Abstract
Background
Patients with post-traumatic stress disorder frequently report persistent problems with social interactions, emerging after a traumatic experience. Chronic social defeat stress is a widely used rodent model of stress that produces robust and sustained social avoidance behavior. The avoidance of other rodents can be reversed by 28 days of treatment with selective serotonin reuptake inhibitors, the only pharmaceutical class approved by the U.S. Food and Drug Administration for treating post-traumatic stress disorder. In this study, the sensitivity of social interaction deficits evoked by 10 days of chronic social defeat stress to prospective treatments for post-traumatic stress disorder was examined.
Methods
The effects of acute and repeated treatment with a low dose of buprenorphine (0.25 mg/kg/d) on social interaction deficits in male C57BL/6 mice by chronic social defeat stress were studied. Another cohort of mice was used to determine the effects of the selective serotonin reuptake inhibitor fluoxetine (10 mg/kg/d), the NMDA antagonist ketamine (10 mg/kg/d), and the selective kappa opioid receptor antagonist CERC-501 (1 mg/kg/d). Changes in mRNA expression of Oprm1 and Oprk1 were assessed in a separate cohort.
Results
Buprenorphine significantly reversed social interaction deficits produced by chronic social defeat stress following 7 days of administration, but not after acute injection. Treatment with fluoxetine for 7 days, but not 24 hours, also reinstated social interaction behavior in mice that were susceptible to chronic social defeat. In contrast, CERC-501 and ketamine failed to reverse social avoidance. Gene expression analysis found: (1) Oprm1 mRNA expression was reduced in the hippocampus and increased in the frontal cortex of susceptible mice and (2) Oprk1 mRNA expression was reduced in the amygdala and increased in the frontal cortex of susceptible mice compared to non-stressed controls and stress-resilient mice.
Conclusions
Short-term treatment with buprenorphine and fluoxetine normalized social interaction after chronic social defeat stress. In concert with the changes in opioid receptor expression produced by chronic social defeat stress, we speculate that buprenorphine’s efficacy in this model of post-traumatic stress disorder may be associated with the ability of this compound to engage multiple opioid receptors.
Keywords: PTSD, buprenorphine, fluoxetine, CERC-501, ketamine, chronic social defeat stress, social interaction
Significance Statement
Post-traumatic stress disorder (PTSD) develops after exposure to a serious threatening event and involves significant, persistent negative changes in mood, arousal, and cognition. Social detachment and lingering social impairment often begin or worsen after the traumatic event. There is a significant medical need to develop better, more effective medications for PTSD. The studies presented here examined the effects of 3 potential new medications for PTSD in mice after chronic social defeat, a rodent model of psychosocial stress that causes enduring social avoidance behavior. The mixed opioid agonist/antagonist buprenorphine, the NMDA receptor antagonist ketamine, and the selective kappa opioid receptor antagonist CERC-501 were studied for the rapid reversal of social interaction deficits induced by chronic social defeat and compared with the SSRI fluoxetine, an approved therapeutic class for PTSD. In this paper, we show that both fluoxetine and buprenorphine reversed the social interaction deficits following 1 week of treatment. Ketamine or CERC-501 did not alter the behaviors induced by defeat. Overall, these data support further investigation of buprenorphine, and its underlying mechanisms, as a potential therapeutic for PTSD.
Introduction
Emotional numbing and social deficits are frequently observed in patients diagnosed with stress-related psychiatric disorders (Hames et al., 2013; Air et al., 2015), such as posttraumatic stress disorder (PTSD) (Cisler et al., 2015; Dutton et al., 2016; LaMotte et al., 2016; Renshaw and Campbell, 2016; Venta et al., 2016). Triggered by exposure to an actual or perceived life-threatening event, serious injury, or sexual violence, PTSD is a debilitating mental health disorder (Goldstein et al., 2016), with a current lifetime prevalence of 3.6% in men and 9.7% in women in the United States (Breslau, 2009). Although behavioral and pharmacological therapies (e.g., selective serotonin reuptake inhibitor [SSRIs]) are available, they are not effective for most patients. As many as 50% of patients are treatment resistant, suffering from chronic unremitted PTSD for years (Green et al., 2006; Ravindran and Stein, 2009; Choi et al., 2010; Bryant et al., 2013). Thus, new pharmacotherapies are urgently needed to alleviate the symptoms of PTSD.
Modulation of the endogenous opioid system presents a potential opportunity for developing novel therapeutics for PTSD. Endogenous κ- (KOR) and μ- (MOR) opioid receptors are critical regulators of mood (Lutz and Kieffer, 2013; Mechling et al., 2016). In response to stressful stimuli, binding of KORs by the endogenous opioid dynorphin (DYN) produces dysphoria, aversion, and negative affect (Land et al., 2008; Knoll and Carlezon, 2010). Conversely, activation of MORs is necessary for social reward (Trezza et al., 2011; Hsu et al., 2013; Resendez et al., 2013) and reinforcement of the rewarding properties of drugs of abuse (Charbogne et al., 2014). Recent clinical reports have highlighted the potential therapeutic impact of opioids in PTSD; morphine administered during early resuscitation and trauma care was associated with reduced risk of a subsequent PTSD diagnosis after serious injury (Holbrook et al., 2010). Similarly, a small study noted that the mixed opioid analgesic buprenorphine alleviated PTSD symptomology in patients diagnosed with comorbid chronic pain and PTSD (Seal et al., 2016).
Buprenorphine is a MOR partial agonist and KOR antagonist that is currently approved by the FDA for the treatment of opioid addiction at high doses and for chronic pain at lower doses. Compelling clinical evidence has demonstrated the potential of low doses of buprenorphine to rapidly alleviate depressive symptoms in treatment-resistant depressed patients (Bodkin et al., 1995; Nyhuis et al., 2008; Ehrich et al., 2014; Karp et al., 2014). Furthermore, buprenorphine produced marked reductions in suicidal ideation (Striebel and Kalapatapu, 2014; Yovell et al., 2016). In parallel with these clinical findings, preclinical evidence from our laboratory has shown that low-dose buprenorphine is highly effective across a range of behavioral tests relevant to anxiety and depression in mice and rats (Browne et al., 2015; Falcon et al., 2015; Robinson et al., 2016; Browne et al., 2017). Moreover, we have shown that buprenorphine effectively reversed stress-induced anhedonia, anxiety, and depressive-like behavior in mice following exposure to unpredictable chronic mild stress within 1 week of treatment (Falcon et al., 2016).
Given the compelling support for buprenorphine’s anti-stress effects, the studies presented here originated by investigating the ability of buprenorphine to reverse social interaction deficits following chronic social defeat stress (CSDS) in mice. CSDS is a well-established preclinical model of stress that produces pronounced and persistent deficits in social interaction that are long-lasting and sensitive to chronic administration of antidepressant drugs (Berton et al., 2006; Nikulina et al., 2008; Golden et al., 2011). Opioid involvement in CSDS has been supported by reports of region-specific increases in mRNA expression of Oprm1 (Nikulina et al., 2008) and DYN concentrations (Berube et al., 2013). Upregulated DYN signaling has been proposed as a key mediator of the behavioral deficits induced following CSDS exposure (McLaughlin et al., 2006). Therefore, we anticipated that compounds that modulate opioidergic tone may have beneficial effects in altering social exploration in this model of stress, which is proposed to be a behavior endpoint that is relevant to PTSD (Flandreau and Toth, 2017). Focusing on the development of rapid treatment effects, mice were tested following acute (24 hours following a single injection) and repeated treatment (once daily for 7 days).
