Abstract
Background
Previous reports suggest that 5-hydroxytryptamine might play a role in the antidepressant actions of (R,S)-ketamine. However, its role in the antidepressant actions of (R)-ketamine, which is more potent than (S)-ketamine, is unknown. This study was conducted to examine whether 5-hydroxytryptamine depletion affects the antidepressant actions of (R)-ketamine in a chronic social defeat stress model.
Methods
An inhibitor of 5-hydroxytryptamine synthesis, para-chlorophenylalanine methyl ester hydrochloride (300 mg/kg, twice daily for 3 consecutive days), or vehicle was administered to control and chronic social defeat stress-susceptible mice. Levels of 5-hydroxytryptamine and its metabolite, 5-hydroxyindoleacetic acid, in mouse brain regions were measured using high-performance liquid chromatography. Furthermore, antidepressant effects of (R)-ketamine (10 mg/kg) in the vehicle- and para-chlorophenylalanine methyl ester hydrochloride-treated susceptible mice were assessed using tail suspension test and 1% sucrose preference test.
Results
para-Chlorophenylalanine methyl ester hydrochloride treatment caused marked reductions of 5-hydroxytryptamine and 5-hydroxyindoleacetic acid in the brain regions of control and chronic social defeat stress susceptible mice. In the tail suspension test, (R)-ketamine significantly attenuated the increased immobility time in the chronic social defeat stress-susceptible mice with or without 5-hydroxytryptamine depletion. In the sucrose preference test (2 and 5 days after a single dose), (R)-ketamine significantly enhanced reduced sucrose consumption in the chronic social defeat stress-susceptible mice with or without 5-hydroxytryptamine depletion.
Conclusions
These findings show that 5-hydroxytryptamine depletion did not affect the antidepressant effects of (R)-ketamine in a chronic social defeat stress model. Therefore, it is unlikely that 5-hydroxytryptamine plays a major role in the antidepressant actions of (R)-ketamine.
Keywords: antidepressant, (R)-ketamine, serotonin, stress
Significance Statement
The rapid and long-lasting antidepressant effects of (R,S)-ketamine in patients with treatment-resistant depression are the most important discovery in the field of depression research in half a century. Although previous studies suggest that 5-hydroixytryptamine (5-HT, serotonin) plays a role in the antidepressant effects of ketamine, the role of 5-HT in the antidepressant effects of (R)-ketamine remain unknown. Here we report that 5-HT depletion by para-chlorophenylalanine did not affect antidepressant actions of (R)-ketamine in a chronic social defeat stress model. It is, therefore, unlikely that 5-HT may play a major role in the antidepressant actions of (R)-ketamine.
Introduction
The N-methyl-D-aspartate receptor antagonist ketamine exhibits rapid and long-lasting antidepressant effects in treatment-resistant patients with major depressive disorder or bipolar disorder (Newport et al., 2015; Kishimoto et al., 2016). At present, ketamine is the most attractive antidepressant for the treatment of treatment-resistant depression (Monteggia and Zarate, 2015; Duman et al., 2016; Hashimoto, 2016a, 2016b, 2017), although the precise mechanisms underlying its antidepressant actions remain unknown.
(R,S)-Ketamine is a racemic mixture containing equal parts of (R)-ketamine and (S)-ketamine. (S)-Ketamine shows approximately 3- to 4-fold greater anesthetic potency and greater undesirable psychotomimetic side effects than (R)-ketamine (Domino, 2010; Hashimoto, 2016a). Meanwhile, (R)-ketamine shows greater potency and longer-lasting antidepressant effects than (S)-ketamine in animal models of depression (Zhang et al., 2014; Yang et al., 2015, Fukumoto et al., 2017; Yang et al., 2017a, 2017b, 2017c). Unlike (S)-ketamine, (R)-ketamine does not induce psychotomimetic side effects or exhibit abuse potential in rodents (Yang et al., 2015, 2016). Furthermore, we reported a marked reduction of dopamine D2/3 receptor binding in conscious monkey striatum after a single infusion of (S)-ketamine but not that of (R)-ketamine (Hashimoto et al., 2017), suggesting that (S)-ketamine-induced dopamine release might be associated with acute psychotomimetic and dissociative side effects in humans (Hashimoto et al., 2017). Therefore, (R)-ketamine could be a safer antidepressant in humans than (S)-ketamine (Hashimoto, 2016a, 2016b, 2017).
