Abstract
High rates of tuberculosis transmission are driving the ongoing global tuberculosis epidemic, and there is a pressing need for research focused on understanding and, ultimately, halting transmission. The ongoing tuberculosis–human immunodeficiency virus (HIV) coepidemic and rising rates of drug-resistant tuberculosis in parts of the world add further urgency to this work. Success in this research will require a concerted, multidisciplinary effort on the part of tuberculosis scientists, clinicians, programs, and funders and must span the research spectrum from biomedical sciences to the social sciences, public health, epidemiology, cost-effectiveness analyses, and operations research. Heterogeneity of tuberculosis disease, both among individual patients and among communities, poses a substantial challenge to efforts to interrupt transmission. As such, it is likely that effective interventions to stop transmission will require a combination of approaches that will vary across different epidemiologic settings. This research roadmap summarizes key gaps in our current understanding of transmission, as laid out in the preceding articles in this series. We also hope that it will be a call to action for the global tuberculosis community to make a sustained commitment to tuberculosis transmission science. Halting transmission today is an essential step on the path to end tuberculosis tomorrow.
Keywords: tuberculosis, transmission, public health
GETTING TO ZERO REQUIRES INTERRUPTING TRANSMISSION TODAY
With the release of the most recent Global Plan to Stop tuberculosis, the attention and efforts of the global tuberculosis community are rightly focused on the necessary goal to end tuberculosis [1]. Achieving this ambitious goal will not be easy. Tuberculosis, often called the oldest disease of mankind, is now the leading global killer among infectious diseases and the leading cause of death for people with HIV [2, 3]. Rising rates of drug resistance in parts of the world bring further urgency to the imperative to end tuberculosis because each case of drug resistance is orders of magnitude more difficult to treat with second- and third-line drug regimens that are more costly for tuberculosis programs, more toxic for patients, and often less effective [4]. The Global Plan calls for a paradigm shift in our approach to combating tuberculosis, including a change in mindset whereby tuberculosis has no place in our societies—with a push for more patient-centered care, programmatic innovations, and sustained increases in funding. Yet a critical component of what will be necessary for success has been too often neglected: an explicit focus on halting transmission.
The analogy of “turning off the tap” of new tuberculosis cases is often upheld as key for decreasing the global burden of tuberculosis [5]; yet our ability to stop new cases hinges directly on our capacity to halt transmission. As with so much of tuberculosis care, our current tools for halting transmission have been around for decades: active case finding, contact investigations, and targeted testing and treatment of latent tuberculosis infection. These interventions have proven quite successful in decreasing the tuberculosis burden at the population level in key historical trials [6–8]. However, as illustrated by several large-scale community-based studies, implementation of these measures in modern high-burden settings is often challenging—and, without substantial scale-up of existing interventions and the addition of novel tools, it is unlikely that we will achieve the goal of ending tuberculosis in those areas [9–12]. For example, in sub-Saharan Africa, transmission in the community is often so intense that household members and other known contacts only account for a minority of transmission events [13–17]. As a result, contact investigation is unlikely to halt tuberculosis transmission on its own in higher-burden contexts, even though it may still avert a high absolute number of transmission events. Additional gaps in our understanding of the biology and local determinants of transmission underscore just how little we know about transmission—and how that ignorance may be undermining our efforts to end tuberculosis.
Although others have advocated for halting transmission [18–22], we believe that this series offers a necessary perspective by focusing on transmission as a unique scientific discipline. While we must continue to deploy tools that are currently available and maximize the benefit from interventions that are known to be effective, we must also mobilize to develop new tools for combating transmission—new tools to understand where, how, and between whom transmission is happening. A roadmap for tuberculosis transmission research will enable us to track the most efficient and effective pathways for studying, understanding, and, ultimately, halting tuberculosis transmission. Only by focusing on transmission as both a sentinel event and an outcome of interest can we become proactive, rather than reactive, to new cases of tuberculosis. The roadmap herein draws from the articles in this series to chart an initial research path across the spectrum of basic sciences to applied and operations research. We hope that this roadmap provides tuberculosis researchers, programmers, policy makers, funders, and advocates with a forecast of research needs and a means for charting progress against milestones. Advances in transmission science will provide us with the necessary means for interrupting transmission wherever it may be occurring. Only then will we be able to design effective biomedical and sociobehavioral interventions to truly turn off the tap.
CURRENT TRANSMISSION RESEARCH NEEDS
In this section, we review and highlight some of the key points from the 3 scientific articles in this series. Within each research area—infectiousness and susceptibility, drivers of tuberculosis transmission, and interventions to halt the transmission of tuberculosis—we have emphasized gaps in our knowledge of transmission science that present immediate opportunities for high-impact research.
