Graphical abstract
1. Introduction
The field of point-of-care (POC) diagnostics offers the tantalizing possibility of providing rapid diagnostic results in non-laboratory settings. Here, we review progress in this research field, with a focus on developments since 2014. First, we provide an overview of significant technological and social trends – notably those concerning data connectivity – which have shifted the underlying landscape for how POC diagnostic devices will be designed, built, and delivered across different healthcare settings. We review important technical advances in fundamental diagnostic components, and increasingly, advances in fully integrated devices designed for specific clinical use cases. While few new classes of POC diagnostic devices have been introduced into the market, continued progress in microfluidics – combined with dramatic advances in connected devices – are bringing the prospects of fulfilling the lofty promises of POC diagnostics closer than ever to reality.
With the rise of connected consumer devices, entire sectors of the economy (including retail, transportation, housing, and freelancing services) have been unmistakably transformed. The potential reach of POC diagnostics into all sectors of healthcare – and increasingly into daily routines of individual patients and consumers – demand that technical advances take into context this broader transformation.
For healthcare providers, the landscape for medicine is poised for a dramatic shift. In any decentralized setting, a connected POC diagnostic device could be available to aid the diagnosis of disease and selection of treatments. Clay Christiansen, a business theorist, predicts a disruption to the healthcare system, “[Disruption will happen]….in practices where the doctor uses these trends — point-of-care imaging and diagnostics, expert systems, telemedicine and personal health records.”1 With therapeutics becoming increasingly tailored to individuals’ precise genetics and biomarkers, the value chain of healthcare delivery could move up to accurate and accessible POC diagnostics as opposed to therapeutics.
For consumers and patients, the impact could be even more dramatic. With the rise of connected consumer electronic devices, patients and consumers are adjusting in how they interact with POC medical technologies alongside mobile devices, and more broadly, in how they manage and seize control of their own health. In due course, POC diagnostic and monitoring devices are expected to become ubiquitous, whether in an at-home setting, in a doctor’s office or hospital, or in low- and middle-income countries.
In this article, we will review recent new developments and trends in POC diagnostics, including work in assay chemistry or microfluidics, and how these developments have been evolving to align with broader trends. In Section 2, we will provide an overview of the components of the evolving POC ecosystem, including how technological components fit into a broader landscape. In Section 3, we will cover recent developments in POC technologies. Because of the increased view on integrated devices in the research community on POC devices, we will review the developments in the context of four common clinical use-case scenarios, as well as discuss fundamental advances. In Section 4, we will provide in-depth reviews for selective technologies which have taken a fundamental technological advance all the way to demonstrations with clinical samples. Finally, in Section 5, we conclude with future trends and directions. We will cover developments in the period between 2012 and 2016, with a focus on 2014 to 2016.
2. The New POC Ecosystem
Although few new classes of POC devices have been introduced into the market2,3, there has been significant progress in POC technologies in recent years. In today’s connected age, this progress is being made within the context of a broader and more diverse POC ecosystem than before.
In this Section, we map out the key components of today’s POC ecosystem (Fig. 1). Some of these components, such as assay chemistry and microfluidics, cover traditional ground for researchers in POC diagnostics, and are the focus of this Review. Other components, ranging from data analytics to regulation, reflect broader forces which are influencing how POC technologies are being designed and developed. Because these broader trends, both technological and non-technological, are progressing relentlessly, there is now more potential than any time in the past for analytical chemists and microfluidics researchers to make a systemic impact, if their technical work can be integrated seamlessly into the broader POC ecosystem.
Figure 1. The POC ecosystem in a connected age.

POC devices require an integration of chemistry, fluidics, hardware and software. Traditional focus areas on POC development have been assay chemistry and microfluidics, but rapid progress is taking place in other technological and non-technological components which need to be considered by researchers in POC diagnostic devices.
Technology Component 1. Assay chemistry: affinity reagents, amplification chemistry, materials
Affinity reagents are essential tools in in vitro diagnostics to isolate, detect and quantify target molecules. While antibodies remain the most widely used affinity reagents, their manufacturing is slow and costly (which is especially concerning for rapidly emerging pathogens4), and there continues to be an opportunity to improve thermal stability for diagnostics deployed in the field. Aptamers, short, single-stranded oligonucleotides with high specificity and affinity target binding, have been identified against various targets (including small molecules, nucleotides, amino acids, proteins, phospholipids, nucleic acids, viruses, bacteria, cell fragments, and whole cells)5. Chemical modifications to aptamers can improve their bioavailability6,7. Advances have also been made on modifications of aptamers with nanoparticles8 and magnetic beads9. Modifications of aptamers with enzymes could be integrated into simple strip tests read by commercial glucose meters10. Developments have also been made in engineering antibodies high affinity and specificity11, antibody-mimetic proteins such as designed ankyrin repeat proteins (DARPins) which are thermally stable12, non-immunoglobulin (non-Ig) scaffolds13, and antigen-specific, single-domain antibodies14–17.
Advances in chemistry and materials are pushing the limits of performance for POC diagnostics. Recent reviews have covered nanoparticles, including fluorescent nanoparticles18 as well as other types of nanoparticles (such as carbon, polymer, silica, and viral)19. Strategies involving upconverting phosphor particles could result in signal enhancement without additional signal amplification steps20. Other directions include polymers and nanoparticles21. This work in amplification and detection chemistries is increasingly being considered in the context of the underlying substrate materials and detector instrument. One platform integrated an aptamer-crosslinked hydrogel for molecular recognition with an enzymatic reaction for signal amplification on a paper-based substrate for fluidic processing and readout22. Use of europium (Eu) (III) chelated nanoparticles were integrated in lateral flow test formats23 (as demonstrated in detection of chlamydia from urethral and endocervical swabs23), and multicolored silver nanoparticles were used for multiplexed detection in a lateral flow format, offering tunable and narrow absorbance spectra compared to gold or latex particles24.
There have also been work to improve the capabilities of materials for integrating sophisticated functions. Advances in paper-based systems have incorporated multiple sample processing steps25, such as sample preconcentration, signal amplification, and electrochemical detection26, in an effort to improve the sensitivity and overall utility. Another class of substrates that has gained attention is flexible polymers, which offer different mechanical and chemical properties compared to hard plastic, paper, or cellulose substrates while being affordable and easily manufactured. When integrated with other components27, flexible substrates could serve as the basis for minimal or non-invasive diagnostic tests28. These materials could be integrated into a wide variety of wearable devices (such as bandage, mouthguard, contact lens, clothes, and temporary tattoo)29 as wearable sensors, as well as tactile, temperature and biochemical sensors30.
