Abstract
Naïve CD4+ helper T (TH) cells, upon activation by antigen-presenting cells (APC), differentiate into different types of effector cells that are characterized by their distinct cytokine production profiles and immune regulatory functions. In addition to TH1 and TH2 cells, a third subset of effector TH cells has recently been described and termed TH17. Since their identification, TH17 cells have emerged as crucial players in infectious, inflammatory, and autoimmune diseases, and cancer. In this review, we summarize the latest discoveries on the cytokine-mediated regulation and transcriptional programming of TH17 cells and their roles in different immune responses and diseases.
Keywords: pro-inflammatory TH17 cells, CD4+ T helper, antigen-presenting cells
Overview
CD4+ T helper (TH) cells play a crucial role in regulating immune responses by orchestrating the function of other immune cell types. Effector TH cell subsets are characterized by their differential cytokine production profiles and immune regulatory functions. For almost two decades, the TH1–TH2 paradigm has prevailed in immunology.1 TH1 cells, through interferon gamma (IFN-γ) production, regulate antigen presentation and immunity against intracellular pathogens, whereas TH2 cells, which produce interleukin-4 (IL-4), IL-5, and IL-13, mediate certain humoral responses and immunity against parasites. However, observations by different groups indicated that pro-inflammatory cytokines IL-17 and IL-17F were expressed by distinct TH cells, which did not express either IFN-γ or IL-4.2,3 Moreover, even though TH1 cells have been linked to the development of autoimmunity, mice deficient in IFN-γ, IFN-γR, or signaling transducer and activator of transcription 1 (STAT1) were still susceptible to experimental autoimmune encephalomyelitis (EAE) or collagen-induced arthritis (CIA) models.4–7 On the other hand, mice deficient in Inducible Costimulator (ICOS) or IL-23 were resistant to the development of CIA, associated with reduced IL-17 expression.8,9 Moreover, IL-23 was found to selectively expand a unique population of TH cells that selectively express IL-17 and IL-17F and play a pathogenic role in EAE.10 These data provided the grounds to the subsequent discovery of TH17 cells as a TH lineage independent of TH1 or TH2 cells.11,12
In the past 2 to 3 years, there has been rapid, exciting progress in understanding the differentiation and function of TH17 cells. TH17 cells produce IL-17, IL-17F, IL-21, and IL-22 and induce the recruitment of neutrophils and macrophages to tissues.13 TH17 cells are regulated by unique cytokine stimuli and transcriptional machinery. In this review, we summarize the latest findings on the developmental regulation of TH17 cells by cytokines and transcription factors and the roles of TH17 cells in different diseases.
Regulation of TH17 Cell Differentiation by Cytokines
As mentioned, TH17 cells develop via an independent lineage from TH1 or TH2 cells. Therefore, the understanding of the generation of these cells has been a major research focus over the past few years. Differentiation of TH cells is steered by the innate immune system, which provides T cell receptor (TCR) and costimulatory signals as well as an appropriate cytokine microenvironment that ultimately leads to the preferential induction of one specific cell lineage over the other (Fig. 1). IFN-γ, IL-12, or IL-4, which are important for TH1 and TH2 differentiation, have been shown to be dispensable for TH17 cell differentiation in vitro and in vivo, providing one of the first clues that TH17 cells are indeed an independent lineage from TH1 or TH2 cells.11,12 In this section we will focus on the role of multiple cytokines in driving or inhibiting the differentiation of TH17 cells (Fig. 1).
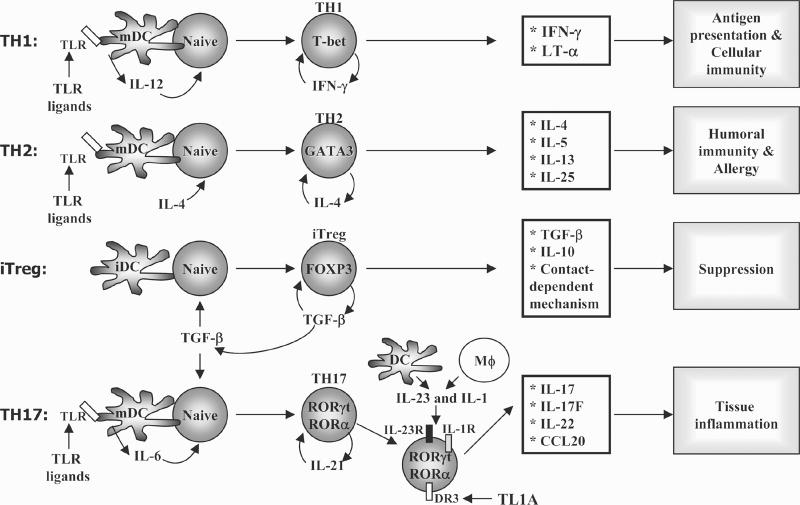
Figure 1.
Differentiation of T helper cell subsets. Naïve CD4+ T cells, upon encountering their cognate antigens presented on professional antigen-presenting cells (APC), differentiate into effector cells (TH1, TH2, TH17, iTreg) that are characterized by their cytokine production profiles and immune regulatory functions. TH1 cells produce IFN-γ and regulate antigen presentation and immunity against intracellular pathogens, whereas TH2 cells, which produce IL-4, IL-5, and IL-13, mediate humoral responses and immunity against parasites, and are important mediators of allergic diseases. TH17 cells express IL-17, IL-17F, IL-21, and IL-22 (and IL-26 in humans) and participate in inflammation and autoimmunity processes. iTregs express Foxp3 transcription factor and mediate immune suppression by secretion of TGF-β and IL-10 and by contact-dependent mechanisms. TGF-β, transforming growth factor-β.
Positive Regulators of TH17 Differentiation
IL-6 and TGF-β
Recent work from several groups indicates that IL-6 and transforming growth factor-β (TGF-β) potently initiate TH17 differentiation.14–16 Veldhoen and colleagues showed that TGF-β and pro-inflammatory cytokine IL-6 support the differentiation of IL-17-producing T cells from naïve cells, and that tumor necrosis factor alpha (TNF-α) and IL-1β further amplified IL-17 expression.15 Involvement of TGF-β in TH17 differentiation was unexpected, since TGF-β was considered an immunosuppressive cytokine with the ability to suppress T cell activation17 and induce the development of Forkhead box P3 (Foxp3+) positive regulatory T cells (Treg).14,18 Using transcription factor Foxp3–green fluorescent protein (GFP) reporter mice to track Treg development, Bettelli and colleagues showed that IL-6 is capable of inhibiting TGF-β-dependent Foxp3+ Treg cell induction.14 When the phenotype of the cells cultured with TGF-β plus IL-6 was examined, the majority of them expressed IL-17. These studies suggested that there is not only functional antagonism between TH17 and Treg cells in autoimmunity, but also a dichotomy in generation of these cells, depending on whether they are activated in the presence of pro-inflammatory conditions.14 However, even though it has been demonstrated that TGF-β is required for induction of TH17 cells, a recent report by Zhou and colleagues shows that increasing concentrations of this cytokine lead to decreased expression of IL-23R and increased levels of Foxp3, shifting the TH17 differentiation pathway to the induction of regulatory T cells.19 Thus, the balance between TGF-β and pro-inflammatory cytokines such as IL-6 might influence the final outcome in the differentiation process from naïve T helper cells to different effector subsets.
The importance of TGF-β and IL-6 in differentiation of TH17 cells was confirmed in vivo. Bettelli and colleagues reported that immunization of TGF-β transgenic mice with myelin oligodendrocyte glycoprotein (MOG) in complete Freund’s adjuvant (CFA) resulted in increased TH17 response and exacerbated EAE.14 Mice whose T cells cannot respond to TGF-β signaling lack TH17 cells and do not develop EAE.15 Moreover, specific deletion of TGF-β in the T cell lineage compartment leads to induction of lethal immunopathology, characterized by increased activation and proliferation of TH1 and TH2 cells.20 Furthermore, these mice failed to mount in vivo TH17 responses and were therefore resistant to EAE, further suggesting an essential role of TGF-β produced by T cells in the control of TH cell differentiation.20 Consistent with the requirement of IL-6 in TH17 cell generation, IL-6-deficient mice failed to develop TH17 responses, had significantly increased numbers of Foxp3+ regulatory T cells, and were resistant to EAE.21,22
A series of recent studies indicate the activity of retinoic acid produced by mucosal dendritic cells (DCs) in inhibiting TGF-β-dependent TH17 cell generation while promoting Foxp3+ Treg cell differentiation.23–27 Retinoic acid induces Foxp3 and inhibits RAR-related orphan receptor gamma (RORγ) expression and IL-17 production. In the intestine and mesenteric lymph nodes, CD103+ DCs secrete retinoic acid and produce TGF-β.28 These CD103+ DCs are strong inducers of Foxp3+ Treg cells, but not IL-17-producing cells. The ability of mucosal DCs to produce retinoic acid may explain why the relative distribution ofTH17 cells in the gut is limited. It is possible that DCs use retinoic acid as a T cell modulator, to suppress excessive inflammation in the gut by promoting Foxp3 expression.
IL-21
It has been recently demonstrated that IL-21 is not only expressed by TH17 cells, but also controls the generation ofTH17 cells in vitro and in vivo.21,29,30 IL-6 induced IL-21 production in a STAT3-dependent and RORγ-independent manner; IL-6-deficient cells did not produce IL-17 or IL-21 in vivo.29 IL-21 in combination with TGF-β induces the expression of IL-23R and RORγ, leading to IL-17 production from naïve CD4 cells.29,30 Similar to IL-6, IL-21 inhibits Foxp3 expression induced by TGF-β. Loss of IL-21 expression or its receptor resulted in defective IL-17 production in vitro and in vivo.21,29 Thus, IL-6 can induce IL-21 production by CD4 T cells, which further amplifies IL-17 responses in an autocrine fashion in a similar way as IFN-γ or IL-4 mediate amplification of TH1 or TH2 responses respectively (Fig. 1). However, Korn and colleagues demonstrated that deletion of regulatory T cells from IL-6-deficient mice leads to the reappearance of TH17, suggesting IL-21 can substitute IL-6 in the generation of TH17 cells and this process is IL-6-independent.21
IL-23
IL-23, composed of IL-12 p40 and p19 chain,31 binds its heterodimeric receptor complex consisting of IL-12Rβ1 and IL-23R.32 IL-23 is required in vivo for sustained inflammation and is involved in pathogenesis of several autoimmune diseases, since IL-23-deficient mice are resistant to experimental autoimmune encephalomyelitis,33 collagen-induced arthritis,9 and inflammatory bowel disease (IBD).34 In all cases, resistance to disease in IL-23-deficient mice is correlated with a defect in IL-17 expression,9,10 demonstrating the link between IL-23, TH17 cells, and autoimmunity (see below).
The precise function of IL-23 in TH17 regulation is still not entirely clear. IL-23 failed to induce the differentiation of naïve T cells into TH17 cells.14–16 This is likely due to the fact that naïve T cells do not express IL-23R and the induction of IL-23R expression depends on factors that initiate TH17 differentiation (IL-6 and IL-21).22,29,30 Moreover, IL-23 synergizes with IL-6 to induce TH17 differentiation.22 IL-23, though not required for the initial differentiation, may thus play a role in the survival and expansion of TH17 cells.
