Abstract
Introduction:
Sickle cell disease guidelines recommend that patients on hydroxyurea receive monitoring at least every 2–3 months, but it is unknown if this occurs in clinical practice. This study aimed to determine if patients with sickle cell disease at Nationwide Children’s Hospital had at least 4, in-person monitoring visits during a 12-month period and if frequent monitoring was associated with hydroxyurea adherence and clinical outcomes.
Methods:
We performed a retrospective analysis of children on hydroxyurea for at least 12 months during 2010–2015. Patients’ demographics, laboratory studies, prescriptions, and number of hydroxyurea and acute visits were recorded from their 12-month period that met eligibility criteria. Patients were considered frequently monitored if they had ≥ 4 hydroxyurea visits and adherent if they had prescriptions for hydroxyurea for ≥ 80% of the days in their 12-month period.
Results:
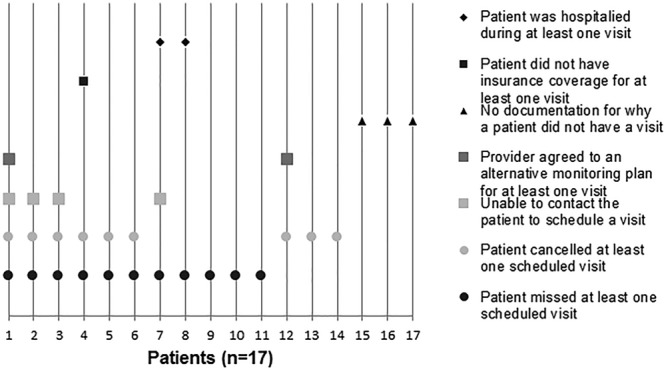
Seventy-four children met the eligibility criteria and 57 (77%) had frequent monitoring. The most common reason for not obtaining frequent monitoring was missing a scheduled appointment. A greater proportion of frequently monitored patients were adherent to hydroxyurea (66.7% versus 17.7%; P < 0.001), and they had significantly fewer acute visits (median 1 versus 2 visits; P = 0.032) compared with infrequently monitored patients.
Conclusions:
Our study shows that most children on hydroxyurea at Nationwide Children’s Hospital received frequent monitoring and that it was associated with improved adherence and outcomes. Our results suggest that frequent in-person monitoring could be an opportunity to identify poorly adherent patients. These data inform our next quality improvement initiative that will maximize adherence to these monitoring guidelines.
INTRODUCTION
Sickle cell disease (SCD) is a chronic red blood cell disorder that results in significant childhood morbidity and premature mortality. Hydroxyurea is the only approved disease-modifying medication for patients with SCD.1 Because hydroxyurea has the potential to cause myelotoxicity (neutropenia, reticulocytopenia, and thrombocytopenia), the 2014 SCD Expert Guidelines from the National Heart, Lung and Blood Institute recommend that all patients who are prescribed hydroxyurea receive frequent monitoring to reduce the toxicity risks and maximize the benefits (e.g., reduced sickling events) of this therapy. A treatment protocol was also included in the guidelines and was informed by past hydroxyurea clinical trials, indirect evidence from hydroxyurea pharmacokinetics studies, and expert panel consensus.2 This protocol recommends that patients receive monthly laboratory monitoring during hydroxyurea dose escalation and at least bimonthly to quarterly monitoring once patients achieve a stable hydroxyurea dose. It does not stipulate if monitoring should occur in-person or if laboratory monitoring is sufficient.
Despite these guidelines, it is unclear if children treated with hydroxyurea in clinical practice are receiving the recommended monitoring and if receiving this monitoring has any impact on patients’ clinical outcomes. Prior studies suggest that it may be unrealistic to assume that children with SCD on hydroxyurea therapy will obtain this frequency of monitoring because only 61% of children with SCD attend their recommended biannual comprehensive hematology appointments.3 Furthermore, children with SCD have a multi-system medical condition, commonly live in urban settings, and frequently have public insurance,4,5 which are all characteristics that are more common in patients who frequently miss clinic appointments in the primary care setting.6,7 Next, it is unclear if frequent monitoring is necessary, particularly for those known to be highly adherent to their hydroxyurea because retrospective studies suggest that it is unlikely that critical toxicities will be missed in these patients.8 Finally, although hydroxyurea monitoring can occur during a scheduled appointment with a provider where side effects, adherence, and hydroxyurea dosing can be discussed openly, it is possible that laboratory checks alone might be sufficient for hydroxyurea monitoring. Considering the increasing number of children on hydroxyurea,9,10 the potential impact that monitoring has on patients’ safety, and the burden that hydroxyurea treatment places on patients, families, and providers, a better understanding of how to monitor children on hydroxyurea is needed.
