Scanner (Siemens Medical, 0.85- x 0.87-mm voxel size, 0.85-mm slice thickness, 230 slices) for co-registration of PET data.
PET Data Analysis
PET images were motion corrected and co-registered to the structural MR using the integral of all frames. Subsequently, the T1-weighted MR image was spatially normalized to a tissue probability map in stereotactic Montreal Neurological Institute space using SPM12 (Wellcome Trust Centre for Neuroimaging; http://www.fil.ion.ucl.ac.uk/spm/). The resulting transformation parameters were applied to the co-registered PET image. To quantify SERT nondisplaceable binding potential (BPND) (Innis et al., 2007), a region of interest (ROI)-based approach and the multilinear reference tissue model (MRTM2; Ichise et al., 2003) was applied in PMOD 3.509 (http://www.pmod.com) with cerebellar gray matter (excluding vermis and venous sinus) used as the reference region. This region only minimally expresses the SERT (Kish et al., 2005; Parsey et al., 2006) and is therefore considered an optimal reference region for [11C]DASB quantification. The 4 ROIS selected comprised the caudate, putamen, thalamus, and midbrain. These subcortical regions show high SERT expression (Saulin et al., 2012; Savli et al., 2012), which optimizes signal-to-noise ratio. Secondly, these regions are relevant to the efficacy of other substances that bind the SERT, such as SSRIs, and SERT occupancy by these substances is thoroughly described in these regions (Meyer et al., 2004). The ROIS were delineated using the automated anatomical labeling (AAL) brain atlas (Tzourio-Mazoyer et al., 2002) with the exception of the midbrain, which is not included in the AAL and was delineated using an in-house template. In addition, a whole-brain ROI was analyzed. The whole-brain ROI consisted of the average BPND of all AAL regions, weighted for size of each individual region, and excluding the cerebellum. This calculation was performed to take into account that ketamine has global effects on the brain (Kavalali and Monteggia, 2015). Occupancy of the SERT by ketamine was computed using the equation:
Ketamine Plasma Levels
After ketamine administration, blood was drawn at 5, 10, 20, 30, 40, 60, and 80 minutes after start of PET measurement. After centrifugation and separation of plasma, plasma samples were frozen at ≤−20°C. Ketamine plasma levels were determined by gas chromatography–tandem mass spectrometry in multiple reaction monitoring at the Department of Laboratory Medicine, Medical University of Vienna, Austria. The analytical method was validated according to the EMA guideline (European Medicines Agency, 2015).
Statistical Analysis
To assess the statistical significance of ketamine binding of the SERT, linear mixed model analyses using measurement (PET 1, PET 2) and region (caudate, putamen, thalamus, midbrain) as fixed factors, subject as random factor, and [11C]DASB BPND as the dependent variable was performed. Analysis was repeated for the whole-brain ROI with measurement as fixed factor (PET 1, PET 2), subject as random factor, and [11C]DASB BPND as the dependent variable.
To assess if potential changes between PET 1 and PET 2 differ from those associated with test-retest variability, linear mixed model analysis incorporating the [11C]DASB test-retest data from a previous study by our group (Kranz et al., 2015) was performed (see supplement).
Correlation analyses between ketamine plasma levels and SERT occupancy were performed to address findings demonstrating diversity in ketamine metabolism (Zarate et al., 2012; Zhao et al., 2012). Ketamine plasma levels at all time points (5, 10, 20, 30, 40, 60, and 80 minutes after start of PET measurement) were correlated with SERT occupancy values within the ROIs showing positive occupancy values. Correlation analyses used Spearman correlation coefficient and correction for multiple comparisons was performed using the Holm-Bonferroni method. Normality of distribution was tested by plotting the individual variables. SPSS 24.0.0.0 (SPSS Inc.; www.spss.com) was used for statistical analyses.
Results
Average occupancy over the group was at (mean±SD) 5.86±21.88, 2.70±13.76, and 1.11±23.27, within the caudate, putamen, and thalamus (Figure 1a), respectively. For the midbrain region, a negative occupancy value of -1.20±13.72 was acquired. In the whole-brain ROI, average occupancy was 7.97±14.40.
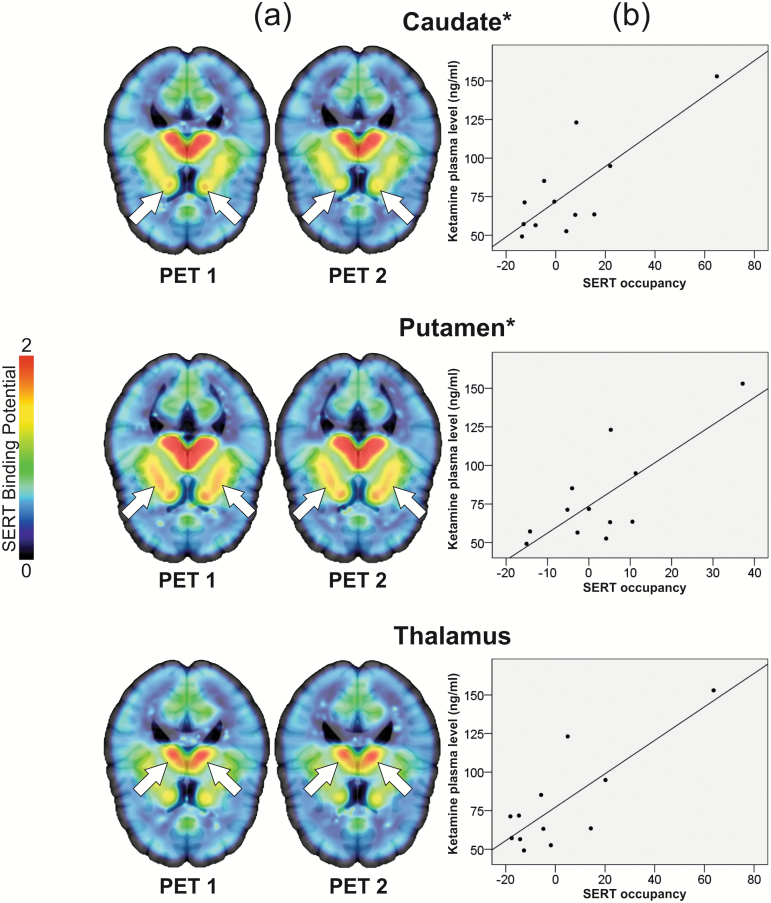
Figure 1.