Infectiousness and Susceptibility
Multiple gaps remain in our understanding of source case infectiousness, aerosolization and airborne survival of Mycobacterium tuberculosis (Mtb), and host susceptibility. Source case infectiousness may be influenced by a number of individual-level factors, including disease site, sex, ethnicity, HIV status, and social risk factors [23–25]. Yet our ability to account for transmission being driven by each of these factors is limited. Furthermore, it is not yet known how subclinical disease or fluctuations in respiratory symptoms might contribute to transmission [26, 27]; nor is it known how variability in respiratory mechanics, tidal breathing, nocturnal breathing, or cough impact the likelihood of producing droplet nuclei with viable Mtb [28–30].
Although it is possible that pathogen-level characteristics, such as organism phenotype or lineage, might affect the aerobiology of tuberculosis, the role of shear forces and air and fluid dynamics is also likely to be quite important for aerosol transmission [29]. Once Mtb is released into the air, it remains unclear what proportion of droplet nuclei contain viable bacilli, how long those bacilli remain viable in the open environment, and whether there are differences between aerosolized versus alveolar Mtb [31, 32]. Elucidating the nature of tuberculosis transmission events by studying patient aerosols, also known as aerobiology, will address critical questions about the physiology and metabolic state of aerosolized Mtb, the viability and persistence of infectious aerosols, and the key characteristics associated with infectiousness.
Once Mtb is aerosolized in an indoor environment, multiple infection control interventions that reduce exposure and inhalation (“environmental controls”) have been shown to be effective, including natural or mechanical ventilation and upper room ultraviolet germicidal irradiation [33]. Reducing crowding in hospital and clinic waiting areas will further limit the effective contact rate between infectious persons and potentially susceptible contacts [34]. Yet, cost of installation, logistics of maintenance, and practical considerations (eg, climate that precludes opening doors and windows) often present barriers to implementation of environmental controls. Innovations are needed that simplify and reduce costs of healthcare facility redesign, with monitoring of airborne infectious particles, alerts when they above a critical level, and direct “cleansing” of shared air.
The determinants behind the probability of aerosol deposition of Mtb in a susceptible host are yet another unknown. There are a number of recent efforts to characterize cough aerosol production and exposure, but there is no current standard for determining whether a particular individual was exposed to Mtb. For close contacts of tuberculosis patients with a negative tuberculin skin test (TST) or interferon-gamma release assay (IGRA), there is no way to know whether they were indeed “adequately” exposed to Mtb, whether they had successful “early clearance” without mounting a T-cell–mediated adaptive immune response, or whether they are simply anergic with a false-negative TST or IGRA. This inability to characterize and quantify recent exposure, including among animal models, poses a substantial impediment to studies of transmission and transmissibility. Efforts to offset host vulnerability and enhance selective immune responses may prove critical for the design of a tuberculosis vaccine that is capable of preventing infection and not simply diminishing bacterial burden.
Drivers of Tuberculosis Transmission—Know Your Epidemic, Know Your Intervention
Mounting evidence from high tuberculosis burden countries, with varying levels of HIV and drug-resistant tuberculosis, implicate ongoing transmission as the driving force maintaining high levels of tuberculosis incidence [17, 35, 36]. Drivers of transmission can be conceived of as 3 stages along a cascade of transmission: (1) contact between infectious and susceptible individuals; (2) infectiousness of a particular individual; and (3) susceptibility to disease of exposed individuals [22]. These stages are heterogeneous at regional and national levels, and the relative role of each stage is heavily dictated by local attributes, including access to health care, population age structure, housing, population density, and migration [37, 38]. Variations in individual host susceptibility, in line with the local prevalence of HIV, malnutrition and low body mass index, diabetes mellitus, tobacco use, alcoholism, and silicosis, can also dramatically alter the transmission cascade [39, 40]. The strength of local tuberculosis control programs’ case finding and treatment strategies is yet another factor that can affect local transmission dynamics; a larger proportion of undiagnosed or untreated patients will contribute to ongoing transmission in a community. This heterogeneity of transmission poses a substantial challenge to tuberculosis control. To design efficacious and efficient tuberculosis control strategies, it will be necessary to delineate the relative contribution of the various drivers of tuberculosis in different settings. Ultimately, drivers of transmission must be studied and understood on a local level.