5-Hydroxytryptamine (5-HT, serotonin) plays a major role in the antidepressant effects of the antidepressant drugs currently being used. In the forced swimming test (FST), depletion of brain 5-HT by treatment with an inhibitor of 5-HT synthesis, para-chlorophenylalanine methyl ester hydrochloride (PCPA), attenuated the sustained (24 hour), but not acute (1 hour), reduction in the immobility time after a single dose of (R,S)-ketamine (25 mg/kg) (Gigliucci et al., 2013). Interestingly, the increase in the immobility time provoked by repeated restraint stress was blocked by a single dose of ketamine (25 mg/kg, 24 hours prior to FST), but ketamine’s effects were not abolished when rats were subjected to 5-HT depletion (Gigliucci et al., 2013). In contrast, Fukumoto et al. (2016) reported that the antidepressant-like effects of (R,S)-ketamine (30 mg/kg, 30 minutes prior to FST) were attenuated by depletion of 5-HT in the brain upon treatment with PCPA. Moreover, Pham et al. (2017) also reported that pretreatment with PCPA abolished (R,S)-ketamine (10 mg/kg, 24 hours prior to FST)-induced antidepressant-like effects in the FST. Although these 3 studies suggest the role of 5-HT in (R,S)-ketamine’s antidepressant effects in control rodents (Gigliucci et al., 2013; Fukumoto et al., 2016; Pham et al., 2017), it is unlikely that 5-HT plays a role in the antidepressant effects of (R,S)-ketamine in rodents with a depression-like phenotype (Gigliucci et al., 2013). Thus, there appears to be variation in the role of 5-HT in the antidepressant-like effects of (R,S)-ketamine depending on the baseline status of the rodents to which it is administered. In addition, the role of 5-HT in the antidepressant effects of (R)-ketamine in animal models such as a chronic social defeat stress (CSDS) model has not been reported.
To elucidate the role of 5-HT in the antidepressant actions of (R)-ketamine, this study was undertaken to examine whether 5-HT depletion in the brain caused by the administration of PCPA could influence the antidepressant effects of (R)-ketamine in a CSDS model.
Methods and Materials
Animals
Male adult C57BL/6 mice (n=72), aged 8 weeks (body weight 20–25 g, Japan SLC, Inc.) and male adult CD1 (ICR) mice (n=20), aged 13 to 15 weeks (body weight >40 g, Japan SLC, Inc.) were used. Animals were housed under controlled temperatures and 12-hour-light/-dark cycles (lights on between 7:00 am and 7:00 pm), with ad libitum food (CE-2; CLEA Japan, Inc) and water. The protocol was approved by the Chiba University Institutional Animal Care and Use Committee (permission no. 29–345). This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, USA. Animals were deeply anaesthetized with isoflurane before being killed by cervical dislocation. All efforts were made to minimize suffering.
Materials
(R)-Ketamine hydrochloride was prepared by recrystallization of (R,S)-ketamine (Ketalar, ketamine hydrochloride, Daiichi Sankyo Pharmaceutical Ltd) and D-(-)-tartaric acid, as described previously (Zhang et al., 2014). The purity of (R)-ketamine was determined by a high-performance liquid chromatography (HPLC) (CHIRALPAK IA, column size: 250x4.6 mm, mobile phase: n-hexane/dichloromethane/diethylamine [75/25/0.1], Daicel Corporation). The dose (10 mg/kg as hydrochloride) of (R)-ketamine dissolved in the physiological saline was used as previously reported (Zhang et al., 2014; Yang et al., 2015, 2016a, 2016b, 2017a, 2017b, 2017c). The dose of PCPA (300 mg/kg, 5-HT synthesis inhibitor, Sigma-Aldrich Co.) was used as previously reported (Fukumoto et al., 2016). Other reagents were purchased commercially.