(a) No measurable occupancy of the serotonin transporter (SERT) after 0.50 mg/kg bodyweight ketamine. [11C]DASB nondisplaceable binding potential (BPND) decreased numerically from positron emission tomography (PET) 1 (left, before ketamine application) to PET 2 (right, after application of the standard antidepressant dose of 0.50 mg/kg bodyweight ketamine) in the caudate, putamen, and thalamus. Mean occupancy values ± SD of the SERT were 5.86±21.88, 2.70±13.76, and 1.11±23.27, respectively. Results were interpreted as a lack of measurable binding, because occupancy values are within [11C]DASB test-retest variability (Kranz et al., 2015) (see supplement). Color bar represents [11C]DASB BPND. Slices at z=10 (caudate), z=6 (putamen), and z=12 (thalamus). For [11C]DASB BPND and occupancy values see Table 1. (b) Correlation between ketamine plasma levels and SERT occupancy. Ketamine plasma levels assessed after ketamine administration during PET correlated with SERT occupancy within the caudate and putamen (caudate 20 min: P=.042; 30 min: P=.018; putamen 30 min: P=.031, all uncorr., min indicate time after start of PET measurement). Though the correlations are influenced by outliers, these findings might suggest that ketamine may bind the SERT when levels are higher, for example, when ketamine is administered at higher doses or depending on differences in metabolism (Zarate et al., 2012; Zhao et al., 2012). Correlation analyses with ketamine plasma levels drawn 30 minutes after PET start are depicted. Correlations may be significant at this time point, as ketamine kinetics are known to switch from the distribution to elimination phase around this time (Hijazi et al., 2003). *Indicates statistical significance of correlations (P<.05, uncorrected). For occupancy values and ketamine plasma levels, see Tables 1 and 2.
Linear mixed-model analyses of caudate, putamen, thalamus, and midbrain did not reveal a measurement by region interaction or a significant effect of measurement, though an effect of region (F3, 27.26 =148.02, P<.001) was revealed. Linear mixed model analyses in the whole-brain ROI also did not reveal a significant effect of measurement (Table 1). [11C]DASB BPND values were not consistently normally distributed within the 4 ROIs or within the whole-brain ROI. However, considering that use of mixed model analyses in a not normally distributed sample has the potential to increase false-positive error, and no significant effect of measurement was found, the mixed-model approach was retained.
Table 1.
Average [11C]DASB BPND
| Region of Interest | BPND PET 1 | BPND PET 2 | Occupancy |
|---|---|---|---|
| Caudate | 0.94±0.22 | 0.89±0.30 | 5.86±21.88 |
| Putamen | 1.56±0.29 | 1.54±0.39 | 2.70±13.76 |
| Thalamus | 1.61±0.30 | 1.61±0.49 | 1.11±23.27 |
| Midbrain | 3.37±0.71 | 3.40±0.80 | n.a. |
| Whole-brain* | 0.39±0.08 | 0.36±0.09 | 7.97±14.40 |
Abbreviations: BPND, nondisplaceable binding potential; PET, positron emission tomography (PET 1=baseline, PET 2=after ketamine).
*All AAL regions (Tzourio-Mazoyer et al., 2002; Savli et al., 2012) weighted for size with the exception of the cerebellum.
Linear mixed model analyses comparing our results with previous test-retest data (Kranz et al., 2015) did not reveal an interaction effect between measurement and study data set, demonstrating that the variability within the 2 PET measurements in the current study does not differ from that between the 2 PET measurements from (Kranz et al., 2015). Therefore, our results can be interpreted as within test-retest variability (see supplement).
SERT occupancy and ketamine plasma levels correlated significantly at 20 and 30 minutes after start of PET measurement within the caudate (20 minutes: P=.042, rho=0.594; 30 minutes: P=.018, rho=0.664) and at 30 minutes after start of PET measurement in the putamen (P=.031, rho=0.622), all uncorr., (Figure 1b). In the whole-brain ROI, occupancy and plasma levels correlated at 20 minutes (P=.015, rho=0.678) and 30 minutes (P=.011, rho=0.699), all uncorr. SERT occupancy and ketamine plasma levels did not correlate in the thalamus. One 5-minute ketamine plasma value was removed from correlation analyses because of a probable blood draw error. Correlation results in the caudate and putamen were influenced by 2 subjects with outlying ketamine and/or occupancy values. While the significance of the correlation in the caudate at 30 minutes survived removal of one of these outliers (P = .043, rho=0.618), removal of the other outlier resulted in loss of significance of all correlations in these regions. Correlation results in the whole-brain ROI were not influenced by removal of outliers.