IL-1 and IL-18
IL-1 has two forms, IL-1α and IL-1β, encoded by separate genes. However, they share similar three-dimensional structures and exhibit almost identical functions.35–37 The major sources of IL-1 are activated myeloid cells, and its production can be rapidly induced by bacterial lipopolysaccharide (LPS), TNF-α, interferons α, β, and γ, and IL-1 itself. IL-1 cytokines bind similarly to the two known IL-1 receptors, IL-1RI and IL-1RII. IL-1RI mediates cell activation with the involvement of the IL-1R accessory protein (IL-1RAcP), while IL-1RII lacks cell-activating properties and acts exclusively as a decoy receptor. Initial studies using IL-1RI-deficient mice showed that IL-1 plays an important role in EAE.38 Recently, IL-1β was found to promote TH17 development/expansion in the presence of IL-6 and TGF-β.15 Sutton and colleagues further demonstrated that the induction of antigen-specific TH17 cells, but not TH1 or TH2 cells, is abrogated in IL-1RI-deficient mice, which is consistent with the low incidence of EAE in these mice compare to wild-type mice.39 Moreover, IL-23 cooperates with IL-1α or IL-1β and enhances IL-17 production, independent of T cell receptor stimulation.39 IL-1 can suppress the inhibitory effects of IL-2 on IL-17 production, through induction of IL-1R, IL-23R, and RORγt.40 However, the exact mechanism by which IL-1 induces or maintains TH17 cells has not been fully elucidated.
IL-18 is another member of the IL-1 proinflammatory cytokine family. IL-18 receptor comprises an IL-18Rα subunit and a signaling IL-18Rβ subunit (also called IL-1RAcPL and IL-1R7). A large body of studies has indicated a critical role for IL-18 in mediating neuroinflammation in the central nervous system under pathological conditions.41 In the EAE model, the disease has been significantly attenuated after administration of neutralizing anti-IL-18 antibodies42 or in IL-18-deficient mice.43 More recently, Gutcher and colleagues demonstrated that, whereas IL-18–deficient mice were susceptible to EAE, IL-18Rα-deficient mice were resistant to the development of the disease.44 Engagement of IL-18Rα on antigen-presenting cells was required for the generation of pathogenic IL-17-producing T cells through an IL-23-dependent mechanism.44 Moreover, IL-18 synergizes with IL-23 in the induction of IL-17-producing CD4 T cells.45
TL1A-DR3
TL1A, a TNF-family cytokine, is produced by endothelial cells, macrophages, lamina propria T cells and plasma cells, and FcγR-activated peripheral blood monocytes and monocyte-derived dendritic cells46–49 and binds to the TNFR family death receptor 3 (DR3). Expression of DR3 was detected in natural killer (NK) cells, macrophages, endothelial cells, and activated T lymphocytes,46,50,51 with a higher expression in TH17 cells compared to TH1 or TH2 cells.52 Interestingly, TL1 A induces the proliferation of effector TH17 cells, and in vivo it is required not only for optimal differentiation but also for the effector function of TH17 cells,52 suggesting that complex regulatory pathways might converge to regulate the function of these cells.
Negative Regulation of TH17 Development
IFN-γ and IL-4
Both TH1 and TH2 subsets negatively regulate TH17 differentiation.11,12 Thus, neutralizing IFN-γ and IL-4 levels greatly increases the differentiation of naïve CD4+ T cells into IL-17-producing cells.11,12 In vivo, mice deficient in IFN-γ exhibited increased numbers of IL-17-producing cells and IL-17 protein expression levels.11,12 These results are consistent with previous observations that IFN- γ- and IFN-γR-deficient mice were highly susceptible to EAE.53–55 On the other hand, many studies of inflammatory diseases have reported the presence of IL-17-producing CD4 T cells that co-express IFN-γ. This may suggest that IFN-γ contributes to the pathogenic function of TH17 cells; however, its role is quite unclear.
IL-27
IL-27, a cytokine that belongs to the IL-12 family, contains two subunits, IL-12 p40–related protein, Epstein-Barr Virus (EBV)-induced gene 3 (EBI-3), and a newly discovered IL-12 p35–related protein, p28. IL-27 receptor comprises a unique unit, termed WSX1 protein (IL-27Rα) and gp130 that is shared by the IL-6 receptor complex.55 Engagement of IL-27 to its receptor promotes TH1 differentiation and increases IL-12Rβ2 and IFN-γ production through activation of STAT1, resulting in induction of T-box expressed in T cells (T-bet) and suppression of GATA binding protein 3 (GATA-3).55 However, in vivo it was shown that either IL-27 receptor knockout mice (WSX1 KO) or EBI-3-deficient mice infected with Leishmania major had only a transient defect in IFN-γ production at early time points, but then production of the cytokine was restored, leading to the ability to control proliferation of the parasite.55 Infection with other TH1-dependent pathogens, such as Mycobacterium tuberculosis, Trypanosoma cruzi, Toxoplasma gondii, and Leishmania donovani, confirmed that TH1 responses were not impaired in the absence of IL-27 signaling.56 Regarding the anti-inflammatory effect of IL-27, it has been shown than IL-27 induces expression of the suppressor protein SOCS3 and suppresses IL-2 production.57,58
Recently, two groups demonstrated IL-27 as a potent suppressor of autoimmunity using disease models mediated by TH17 cells.59,60 In EAE or chronic infection with T. gondii, IL-27 receptor (WSX1)-deficient mice develop more severe disease accompanied with increased numbers of IL-17-producing cells infiltrated in the central nervous system.59,60 In vitro, IL-27 suppressed IL-6 and TGF-β-induced TH17 differentiation in a STAT1-dependent manner.59,60 In addition, IL-27 also promoted IL-10 expression in effector T cells that produced IFN-γ and expressed T-bet, but did not express the transcription factor Foxp3.60–62 Moreover, IL-10 production induced by IL-27 was associated with reduced secretion of IL-17.60 The ability of IL-27 to stimulate IL-10 production was independent of STAT4 and T-bet, but was dependent on activation of STAT1 and STAT3.60 Confirming these results, IL-27ra-deficient mice chronically infected with T. gondii had less capacity to make IL-10. Examination of the phenotype of CD4 cells from the brain showed that, whereas no differences were detected in the percentage of cells making IFN-γ, few cells producing both IFN-γ and IL-10 were observed in IL-27ra deficient mice compared to wild-type controls. Conversely, higher numbers of IL-17-producing cells in the brain of IL-27ra-deficient mice compared to control animals were detected.60 Furthermore, addition of exogenous IL-27 reduced the severity of adoptively transferred EAE by an IL-10-dependent mechanism.62
IL-2
Recently, IL-2, a growth factor for most T cells, was shown to suppress TH17 development. Laurence and colleagues demonstrated that IL-2 suppresses IL-17 production and shifts the balance toward expression of Foxp3.63 Mechanistically, IL-2 activates STAT5, which suppresses IL-17 expression by directly binding to the IL-17 gene promoter.63 The addition of IL-2 resulted in marked reduction in the expression of RORγ and enhanced TGF-β−induced Foxp3 expression, suggesting that IL-2/STAT5 signaling may influence the balance of Treg and TH17 cells. However, in humans, the role of IL-2 in inhibiting TH17 responses remains controversial (see below).
Transcriptional Regulation of TH17 Cell Lineage
TH lineage specification is determined by cytokine signaling and subsequent activation of specific transcription factors. These factors individually or coordinately initiate and sustain the expression of lineage-specific genes. It has been well established that during TH1 and TH2 differentiation, cytokines function through selective STAT proteins to further activate the expression of lineage-specific transcription factors. Recent work has revealed similar regulatory circuits in TH17 differentiation. In this section, we will discuss the role of transcription factors in either inducing or inhibiting development of TH17 cells (summarized in Fig. 2).
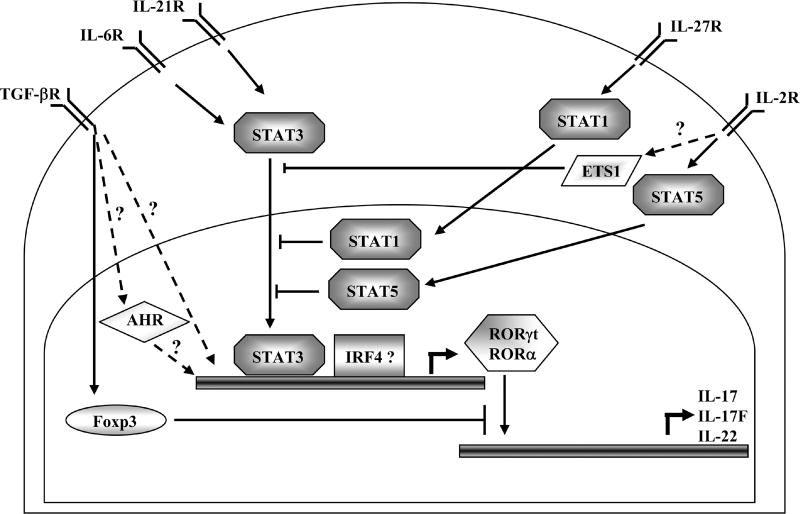
Figure 2.
Transcriptional regulation of TH17-cell differentiation. Naïve CD4+ T cells stimulated under the presence of IL-6 and/or IL-21 induce activation of the signal transducer and activator of transcription 3 (STAT3). Activation of STAT3 induces the expression of retinoic-acid-receptor-related orphan receptor-α (RORα) and RORγt, which establish the expression of TH-17-cell specific gene program. The role of STAT3 in directly inducing IRF4 remains unclear. STAT1, downstream of IFN-γ and IL-27 signaling, or STAT5, which is downstream of IL-2 signaling, as well as ETS1, negatively regulate TH17 differentiation. Moreover, the transcription factor forkhead box P3 (Foxp3), induced by transforming growth factor-β (TGF-β) signaling, antagonizes the TH17-cell developmental program by directly binding to RORα or RORγt. Whether TGF-β-induced Smads or MAPKs participate in TH17 differentiation needs to be demonstrated.
STATs
The STAT proteins selectively regulate TH differentiation. STAT1, delivering signals from type I and II interferons or IL-27, and STAT5, signaling from IL-2, appear to negatively regulate TH17 differentiation,12,59,60,63,64 while STAT4 or STAT6 are not involved.11,12 However, a recent report suggested that STAT4 might be partially required for the generation of IL-23-primed IL-17-secreting cells, whereas it is almost absolutely required for IL-17 production in response to the synergistic effect of IL-23 and IL-18.45
The importance of STAT3 in TH17 differentiation was first suggested by suppressor of cytokine signaling SOCS3-deficient mice that were found to exhibit enhanced IL-17 expression, associated with increased activity of STAT3 in response to IL-23 that could bind to IL-17 and IL-17F promoters.65 More recently, several groups have identified STAT3 as a crucial transcription factor regulating TH17 lineage development. Over-expression of a hyperactive STAT3 enhanced TH17 differentiation, while STAT3 deficiency impaired TH17 differentiation in vitro22,63 and in vivo.30 In addition, STAT3 is necessary for IL-6 induction of IL-21 expression and is required for IL-21-mediated TH17 differentiation.29 The precise biochemical function of STAT3 is unclear at this point. Although STAT3 has been shown to bind to the Il17 gene promoter,65 STAT3 appears to control more than just il17 gene expression. It is likely that STAT3, similar to STAT1 and STAT6, transducing signals from IL-6, IL-21, and IL-23, regulated the expression of lineage-specific master transcription factors RORγt22,63,66 and RORα67 (see below). In humans, it has also been demonstrated that patients with mutations in STAT3 fail to produce TH17 responses.68
RORγt and RORα
Both retinoic acid-related (RAR) orphan receptor gamma (RORγ) and T cell-expressed RORγ (RORγt) genes, encoded by the Rorc locus using different promoters, belong to the RAR orphan nuclear receptor family that also includes RORα and RORβ.69 The specific isoform RORγt induced by TGF-β or IL-6, was recently shown as the first transcription factor to be selectively expressed in TH17 cells.66 Expression of IL-17 and IL-17F require the transcription factor RORγt,66 which is regulated by STAT3.22,63 In the intestinal lamina propria, TH17 cells are constitutively present and express RORγt. In RORγt-deficient mice, this population is greatly reduced.66 Overexpression of RORγt promotes TH17 differentiation when TH1 and TH2 development is inhibited.66 Conversely, RORγt deficiency results in profound reduction of TH17 cells and leads to a partial protection from experimental autoimmune encephalomyelitis.66 However, the RORγt defect does not completely abolish TH17 differentiation or totally inhibit EAE, implicating additional factors may be involved.