At present, measuring levels of tuberculosis transmission is also exceedingly difficult. The natural history and long latency period of tuberculosis means that observed cases of disease reflect a mix of recent transmission and reactivation disease from a remote infection. In addition, most cases observed through passive case-finding systems underestimate the number of undiagnosed, but diagnosable, infectious individuals in the community. Current methods to measure transmission include examining case notification rates and trends in TST or IGRA. However, real-time, definitive diagnosis of recent exposure to Mtb at the individual level is not possible with any of the currently available tests (eg, TST, IGRA, chest X-ray, sputum analysis). Yet there are several recent reports of promising new approaches to identifying recent exposure, including a T-cell immune signature capable of differentiating recent from remote tuberculosis infection and a blood transcriptomic signature associated with greater risk of progression to active tuberculosis disease [41, 42]. Although these signatures need to be validated in other settings, this type of biomarker of recent exposure would enable precisely targeted preventive therapy (akin to ring prophylaxis) and accelerate identification of tuberculosis “hotspots”—areas of high tuberculosis incidence—for earlier and more effective implementation of infection control measures [43]. Such hotspot detection and elimination represents a stop-gap measure to interrupt transmission and reduce the global burden of tuberculosis while new drugs, diagnostics, and vaccines are progressing through the product development pipeline. These hotspot communities could also serve as priority areas for piloting new transmission interventions as they become available [44].
More recently, molecular epidemiology using whole genome sequencing has greatly facilitated studies of patient- and population-level transmission [13, 14]. Interestingly, in a number of studies that used genotyping in the late 1990s and early 2000s, cases with genetic links often did not have traditional epidemiologic links [16]. Several studies have aimed to estimate the proportion of transmission that occurs within households by comparing genotypes of cases occurring within households or community settings. These studies have found that in medium and high tuberculosis burden settings, the majority of tuberculosis transmission occurs outside of households or known close contacts [15, 45]. As such, although contact investigations remain a critical component of any effort to identify and eliminate tuberculosis in the community, the proportion of tuberculosis transmission that can be halted through contact investigations alone may be lower in some settings than traditionally anticipated [46]. Furthermore, the location where infections are occurring within these communities remains a major unknown in the transmission ecology of tuberculosis. Congregate locations that facilitate air exchange between infectious cases with noninfected individuals, such as in transport hubs, school classrooms, and prisons, have been implicated as hotspots of transmission and may offer opportunities for targeted interventions [47, 48]. However, it is unknown whether other, less obvious sites of congregation may also be driving ongoing transmission. An integrated approach that combines traditional epidemiology with methods drawn from spatial, demographic, network, and whole genome analysis will undoubtedly be required to identify such drivers.
Interventions to Halt the Transmission of Tuberculosis
Ultimately, it is not simply enough to understand where and how tuberculosis transmission occurs; we must intervene to interrupt that transmission if we hope to have an impact on incidence. To be effective in this effort, it is critical to understand which interventions, implemented in what fashion, are most likely to avert the largest proportion of transmission events (specifically, those events that result in secondary cases of infectious tuberculosis) at the population level. For example, in settings where transmission originates largely from specific and identifiable populations (eg, young adult men [49]), it may be feasible to target case-finding efforts at those groups. Where reactivation is common in high-risk groups (eg, HIV-positive individuals [50], people with risk factors such as diabetes or malnutrition [51], elderly populations [52]), it may be critical to target preventive therapy to those populations. In congregate settings (eg, prisons or healthcare institutions), infection control and modification of the built environment can have a major impact in reducing transmission.
In prioritizing between different potential interventions in a given setting, creating “snapshots” of prevalent tuberculosis to understand who has active tuberculosis at a given time and how each case of infectious tuberculosis might have been averted can be helpful because these cases represent the tuberculosis transmission potential in a community. Novel study designs and statistical techniques (such as adaptive trials [53] and linkage of trial outcomes with transmission models [54]) can aid in developing an evidence base that speaks not just to the effectiveness of specific interventions but also to their comparative impact on transmission at the population level.
Although research to understand tuberculosis transmission is essential, we must link those efforts with rigorous evaluation of the efficacy and cost-effectiveness of transmission-halting interventions so that we can prioritize those activities most likely to prevent the greatest number of transmission events as rapidly as possible given available resources. Meanwhile, our armamentarium for halting tuberculosis transmission need not be limited to biomedical interventions. Historical data from Western Europe, where tuberculosis incidence declined 10% annually after World War II, tells us that socioeconomic development can reduce tuberculosis transmission. We should hypothesize broadly about how socioeconomic development may interrupt transmission and systematically test interventions for impact, including nutrition support and direct cash transfer programs to household or individuals in tuberculosis-affected communities [55]. An additional benefit of interventions aimed at reducing tuberculosis transmission through poverty alleviation is that they may also reduce the spread of emerging infectious diseases such as pandemic influenza and Ebola. Finally, when evaluations to halt transmission do not turn out as expected, as with the Thibela tuberculosis trial of isoniazid preventive therapy in South African gold mines [12], rigorous efforts must be undertaken to understand the reasons behind the negative findings so that any pitfalls can be adequately addressed or avoided in future studies [54].