A follow-up study compared the effects of buprenorphine with those of the SSRI fluoxetine, the analgesic/anesthetic ketamine, and the selective KOR antagonist CERC-501 (formerly LY2456302). There was a clear rationale for choosing each one of these comparator compounds. Because fluoxetine had only been tested in CSDS following 28 days of treatment (Berton et al, 2006) and SSRIs are the only class of drugs currently approved by the FDA for PTSD, it would be informative to examine the effects of an SSRI on social deficits in a rapid time frame like that of buprenorphine. Second, ketamine was included as a comparator, because recent clinical studies have demonstrated rapid reductions in symptom severity following ketamine infusion in patients with treatment-resistant depression (Zarate et al., 2006; Aan Het Rot et al., 2010; DiazGranados et al., 2010; Ibrahim et al., 2011; Murrough et al., 2013; Ionescu et al., 2016) and possibly in patients with chronic PTSD (Feder et al., 2014). Finally, as KORs have been implicated in the emergence of affective behaviors following CSDS (McLaughlin et al., 2006) and buprenorphine’s antidepressant-like effects are known to involve KORs (Falcon et al., 2016), the selective KOR antagonist CERC-501 was expected to provide valuable information regarding the therapeutic potential of this class of compounds in the treatment of PTSD.
Methods
Animals
Male C57BL/6J mice, age 8 to 9 weeks, and retired breeder CD-1 mice 4 to 6 months of age were obtained from Jackson Laboratories and allowed 1 week to adjust to the vivarium prior to the onset on behavioral experiments. Mice were maintained under a 12-h-light/-dark cycle (lights on at 7:30 am) in temperature- and humidity-controlled rooms. Food and water were provided ad libitum. The first cohort of mice was used to assess the effect of buprenorphine treatment on CSDS-induced social deficits. Following this study, another cohort was used to establish the optimal dose of CERC-501 and ketamine for the subsequent CSDS study conducted in an additional cohort. Gene expression analysis was performed using tissue obtained from a separate cohort of mice exposed to the CSDS procedure. All studies were approved by the Institutional Animal Care and Use Committee for the University of Pennsylvania and conducted in accordance with the PHS Policy on Humane Care and Use of Laboratory Animals.
Drugs
Buprenorphine hydrochloride (0.25 mg/kg; RTI, National Institute on Drug Abuse), fluoxetine hydrochloride (10 mg/kg; AK Scientific), ketamine (Ketaset), and CERC-501 (formerly LY2456302; Eli Lily) were prepared freshly on the morning of each experimental day and administered i.p. using a 10-mL/kg injection volume. Fluoxetine and buprenorphine were dissolved in water (sterile HPLC grade water, MilliQ system, Millipore); ketamine was dissolved in 0.9% sterile saline (Hospira Inc.), and CERC-501 in vehicle (sterile water, 1% lactic acid [85%], sonicated for 5 minutes and titrated to pH 5). Mice in the nonstress control groups received 0.9% saline injections. Drugs were administered on the day following the first baseline social interaction test, and mice were then testing 24 hours after drug treatment on day 1 and day 7. Prior to the CSDS study, dose-response curves were determined for ketamine and CERC-501 in the forced swim test (FST) and novelty-induced hypophagia (NIH) test to determine an appropriate behaviorally active dose 24 hours post injection for use in the CSDS study.
CSDS
The CSDS studies were conducted according to a previously established protocol (Berton et al., 2006) with defeat bouts lasting 5 minutes. Resident CD-1 mice were prescreened for aggression and housed 1 per cage in hamster cages (26.7 x48.3 x15.2 cm) with a Plexiglas perforated divider. C57BL/6J mice were initially housed 5 per cage until the beginning of the stress paradigm. For 10 consecutive days, each C57BL/6J mouse was placed into the cage of a different resident CD-1 mouse for 5 minutes, where they engaged in aggressive physical contact. Once the defeat bout was over, the intruder C57BL/6J was transferred to the other side of a cage divider for the remainder of the 24-hour period, allowing the mice to see, smell, and hear but not touch one another. This continuous sensory interaction comprises the psychological component of the stressor, as the C57BL/6J mouse was unable to escape the presence of the aggressor. Nonstressed control mice did not experience social defeat. They were housed in pairs in the same experimental cages (including divider) and were handled daily. Following the cessation of the CSDS exposure, mice were housed in pairs as per control animals.
Social Interaction Test
Social approach behaviors of susceptible and control mice were tested following CSDS in a social interaction open-field arena (42 cm×42cm×42 cm), in which a wire-mesh cage was placed at the end of the arena (Challis et al., 2013). An area of 14 cm×24 cm surrounding the wire mesh cage was designated as the “interaction zone”. Each mouse was allowed to freely explore the arena for 150 seconds in the absence of a conspecific target mouse. The mice were returned to their home cage briefly for ~1 minute, during which time the experimenter introduced the target CD-1 mouse into the wire-mesh cage at the end of the box. The second 150-second session started immediately. Using a video-tracking system, the time spent interacting with the unfamiliar target mouse was recorded. Social interaction ratios were determined as: time in interaction zone with mouse / time in interaction zone without mouse. The performance of defeated mice was split into 2 categories: susceptible mice exhibit pronounced social avoidance (interaction ratios <1) and resilient mice that sustained social interaction (interaction ratios >1).
For these studies, drug treatments were evaluated in susceptible mice only. For the buprenorphine study, 50 mice were exposed to the social defeat procedure, and 2 were excluded due to wounding; 42% were resilient and 58% were susceptible from a total of 48 mice. For the second experiment, 80 mice were exposed to the CSDS procedure and 6 were excluded due to wounding. Of the remaining 74 mice, 48% were resilient and 52% were susceptible. Mice were retested in the same apparatus on the first day after the cessation of the social defeat paradigm (baseline) and then again 24 hours following drug administration on day 1 and day 7 of treatment.
Selection of Doses for the CSDS Study
The doses of ketamine and CERC-501 used in the CSDS model were selected based on 2 behavioral screens for antidepressant activity: the FST and the NIH tests. We have previously shown that the 0.25-mg/kg buprenorphine dose was active in both the FST and NIH test and could be injected in mice chronically without causing toxicity (Falcon et al., 2015, 2016; Robinson et al., 2016). Similarly, the selected 10-mg/kg/d dose of fluoxetine was previously shown to reduce immobility in the FST (Lucki et al., 2001), reduce approach latency in the NIH test (Hodes et al., 2010), and bind to SERT in brain and was administered to C57BL/6 mice chronically without development of toxicity (Hirano et al., 2005; Balu et al., 2009). As we did not have prior experience with CERC-501 and ketamine, we conducted dose-response curves for these compounds to identify comparable behaviorally active doses on these tests.
FST
The FST was conducted in our laboratory as described previously (Lucki et al., 2001; Balu et al., 2009; Falcon et al., 2015). Mice were gently placed in a cylinder of water (21 cm in diameter), filled with 15 cm of water (25°C±1°C), for 6 minutes. Water was changed between each animal. A rater blind to the treatment conditions evaluated the full 6 minutes of the test from video recordings, as C57BL/6J mice develop immobility during the first 2 minutes of this test. The data are presented as immobility(s). Drugs were administered 24 hours prior to testing.
NIH
The NIH test measures approach latencies of rodents for palatable food in a novel arena. Approach latencies in the NIH test are diminished following acute administration of anxiolytics or chronic administration of antidepressants (Dulawa and Hen, 2005). Mice were trained to rapidly approach and readily consume a palatable food (3 peanut butter chips in a clear petri dish) as described previously (Balu et al., 2009; Falcon et al., 2015). Daily training sessions were performed until mice met the criteria of 3 consecutive days with approach latencies of ≤30 seconds. Testing in the novel arena was conducted in a different room from that of training. On the test day, mice were placed in a clear polycarbonate cage (25.5×46×20 cm) that was brightly lit (800 Lux) and scented with lemon (20% Lemon Joy solution). Latency, defined as the time to approach and start consuming the peanut butter chips located in the dish in the center of the arena, was scored for each mouse. The 15-minute test session duration was recorded with a digital camera.
