Abstract
Background
Major depressive disorder is a common condition that often includes cognitive dysfunction. A systematic literature review of studies and a network meta-analysis were carried out to assess the relative effect of antidepressants on cognitive dysfunction in major depressive disorder.
Methods
MEDLINE, Embase, Cochrane, CDSR, and PsychINFO databases; clinical trial registries; and relevant conference abstracts were searched for randomized controlled trials assessing the effects of antidepressants/placebo on cognition. A network meta-analysis comparing antidepressants was conducted using a random effects model.
Results
The database search retrieved 11337 citations, of which 72 randomized controlled trials from 103 publications met the inclusion criteria. The review identified 86 cognitive tests assessing the effect of antidepressants on cognitive functioning. However, the Digit Symbol Substitution Test, which targets multiple domains of cognition and is recognized as being sensitive to change, was the only test that was used across 12 of the included randomized controlled trials and that allowed the construction of a stable network suitable for the network meta-analysis. The interventions assessed included selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, and other non-selective serotonin reuptake inhibitors/serotonin-norepinephrine reuptake inhibitors. The network meta-analysis using the Digit Symbol Substitution Test showed that vortioxetine was the only antidepressant that improved cognitive dysfunction on the Digit Symbol Substitution Test vs placebo {standardized mean difference: 0.325 (95% CI = 0.120; 0.529, P=.009}. Compared with other antidepressants, vortioxetine was statistically more efficacious on the Digit Symbol Substitution Test vs escitalopram, nortriptyline, and the selective serotonin reuptake inhibitor and tricyclic antidepressant classes.
Conclusions
This study highlighted the large variability in measures used to assess cognitive functioning. The findings on the Digit Symbol Substitution Test indicate differential effects of various antidepressants on improving cognitive function in patients with major depressive disorder.
Keywords: major depressive disorder, vortioxetine, cognitive dysfunction, systematic literature review, network meta-analysis
Significance Statement
Cognitive dysfunction is a common impairment for patients with major depressive disorder (MDD) and leads to debilitating problems such as missed workdays, poor academic performance, and reduced ability to perform day-to-day tasks. Numerous studies have investigated cognitive function in depression in a variety of domains, including attention, processing speed, executive function, and memory. Nevertheless, there is limited evidence on comparative effectiveness of antidepressants on cognitive symptoms, mainly due to the diversity of tools used in clinical trials. This study compared the effects of antidepressants on a commonly used cognitive outcome, the Digit Symbol Substitution Test (DSST), and showed that vortioxetine had the largest improvement in DSST vs all investigated classes of single antidepressants. It was the only antidepressant demonstrating statistically significant improvement vs placebo and vs specific antidepressants. The findings support the effect of vortioxetine in improving cognitive function in MDD patients measured by the DSST.
Introduction
Background
Major depressive disorder (MDD) is one of the most common psychiatric disorders, affecting more than 350 million people worldwide (World Health Organization, 2016). MDD is characterized by psychological, physical, and behavioral symptoms that can be complex and vary widely between individuals. In general, patients with MDD experience a prolonged period of low mood often accompanied by low self-esteem, loss of interest in usually enjoyable activities, feelings of hopelessness, and low energy (World Health Organization, 2016). MDD exerts a substantial burden on the patient, including a negative impact on health-related quality of life (Daly et al., 2010; Fournier et al., 2013), impairments in multiple domains of cognitive function, premature mortality due to a range of physical disorders, and suicide in about 4% to 15% of patients (Seguin et al., 2006; Gonda et al., 2007; American Psychiatric Association, 2013). As of 2010, the World Health Organization has listed MDD as the second leading cause of disability worldwide, and it is expected to become the leading cause of disease burden in high-income countries by 2030 (Mathers and Loncar, 2006; Ferrari et al., 2013).
Patients with MDD often experience impairment in cognitive function in several domains, including executive functioning, processing speed, concentration/attention, learning, and memory (Porter et al., 2007; Hammar and Ardal, 2009; Baune et al., 2010; Beblo et al., 2011; National Academies of Sciences, 2015). Patients with MDD may experience cognitive impairments not only before and during depressive episodes but also after remission of mood symptoms (Baune et al., 2010; Papakostas and Culpepper, 2015). A 3-year prospective study of 267 patients found that cognitive problems were present 94% of the time during depressive episodes and 44% of the time during remission (Conradi et al., 2011). In addition to the burden for patients, cognitive dysfunction in mood disorders including depression is also associated with economic and psychosocial consequences (Baune et al., 2010, 2013; Baune and Malhi, 2015). These impairments may lead to debilitating problems for patients such as missed workdays, poor academic performance, and a reduced ability to carry out day-to-day tasks (McIntyre et al., 2015). This, in turn, can lead to elevated costs due to absenteeism and reduced productivity, which are the main drivers of the economic burden due to MDD (Marazziti et al., 2010; National Academies of Sciences, 2015). Cognitive dysfunction is increasingly becoming recognized as an important symptom dimension of MDD. The Diagnostic and Statistical Manual 5 lists impairment in cognition (i.e., diminished ability to think or concentrate, or indecisiveness) as a diagnostic criterion for MDD (American Psychiatric Association, 2013). In addition, cognitive dysfunction has recently been identified by the Food and Drug Administration as a target for pharmacological treatments in patients with MDD (Food and Drug Administration, 2016).
Many clinical studies of antidepressants have shown improvements in cognition; however, there are several unanswered questions, including whether certain classes of antidepressants are superior to others in improving neuropsychological function. Studies suggest that serotonin norepinephrine reuptake inhibitors (SSRIs), including duloxetine and other antidepressants such as vortioxetine, bupropion, and moclobemide, may improve cognitive function in depression (Baune and Renger, 2014). Vortioxetine, a novel antidepressant with multimodal activity, has shown evidence of cognitive benefit in animal models (Wallace et al., 2014; Sanchez et al., 2015) as well as in patients with MDD (Katona et al., 2012; McIntyre et al., 2014; Mahableshwarkar et al., 2015). Clinical effect of vortioxetine on cognitive function is reported in the product characteristics in many countries, including in Europe. In addition, a recent meta-analysis by McIntyre et al. included 3 randomized controlled trials (RCTs) and showed that vortioxetine significantly improved cognition measured by the Digit Symbol Substitution Test (DSST), independent of changes in overall depressive symptoms (McIntyre et al., 2016).