In addition to RORγt, TH17 cells also highly express the orphan nuclear receptor RORα, which is induced by TGF-β and IL-6 in a STAT3-dependent manner. Over-expression of RORα promotes TH17 differentiation and substantially upregulates IL-17 and IL-17F expression.67 RORα deficiency results in reduced IL-17 expression in vitro and in vivo. Furthermore, RORα and RORγt co-expression synergistically drove greater TH17 differentiation especially under nonfavorable conditions. Moreover, double deficiencies in RORα and RORγ entirely impair TH17 generation in vitro and completely inhibit EAE disease.67 Therefore, RORα is another TH17-specific factor that, together with RORγt, directs TH17 lineage differentiation.
IRF4
Interferon-regulatory factor 4 (IRF4) was previously shown to regulate the development of TH2 cells through activation of GATA3, a TH2-restricted transcription factor, and interacts with transcription factor Nuclear Factor of Activated T cells (NFAT).70–72 Recently, Brustle and colleagues showed that IRF4 was also critical in directing TH17 differentiation.73 IRF4-deficient or IRF4 siRNA-treated TH cells failed to differentiate into TH17 cells. Irf4−/− TH cells exhibited impaired expression of RORγt, while Foxp3 levels were enhanced.73 Mice deficient in IRF4 were resistant to EAE, while transfer of wild-type T helper cells into Irf4−/− mice rescued TH17 defect and rendered the mice susceptible to EAE.73 Therefore, IRF4 contributes not only to TH2 but also to TH17 differentiation.
Aryl Hydrocarbon Receptor
The aryl hydrocarbon receptor (AHR) is a type I nuclear receptor that interacts with heat shock protein 90 (Hsp90) upon ligand binding.74 It has been recently reported by two groups that AHR plays a crucial role in TH17 differentiation. Both regulatory T cells and TH17 cells express AHR,75,76 although the expression of this receptor is significantly higher in TH17 cells compared to Tregs or any other TH subset.75 Interestingly, neither Treg nor TH17 differentiation is impaired in AHR-deficient mice. However, TH17 cells from AHR-deficient mice do not express IL-22.75 In fact, mice deficient in AHR show hepatic defects,74 and it has been reported that IL-22 protects against liver damage in an acute inflammation model.77
Even though TH17 and Treg levels were not affected in the absence of AHR, activation of this receptor by different ligands leads to increased Tregs or TH17 functions. Ligation of AHR with 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) augments Foxp3 levels on CD4 T cells stimulated with anti-CD3 and anti-CD28, through direct binding of AHR to Foxp3 promoter. Moreover, in vivo administration of a single dose of TCDD before MOG immunization significantly reduced EAE onset and disease severity.76 Conversely, activation of AHR with 6-formylindolo[3,2-b]carbazole (FICZ) during TH17 differentiation significantly enhances IL-17, IL-17F, and most strikingly IL-22 levels in vitro.75,76 Furthermore, in vivo administration of FICZ during MOG immunization leads to increased TH17-specific gene expression and more severe EAE induction.75,76 Thus, these results provide a new level of complexity in the reciprocal regulation of TH17 and Treg cells by demonstrating that AHR can regulate differentiation of both cell types in a ligand-dependent manner.
Foxp3
TGF-β is essential in regulating both TH17 and regulatory T cell differentiation. Upon binding to its tetrameric receptor composed of two subunits of TGF-βRI and two subunits of TGF-βRII, TGF-β induces phosphorylation of Smad2/3 molecules. Phosphorylated Smad2/3 can then bind to the Common-Smad (C-Smad), Smad4, and translocate to the nucleus to induce transcription of target genes.78 Smad-independent signaling pathways have also been described, involving the activation of Mitogen-Activated Protein Kinase (MAPK) signaling pathways.78 Although TGF-β receptor signaling is required for both TH17 and inducible regulatory T (iTreg) cells generation, Smad4 was partially required for iTreg generation, while it was dispensable for generation of TH17 cells.79 However, the activation of other Smads or MAPKs by TGF-β for development of TH17 lineage has not been established. Moreover, whether MAPK or Smad proteins crosstalk with STAT3 and regulate the lineage-specific transcription factors, RORα and RORγt needs to be determined.
As mentioned in the previous section, increasing concentrations of TGF-β can augment Foxp3 levels and reduce IL-23R expression, even in the presence of low concentrations of IL-6, shifting the differentiation of TH cells from TH17 toward regulatory T cells.19 Recently, it was demonstrated that once the expression of Foxp3 increases, it can directly interact with RORγt, leading to inhibition of its transcriptional activity.19 Indeed, cells co-expressing Foxp3 and RORγt in lamina propria had lower IL-17 production compared with cells expressing RORγt alone.19 Meanwhile, Foxp3 LxxLL sequence in exon 2 was shown to associate with the newly identified TH17-specific transcription factor RORα,80 suggesting a potential role of Foxp3 in suppression of TH17 development through inhibition of both RORα and RORγt. Indeed, Foxp3 overexpression under TH17 polarizing conditions inhibited IL-17, IL-17F, IL-21, and IL-22 cytokine expression but did not affect RORα or RORγ mRNA levels. Furthermore, it was found that not only the LxxLL sequence, but also the TIP60/HDAC7 domain of Foxp3 is required for its inhibitory effect on RORγt and RORα, suggesting that Foxp3 interacts with RORs and recruits histone deacetylases to TH17-specific genes, thus inhibiting the transcription of those genes.79
T-bet and Ets1
Terminal differentiation of T cell subsets is characterized by selective expression and action of lineage-specific transcription factors. T-bet is a master regulator of TH1 cells, whereas GATA3 expression activates TH2 specific genes.1
In T-bet-deficient mice in comparison to wild-type counterparts, elevated IL-17 expression levels and increased numbers of IL-17-producing cells were observed upon MOG/CFA immunization.11 In a mouse model for human autoimmune myocarditis, mice lacking T-bet developed more severe disease compared to T-bet+/+ control mice.81 Moreover, the T-bet−/− mice demonstrated a marked increase in production of the IL-23-dependent cytokine IL-17 by heart-infiltrating lymphocytes.81 Thus, these results suggest that T-bet might serve as a negative regulator for TH17 cell differentiation. However, since mice deficient in T-bet have reduced levels of IFN-γ, it is still possible that IFN-γ through STAT1, but not T-bet, inhibits the differentiation of TH17 cells.
In addition to STAT1 and STAT5,V-ets erythroblastosis virus 26 oncogene homologue 1 (Ets1) appears as another negative regulator during TH17 differentiation.82 Although overexpression of Ets1 minimally affected the differentiation of TH17 cells, Ets1 deficiency greatly enhanced TH17 cell differentiation in response to TGF-β and IL-6, as characterized by the increased expression of RORγt, IL-17, IL-17F, IL-22, and IL-23R. in vivo, Ets1-deficient mice had increased TH17-cell-associated cytokines and developed IL-17-dependent mucus hyperplasia in the lung epithelium.82 It is unclear how Ets1 inhibits TH17-cell differentiation. The data presented by Moisan and colleagues indicate that Ets1 is required for IL-2-mediated inhibition of TH17-cell development.82
TH17 Differentiation and Phenotypic Characteristics in Humans
IL-17 expression has been found to be associated with many inflammatory diseases in humans. However at this time, understanding of TH17 differentiation in humans is limited and it remains to be established whether the factors that promote or inhibit mouse TH17 differentiation have similar effects in humans. In this section, we will discuss the current knowledge on human TH17 cell generation and their phenotypic characteristics.
Memory TH17 Cells and Their Phenotypic Characterization
By studying the response of memory T cells to antigens from different pathogens that require distinct types of immune response, Acosta-Rodriguez and colleagues have identified that Candida albicans-specific memory T cells were capable of producing IL-17, whereas Mycobacterium tuberculosis-specific memory cells expressed IFN-γ.83 Interestingly, IL-17-producing memory cells selectively expressed CCR6 and CCR4 (and also express RORγt), whereas IFN-γ or IFN-γ+IL-17+-producing T helper cells expressed CCR6 and CXCR3.83 This differential expression of chemokine receptors on memory T cell subsets then suggests that these cells might have distinct migratory capacities and effector functions.84 Indeed, CCR4 is important for homing to gut, where most RORγt+IL-17+ T cells are found.66 On the other hand, Lim and colleagues found that activated tonsilar IL-17-producing T cells express CCR5, CXCR6, CCR6, CCR2, and CXCR3, as well.85 Surprisingly, human TH17 cells share trafficking chemokine receptors with regulatory T cells.85 Also, human memory TH17 cells were found to express only CCR2 compared to CCR2+CCR5+ TH1 cells.86 These results clearly demonstrate the need of additional markers to better define different T helper cell subsets. Indeed, recently Toscano and colleagues found differential glycosylation patterns between TH2 and TH1 or TH17 cells, which have an impact on the differential susceptibility to cell death.87 Moreover, Nakae and colleagues found that even though TH1 and TH17 cells share similar cell surface markers, there were differences in the intensity of expression of those markers.88 Thus, the identification of specific markers for each TH cell type might help us understand the physiology and regulation of these cells.
De Novo Generation of Human TH17 Cells from Naïve Precursors
Last year, two groups reported simultaneously on the cytokines that drive the differentiation of naïve human CD4+CD45RA+ T cells into TH17 effector cells. IL-6 alone did not induce IL-17 production or RORγt expression.89,90 However, IL-23 or IL-1β alone transiently induced TH17 cytokine production, such as IL-17 A, IL-17F, IL-22, IL-26, CCL20, and RORγt expression, and this commitment was further enhanced and sustained by IL-6.89,90 Interestingly, IFN-γ levels were not affected, or slightly reduced, by these cytokines as compared to IL-12 stimulation.89
Under the mentioned stimulation conditions, both RORγt and the aryl hydrocarbon receptor (AHR) were highly expressed in human TH17 cells compared to human TH1 cells, and the TH17 cytokine levels were even further enhanced by the AHR ligand β-naftoflavone.75 Also, STAT3 transcription factor has been shown to be required for human TH17 differentiation since patients with mutations in this transcription factor have impaired TH17 generation.68
Moreover, IL-2, in contrast to the murine system, did not inhibit TH17 cell generation, unless added at high doses at the beginning of the stimulation.89,90 In fact, IL-2 was required for normal proliferation of these cells. However, Annunziato and colleagues recently suggested that IL-2 is able to downregulate RORγt levels in both TH17 and TH17/TH1 cells (expressing both IL-17 and IFN-γ) and increase the expression of T-bet, thus enhancing IFN-γ production.91 Remarkably, this effect could be partially prevented by IL-23, suggesting some plasticity and regulatory roles between these transcription factors.91 In fact, another group also suggested some plasticity between different TH cell types. They showed that TH0, TH1, and TH2 cells were capable of producing IL-17 when restimulated after 7 days of culture, suggesting that IL-17 locus might not be entirely repressed in other differentiated TH subsets.92
Many controversies have arisen regarding the role of TGF-β on human TH17 differentiation. It has been previously suggested that TGF-β by itself or in combination with IL-6 and IL-23 did not induce TH17 differentiation.89 In addition, Chen and colleagues recently reported that IL-6 did not inhibit Foxp3 expression when cultured in the presence of TGF-β, suggesting a different mechanism for Foxp3 downregulation in human versus mouse systems.92 However, recent papers demonstrated that TGF-β is required for human TH17 differentiation.93,94 Manel and colleagues demonstrated that under serum-free conditions, naïve T cells from cord blood when stimulated with TGF-β together with IL-23 and IL-1 became TH17 cells.94 Interestingly, IL-6 or IL-21 together with TGF-β alone was not capable of inducing IL-17-producing T cells. However, Yang and collaborators showed that CD4+CD25−CD62L+CD45RA+ human naïve T cells stimulated only with TGF-β and IL-21 in serum-free conditions were capable of producing IL-17, whereas TGF-β plus IL-6 was not. Not only IL-17A, but also RORC2, the human homologue of mouse RORγt, was induced upon TGF-β and IL-21 stimulation, accompanied with inhibition of other TH-specific transcription factors GATA-3, T-bet and Foxp3.95 On the other hand, Volpe and colleagues showed that only the full combination of TGF-β with IL-1β, IL-6, TNF-α, and IL-23, but not each treatment individually, was able to induce IL-17 production from human naïve CD4 T cells. In addition, they demonstrated that there was no difference in serum-free versus serum-containing media in the induction of IL-17-producing cells under this full polarizing condition containing TGF-β, IL-1β, IL-6, TNF-α, and IL-23.93
Still many questions remain regarding the regulation/generation of human TH17 cells, and thus further deep analysis of these cells is required, which will ultimately help us understand the physiological role of TH17 cells in human diseases.