INTERRUPTING TRANSMISSION TO END TUBERCULOSIS
The global burden of tuberculosis cannot be understated. In addition to being the leading infectious disease killer and leading killer among people with HIV, the costs to healthcare systems, communities, families, and patients is unsustainable. For example, in Myanmar it is estimated that 65% of tuberculosis-affected households face catastrophic costs (ie, >20% of their annual household income) [3]. The World Health Assembly member states have endorsed the END TB Strategy, which prioritizes intensified research and innovation. Here we propose that interrupting transmission become a central focus of that intensified research and innovation.
As we have described herein and in this series, measuring tuberculosis transmission in high-burden settings where each case is not an isolated event represents a significant methodological challenge (Table 1). The dynamic interaction between the tuberculosis and HIV epidemics in many parts of the world further confounds this challenge. Addressing this challenge will require innovative, high-resolution tools, such as geospatial and whole genome sequencing–based analyses of transmission networks [56, 57]. Understanding who transmits to whom, where, and when using new molecular methods will inform the development, deployment, and assessment of precision public health interventions aimed at ending tuberculosis [58]. Countries can and should use such tools to “understand their epidemic” [59] in order to maximize the impact of current interventions and accelerate the application of new tools for the benefit of at-risk populations. For example, the recent decision of Public Health England to institute routine whole genome sequencing for all mycobacterial infections will undoubtedly contribute to advances in our understanding of transmission in the United Kingdom and other low-burden settings [60]. A deeper understanding of tuberculosis transmission may well lead to new interventions to interrupt it, as was the case for HIV with condoms, preexposure prophylaxis, and the dapivirine ring, for example.
Table 1.
Tuberculosis Transmission Research Needs, Potential Obstacles, and Anticipated Impact and Benefit.
| Research needs | Potential obstacles | Anticipated impact and benefit | |
|---|---|---|---|
| Infectiousness and susceptibility | Aerobiology: variability in cough aerosol production, role of tidal breathing, airborne survival of Mtb particles | Heterogeneity among patients and limited tools for evaluating aerosol particles | Better tools for preventing aerosol transmission, particularly in nosocomial settings |
| Degree of source case infectiousness (eg, for subclinical disease, people with HIV) | Identification of people with subclinical disease prior to onset of symptoms | Understanding of relative contributions from different source cases and ability to target prevention efforts accordingly | |
| Means to reduce effective contact rates and shared air | Cost and logistics of overhauling congregate facilities; need to engage nonmedical disciplines (eg, engineering, biotechnology) | Reduction of transmission in congregate settings | |
| Correlates of resistance to tuberculosis infection | Inadequate animal models | Vaccine to prevent infection | |
| Drivers of transmission | Local epidemiology and relative contribution of various factors in a given setting | Multidisciplinary approach needed to fully illustrate drivers and catalysts of transmission | Evidence to guide use of limited public health resources for targeted interventions |
| Better measures and markers of transmission | TST/IGRA does not differentiate recent from remote infection | Accurate measures of impact of interventions designed to halt transmission | |
| Community locations of transmission | Difficult to identify epidemiologic links among casual contacts | Identification of congregate areas that may be driving nonhousehold transmission | |
| Real-time molecular epidemiology and whole genome sequencing to identify linked cases | High cost and technical capacity for molecular epidemiology; transmission occurring from undiagnosed cases | Rapid recognition of outbreaks and potential to intervene and prevent further transmission | |
| Interventions to halt tuberculosis transmission | Detailed cross-sectional snapshots of tuberculosis prevalence and transmission at the community level | Difficulty in identifying/diagnosing the tuberculosis cases most associated with transmission | Understanding the sources of tuberculosis transmission in communities (ie, who needs to be evaluated and diagnosed) |
| Models and decision aids to prioritize those interventions likely to have greatest impact on transmission in different settings | Assumptions needed for decision making in the absence of complete data | Ability to prioritize those interventions most likely to reduce transmission, given current resource availability | |
| Clinical trials of interventions designed to halt tuberculosis transmission in populations | Need for preliminary evidence of ability to curb transmission at the population level | Novel evidence-based interventions proven to reduce population-level tuberculosis transmission |
Abbreviations: HIV, human immunodeficiency virus; IGRA, interferon-gamma release assay; Mtb, Mycobacterium tuberculosis; TST, tuberculin skin test.