Numerous studies have investigated cognitive function in depression in a variety of cognitive domains, including attention, processing speed, executive function, and memory. Nevertheless, there is limited evidence on the comparative effectiveness of antidepressants on cognitive symptoms, mainly due to the diversity of tools used in clinical trials creating heterogeneous outcomes. At least 3 recent reviews were conducted to investigate the effects of antidepressants on cognitive dysfunction (Baune and Renger, 2014; Keefe et al., 2014; Rosenblat et al., 2016). These reviews highlighted the fact that antidepressants may reduce cognitive dysfunction in MDD. However, the variability in the study design and the high level of heterogeneity of cognitive tests are important limitations in assessing the relative effect of antidepressants on cognitive dysfunction in MDD.
Objectives
The objective of this study was to assess the comparative effect of a variety of antidepressants on cognitive dysfunction, as measured by DSST, in patients with MDD through a systematic literature review and a network meta-analysis (NMA). The DSST is the most extensively used and validated cognitive test in neuropsychology (Jaeger and Zaragoza Domingo, 2016). The current analysis presents the results of the comparison of various classes and single antidepressants vs placebo on improving cognitive dysfunction as assessed on the DSST.
Methods
Systematic Literature Review
A systematic literature review (SLR) was carried out to appraise the clinical evidence currently available, focusing on RCTs for interventions treating cognitive dysfunction in adult patients with MDD. Studies were obtained from a comprehensive search of Embase, MEDLINE, MEDLINE In-Process, Cochrane Central Register of Controlled Trials (CENTRAL), Cochrane Database for Systematic Reviews (CDSR), and PsychINFO, from database inception date to 13 November 2014. Hand-searching of conference abstracts and trial registries (American Psychiatric Association, International College of Neuropsychology, European College of Neuropsychopharmacology, International College of Neuropsychopharmacology, Clinical.trials.gov, and European Union Clinical Trials Register) was also conducted to retrieve clinical studies that are unpublished in journals as full-text articles or supplement results of previously published studies.
To be included in this review, trials had to meet the predefined eligibility criteria provided in Table 1. The review focused on evidence from RCTs assessing the impact of antidepressants or placebo on cognitive dysfunction in adult patients with MDD, with no restrictions on gender, race, or publication language. Studies that evaluated the effect of the interventions included in Table 1 were eligible for inclusion.
Table 1.
Eligibility Criteria for Trials to Be Included in the SLR
| Parameter | Inclusion/exclusion criteria in current review | |
|---|---|---|
| Patient population | • Age: adult patients (≥18 years of age) • Gender: any • Race: any • Disease: major depressive disorder |
|
| Study design | • RCTs (irrespective of blinding status) • Comparative controlled trials (including nonrandomized studies, retrospective and prospective controlled cohort studies) will be included during screening stage to supplement the RCTs in case of limited evidence |
|
| Intervention |
Pharmacological interventions
• SSRIs (citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline) • SNRIs (desvenlafaxine, duloxetine, venlafaxine, milnacipran, levomilnacipran) • TCAs (desipramine, imipramine, clomipramine, nortriptyline, tianeptine, dothiepin, opipramol, trimipramine, lofepramine, dibenzepin, amitriptyline, protriptyline, doxepin, melitracen, butriptyline, dimetacrine, quinupramine) • TeCA (mirtazapine, maprotiline, mianserin, amoxapine) • MAOI (moclobemide, isocarboxazid, tranylcypromine, phenelzine, toloxatone) Nonpharmacological interventions • Cognitive therapy/remediation therapy • Exercise therapy |
• Other antidepressants o Bupropion o Reboxetine o Viloxazine o Trazodone o Vortioxetine o Etoperidone o Nefazodone o Bifemelane o Agomelatine o Vilazodone Alternative therapy • Diet therapy • S-adenosylmethionine • Vitamins • Omega 3 fatty acid • Tryptophan • 5-hydroxytryptophan • Hypericum perforatum |
| Effect on cognition and cognitive impact assessment | • Studies evaluating the effect of above listed interventions on cognition in MDD patients were included • Studies that assess the impact of cognitive dysfunction on patient’s daily functioning, work productivity, and quality of life were also of interest in the review |
|
| Comparator | • Any of the above included interventions • Placebo/best supportive care • Any other pharmacological/nonpharmacological therapy |
|
| Publication timeframe | • From database inception till 13 November 2014 | |
| Language | • English language articles | |
Abbreviations: MAOI, monoamine oxidase inhibitor; RCT, randomized controlled trial; SNRI, serotonin norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant; TeCA, tetracyclic antidepressant.
A first-pass screening of each citation was conducted based on the abstracts. Citations that did not match the eligibility criteria and duplicates (due to overlap in the coverage of the databases) were excluded at the first-pass stage. Full-text copies of all included references were then assessed, and citations that did not match the eligibility criteria were excluded at the second-pass screening.
During data extraction, publications describing the same trial were compiled into a single entry to avoid double counting of patients and studies. Data were extracted on methodological and clinical characteristics of the studies, including sample size, age, gender, race, disease duration, disease severity, interventions assessed, study duration, and assessment time points. Both stages of screening and the data extraction were carried out by 2 independent reviewers, and any discrepancies between reviewers were reconciled by a third independent reviewer.
Studies were critically appraised using comprehensive assessment criteria based on the recommendations in the NICE guidelines (NICE, 2013) according to 7 categories: (1) statistical analyses, (2) outcome selection and reporting, (3) withdrawals, (4) blinding, (5) baseline comparability, (6) allocation concealment, and (7) randomization.
Evidence Network Development
A feasibility assessment was carried out to determine possible approaches for developing an NMA to compare the comparative effect of antidepressants on cognitive dysfunction. Two approaches were considered: (1) Assessing studies evaluating the same cognitive test, irrespective of cognitive domain; and (2) assessing studies evaluating the same cognitive domain, irrespective of the test. The second approach was not pursued due to the lack of consensus for categorizing cognitive symptoms into cognitive domains. In addition, considering the large variety of 86 cognitive tests used as endpoints in the reviewed studies, collapsing these into domain-specific groups would introduce a validity bias, since different cognitive tests measure different cognitive abilities, even within the same cognitive domain. Moreover, because certain individual tests would qualify to be assigned to different cognitive domains, this would introduce uncertainty when interpreting the results. To overcome several of these problems and allow for the generation of a stable NMA, the first approach evaluating studies with a common cognitive test was chosen for the NMA.