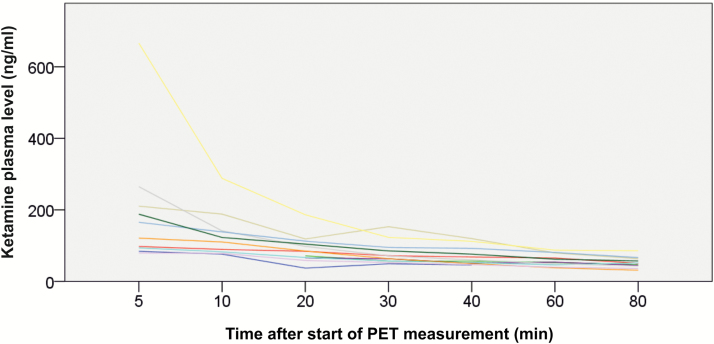
Ketamine plasma levels were in accordance with those previously described in the literature (Zarate et al., 2012) (Table 2, Figure 2). For information on vital signs during ketamine infusion and PET measurement, please see Table 3.
Table 2.
Average Ketamine Plasma Levels
| Time point | Ketamine plasma level (mean±SD) |
|---|---|
| 05 min (n=10)a | 197.06±176.04 ng/mL |
| 10 min (n=10) | 131.54±65.40 ng/mL |
| 20 min (n=12) | 90.71±38.33 ng/mL |
| 30 min (n=12) | 78.46±31.44 ng/mL |
| 40 min (n=12) | 69.28±25.65 ng/mL |
| 60 min (n=11) | 59.56±17.02 ng/mL |
| 80 min (n=11) | 52.46±15.35 ng/mL |
a1 aberrant value removed from analysis (see Methods).
n=available measurements.
min indicates time after start of PET measurement.
Figure 2.
Ketamine plasma levels. Ketamine plasma levels were in accordance with those previously described in the literature (Zarate et al., 2012). PET measurement began 5 minutes after completion of the ketamine infusion (0.50 mg/kg bodyweight over 40 min) and ketamine plasma levels were drawn 5, 10, 20, 30, 40, 60, and 80 minutes after the start of PET. The 5-minute ketamine value from 1 subject was removed from analyses due to probable blood draw error. For ketamine plasma levels see Table 2.
Table 3.
Vital Signs during Ketamine Administration and PET Measurement
| Parameter | Mean±SD | Min | Max | % increase (mean±SD) | % decrease (mean±SD) |
|---|---|---|---|---|---|
| Heart rate (bpm) | 69.83±10.88 | 43 | 104 | 9.31±8.05 | 10.72±7.55 |
| Sa02 | 97.29±0.94 | 94 | 99 | 1.31±0.47 | 1.33±0.51 |
| Systolic RR | 134.62±15.50 | 106 | 177 | 7.80±5.97 | 4.35±2.69 |
| Diastolic RR | 79.52±10.94 | 64 | 105 | 11.53±8.55 | 8.86±6.05 |
Abbreviation: RR,Riva-Rocci blood pressure.
Parameters were assessed every 10 minutes.
Discussion
We used [11C]DASB PET to investigate cerebral SERT occupancy after a single antidepressant dose of ketamine. Subcortical regions with high SERT expression (Saulin et al., 2012; Savli et al., 2012) shown to be relevant in other SERT occupancy studies were investigated (Meyer et al., 2001, 2004). Occupancy values were between approximately 1% and 8% in the caudate, putamen, thalamus, and whole-brain ROI, which is within the test-retest variability of [11C]DASB (Kranz et al., 2015) (see supplement). Therefore, no measurable occupancy of the serotonin transporter was detected after administration of an antidepressant ketamine dose.
A positive relationship between ketamine plasma levels and SERT occupancy values was found in the caudate, putamen, and whole-brain ROI. This finding suggests that ketamine may bind the SERT when administered at higher doses. After falling for the first 20 to 30 minutes after start of PET, ketamine levels began to asymptotically approach zero (Figure 2). This time-point may be when ketamine kinetics switch from the distribution to the elimination phase (Hijazi et al., 2003). Starting at this time, ketamine plasma levels may most closely correspond with levels in the brain compartment, which would explain why plasma levels and occupancy correlated. Based on this explanation, one would expect plasma levels and occupancy to correlate at all further time-points. However, as ketamine levels fall, signal to noise ratio decreases, which may explain why this was not the case in our study. These correlation results must be interpreted with caution, as they are influenced in the caudate and putamen by the inclusion of 2 subjects with outlying ketamine and occupancy values (Figure 1b). However, the lack of normal distribution in occupancy and ketamine data and the presence of outliers in our small data set may in fact reflect previous studies showing diversity in ketamine metabolism (Zarate et al., 2012; Zhao et al., 2012). Investigation in a larger data set at a wide dose range is necessary for confirmation of this pattern. We thus highlight that SERT binding by ketamine is not detectable after administration of low doses, but that occupancy increase after higher doses might be possible. These results provide valuable insight into the nature of ketamine’s effects on the serotonergic system in humans in vivo.