The ongoing transmission of tuberculosis in clinics and hospitals is another area of grave concern that directly impacts patients, their families, and the global healthcare workforce. Tuberculosis has become an occupational lung disease for providers from high-burden countries, including frontline community health workers, nurses, medical students, and physicians [61–63]. Tuberculosis as a personal risk associated with a professional choice is unacceptable in the 21st century and must be addressed by national and international professional associations, lest their workers succumb to tuberculosis.
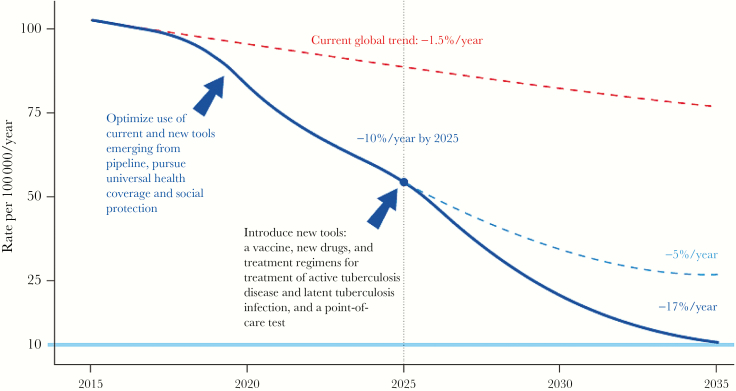
Ultimately, both established and new interventions will be required in order to end tuberculosis (Figure 1). Although established interventions such as isoniazid preventive therapy have been quite successful at reducing tuberculosis rates in many settings, several recent studies did not demonstrate a sustained impact for isoniazid preventive therapy in settings where transmission and force of infection is very high [6, 7, 9, 12, 64]. The same may be true for new interventions such as vaccines, which may protect against a single exposure or a low inoculum but be ineffective against repeated, high-dose exposures. The tuberculosis control community should therefore reduce ongoing transmission using all available methods such that any new tools can be effectively deployed. Only by combining the effective use of our existing tools with novel biomedical and social interventions will we be achieve the dramatic declines in incidence necessary to achieve our goals of tuberculosis elimination. And only then will we know that we have reached the end of our research roadmap for tuberculosis transmission science.
Figure 1.
Projected acceleration in the decline of global tuberculosis incidence rates to target levels. From WHO END TB Strategy [62].
In conclusion, continued research in basic discovery, clinical epidemiology, programmatic and population interventions, and cost-effectiveness are required to interrupt transmission and end tuberculosis. We hope that this series furthers that call by highlighting both the gaps and the enormous potential in transmission research. The success of the Global Plan and our collective ability to bend the curve of tuberculosis incidence to zero will require a concerted effort to interrupt transmission today.
Notes
Disclaimer. The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, the National Institute of Allergy and Infectious Diseases, the National Institutes of Health, or the US Department of Health and Human Services.
Financial support. This publication and workshop have been funded in whole or in part with federal funds from the Division of AIDS, National Institute of Allergy and Infectious Diseases (NIAID), US National Institutes of Health (NIH), Department of Health and Human Services under contract number HHSN272201100001G—Research Support Services for the Division of AIDS. Funding support was also provided in part by NIH grants: NIAID R01AI116787 (to D. W. D.) NIAID R01AI089349 (to N. R. G.), NIAID R01AI087465 (to N. R. G.), NIAID K24AI114444 (to N. R. G.), Emory CFAR P30AI050409 (principal investigator: J. W. Curran), and NHLBI T32 HL116271 (principal investigator: D. M. Guidot). The Bill and Melinda Gates Foundation provided funding for participant travel to the workshop. The Centers for Disease Control and Prevention and the South African Medical Research Council co-hosted the corresponding meeting.
Supplement sponsorship. This work is part of a supplement sponsored by the Centers for Disease Control and Prevention and the National Institutes of Health.