To provide baseline data to inform a quality improvement initiative for children on hydroxyurea at Nationwide Children’s Hospital (NCH), the aim of this study was to determine the prevalence of patients who receive frequent in-person monitoring for hydroxyurea at NCH. Our goal was to identify common barriers to obtaining the recommended frequency of hydroxyurea monitoring and to determine if frequent hydroxyurea monitoring was associated with hydroxyurea adherence and laboratory and clinical outcomes.
METHODS
NCH Hydroxyurea Monitoring Guidelines and Clinical Setting
NCH is an urban, tertiary-care children’s hospital located in Franklin County, OH. NCH provides care for approximately 380 children with SCD who are 0–21 years of age and most (> 90%) of these patients live within Franklin County. The SCD team at NCH includes 2 pediatric hematologists, 2 advanced practice registered nurses, and 2 nurse clinicians, and these individuals provide the majority of outpatient medical care to children with SCD at NCH. Given the positive clinical trial results in young children in 2011 and the 2014 SCD Expert Guideline recommendations,2,11 the number of patients who are eligible, offered, and treated with hydroxyurea at NCH has increased substantially over the past few years and currently, 115 children (30% of the total population) with SCD are prescribed hydroxyurea. These children primarily have severe SCD genotypes (hemoglobin SS or Sβ0), but based on the guidelines, hydroxyurea is also used in patients with other less severe genotypes, if they have significant symptoms.2
Before 2010, the SCD team at NCH created hydroxyurea monitoring guidelines for patients similar to those published in 2014.2 Specifically, NCH guidelines recommend that children treated with hydroxyurea have a minimum of 4 hydroxyurea monitoring appointments with a SCD provider each year, and more often if their hydroxyurea doses are adjusted. All of these monitoring visits occur in-person with a hematologist or advanced practice registered nurse and during patients’ biannual SCD comprehensive appointments or separate hydroxyurea-only appointments. During these visits, providers review patients’ SCD and hydroxyurea-related concerns, hydroxyurea adherence, clinical and laboratory results, and they can adjust patients’ hydroxyurea dose. Hydroxyurea prescription refills are provided and patients are informed when to schedule their next appointment. If a patient misses a scheduled appointment, the SCD nurse clinicians attempt to contact the patient to reschedule it. Patients can also request hydroxyurea refills at any hospitalization discharge, by contacting the nurse clinicians, or during any other contact they have with the hematology team. If a patient requests a hydroxyurea refill by telephone but has not had a hydroxyurea monitoring appointment according to the NCH guidelines, hydroxyurea is refilled but the patient is instructed to schedule an appointment and may be instructed to have laboratory work completed. To facilitate patient access, appointments are available either at the main hospital clinic in downtown Columbus, Ohio, or an offsite clinic on the east side of Columbus.
Patient Population and Study Design
We performed a retrospective chart review of children ages 2–20 years with SCD at NCH. We used the NCH SCD patient database to identify all previous and current NCH patients who may have been eligible from January 1, 2010, to December 31, 2015. Eligibility criteria, confirmed from patients’ electronic medical records (EMRs), included (1) any SCD genotype, (2) provider documentation that the patient was treated with hydroxyurea for at least 1 consecutive 12-month period from January 1, 2010 to December 31, 2015, and (3) at least 1 electronic prescription for hydroxyurea in the EMR. We excluded 12-month periods when patients were hospitalized 6 or more times for SCD-related complications, received care at another institution, participated in a hydroxyurea adherence study, or received chronic transfusion therapy because these events had the potential to affect patients’ hydroxyurea adherence, hydroxyurea monitoring, and laboratory and clinical outcomes. Patients could have multiple 12-month periods that met the inclusion and exclusion criteria, but only their most recent 12-month period was extensively reviewed and included in the analysis (Fig. 1). This study was approved by the NCH Institutional Review Board (IRB16-00033) on January 26, 2016.