Across the 72 included RCTs, 86 different cognitive tests were used to assess the effect of antidepressants on cognitive dysfunction in patients with MDD. Most of the tests were used in only one study. A total of 12 tests were reported in 4 or more studies, with the Mini-Mental State Examination (MMSE) and DSST being the most commonly reported outcomes (13 studies each). Even across studies that evaluated cognitive function using the same test, there were variations in reporting outputs. For example, some studies reported the mean score as an endpoint, while others reported either the percentage of correct answers or a time estimate. Additionally, some studies reported different and/or multiple domains for the same test, and there was variation in whether the cognitive endpoint was a primary or secondary outcome. The MMSE is considered to be a poor choice to measure cognitive function in MDD, because it broadly measures global cognitive function, has no alternate form, and has extreme ceiling effects (Keefe et al., 2014). In addition, the MMSE is most commonly used for evaluating cognition in late-life depression due to evidence of its validity for dementia (Rajji et al., 2009). Furthermore, the MMSE did not allow for a comparison of all antidepressant drug classes. Other tests were also not appropriate for the network in our analyses: Stroop, Trail Making Test A (TMT A) and Trail Making Test B (TMT B), and Rey Auditory Verbal Learning Test (RAVLT) were each reported in only 8 to 9 trials, and their networks were all smaller than the DSST network. The networks of these 4 tests decomposed into 2, 4, 3, and 4 subnetworks for Stroop, TMT A and TMT B, and RAVLT, respectively. Since these networks decomposed into small subnetworks, only a limited comparison of 1 to 3 (depending on the subnetwork) other antidepressants vs placebo, duloxetine, and vortioxetine was possible. In contrast, for the DSST, it was possible to construct a single “connected” network that allowed for multiple comparisons across various antidepressants as shown in the Results. As a conclusion, the subsequent analyses and results are presented for the NMA using published clinical trials that utilized the DSST.
The DSST is a “pencil and paper” cognitive test that assesses several aspects of the cognitive function that are most impaired in patients with MDD, such as components of executive function, processing speed, attention, and working memory. It is recognized as being sensitive to change during effective treatment of MDD (Jaeger and Zaragoza Domingo, 2016; McIntyre et al., 2016). The DSST is sensitive to both the presence of cognitive dysfunction and the change in cognitive function across a wide range of clinical populations. Due to its brief administration time and high discriminant (known group) validity, the DSST is a frequently used test that allows the opportunity to benchmark clinical effects. DSST performance is one of the most robust predictors of outcomes in patients with severe illness and has been shown to correlate with functional outcomes.
NMA
To simultaneously assess the comparative effects of more than 2 treatments, an NMA was performed. An NMA synthesizes direct and indirect comparisons over an entire network of treatments, allowing for all available evidence to be considered in one analysis. Based on the network development process as outlined above, the outcome variable for the NMA was the standardized mean change in the DSST (measured using Hedge’s G) from baseline to end of study. The standardization was based on the pooled (across treatment arms within study) estimate of the SDs. The NMA was carried out using a frequentist’s approach, and a 2-way ANOVA model was used. As the residual variances between treatment groups are known, it was possible for random effect estimates to be produced, which account for the between-trial heterogeneity. The model was first used to perform ordinary pairwise meta-analysis comparing the antidepressants to placebo based on direct evidence from the clinical studies. Secondly, for the NMA, 2 networks were developed: one network by drug class and another by type of antidepressant. Ranking probabilities were calculated based on the joint distribution of the estimates of relative efficacy.
Consistency was addressed through the principle of node-splitting by using a network meta-regression model. The purpose of node-splitting is to investigate if the relative effect of 2 treatments based on direct comparisons is comparable with the same effect based on indirect comparisons. Statistically, the model is an extension of the NMA, which allows for a different relative effect between the 2 treatments that are being split in head-to-head trials compared with all other trials.
Results
SLR
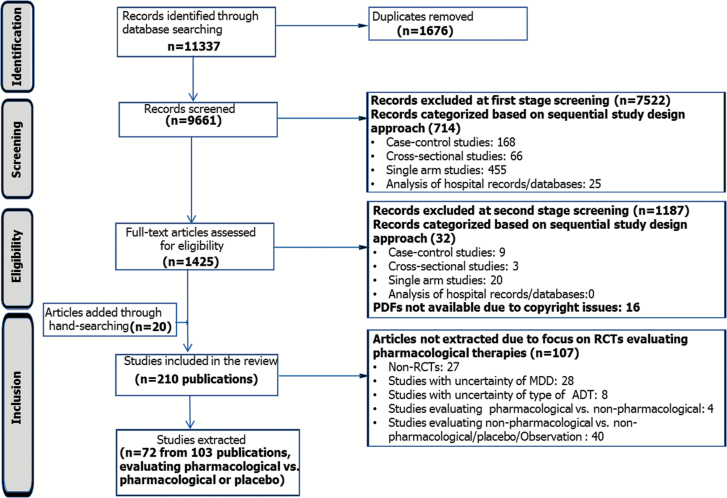
Figure 1 shows the flow of studies through the systematic review process. Searching of literature databases yielded 11337 references. Due to the overlap of coverage between the databases, 1676 abstracts were found to be duplicates and were removed. First-stage screening of the citations identified 1425 potentially relevant references based on their titles/abstracts. Full-text reports of these citations were obtained for more detailed evaluation, following which 190 references remained. Hand-searching identified 20 additional relevant citations, resulting in a total of 210 publications prior to extraction.
Figure 1.
Flow diagram for the identification and selection of studies. Note: 12 studies that assessed Digit Symbol Substitution Test (DSST) were included in the final network of evidence. Abbreviations: ADT, antidepressant therapy; MDD, major depressive disorder; RCT, randomized controlled trial.
The review focused on RCTs assessing pharmacological interventions, as RCTs are considered the gold standard of clinical evidence and they minimize the risk of confounding factors. Of the 210 references screened, 72 RCTs from 103 publications were identified based on prespecified eligibility criteria and included for data extraction.
The interventions assessed across the studies included SSRIs (citalopram, escitalopram, fluoxetine, paroxetine, sertraline, or fluvoxamine), serotonin and norepinephrine reuptake inhibitors (SNRIs) (duloxetine, venlafaxine, desvenlafaxine, or levomilnacipran), monoamine oxidase inhibitors (phenelzine or tranylcypromine), tricyclic antidepressants (desipramine, amitriptyline, imipramine, trimipramine, or tianeptine), tetracyclic antidepressants (mianserin or mirtazapine), or non-SSRI/SNRI antidepressants (agomelatine, bupropion, reboxetine, or vortioxetine).