Quantitative RT-PCR
Brain tissue from the frontal cortex (FC), hippocampus (Hp), striatum (Str), and amygdala (Amy) was collected by gross dissection from non-stressed (NS) controls, stress resilient and stress susceptible male C57BL/6J mice. Briefly, a TriZol (cat. no. 15596-026, Ambion, ThermoFisher Scientific) chloroform-based extraction method was used to isolate total RNA. Samples were homogenized using a Kontes Pellet Pestle. Quantification of the isolated RNA was performed using the NanoDrop spectrophotometer and ND-1000 software (Thermo Fisher Scientific) at the optical densities of 260 and 280 nm. Samples with poor RNA quality/degradation were excluded at this stage. Reverse transcriptase amplification of cDNA from total RNA was performed using the High-Capacity RNA-to-cDNA Kit (Applied Biosystems, ThermoFisher Scientific) conducted using a MJ, Research PTC-100 thermal cycler. Taqman Gene Expression Assays (Applied Biosystems, ThermoFisher Scientific) were used in a CFX96 Touch Real-Time PCR Detection System (BioRad) to quantify the following target genes during amplification: Oprk1 Mm01230885_m1, Oprm1 Mm01188089_m1, and the endogenous control Rn18S Mm04277571_s1. For each sample, the average of the triplicate cycle numbers at threshold crossing (CT) value for the endogenous control was subtracted from the average CT values for the target gene, generating ΔCT values. As the PCR efficiencies for both the endogenous control gene and target gene were equal (~1), the changes in expression of the target gene were expressed as 2-ΔΔCT for each sample calculated. All data were normalized to the control/saline group and expressed as fold change for statistical analysis and presentation.
Statistical Analysis
Data are expressed as mean ± SEM. Statistical analysis was performed using GraphPad Prism version 7.00 for Windows (GraphPad Software). One-way ANOVAs with Dunnett’s multiple comparisons were used to evaluate the effects of CERC-501 and ketamine in the FST and NIH, and to determine any CSDS-induced alterations in gene expression. Two-way repeated-measures ANOVA were used to evaluate a treatment*stress interaction on social interaction scores. Bonferroni multiple comparisons tests were used where appropriate.
Results
Buprenorphine Reversed CSDS-Induced Social Interaction Deficits
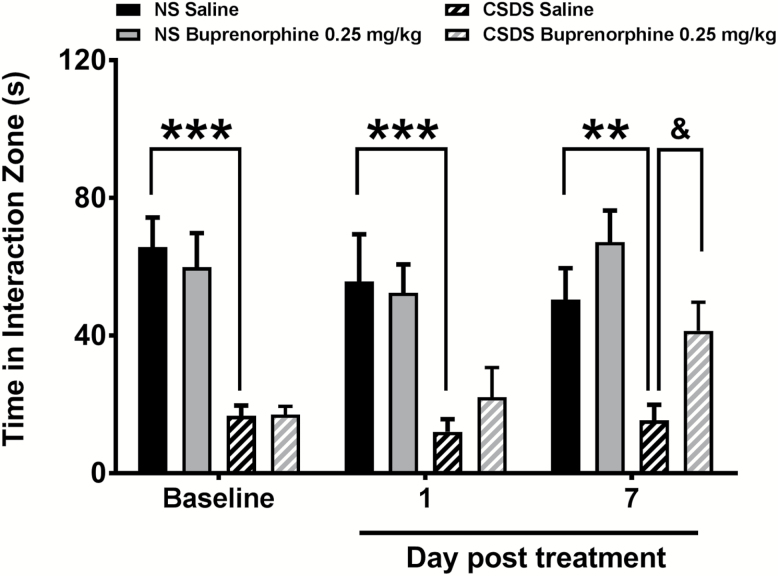
Social interaction was measured after exposure to 10 sessions of CSDS (baseline) and again after acute (1 day) and repeated treatment (7 days) with buprenorphine or saline (Figure 1). Behavioral scores after drug treatment were compared with nonstress control groups that received treatment with buprenorphine or saline. Group sizes were n=9 for both control groups, n=13 for susceptible saline, and n=15 for susceptible buprenorphine.
Figure 1.
Buprenorphine reversed social interaction deficits in susceptible mice. Across the 3 testing exposures, non-stressed (NS) mice spent a greater amount of time interacting with the target mouse compared with susceptible CSDS mice (***P<.001, **P<.01). Social interaction scores of susceptible CSDS mice were normalized following 7 days of treatment with buprenorphine compared with mice treated with saline (&P<.05).
Test-retest conditions did not induce alterations in the social interaction values in untreated control and susceptible mice. A significant stress*treatment interaction was observed on social interaction (F6, 84=2.321, P=.04). Tukey’s multiple comparisons tests determined that susceptible mice exhibited lower social interaction values than the corresponding control groups at baseline (P<.001), day 1 (P<.001), and day 7 (P<.01). CSDS-stressed mice treated with buprenorphine did not show changes in social interaction scores 24 hours after a single injection. However, these mice exhibited significantly higher interaction scores compared with saline-treated controls following 7 days of treatment with buprenorphine (P<.05).
Dose Response Curves for CERC-501 and Ketamine in the NIH Test and the FST
To establish the optimal dose of CERC-501 and ketamine for repeated treatment in the CSDS model, dose-response curves were determined on two behavioral tests used to screen compounds for antidepressant-like activity: the NIH test and the FST.
FST
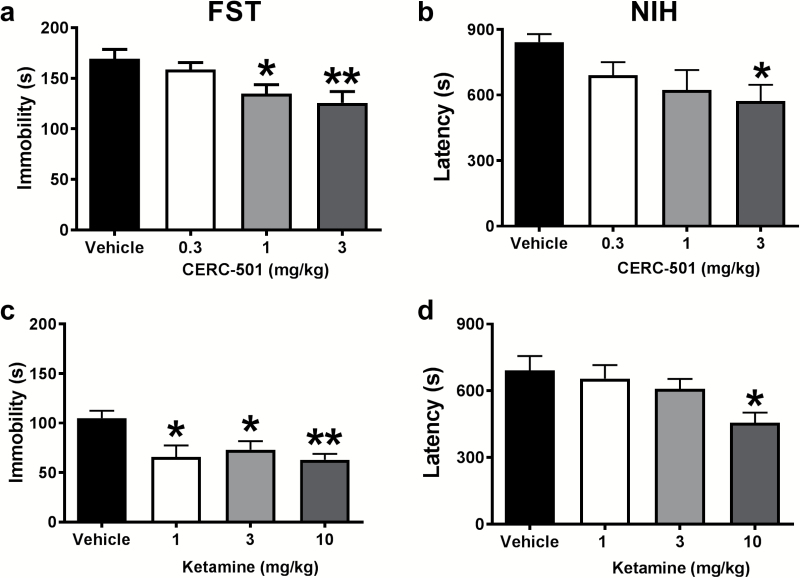
A significant effect of treatment with CERC-501 was observed on immobility scores (F3, 34=4.775 P<.007; n=9–10/group) (Figure 2a). More specifically, mice displayed reductions in the time spent immobile following administration of CERC-501 at doses of 1 (P<.05) and 3 mg/kg (P<.01). A similar effect of ketamine treatment was detected for immobility (F3, 26=5.138, P<.007; n=7–8/group) (Figure 2c). All the doses tested effectively reduced the time spent immobile [1 (P<.05), 3 (P<.05), and 10 mg/kg (P<.01)] compared with the vehicle-treated control group.
Figure 2.
Acute behavioral effects of ketamine and CERC-501 tested at 24 hours post injection. In the FST, immobility time was reduced significantly following administration of 1 (P<.05) and 3 mg/kg (P<.01) of CERC-501 (Figure 2a). Additionally, ketamine treatment significantly reduced the time spent immobile at all doses [1 (P<.05), 3 (P<.05), and 10 mg/kg (P<.01)] compared with vehicle (Figure 2c). In the NIH test, mice treated with CERC-501 (3 mg/kg) significantly decreased latencies to approach and consume food compared with vehicle (P < .01) (Figure 2b). Similarly, ketamine (10 mg/kg) significantly decreased approach latencies compared with vehicle-treated controls in the NIH test (Figure 2d).