CSDS Model
The procedure of CSDS was performed as previously reported (Zhang et al., 2015; Yang et al., 2015, 2016a, 2016b, Yang et al., 2017a, 2017b, 2017c; Ren et al., 2016). The C57BL/6 mice were exposed to a different CD1 aggressor mouse for 10 min/d for consecutive 10 days. When the social defeat session ended, the resident CD1 mouse and the intruder mouse were housed in one-half of the cage separated by a perforated Plexiglas divider to allow visual, olfactory, and auditory contact for the remainder of the 24-hour period. At 24 hours after the last session, all mice were housed individually. On day 11, a social interaction test was performed to identify subgroups of mice that were susceptible and unsusceptible to social defeat stress. This was accomplished by placing mice in an interaction test box (42×42 cm) with an empty wire-mesh cage (10×4.5 cm) located at one end. The movement of the mice was tracked for 2.5 minutes, followed by 2.5 minutes in the presence of an unfamiliar aggressor confined in the wire-mesh cage. The duration of the subject’s presence in the “interaction zone” (defined as the 8-cm-wide area surrounding the wiremesh cage) was recorded by a stopwatch. The interaction ratio was calculated as time spent in an interaction zone with an aggressor/time spent in an interaction zone without an aggressor. An interaction ratio of 1 was set as the cutoff: mice with scores <1 were defined as “susceptible” to social defeat stress and those with scores ≥1 were defined as “resilient.” Approximately 70% to 80% of mice were susceptible after CSDS. Susceptible mice were randomly divided in the subsequent experiments. Control C57BL/6 mice not exposed CSDS were housed in the cage before the behavioral tests.
Treatment and Measurement of 5-HT and 5-HIAA in Mouse Brain by HPLC
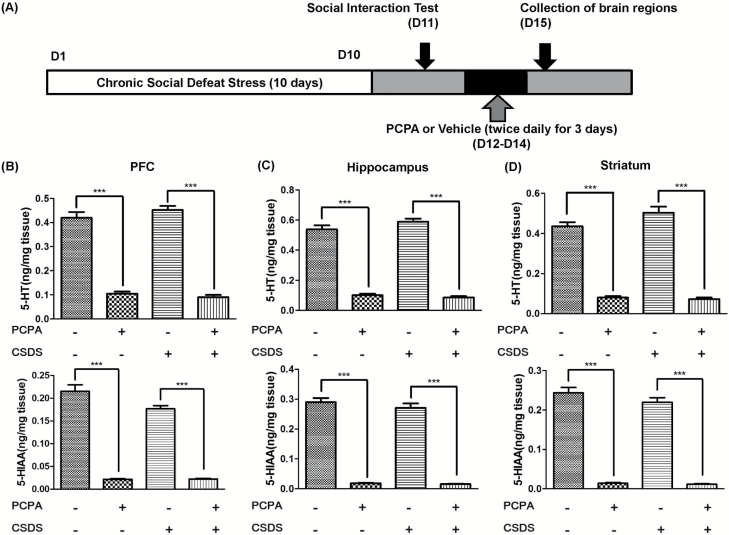
Control mice and CSDS susceptible mice were divided into 2 groups, respectively. Vehicle [0.5% carboxymethylcellulose (CMC)] or PCPA (300 mg/kg, twice daily (9:00 am and 7:00 pm) for 3 consecutive days) was administered i.p. into the control and CSDS susceptible mice (day 12–14). On day 15, all mice were killed by decapitation after isoflurane anesthesia, and brain regions including prefrontal cortex (PFC), hippocampus, and striatum were collected. These brain samples were stored at -80℃ before assay (Figure 1A).
Figure 1.
Schedule of a chronic social defeat stress (CSDS) model, treatment, and high-performance liquid chromatography (HPLC) measurement. (A) CSDS was performed from day 1 to day 10, and the social interaction test (SIT) was performed on day 11. Vehicle (0.5% carboxymethylcellulose [CMC]) or para-chlorophenylalanine methyl ester hydrochloride (PCPA) (300 mg/kg, twice daily [9:00 am and 7:00 pm] for 3 consecutive days) was administered i.p. in the susceptible mice from day 12 to day 14. All mice were sacrificed by decapitation, and brain samples were collected on day 15. (B) Prefrontal cortex (PFC), (C) hippocampus, (D) striatum. The values represent the mean ± SEM (n=8). *P<.05, **P<.01, ***P<.001.
The brain samples were homogenized in 0.2 M perchloric acid (HClO4) containing 100 μM disodium EDTA and 100 ng/mL isoproterenol (internal standard) and were then centrifuged at 20 000 × g for 15 minutes at 4°C. The supernatants were filtered through a 0.45-μm-pore membrane (Millex-LH, 4 mm; Millipore) and analyzed for 5-HT and 5-HIAA by HPLC coupled with electrochemical detection. The HPLC system consisted of a liquid chromatograph pump (HTEC-500, Eicom), a reversed phase column (Eicompak SC-5ODS 150 × 3.0 mm; Eicom), and a data processor (EPC-500, Eicom). The mobile phase was 0.1 M acetate-citric acid buffer (pH 3.5) containing 13% methanol, 5 mg/L disodium EDTA, and 190 mg/L sodium octyl sulfate.