DSST As the Single Cognitive Measure for Network Development
Although there was large variation in the cognitive measures used in the RCTs, the DSST was the only cognitive endpoint from the reviewed studies that could be used as a test of cognitive dysfunction for developing a homogeneous and “stable” network of evidence to compare various antidepressants (see Methods). One study was excluded in the absence of a common link with the other antidepressants, resulting in 12 studies (3738 patients) in the final network (Tignol et al., 1998). DSST was the primary endpoint in 2 of the trials included in the network, and these both assessed vortioxetine (McIntyre et al., 2014; Mahableshwarkar et al., 2015); all other studies in the network assessed DSST as a secondary outcome.
The total number of patients in the RCTs where the DSST was employed as a primary or secondary cognitive endpoint ranged from 27 to 602, the mean age ranged from 36.6 to 79.6 years, and the percentage of males ranged from 24% to 58%. The time of DSST assessment in the studies included in the network varied from 3 to 24 weeks after baseline assessment. The antidepressants assessed (with analyzable number of patients in brackets) were SNRIs (duloxetine [707 patients]), SSRIs (citalopram [84 patients], escitalopram [54 patients], fluoxetine [127 patients], sertraline [240 patients]), MAOIs (phenelzine [28 patients]), tricyclic antidepressants (TCAs) (desipramine [9 patients], nortriptyline [102 patients]), and non-SSRI/SNRIs (vortioxetine [725 patients]). Vortioxetine and duloxetine have the most subjects in which cognition was assessed by the DSST in clinical trials. The majority (9 of 12) of the studies included a placebo control. The studies included in the DSST network of evidence and their characteristics are presented in Supplementary Table S1.
Critical appraisal of the included studies was conducted using comprehensive assessment criteria based on the recommendations in the NICE guidelines (NICE, 2013). The studies were generally of good quality with around 4 of the 7 categories being assessed as low risk on average. The best scoring category was the baseline comparability (83% low risk) and the worst scoring category was outcome selection and reporting (25% low risk). The FOCUS trial was the best quality, with all 7 aspects of the assessment deemed to be low risk (McIntyre et al., 2014). However, the overall risk of bias was unclear in the majority of the categories for 5 of the 12 studies. A summary of the qualitative assessment is provided in Supplementary Table S2.
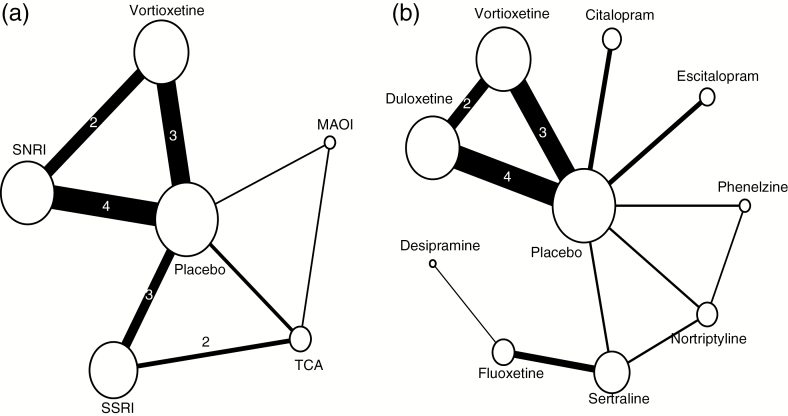
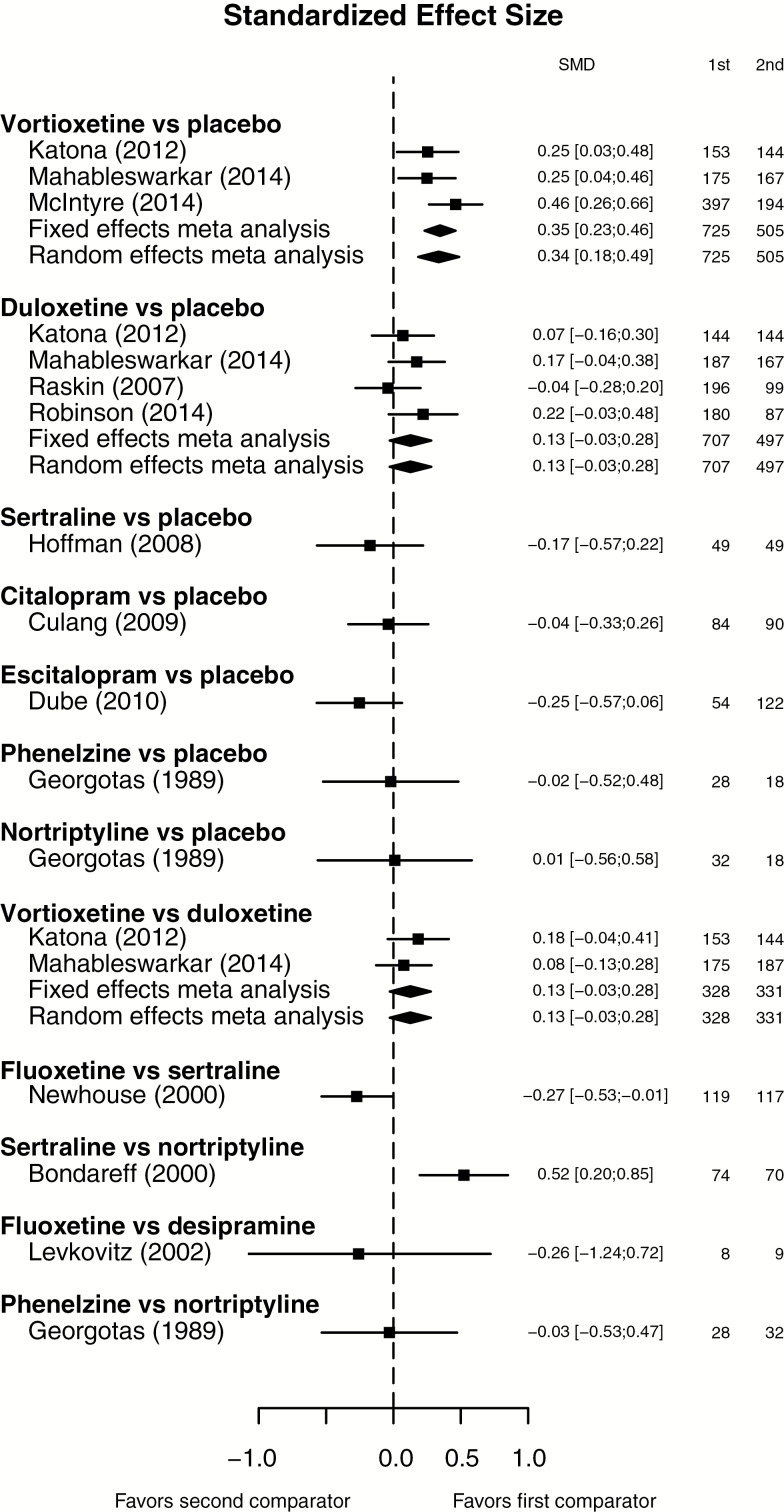
As a result, 2 networks for the studies using the DSST were developed: one network by drug class and another by type of antidepressant. The network diagrams in Figure 2 provide a graphical representation of how each intervention is connected to the others through direct comparisons. Each line depicts a direct comparison between 2 intervention nodes. A forest plot showing the standardized mean differences for each study in direct comparisons, based on data reported in each of the included studies, is shown in Figure 3.