Based on previous literature, one possible explanation for the role of the 5-HT system in ketamine’s antidepressant effects would be via SERT binding and reuptake inhibition. In this model, increased prefrontal 5-HT following ketamine binding of the SERT (Yamamoto et al., 2013) would be a result of 5-HT reuptake blockade, as has been shown in vitro (Zhao and Sun, 2008; Barann et al., 2015). This would result in increased serotonergic activity in projection regions. This theory is supported by an animal study demonstrating that injection of ketamine into the prefrontal cortex resulted in a local 5-HT increase, which correlated with antidepressant response (Pham et al., 2017). Along this line, animal studies have shown in vivo that ketamine binds the SERT (Yamamoto et al., 2013; Yamanaka et al., 2014). However, it was demonstrated that while 0.50 mg/kg bodyweight ketamine resulted in a numeric reduction in available SERT, only 1.5 mg/kg bodyweight ketamine showed statistically significant SERT occupancy (Yamamoto et al., 2013). Dosing, pharmacokinetics, and clinical effects differ between animals and humans (Radford et al., 2017), making translation from animal to human models problematic and investigations in the latter essential. For example, 0.75 mg/kg bodyweight ketamine/h in monkeys was suggested as an equivalent dose to 0.50 mg/kg bodyweight/40 min in humans (Yamanaka et al., 2014). In this case, the general pattern shown in monkeys seems to be mirrored in humans. We do not find measureable occupancy of the SERT after administration of an antidepressant dose. However, based on extrapolation of the positive trend we demonstrate between SERT occupancy and ketamine plasma levels, it can be postulated that ketamine may bind the SERT at higher doses.
The occupancy levels shown in this study are only a fraction of those shown after antidepressant doses of SSRIs, which exhibit approximately 70% to 80% SERT occupancy and for which 5-HT reuptake is thought to be the initial, primary antidepressant mechanism (Meyer et al., 2001, 2004). Therefore, it seems improbable that ketamine’s binding of the SERT serves as a main facilitator of antidepressant effects as it does with SSRIs. However, it remains to be investigated whether SERT binding plays a likely subordinate but possibly potentiating role. Furthermore, future studies should address whether ketamine has more long-term effects on the serotonergic system, including the SERT.
The phenotypical correlate of the, at most discrete, SERT binding we show at low doses is not entirely clear, though a recent publication on co-medication of ketamine and the irreversible MOA-inhibitor tranylcypromine promotes skepticism (Bartova et al., 2015). However, this report is a set of case studies, and ketamine metabolism and plasma levels have been shown to vary after administration of a constant dose (Zarate et al., 2012; Zhao et al., 2012). Therefore, the generalizability of these findings is questionable. Furthermore, potentially increased SERT binding at higher ketamine doses, which are increasingly being administered to depressed patients (Cusin et al., 2017) and have long been state of the art in anesthesiology, may indeed carry clinical consequences.
Another possibility is that 5-HTs relevance in ketamine’s effects (du Jardin et al., 2016b) is through secondary mechanisms, including interaction with other neurotransmitter systems. In this regard, interface with the glutamatergic system appears relevant. For example, injection of an AMPA blocker prior to ketamine administration in a mouse model inhibited the substance’s antidepressant effects (Fukumoto et al., 2016). Based on results gleaned from microinjection of these 2 substances, it was postulated that binding of the AMPA receptor by ketamine in the medial prefrontal cortex stimulates serotonergic neurons in the dorsal raphe nuclei (Fukumoto et al., 2016). Furthermore, it was demonstrated that blockade of AMPA receptors inhibited the effects of anesthetic doses of ketamine on the 5-HT1B receptor (Yamanaka et al., 2014). Theoretically, ketamine’s modulation of the serotonergic system may also be via direct binding of serotonergic receptors, though the extent to which this is the case has been insufficiently investigated. It has, however, been suggested that ketamine may bind 5-HT2 receptors (Kapur and Seeman, 2002; Waelbers et al., 2013).
We applied ketamine as the entire racemat, as this is the more thoroughly investigated form that has repeatedly been shown to be clinically effective as an antidepressant (Romeo et al., 2015), and it was our goal to elucidate the role of the 5-HT system in this context. However, differences between the individual ketamine enantiomers in regards to their pharmacodynamic (Nishimura and Sato, 1999) and clinical (Yang et al., 2015) properties are a current topic of intense research (Andrade, 2017). Differing binding affinities would be a potential explanation for why the 2 enantiomers may display differential clinical effects. However, in vitro studies have shown that the ketamine enantiomers do not show stereo-selectivity for the SERT (Nishimura and Sato, 1999). Therefore, the results of our study are not likely affected by the use of the entire racemat.
It has been suggested that ketamine binds the SERT at the binding site likely bound by 5-HT and SSRIs (Martin et al., 1990; Andersen et al., 2009). It could therefore be questioned whether the reduction in SERT BPND shown in animal studies and numerically in our study is truly a reflection of ketamine binding on the transporter or rather of changes to endogenous 5-HT levels elicited by ketamine. [11C]DASB may to a certain extent be sensitive to endogenous 5-HT levels (Yamamoto et al., 2007), though this has been negated by others (Talbot et al., 2005). With the exception of the hypothalamus, a substantial influence of endogenous 5-HT levels on [11C]DASB binding due to tranylcypromine administration was only significant at a dose of 15 mg/kg bodyweight (Lundquist et al., 2005), which was associated with a 11- to 63-fold increase in 5-HT (Celada and Artigas, 1993). Considering that only about 2.5-fold increase in 5-HT was shown after ketamine administration (Yamamoto et al., 2013), we assume that changes to endogenous 5-HT levels likely do not play a relevant role in our results.