Technology Component 2. Microfluidics: assay integration
Recent advances microfluidics have emphasized the need for practicality31. For POC diagnostic devices to be accessible to end users, the disparate steps of a multi-step diagnostic assay – including fluid handling, sample processing, signal amplification, washings, and detection – must be seamlessly integrated. In this regard, microfluidics serves as an integrating force. In Section 3, we will review the latest advances from researchers in microfluidics, with trends in producing integrated devices which are self-contained, automated, easy-to-use, and rapid32–35. Applications of focus include molecular diagnostics36,37, infectious diseases38, chronic diseases39, and resource-limited settings40.
To demonstrate the promise of an integrated POC diagnostics device, which contains a coherent and mutually compatible set of POC technologies3, it is critical to determine the sensitivity and specificity using clinical specimens. Successful evaluations require formation of partnerships, development of early versions of manufacturing processes, and proper design of field study41. Increasing attention is also being paid to the source of clinical specimens; for example, fingerstick measurements hold great promise, but care must be taken to ensure proper performance42.
As a technique to build integrated devices, microfluidic is also becoming more accessible to non-specialists. In recent years, opportunities for collaboration and outsourcing have increased for researchers43. This trend extends to microfluidic foundries and contract research organizations, which make off-the-shelf or customized microfluidic chips. There is now a robust set of offerings (Table 1), such that other researchers in POC diagnostics (including chemists, biochemists, biologists, and clinicians), can design, build, and test custom integrated devices.
Table 1. Companies offering manufacturing of microfluidic chips.
Overview of foundry and contract manufacturing options for microfluidic chip manufacturing, offering services for different substrate materials, integration of additional optical or electrical components, as well as options in scale of manufacturing (e.g. prototyping vs. mass production). Adapted from Elveflow Microfluidic Tutorial: “Microfluidic Foundry: How to Choose the Right Manufacturer to Fabricated Your Glass, Polymer or PDMS Microfluidic Device”. http://www.elveflow.com/microfluidic-tutorials/soft-lithography-reviews-and-tutorials/microfluidic-device-fabrication/microfluidic-foundries/
| Glass | PDMS | Polymer | Thermo- plastic |
Quartz | Silicon | Electrodes | Optical Components |
Mass Production |
Prototyping | Customization | Other | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AchiraLabs | + | + | + | + | Silicon master with SU-8; PMMA, COC, etc. | |||||||
| microfluidic | + | + | + | + | + | + | + | Standard catalog chips or custom design; Topas, Zeonex | ||||
| ChipShop | ||||||||||||
| Dolomite | + | + | + | + | Expertise in droplet microfluidics; Off the shelf chips | |||||||
| Microfluidics | ||||||||||||
| Epigem | + | + | + | + | + | + | PMMA, PEEK, PET, PC, FR4 | |||||
| FlowJEM | + | + | + | + | + | PC, COC, Acrylic | ||||||
| iX Factory | + | + | + | + | + | + | Specializes in microreactors, LOC, integrated optics, MEMS | |||||
| Klearia | + | + | + | + | Attractive prices | |||||||
| LioniX BV | + | + | + | + | + | + | + | Customized glass (fused silica and borosilicate) | ||||
| Micralyne | + | + | + | + | + | + | + | + | Expertise in sensors and optical MEMS; additional materials: alumina/sapphire, piezoelectric crystas, ceramic, diamond | |||
| microLIQUID | + | + | + | + | + | + | + | + | COC, COP, PMMA, PC, PS; PDMS and SU8 mold | |||
| Micronics, Inc. | + | + | + | + | + | Injection molding; Custom and off-shelf cards | ||||||
| Micronit | + | + | + | + | + | + | + | + | + | Custom or off-shelf; COC, COP, PC, Fused silica (Quartz glass) | ||
| MicruX | + | + | + | + | Long lifetime; manufactured on SU-8/Pyrex material | |||||||
| MiniFAB | + | + | + | + | + | + | + | + | PMMA, ABS, PVC, PET, COC, COP, PI, PEEK, PP, PU, PS; integrated thin films: Al, Cu, Au, Cr, Ti, Ni, W or parylene | |||
| NIL Technology | + | + | + | + | Injection molding | |||||||
| SIMTech | + | + | + | + | + | PMMA, PS; standard or custom | ||||||
| Microfluidics | ||||||||||||
| Foundry | ||||||||||||
| STRATEC | + | + | + | + | + | Leading supplier of “smart” polymer-based consumables | ||||||
| Consumables GmbH thinXXS | + | + | + | + | Mainly for commercial applications | |||||||
| Trianja | + | + | + | + | Also provides glass mold for PDMS casting and hot embossing | |||||||
| z-microsystems | + | + | + | + | Offers microtooling and micro injection molding, and post-molding processes like bonding and coating |
Technology Component 3. Connected instrumentation
The uptake of smart consumer electronic devices has moved forward with breathtaking speed. Their omnipresence has transformed how services are delivered for entire sectors of the economy, with at least five consequences for POC diagnostics:
Adaptation of existing devices. Consumers are increasingly using smartphones as platform devices. Towards health and monitoring, smartphones are increasingly adapted for imaging, sensing, and diagnostics44. Interestingly, while glucose meters are widely used in home settings by diabetic patients, they could potentially be re-purposed into a general measurement device via novel chemistries, to perform many common clinical (such as immunoassays, molecular diagnostics, and enzymatic assays)45.
Hardware components. Miniaturized components used in consumer electronic devices can be adapted for use in POC diagnostic devices. The camera lens in smartphones can be used for microscopy, cytometry, and optical readouts for different detection methods32, low-energy bluetooth modules for data connectivity are becoming standardized and prevalent, and batteries are becoming longer lasting. The use of these components for POC devices can be extended from prototyping to large-scale manufacturing by leveraging the established supply chains for smartphones and smart devices.