Treatment and Behavioral Tests
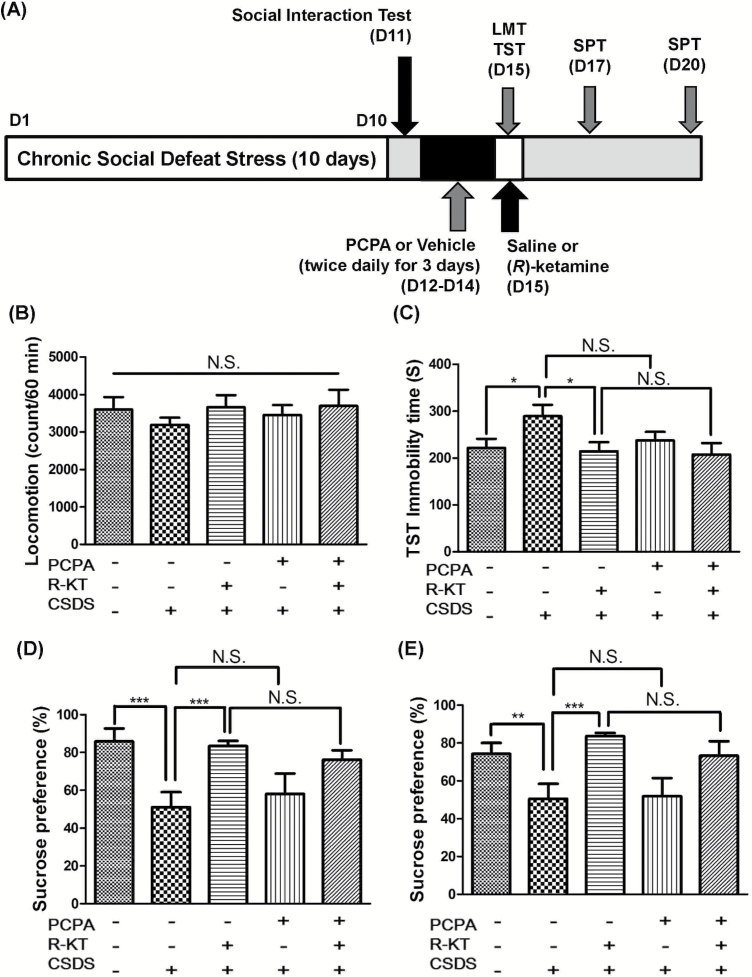
The CSDS susceptible mice were divided into 4 groups; vehicle + saline group, vehicle + (R)-ketamine group, PCPA + saline group, and PCPA + (R)-ketamine group. Vehicle (0.5% CMC) or PCPA (300 mg/kg twice daily [9:00 am and 7:00 pm] for 3 consecutive days) was administered i.p. to the susceptible mice after CSDS (days 12–14). Subsequently, saline (10 mL/kg) or (R)-ketamine (10 mg/kg) was administered i.p. into mice (day 15) (Figure 2A). Behavioral tests, including locomotion test (LMT), tail suspension test (TST), and 1% sucrose preference test (SPT), were performed as reported previously (Zhang et al., 2015; Yang et al., 2015, 2017a, 2017b, 2017c; Ren et al., 2016). LMT and TST were performed 2 and 4 hours after a single injection of (R)-ketamine or saline, respectively. SPT was performed 2 and 5 days after a single injection of (R)-ketamine or saline. Behavioral tests were performed by 2 observers who were blinded to the group assignment of mice.
Figure 2.