Figure 2.
Network for the (a) by-class analysis and (b) by-treatment analysis. Note: The size (area) of the nodes is proportional to the number of patients on treatment. The width of the lines is proportional to the number of patients in trials with direct comparison between the nodes. The numbers on the lines indicate the number of trials with direct comparisons, if it is more than one. Abbreviations: MOAI, monoamine-oxidase inhibitor; SNRI, serotonin and norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant.
Figure 3.
Standardized mean differences in Digit Symbol Substitution Test (DSST) of antidepressants based on direct evidence from clinical studies included in the network of evidence. Abbreviations: SMD, standardized mean difference.
NMA
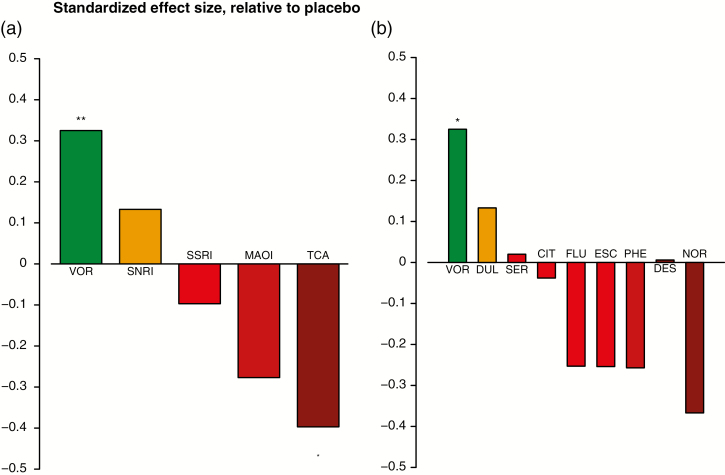
Figure 4 shows the standardized mean differences on DSST comparing antidepressant classes as well as individual antidepressants vs placebo. In the by-class analysis (Figure 4a), SSRIs, MAOIs, and TCAs showed a smaller effect on DSST vs placebo, with TCAs showing a significantly worse effect on DSST than placebo. Vortioxetine and SNRIs were the only antidepressant classes showing an improvement in DSST vs placebo, and this difference was statistically significant in the comparison of vortioxetine vs placebo.
Figure 4.
Standardized mean difference vs placebo (a) by-class analysis and (b) by-treatment analysis. Abbreviations: CIT, citalopram; DES, desipramine; DUL, duloxetine; ESC, escitalopram; FLU, fluoxetine; MOAI, monoamine-oxidase inhibitor; NOR, nortriptyline; PHE, phenelzine; SER, sertraline; SNRI, serotonin and norepinephrine reuptake inhibitors; SSRI, selective serotonin reuptake inhibitors; TCA, tricyclic antidepressants; VOR, vortioxetine. *P<.05; ** P<.01.
When comparing individual antidepressants vs placebo, vortioxetine, duloxetine and sertraline showed an improvement in the DSST vs placebo, with vortioxetine being the only antidepressant showing a statistically significant difference. The standardized mean difference on the change in the DSST from baseline for vortioxetine vs placebo was 0.325 [95% CI=0.120; 0.529, P=.009]. The differences for duloxetine and sertraline vs placebo were not statistically significant. All other antidepressants (citalopram, desipramine, escitalopram, fluoxetine, nortriptyline, and phenelzine) demonstrated a smaller effect on cognitive dysfunction vs placebo (Figure 4b).
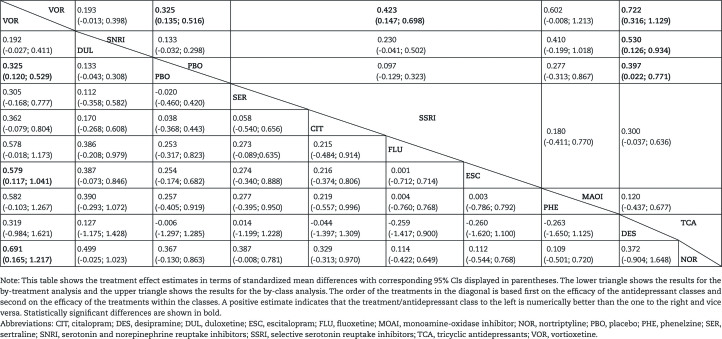
Table 2 shows the comparative effects between antidepressants in terms of standardized mean differences in the DSST for both the treatment class analysis (upper triangle) and the individual treatment analysis (lower triangle). The order of the treatments in the diagonal is based on the efficacy of the treatment classes as well as the individual antidepressants. The 2 most efficacious antidepressants in terms of improvement in the DSST were vortioxetine and duloxetine, but duloxetine was not statistically significantly different from placebo. Placebo was the third most efficacious, highlighting the fact that many of the antidepressants have less impact on cognitive function vs placebo.
Table 2.
Treatment Effect Estimates (standardized mean differences of DSST change from baseline)
The analysis showed that vortioxetine was numerically more efficacious in terms of change in DSST from baseline than all other antidepressants included in the analysis. The difference in DSST was statistically significant for vortioxetine vs SSRIs and TCAs with standardized mean differences of 0.423 [95% CI=0.147; 0.698, P=.006] and 0.722 [95% CI=0.316; 1.129, P=.002], respectively. In the by-treatment analysis, vortioxetine was statistically significantly better than escitalopram and nortriptyline with standardized mean differences of 0.579 [95% CI=0.117; 1.041, P=.021] and 0.691 [95% CI=0.165; 1.217, P=.017], respectively. Moreover, based on the by-class ranking analysis, the probability of vortioxetine having a higher change in DSST from baseline than all other classes of antidepressants (including placebo) is 97%.