TH17 Cells and Diseases
Since their discovery, TH17 cells have been shown to play important roles in multiple types of diseases, ranging from inflammation and autoimmunity to infectious diseases and cancer. TH17 cytokines regulate the secretion of granulopoietic factors (G-CSF and SCF), CXCL and CCL chemokines, matrix metalloproteases, pro-inflammatory cytokines, and antimicrobial peptides, depending on the target cells,13 and thus lead to increased recruitment of neutrophils and other immune cells together with the generation of local inflammation and/or antimicrobial immune response, as summarized in Figure 3. In this section, we will discuss the latest findings on the roles of TH17 cells in different disease models.
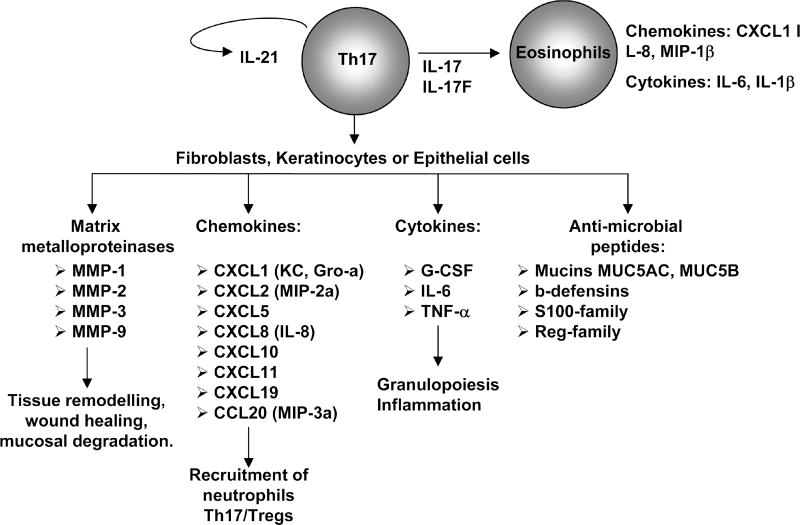
Figure 3.
Role of TH17 cells cytokines. On immune cells, TH17- produced IL-21 further induces TH17 differentiation creating a positive feedback loop in an autocrine manner. Moreover, both IL-17 and IL-17F induce production of cytokines and chemokines by eosinophils, leading to allergic inflammation. In nonimmune cells, TH17 cytokines collectively induce the secretion of cytokines, chemokines, antimicrobial compounds, and matrix metalloproteases from epithelial cells, fibroblasts, and keratinocytes, resulting in an increase in inflammation by recruitment of neutrophils and macrophages and tissue remodeling.
TH17 Cells in Infectious Diseases
Intracellular Bacteria
In Mycobacterium tuberculosis model, IL-12 p40–deficient mice are more susceptible to mycobacterial infections than IL-12 p35.96 Moreover, Happel and colleagues demonstrated that local adenoviral delivery of IL-23 greatly enhanced IFN-γ and IL-17 production by activated CD4+ T cells in lung-draining lymph nodes, and controls growth of M. tuberculosis in the lung.97 However, IL-23p19−/− mice showed a severe defect in antigen-specific IL-17 production that was, however, not associated with altered resistance to infection in these mice.98 Also, Aujla and colleagues recently demonstrated that IL-23 or IL-17RA-deficient mice have no differences in the susceptibility to H37Rv M. tuberculosis (Mtb) infection compared to WT (wild-type), and neither IL-17RA is required for immune response against another intracellular pathogen Listeria monocytogenes.99 Furthermore, IL-22-deficient mice were capable of mounting a normal innate and adaptive immune response against L. monocytogenes.77
Even though deficiency in TH17 cytokines seems to be dispensable for protection against mycobacteria, after antigen vaccination, IL-17-producing cells were detected in the lung, prior to the recruitment of IFN-γ-producing cells.100 Interestingly, IL-23 but not IL-12 was required for the protective recall response, through the generation of granulomas structures and recruitment of activated CD4 T cells. This effect was due to IL-17-induced chemokine expression of CXCL19, CXCL10, and CXCL11, the ligands for CXCR3, inducing the recruitment of neutrophils, facilitating the formation of the granulomas.100,101 Moreover, humans that had been exposed to M. tuberculosis possessed IL-17- and IL-22-producing cells in peripheral blood mononuclear cells (PBMCs) stimulated with Mtb antigens (Ags). Interestingly, even though IL-17 and IL-22 are produced by the same TH17 cells in mice,102,103 the authors failed to find IL-17 and IL-22 double producers cells.104 In fact, IL-22-producing T cells were more frequent than IL-17+CD4+ T cells.104
Extracellular Bacteria
The role of TH17 cells in the host immune response against many extracellular bacteria, including Klebsiella pneumoniae, Pseudomonas aeruginosa, Helicobacter pylori, Mycoplasma pneumoniae, and Citrobacter rodentium among others, has been investigated. It has been shown that T cells producing IL-17 are induced upon K. pneumoniae infection.105–107 Recently, Aujla and colleagues provided some evidence for the role of IL-22, another TH17-specific cytokine, in protection against this pathogen.99 Mice infected with K. pneumoniae showed an IL-23-dependent IL-22 expression in lung tissue, with similar kinetics to IL-17 and IL-17F.99,105 Neutralization of endogenous IL-22 levels lead to a severe dissemination of the bacteria to spleen, and to reduced survival as compared to isotype control treatment in either WT or IL-17-deficient mice, suggesting a more crucial role of IL-22 in mucosal host defense.99
It was also demonstrated that the TH17 cytokines IL-17 and IL-22 had a direct role in human bronchial epithelial (HBE) cells, since they could induce a significant increase in host defense genes. Moreover, IL-22 was capable of inducing recovery of epithelial cells after injury,99 further demonstrating the dual effect of IL-22 in participating not only in the induction of increased immune responses but also in the protection of the tissue from excessive damage. Furthermore, direct killing of K. pneumoniae by primary mouse tracheal epithelial cells required IL-22-induced expression of lipocalin-2, a protein with innate immune response functions.99
It has also been shown that patients with cystic fibrosis infected with Pseudomonas aeruginosa had higher levels of IL-17 and IL-17F in bronchoalveolar lavage fluids, and also had increased production of these cytokines together with IL-22 in cells from draining lymph nodes compared to non-infected individuals.99
In a Mycoplasma pneumoniae infection model, Wu and colleagues showed increased IL-17A and IL-17F expression in lung from CD4+ T cells, which was impaired in IL-23-deficient mice.108 A reduction, not only in the levels of this cytokine, but also in neutrophil recruitment to the lung, was observed after IL-23 blockade in vivo, which lead to a decrease in bacterial clearance.108 Thus, these results demonstrate the important role of TH17 cells in the protection against pathogens in lung.
The role of TH17 cells in Helicobacter pylori–infected patients has also been evaluated.109 Gastric biopsies from H. pylori–infected individuals compared to gastric biopsies from uninfected patients or with controls showed increased IL-17 and IL-23 mRNA levels. Moreover, increased CD3+IL-17+ T cells in freshly isolated gastric lamina propria mononuclear cells were found. Furthermore, not only IL-17, but also IFN-γ expression, was increased by IL-23 on distinct CD4 T cells.109
The induction of TH17 cells in infection with Citrobacter rodentium has been previously demonstrated.16 Recently, Zheng and colleagues showed the role of IL-22 using the same infection model.110 In this report, the authors demonstrated that IL-22 expression in colon, induced by IL-23 and IL-6, was required for a protective immune response against this pathogen. Interestingly, whereas IL-22 had a protective role in this infection model, both IL-17 and IL-17F were dispensable, since IL-17RC KO mice did not have any significant differences in the disease behavior compared to WT littermates.110 However, by using RAG KO mice the authors identified that IL-22 production was due to DC, but not to CD4 T cells, suggesting a source of IL-22 independent of TH17 cells. Therefore, whereas Mangan and colleagues demonstrated the generation of TH17 cells in this model, and the importance of IL-23 in the protection against the bacteria, these new results from Zheng and colleagues suggest a more important role of the innate rather than the adaptive source of IL-22 in the clearance of this infectious agent.16,110
Virus
Although the role of IL-17 has been evaluated in some viral infections by overexpression of IL-17 by different viruses,111,112 the participation of TH17 cells in immune responses against viruses remains controversial. Recently, Smiley and colleagues demonstrated that after immunization, mice challenged with rotavirus produced IFN-γ and IL-17 by Ag-specific CD4 T cells, which was associated with protection against infection.113 However, IFN-γR or IL-17R KO mice remained protected after immunization, suggesting that other factors may play direct or indirect roles in protection against rotavirus.113
In HIV infection, two groups recently evaluated the expression of both IL-17 and IL-22. In the first report, higher levels of IL-17+CD3+CD4+ and IL-17+CD3+CD4− T cells in peripheral blood were found in HIV+ patients compared to controls.114 Misse and colleagues showed that activated T cells from HIV-exposed, uninfected individuals expressed high levels of IL-22 and induced acute phase proteins, which are associated with host resistance to HIV infection.115
In a recent report, IL-23 p19−/− mice showed increased viral inflammatory lesions and higher levels of IFN-γ-producing cells after ocular infection with herpes simplex virus (HSV). Thus, a more robust TH1 immune response in IL-23-deficient mice, rather than lack of TH17 responses, is responsible for the enhanced immunopathology.116
Parasites
The role of TH17 cells in the immune response against parasites is also limited. It has been previously shown that IL-17R-deficient mice infected with Toxoplasma gondii have increased mortality, which correlated to a decrease in neutrophil recruitment to different tissues and CXCL8 expression, and increased parasites burden. Moreover, IL-17R KO mice developed less severe tissue damage, indicating that IL-17 induces more tissue damage but is required for infection resolution.117
Recently, it has been demonstrated that exacerbation of pathology following immunization with schistosome egg antigens in CFA correlates with increase in TH17 cells.118 In order to further corroborate those results, the same group showed that IL-23p19−/− mice immunized with schistosome egg antigens in CFA have reduced severe immunopathology, which is associated with a reduction of IL-17-producing T cells in granulomas and with a decrease in neutrophil recruitment due to reduced chemokine levels.119 Although these reports might suggest a role of TH17 cells in the immune response against parasites, it needs to be further confirmed using other infectious models.