NIH
Approach latency in the NIH was significantly altered following CERC-501 treatment (F3, 35=3.668, P<.05; n=9–10/group) (Figure 2b). Multiple comparisons revealed that CERC-501 (3 mg/kg) significantly decreased latencies to consume the peanut butter chips compared with the vehicle-treated group (P<.05). A trend towards decreased approach latencies was observed for the 1-mg/kg dose (P=.07). Ketamine also produced a significant reduction of latency values (F3, 51=3.212, P<.04; n=12–15/group) (Figure 2d). Only the highest dose of ketamine (10 mg/kg) decreased the latencies of mice to approach and begin eating the food in the novel cage (P<.05).
Social Avoidance Was Reversed by Fluoxetine, but Not Ketamine, or CERC-501
Based on the results of the acute behavioral tests, the doses of CERC-501 (1 mg/kg) and ketamine (10 mg/kg) were chosen for the CSDS study. The dose of fluoxetine (10 mg/kg) was selected based on previous studies conducted in our laboratory (Lucki et al., 2001; Hodes et al., 2010) and the published relationship between plasma concentrations of fluoxetine and SERT binding in mouse brain (Hirano et al. 2005).
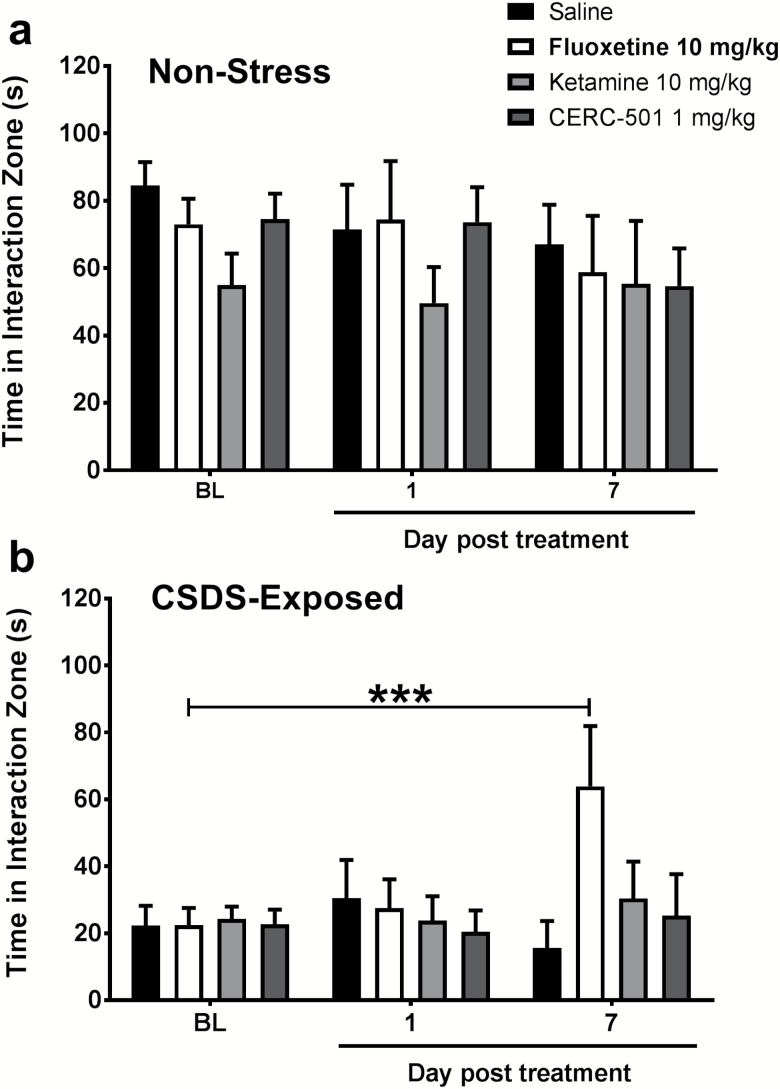
No significant interaction or main effect of stress or treatment was observed on social interaction scores of control mice across the test days (Figure 3a; n=6–8/group). CSDS produced significant reductions in social interaction of susceptible mice at baseline, showing mean interaction values (22.31seconds ±5.92) (Figure 3b) that were nearly 4-fold lower than controls (84.51 seconds±6.97). A significant stress*treatment interaction was noted on social interaction scores of susceptible mice (F6,52=3.573, P=.005; n=6–8/group). None of the drugs produced a notable change in social interaction following a single drug treatment. However, on day 7, there was a significant increase in social interaction scores in mice treated with fluoxetine compared with their corresponding baseline scores (P<.001). No effect of treatment with ketamine or CERC-501 was observed.
Figure 3.
Social interaction deficits in susceptible mice were reversed by fluoxetine. Non-stress mice did not exhibit any significant differences in the amount of time in the interaction zone over the course of the experiment (Figure 3a). Susceptible mice exhibited less time interacting with the conspecific target on all 3 test days compared with control mice (Figure 3b). Following 7 days but not 24 hours of treatment with fluoxetine (10 mg/kg), social interaction scores of susceptible C57BL/6J mice were restored to a level comparable with control mice (***P<.001 compared with baseline values). No effect was observed with ketamine (10 mg/kg) or CERC-501 (1 mg/kg) treatment.
Oprm1 and Oprk1 Expression in Limbic and Cortical Regions of Susceptible Mice
The most potent effects of buprenorphine are as a MOR partial agonist and KOR antagonist (Cowan, 2007), and we previously noted the reversal of stress-induced alterations in opioid receptor mRNA expression post buprenorphine (Falcon et al., 2016). We hypothesized that the ability of buprenorphine but not CERC-501 to produce beneficial behavioral changes in the CSDS model was due to buprenorphine’s capacity to modulate MORs in addition to blockade of KORs. To determine whether MORs and KORs were equally altered in the CSDS model, a separate cohort of mice exposed to the CSDS procedure was used to evaluate Oprm1 and Oprk1 gene expression in nonstressed control, susceptible, and resilient mice. This cohort consisted of 40% resilient mice and 60% susceptible mice (n=5–8/group).
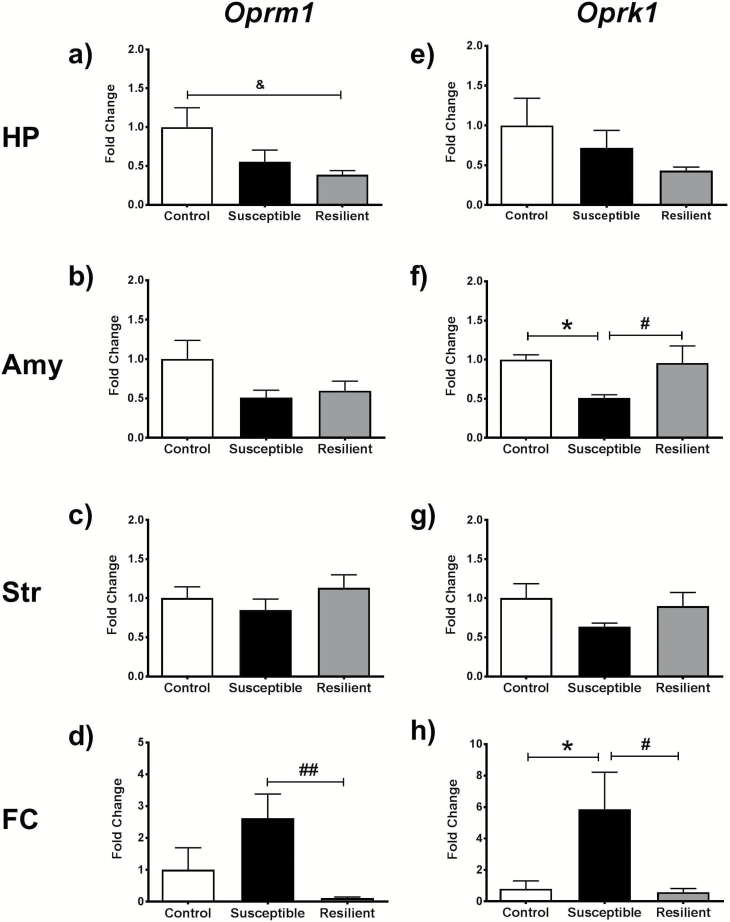
A significant effect of stress was detected for Oprm1 mRNA expression in the HP (F2,17=3.672, P=.047; Figure 4a), where resilient mice exhibited lower levels of expression compared with controls (P<.05). Susceptible mice also exhibited a nonsignificant decrease in Oprm1 mRNA levels in this region. No significant effect of stress was distinguished for Oprk1 expression in the Hp (Figure 4b). In the Amy, only a trend towards a stress effect was noted for Oprm1 mRNA expression (Figure 4c), whereas Oprk1 mRNA expression was dramatically altered by stress in this region (F2, 12=7.28, P=.008; Figure 4d). Tukey multiple comparison tests detected a decrease in amygdalar Oprk1 mRNA expression in susceptible mice that was significantly lower than both control and resilient mice (P<.05). Although no significant changes in gene expression were apparent in the Str (Figure 4e, f), robust alterations in the expression of both genes were detected in the FC. Cortical Oprm1 mRNA was significantly altered by stress (F2,16=6.486, P=.008; Figure 4g), where the levels of Oprm1 in resilient mice were considerably lower than those measured in susceptible animals (P<.01). Similarly, a main effect of stress was observed for Oprk1 in the FC (F2, 14=4.233, P=.037) (Figure 4h). Multiple comparisons test revealed that the elevation in Oprk1 mRNA expression in susceptible mice was higher than the levels determined in resilient and control mice (P<.05).