Potential conflicts of interest. R. R. is contracted by Kelly Services, which receives federal funds through a government contract with the National Institutes of Health. All other authors declare no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Global Plan to End TB: The Paradigm Shift, 2016–2020. Geneva, Switzerland: STOP TB Partnership, UNOPS; 2015. http://www.stoptb.org/assets/documents/global/plan/GlobalPlanToEndTB_TheParadigmShift_2016-2020_StopTBPartnership.pdf. [Google Scholar]
- 2. Zink AR, Sola C, Reischl U et al. Characterization of Mycobacterium tuberculosis complex DNAs from Egyptian mummies by spoligotyping. J Clin Microbiol 2003; 41:359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Global Tuberculosis Report. Geneva, Switzerland: World Health Organization, 2016. http://www.who.int/tb/publications/global_report/en/. [Google Scholar]
- 4. Gandhi NR, Nunn P, Dheda K et al. Multidrug-resistant and extensively drug-resistant tuberculosis: a threat to global control of tuberculosis. Lancet 2010; 375:1830–43. [DOI] [PubMed] [Google Scholar]
- 5. Yuen CM, Amanullah F, Dharmadhikari A et al. Turning off the tap: stopping tuberculosis transmission through active case-finding and prompt effective treatment. Lancet 2015; 386:2334–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Comstock GW, Ferebee SH, Hammes LM. A controlled trial of community-wide isoniazid prophylaxis in Alaska. Am Rev Respir Dis 1967; 95:935–43. [DOI] [PubMed] [Google Scholar]
- 7. Comstock GW, Baum C, Snider DE Jr. Isoniazid prophylaxis among Alaskan Eskimos: a final report of the bethel isoniazid studies. Am Rev Respir Dis 1979; 119:827–30. [DOI] [PubMed] [Google Scholar]
- 8. Smieja MJ, Marchetti CA, Cook DJ, Smaill FM. Isoniazid for preventing tuberculosis in non-HIV infected persons. Cochrane Database Syst Rev 2000; 2:Cd001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ayles H, Muyoyeta M, Du Toit E et al. ; ZAMSTAR Team Effect of household and community interventions on the burden of tuberculosis in southern Africa: the ZAMSTAR community-randomised trial. Lancet 2013; 382:1183–94. [DOI] [PubMed] [Google Scholar]
- 10. Cavalcante SC, Durovni B, Barnes GL et al. Community-randomized trial of enhanced DOTS for tuberculosis control in Rio de Janeiro, Brazil. Int J Tuberc Lung Dis 2010; 14:203–9. [PMC free article] [PubMed] [Google Scholar]
- 11. Corbett EL, Bandason T, Duong T et al. Comparison of two active case-finding strategies for community-based diagnosis of symptomatic smear-positive tuberculosis and control of infectious tuberculosis in Harare, Zimbabwe (DETECTB): a cluster-randomised trial. Lancet 2010; 376:1244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Churchyard GJ, Fielding KL, Lewis JJ et al. ; Thibela TB Study Team. A trial of mass isoniazid preventive therapy for tuberculosis control. N Engl J Med 2014; 370:301–10. [DOI] [PubMed] [Google Scholar]
- 13. Glynn JR, Guerra-Assunção JA, Houben RM et al. Whole genome sequencing shows a low proportion of tuberculosis disease is attributable to known close contacts in rural Malawi. PLoS One 2015; 10:e0132840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Verver S, Warren RM, Munch Z et al. Proportion of tuberculosis transmission that takes place in households in a high-incidence area. Lancet 2004; 363:212–4. [DOI] [PubMed] [Google Scholar]
- 15. Chamie G, Wandera B, Marquez C et al. Identifying locations of recent TB transmission in rural Uganda: a multidisciplinary approach. Trop Med Int Health 2015; 20:537–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mathema B, Kurepina NE, Bifani PJ, Kreiswirth BN. Molecular epidemiology of tuberculosis: current insights. Clin Microbiol Rev 2006; 19:658–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shah NS, Auld SC, Brust JC et al. Transmission of extensively drug-resistant tuberculosis in South Africa. N Engl J Med 2017; 376:243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Das P, Horton R. Tuberculosis—getting to zero. Lancet 2015; 386:2231–2. [DOI] [PubMed] [Google Scholar]
- 19. Yates TA, Khan PY, Knight GM et al. The transmission of Mycobacterium tuberculosis in high burden settings. Lancet Infect Dis 2016; 16:227–38. [DOI] [PubMed] [Google Scholar]
- 20. Nardell E, Dharmadhikari A. Turning off the spigot: reducing drug-resistant tuberculosis transmission in resource-limited settings. Int J Tuberc Lung Dis 2010; 14:1233–43. [PMC free article] [PubMed] [Google Scholar]