Collection of multimodal data. A range of different health-monitoring devices are now actively being used, streaming different types of health data. Fitness trackers are used by consumers for tracking movement and heart rate, pulse oximeters are used by patients to measure blood oxygen as an indicator for heart and lung disease, and portable ultrasound by health professionals46 for different uses, including to guide injections and line placements. In the near future, devices equipped with “Internet of Things” technologies will stream other types of health data from diverse sources. For POC diagnostic devices, the value of health biomarkers could be augmented by interpreting the information alongside these ever expanding data sets.
Transmission of health information. With a rising number of cellphone users across the world47, mobile health (“mHealth”) demonstrations in pilot projects have proliferated. Unlike the rapidly evolving modes of real-time communication in consumer technology, many of the underlying technologies in mHealth still rely on primitive systems. Methods for transmitting health information include web browsers on computers, and short message service and interactive voice recording48,49 on cell phones. Efforts have been made to automate the transmission of data garnered from POC diagnostics on cell-phone44 and satellite networks50.
Self-running diagnostic machines. The rise of consumer electronics, alongside advances in robotics and artificial intelligence, is leading the ambitious development of self-operating devices in a variety of highly independent field settings. Microfluidics continues to be explored for these frontier settings, including military51, postal service anthrax52, drones53, deep-sea exploration54, and space travel and colonization55,56. In these increasingly isolated and autonomous settings, the need for POC sensors and medical diagnostics will persist. As POC technologies mature into integrated devices, we should expect to see increasing deployment of POC diagnostics in these settings.
Technology Component 4. Data analytics
Consumer-led health data platforms
Health information collected from consumers (from Internet, mobile phones and social media platforms) are increasingly being entered into a central database for analysis. For a patient’s health, information being collected into the ResearchKit (a database from Apple)57 could link users to studies on Parkinson’s disease, diabetes, asthma, breast cancer and heart disease58. Towards public health and epidemiology59, search engine queries and page views to websites could be used to infer behaviors associated with medical conditions (such as cardiovascular disease, sexually transmitted infectious, pregnancy and mental health conditions60, or outbreaks of communicable diseases61). However, care must be taken to avoid over interpretation, by using multiple data sources and analysis methods and cross-validation with public health data61, and by addressing vulnerabilities in algorithms for overfitting62. Future analysis of large sets of health and medical data will be augmented by artificial intelligence (for example by leveraging IBM Watson Health’s cloud-based healthcare analytic platform63). Already, interesting concepts are being proposed for how POC systems can be integrated with machine learning, such as the generation of a predictive model capable of assessing risks for heart attack and heart failure patients64. An intriguing (but less tested) approach for analyzing large sets of data is crowdsourcing65.
Genomics
Personalized genomic information will be increasingly available as the price of next-generation sequencing has lowered dramatically. To assist cancer researchers in analyzing this information, several private companies (such as Flatiron Health and Foundation Medicine) have launched databases where tumor sequencing data are continuously updated with clinical and outcomes data from electronic health records, to allow researchers to have an integrated, longitudinal view of a patient’s clinical, diagnostic and therapeutic outcomes66.
Technology Component 5. Systems integration: chemistry, fluidics, hardware, software
Even if a microfluidics diagnostics device displays high sensitivity and specificity with clinical specimens, it is still not ready for the market. Rather, it must be additionally integrated with hardware and software to ensure an appropriate user experience. We have previously discussed the importance of systems design for specific use-case scenarios67. The application of design for health devices has heightened in recent years, as shown in fitness and activity trackers for consumers, and portable ultrasound and optical imaging devices for medical professionals46,68. For systems integration for POC diagnostic devices, it will be critical to recognize that different POC settings (which include emergency room, ambulance, physician’s office, pharmacy, home, or in the field) hold vastly different constraints and requirements. (We will discuss these constraints in the next Section.)
Non-technology Component 1. Clinical workflow
As healthcare delivery becomes increasingly decentralized, healthcare professionals and patients are adapting their workflows to fit in the use of POC diagnostic devices. For pathologists and clinicians, adoption of POC testing continues to grow69. Effective implementation of POC testing eliminates transport, processing, and aliquoting processes that take place in core laboratories, thereby creating a more streamlined and faster workflow70 and enabling more face-to-face interactions between providers and patients to understand test results and plan treatment options70. Nevertheless, because more CLIA-waived tests means expansion of POC tests in diverse settings71, it is often difficult to ensure proper oversight and quality assurance72 over minimally trained or untrained users. Some hospitals and clinics appoint point-of-care coordinators or management teams to ensure consistent procedures, regulatory compliance, correct documentation of results, and end-user assistance72,73. It is important for developers of POC devices to take advantage of advances in wireless connectivity and electronic health records to achieve ease of use and quality assurance.
Patients and consumers are increasingly adapting POC tests. Examples include genetic testing services (such as 23 and me)71. They are also expanding their use of on-demand home visits by doctors74, which are being offered by a number of startup companies (including Heal, Pager, Doctors Making Housecalls). Many of these visits entail the use of a large range of connected smart devices for POC or remote diagnosis74, in the context of primary and urgent care as well as wellness assessments and annual physical exams. Examples include POC assessments of sexually transmitted infections, and measurement of diabetes and cholesterol levels75. The use of such POC tests will only increase, spurred by the growth of more CLIA-waived POC diagnostic tests (including approval of the first direct-to-consumer HIV test in the U.S. in 2012), increased self-monitoring via fitness trackers and smartphone apps, and the growth of on-demand clinical services and telemedicine.
Non-technology Component 2. Regulatory guidance
Regulatory procedures for consumer-targeted health devices and apps are evolving76. How the Food and Drug Administration regulates the products depends on the intended functionality and claims. In recent years, some products have claimed that their information is for entertainment purposes only, but simultaneously promise medical benefits76. POC diagnostic devices which measure biomarker levels are often classified as Class II devices posing “moderate risk” (requiring 510(k) approval), with some devices classified as Class III devices posing “high risk” (requiring the more burdensome premarket approval). As POC diagnostic devices are increasingly used outside of a centralized laboratory, their benefits will continue to be weighed against ensuring privacy and security of protected health-related information (including compliance with Health Insurance Portability and Accountability Act) and legal liability in case of inaccuracies or misuse. Researchers in POC diagnostic devices should be cognizant of the evolving regulations which strive to protect patient health without discouraging technological innovation.