Fungus
To support the role of TH17 cells in protective immunity, several groups have studied the role of IL-23 or IL-17 in fungal infections. Macrophages stimulated with Pneumocystis carinii expressed IL-1β, IL-6, and IL-23, cytokines that participate in the induction of TH17 differentiation, suggesting a possible role of these cells in the immune response against the fungus. Indeed, blockade of IL-23 or IL-17 by neutralizing antibodies significantly increased the burden of P. carinii.120 Moreover, IL-23-deficient mice showed greater susceptibility to systemic Cryptococcus neoformans and pulmonary P. carinii infection.120,121 Also, Candida albicans induces IL-23 expression by monocyte-derived DCs, and memory T cells against C. albicans, expressing CCR6 and CCR4 and producing IL-17, were detected in humans.83 Also, it was reported that patients with autosomal dominant hyper-IgE syndrome, which have mutations in the STAT3, fail to generate TH17 responses.68 Thus, these results suggest a possible role of TH17 cells in the control of infections since these patients present recurrent and often severe pulmonary infections, staphylococcal abscesses, and mucocutaneous candidiasis.68
However, the IL-23/IL-17 pathway was shown to promote inflammation, inhibiting the protective TH1 response against Candida and Aspergillus.122,123 Moreover, in Toll IL-1R8 (Tir8)-deficient mice, increased TH1 and TH17 responses were observed, leading to increased susceptibility to infection against C. albicans and A. fumigatus. However, blocking IL-17 or IL-23, but not TH1 cytokines, can control the infection, demonstrating a negative role of TH17 cells in the protective immunity against these pathogens.124
From above, although TH17 cells and TH17 cytokines have been shown or implicated in immunity against various infectious agents, the source of these cytokines in innate host defenses has yet to be well defined. The contribution of TH17 cells in chronic infection or memory responses requires more research.
TH17 Cells in Cancer
Certain cancers can use inflammatory mediators for their own benefit to induce angiogenesis and tissue remodeling.125 For instance, it has been demonstrated that in non-small cell lung carcinoma, IL-17 can promote tumor growth through the enhancement of angiogenesis-mediating factor production.126 In addition, previous reports have demonstrated the requirement of IL-23 for tumor development.127 However, it has also been shown that IL-17 production can inhibit tumor cell growth due to the recruitment of CD8+ T lymphocytes with cytotoxic activity against the tumor.128 Therefore, the role of inflammation in cancer remains a controversial issue.
Recently, Kottke and colleagues demonstrated that intraprostatic injection of adenoviruses induced a TH17 autoimmune response in the mouse prostate, which was capable of rejecting an established tumor of pancreatic TC2 cells growing elsewhere in the mice.129 Muranski and colleagues found that adoptive transfer of TH17 cells was most potent in mediating tumor regression, and animals receiving TH17 cells remained tumor-free compared to animals treated with TH0 or TH1 cells which developed relapsing disease.130 Interestingly, the ability of TH17 cells to mediate tumor rejection was due to IFN-γ production in vivo.130 These reports suggest that TH17 cells might have an important contribution in the cancer immunology field. However, given that IFN-γ seems to be required for tumor rejection, whether contaminating TH1 or IL-17/IFN-γ double producer cells participate in such response needs further clarification.
TH17 Cells in Autoimmune and Inflammatory Diseases
Multiple Sclerosis
Multiple sclerosis is a human inflammatory demyelinating autoimmune disease with ascending paralysis, in which CD4+ T cells play a crucial role. Many reports have identified the key role of TH17 rather than TH1 cells in mediating experimental autoimmune encephalomyelitis (EAE), mouse model of multiple sclerosis.4,5,10–12,33,131
Recently, Thakker and colleagues demonstrated that IL-23 plays a crucial role in the induction/priming phase of the disease, whereas it has no distinct function in the effector stage once encephalitogenic cells, capable of producing IL-17, IFN-γ, and TNF-α are generated.132 Interestingly, whereas IL-17 deficiency or blockade of this cytokine leads to partial susceptibility to EAE, IL-23 p19 deficiency or blockade of IL-23 produces complete resistance to this disease, suggesting that other TH17 cytokines are also important for mediating this disease.11,33,132,133 Analysis of IL-17- and IL-17F-deficient animals reveals that IL-17 is more important than IL-17F in initiating EAE.134 In addition, although TH17 cells co-expressed IL-17 and IL-22 in the CNS, IL-22 was not found to be directly required for the induction of EAE.135 Nevertheless, high expression of both IL-17R and IL-22R was observed on CNS vessels within highly infiltrated brain lesions.136 Indeed, human TH17 cells migrated more avidly across human brain-derived microvascular endothelial cells compared to TH1 cells, suggesting that through the production of both IL-17 and IL-22, TH17 cells are able to access the CNS.136 Moreover, high CD4+CD45RO+ cells expressing IL-17 and IL-22 in highly infiltrated multiple sclerosis lesions were detected, compared to non-inflamed brain tissues.136,137 Interestingly, cells producing IL-17 included not only CD4+ T cells, but also CD8+ T cells, astrocytes, and oligodendrocytes. Recently, Calson and colleagues show that transfer of encephalitogenic TH17 cells is sufficient to induce ELR+ CXC chemokines,CXCL1 andCXCL2, in the spinal cords of naïve, syngeneic recipients.138 Not only the chemokines CXCL1 and CXCL2, but also their receptor CXCR2, were increased during the course of EAE. Furthermore, blockade of CXCR2 or depletion of polymorphonuclear leukocytes (PMN) cells during the remission stage inhibited subsequent relapses,138 demonstrating that TH17-induction of PMN cells is important for disease onset.
Recently, it was shown that the ratio of antigen-specific TH17:TH1 cells determines the extent of inflammation in brain or spinal cord.139 This report benefits our understanding on the differential roles of TH1 and TH17 cells in the pathogenesis of multiple sclerosis and may provide insight into possible therapeutics depending on the type of disease in which infiltration of cells is mainly observed in brain versus mainly in the spinal cord parenchyma.
Arthritis
Rheumatoid arthritis is a chronic inflammatory disease that primarily affects joints, causing cartilage degradation and bone erosion, which ultimately leads to joint destruction. Several studies have demonstrated a key role of IL-17 or IL-23 in the progression of arthritis.9,140–143 Indeed, blockade of IL-17 after disease onset was able to prevent cartilage and bone destruction, leading to amelioration of the clinical symptoms of the disease.142 Interestingly, it was found that human TH17 cells in arthritic synovium expressed the nuclear factor kappa B ligand RANKL,144 which induces osteoclastogenesis.145 Also, mice deficient in the IL-23 p19 subunit did not develop collagen-induced arthritis and had reduced levels of IL-17, IL-6, and TNF-α.9 Recently, Sakaguchi’s group has demonstrated that CD4 T cells from a spontaneous arthritis model can spontaneously differentiate into arthritogenic TH17 cells in an IL-6-dependent manner.146 Moreover, those TH17 cells expressed CCR6, which was required for the onset and severity of the disease.147 Interestingly, not only TH17 cells, but also synoviocytes, were able to produce CCL20, the ligand for CCR6. Indeed, synovial fluids from human samples contained high levels of both CCL20 and IL-17, suggesting they are required for the disease progression.147 Other reports also demonstrated the presence of IL-17 in synovial fluids.148–150 The amount of TH17 cells augmented as the disease progressed from persistent oligoarthritis (mild form) to extended oligoarthritis (more severe form), and an inverse correlation was found between TH17 and Foxp3+ regulatory T cells.149 Moreover, IL-17 induced synovial fibroblasts to produce IL-6, IL-8, MMP-1, and MMP-3, contributing to the destruction of the joint.148
A recent report by Joosten and colleagues developed a mouse model of chronic destructive arthritis induced by reiterative intra-auricular exposure to Streptococcus pyogens cell wall components.151 Using this system in cytokine-deficient mice, the authors demonstrated a key role of IL-17 and IL-1β in the chronic stage of the disease, whereas no role was observed for TNF-α. Moreover, they showed that RAG1 KO as well as IL-17R−/− or IFN-γ−/− mice do not develop chronic arthritis.151
Psoriasis
Many reports have identified the presence of TH17 cytokines in psoriatic lesions.152–156 Wilson and coworkers recently confirmed that psoriatic skin lesions contained IL-23-producing DCs and were enriched in the cytokines produced by human TH17 cells that promote the production of antimicrobial peptides in human keratinocytes.89 Also, Ma and colleagues demonstrated that in a psoriatic-like disease, induced by transfer of CD4+CD45RBhiCD25− T cells, high levels of TH17 and TH1 cytokines were observed in lesions, and blockade of either the IL-12/IL-23 p40 subunit or IL-22 significantly prevented the development of the skin lesions.157 Interestingly, blockade of IL-22 decreases not only IL-22 levels but also all pro-inflammatory cytokines associated with TH17 cells, such as IL-1α, IL-1β, IL-6, IL-17, IL-17F, and TNF-α, and increase TH1 IFN-γ in psoriatic lesions.157 Indeed, other reports also recently demonstrated the direct role of IL-22 in the induction of dermal inflammation and acanthosis.158,159 Zheng and colleagues demonstrated that IL-23-induced acanthosis and inflammation were reduced in IL-22-deficient mice compared to wild-type controls,158 while Boniface and colleagues showed that infiltrating T lymphocytes from psoriatic lesions produced more IL-22 than peripheral blood T lymphocytes.159
Different treatments are currently being developed in psoriatic patients. Zaba and colleagues recently demonstrated that 1 week of treatment with etanercept, a TNF-α receptor-Ig fusion protein that blocks TNF-α signaling, resulted in decreased keratinocytes acanthosis, proliferation and differentiation was observed in almost all the patients.160 This reduced disease correlated with a rapid downregulation of IL-17, IL-22, CCL20, and β-defensin 4. Moreover, decreases in other pro-inflammatory cytokines such as IL-8, IL-1β, IL-6, IL-23 p19 and p40 were also observed at earlier time points during etanercept treatment.160 Another possible treatment is administration of cyclosporine. Lowes and colleagues recently reported that after treatment with cyclosporine, expression of IL-17, IL-22, IFN-γ, and keratin 16 was reduced to baseline levels, demonstrating the functional role of TH17 cells in psoriatic phenotype.161 Also, a clinical trial using anti-IL-12/IL-23 p40 neutralizing antibodies has been carried out. In this study, patients receiving different doses of the neutralizing antibodies had a significant improvement in psoriatic areas and disease index, demonstrating a crucial role in these cytokines in the pathogenesis of the disease.162
Inflammatory Bowel Disease
Patients with inflammatory bowel disease display an increase in TH17 and TH1/TH17 cells in gut compared to normal controls. These memory cells express CCR4, CCR5, CCR6, and IL-23R. However, IL-23 did not increase the proliferative response of these cells.91
Using a trinitrobenzenesulfonic acid (TNBS)-induced colitis model, Zhang and colleagues showed that IL-17R KO mice had lower levels of neutrophils in the colon, which correlated with a decrease in weight loss, inflammation, MIP2, and IL-6 levels.163 Moreover, neutralization of IL-23 levels in IL-10 KO mice can also lead to prevention in the induction of colitis, due to a decrease in IL-6 and IL-17 levels produced by memory T cells,34 further demonstrating the role of TH17 cytokines in the pathogenesis of this disease. However, recently Yang and colleagues demonstrated that IL-17F-deficient mice had reduced dextran sulphate sodium (DSS)-induced colitis, whereas IL-17 KO animals developed enhanced disease compared to WT controls, suggesting that IL-17F plays a pathogenic role in this disease model, while IL-17 may protect lamina propria from excessive damage.134
Also, the role of IL-21 in gut inflammation has been recently addressed by Monteleone’s group. IL-21 is able to induce production of matrix metalloproteases by human intestinal fibroblasts164 and the chemokine MIP-3α by epithelial cells.165 Moreover, higher levels of IL-21 in lesions from patients with Crohn disease were detected.166 In addition, IL-21-deficient mice were protected against DSS or TNSB-induced colitis, and were incapable of upregulating TH17 associated genes during inflammation. Moreover, decreased IL-17 production was detected by lamina propria lymphocytes from IBD mice when IL-21 levels were blocked.167
Not only IL-17 or IL-21, but also IL-22, levels were found increased in patients with IBD. Indeed, serum IL-22 levels were increased in patients with Crohn disease compared to healthy controls.168,169 Moreover, IL-22 levels were higher in patients with high susceptibility risk IL-23R polymorphisms, suggesting a direct correlation between higher IL-22 levels and TH17 generation.168 Interestingly, IL-23R polymorphisms have been detected not only in Crohn disease168,170 but also in psoriasis,171–173 multiple sclerosis,174,175 and Graves disease,176 although the role of TH17 cells in the latter has not been entirely elucidated.177 Also, IL-22 was found to induce LPS-binding protein (LBP) expression in liver using a mouse system, which might prevent systemic inflammation induced by LPS. These data correlate with the increased LBP levels in the blood of patients with Crohn disease.169 Moreover, not only IL-22 but also increased IL-17F mRNA levels were detected in inflamed biopsied compared to noninflamed tissues in patients with Crohn disease.178
Concluding Remarks
Since TH17 cells were described as a third lineage of effector T helper cells, generation of TH17 cells and their role in the immune system has been extensively studied. TH17 cells were shown to express IL-17, IL-17F, IL-21, IL-22, and IL-26 in humans, and possibly CCL20. These cells can be generated from naive TH cells, upon TCR and costimulatory receptor signaling in the presence of TGF-β and IL-6, leading to induction and/or activation of STAT3, IRF4, AHR, RORα, and RORγt, which might be required for the establishment of a TH17 program. However, whether other transcription factors are required for induction of TH17-specific genes or through inhibition of other T cell genetic programs remains to be established. Moreover, the role of each cytokine and the cells that produce them will help us answer many unsolved issues in the field.