Effects of 5-hydroxytryptamine (5-HT) deletion in the antidepressant effects of (R)-ketamine in a chronic social defeat stress (CSDS) model. (A) CSDS was performed from day 1 to day 10, and the social interaction test (SIT) was performed on day 11. Vehicle (0.5% carboxymethylcellulose [CMC]) or para-chlorophenylalanine methyl ester hydrochloride (PCPA) (300 mg/kg, twice daily [9:00 am and 7:00 pm] for 3 consecutive days) was administered i.p. in the susceptible mice from day 12 to day 14. Saline (10 mL/kg) or (R)-ketamine (10 mg/kg) was administered i.p. into mice on day 15. LMT and TST were performed 2 and 4 hours after a single injection of (R)-ketamine or saline, respectively. SPT was performed 2 and 5 days after a single injection of (R)-ketamine or saline. (B) Locomotion test (LMT) (day 15). (C) Tail suspension test (TST) (day 15). (D) Sucrose preference test (SPT) (day 17). (E) SPT (day 20). The values represent the mean ± SEM (n = 8). *P<.05, **P<.01, ***P<.001. N.S., not significant; R-KT, (R)-ketamine.
Locomotion
The locomotor activity was measured by an animal movement analysis system SCANETMV-40 (MELQUEST Co., Ltd). The mice were placed in experimental cages (length × width × height: 560×560×330 mm). The cumulative locomotor activity counts were recorded for 60 minutes. Cages were cleaned between testing session.
TST
A small piece of adhesive tape was placed approximately 2 cm from the tip of the tail for mouse. A single hole was punched in the tape, and mice were hung individually on a hook. The immobility time was recorded for 10 minutes. Mice were considered immobile only when they hung passively and completely motionless.
SPT
Mice were exposed to water and 1% sucrose solution for 48 hours, followed by 4 hours of water and food deprivation and a 1-hour exposure to 2 identical bottles (water and 1% sucrose solution). The bottles containing water and sucrose were weighed before and at the end of this period. The sucrose preference was calculated as a percentage of sucrose solution consumption to the total liquid consumption.
Statistical Analysis
The data show as the mean ± SEM. Analysis was performed using PASW Statistics 20 (formerly SPSS Statistics; SPSS). The data of 5-HT and 5-HIAA were analyzed using the 2-way ANOVA followed by posthoc Tukey test. The behavioral data were analyzed using the 1-way ANOVA followed by posthoc Tukey test. P < .05 was considered statistically significant.
Results
Effects of PCPA on the Levels of 5-HT and 5-HIAA in the Brain Regions
The effects of PCPA treatment on the tissue levels of 5-HT and 5-HIAA in the PFC, hippocampus, and striatum were examined. PCPA treatment caused significant reduction of 5-HT and 5-HIAA in these 3 regions (Figure 1B–D). Two-way ANOVA (PCPA and stress) showed statistical results (PFC: 5-HT, PCPA, F1,32 =452.76, P<.001, stress, F1,32=2.27, P=.14, interaction, F1,32=0.30, P=.59; 5-HIAA, PCPA, F1,32 =460.75, P<.001, stress, F1,32 =5.80, P=.02, interaction, F1,32 =5.53, P=.03; hippocampus: 5-HT, PCPA, F1,32=669.57, P<.001, stress, F1,32=3.37, P=.08, interaction, F1,32=0.95, P=.34, 5-HIAA, PCPA, F1,32 =680.08, P<.001, stress, F1,32 =0.63, P=.44, interaction, F1,32 =1.15, P=.29; striatum: 5-HT, PCPA, F1,32=434.70, P<.001, stress, F1,32=3.96, P=.06, interaction, F1,32=2.56, P=.12, 5-HIAA, PCPA, F1,32 =609.74, P<.001, stress, F1,32 =1.49, P=.23, interaction, F1,32 =2.20, P=.15). These data suggest that the reductions of 5-HT in the brain regions by PCPA were not affected by CSDS.
Effects of 5-HT Reduction in the Antidepressant Effect of (R)-Ketamine in a CSDS Model
Next, we examined the effects of PCPA-induced 5-HT reduction in the antidepressant effects of (R)-ketamine (10 mg/kg) in a CSDS model (Figure 2A). Locomotion showed no difference (F4,40 =0.43, P=.79) among the 5 groups (Figure 2B). One-way ANOVA of TST data showed a statistical significance (F4,40 =2.41, P<.01) among the 5 groups (Figure 2C). Posthoc tests showed that (R)-ketamine (10 mg/kg) significantly attenuated the increased immobility times in susceptible mice after CSDS (Figure 2C). However, there were no differences between vehicle + (R)-ketamine group and PCPA + (R)-ketamine group in the TST immobility times (Figure 2C). One-way ANOVA of SPT data showed statistical significances (2 days after a single injection: F4,40 =5.06, P=.003, 5 days after a single injection: F4,40=4.36, P=.01) among 5 groups (Figure 2D–E). Posthoc tests showed that sucrose preference in vehicle + (R)-ketamine-treated group was significantly higher than in the vehicle + saline-treated group (Figure 2D–E). Furthermore, sucrose preference in the vehicle + (R)-ketamine-treated group was not different from the PCPA + (R)-ketamine-treated group (Figure 2D–E). These results suggest that depression-like phenotype in the susceptible mice after CSDS was not affected by 5-HT depletion and that depletion of 5-HT in the brain by PCPA did not abolish the antidepressant effects of (R)-ketamine in a CSDS model.