Several limitations should be discussed. Firstly, the sample size (n=12) of this study is small. However, it is in accordance with previous pharmaco-PET studies investigating SERT occupancy by other psychopharmacologic substances (Meyer et al., 2001). In addition, this study investigates healthy subjects rather than depressed patients, and a relationship between SERT binding and antidepressant response can therefore not be drawn. As a result, our interpretation that SERT binding by ketamine is not a likely primary antidepressant mechanism is made with caution and is based on existing literature on SSRI SERT occupancy (Meyer et al., 2001, 2004). Furthermore, it is important to note that the occupancy levels shown were <10% and therefore within the test-retest variability of [11C]DASB PET, which was validated through comparison with a test-retest data set from a previous study by our group (Kranz et al., 2015). Therefore, we argue that the low numeric occupancy values attained can be understood as a lack of measureable binding of the SERT. Furthermore, we based the infusion protocol on a primate study demonstrating ketamine’s occupancy of the SERT, which reported starting PET measurement after infusion of ketamine over 40 minutes (Yamamoto et al., 2013). However, due to this set-up, ketamine levels fell over the course of PET measurement as the substance was eliminated (Table 2; Figure 2). This may result in a bias in the direction of underestimation of ketamine’s SERT occupancy, particularly as ketamine levels towards the end of PET measurement more accurately reflect the occupancy values attained. Occupancy might therefore be higher during or immediately after ketamine infusion.
In summary, the results from our study provide insight into the effects of ketamine on the serotonergic system in humans in vivo. We did not find measureable binding of the SERT after administration of an antidepressant dose of ketamine. This is in contrast with SSRIs, which show 70% to 80% SERT occupancy (Meyer et al., 2001, 2004). As 0.50 mg/kg bodyweight ketamine is nevertheless clinically effective (Zarate et al., 2006), this might suggest that binding of the SERT is unlikely to be a main antidepressant mechanism in ketamine treatment. The increase in 5-HT levels following ketamine application (Yamamoto et al., 2013) and the dependency of ketamine’s efficacy on the presence of 5-HT (du Jardin et al., 2016a) as recently shown in the literature, may more likely be a reflection of secondary modulation of the serotonergic system. We find a positive trend between ketamine plasma levels and SERT occupancy. Assessment of ketamine’s binding of the SERT at higher doses is therefore of importance considering that dose escalation will inevitably increasingly be utilized in depressed patients (Cusin et al., 2017) and is already relevant in ketamine’s administration as an anesthetic.
Supplementary Material
Supplementary data are available at International Journal of Neuropsychopharmacology online.
Funding
This research is supported by a NARSAD Young Investigator Grant from the Brain and Behavior Research Foundation (grant no. 23741) to M. Spies.
Statement of Interest
M. Spies has received travel grants from Janssen, Eli Lilly, and AOP Orphan Pharmaceuticals AG; speaker honoraria from Janssen, workshop participation from Eli Lilly, and is recipient of a NARSAD Young Investigator Grant from the Brain and Behavior Research Foundation. G.S. Kranz received travel grants from Roche Austria GmbH and Pfizer. G. Gryglewski is recipient of a DOC Fellowship of the Austrian Academy of Sciences at the Medical University of Vienna. M. Hienert has received financial support from the Austrian Science Fund and the Jubilee Fund of the Austrian National Bank and the Austrian Society for Neuropsychopharmacology and Biological Psychiatry. D. Winkler has received lecture fees from Angelini, Lundbeck, and Pfizer. Siegfried Kasper has received grants/research support, consulting fees, and/or honoraria within the last 3 years from Angelini, AOP Orphan Pharmaceuticals AG, AstraZeneca, Eli Lilly, Janssen, KRKA-Pharma, Lundbeck, Neuraxpharm, Pfizer, Pierre Fabre, Dr. Willmar Schwabe GmbH, and Servier. R. Lanzenberger has received travel grants and/or conference speaker honoraria from AstraZeneca, Lundbeck, Dr. Willmar Schwabe GmbH, AOP Orphan Pharmaceuticals AG, Janssen, and Roche Austria GmbH. All other authors report no conflict of interest with regards to this paper.
Supplementary Material
Acknowledgments
We thank Andreas Hahn, Sebastian Ganger, Manfred Kloebl, and Volker Weiss for technical assistance, Benjamin Spurny for administrative assistance, and Daniela Haeusler for set up of radioligand synthesis.
Clinicaltrials.gov: NCT02717052.