TH17 cells have been identified to be crucial in the induction/maintenance of a variety of diseases, ranging from autoimmune and inflammatory diseases to cancer and infectious diseases. How TH17 cells interact with or inhibit other TH cells during these diseases needs to be further investigated. In different mouse and human disease models, cells co-expressing IL-17 and IFN-γ have been identified. Whether these cells represent TH1 cells becoming TH17 cells or vice versa remains to be elucidated. Moreover, this raises the possibility of an inter-conversion between different T helper subsets, suggesting a higher plasticity than previously thought. Thus, understanding not only how TH17 cells are regulated, but also how different T helper subsets interact in vivo, might help design new therapeutic approaches to specifically target TH17 cells in these diseases.
Acknowledgments
We thank our colleagues and collaborators for their scientific contribution. The work is supported by research grants from NIH (to CD). RN received a postdoctoral fellowship from the Arthritis Foundation and is a recipient of a Scientist Development Grant from the American Heart Association. CD is a Trust Fellows of the MD Anderson Cancer Center, a Cancer Research Institute Investigator, and an American Lung Association Career Investigator.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Dong C, Flavell RA. Cell fate decision: T-helper 1 and 2 subsets in immune responses. Arthritis Res. 2000;2:179–188. doi: 10.1186/ar85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Infante-Duarte C, et al. Microbial lipopeptides induce the production of IL-17 in Th cells. J. Immunol. 2000;165:6107–6115. doi: 10.4049/jimmunol.165.11.6107. [DOI] [PubMed] [Google Scholar]
- 3.Aggarwal S, et al. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 2003;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 4.Bettelli E, et al. Loss of T-bet, but not STAT1, prevents the development of experimental autoimmune encephalomyelitis. J. Exp. Med. 2004;200:79–87. doi: 10.1084/jem.20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferber IA, et al. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) J. Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- 6.Kageyama Y, et al. Reduced susceptibility to collagen-induced arthritis in mice deficient in IFN-gamma receptor. J. Immunol. 1998;161:1542–1548. [PubMed] [Google Scholar]
- 7.Willenborg DO, et al. IFN-gamma plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J. Immunol. 1996;157:3223–3227. [PubMed] [Google Scholar]
- 8.Dong C, Nurieva RI. Regulation of immune and autoimmune responses by ICOS. J. Autoimmun. 2003;21:255–260. doi: 10.1016/s0896-8411(03)00119-7. [DOI] [PubMed] [Google Scholar]
- 9.Murphy CA, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J. Exp. Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langrish CL, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrington LE, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 13.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat. Rev. Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 14.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 15.Veldhoen M, et al. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Mangan PR, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 17.Gorelik L, Flavell RA. Transforming growth factor-beta in T-cell biology. Nat. Rev. Immunol. 2002;2:46–53. doi: 10.1038/nri704. [DOI] [PubMed] [Google Scholar]
- 18.Chen W, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou L, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li MO, Wan YY, Flavell RA. T cell-produced transforming growth factor-beta1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity. 2007;26:579–591. doi: 10.1016/j.immuni.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 21.Korn T, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang XO, et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J. Biol. Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 23.Elias KM, et al. Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independent signaling pathway. Blood. 2008;111:1013–1020. doi: 10.1182/blood-2007-06-096438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mucida D, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 25.Schambach F, et al. Activation of retinoic acid receptor-alpha favours regulatory T cell induction at the expense of IL-17-secreting T helper cell differentiation. Eur. J. Immunol. 2007;37:2396–2399. doi: 10.1002/eji.200737621. [DOI] [PubMed] [Google Scholar]
- 26.Kang SG, et al. Vitamin A metabolites induce gut-homing FoxP3+ regulatory T cells. J. Immunol. 2007;179:3724–3733. doi: 10.4049/jimmunol.179.6.3724. [DOI] [PubMed] [Google Scholar]
- 27.Sun CM, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J. Exp. Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nurieva R, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 30.Zhou L, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 31.Oppmann B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 32.Parham C, et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J. Immunol. 2002;168:5699–5708. doi: 10.4049/jimmunol.168.11.5699. [DOI] [PubMed] [Google Scholar]
- 33.Cua DJ, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 34.Yen D, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J. Clin. Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moller B, Villiger PM. Inhibition of IL-1, IL-6, and TNF-alpha in immune-mediated inflammatory diseases. Springer Semin Immunopathol. 2006;27:391–408. doi: 10.1007/s00281-006-0012-9. [DOI] [PubMed] [Google Scholar]
- 36.Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev. 2002;13:323–340. doi: 10.1016/s1359-6101(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 37.Braddock M, Quinn A. Targeting IL-1 in inflammatory disease: new opportunities for therapeutic intervention. Nat. Rev. Drug Discov. 2004;3:330–339. doi: 10.1038/nrd1342. [DOI] [PubMed] [Google Scholar]
- 38.Schiffenbauer J, et al. The induction of EAE is only partially dependent on TNF receptor signaling but requires the IL-1 type I receptor. Clin. Immunol. 2000;95:117–123. doi: 10.1006/clim.2000.4851. [DOI] [PubMed] [Google Scholar]
- 39.Sutton C, et al. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J. Exp. Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kryczek I, et al. Cutting edge: opposite effects of IL-1 and IL-2 on the regulation of IL-17+ T cell pool IL-1 subverts IL-2-mediated suppression. J. Immunol. 2007;179:1423–1426. doi: 10.4049/jimmunol.179.3.1423. [DOI] [PubMed] [Google Scholar]
- 41.Felderhoff-Mueser U, et al. IL-18: a key player in neuroinflammation and neurodegeneration? Trends Neurosci. 2005;28:487–493. doi: 10.1016/j.tins.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 42.Wildbaum G, et al. Neutralizing antibodies to IFN-gamma-inducing factor prevent experimental autoimmune encephalomyelitis. J. Immunol. 1998;161:6368–6374. [PubMed] [Google Scholar]
- 43.Shi FD, et al. IL-18 directs autoreactive T cells and promotes autodestruction in the central nervous system via induction of IFN-gamma by NK cells. J. Immunol. 2000;165:3099–3104. doi: 10.4049/jimmunol.165.6.3099. [DOI] [PubMed] [Google Scholar]
- 44.Gutcher I, et al. Interleukin 18-independent engagement of interleukin 18 receptor-alpha is required for autoimmune inflammation. Nat. Immunol. 2006;7:946–953. doi: 10.1038/ni1377. [DOI] [PubMed] [Google Scholar]
- 45.Mathur AN, et al. Stat3 and Stat4 direct development of IL-17-secreting Th cells. J. Immunol. 2007;178:4901–4907. doi: 10.4049/jimmunol.178.8.4901. [DOI] [PubMed] [Google Scholar]
- 46.Kang YJ, et al. Involvement of TL1 A and DR3 in induction of pro-inflammatory cytokines and matrix metalloproteinase-9 in atherogenesis. Cytokine. 2005;29:229–235. doi: 10.1016/j.cyto.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 47.Bamias G, et al. Expression, localization, and functional activity of TL1 A, a novel Th1-polarizing cytokine in inflammatory bowel disease. J. Immunol. 2003;171:4868–4874. doi: 10.4049/jimmunol.171.9.4868. [DOI] [PubMed] [Google Scholar]
- 48.Prehn JL, et al. Potential role for TL1 A, the new TNF-family member and potent costimulator of IFN-gamma, in mucosal inflammation. Clin. Immunol. 2004;112:66–77. doi: 10.1016/j.clim.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 49.Prehn JL, et al. The T cell costimulator TL1 A is induced by FcgammaR signaling in human monocytes and dendritic cells. J. Immunol. 2007;178:4033–4038. doi: 10.4049/jimmunol.178.7.4033. [DOI] [PubMed] [Google Scholar]
- 50.Al-Lamki RS, et al. Expression of silencer of death domains and death-receptor-3 in normal human kidney and in rejecting renal transplants. Am. J. Pathol. 2003;163:401–411. doi: 10.1016/S0002-9440(10)63670-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang EC, et al. Genomic structure, expression, and chromosome mapping of the mouse homologue for the WSL-1 (DR3, Apo3, TRAMP, LARD, TR3, TNFRSF12) gene. Immunogenetics. 2001;53:59–63. doi: 10.1007/s002510000290. [DOI] [PubMed] [Google Scholar]
- 52.Pappu BP, et al. TL1A-DR3 interaction regulates Th17 cell function and Th17-mediated autoimmune disease. J. Exp. Med. 2008;205:1049–1062. doi: 10.1084/jem.20071364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J. Clin. Invest. 2006;116:1218–1222. doi: 10.1172/JCI28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McKenzie BS, Kastelein RA, Cua DJ. Understanding the IL-23-IL-17 immune pathway. Trends Immunol. 2006;27:17–23. doi: 10.1016/j.it.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 55.Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat. Rev. Immunol. 2005;5:521–531. doi: 10.1038/nri1648. [DOI] [PubMed] [Google Scholar]
- 56.Batten M, Ghilardi N. The biology and therapeutic potential of interleukin 27. J. Mol. Med. 2007;85:661–672. doi: 10.1007/s00109-007-0164-7. [DOI] [PubMed] [Google Scholar]
- 57.Owaki T, et al. IL-27 suppresses CD28-mediated [correction of medicated] IL-2 production through suppressor of cytokine signaling 3. J. Immunol. 2006;176:2773–2780. doi: 10.4049/jimmunol.176.5.2773. [DOI] [PubMed] [Google Scholar]
- 58.Villarino AV, et al. IL-27 limits IL-2 production during Th1 differentiation. J. Immunol. 2006;176:237–247. doi: 10.4049/jimmunol.176.1.237. [DOI] [PubMed] [Google Scholar]
- 59.Batten M, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat. Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 60.Stumhofer JS, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat. Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 61.Batten M, et al. Cutting Edge: IL-27 Is a Potent Inducer of IL-10 but Not FoxP3 in Murine T Cells. J. Immunol. 2008;180:2752–2756. doi: 10.4049/jimmunol.180.5.2752. [DOI] [PubMed] [Google Scholar]