Fig. 1.
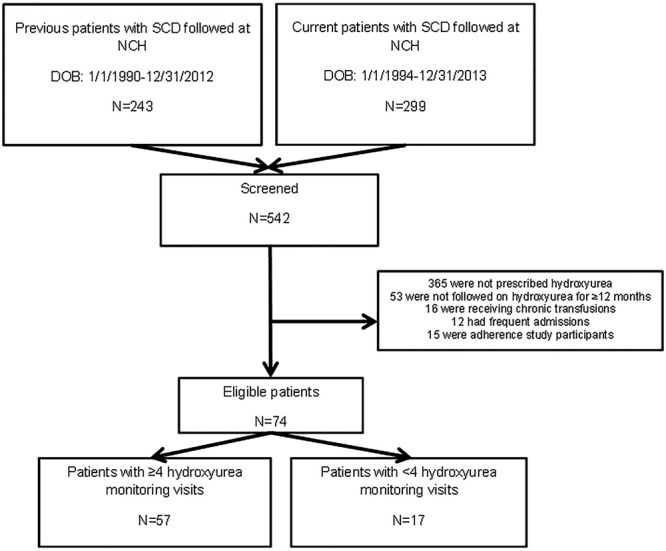
Prescreening schema.
Data Collection
Participants’ age, SCD genotype, primary insurance, sex, total number of hydroxyurea monitoring appointments, reasons for not obtaining the recommended number of hydroxyurea visits, hydroxyurea dose, and duration of hydroxyurea therapy were recorded from their EMR. The total number of pain and acute chest syndrome episodes that resulted in visits to the emergency room or a hospitalization during the included year were also noted because clinical trials show that hydroxyurea reduces these complications specifically.11 Finally, because hydroxyurea exposure causes many hematologic changes that contribute to its clinical effects,12 we also recorded hemoglobin, mean corpuscular volume (MCV), fetal hemoglobin, and also patients’ hydroxyurea doses documented during their hydroxyurea monitoring visits. We defined patients as frequently monitored if they had at least 4 in-person hydroxyurea appointments during their included 12-month period.
Hydroxyurea Prescriptions and Adherence
NCH started using electronic medication prescribing in 2010. Hydroxyurea adherence was measured using electronically written hydroxyurea prescriptions and calculated as the number of days patients had access to hydroxyurea during their included 12-month period based on these records divided by 365 days and multiplied by 100%. Hydroxyurea doses that were written and may have remained from a prescription just before their included year and hydroxyurea doses written during their included year were used in this calculation. Because most patients achieved at least 80% adherence in the large pediatric hydroxyurea clinical trial that showed an improvement in clinical outcomes,13 we defined patients who had access to hydroxyurea on at least 80% of the days during their 12-month period based on these records as adherent.
Statistical Analyses
Descriptive statistics were used to summarize all independent variables in our study cohort. Differences in demographic and clinical information were assessed between frequently and infrequently monitored patients using nonparametric methods. Chi-square or Fisher’s exact tests were used for categorical variables (e.g., insurance provider, SCD genotype), and Wilcoxon Rank-Sum tests were used for continuous variables (e.g., age). P values less than 0.05 were considered significant. All statistical analyses were performed using SAS software, version 9.3 (SAS Institute, Cary, N.C.).
RESULTS
Hydroxyurea Monitoring
We prescreened 542 NCH patients (Fig. 1) and identified 74 unique children who met our eligibility criteria (Table 1). Of these, 57 (77%) patients had frequent hydroxyurea monitoring, with most of these (n = 33) having 4 in-person hydroxyurea monitoring visits and a few (n = 3) having as many as 7 monitoring visits. Of those who did not have frequent monitoring (n = 17), 6 had 2 hydroxyurea monitoring visits and 11 had 3 visits.
Table 1.