Figure 4.
This panel of graphs depicts the changes in gene expression of Oprm1 (a–d) and Oprk1 (e–h) in the hippocampus (HP), amygdala (Amy), striatum (Str), and frontal cortex (FC) of nonstressed control, susceptible, and resilient mice. *P<.05 between control and susceptible mice, &P<.05 between control and resilient; #P<.05, ##P<.01 for difference between susceptible and resilient mice.
Discussion
These experiments examined the ability of buprenorphine, and a series of related compounds, to reverse social interaction deficits in susceptible mice following CSDS. CSDS is a well-established preclinical model of stress that produces distinctive behavioral deficits. In the initial study, repeated treatment with buprenorphine was demonstrated to reverse the social interaction deficits that are characteristic of CSDS without altering social interaction in nonstressed control mice. A follow-up study evaluated the effects of fluoxetine, ketamine, and the selective KOR antagonist CERC-501 for comparison. Chronic administration of fluoxetine reversed the deficits produced by CSDS, whereas chronic administration of ketamine and CERC-501 were ineffective.
Recent studies by our laboratory and others have shown that buprenorphine produces behavioral changes in numerous tests in rodents that are associated with the effects of antidepressant and anxiolytic drugs in humans. In addition, using unpredictable chronic mild stress as a model of depression, buprenorphine produced reversal of behavioral deficits in anhedonia, stress coping, and anxiety with repeated treatment for 1 week (Falcon et al., 2016). Our data parallel the reduced responses to stress and subjective level of threat (Bershad et al., 2015) and decreased responses to negative social stimuli while enhancing positive responses to social stimuli (Bershad et al., 2016) produced by buprenorphine in healthy volunteers exposed to psychosocial stress challenges. These results are noteworthy, because they correspond with the results from clinical evaluation of buprenorphine in numerous studies in depressed patients (Karp et al., 2014; Yovell et al., 2016; Stanciu et al., 2017). Furthermore, ALKS-5461, a combination of buprenorphine and the mu opioid receptor antagonist samidorphan, also induced positive effects within 1 week of treatment for treatment-resistant depression (Ehrich et al., 2014).
Fluoxetine and imipramine were shown previously to ameliorate CSDS-induced social avoidance following chronic (28 days), but not after acute (1 day), administration and this suggested that long-term treatment with antidepressant drugs was necessary to impact social behavior (Berton et al., 2006; Tsankova et al., 2006). Although somewhat unexpected, the present results indicate that 1 week of fluoxetine treatment was sufficient for improving social avoidance scores following CSDS. To our knowledge, 1 week of fluoxetine treatment has not been examined in previous studies applying the CSDS protocol. These data suggest that both buprenorphine and fluoxetine can modulate social deficits within a relatively short period. Although buprenorphine and fluoxetine produced comparable amelioration of the negative outcomes of the CSDS, buprenorphine may produce its effects through mechanisms that are distinct from SSRIs. The SSRIs sertraline and paroxetine are treatments approved by the FDA for PTSD, and a recent meta-analysis established an overall “small positive impact” for fluoxetine, paroxetine, and venlafaxine on PTSD symptoms (Hoskins et al., 2015). Repeated exposure to CSDS induced social avoidance in mice, a behavioral change that resembles one of the key symptoms of PTSD (Flandreau and Toth, 2017). Therefore, the similar effects obtained between buprenorphine and fluoxetine could be viewed as supporting an extension of indications for buprenorphine for the treatment of PTSD.
KOR blockade has been associated with anti-stress effects (Carlezon and Krystal, 2016) and could underlie some of buprenorphine’s ability to alleviate negative affect and anhedonia (Falcon et al., 2015, 2016; Robinson et al., 2016; Browne et al., 2017). The selective KOR antagonist CERC-501 was examined, anticipating that it might produce many similar effects to buprenorphine. Accordingly, CERC-501 produced clear behavioral effects in the FST and NIH tests, behavioral assays in mice that are also sensitive to many antidepressant drugs (Lucki et al., 2001; Dulawa and Hen, 2005). Others have shown that CERC-501 produces robust behavioral effects in tests relevant to motivation, affect, and addiction in both humans and rodents (Rorick-Kehn et al., 2014, 2015; Jackson et al., 2015). As CERC-501 produced significant effects in the NIH test following a single injection, these actions appeared like those produced by other rapid-acting clinical therapeutics, like ketamine. Despite establishing evidence of its behavioral activity, our studies presented in this manuscript did not find CERC-501 to be effective in ameliorating social interaction deficits induced by CSDS. To our knowledge, only 3 other studies have previously evaluated selective KOR antagonists in the CSDS model, and they differed from the present study by administering drug treatment during defeat sessions. In the first of these studies, the KOR antagonist nor-binaltorphimine given to mice during exposure to social defeat for 3 days prevented the development of the stress-induced increases in immobility in the FST, analgesia, and defeat postures (McLaughlin et al., 2006). A second study described the effective prevention of social defeat postures during stress by cotreatment with PF-04455242, a high-affinity KOR antagonist and moderate MOR antagonist (Grimwood et al., 2011). Social avoidance behavior and reversal of the effects of stress were not directly examined in this study. In a third study, pretreatment with the KOR antagonist JDTic and genetic disruption of KORs on DAT containing neurons prevented increased intra-cranial self-stimulation thresholds but did not alter social interaction deficits in mice measured following exposure to CSDS (Donahue et al., 2015). Taken together, this suggests that in contrast to consistent effects of KOR antagonism on measures of depression, anxiety, and anhedonia, selective KOR antagonism did not reverse established patterns of social avoidance, although it may prevent effects of stress if delivered prior to its presentation.
Ketamine produced robust antidepressant action in the FST and anxiolytic-like effects in the NIH but was ineffective in the CSDS model. Reversal of social interaction deficits after CSDS in mice has been previously reported with ketamine (Donahue et al., 2014; Brachman et al., 2016), but both studies used higher doses (20–30 mg/kg) than used in this study (10 mg/kg). From our data and others, low doses of ketamine (1–10 mg/kg) can alter mouse behavior on tests related to antidepressant activity, such as the FST, NIH test, and reversed the behavioral deficits induced following chronic mild stress (Autry et al., 2011; Browne and Lucki, 2013). Ketamine produces a variety of pharmacological effects as doses are gradually increased, ranging from affective change, modeling symptoms of schizophrenia, analgesia to ataxia, and anesthesia. It is unclear which effects of ketamine would mediate the effects on social interaction deficits if much higher doses are required than for antidepressant-like behavioral outcomes. Despite the negative findings presented here, further research into the beneficial effects of ketamine for PTSD with other models is warranted, particularly considering the emerging clinical evidence that ketamine infusion can rapidly reduce symptoms in patients diagnosed with chronic PTSD (Feder et al., 2014).