- 62.Fitzgerald DC, et al. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat. Immunol. 2007;8:1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 63.Laurence A, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 64.Amadi-Obi A, et al. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat. Med. 2007;13:711–718. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 65.Chen Z, et al. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc. Natl. Acad. Sci. USA. 2006;103:8137–8142. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ivanov II, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 67.Yang XO, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Milner JD, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452:773–776. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jetten AM. Recent advances in the mechanisms of action and physiological functions of the retinoid-related orphan receptors (RORs) Curr. Drug Targets Inflamm. Allergy. 2004;3:395–412. doi: 10.2174/1568010042634497. [DOI] [PubMed] [Google Scholar]
- 70.Hu CM, et al. Modulation of T cell cytokine production by interferon regulatory factor-4. J. Biol. Chem. 2002;277:49238–49246. doi: 10.1074/jbc.M205895200. [DOI] [PubMed] [Google Scholar]
- 71.Lohoff M, et al. Dysregulated T helper cell differentiation in the absence of interferon regulatory factor 4. Proc. Natl. Acad. Sci. USA. 2002;99:11808–11812. doi: 10.1073/pnas.182425099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rengarajan J, et al. Interferon regulatory factor 4 (IRF4) interacts with NFATc2 to modulate interleukin 4 gene expression. J. Exp. Med. 2002;195:1003–1012. doi: 10.1084/jem.20011128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brustle A, et al. The development of inflammatory T(H)-17 cells requires interferon-regulatory factor 4. Nat. Immunol. 2007;8:958–966. doi: 10.1038/ni1500. [DOI] [PubMed] [Google Scholar]
- 74.Schmidt JV, et al. Characterization of a murine Ahr null allele: involvement of the Ah receptor in hepatic growth and development. Proc. Natl. Acad. Sci. USA. 1996;93:6731–6736. doi: 10.1073/pnas.93.13.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Veldhoen M, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 76.Quintana FJ, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 77.Zenewicz LA, et al. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity. 2007;27:647–659. doi: 10.1016/j.immuni.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 79.Yang XO, et al. Analysis of inflammatory and regulatory T cell lineage determination assisted with an IL-17F knockin reporter mouse. Immunity. 2008;29:44–56. [Google Scholar]
- 80.Du J, et al. Isoform-specific inhibition of ROR{alpha}-mediated transcriptional activation by human FOXP3. J. Immunol. 2008;180:4785–4792. doi: 10.4049/jimmunol.180.7.4785. [DOI] [PubMed] [Google Scholar]
- 81.Rangachari M, et al. T-bet negatively regulates autoimmune myocarditis by suppressing local production of interleukin 17. J. Exp. Med. 2006;203:2009–2019. doi: 10.1084/jem.20052222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moisan J, et al. Ets-1 is a negative regulator of Th17 differentiation. J. Exp. Med. 2007;204:2825–2835. doi: 10.1084/jem.20070994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Acosta-Rodriguez EV, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat. Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 84.Sallusto F, Mackay CR, Lanzavecchia A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu. Rev. Immunol. 2000;18:593–620. doi: 10.1146/annurev.immunol.18.1.593. [DOI] [PubMed] [Google Scholar]
- 85.Lim HW, et al. Human Th17 cells share major trafficking receptors with both polarized effector T cells and FOXP3+ regulatory T cells. J. Immunol. 2008;180:122–129. doi: 10.4049/jimmunol.180.1.122. [DOI] [PubMed] [Google Scholar]
- 86.Sato W, Aranami T, Yamamura T. Cutting edge: Human Th17 cells are identified as bearing CCR2+CCR5- phenotype. J. Immunol. 2007;178:7525–7529. doi: 10.4049/jimmunol.178.12.7525. [DOI] [PubMed] [Google Scholar]
- 87.Toscano MA, et al. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat. Immunol. 2007;8:825–834. doi: 10.1038/ni1482. [DOI] [PubMed] [Google Scholar]
- 88.Nakae S, et al. Phenotypic differences between Th1 and Th17 cells and negative regulation of Th1 cell differentiation by IL-17. J. Leukoc. Biol. 2007;81:1258–1268. doi: 10.1189/jlb.1006610. [DOI] [PubMed] [Google Scholar]
- 89.Wilson NJ, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat. Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 90.Acosta-Rodriguez EV, et al. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat. Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 91.Annunziato F, et al. Phenotypic and functional features of human Th17 cells. J. Exp. Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen Z, et al. Distinct regulation of interleukin-17 in human T helper lymphocytes. Arthritis Rheum. 2007;56:2936–2946. doi: 10.1002/art.22866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Volpe E, et al. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat. Immunol. 2008;9:650–657. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- 94.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat. Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang L, et al. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454:350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cooper AM, et al. Mice lacking bioactive IL-12 can generate protective, antigen-specific cellular responses to mycobacterial infection only if the IL-12 p40 subunit is present. J. Immunol. 2002;168:1322–1327. doi: 10.4049/jimmunol.168.3.1322. [DOI] [PubMed] [Google Scholar]
- 97.Happel KI, et al. Pulmonary interleukin-23 gene delivery increases local T-cell immunity and controls growth of Mycobacterium tuberculosis in the lungs. Infect. Immun. 2005;73:5782–5788. doi: 10.1128/IAI.73.9.5782-5788.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Khader SA, et al. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-gamma responses if IL-12p70 is available. J. Immunol. 2005;175:788–795. doi: 10.4049/jimmunol.175.2.788. [DOI] [PubMed] [Google Scholar]
- 99.Aujla SJ, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat. Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Khader SA, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4 +T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat. Immunol. 2007;8:369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 101.Umemura M, et al. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J. Immunol. 2007;178:3786–3796. doi: 10.4049/jimmunol.178.6.3786. [DOI] [PubMed] [Google Scholar]
- 102.Liang SC, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chung Y, et al. Expression and regulation of IL-22 in the IL-17-producing CD4+ T lymphocytes. Cell Res. 2006;16:902–907. doi: 10.1038/sj.cr.7310106. [DOI] [PubMed] [Google Scholar]
- 104.Scriba TJ, et al. Distinct, specific IL-17- and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. J. Immunol. 2008;180:1962–1970. doi: 10.4049/jimmunol.180.3.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Happel KI, et al. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J. Exp. Med. 2005;202:761–769. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Happel KI, et al. Cutting edge: roles of Toll-like receptor 4 and IL-23 in IL-17 expression in response to Klebsiella pneumoniae infection. J. Immunol. 2003;170:4432–4436. doi: 10.4049/jimmunol.170.9.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ye P, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wu Q, et al. IL-23-dependent IL-17 production is essential in neutrophil recruitment and activity in mouse lung defense against respiratory Mycoplasma pneumoniae infection. Microbes. Infect. 2007;9:78–86. doi: 10.1016/j.micinf.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Caruso R, et al. IL-23-mediated regulation of IL-17 production in Helicobacter pylori-infected gastric mucosa. Eur. J. Immunol. 2008;38:470–478. doi: 10.1002/eji.200737635. [DOI] [PubMed] [Google Scholar]
- 110.Zheng Y, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 111.Patera AC, et al. Interleukin 17 modulates the immune response to vaccinia virus infection. Virology. 2002;299:56–63. doi: 10.1006/viro.2002.1400. [DOI] [PubMed] [Google Scholar]
- 112.Maertzdorf J, Osterhaus AD, Verjans GM. IL-17 expression in human herpetic stromal keratitis: modulatory effects on chemokine production by corneal fibroblasts. J. Immunol. 2002;169:5897–5903. doi: 10.4049/jimmunol.169.10.5897. [DOI] [PubMed] [Google Scholar]
- 113.Smiley KL, et al. Association of gamma interferon and interleukin-17 production in intestinal CD4+ T cells with protection against rotavirus shedding in mice intranasally immunized with VP6 and the adjuvant LT(R192G) J. Virol. 2007;81:3740–3748. doi: 10.1128/JVI.01877-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Maek ANW, et al. Increased interleukin-17 production both in helper T cell subset Th17 and CD4-negative T cells in human immunodeficiency virus infection. Viral Immunol. 2007;20:66–75. doi: 10.1089/vim.2006.0063. [DOI] [PubMed] [Google Scholar]
- 115.Misse D, et al. IL-22 participates in an innate anti-HIV-1 host-resistance network through acute-phase protein induction. J. Immunol. 2007;178:407–415. doi: 10.4049/jimmunol.178.1.407. [DOI] [PubMed] [Google Scholar]
- 116.Kim B, et al. Enhanced viral immunoinflammatory lesions in mice lacking IL-23 responses. Microbes. Infect. 2008;10:302–312. doi: 10.1016/j.micinf.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kelly MN, et al. Interleukin-17/interleukin-17 receptor-mediated signaling is important for generation of an optimal polymorphonuclear response against Toxoplasma gondii infection. Infect. Immun. 2005;73:617–621. doi: 10.1128/IAI.73.1.617-621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rutitzky LI, Lopes da Rosa JR, Stadecker MJ. Severe CD4 T cell-mediated immunopathology in murine schistosomiasis is dependent on IL-12p40 and correlates with high levels of IL-17. J. Immunol. 2005;175:3920–3926. doi: 10.4049/jimmunol.175.6.3920. [DOI] [PubMed] [Google Scholar]
- 119.Rutitzky LI, et al. IL-23 is required for the development of severe egg-induced immunopathology in schistosomiasis and for lesional expression of IL-17. J. Immunol. 2008;180:2486–2495. doi: 10.4049/jimmunol.180.4.2486. [DOI] [PubMed] [Google Scholar]
- 120.Rudner XL, et al. Interleukin-23 (IL-23)-IL-17 cytokine axis in murine Pneumocystis carinii infection. Infect. Immun. 2007;75:3055–3061. doi: 10.1128/IAI.01329-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kleinschek MA, et al. IL-23 enhances the inflammatory cell response in Cryptococcus neoformans infection and induces a cytokine pattern distinct from IL-12. J. Immunol. 2006;176:1098–1106. doi: 10.4049/jimmunol.176.2.1098. [DOI] [PubMed] [Google Scholar]
- 122.Zelante T, et al. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur. J. Immunol. 2007;37:2695–2706. doi: 10.1002/eji.200737409. [DOI] [PubMed] [Google Scholar]
- 123.De Luca A, et al. Functional yet balanced reactivity to Candida albicans requires TRIF,MyD88, and IDO-dependent inhibition of Rorc. J. Immunol. 2007;179:5999–6008. doi: 10.4049/jimmunol.179.9.5999. [DOI] [PubMed] [Google Scholar]
- 124.Bozza S, et al. Lack of Toll IL-1R8 Exacerbates Th17 Cell Responses in Fungal Infection. J. Immunol. 2008;180:4022–4031. doi: 10.4049/jimmunol.180.6.4022. [DOI] [PubMed] [Google Scholar]