- 21. Kompala T, Shenoi SV, Friedland G. Transmission of tuberculosis in resource-limited settings. Curr HIV/AIDS Rep 2013; 10:264–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dowdy DW, Azman AS, Kendall EA, Mathema B. Transforming the fight against tuberculosis: targeting catalysts of transmission. Clin Infect Dis 2014; 59:1123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang Z, Kong Y, Wilson F et al. Identification of risk factors for extrapulmonary tuberculosis. Clin Infect Dis 2004; 38:199–205. [DOI] [PubMed] [Google Scholar]
- 24. Peto HM, Pratt RH, Harrington TA, LoBue PA, Armstrong LR. Epidemiology of extrapulmonary tuberculosis in the United States, 1993–2006. Clin Infect Dis 2009; 49:1350–7. [DOI] [PubMed] [Google Scholar]
- 25. Musellim B, Erturan S, Sonmez Duman E, Ongen G. Comparison of extra-pulmonary and pulmonary tuberculosis cases: factors influencing the site of reactivation. Int J Tuberc Lung Dis 2005; 9:1220–3. [PubMed] [Google Scholar]
- 26. Dowdy DW, Basu S, Andrews JR. Is passive diagnosis enough? The impact of subclinical disease on diagnostic strategies for tuberculosis. Am J Respir Crit Care Med 2013; 187:543–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Perrin FM, Woodward N, Phillips PP et al. Radiological cavitation, sputum mycobacterial load and treatment response in pulmonary tuberculosis. Int J Tuberc Lung Dis 2010; 14:1596–602. [PubMed] [Google Scholar]
- 28. Fennelly KP, Jones-López EC, Ayakaka I et al. Variability of infectious aerosols produced during coughing by patients with pulmonary tuberculosis. Am J Respir Crit Care Med 2012; 186:450–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Turner RD, Bothamley GH. Cough and the transmission of tuberculosis. J Infect Dis 2015; 211:1367–72. [DOI] [PubMed] [Google Scholar]
- 30. Wurie FB, Lawn SD, Booth H, Sonnenberg P, Hayward AC. Bioaerosol production by patients with tuberculosis during normal tidal breathing: implications for transmission risk. Thorax 2016; 71:549–54. [DOI] [PubMed] [Google Scholar]
- 31. Garton NJ, Waddell SJ, Sherratt AL et al. Cytological and transcript analyses reveal fat and lazy persister-like bacilli in tuberculous sputum. PLoS Med 2008; 5:e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nardell EA. Catching droplet nuclei: toward a better understanding of tuberculosis transmission. Am J Respir Crit Care Med 2004; 169:553–4. [DOI] [PubMed] [Google Scholar]
- 33. Escombe AR, Moore DA, Gilman RH et al. Upper-room ultraviolet light and negative air ionization to prevent tuberculosis transmission. PLoS Med 2009; 6:e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fennelly KP, Jones-López EC. Quantity and quality of inhaled dose predicts immunopathology in tuberculosis. Front Immunol 2015; 6:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Becerra MC, Appleton SC, Franke MF et al. Tuberculosis burden in households of patients with multidrug-resistant and extensively drug-resistant tuberculosis: a retrospective cohort study. Lancet 2011; 377:147–52. [DOI] [PubMed] [Google Scholar]
- 36. Li X, Zhang Y, Shen X et al. Transmission of drug-resistant tuberculosis among treated patients in Shanghai, China. J Infect Dis 2007; 195:864–9. [DOI] [PubMed] [Google Scholar]
- 37. Wood R, Liang H, Wu H et al. Changing prevalence of tuberculosis infection with increasing age in high-burden townships in South Africa. Int J Tuberc Lung Dis 2010; 14:406–12. [PMC free article] [PubMed] [Google Scholar]
- 38. Liu L, Wu J, Zhao XQ. The impact of migrant workers on the tuberculosis transmission: general models and a case study for China. Math Biosci Eng 2012; 9:785–807. [DOI] [PubMed] [Google Scholar]
- 39. Hanifa Y, Grant AD, Lewis J, Corbett EL, Fielding K, Churchyard G. Prevalence of latent tuberculosis infection among gold miners in South Africa. Int J Tuberc Lung Dis 2009; 13:39–46. [PubMed] [Google Scholar]
- 40. Murray J, Kielkowski D, Reid P. Occupational disease trends in black South African gold miners. An autopsy-based study. Am J Respir Crit Care Med 1996; 153:706–10. [DOI] [PubMed] [Google Scholar]