Discussion
In the present study, we demonstrated that reductions of 5-HT and 5-HIAA in mouse brain upon the administration of PCPA did not affect the depression-like phenotype in the susceptible mice after CSDS and that PCPA-induced 5-HT depletion did not abolish the antidepressant effects of (R)-ketamine in the CSDS susceptible mice. These findings suggest that 5-HT does not play a major role in the antidepressant actions of (R)-ketamine in a CSDS model. To our knowledge, this is the first report showing that 5-HT depletion in the brain does not affect the antidepressant effects of (R)-ketamine in mice with a depression-like phenotype.
A previous study showed that the depletion of 5-HT by PCPA did not abolish the acute antidepressant effects of (R,S)-ketamine (25 mg/kg, 1 h prior to FST) in control SD rats (Gigliucci et al., 2013). In contrast, depletion of 5-HT by PCPA abolished the acute antidepressant effects of (R,S)-ketamine (30 mg/kg, 30 minutes prior to FST) in control C57BL/6J mice (Fukumoto et al., 2016). The reasons underlying this discrepancy are currently unknown. First, the dose (25 mg/kg for rats vs 30 mg/kg for mice) of (R,S)-ketamine may have contributed to this discrepancy, since these doses can increase locomotor activity after a single administration (Yang et al., 2015; Fukumoto et al., 2017). Second, the species difference (rats vs mice) may have contributed to this discrepancy. Therefore, acute hyperactivity should be taken into consideration to examine the acute antidepressant effects of (R,S)-ketamine and its enantiomers.
Using Flinders Sensitive Line (FSL) rats (a genetic model of depression), du Jardin et al. (2016b) examined the effects of 5-HT depletion by PCPA on the antidepressant effects of (S)-ketamine (15 mg/kg). The depression-like phenotype in FSL rats was not associated with 5-HT depletion. Interestingly, the acute (1 hour) and sustained (24 hours) antidepressant effects of (S)-ketamine (15 mg/kg) in FSL rats were abolished by 5-HT depletion, suggesting that the acute and sustained antidepressant-like effects of (S)-ketamine in FSL rats depend on the endogenous 5-HT concentration (du Jardin et al., 2016a, 2016b). However, our present results indicate that acute and long-lasting (5 days after a single dose) antidepressant effects of (R)-ketamine (10 mg/kg) in a CSDS model in male C57BL/6 mice occur independently of brain 5-HT depletion. Interestingly, 5-HT depletion in the brain did not affect the sustained antidepressant effects of (R,S)-ketamine (25 mg/kg, 24 hours prior to FST) on the increased immobility time caused by repeated restraint stress (Gigliucci et al., 2013). Although the reasons for these discrepancies are currently unknown, there are several factors that could have contributed to them, such as different models (control animals vs genetic rat model vs stress model) and different doses and isomers of ketamine (25 mg/kg vs 30 mg/kg vs 10 mg/kg; (R,S)-ketamine vs (S)-ketamine vs (R)-ketamine). Therefore, further detailed studies on the role of 5-HT in the acute and sustained antidepressant effects of (R,S)-ketamine and its enantiomers in rodents with a depression-like phenotype are needed.