Although the mechanisms underlying buprenorphine’s behavioral effects on social interaction after CSDS are unknown, evidence suggests that MORs could be involved. Mice with genetic deletion of MORs do not exhibit social interaction deficits following exposure to stress (Komatsu et al., 2011). Additionally, our laboratory has recently demonstrated that the effect of buprenorphine in the NIH test is mediated by functional blockade of MORs (Robinson et al., 2016). Together, these findings suggest that the development of compounds with a combination of MOR and KOR blockade, as achieved by buprenorphine, should be considered as providing a beneficial therapeutic profile for stress-related disorders such as PTSD.
As buprenorphine, but not CERC-501 or ketamine at the doses used in this study, reversed the social interaction deficits induced by CSDS, we hypothesized that the contribution of opioid receptors should be considered in the emergence of social avoidance behaviors. Our hypothesis was bolstered by earlier studies that reported increased release of endogenous opioids, and subsequent analgesic effects equivalent to morphine (75 mg/kg/d), in response to social defeat stress (Miczek et al., 1982; Vivian and Miczek, 1998; Boyson et al., 2016). This effect was blocked by pretreatment with the opioid antagonist naltrexone (Miczek et al., 1982). Thus, given that buprenorphine mediates antidepressant actions via KORs and anxiolytic effects with MORs, we hypothesized that both Oprk1 and Oprm1 mRNA expression would be altered in brain regions relevant to social interaction. Although the mRNA expression analysis is limited by the large and heterogeneous nature of gross dissection, which may mask key variations in discrete nuclei of interest, comparing susceptible mice, that is, those mice that exhibited significant social avoidance following CSDS with resilient and nonstressed control mice allowed for the detection of 2 compelling findings.
Oprm1 mRNA expression was decreased in hippocampus and increased in the frontal cortex following CSDS. Furthermore, a trend towards a significant decrease in Oprm1 mRNA levels was detected in the amygdala. Previous findings from other studies using resident intruder and social defeat models in mice and rats have reported alterations in Oprm1 expression, MOR availability, and function following social stress (Nikulina et al., 1999, 2005, 2008; Komatsu et al., 2011; Miczek et al., 2011; Berube et al., 2013; Johnston et al., 2015), especially decreased Oprm1 mRNA expression in the basolateral amygdala of defeated rats (Berube et al., 2013). Additionally, decreased binding potential of MORs in the extended amygdala and increased binding in the orbitofrontal cortex has been reported in PTSD patients and combat-exposed controls compared with healthy controls without combat exposure (Liberzon et al., 2007). These studies agree with our findings, suggesting that the Amy and discreet regions of the cortex are stress-sensitive regions for MORs that may be of interest in PTSD.
Furthermore, expression of Oprk1 mRNA was reduced in the Amy and increased in the FC after CSDS. Previously, we found similar changes in Oprk1 mRNA expression following exposure to unpredictable chronic mild stress (Falcon et al., 2016). These findings highlight the importance of these brain regions in mediating changes in affective behavior following stress. These data are also in accord with the clinical literature pertaining to aberrant prodynorphin signaling and KOR activation in depressed patients and the utilization of PET ligands to assay KOR availability as a transdiagnostic maker of dysphoria in patients diagnosed with depression, anhedonia, and PTSD (Hurd et al., 1997; Hurd, 2002; Anderson et al., 2013; Pietrzak et al., 2014). Going forward, studies will interrogate the influence of MOR and KOR signaling in discreet areas of the Amy and FC in regulating dysphoria and negative affect that are relevant to the development and maintenance of PTSD.
In conclusion, these data highlight the ability of low-dose buprenorphine in reversing avoidance behavior following social defeat and the potential role of both MORs and KORs in regulating behavior following exposure to CSDS. Taken together, the clinical and preclinical literature have demonstrated many instances of low-dose buprenorphine in reversing negative affect or social inhibition (Karp et al., 2014; Falcon et al., 2016; Yovell et al., 2016), supporting the need for clinical evaluation of buprenorphine as a therapeutic for PTSD. These preclinical studies advocate for the continued investigation of compounds capable of modulating both MOR and KOR activity, as these compounds may have the most beneficial therapeutic profile for social deficits, as indicated for PTSD and depression.
Funding
This research effort was supported by US Public Health Service (USPHS) grants R01 MH92412, R01 MH105623, and T32 MH14654.
Statement of Interest
None.
Acknowledgments
We thank Dr. Jeanine Jochems for her invaluable instruction and advice in performing the social defeat procedure. Ann Charlot for assistance in the RT-PCR assays and Dr. Linda Rorick-Kehn for consultation
References
- Aan Het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, Mathew SJ(2010)Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry 67:139–145. [DOI] [PubMed] [Google Scholar]
- Air T, Weightman MJ, Baune BT(2015)Symptom severity of depressive symptoms impacts on social cognition performance in current but not remitted major depressive disorder. Front Psychol 6:1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SA, Michaelides M, Zarnegar P, Ren Y, Fagergren P, Thanos PK, Wang GJ, Bannon M, Neumaier JF, Keller E, Volkow ND, Hurd YL(2013)Impaired periamygdaloid-cortex prodynorphin is characteristic of opiate addiction and depression. J Clin Invest 123:5334–5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM(2011)NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475:91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balu DT, Hodes GE, Anderson BT, Lucki I(2009)Enhanced sensitivity of the MRL/MpJ mouse to the neuroplastic and behavioral effects of chronic antidepressant treatments. Neuropsychopharmacology 34:1764–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bershad AK, Jaffe JH, Childs E, de Wit H(2015)Opioid partial agonist buprenorphine dampens responses to psychosocial stress in humans. Psychoneuroendocrinology 52:281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bershad AK, Seiden JA, de Wit H(2016)Effects of buprenorphine on responses to social stimuli in healthy adults. Psychoneuroendocrinology 63:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ(2006)Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311:864–868. [DOI] [PubMed] [Google Scholar]
- Berube P, Laforest S, Bhatnagar S, Drolet G(2013)Enkephalin and dynorphin mRNA expression are associated with resilience or vulnerability to chronic social defeat stress. Physiology Behav 122:237–245. [DOI] [PubMed] [Google Scholar]
- Bodkin JA, Zornberg GL, Lukas SE, Cole JO(1995)Buprenorphine treatment of refractory depression. J Clin Psychopharm 15:49–57. [DOI] [PubMed] [Google Scholar]
- Boyson CO, Holly EN, Burke AR, Montagud-Romero S, DeBold JF, Miczek KA(2016)Maladaptive choices by defeated rats: link between rapid approach to social threat and escalated cocaine self-administration. Psychopharmacology 233:3173–3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachman RA, McGowan JC, Perusini JN, Lim SC, Pham TH, Faye C, Gardier AM, Mendez-David I, David DJ, Hen R, Denny CA(2016)Ketamine as a prophylactic against stress-induced depressive-like behavior. Biol Psychiatry 79:776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N.(2009)The epidemiology of trauma, PTSD, and other posttrauma disorders. Trauma Violence Abuse 10:198–210. [DOI] [PubMed] [Google Scholar]