- 41. Halliday A, Whitworth H, Kottoor SH et al. Stratification of latent Mycobacterium tuberculosis infection by cellular immune profiling. J Infect Dis 2017; 215:1480–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zak DE, Penn-Nicholson A, Scriba TJ et al. ; ACS and GC6-74 cohort study groups. A blood RNA signature for tuberculosis disease risk: a prospective cohort study. Lancet 2016; 387:2312–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wood R, Morrow C, Ginsberg S et al. Quantification of shared air: a social and environmental determinant of airborne disease transmission. PLoS One 2014; 9:e106622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lee RS, Radomski N, Proulx J- F et al. Population genomics of Mycobacterium tuberculosis in the Inuit. Proc Nat Acad Sci U S A 2015; 112:13609–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Guerra-Assuncao JA, Crampin AC, Houben RM et al. Large-scale whole genome sequencing of M. tuberculosis provides insights into transmission in a high prevalence area. Elife 2015; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. World Health Organization. Recommendations for investigating contacts of persons with infectious tuberculosis in low and middle-income countries. Geneva, Switzerland: WHO, 2012. [PubMed] [Google Scholar]
- 47. Dowdy DW, Golub JE, Chaisson RE, Saraceni V. Heterogeneity in tuberculosis transmission and the role of geographic hotspots in propagating epidemics. Proc Natl Acad Sci U S A 2012; 109:9557–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zelner JL, Murray MB, Becerra MC et al. Identifying hotspots of multidrug-resistant tuberculosis transmission using spatial and molecular genetic data. J Infect Dis 2016; 213:287–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dodd PJ, Looker C, Plumb ID et al. Age- and sex-specific social contact patterns and incidence of Mycobacterium tuberculosis infection. Am J Epidemiol 2016; 183:156–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pretorius C, Menzies NA, Chindelevitch L et al. The potential effects of changing HIV treatment policy on tuberculosis outcomes in South Africa: results from three tuberculosis-HIV transmission models. AIDS 2014; 28(suppl 1):S25–34. [DOI] [PubMed] [Google Scholar]
- 51. Odone A, Houben RM, White RG, Lonnroth K. The effect of diabetes and undernutrition trends on reaching 2035 global tuberculosis targets. Lancet Diabetes Endocrinol 2014; 2:754–64. [DOI] [PubMed] [Google Scholar]
- 52. Prince MJ, Wu F, Guo Y et al. The burden of disease in older people and implications for health policy and practice. Lancet 2015; 385:549–62. [DOI] [PubMed] [Google Scholar]
- 53. Davies GR, Phillips PP, Jaki T. Adaptive clinical trials in tuberculosis: applications, challenges and solutions. Int J Tuberc Lung Dis 2015; 19:626–34. [DOI] [PubMed] [Google Scholar]
- 54. Vynnycky E, Sumner T, Fielding KL et al. Tuberculosis control in South African gold mines: mathematical modeling of a trial of community-wide isoniazid preventive therapy. Am J Epidemiol 2015; 181:619–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Boccia D, Pedrazzoli D, Wingfield T et al. Towards cash transfer interventions for tuberculosis prevention, care and control: key operational challenges and research priorities. BMC Infect Dis 2016; 16:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Baumgartner L. Urban reservoirs of tuberculosis. J Chronic Dis 1959; 9:688–92. [DOI] [PubMed] [Google Scholar]
- 57. Gardy JL, Johnston JC, Ho Sui SJ et al. Whole-genome sequencing and social-network analysis of a tuberculosis outbreak. N Engl J Med 2011; 364:730–9. [DOI] [PubMed] [Google Scholar]
- 58. Dowell SF, Blazes D, Desmond-Hellman S. Four steps to precision public health. Nature 2016; 540:189–91. [Google Scholar]
- 59. World Health Organization. A global action framework forTB reserach in support of the third pillar of WHO’s End TB strategy. Geneva, Switzerland: WHO, 2015. [Google Scholar]
- 60. Walker TM, Cruz ALG, Peto TE, Smith EG, Esmail H, Crook DW. Tuberculosis is changing. Lancet Infect Dis 2017; 17:359–61. [DOI] [PubMed] [Google Scholar]
- 61. Naidoo S, Jinabhai CC. TB in health care workers in KwaZulu-Natal, South Africa. Int J Tuberc Lung Dis 2006; 10:676–82. [PubMed] [Google Scholar]
- 62. Barnagarwala T. TB hospital staff live under shadow of dreaded disease. The Indian Express. Uttar Pradesh, India: IE Online Media Services, 2014. [Google Scholar]
- 63. TB Proof: Agents for Change http://www.tbproof.org/.
- 64. Hanson ML, Comstock GW, Haley CE. Community isoniazid prophylaxis program in an underdeveloped area of Alaska. Public Health Rep 1967; 82:1045–56. [PMC free article] [PubMed] [Google Scholar]