Fukumoto et al. (2016) reported the role of 5-HT neurons in the dorsal raphe nucleus (DRN) regulated by the medial PFC (mPFC)–DRN projections in the acute antidepressant effects of (R,S)-ketamine (30 mg/kg, 30 minutes prior to FST). In addition, antidepressant effects of (R,S)-ketamine were reported to be blocked by an α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor antagonist, NBQX (10 mg/kg). Interestingly, microinjection of (R,S)-ketamine into mPFC significantly increased the c-Fos expression in 5-HT neurons in the DRN, and the effect of (R,S)-ketamine was attenuated by the microinjection of NBQX into the mPFC. These results suggest that the activation of 5-HT neurons in the DRN regulated by stimulation of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor in the mPFC may be involved in the antidepressant effects of (R,S)-ketamine (Fukumoto et al., 2016). Moreover, Pham et al. (2017) reported an increase (144%) in the extracellular 5-HT levels in the mPFC after a single dose of (R,S)-ketamine (10 mg/kg). In addition, pretreatment with NBQX blunted the effects of intra-mPFC ketamine on both the swimming duration in the FST and extracellular 5-HT in the mPFC, suggesting a key role of cortical 5-HT release in (R,S)-ketamine’s antidepressant-like activity (Pham et al., 2017). However, these 2 studies used control animals, but not animals with a depression-like phenotype. Therefore, further detailed studies on the role of 5-HT in the mPFC–DRN projections on the antidepressant effects of (R,S)-ketamine and its 2 enantiomers in animals with a depression-like phenotype are needed.
This paper has some limitations. Although we show 5-HT-independent antidepressant actions of (R)-ketamine in a CSDS model, the precise molecular mechanisms underlying (R)-ketamine’s antidepressant actions are currently unknown. A recent study showed that mTORC1 signaling does not play a role in the antidepressant actions of (R)-ketamine, but not (S)-ketamine, in a CSDS model (Yang et al., 2017c). In addition, it is reported that the gut-microbiota-brain axis may play a role in the antidepressant actions of (R)-ketamine (Yang et al., 2017b). Further studies on molecular mechanisms of (R)-ketamine’s antidepressant actions are necessary.
In conclusion, this study suggests that 5-HT depletion by PCPA did not affect the acute and long-lasting antidepressant effects of (R)-ketamine in a CSDS model. Therefore, it is unlikely that 5-HT plays a major role in the antidepressant actions of (R)-ketamine.
Statement of Interest
Dr. Hashimoto is an inventor on a filed patent application on “The use of (R)-ketamine in the treatment of psychiatric diseases” by Chiba University. Dr. Hashimoto has received research support from Mochida, Otsuka, and Taisho. Other authors declare no conflict of interest.
Acknowledgments
This study was supported by the Strategic Research Program for Brain Sciences from Japan Agency for Medical Research and Development, AMED (to K.H.). Dr. Chao Dong was supported by the Uehara Memorial Foundation (Tokyo, Japan).
References
- du Jardin KG, Liebenberg N, Müller HK, Elfving B, Sanchez C, Wegener G (2016a) Differential interaction with the serotonin system by S-ketamine, vortioxetine, and fluoxetine in a genetic rat model of depression. Psychopharmacology (Berl) 233:2813–2825. [DOI] [PubMed] [Google Scholar]
- du Jardin KG, Müller HK, Elfving B, Dale E, Wegener G, Sanchez C (2016b) Potential involvement of serotonergic signaling in ketamine’s antidepressant actions: a critical review. Prog Neuropsychopharmacol Biol Psychiatry 71:27–38. [DOI] [PubMed] [Google Scholar]
- Domino EF.(2010)Taming the ketamine tiger. Anesthesiology 113:678–684. [DOI] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK, Sanacora G, Krystal JH(2016)Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med 22:238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto K, Iijima M, Chaki S(2016)The antidepressant effects of an mGlu2/3 receptor antagonist and ketamine require AMPA receptor stimulation in the mPFC and subsequent activation of the 5-HT neurons in the DRN. Neuropsychopharmacol 41:1046–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto K, Toki H, Iijima M, Hashihayata T, Yamaguchi JI, Hashimoto K, Chaki S(2017)Antidepressant potential of (R)-ketamine in rodent models: comparison with (S)-ketamine. J Pharmacol Exp Ther 361:9–16. [DOI] [PubMed] [Google Scholar]