Consistency was addressed by node-splitting. Due to the size of the network, the risk of inconsistency was relatively small, and only 2 potential loops were identified in each of the by-treatment and by-class analyses. Although there were mild inconsistencies in the vortioxetine/SNRI and the placebo/SSRI/TCA comparisons, none were considered significant. Heterogeneity was mainly driven by the vortioxetine/placebo comparison. This was accounted for by using a random effects model for estimating both relative mean differences and their CIs in the NMA.
Discussion
Cognitive dysfunction is a well-known impairment associated with MDD and causes a significant additional burden to patients and society. The effects of antidepressants on cognitive function are still not fully understood, but cognitive dysfunction has recently been identified by the FDA as a target for pharmacological treatments in patients with MDD and is increasingly becoming of clinical importance.
In the current review, 72 RCTs assessing cognitive function using 86 different cognitive measures in MDD were identified. Of these 72 RCTs and 68 cognitive measures, 12 studies were included in the network of evidence for evaluating the effect of antidepressants. A single measure of cognitive function, the DSST, was used for the NMA based on results of the network development process. The findings of the NMA showed that vortioxetine, duloxetine, sertraline, and the SNRI class improved cognitive function measured with the DSST vs placebo, with vortioxetine being the only antidepressant or non-SSRI/SNRI showing a statistically significant effect vs placebo. All other antidepressants or classes of antidepressants included in the analysis demonstrated no effect on the DSST. The comparative analysis showed that vortioxetine was statistically significantly more efficacious in terms of change in the DSST from baseline compared with escitalopram and nortriptyline. Placebo was the third most efficacious, further highlighting the fact that many of the antidepressants have less impact on the DSST than placebo.
These findings confirm previous research by showing that some antidepressants improve cognitive function, and it also highlights that many antidepressants and classes of antidepressants may have less impact on cognition than placebo (Baune and Renger, 2014; Keefe et al., 2014; McIntyre et al., 2016; Rosenblat et al., 2016). Vortioxetine showed the largest improvement on the DSST, which is in line with the results of previous studies (McIntyre et al., 2016; Rosenblat et al., 2016). In addition, the current analysis showed that vortioxetine was the only antidepressant that demonstrated a statistically significant improvement on the DSST vs placebo. The European Medicines Agency has also recognized vortioxetine’s improvement on the DSST, stating that it has a statistically significant effect vs placebo according to 2 studies and a meta-analysis (European Medicines Agency, 2016).
The magnitude of the cognitive deficit in MDD is typically between 0.2 and 0.7 standardized mean differences below what would be normal, depending on disease state and cognitive domain (Rund et al., 2006; Lee et al., 2012; Rock et al., 2014). For comparison, cognitive dysfunction in disorders such as Alzheimer’s dementia is greater than in MDD and usually up to several SDs greater than what would be considered normal (Buchanan et al., 2011). A meta-analysis of 22 studies in patients with MDD showed that patients were impaired on the DSST with a standardized mean difference of 0.55 compared with healthy controls. The DSST was shown to be strongly associated with the level of functioning at work, school, and home. These findings suggest that the DSST provides an effective means to detect clinically relevant treatment effects of antidepressants on important components of cognitive function in patients with MDD (Jaeger and Zaragoza Domingo, 2016).
Vortioxetine’s statistically significant improvement on the DSST is likely to be due to its unique pharmacological profile vs other antidepressants. These mechanisms include increased glutamate neurotransmission (via inhibition of gamma-aminobutyric acid interneurons expressing 5-HT3 heteroreceptors) and neuroplasticity in brain regions such as hippocampus and prefrontal cortex (Haddjeri et al., 2012; Riga et al., 2013; Sanchez et al., 2015; Pehrson et al., 2015). For example, vortioxetine significantly enhances excitatory synaptic transmission and neuroplasticity (increased cell proliferation and maturation) compared with SSRIs (Dale et al., 2014). In addition, cognitive improvements with vortioxetine may be due to direct and/or indirect effects via serotonergic, noradrenergic, cholinergic, dopaminergic, and histaminergic systems (Elmaadawi et al., 2015; Sanchez et al., 2015). Further research is needed to understand if pharmacological differences translate into differential effects on cognition.
To the best of our knowledge, the current NMA included the largest network of evidence published to date in assessing the impact of antidepressants on cognitive function in MDD and significantly extends a recently published meta-analysis that included only 3 RCTs (McIntyre et al., 2016). Importantly, none of the previous publications have quantitatively assessed the comparative effects of antidepressant classes, and therefore this analysis provides new insights into the effects of the classes as well as the individual antidepressants. The strengths of our analysis include a robust and thorough SLR obtaining high-quality RCTs for inclusion in the analysis and consideration of heterogeneity within the NMA by using random effects models. In addition, the important issue of consistency between direct and indirect effects in the network was also addressed. Although the SLR was initially carried out in November 2014, an additional search was carried out in October 2016, which found no new studies assessing the effects on cognition using the DSST.
There are also some limitations of the current analysis. The evidence retrieved from the SLR suggests that there is ample clinical data evaluating the effect of antidepressants on cognitive functioning. However, due to the lack of defined clinical recommendations for the management of cognitive dysfunction in patients with MDD, a high variability is observed in reporting of cognitive outcomes. There are also several methodological constraints in the studies with regards to large variability in the outcomes, domains, time points of assessments, reporting of outputs, and patient numbers. These variations limit the generalizability of the results and caution the interpretation. In particular, vortioxetine and duloxetine had the most patients included in the trials in the network (725 and 707, respectively), whereas the other antidepressants had between 9 and 240 patients. Although a lower number of patients treated with a specific compound does not in itself bias the relative effect of that compound, it will decrease the likelihood of finding a significant difference between that compound and any of the other compounds, because fewer patients means wider CIs in the results of the NMA. It should also be noted that vortioxetine is very clearly significantly better than placebo, due to the relatively large number of patients in trials with both vortioxetine and placebo. One of the reasons for vortioxetine showing a significant difference vs other antidepressants (despite the relatively few patients in the trials of the other antidepressants) is the generally poor performance of the SSRIs and TCAs. In addition, the DSST was only a primary endpoint in 2 of the trials, and 3 of the studies did not include a placebo control arm.