- Browne CA, Lucki I(2013)Antidepressant effects of ketamine: mechanisms underlying fast-acting novel antidepressants. Front Pharmacol 4:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne CA, van Nest DS, Lucki I(2015)Antidepressant-like effects of buprenorphine in rats are strain dependent. Behav Brain Res 278C:385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne CA, Erickson RL, Blendy JA, Lucki I(2017)Genetic variation in the behavioral effects of buprenorphine in female mice derived from a murine model of the OPRM1 A118G polymorphism. Neuropharmacology 117:401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant RA, Mastrodomenico J, Hopwood S, Kenny L, Cahill C, Kandris E, Taylor K(2013)Augmenting cognitive behaviour therapy for post-traumatic stress disorder with emotion tolerance training: a randomized controlled trial. Psych Med 43:2153–2160. [DOI] [PubMed] [Google Scholar]
- Carlezon WA Jr, Krystal AD(2016)Kappa-opioid antagonists for psychiatric disorders: From bench to clinical trials. Depress Anxiety 33:895–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challis C, Boulden J, Veerakumar A, Espallergues J, Vassoler FM, Pierce RC, Beck SG, Berton O(2013)Raphe GABAergic neurons mediate the acquisition of avoidance after social defeat. J Neurosci 33:13978–13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbogne P, Kieffer BL, Befort K(2014)15 years of genetic approaches in vivo for addiction research: Opioid receptor and peptide gene knockout in mouse models of drug abuse. Neuropharmacology 76Pt B:204–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DC, Rothbaum BO, Gerardi M, Ressler KJ(2010)Pharmacological enhancement of behavioral therapy: focus on posttraumatic stress disorder. Curr Top Behav Neurosci 2:279–299. [DOI] [PubMed] [Google Scholar]
- Cisler JM, Bush K, Scott Steele J, Lenow JK, Smitherman S, Kilts CD(2015)Brain and behavioral evidence for altered social learning mechanisms among women with assault-related posttraumatic stress disorder. Journal Psych Res 63:75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan A.(2007)Buprenorphine: the basic pharmacology revisited. J Add Med 1:68–72. [DOI] [PubMed] [Google Scholar]
- DiazGranados N, Ibrahim LA, Brutsche NE, Ameli R, Henter ID, Luckenbaugh DA, Machado-Vieira R, Zarate CA Jr(2010)Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry 71:1605–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue RJ, Muschamp JW, Russo SJ, Nestler EJ, Carlezon WA Jr(2014)Effects of striatal DeltaFosB overexpression and ketamine on social defeat stress-induced anhedonia in mice. Biol Psychiatry 76:550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue RJ, Landino SM, Golden SA, Carroll FI, Russo SJ, Carlezon WA Jr(2015)Effects of acute and chronic social defeat stress are differentially mediated by the dynorphin/kappa-opioid receptor system. Behav Pharmacol 26:654–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulawa SC, Hen R(2005)Recent advances in animal models of chronic antidepressant effects: the novelty-induced hypophagia test. Neurosci Biobehav Rev 29:771–783. [DOI] [PubMed] [Google Scholar]
- Dutton CE, Rojas SM, Badour CL, Wanklyn SG, Feldner MT(2016)Posttraumatic stress disorder and suicidal behavior: indirect effects of impaired social functioning. Arch Suicide Res 20:567–579. [DOI] [PubMed] [Google Scholar]
- Ehrich E, Turncliff R, Du Y, Leigh-Pemberton R, Fernandez E, Jones R, Fava M(2014)Evaluation of opioid modulation in major depressive disorder. Neuropsychopharmacology 40:1448–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcon E, Browne CA, Leon RM, Fleites VC, Sweeney R, Kirby LG, Lucki I (2016) Antidepressant-like effects of buprenorphine are mediated by kappa opioid receptors. Neuropsychopharmacology 41:2344–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcon E, Maier K, Robinson SA, Hill-Smith TE, Lucki I (2015) Effects of buprenorphine on behavioral tests for antidepressant and anxiolytic drugs in mice. Psychopharmacology 232:907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder A, Parides MK, Murrough JW, Perez AM, Morgan JE, Saxena S, Kirkwood K, Aan Het Rot M, Lapidus KA, Wan LB, Iosifescu D, Charney DS(2014)Efficacy of intravenous ketamine for treatment of chronic posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry 71:681–688. [DOI] [PubMed] [Google Scholar]
- Flandreau EI, Toth M(2017)Animal models of PTSD: a critical review. Curr Top Behav Neurosci doi: 10.1007/7854_2016_65 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- Golden SA, Covington HE 3rd, Berton O, Russo SJ(2011)A standardized protocol for repeated social defeat stress in mice. Nat Prot 6:1183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RB, Smith SM, Chou SP, Saha TD, Jung J, Zhang H, Pickering RP, Ruan WJ, Huang B, Grant BF(2016)The epidemiology of DSM-5 posttraumatic stress disorder in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions-III. Social Psychiatry Psychiatr Epidemiol 51:1137–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BL, Krupnick JL, Chung J, Siddique J, Krause ED, Revicki D, Frank L, Miranda J(2006)Impact of PTSD comorbidity on one-year outcomes in a depression trial. J Clin Psychol 62:815–835. [DOI] [PubMed] [Google Scholar]
- Grimwood S, Lu Y, Schmidt AW, Vanase-Frawley MA, Sawant-Basak A, Miller E, McLean S, Freeman J, Wong S, McLaughlin JP, Verhoest PR(2011)Pharmacological characterization of 2-methyl-N-((2’-(pyrrolidin-1-ylsulfonyl)biphenyl-4-yl)methyl)propan-1-amine (PF-04455242), a high-affinity antagonist selective for kappa-opioid receptors. J Pharmacol Exp Therap 339:555–566. [DOI] [PubMed] [Google Scholar]
- Hames JL, Hagan CR, Joiner TE(2013)Interpersonal processes in depression. Annu Rev Clin Psychol 9:355–377. [DOI] [PubMed] [Google Scholar]
- Hirano K, Kimura R, Sugimoto Y, Yamada J, Uchida S, Kato Y, Hashimoto H, Yamada S(2005)Relationship between brain serotonin transporter binding, plasma concentration and behavioural effect of selective serotonin reuptake inhibitors. Br J Pharmacol 144:695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes GE, Hill-Smith TE, Lucki I(2010)Fluoxetine treatment induces dose dependent alterations in depression associated behavior and neural plasticity in female mice. Neurosci Lett 484:12–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook TL, Galarneau MR, Dye JL, Quinn K, Dougherty AL(2010)Morphine use after combat injury in Iraq and post-traumatic stress disorder. New Eng J Med 362:110–117. [DOI] [PubMed] [Google Scholar]
- Hoskins M, Pearce J, Bethell A, Dankova L, Barbui C, Tol WA, van Ommeren M, de Jong J, Seedat S, Chen H, Bisson JI(2015)Pharmacotherapy for post-traumatic stress disorder: systematic review and meta-analysis. Br J Psychiatry 206:93–100. [DOI] [PubMed] [Google Scholar]
- Hsu DT, Sanford BJ, Meyers KK, Love TM, Hazlett KE, Wang H, Ni L, Walker SJ, Mickey BJ, Korycinski ST, Koeppe RA, Crocker JK, Langenecker SA, Zubieta JK(2013)Response of the mu-opioid system to social rejection and acceptance. Mol Psychiatry 18:1211–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd YL.(2002)Subjects with major depression or bipolar disorder show reduction of prodynorphin mRNA expression in discrete nuclei of the amygdaloid complex. Mol Psychiatry 7:75–81. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Herman MM, Hyde TM, Bigelow LB, Weinberger DR, Kleinman JE(1997)Prodynorphin mRNA expression is increased in the patch vs matrix compartment of the caudate nucleus in suicide subjects. Mol Psychiatry 2:495–500. [DOI] [PubMed] [Google Scholar]
- Ibrahim L, Diazgranados N, Luckenbaugh DA, Machado-Vieira R, Baumann J, Mallinger AG, Zarate CA Jr(2011)Rapid decrease in depressive symptoms with an N-methyl-d-aspartate antagonist in ECT-resistant major depression. Prog Neuropsychopharmacol Biol Psychiatry 35:1155–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionescu DF, Swee MB, Pavone KJ, Taylor N, Akeju O, Baer L, Nyer M, Cassano P, Mischoulon D, Alpert JE, Brown EN, Nock MK, Fava M, Cusin C(2016)Rapid and sustained reductions in current suicidal ideation following repeated doses of intravenous ketamine: secondary analysis of an open-label study. J Clin Psychiatry 77:e719–e725. [DOI] [PubMed] [Google Scholar]