References
- Anderson IM, et al. (2017)Ketamine augmentation of electroconvulsive therapy to improve neuropsychological and clinical outcomes in depression (Ketamine-ECT): a multicentre, double-blind, randomised, parallel-group, superiority trial. Lancet Psychiatry 4:365–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen J, Taboureau O, Hansen KB, Olsen L, Egebjerg J, Stromgaard K, Kristensen AS(2009)Location of the antidepressant binding site in the serotonin transporter: importance of Ser-438 in recognition of citalopram and tricyclic antidepressants. J Biol Chem 284:10276–10284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade C.(2017)Ketamine for depression, 3: does chirality matter?J Clin Psychiatry 78:e674–e677. [DOI] [PubMed] [Google Scholar]
- Barann M, Stamer UM, Lyutenska M, Stuber F, Bonisch H, Urban B(2015)Effects of opioids on human serotonin transporters. Naunyn Schmiedebergs Arch Pharmacol 388:43–49. [DOI] [PubMed] [Google Scholar]
- Bartova L, Vogl SE, Stamenkovic M, Praschak-Rieder N, Naderi-Heiden A, Kasper S, Willeit M(2015)Combination of intravenous S-ketamine and oral tranylcypromine in treatment-resistant depression: a report of two cases. Eur Neuropsychopharmacol 25:2183–2184. [DOI] [PubMed] [Google Scholar]
- Bel N, Artigas F(1992)Fluvoxamine preferentially increases extracellular 5-hydroxytryptamine in the raphe nuclei: an in vivo microdialysis study. Eur J Pharmacol 229:101–103. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH(2000)Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47:351–354. [DOI] [PubMed] [Google Scholar]
- Celada P, Artigas F(1993)Monoamine oxidase inhibitors increase preferentially extracellular 5-hydroxytryptamine in the midbrain raphe nuclei. A brain microdialysis study in the awake rat. Naunyn Schmiedebergs Arch Pharmacol 347:583–590. [DOI] [PubMed] [Google Scholar]
- Chiu CT, Scheuing L, Liu G, Liao HM, Linares GR, Lin D, Chuang DM(2014)The mood stabilizer lithium potentiates the antidepressant-like effects and ameliorates oxidative stress induced by acute ketamine in a mouse model of stress. Int J Neuropsychopharmacol 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusin C, Ionescu DF, Pavone KJ, Akeju O, Cassano P, Taylor N, Eikermann M, Durham K, Swee MB, Chang T, Dording C, Soskin D, Kelley J, Mischoulon D, Brown EN, Fava M(2017)Ketamine augmentation for outpatients with treatment-resistant depression: preliminary evidence for two-step intravenous dose escalation. Aust N Z J Psychiatry 51:55–64. [DOI] [PubMed] [Google Scholar]
- du Jardin KG, Liebenberg N, Muller HK, Elfving B, Sanchez C, Wegener G (2016a) Differential interaction with the serotonin system by S-ketamine, vortioxetine, and fluoxetine in a genetic rat model of depression. Psychopharmacology (Berl) 233:2813–2825. [DOI] [PubMed] [Google Scholar]
- du Jardin KG, Muller HK, Elfving B, Dale E, Wegener G, Sanchez C (2016b) Potential involvement of serotonergic signaling in ketamine’s antidepressant actions: a critical review. Prog Neuropsychopharmacol Biol Psychiatry 71:27–38. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency, Committee for Medicinal Products for Human Use (CHMP) (2015) Guideline on bioanalytical method validation. London, United Kingdom . [Google Scholar]
- Fukumoto K, Iijima M, Chaki S(2014)Serotonin-1A receptor stimulation mediates effects of a metabotropic glutamate 2/3 receptor antagonist, 2S-2-amino-2-(1S,2S-2-carboxycycloprop-1-yl)-3-(xanth-9-yl)propanoic acid (LY341495), and an N-methyl-D-aspartate receptor antagonist, ketamine, in the novelty-suppressed feeding test. Psychopharmacology (Berl) 231:2291–2298. [DOI] [PubMed] [Google Scholar]
- Fukumoto K, Iijima M, Chaki S(2016)The antidepressant effects of an mGlu2/3 receptor antagonist and ketamine require AMPA receptor stimulation in the mPFC and subsequent activation of the 5-HT neurons in the DRN. Neuropsychopharmacology 41:1046–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigliucci V, O’Dowd G, Casey S, Egan D, Gibney S, Harkin A(2013)Ketamine elicits sustained antidepressant-like activity via a serotonin-dependent mechanism. Psychopharmacology (Berl) 228:157–166. [DOI] [PubMed] [Google Scholar]
- Gryglewski G, Lanzenberger R, Kranz GS, Cumming P(2014)Meta-analysis of molecular imaging of serotonin transporters in major depression. J Cereb Blood Flow Metab 34:1096–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeusler D, Mien LK, Nics L, Ungersboeck J, Philippe C, Lanzenberger RR, Kletter K, Dudczak R, Mitterhauser M, Wadsak W(2009)Simple and rapid preparation of [11C]DASB with high quality and reliability for routine applications. Appl Radiat Isot 67:1654–1660. [DOI] [PubMed] [Google Scholar]
- Hevers W, Hadley SH, Luddens H, Amin J(2008)Ketamine, but not phencyclidine, selectively modulates cerebellar GABA(A) receptors containing alpha6 and delta subunits. J Neurosci 28:5383–5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijazi Y, Bodonian C, Bolon M, Salord F, Boulieu R(2003)Pharmacokinetics and haemodynamics of ketamine in intensive care patients with brain or spinal cord injury. Br J Anaesth 90:155–160. [DOI] [PubMed] [Google Scholar]