Patient Characteristics
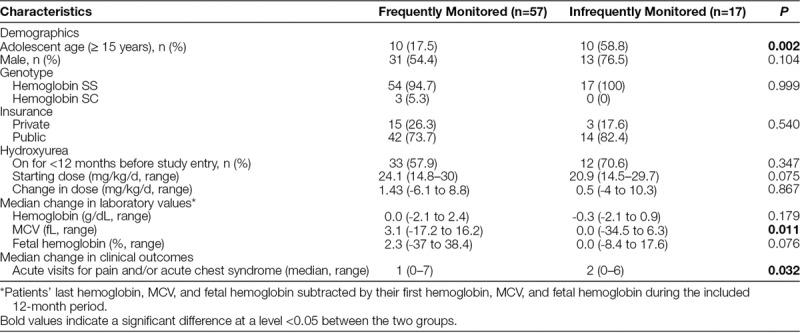
Barriers to Monitoring
The most common reason documented for not obtaining frequent hydroxyurea monitoring was missing a scheduled appointment (n = 11; Fig. 2). The proportion of publicly insured patients did not significantly differ between the 2 groups (Table 1), but the median age of the patients with infrequent hydroxyurea monitoring (15 years; range, 5–20 years) was significantly older than those with frequent monitoring (9 years; range, 2–20 years; P = 0.002).
Fig. 2.
Documented reason(s) for why infrequently monitored patients did not have hydroxyurea monitoring visits.
Hydroxyurea Adherence and Laboratory and Clinical Outcomes
The frequently monitored patients had a significantly higher hydroxyurea adherence (median 88% adherence; range, 36–100%) measured by written prescriptions compared with the infrequently monitored patients (median 57% adherence; range, 15–98%; P < 0.001). Only 55% of all the patients in this study achieved the 80% adherence threshold during their 12-month period, but a significantly higher proportion of the frequently monitored patients (n = 38; 66.7%) achieved this threshold compared with the infrequently monitored patients (n = 3; 17.7%; P < 0.001). Frequently monitored patients had a significantly larger increase in their median MCV and fewer acute visits for pain and acute chest syndrome episodes compared with the infrequently monitored patients (Table 1).
DISCUSSION
This study suggests that most children on hydroxyurea at NCH obtain frequent hydroxyurea monitoring and that frequent monitoring is associated with improved hydroxyurea adherence and clinical outcomes in children with SCD. These findings are important because they suggest that it is possible for children to receive frequent in-person monitoring in the clinical setting, despite prior studies suggesting that many children with SCD obtain less frequent routine care.3 We suspect the patients in this retrospective review received frequent hydroxyurea monitoring because of the case management resources and size of the SCD team at NCH, but a multiinstitutional study that includes sites with fewer resources than NCH is needed to confirm this hypothesis.
Hydroxyurea monitoring at NCH occurs during an in-person clinic appointment with a hematology provider, whereas the National Heart, Lung, and Blood Institute guidelines do not specify if hydroxyurea monitoring should include an in-person appointment. It is important to note that these in-person visits may be a barrier to hydroxyurea monitoring because laboratory-only monitoring could allow patients to have blood work done closer to home and during hours that may be more flexible than the NCH clinic hours. However, laboratory-only monitoring does not provide the same opportunity to have ongoing discussions with patients and families about patients’ treatment response, hydroxyurea’s potential side effects, or to address hydroxyurea nonadherence that occurs in approximately half of patients.14 These in-person discussions may also have the potential to impact long-term acceptance and willingness to continue hydroxyurea because patients and families consider these factors when they are deciding to start or continue hydroxyurea.15,16
We found that the frequently monitored patients had a significantly larger median MCV increase during their included year compared with the infrequently monitored patients. We suspect this was primarily due to higher medication adherence in this group because the 2 groups did not significantly differ between other factors that can influence MCV, such as hydroxyurea dose.17 We did not find significant differences in hemoglobin or fetal hemoglobin change between the 2 groups, and this may be due to the short study duration, small sample size, or because increased hydroxyurea exposure results in more rapid MCV increases compared with hemoglobin or fetal hemoglobin increases.18