- 125.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Numasaki M, et al. IL-17 enhances the net angiogenic activity and in vivo growth of human non-small cell lung cancer in SCID mice through promoting CXCR-2-dependent angiogenesis. J. Immunol. 2005;175:6177–6189. doi: 10.4049/jimmunol.175.9.6177. [DOI] [PubMed] [Google Scholar]
- 127.Langowski JL, et al. IL-23 promotes tumour incidence and growth. Nature. 2006;442:461–465. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- 128.Benchetrit F, et al. Interleukin-17 inhibits tumor cell growth by means of a T-cell-dependent mechanism. Blood. 2002;99:2114–2121. doi: 10.1182/blood.v99.6.2114. [DOI] [PubMed] [Google Scholar]
- 129.Kottke T, et al. Induction of hsp70-mediated Th17 autoimmunity can be exploited as immunotherapy for metastatic prostate cancer. Cancer Res. 2007;67:11970–11979. doi: 10.1158/0008-5472.CAN-07-2259. [DOI] [PubMed] [Google Scholar]
- 130.Muranski P, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–373. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Segal BM, Dwyer BK, Shevach EM. An interleukin (IL)-10/IL-12 immunoregulatory circuit controls susceptibility to autoimmune disease. J. Exp. Med. 1998;187:537–546. doi: 10.1084/jem.187.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Thakker P, et al. IL-23 is critical in the induction but not in the effector phase of experimental autoimmune encephalomyelitis. J. Immunol. 2007;178:2589–2598. doi: 10.4049/jimmunol.178.4.2589. [DOI] [PubMed] [Google Scholar]
- 133.Komiyama Y, et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J. Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 134.Yang XO, et al. Regulation of inflammatory responses by IL-17F. J. Exp. Med. 2008;205:1063–1075. doi: 10.1084/jem.20071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kreymborg K, et al. IL-22 is expressed by Th17 cells in an IL-23-dependent fashion, but not required for the development of autoimmune encephalomyelitis. J. Immunol. 2007;179:8098–8104. doi: 10.4049/jimmunol.179.12.8098. [DOI] [PubMed] [Google Scholar]
- 136.Kebir H, et al. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat. Med. 2007;13:1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tzartos JS, et al. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am. J. Pathol. 2008;172:146–155. doi: 10.2353/ajpath.2008.070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Carlson T, et al. The Th17-ELR+ CXC chemokine pathway is essential for the development of central nervous system autoimmune disease. J. Exp. Med. 2008;205:811–823. doi: 10.1084/jem.20072404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Stromnes IM, et al. Differential regulation of central nervous system autoimmunity by T(H)1 and T(H)17 cells. Nat. Med. 2008;14:337–342. doi: 10.1038/nm1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nakae S, et al. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J. Immunol. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 141.Nakae S, et al. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc. Natl. Acad. Sci. USA. 2003;100:5986–5990. doi: 10.1073/pnas.1035999100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lubberts E, et al. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheum. 2004;50:650–659. doi: 10.1002/art.20001. [DOI] [PubMed] [Google Scholar]
- 143.Ruddy MJ, et al. Interleukin-17 regulates expression of the CXC chemokine LIX/CXCL5 in osteoblasts: implications for inflammation and neutrophil recruitment. J. Leukoc. Biol. 2004;76:135–144. doi: 10.1189/jlb.0204065. [DOI] [PubMed] [Google Scholar]
- 144.Sato K, et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J. Exp. Med. 2006;203:2673–2682. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nakashima T, Takayanagi H. The dynamic interplay between osteoclasts and the immune system. Arch. Biochem. Biophys. 2008;473:166–171. doi: 10.1016/j.abb.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 146.Hirota K, et al. T cell self-reactivity forms a cytokine milieu for spontaneous development of IL-17+ Th cells that cause autoimmune arthritis. J. Exp. Med. 2007;204:41–47. doi: 10.1084/jem.20062259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hirota K, et al. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J. Exp. Med. 2007;204:2803–2812. doi: 10.1084/jem.20071397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Agarwal S, Misra R, Aggarwal A. Interleukin 17 levels are increased in juvenile idiopathic arthritis synovial fluid and induce synovial fibroblasts to produce proinflammatory cytokines and matrix metalloproteinases. J. Rheumatol. 2008;35:515–519. [PubMed] [Google Scholar]
- 149.Nistala K, et al. Interleukin-17-producing T cells are enriched in the joints of children with arthritis, but have a reciprocal relationship to regulatory T cell numbers. Arthritis Rheum. 2008;58:875–887. doi: 10.1002/art.23291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kohno M, et al. Interleukin-17 gene expression in patients with rheumatoid arthritis. Mod. Rheumatol. 2008;18:15–22. doi: 10.1007/s10165-007-0015-y. [DOI] [PubMed] [Google Scholar]
- 151.Joosten LA, et al. T cell dependence of chronic destructive murine arthritis induced by repeated local activation of Toll-like receptor-driven pathways: crucial role of both interleukin-1beta and interleukin-17. Arthritis Rheum. 2008;58:98–108. doi: 10.1002/art.23152. [DOI] [PubMed] [Google Scholar]
- 152.Li D, et al. Expression of LL-37, human beta defensin-2, and CCR6 mRNA in patients with psoriasis vulgaris. J. Huazhong Univ. Sci. Technolog Med. Sci. 2004;24:404–406. doi: 10.1007/BF02861879. [DOI] [PubMed] [Google Scholar]
- 153.Li J, Li D, Tan Z. The expression of interleukin-17, interferon-gamma, and macrophage inflammatory protein-3 alpha mRNA in patients with psoriasis vulgaris. J. Huazhong Univ. Sci. Technolog Med. Sci. 2004;24:294–296. doi: 10.1007/BF02832018. [DOI] [PubMed] [Google Scholar]
- 154.Piskin G, et al. In vitro and in situ expression of IL-23 by keratinocytes in healthy skin and psoriasis lesions: enhanced expression in psoriatic skin. J. Immunol. 2006;176:1908–1915. doi: 10.4049/jimmunol.176.3.1908. [DOI] [PubMed] [Google Scholar]
- 155.Lee E, et al. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J. Exp. Med. 2004;199:125–130. doi: 10.1084/jem.20030451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wolk K, et al. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur. J. Immunol. 2006;36:1309–1323. doi: 10.1002/eji.200535503. [DOI] [PubMed] [Google Scholar]
- 157.Ma HL, et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J. Clin. Invest. 2008;118:597–607. doi: 10.1172/JCI33263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zheng Y, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 159.Boniface K, et al. A role for T cell-derived interleukin 22 in psoriatic skin inflammation. Clin. Exp. Immunol. 2007;150:407–415. doi: 10.1111/j.1365-2249.2007.03511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zaba LC, et al. Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses. J. Exp. Med. 2007;204:3183–3194. doi: 10.1084/jem.20071094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lowes MA, et al. Psoriasis Vulgaris Lesions Contain Discrete Populations of Th1 and Th17 T Cells. J. Invest. Dermatol. 2008;128:1207–1211. doi: 10.1038/sj.jid.5701213. [DOI] [PubMed] [Google Scholar]
- 162.Krueger GG, et al. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N. Engl. J. Med. 2007;356:580–592. doi: 10.1056/NEJMoa062382. [DOI] [PubMed] [Google Scholar]
- 163.Zhang Z, et al. Critical role of IL-17 receptor signaling in acute TNBS-induced colitis. Inflamm. Bowel Dis. 2006;12:382–388. doi: 10.1097/01.MIB.0000218764.06959.91. [DOI] [PubMed] [Google Scholar]
- 164.Monteleone G, et al. Control of matrix metalloproteinase production in human intestinal fibroblasts by interleukin 21. Gut. 2006;55:1774–1780. doi: 10.1136/gut.2006.093187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Caruso R, et al. A functional role for interleukin-21 in promoting the synthesis of the T-cell chemoattractant,MIP-3alpha, by gut epithelial cells. Gastroenterology. 2007;132:166–175. doi: 10.1053/j.gastro.2006.09.053. [DOI] [PubMed] [Google Scholar]
- 166.Monteleone G, et al. Interleukin-21 enhances T-helper cell type I signaling and interferon-gamma production in Crohn’s disease. Gastroenterology. 2005;128:687–694. doi: 10.1053/j.gastro.2004.12.042. [DOI] [PubMed] [Google Scholar]
- 167.Fina D, et al. Regulation of gut inflammation and th17 cell response by interleukin-21. Gastroenterology. 2008;134:1038–1048. doi: 10.1053/j.gastro.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 168.Schmechel S, et al. Linking genetic susceptibility to Crohn’s disease with Th17 cell function: IL-22 serum levels are increased in Crohn’s disease and correlate with disease activity and IL23R genotype status. Inflamm. Bowel Dis. 2008;14:204–212. doi: 10.1002/ibd.20315. [DOI] [PubMed] [Google Scholar]
- 169.Wolk K, et al. IL-22 induces lipopolysaccharide-binding protein in hepatocytes: a potential systemic role of IL-22 in Crohn’s disease. J. Immunol. 2007;178:5973–5981. doi: 10.4049/jimmunol.178.9.5973. [DOI] [PubMed] [Google Scholar]
- 170.Amre DK, et al. Association Between Genetic Variants in the IL-23R Gene and Early-Onset Crohn’s Disease: Results From a Case-Control and Family-Based Study Among Canadian Children. Am. J. Gastroenterol. 2008;103:615–620. doi: 10.1111/j.1572-0241.2007.01661.x. [DOI] [PubMed] [Google Scholar]
- 171.Capon F, et al. Sequence variants in the genes for the interleukin-23 receptor (IL23R) and its ligand (IL12B) confer protection against psoriasis. Hum. Genet. 2007;122:201–206. doi: 10.1007/s00439-007-0397-0. [DOI] [PubMed] [Google Scholar]
- 172.Cargill M, et al. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am. J. Hum. Genet. 2007;80:273–290. doi: 10.1086/511051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Smith RL, et al. Polymorphisms in the IL-12beta and IL-23R genes are associated with psoriasis of early onset in a UK cohort. J. Invest. Dermatol. 2008;128:1325–1327. doi: 10.1038/sj.jid.5701140. [DOI] [PubMed] [Google Scholar]
- 174.Burton PR, et al. Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat. Genet. 2007;39:1329–1337. doi: 10.1038/ng.2007.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Illes Z, et al. 3′UTR C2370 A allele of the IL-23 receptor gene is associated with relapsing-remitting multiple sclerosis. Neurosci. Lett. 2008;431:36–38. doi: 10.1016/j.neulet.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 176.Huber AK, et al. Interleukin (IL)-23 receptor is a major susceptibility gene for Graves’ ophthalmopathy: the IL-23/T-helper 17 axis extends to thyroid autoimmunity. J. Clin. Endocrinol. Metab. 2008;93:1077–1081. doi: 10.1210/jc.2007-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Borgogni E, et al. Elocalcitol inhibits inflammatory responses in human thyroid cells and T cells. Endocrinology. 2008;149:3626–3634. doi: 10.1210/en.2008-0078. [DOI] [PubMed] [Google Scholar]
- 178.Seiderer J, et al. Role of the novel Th17 cytokine IL-17F in inflammatory bowel disease (IBD): Upregulated colonic IL-17F expression in active Crohn’s disease and analysis of the IL17 F p.His161Arg polymorphism in IBD. Inflamm. Bowel Dis. 2008;14:437–445. doi: 10.1002/ibd.20339. [DOI] [PubMed] [Google Scholar]