Non-technology Component 3. Reimbursement
While the future of the Affordable Care Act is uncertain, reimbursement for healthcare services in the U.S. and around the world are experiencing a structural shift towards a value-based model rather than fee-for-service77. In the traditional fee-for-service model, providers are paid for the number of tests and procedures performed. By contrast, in a value-based model, providers are offered incentives (and penalties) depending on how they perform based on quality metrics. This model encourages providers to measure the health of patients on a continuous basis, shifting the emphasis to prevention and early detection of disease78, and effective continuous management of chronic diseases. With additional motivation for screening and monitoring (Christiansen hypothesizes that “diagnostics is where the most attractive profits will be made in the future”1), the effectiveness of POC diagnostic devices in cost reduction is being evaluated. For example, one study showed the use of POC diagnostic devices in ambulatory practices resulted in improvements in clinical operations and reductions in cost79. On the other hand, another study examined consumer usage of POC devices (such as blood pressure monitor, glucose meter, and heart monitor), and observed no short-term reductions in visits to emergency rooms or office visits80. While such cost-benefit studies will be important in determining whether POC products will be reimbursed by public and private insurers, consumers and patients are also exhibiting an increasing willingness to pay out of pocket for POC health products and services.
Non-technology Component 4. Legislation
In response to consumers’ increasing interest in self-management and self-monitoring, laws are being passed in the U.S. and around the world to define the boundaries of direct-to-consumer diagnostic services. Traditionally, POC tests can only be ordered by a physician, and the results passed directly to the physician. More recently, diagnostic test results have been more accessible to patients; for example, Quest Diagnostics and LabCorp have both launched their own patient portals (MyQuest and Beacon, respectively), but the proprietary nature of the portals have limited the impact. Going further, legislation in the U.S. and around the world81 are defining the possibilities of which genetic tests can be ordered directly by consumers and under which constraints (e.g. with the availability of genetic counselling). An early study on consumers selecting to undergo genome-wide profiling did not show short-term changes in psychological health, behavior (diet or exercise), or use of follow-on screening tests82.
Beyond genomics, direct-to-consumer access for blood tests and services is also increasing. For example, corporate wellness programs are signing up for enhanced diagnostic services (such as WellnessFx) as a benefit for employees to promote wellness. In 2015, the state legislature in Arizona passed a law to allow consumers to directly order tests from a licensed clinical laboratory without physician orders. While laboratory test results from the company Theranos have shown disparity against traditional laboratories83 (and whose services have since shut down), other companies (such as Sonora Quest Laboratories) are expanding their diagnostic services to consumers. There continues to be a vigorous discussion over the right balance84,85 between consumer access and protection, with federal and state laws continuing to evolve.
3. Recent Developments in POC Diagnostics
In this Section, we will review recent advances in POC diagnostics. While it is widely acknowledged that POC testing is completed at or near the patient, this definition spans a large range of possible POC settings, each of which imposes a different set of specific design constraints on POC devices. Previously, different levels of healthcare delivery have been described. For example, one popular public-health model describes different “levels” (Level 1 being community outreach, Level 2 being primary health, and Level 3 being district level lab)86. Another model describing POC diagnostics for infectious diseases in low- and middle-income countries presents five different scenarios, from home to hospital87. Although these distinctions are useful in certain areas of POC diagnostics (i.e. global health), in our view, another description is needed to distinguish the key features across all POC settings. Specifically, a distinction should be made to uncouple infrastructure and budget. For example, a “clinic” which is a non-profit entity in developing countries could be as geographically remote as a “clinic” in a military setting, but their cost constraints for the POC device are very different. In another example, a device in the “field” for global health is as similarly removed from lab testing facilities as a monitoring device used at home in the U.S., but their cost constraints are again very different. For developers of POC diagnostic devices, infrastructure and cost are two important and independent sets of constraints.
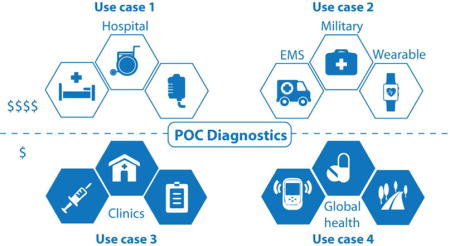
Hence, we propose a general description of POC uses cases in a 2 × 2 matrix, which separates budget (low and moderate) from infrastructure (clinic and home) (Fig. 2). Design of POC devices can be targeted towards four distinct use cases. Use Case 1 is the least-constrained category, allowing for higher material costs, accessory equipment, moderately trained personnel to assist in running the test, ground electricity, refrigeration, controlled ambient temperature, and wireless connectivity. Examples include hospitals, emergency rooms, operating rooms, intensive care units, private pharmacies, and military base clinics. At the other end of the spectrum, Use Case 4 is the most constrained for a developer of POC device, operating at a low budget and minimal to no infrastructure, with design constraints on material, power, storage, personnel, detection and data. Examples include global health, remote clinics, and self-testing at low cost and with minimal accessory resources. Use Cases 2 and 3 are intermediate scenarios. Use Case 2 is constrained by its portability in the field but maintains a moderate budget, encompassing military personnel, consumer devices for self-testing and healthcare monitoring, space travel, ambulance, and industrial and agricultural field testing. Use Case 3 has the amenities of a basic clinical setting, but is constrained by its budget. Examples include non-profit health centers and some primary care clinics.
Figure 2. POC use cases, uncoupling cost from infrastructure.

Differences in budget and infrastructure level play a role in categorizing four distinct use cases for POC devices. Each setting imposes specific constraints on the design of POC devices.
With increased recognition that POC diagnostic devices must be designed differently across different use cases, we will review recent advances in POC diagnostics primarily in the context of these four use cases. We will also review fundamental advances in a separate section.
Use Case 1. Clinic level with moderate budget
Use Case 1 encompasses settings with access to a moderate budget and some level of clinical infrastructure such as hospital emergency rooms, operating rooms, or intensive care units. Here, there are personnel and resources such as electricity and refrigeration which support operation of POC devices, as well as a moderate budget which can accommodate accessory equipment necessary to help run the test. (At Columbia University Medical Center, the number of different platforms used for POC testing grew from 7 in 1995 to 29 in 2015, and the number of patient tests grew from about 500,000 in 1995 to more than 2,000,000 tests as performed by 4000 users in 201488.) In these settings, a POC diagnostic device can be used at the patient’s bedside and return rapid results to help direct treatment. Moderate throughput in testing of samples is sometimes desirable as a feature of the POC device.