- Jackson KJ, Jackson A, Carroll FI, Damaj MI(2015)Effects of orally-bioavailable short-acting kappa opioid receptor-selective antagonist LY2456302 on nicotine withdrawal in mice. Neuropharmacology 97:270–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston CE, Herschel DJ, Lasek AW, Hammer RP Jr, Nikulina EM(2015)Knockdown of ventral tegmental area mu-opioid receptors in rats prevents effects of social defeat stress: implications for amphetamine cross-sensitization, social avoidance, weight regulation and expression of brain-derived neurotrophic factor. Neuropharmacology 89:325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp JF, Butters MA, Begley AE, Miller MD, Lenze EJ, Blumberger DM, Mulsant BH, Reynolds CF 3rd (2014) Safety, tolerability, and clinical effect of low-dose buprenorphine for treatment-resistant depression in midlife and older adults. J Clin Psychiatry 75:e785–e793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll AT, Carlezon WA Jr(2010)Dynorphin, stress, and depression. Brain Res 1314:56–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu H, Ohara A, Sasaki K, Abe H, Hattori H, Hall FS, Uhl GR, Sora I(2011)Decreased response to social defeat stress in mu-opioid-receptor knockout mice. Pharmacol Biochem Behav 99:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMotte AD, Taft CT, Weatherill RP, Eckhardt CI(2016)Social skills deficits as a mediator between PTSD symptoms and intimate partner aggression in returning veterans. J Family Psychol 31:105–110. [DOI] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C(2008)The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci 28:407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, Taylor SF, Phan KL, Britton JC, Fig LM, Bueller JA, Koeppe RA, Zubieta JK(2007)Altered central micro-opioid receptor binding after psychological trauma. Biol Psychiatry 61:1030–1038. [DOI] [PubMed] [Google Scholar]
- Lucki I, Dalvi A, Mayorga AJ(2001)Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice. Psychopharmacology 155:315–322. [DOI] [PubMed] [Google Scholar]
- Lutz PE, Kieffer BL(2013)Opioid receptors: distinct roles in mood disorders. Trends Neurosci 36:195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Li S, Valdez J, Chavkin TA, Chavkin C(2006)Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacology 31:1241–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechling AE, Arefin T, Lee HL, Bienert T, Reisert M, Ben Hamida S, Darcq E, Ehrlich A, Gaveriaux-Ruff C, Parent MJ, Rosa-Neto P, Hennig J, von Elverfeldt D, Kieffer BL, Harsan LA(2016)Deletion of the mu opioid receptor gene in mice reshapes the reward-aversion connectome. Proc Natl Acad Sci USA 113:11603–11608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Thompson ML, Shuster L(1982)Opioid-like analgesia in defeated mice. Science 215:1520–1522. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Nikulina EM, Takahashi A, Covington HE 3rd, Yap JJ, Boyson CO, Shimamoto A, de Almeida RM(2011)Gene expression in aminergic and peptidergic cells during aggression and defeat: relevance to violence, depression and drug abuse. Behav Genet 41:787–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, Aan Het Rot M, Collins KA, Mathew SJ, Charney DS, Iosifescu DV(2013)Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry 74:250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikulina EM, Hammer RP Jr, Miczek KA, Kream RM(1999)Social defeat stress increases expression of mu-opioid receptor mRNA in rat ventral tegmental area. Neuroreport 10:3015–3019. [DOI] [PubMed] [Google Scholar]
- Nikulina EM, Miczek KA, Hammer RP Jr(2005)Prolonged effects of repeated social defeat stress on mRNA expression and function of mu-opioid receptors in the ventral tegmental area of rats. Neuropsychopharmacology 30:1096–1103. [DOI] [PubMed] [Google Scholar]
- Nikulina EM, Arrillaga-Romany I, Miczek KA, Hammer RP Jr(2008)Long-lasting alteration in mesocorticolimbic structures after repeated social defeat stress in rats: time course of mu-opioid receptor mRNA and FosB/DeltaFosB immunoreactivity. Eur J Neurosci 27:2272–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyhuis PW, Gastpar M, Scherbaum N(2008)Opiate treatment in depression refractory to antidepressants and electroconvulsive therapy. J Clin Psychopharm 28:593–595. [DOI] [PubMed] [Google Scholar]
- Pietrzak RH, Naganawa M, Huang Y, Corsi-Travali S, Zheng MQ, Stein MB, Henry S, Lim K, Ropchan J, Lin SF, Carson RE, Neumeister A(2014)Association of in vivo kappa-opioid receptor availability and the transdiagnostic dimensional expression of trauma-related psychopathology. JAMA Psychiatry 71:1262–1270. [DOI] [PubMed] [Google Scholar]
- Ravindran LN, Stein MB(2009)Pharmacotherapy of PTSD: premises, principles, and priorities. Brain Res 1293:24–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renshaw KD, Campbell SB(2016)Deployment-related benefit finding and postdeployment marital satisfaction in military couples. Fam Process doi: 10.1111/famp.12249 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resendez SL, Dome M, Gormley G, Franco D, Nevarez N, Hamid AA, Aragona BJ(2013)mu-Opioid receptors within subregions of the striatum mediate pair bond formation through parallel yet distinct reward mechanisms. J Neurosci 33:9140–9149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SA, Erickson RL, Browne CA, Lucki I(2016)A role for the mu opioid receptor in the antidepressant effects of buprenorphine. Behav Brain Res 319:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorick-Kehn LM, et al. (2014)LY2456302 is a novel, potent, orally-bioavailable small molecule kappa-selective antagonist with activity in animal models predictive of efficacy in mood and addictive disorders. Neuropharmacology 77:131–144. [DOI] [PubMed] [Google Scholar]
- Rorick-Kehn LM, Witcher JW, Lowe SL, Gonzales CR, Weller MA, Bell RL, Hart JC, Need AB, McKinzie JH, Statnick MA, Suico JG, McKinzie DL, Tauscher-Wisniewski S, Mitch CH, Stoltz RR, Wong CJ(2015)Determining pharmacological selectivity of the kappa opioid receptor antagonist LY2456302 using pupillometry as a translational biomarker in rat and human. Int J Neuropsychopharm 18 pii: pyu036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal KH, Maguen S, Bertenthal D, Batki SL, Striebel J, Stein MB, Madden E, Neylan TC(2016)Observational evidence for buprenorphine’s impact on posttraumatic stress symptoms in veterans with chronic pain and opioid use disorder. J Clin Psychiatry 77:1182–1188. [DOI] [PubMed] [Google Scholar]
- Stanciu DN, Glass OM, Penders TM(2017)Use of buprenorphine in treatment of refractory depression: a review of current literature. Asian J Psychiatry 26:94–98. [DOI] [PubMed] [Google Scholar]
- Striebel JM, Kalapatapu RK(2014)The anti-suicidal potential of buprenorphine: a case report. Int J Psych Med 47:169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Damsteegt R, Achterberg EJ, Vanderschuren LJ(2011)Nucleus accumbens mu-opioid receptors mediate social reward. J Neurosci 31:6362–6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ(2006)Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci 9:519–525. [DOI] [PubMed] [Google Scholar]
- Venta A, Hatkevich C, Mellick W, Vanwoerden S, Sharp C(2016)Social cognition mediates the relation between attachment schemas and posttraumatic stress disorder. Psychol trauma 9:88–95. [DOI] [PubMed] [Google Scholar]
- Vivian JA, Miczek KA(1998)Effects of mu and delta opioid agonists and antagonists on affective vocal and reflexive pain responses during social stress in rats. Psychopharmacology 139:364–375. [DOI] [PubMed] [Google Scholar]
- Yovell Y, Bar G, Mashiah M, Baruch Y, Briskman I, Asherov J, Lotan A, Rigbi A, Panksepp J(2016)Ultra-low-dose buprenorphine as a time-limited treatment for severe suicidal ideation: a randomized controlled trial. Am J Psychiatry 173:491–498. [DOI] [PubMed] [Google Scholar]
- Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK(2006)A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63:856–864. [DOI] [PubMed] [Google Scholar]