- Ichise M, Liow JS, Lu JQ, Takano A, Model K, Toyama H, Suhara T, Suzuki K, Innis RB, Carson RE(2003)Linearized reference tissue parametric imaging methods: application to [11C]DASB positron emission tomography studies of the serotonin transporter in human brain. J Cereb Blood Flow Metab 23:1096–1112. [DOI] [PubMed] [Google Scholar]
- Innis RB, et al. (2007)Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 27:1533–1539. [DOI] [PubMed] [Google Scholar]
- Kapur S, Seeman P(2002)NMDA receptor antagonists ketamine and PCP have direct effects on the dopamine D(2) and serotonin 5-HT(2)receptors-implications for models of schizophrenia. Mol Psychiatry 7:837–844. [DOI] [PubMed] [Google Scholar]
- Kavalali ET, Monteggia LM(2015)How does ketamine elicit a rapid antidepressant response?Curr Opin Pharmacol 20:35–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kish SJ, Furukawa Y, Chang LJ, Tong J, Ginovart N, Wilson A, Houle S, Meyer JH(2005)Regional distribution of serotonin transporter protein in postmortem human brain: is the cerebellum a SERT-free brain region?Nucl Med Biol 32:123–128. [DOI] [PubMed] [Google Scholar]
- Kranz GS, Wadsak W, Kaufmann U, Savli M, Baldinger P, Gryglewski G, Haeusler D, Spies M, Mitterhauser M, Kasper S, Lanzenberger R(2015)High-dose testosterone treatment increases serotonin transporter binding in transgender people. Biol Psychiatry 78:525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus C, Rabl U, Vanicek T, Carlberg L, Popovic A, Spies M, Bartova L, Gryglewski G, Papageorgiou K, Lanzenberger R, Willeit M, Winkler D, Rybakowski JK, Kasper S(2017)Administration of ketamine for unipolar and bipolar depression. Int J Psychiatry Clin Pract 21:2–12. [DOI] [PubMed] [Google Scholar]
- Lanzenberger R, Baldinger P, Hahn A, Ungersboeck J, Mitterhauser M, Winkler D, Micskei Z, Stein P, Karanikas G, Wadsak W, Kasper S, Frey R(2013)Global decrease of serotonin-1A receptor binding after electroconvulsive therapy in major depression measured by PET. Mol Psychiatry 18:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lener MS, Niciu MJ, Ballard ED, Park M, Park LT, Nugent AC, Zarate CA Jr(2016)Glutamate and gamma-aminobutyric acid systems in the pathophysiology of major depression and antidepressant response to ketamine. Biol Psychiatry 81:886–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhu ZR, Ou BC, Wang YQ, Tan ZB, Deng CM, Gao YY, Tang M, So JH, Mu YL, Zhang LQ(2015)Dopamine D2/D3 but not dopamine D1 receptors are involved in the rapid antidepressant-like effects of ketamine in the forced swim test. Behav Brain Res 279:100–105. [DOI] [PubMed] [Google Scholar]
- Lundquist P, Wilking H, Hoglund AU, Sandell J, Bergstrom M, Hartvig P, Langstrom B(2005)Potential of [11C]DASB for measuring endogenous serotonin with PET: binding studies. Nucl Med Biol 32:129–136. [DOI] [PubMed] [Google Scholar]
- Martin DC, Introna RP, Aronstam RS(1990)Inhibition of neuronal 5-HT uptake by ketamine, but not halothane, involves disruption of substrate recognition by the transporter. Neurosci Lett 112:99–103. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Wilson AA, Ginovart N, Goulding V, Hussey D, Hood K, Houle S(2001)Occupancy of serotonin transporters by paroxetine and citalopram during treatment of depression: a [(11)C]DASB PET imaging study. Am J Psychiatry 158:1843–1849. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Wilson AA, Sagrati S, Hussey D, Carella A, Potter WZ, Ginovart N, Spencer EP, Cheok A, Houle S(2004)Serotonin transporter occupancy of five selective serotonin reuptake inhibitors at different doses: an [11C]DASB positron emission tomography study. Am J Psychiatry 161:826–835. [DOI] [PubMed] [Google Scholar]
- Moller HJ, Seemuller F, Schennach-Wolff R, Stubner S, Ruther E, Grohmann R(2014)History, background, concepts and current use of comedication and polypharmacy in psychiatry. Int J Neuropsychopharmacol 17:983–996. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, aan het Rot M, Collins KA, Mathew SJ, Charney DS, Iosifescu DV(2013)Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry 74:250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M, Sato K(1999)Ketamine stereoselectively inhibits rat dopamine transporter. Neurosci Lett 274:131–134. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Kent JM, Oquendo MA, Richards MC, Pratap M, Cooper TB, Arango V, Mann JJ(2006)Acute occupancy of brain serotonin transporter by sertraline as measured by [11C]DASB and positron emission tomography. Biol Psychiatry 59:821–828. [DOI] [PubMed] [Google Scholar]
- Perry EB Jr, Cramer JA, Cho HS, Petrakis IL, Karper LP, Genovese A, O’Donnell E, Krystal JH, D’Souza DC, Yale Ketamine Study G (2007)Psychiatric safety of ketamine in psychopharmacology research. Psychopharmacology (Berl) 192:253–260. [DOI] [PubMed] [Google Scholar]
- Pham TH, Mendez-David I, Defaix C, Guiard BP, Tritschler L, David DJ, Gardier AM(2017)Ketamine treatment involves medial prefrontal cortex serotonin to induce a rapid antidepressant-like activity in BALB/cJ mice. Neuropharmacology 112:198–209. [DOI] [PubMed] [Google Scholar]