As an example, a lab-on-a-disc was developed for automated molecular diagnosis of bacterial sepsis infection89. The intended application of this device is in an intensive care unit, where rapid diagnosis of and response to sepsis infection is crucial. Further, the cost, size, and requirement for a centrifuge make this POC device appropriate for this use case. In another example for sepsis in intensive care units, a POC device for rapid quantification of cell free DNA for early diagnosis of sepsis using 10 uL of plasma or whole blood90. This rapid test requires DC voltage to run gel electrophoresis to detect cell-free DNA which is traditionally difficult to capture and characterize90. Another POC microfluidic chip was designed using iso-dielectric separation of immune cells based on varying electrical properties, also with an intended future application for sepsis monitoring91. Proof-of-concept functionality was demonstrated on primary immune cells91, and the device holds potential to replace traditional laboratory-based flow cytometry assays with a fast, label-free solution for monitoring sepsis progression at the POC.
Additional examples of POC devices useful in this use case include a microfluidic device to quantify B-type natriuretic peptide (BNP) in plasma samples from heart failure patients92, a device that uses Dean flow and nucleic acid amplification to rapidly identify antibiotic-responsive bacteria for guiding treatment decisions in patients with bacterial infections93, and a microfluidic blood cell counting device for PET and SPECT preclinical pharmacokinetic studies94.
Sample type can act as a design constraint. In this use case, there are adequate resources and trained personnel to help obtain difficult samples such as amniotic fluid or cerebral spinal fluid. For instance, a paper lateral flow test was developed to detect interleukin-6 (IL-6) and interferon-γ inducible protein 10 (IP-10) in amniotic fluid in less than 20 minutes, for diagnosis of acute intra-amniotic inflammation/infection95. This device used a paper-based immunoassay to replace a traditional laboratory-based ELISA, the gold standard in IL-6 measurements95. Other interesting examples include analysis of brain tissue from dialysate for traumatic brain injury96, a lateral flow test for invasive pulmonary aspergillosis (IPA) using bronchoalveolar lavage samples97, continuous glucose monitoring for ICU patients using POC intravascular sensing98, and a disposable, POC diagnostic device for measuring the electric, thermal, and mechanical properties of breast tissue from a tissue biopsy sample99.
Plasma and serum samples which are difficult to obtain or process could also be best analyzed in this use case, if their preparation requires large equipment and significant laboratory resources and personnel. A microfluidic device for on-chip isolation, quantification, and characterization of cancer exosomes from serum samples was built, in a manner that simplified traditional methods for extracting exosomes by combining these modalities into one PDMS chip100. The preparation of serum samples to use on the chip required centrifugation steps as well as laboratory mixing and incubation with Thromboplastin D reagent (a clotting reagent)100, and the chip was read by a plate reader100. Another device, using laboratory separation of whole blood to obtain serum, could detect gastric cancer at the POC101. These devices, as they stand, require a degree of sample preparation which is best performed in a setting with infrastructure and moderate budget, but could make an important impact in POC monitoring and diagnostics of patients.
Devices that require some hands-on operation from trained personnel would also fall into this use case. A POC immunoassay was developed for detection of thyroid stimulating hormone (TSH) using digital microfluidics and electrochemical sensing102. The device was fabricated on indium tin oxide-coated glass substrates, with a patterned coating of Teflon-AF and gold or silver electrodeposited electrodes102. In another example, a low-cost device that uses localized surface plasmon resonance was built for detection of various biomolecules103. While these devices greatly simplify traditionally difficult laboratory processes, they do require a few steps of pipetting which are best performed by trained personnel.
Further, many POC devices require accessory equipment that can be moderately expensive or moderately complex to use, constraining the use of these devices to Use Case 1 settings. For instance, a centrifugal microfluidic device was built for detection of bacteria in urine samples104. The detection method was Raman spectroscopy, performed on a fluorescent microscope for imaging and subsequent Raman spectroscopic fingerprinting104. In another advance, a microfluidic chip was developed for thrombosis detection from whole blood105. This device used endothelial cells to support formation of platelet-rich thrombi, a design choice made to increase longevity and storage of the device105. Detection was based on fluorescence microscopy and computer algorithms105. Other POC examples performed with accessory equipment by moderately trained personnel include a platform for real-time detection of airborne microorganisms using optofluidics106, and a device for quantification of platelet adhesion for monitoring cardiovascular disease107.
An attractive capability for many instances of this use case, compared to devices in the field, would be to increase throughput for diagnostic testing. For instance, a POC device for rapid immunoassays was developed that can multiplex up to 8 samples in 45 minutes with a limit of detection down to 10 pg/mL108. Similarly, an integrated, high-resolution microfluidic TaqMan chip was developed, capable of multiplexing numerous mutations across 10 genes for tuberculosis diagnostics109. Finally, a paper-based microfluidic device with electrochemical sensing was developed for multiplexed detection of cancer biomarkers110. These examples demonstrate steps toward attaining a higher throughput of analysis while decentralizing testing.
With the advent of genomic technologies like next-generation sequencing, combined with an increased interest in precision medicine, there have been significant developments in POC tests involving genomic methods. In particular, analysis of circulating tumor cells (CTCs) and cell-free DNA (cfDNA) is becoming increasingly popular for POC devices because of their potential role in a future “liquid biopsy” POC test. A microfluidic chip designed to capture CTC clusters from unprocessed whole blood was developed for use at the POC111. The device captured unlabeled CTC clusters using bifurcating traps with minimal shear stress, and was tested in a variety of patients with metastatic breast cancer, prostate cancer, and melanoma111. The intent was for downstream RNA sequencing111, best performed under this use case. In another development, a microfluidic chip for mutation analysis of circulating cfDNA in serum112. The device used an electrochemical clamp assay that detected single nucleotide changes in cfDNA fragments in serum, without any enzymatic amplification and minimal sample preparation112. This device presented a POC alternative for traditionally labor-intensive genotyping procedures conducted in a laboratory. Finally, precision medicine also involves trends in grouping patients into different subtypes in order to more accurately monitor their individual responses to drugs. A vertical-flow paper-based POC assay was developed to be used with a low-cost, bench-top fluorometer for monitoring therapeutic response via surface enhanced Raman detection113. The device operated on undiluted serum and provided a result in under 15 minutes113. Such near-patient devices hold potential in implementing precision medicine across a broad range of clinical settings.