Across the 72 RCTs identified in our review, 86 different cognitive tests were employed and hence 2 possible approaches for the NMA were considered: assessing studies that used a common cognitive test or a common cognitive domain. It was decided that the network of evidence would be developed using the DSST as a common single cognitive test to reduce the amount of variability between studies as much as possible. The feasibility of the alternative approach, using a common cognitive domain, was investigated but was not pursued due to the lack of standardization for classifying the different symptoms into commonly accepted domains. It was also considered whether multiple tests could be included in the network; however, it was not considered prudent to do so, because different tests measure different aspects of cognitive function and may span across various cognitive domains. In addition, the studies that could have been included would all sit as “appendices” to the network, and there would be no change to the estimates of the already included studies. Further, the additions would each be connected to the network only through one trial, which yields very wide CIs, and hence it was not deemed reasonable to introduce more than one test in the NMA in this paper. As a consequence, our results have to be interpreted as an effect on the DSST only, and results should not be generalized to other cognitive tests during interpretation.
An important limitation of the underlying RCTs is the large variability of the reported cognitive outcomes. Although there is an abundance of studies exploring the effects of antidepressants on cognition, the heterogeneity of cognitive tests and outcomes used limits the analysis that can be performed in a meta-analysis such as ours. The advantage of selecting the DSST as a single cognitive test for the NMA is that like-for-like comparisons between treatments could be made and that a “stable” network was generated for the DSST; however, the selection of the DSST also means that a smaller number of RCTs was used in the analysis. Further research using other cognitive scales is needed in the future, and recommendations for using a standardized cognitive test battery would be highly useful for future clinical research and would help to overcome some of the limitations of this type of research. In addition, further research into the effects within different subpopulations, for example based on age and gender, would be valuable.
In summary, although some antidepressants have shown improvements in cognitive function in patients with MDD, the majority of antidepressants have not shown an effect on cognition. Comparing the effects of a large group of antidepressants across classes on single cognitive measures, the DSST indicated that vortioxetine was the only antidepressant that exerted statistically significant effects on the DSST between baseline and follow-up when compared with both placebo and all other antidepressants analyzed. Further research is needed to overcome the limitations associated with the large amount of heterogeneity of cognitive measures in MDD, and future analyses would benefit from a standardized cognitive test battery in MDD.
Funding
This work was supported by H. Lundbeck A/S and Takeda Pharmaceutical Company, Ltd.
Statement of Interest
Mélanie Brignone was an employee of Lundbeck SAS at time of study and Klaus Groes Larsen is an employee of H. Lundbeck A/S. Professor Baune is a member of advisory boards and/or gave presentations for the following companies: AstraZeneca, Lundbeck, Pfizer, Servier, and Wyeth. He receives funding from the National Health and Medical Research Council, Australia.
Supplementary Material
Acknowledgments
Assistance with manuscript preparation was provided by PAREXEL and was paid for by the Takeda Pharmaceutical Company, Ltd and H. Lundbeck A/S.
References
- American Psychiatric Association (2013)Diagnostic and statistical manual of mental disorders, fifth edition Washington, DC. [Google Scholar]
- Baune BT, Li X, Beblo T(2013)Short- and long-term relationships between neurocognitive performance and general function in bipolar disorder. J Clin Exp Neuropsychol 35:759–774. [DOI] [PubMed] [Google Scholar]
- Baune BT, Malhi GS(2015)A review on the impact of cognitive dysfunction on social, occupational, and general functional outcomes in bipolar disorder. Bipolar Disord 17:41–55. [DOI] [PubMed] [Google Scholar]
- Baune BT, Miller R, McAfoose J, Johnson M, Quirk F, Mitchell D(2010)The role of cognitive impairment in general functioning in major depression. Psychiatry Res 176:183–189. [DOI] [PubMed] [Google Scholar]
- Baune BT, Renger L(2014)Pharmacological and non-pharmacological interventions to improve cognitive dysfunction and functional ability in clinical depression--a systematic review. Psychiatry Res 219:25–50. [DOI] [PubMed] [Google Scholar]
- Beblo T, Sinnamon G, Baune BT(2011)Specifying the neuropsychology of affective disorders: clinical, demographic and neurobiological factors. Neuropsychol Rev 21:337–359. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Keefe RS, Umbricht D, Green MF, Laughren T, Marder SR(2011)The FDA-NIMH-MATRICS guidelines for clinical trial design of cognitive-enhancing drugs: what do we know 5 years later?Schizophr Bull 37:1209–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradi HJ, Ormel J, de JP(2011)Presence of individual (residual) symptoms during depressive episodes and periods of remission: a 3-year prospective study. Psychol Med 41:1165–1174. [DOI] [PubMed] [Google Scholar]
- Dale E, Zhang H, Leizer SC, Xiao Y, Lu D, Yang CR, Plath N, Sanchez C(2014)Vortioxetine disinhibits pyramidal cell function and enhances synaptic plasticity in the rat hippocampus. J Psychopharmacol 28:891–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly EJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Gaynes BN, Warden D, Morris DW, Luther JF, Farabaugh A, Cook I, Rush AJ(2010)Health-related quality of life in depression: a STAR*D report. Ann Clin Psychiatry 22:43–55. [PubMed] [Google Scholar]
- Elmaadawi A, Singh N, Reddy J(2015)Prescriber’s guide to using 3 new antidepressants: vilazodone, levomilnacipran, vortioxetine. Current Psychiatry 14:28–29, 32–26. [Google Scholar]
- European Medicines Agency (2016)Brintellix summary of product characteristics. [Google Scholar]
- Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJ, Vos T, Whiteford HA(2013)Burden of depressive disorders by country, sex, age, and year: findings from the global burden of dizease study 2010. PLoS Med 10:e1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration (2016)Psychopharmacologic Drugs Advisory Committee: (PDAC) Meeting. [Google Scholar]