We evaluated if infrequently monitored patients were more likely to have public health insurance, poor access to care, or if they were older because these are known risk factors of poor appointment adherence in other populations.19,20 We did not find that a significantly higher proportion of infrequently monitored patients had public insurance, but this may have been because of our small sample size and because most children in our cohort were publicly insured. We could not definitively determine if clinic access was a barrier because the EMR did not include documentation about the reasons why patients missed or cancelled appointments. Finally, we did find that significantly more infrequently monitored patients were adolescents. This finding could support that adolescents with SCD face unique monitoring barriers to be addressed, such as prioritizing other responsibilities and poor self-management skills because these are factors known to impact adolescents’ adherence to medications and other aspects of their medical care and also their ability to successfully transition from pediatric to adult care.21,22
Although our results inform the next steps of our quality improvement project, our study has a few limitations. First, it was retrospective and limited by what was documented in the EMR. Second, using written hydroxyurea prescriptions to calculate patients’ hydroxyurea adherence is not a validated adherence measure and it has the potential to overestimate patients’ adherence. We used this measure in this study because written prescriptions can provide an assessment of the maximum possible adherence that patients could achieve during their 12-month period because prescriptions are required to access hydroxyurea. Third, we may have excluded a few severely affected patients from our study by excluding those with frequent hospitalizations. This was done because these patients were more likely to miss their outpatient hydroxyurea monitoring visits because they were hospitalized and because we were less confident that their outpatient written prescription records would provide an accurate adherence estimate because these patients receive a larger number of hydroxyurea doses in the hospital. Lastly, because this was a retrospective study, we cannot determine why frequent hydroxyurea monitoring and hydroxyurea adherence were associated. It is possible that this relationship may only reflect that patients who attend frequent in-person hydroxyurea monitoring are more likely to adhere to hydroxyurea. If so, interventions that increase the number of patients who receive frequent monitoring could provide more opportunities to identify poorly adherent patients. However, to affect outcomes, these interventions will likely need to be paired with additional adherence-targeted interventions. Using the results of our baseline assessment, we created a key driver diagram (Fig. 3) that outlines the intervention, key drivers, and the overall aim of our next quality improvement project. This study also identified that future prospective investigation is needed to identify the specific reasons for why patients, and especially adolescents, miss or cancel their hydroxyurea monitoring visits so that these can also be addressed.
Fig. 3.
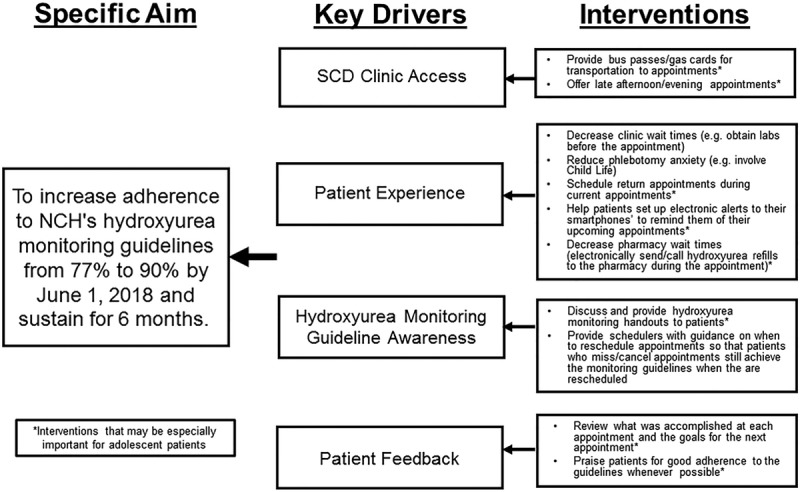
Proposed key drivers to promote adherence to NCH’s hydroxyurea montioring guidelines.
In summary, we found that frequent in-person hydroxyurea monitoring was associated with improved adherence and outcomes. This retrospective review informs our future quality improvement initiative that will aim to promote adherence to NCH’s Hydroxyurea Monitoring Guidelines. Although in-person monitoring can be labor-intensive, our results suggest it is feasible for large pediatric hospitals that care for children that primarily live in urban settings. It also provides opportunities to identify poorly adherent patients and address factors that may influence patients’ long-term acceptance of this therapy.
ACKNOWLEDGMENTS
A grant from the National Heart, Lung and Blood Institute of the National Institutes of Health (1 K23 HL127303-01) was used to support this project.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1. Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/016295s041s042lbl.pdf. Accessed January 26, 2017.