In summary, numerous advances in this use case have been made, across many different types of applications, sample types, and equipment. With a moderately sized budget and availability of personnel and resources (to help run slightly more difficult assays), this use case is the least restrictive in terms of design constraints, with a result of many commercially available POC devices on the market. For example, Alere’s epoc® POC blood analysis system has been validated on samples obtained from cardiopulmonary bypass patients in hospitals114. The epoc® system is a handheld, wireless in-vitro diagnostic system that uses disposable cards containing sensing electrodes to provide blood gas, electrolyte, and metabolite information at the patient’s bedside. The cards are coupled with a small card reader and computer host system, which can wirelessly transmit information back and forth via Bluetooth. The system is currently being used in clinical settings to enable POC blood analysis in about 30 seconds. Other POC systems are in use for near-patient care settings such as hospitals, intensive care units, and operating rooms. Examples include Alere’s Triage meter, Abbott’s i-STAT, Philips’ Minicare I-20, Cepheid’s Gene Xpert, BD’s Max System, Sony’s Micronics ABORhCard, Luminex devices and others26–30,115. Many of these companies have other POC devices on the market or in the pipeline for hospitals, ambulatory care, urgent care, and government facilities.
Use Case 2. In the field with moderate budget
Use Case 2, as in the previous use case, allows for a relatively flexible and moderate budget for the POC device, but it is set apart by differences in infrastructure available to run the test. In this use case, there are often no trained users and no accessory laboratory equipment, such that a self-contained, portable device is vital, with the ability to withstand rough handling and provide rapid analysis. POC devices in this use case are intended for the field, and can contain sophisticated POC technologies to accomplish their analytical functions. For example, POC devices used at home, what we commonly refer to as consumer electronics or consumer devices, may cost a significantly greater amount to the insurer or patient (compared to other field settings such as global health), but need to be operable by untrained users and are self-contained in terms of reagents and disposal. This use case also encompasses many devices currently on the market and sold in pharmacies in the U.S., Europe, and other developed settings.
One class of consumer POC diagnostics devices that has stood out in adoption is self-glucose monitoring. Self-testing via paper test strips and associated handheld readers has long been a standard of care for patients with pre-diabetes, type-1 diabetes, and type-2 diabetes. In 2014, the global market for self-monitoring blood glucose devices was about $11.2 billion, with a projected 5.7% compound annual growth rate through 2022116 (Johnson and Johnson, Roche, and BD Bioscience are all market leaders in this field, with many other companies offering similar products). The past two years have shown sizeable advances in POC glucose self-testing devices. A large trend in glucose self-monitoring over the past few years has been minimally invasive, continuous glucose monitoring (CGM) devices. One method to achieve this is through microneedles, transdermal devices that sample interstitial fluid for continuous glucose sensing. A CGM device comprised of a 200 silicon microneedle array was developed, which samples interstitial fluid that can passively diffuse to an amperometric glucose sensor outside the body117. The device was tested in clinical studies at two different site locations in the United States, and showed continuous sampling up to 72 hours117. Similarly, an additional microneedle device for CGM was developed, using bulk micromachining to fabricate needles from stainless steel, which were subsequently electroplated with Pt black for sensing118. Finally, microneedles made from arrays of nitrogen-incorporated ultrananocrystalline diamond were developed for dopamine detection in uric acid119.
Other approaches to POC CGMs aim to avoid skin penetration, in an effort towards developing minimally invasive or non-invasive sensing. For instance, a wearable electrochemical biosensor was developed able to detect glucose from sweat120. The device used metal/metal-oxide thin films contained within porous polyamide substrates to detect glucose via impedance changes120. The device was also able to simultaneously measure cortisol levels, which have been known to correlate physiologically to changes in glucose levels120. Beyond glucose, a device exploiting personal glucose meters was developed in a manner that measured non-glucose targets (such as nicotinamide coenzymes such as NADH121, or analytes in a complex matrix such as milk122) by triggering redox reactions found on most glucose test strips. This set of interesting work holds potential to diversify the use of personal glucose meters to detect additional targets.
Advances in materials and flexible printed electronics are pushing the frontiers of wearable sensors beyond applications involving personal health tracking and fitness monitoring, and towards molecular-level analysis. A wearable sensor for multiplexed analysis of metabolites and electrolytes such as sodium, potassium, glucose and lactate from sweat was developed123. The device used multiple individual sensors consolidated via printing on commercially-available flexible printed circuit board123. Similarly, a temporary tattoo sensor was designed for real-time monitoring of trace metals such as zinc in sweat for physiological monitoring124. Also, a wearable mouth guard to continuously monitor salivary metabolites, such as lactate or uric acid, was developed for physical health monitoring by using printable amperometric enzymatic biosensors125,126. Such devices could be useful for diverse applications in gathering increasing amounts of personal health data.
Other prototypes of sophisticated wearable POC devices have been developed. A “smart bandage” to track wound healing was developed, based on changes in uric acid levels127. Here, an amperometric biosensor was printed directly onto a wound dressing, which interfaces with a wearable potentiostat to enable wireless connectivity127. This device holds potential for patients (such as elderly patients) who have difficulty traveling to the doctor for wound assessment. Additionally, a wearable device was developed to monitor macro and microvasculature blood flow128. This device offered a near-patient solution to current blood flow mapping technologies, mainly imaging techniques, which typically require immobilization of the patient in a hospital setting.
This use case encompasses other sophisticated forms of POC tests for disease monitoring. For example, a paper-based device was developed for at-home testing of urinary tract infections and gonorrhea129. Requiring some manual steps in the current setup, the device could replace traditional bacterial cultures on agar plates with a consumer device that operates in about 30 seconds129. Additionally, a device for label-free blood analysis (white and red blood cell detection and quantification) was designed, using smartphone imaging and magnetic levitation130. The potential for this device, which provides clinical information traditionally captured via a hemocytometer or other labor-intensive techniques, could provide important pre-clinical information in an at home or ambulatory setting130. Similar examples include a microfluidic chip capable of a partial CBC from microliter volumes of whole blood was developed131, as well as a chip for detecting coagulopathy132. Both of these devices have implications in pre-hospital emergency care settings, providing the doctor with information upon arrival in order to more quickly execute treatments.