- Radford KD, Park TY, Lee BH, Moran S, Osborne LA, Choi KH(2017)Dose-response characteristics of intravenous ketamine on dissociative stereotypy, locomotion, sensorimotor gating, and nociception in male Sprague-Dawley rats. Pharmacol Biochem Behav 153:130–140. [DOI] [PubMed] [Google Scholar]
- Romeo B, Choucha W, Fossati P, Rotge JY(2015)Meta-analysis of short- and mid-term efficacy of ketamine in unipolar and bipolar depression. Psychiatry Res 230:682–688. [DOI] [PubMed] [Google Scholar]
- Saulin A, Savli M, Lanzenberger R(2012)Serotonin and molecular neuroimaging in humans using PET. Amino Acids 42:2039–2057. [DOI] [PubMed] [Google Scholar]
- Savli M, Bauer A, Mitterhauser M, Ding YS, Hahn A, Kroll T, Neumeister A, Haeusler D, Ungersboeck J, Henry S, Isfahani SA, Rattay F, Wadsak W, Kasper S, Lanzenberger R(2012)Normative database of the serotonergic system in healthy subjects using multi-tracer PET. Neuroimage 63:447–459. [DOI] [PubMed] [Google Scholar]
- Spies M, Knudsen GM, Lanzenberger R, Kasper S(2015)The serotonin transporter in psychiatric disorders: insights from PET imaging. Lancet Psychiatry 2:743–755. [DOI] [PubMed] [Google Scholar]
- Spindelegger C, Lanzenberger R, Wadsak W, Mien LK, Stein P, Mitterhauser M, Moser U, Holik A, Pezawas L, Kletter K, Kasper S(2009)Influence of escitalopram treatment on 5-HT 1A receptor binding in limbic regions in patients with anxiety disorders. Mol Psychiatry 14:1040–1050. [DOI] [PubMed] [Google Scholar]
- Talbot PS, Frankle WG, Hwang DR, Huang Y, Suckow RF, Slifstein M, Abi-Dargham A, Laruelle M(2005)Effects of reduced endogenous 5-HT on the in vivo binding of the serotonin transporter radioligand 11C-DASB in healthy humans. Synapse 55:164–175. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M(2002)Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15:273–289. [DOI] [PubMed] [Google Scholar]
- Ungersboeck J, Philippe C, Haeusler D, Mitterhauser M, Lanzenberger R, Dudczak R, Wadsak W(2012)Optimization of [11C]DASB-synthesis: vessel-based and flow-through microreactor methods. Appl Radiat Isot 70:2615–2620. [DOI] [PubMed] [Google Scholar]
- Waelbers T, Polis I, Vermeire S, Dobbeleir A, Eersels J, De Spiegeleer B, Audenaert K, Slegers G, Peremans K(2013)5–HT2A receptors in the feline brain: 123I-5-I-R91150 kinetics and the influence of ketamine measured with micro-SPECT. J Nucl Med 54:1428–1433. [DOI] [PubMed] [Google Scholar]
- Wilson AA, Ginovart N, Schmidt M, Meyer JH, Threlkeld PG, Houle S(2001)Novel radiotracers for imaging the serotonin transporter by positron emission tomography: synthesis, radiosynthesis, and in vitro and ex vivo evaluation of (11)C-labeled 2-(phenylthio) araalkylamines. J Med Chem 44:280. [DOI] [PubMed] [Google Scholar]
- Xu AJ, Niciu MJ, Lundin NB, Luckenbaugh DA, Ionescu DF, Richards EM, Vande Voort JL, Ballard ED, Brutsche NE, Machado-Vieira R, Zarate CA Jr(2015)Lithium and valproate levels do not correlate with ketamine’s antidepressant efficacy in treatment-resistant bipolar depression. Neural Plast 2015:858251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Onoe H, Tsukada H, Watanabe Y(2007)Effects of increased endogenous serotonin on the in vivo binding of [11C]DASB to serotonin transporters in conscious monkey brain. Synapse 61:724–731. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Ohba H, Nishiyama S, Harada N, Kakiuchi T, Tsukada H, Domino EF(2013)Subanesthetic doses of ketamine transiently decrease serotonin transporter activity: a PET study in conscious monkeys. Neuropsychopharmacology 38:2666–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka H, Yokoyama C, Mizuma H, Kurai S, Finnema SJ, Halldin C, Doi H, Onoe H(2014)A possible mechanism of the nucleus accumbens and ventral pallidum 5-HT1B receptors underlying the antidepressant action of ketamine: a PET study with macaques. Transl Psychiatry 4:e342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Shirayama Y, Zhang JC, Ren Q, Yao W, Ma M, Dong C, Hashimoto K(2015)R-ketamine: a rapid–onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry 5:e632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK(2006)A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63:856–864. [DOI] [PubMed] [Google Scholar]
- Zarate CA Jr, Brutsche N, Laje G, Luckenbaugh DA, Venkata SL, Ramamoorthy A, Moaddel R, Wainer IW(2012)Relationship of ketamine’s plasma metabolites with response, diagnosis, and side effects in major depression. Biol Psychiatry 72:331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GF, Liu WX, Qiu LL, Guo J, Wang XM, Sun HL, Yang JJ, Zhou ZQ(2015)Repeated ketamine administration redeems the time lag for citalopram’s antidepressant-like effects. Eur Psychiatry 30:504–510. [DOI] [PubMed] [Google Scholar]
- Zhao X, Venkata SL, Moaddel R, Luckenbaugh DA, Brutsche NE, Ibrahim L, Zarate CA Jr, Mager DE, Wainer IW(2012)Simultaneous population pharmacokinetic modelling of ketamine and three major metabolites in patients with treatment-resistant bipolar depression. Br J Clin Pharmacol 74:304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Sun L(2008)Antidepressants modulate the in vitro inhibitory effects of propofol and ketamine on norepinephrine and serotonin transporter function. J Clin Neurosci 15:1264–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.