- 2.Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 2014;312:1033–1048.. [DOI] [PubMed] [Google Scholar]
- 3.Modi AC, Crosby LE, Hines J, et al. Feasibility of web-based technology to assess adherence to clinic appointments in youth with sickle cell disease. J Pediatr Hematol Oncol. 2012;34:e93–e96.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.United States Census Bureau. Income, poverty and health insurance coverage in the United States: 2015. Available at http://www.census.gov/newsroom/press-releases/2016/cb16-158.html. Accessed September 14, 2016.
- 5.Grosse SD, Boulet SL, Amendah DD, et al. Administrative data sets and health services research on hemoglobinopathies: a review of the literature. Am J Prev Med. 2010;38(Suppl 4):S557–S567.. [DOI] [PubMed] [Google Scholar]
- 6.DuMontier C, Rindfleisch K, Pruszynski J, et al. A multi-method intervention to reduce no-shows in an urban residency clinic. Fam Med. 2013;45:634–641.. [PubMed] [Google Scholar]
- 7.Hixon AL, Chapman RW, Nuovo J. Failure to keep clinic appointments: implications for residency education and productivity. Fam Med. 1999;31:627–630.. [PubMed] [Google Scholar]
- 8.Nevin J, Myers L, Osunkwo I, et al. A retrospective study to assess the utility of frequent laboratory monitoring of pediatric patients with sickle cell disease on hydroxyurea. J Pediatr Hematol Oncol. 2014;36:e180–e184.. [DOI] [PubMed] [Google Scholar]
- 9.Creary SE, Chisolm DJ, Koch TL, et al. Hydroxyurea use in children with sickle cell disease: do severely affected patients use it and does it impact hospitalization outcomes? Pediatr Blood Cancer. 2016;63:844–847.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anders DG, Tang F, Ledneva T, et al. Hydroxyurea use in young children with sickle cell anemia in New York state. Am J Prev Med. 2016;51(1 Suppl 1):S31–S38.. [DOI] [PubMed] [Google Scholar]
- 11.Wang WC, Ware RE, Miller ST, et al. ; BABY HUG investigators. Hydroxycarbamide in very young children with sickle-cell anaemia: a multicentre, randomised, controlled trial (BABY HUG). Lancet. 2011;377:1663–1672.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ware RE. How I use hydroxyurea to treat young patients with sickle cell anemia. Blood. 2010;115:5300–5311.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thornburg CD, Rogers ZR, Jeng MR, et al. ; BABY HUG Investigators. Adherence to study medication and visits: data from the BABY HUG trial. Pediatr Blood Cancer. 2010;54:260–264.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Candrilli SD, O’Brien SH, Ware RE, et al. Hydroxyurea adherence and associated outcomes among Medicaid enrollees with sickle cell disease. Am J Hematol. 2011;86:273–277.. [DOI] [PubMed] [Google Scholar]
- 15.Crosby LE, Shook LM, Ware RE, et al. Shared decision making for hydroxyurea treatment initiation in children with sickle cell anemia. Pediatr Blood Cancer. 2015;62:184–185.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Creary S, Zickmund S, Ross D, et al. Hydroxyurea therapy for children with sickle cell disease: describing how caregivers make this decision. BMC Res Notes. 2015;8:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ware RE, Eggleston B, Redding-Lallinger R, et al. Predictors of fetal hemoglobin response in children with sickle cell anemia receiving hydroxyurea therapy. Blood. 2002;99:10–14.. [DOI] [PubMed] [Google Scholar]
- 18.Wang WC, Wynn LW, Rogers ZR, et al. A two-year pilot trial of hydroxyurea in very young children with sickle-cell anemia. J Pediatr. 2001;139:790–796.. [DOI] [PubMed] [Google Scholar]
- 19.Davis AM, Taitel MS, Jiang J, et al. A national assessment of medication adherence to statins by the racial composition of neighborhoods. J. Racial and Ethnic Health Disparities. 2016. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neal RD, Lawlor DA, Allgar V, et al. Missed appointments in general practice: retrospective data analysis from four practices. Br J Gen Pract. 2001;51:830–832.. [PMC free article] [PubMed] [Google Scholar]
- 21.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497.. [DOI] [PubMed] [Google Scholar]
- 22.Pai AL, Ostendorf HM. Treatment adherence in adolescents and young adults affected by chronic illness during the health care transition from pediatric to adult care: a literature review. 2011;40:16–33.. [Google Scholar]