This use case is also interesting for use settings beyond healthcare, such as food testing and safety, law enforcement, environmental testing, military and defense agencies. While these devices can be designed with fewer budgetary constraints than some of the lowest-cost POC devices, it is paramount that they are portable for field use and operated by minimally-trained users. For instance, a multiplexed meter chip for quantitative detection of bovine catalase in dairy products was developed for food safety monitoring133. Such a device could potentially be deployed in production facilities without accessory laboratory equipment. Further, a POC device for food safety was built for diagnosis of subclinical ketosis in dairy cows134. Such a use would have important implications in decreasing economic loss for farmers and ensuring safety of products. An advance in POC device that was made for law enforcement groups included a paper-based device for ketamine detection using zeolites and graphene oxide for electrochemical sensing; such a device could enable drug testing at the scene without having to send samples to a laboratory and wait for results135. Another example involves using magnetic biosensors to detect tetrahydrocannabinol (THC – i.e. marijuana) from saliva136. Other law enforcement applications include POC detection of explosives in the field137, and POC detection of bioweapons such as anthrax on the scene138.
A final example of a POC device that would be useful for deployment in a government or military setting is the “lab-on-a-drone” device53. The ability to send a laboratory test to many different locations via a drone holds intriguing applications in both military and defense testing, where the setting may be remote with few traditional resources. Finally, we should note for this use case that improvements in the integration and manufacturing of POC technologies could reduce the costs of the devices highlighted in this Section, such that they become appropriate for the lower-cost settings of Use Case 4.
Use Case 3. Clinic level with constrained budget
Use Case 3 presents settings with access to laboratory facilities and trained personnel, but where the resources and budget are more constrained than Use Case 1. Examples include primary care clinics in developing countries, and nonprofit and non-governmental organization health centers. For researchers intending to develop POC devices for this use case, significant design considerations must be given to cost; in return, full integration (e.g. sample preparation steps), portability, and sample throughout may not be as critical. In addition, while the goal remains to still provide diagnostic within a single clinical visit to prompt a treatment decision, the turnaround time for results could be extended from minutes to hours.
Microtiter plate based assays, such as ELISAs, are a workhorse of clinical diagnostic laboratories. These assays typically require spectrophotometers, fluorometers, or plate readers which are expensive and bulky. To bridge the gaps between high-resource central laboratories and smaller, lower-resource clinics where the ability to expand diagnostic testing capabilities is desirable, many groups have worked on developing miniaturized smartphone spectrometers which use detection modalities of absorption, fluorescence or photonic crystal based detection139–142. Most of these methods report single channel detection, or one sample monitoring per measurement. To increase sample throughput, an 8-channel smartphone spectrometer suitable for 96 well plates was developed, which included a cradle to hold the smartphone, and a custom app to control optical sensing parameters and to align each sample to the corresponding channel142. Another device introduced a smartphone-based colorimetric reader platform for 96-well plate based colorimetric ELISA immunoassays143. Unlike previous approaches to use the camera of a smartphone to capture images of the well plate with no optical modification, this device limited optical aberrations (from differential ambient light and field-of-view incongruences) by using an optical fiber array to capture the transmitted light from each well of the microtiter plate. The battery powered device was paired with an app that returned quantitative clinical results in about 1 minute for every 96-well plate143. Also, work has been done to redesign the microtiter plate itself, such as a miniaturized 56-well paper/PMMA hybrid microplate which facilitates rapid antibody/antigen immobilization and efficient washing, and provides colorimetric ELISA results in an hour144. Integration of new materials such as nanoporous glasses as ELISA substrates on microfluidic chips have increased sensitivity and reduced assay time, such as an NPG V-Chip platform for NSCLC biomarker detection which can take place in 30 min using serum samples145.
Much effort has been made to make POC versions of high-resource, clinical lab technologies. Examples included a microfluidic surface plasmon resonance (SPR) platform for bacterial pathogen detection146, a miniaturized nuclear magnetic resonance device (VNMR) for detecting biomarkers in urine147, a lateral electrophoretic flow platform paired with a CellScope mobile reader for antibody detection148, and an optofluidic flow analyzer to measure optical absorbance of RBCs149. Work has also been done to expand the types of analytes which can be detected in a clinic setting, such as the use of smartphone-coupled imaging for DNA strand imaging and length quantification150, and sensitive detection of prostate specific antigen (PSA) from whole-blood samples151. A portable, centrifugal sedimentation based immunoassay platform was developed for multiplexed detection of pathogenic bacteria (E. coli, Listeria, Salmonella, and Shigella) from a variety of sample matrices such as urine, blood and stool152. While the device itself may be portable and battery-powered, sample preparation and manual handling steps, along with the need for a connected computer for fluorescence quantification and interpretation keeps it most appropriate for this use case. An automated, integrated platform for multiplexed protein profiling from saliva samples yielded results in 70 minutes, and could be used by more minimally trained personnel153. Centrifugation of saliva samples before introduction to the disposable microfluidic chip, along with device powering requirements through AC adapter153 make the device as it stands, appropriate for this use case.