- Fournier JC, Keener MT, Almeida J, Kronhaus DM, Phillips ML(2013)Amygdala and whole-brain activity to emotional faces distinguishes major depressive disorder and bipolar disorder. Bipolar Disord 15:741–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda X, Fountoulakis KN, Kaprinis G, Rihmer Z(2007)Prediction and prevention of suicide in patients with unipolar depression and anxiety. Ann Gen Psychiatry 6:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddjeri N, Etievant A, Pehrson AL, Sanchez C, Betry C(2012)Effects of the multimodal antidepressant Lu AA21004 on rat synaptic and cellular hippocampal plasticity and memory recognition. In: European Neuropsychopharmacology 22:S303. [Google Scholar]
- Hammar A, Ardal G(2009)Cognitive functioning in major depression--a summary. Front Hum Neurosci 3:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger J, Zaragoza Domingo S(2016)The digit symbol substitution test (DSST): psychometric properties and clinical utility in major depressive disorder. Abstract presented at the 29th ECNP Congress, Vienna, Austria. [Google Scholar]
- Katona C, Hansen T, Olsen CK(2012)A randomized, double-blind, placebo-controlled, duloxetine-referenced, fixed-dose study comparing the efficacy and safety of Lu AA21004 in elderly patients with major depressive disorder. Int Clin Psychopharmacol 27:215–223. [DOI] [PubMed] [Google Scholar]
- Keefe RS, McClintock SM, Roth RM, Doraiswamy PM, Tiger S, Madhoo M(2014)Cognitive effects of pharmacotherapy for major depressive disorder: a systematic review. J Clin Psychiatry 75:864–876. [DOI] [PubMed] [Google Scholar]
- Lee RS, Hermens DF, Porter MA, Redoblado-Hodge MA(2012)A meta-analysis of cognitive deficits in first-episode Major Depressive Disorder. J Affect Disord 140:113–124. [DOI] [PubMed] [Google Scholar]
- Mahableshwarkar AR, Zajecka J, Jacobson W, Chen Y, Keefe RS(2015)A randomized, placebo-controlled, active-reference, double-blind, flexible-dose study of the efficacy of vortioxetine on cognitive function in major depressive disorder. Neuropsychopharmacology 40:2025–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marazziti D, Consoli G, Picchetti M, Carlini M, Faravelli L(2010)Cognitive impairment in major depression. Eur J Pharmacol 626:83–86. [DOI] [PubMed] [Google Scholar]
- Mathers CD, Loncar D(2006)Projections of global mortality and burden of dizease from 2002 to 2030. PLoS Med 3:e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre RS, Lophaven S, Olsen CK(2014)A randomized, double-blind, placebo-controlled study of vortioxetine on cognitive function in depressed adults. Int J Neuropsychopharmacol 17:1557–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre RS, Soczynska JZ, Woldeyohannes HO, Alsuwaidan MT, Cha DS, Carvalho AF, Jerrell JM, Dale RM, Gallaugher LA, Muzina DJ, Kennedy SH(2015)The impact of cognitive impairment on perceived workforce performance: results from the International Mood Disorders Collaborative Project. Compr Psychiatry 56:279–282. [DOI] [PubMed] [Google Scholar]
- McIntyre RS, Harrison J, Loft H, Jacobson W, Olsen CK(2016)The effects of vortioxetine on cognitive function in patients with major depressive disorder (MDD): a meta-analysis of three randomized controlled trials. Int J Neuropsychopharmacol 19:pyw055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences EaM (2015)Enabling discovery, development, and translation of treatments for cognitive dysfunction in depression. Workshop summary. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- NICE (2013)Specification for manufacturer/sponsor submission of evidence June 2012. [Google Scholar]
- Papakostas GI, Culpepper L(2015)Understanding and managing cognition in the depressed patient. J Clin Psychiatry 76:418–425. [DOI] [PubMed] [Google Scholar]
- Pehrson AL, Leizer SC, Gulinello M, Dale E, Li Y, Waller JA, Sanchez C(2015)Treatment of cognitive dysfunction in major depressive disorder--a review of the preclinical evidence for efficacy of selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors and the multimodal-acting antidepressant vortioxetine. Eur J Pharmacol 753:19–31. [DOI] [PubMed] [Google Scholar]
- Porter RJ, Bourke C, Gallagher P(2007)Neuropsychological impairment in major depression: its nature, origin and clinical significance. Aust N Z J Psychiatry 41:115–128. [DOI] [PubMed] [Google Scholar]
- Rajji TK, Miranda D, Mulsant BH, Lotz M, Houck P, Zmuda MD, Bensasi S, Reynolds CF III, Butters MA(2009)The MMSE is not an adequate screening cognitive instrument in studies of late-life depression. J Psychiatr Res 43:464–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riga MS, Celada P, Sanchez C, Artigas F(2013)Role of 5-HT3 receptors in the mechanism of action of the investigational antidepressant vortioxetine. European Neuropsychopharmacology 23:S393–394. [Google Scholar]
- Rock PL, Roizer JP, Riedel WJ, Blackwell AD(2014)Cognitive impairment in depression: a systematic review and meta-analysis. Psychol Med 44:2029–2040. [DOI] [PubMed] [Google Scholar]
- Rosenblat JD, Kakar R, McIntyre RS(2016)The cognitive effects of antidepressants in major depressive disorder: a systematic review and meta-analysis of randomized clinical trials. Int J Neuropsychopharmacol 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rund BR, Sundet K, Asbjornsen A, Egeland J, Landro NI, Lund A, Roness A, Stordal KI, Hugdahl K(2006)Neuropsychological test profiles in schizophrenia and non-psychotic depression. Acta Psychiatr Scand 113:350–359. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Asin KE, Artigas F(2015)Vortioxetine, a novel antidepressant with multimodal activity: review of preclinical and clinical data. Pharmacol Ther 145:43–57. [DOI] [PubMed] [Google Scholar]
- Seguin M, Lesage A, Chawky N, Guy A, Daigle F, Girard G, Turecki G(2006)Suicide cases in New Brunswick from April 2002 to May 2003: the importance of better recognizing substance and mood disorder comorbidity. Can J Psychiatry 51:581–586. [DOI] [PubMed] [Google Scholar]
- Tignol J, Pujol-Domenech J, Chartres JP, Leger JM, Pletan Y, Tonelli I, Tournoux A, Pezous N(1998)Double-blind study of the efficacy and safety of milnacipran and imipramine in elderly patients with major depressive episode. Acta Psychiatr Scand 97:157–165. [DOI] [PubMed] [Google Scholar]
- Wallace A, Pehrson AL, Sanchez C, Morilak DA(2014)Vortioxetine restores reversal learning impaired by 5-HT depletion or chronic intermittent cold stress in rats. Int J Neuropsychopharmacol 17:1695–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2016)Fact sheet N°369. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.