- Gigliucci V, O’Dowd G, Casey S, Egan D, Gibney S, Harkin A(2013)Ketamine elicits sustained antidepressant-like activity via a serotonin-dependent mechanism. Psychopharmacology (Berl) 228:157–166. [DOI] [PubMed] [Google Scholar]
- Hashimoto K. (2016a) R-ketamine: a rapid-onset and sustained antidepressant without risk of brain toxicity. Psychol Med 46:2449–2451. [DOI] [PubMed] [Google Scholar]
- Hashimoto K. (2016b) Ketamine’s antidepressant action: beyond NMDA receptor inhibition. Expert Opin Ther Targets 20:1389–1392. [DOI] [PubMed] [Google Scholar]
- Hashimoto K.(2017)Chapter 4. Rapid antidepressant activity of ketamine beyond NMDA receptor. In: The NMDA receptors (Hashimoto K, ed), pp 69–81. New York: Humana Press. [Google Scholar]
- Hashimoto K, Kakiuchi T, Ohba H, Nishiyama S, Tsukada H(2017)Reduction of dopamine D2/3 receptor binding in the striatum after a single administration of esketamine, but not R-ketamine: a PET study in conscious monkeys. Eur Arch Psychiatry Clin Neurosci 267:173–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T, Chawla JM, Hagi K, Zarate CA, Kane JM, Bauer M, Correll CU(2016)Single-dose infusion ketamine and non-ketamine N-methyl-D-aspartate receptor antagonists for unipolar and bipolar depression: a meta-analysis of efficacy, safety and time trajectories. Psychol Med 46:1459–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteggia LM, Zarate C Jr(2015)Antidepressant actions of ketamine: from molecular mechanisms to clinical practice. Curr Opin Neurobiol 30:139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport DJ, Carpenter LL, McDonald WM, Potash JB, Tohen M, Nemeroff CB, APA Council of Research Task Force on Novel Biomarkers and Treatments (2015)Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry 172:950–966. [DOI] [PubMed] [Google Scholar]
- Pham TH, Mendez-David I, Defaix C, Guiard BP, Tritschler L, David DJ, Gardier AM(2017)Ketamine treatment involves medial prefrontal cortex serotonin to induce a rapid antidepressant-like activity in BALB/cJ mice. Neuropharmacology 112:198–209. [DOI] [PubMed] [Google Scholar]
- Ren Q, Ma M, Ishima T, Morisseau C, Yang J, Wagner KM, Zhang JC, Yang C, Yao W, Dong C, Han M, Hammock BD, Hashimoto K(2016)Gene deficiency and pharmacological inhibition of soluble epoxide hydrolase confers resilience to repeated social defeat stress. Proc Natl Acad Sci USA 2113:E1944–E1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Han M, Zhang JC, Ren Q, Hashimoto K (2016a) Loss of parvalbumin-immunoreactivity in mouse brain regions after repeated intermittent administration of esketamine, but not R-ketamine. Psychiatric Res 239:281–283. [DOI] [PubMed] [Google Scholar]
- Yang B, Zhang JC, Han M, Yao W, Yang C, Ren Q, Ma M, Chen QX, Hashimoto K (2016b) Comparison of R-ketamine and rapastinel antidepressant effects in the social defeat stress model of depression. Psychopharmacology (Berl) 233: 3647–3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Qu Y, Abe M, Nozawa D, Chaki S, Hashimoto K (2017a) (R)-ketamine shows greater potency and longer lasting antidepressant effects than its metabolite (2R,6R)-hydroxynorketamine. Biol Psychiatry 82:e43–e44. [DOI] [PubMed] [Google Scholar]
- Yang C, Qu Y, Fujita Y, Ren Q, Ma M, Dong C, Hashimoto K (2017b) Possible role of gut-microbiota in the antidepressant effects of (R)-ketamine in a social defeat stress model. Transl Psychiatry. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Ren Q, Qu Y, Zhang JC, Ma M, Dong C, Hashimoto K (2017c) Mechanistic target of rapamycin-independent antidepressant effects of (R)-ketamine in a social defeat stress model. Biol Psychiatry doi: 10.1016/j.biopsych.2017.05.016. [DOI] [PubMed] [Google Scholar]
- Yang C, Shirayama Y, Zhang JC, Ren Q, Yao W, Ma M, Dong C, Hashimoto K(2015)R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry 5:e632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JC, Li SX, Hashimoto K(2014)R(-)-ketamine shows greater potency and longer lasting antidepressant effects than S(+)-ketamine. Pharmacol Biochem Behav 116:137–141. [DOI] [PubMed] [Google Scholar]
- Zhang JC, Yao W, Dong C, Yang C, Ren Q, Ma M, Han M, Hashimoto K(2015)Comparison of ketamine, 7,8-dihydroxyflavone, and ANA-12 antidepressant effects in the social defeat stress model of depression. Psychopharmacology (Berl) 232:4325–4335. [DOI] [PubMed] [Google Scholar]