Infectious diseases represent a large category of diagnostic need in this use case, with a single local clinic serving a large number of patients per day. For HIV, testing is required for initial diagnosis, staging and ongoing monitoring throughout treatment. In low-resource settings, it can be challenging to achieve timely early infant diagnosis (EID) of infants under 18 months, viral load (VL) monitoring and CD4 staging154. For example, CD4 staging is important in implementing and monitoring antiretroviral therapy, but gold-standard methods such as flow cytometry are costly and technologically complex for decentralized settings. Several POC diagnostics for CD4 testing are on the market including the Pima™ Analyser (Alere), Partec CyFlow® CD4 miniPOC (Sysmex Partec) and the BD FACSPresto™ (BD Biosciences)154. Others in development include the Millipore Muse® (Merck KGaA) and Visitect CD4 (Burnet Institute & Omega Diagnostics Ltd). Most of these diagnostic systems are benchtop-sized with the analyzer costing several thousand U.S. dollars (~$3000-$10,000 USD)154. The Daktari CD4 Counter, had notably eliminated sample preparation steps with no pipetting, labels or reagents and reported CD4 counts in <15 min, but the company has discontinued the CD4 Counter cartridges as a result of shifting global landscape around CD4 testing154. Academic groups have worked on making POC CD4 tests more amenable for global health, such as a microfluidic chip capable of generating CD4+ and CD8+ T-cell counts from 10 μL of unprocessed whole blood155. Sample preparation was integrated into the chip, which used differences in impedance to count cells, a design based off of the Coulter counting principle156. A miniaturized platform for CD4 cell count was also introduced, which is achieved by eliminating operational fluid flow and “moving the substrate” vs flowing liquid157. Fingerprick whole blood is loaded onto the chip along with antibody functionalized magnetic beads, after which the chip is placed on magnet actuated motorized stage, and colorimetric image analysis can be performed on a smartphone157.
Continuing with HIV analysis, early-infant diagnosis and virological testing for HIV DNA or RNA hold clinical value, with laboratory-based nucleic acid tests serving as the standard. Some commercially available POC platforms include the SAMBA Platforms (Diagnostics for the Real World Ltd), Alere q HIV-1/2 Detect (Alere), and Xpert HIV-1 Qual assay and HIV-1 Viral Load kits (Cepheid)154. The SAMBA HIV tests use 100 μL whole blood and require 2 hours assay time, lab technicians and electricity154. The Alere q HIV-1/2 Detect uses 25 μL of whole blood with a portable device that has battery capabilities and requires no manual sample preparation154. Cepheid has recently developed a portable version (1kg) of the GeneXpert instrument called the GeneXpert Omni, which can operate without direct power supply or Internet connectivity, at a list cost of ~$3000 USD154. Xpert HIV-1 Qual assay and HIV-1 Viral Load kits use 100μL sample and provide results in 90 min154.
Translating nucleic acid tests into POC platforms for this use case remains a challenge. A compact polarization anisotropy diagnostic device for bacterial nucleic-acid detection was developed consisting of a disposable sample processing cartridge and compact reader for fluorescence anisotropy detection (overall system 8 × 8 × 8 cm3, ~400g)158. Although not yet a fully contained system in which sample preparation, thermocycling, and detection functions are housed in one device, the device accuracy was comparable to bacterial culture with a faster assay time (~2hr), multiplexing capabilities, and lower cost (< $2/assay)158. Magnetic beads have been used in a centrifugal microfluidic disk-based platform, showing total nucleic acid extraction from whole blood, Gram-positive Bacillus subtilis, Gram-negative Escherichia coli, and Rift Valley fever RNA virus159. This system combined lysis and nucleic acid purification on one cartridge159. Integration of sample preparation through immunomagnetic separation has been shown through development of a series of electrochemical DNA sensors (E-DNA) into microfluidic chip formats including both PCR and LAMP designs, which allowed for detection of influenza virus directly from throat swabs160.
Previous examples of paper-based POC devices mainly focused on protein tests, due to the higher analyte concentration, which required minimal off-chip sample preparation and rendered the targets easier to detect. For nucleic acids, the growth of isothermal amplification techniques as well as paper printing have spurred an increase in the use of paper for nucleic acid-based tests. One advance featured a test which amplified the malB gene found in Escherichia coli161. In another example, a paper device was developed that carries out rolling-circle amplification (RCA) to detect DNA or microRNA162. In this interesting study, the authors were able to actually print a DNA molecule complementary to a template sequence for hybridization162. Another notable paper based device integrated nucleic-acid extraction and purification on various paper layers prior to an isothermal amplification step (loop-mediated amplification, LAMP, in this case)163. Overall, advances in techniques for manufacturing paper-based tests have enabled increasingly widespread development of nucleic-acid testing.
For this use case, other microfluidic systems for nucleic-acid testing are also being developed, often with LAMP as an amplification technique. For example, the Loopamp MALARIA Pan Detection Kit (Eiken Chemical Company) has shown good performance compared to PCR in fingerprick samples from 1000 subjects in Zanzibar, Tanzania164. In this system, two heat blocks, a UV lamp and one centrifuge were required for LAMP testing, with the result that two lab technicians with limited experience in these assays were able to complete testing after 3 days training164. A proof of concept RT-LAMP system on a microfluidic and silicon microchip platform showed detection of minimally processed HIV-spiked whole blood samples using fluorescence measurements taken with a smartphone165. Valve-assisted sample metering and microfluidic mixing for on chip lysis were integrated into the chip, although additional steps to reduce manual handling steps and integration of fluidic control elements are needed for full automation165. Other proof of concept POC LAMP designs included a flexible ribbon polyethylene substrate in a reel-to-reel cassette with colorimetric readout for simplified result interpretation, and a chip (paired with a separate plasma separator) that housed an array of amplification reactors, all of which can be monitored concurrently with a single camera166,167.
Recent epidemics of infectious diseases such as Ebola and Zika have highlighted the importance of large-scale, rapid testing for public health needs and patient outcomes as well as gaps in current diagnostic testing methods. In the 2014-2015 outbreak of Ebola virus disease in West Africa, diagnosis relied primarily on venipuncture blood samples from symptomatic individuals but expanded to field diagnostics168. The current standard for diagnosing acute Ebola in an outbreak setting is real-time RT-PCR from blood samples (or oral fluid samples for postmortem testing)168. Due to the unprecedented scope of the West African Ebola epidemic, about 40 field laboratories were deployed to West Africa from agencies across the globe, and were all equipped to run RT-PCR with trained personnel168. The need for more rapid turnaround time for outbreak control and clinical management as well as significant challenges of collecting and transporting samples, led to the development of novel Ebola virus diagnostic tests, such as automated nucleic-acid tests and rapid antigen detection tests for the field.
A number of commercial cartridge-based, sample-to-answer real-time RT-PCR systems have been developed for detection of the EBOVL gene of Ebola. The Xpert Ebola system (from the company Cepheid) adapted the company’s previous work on tuberculosis to integrate sample preparation, virus inactivation, nucleic acid amplification, and detection169. In Sierra Leone, the Xpert Ebola assay showed high sensitivity and specificity for both venipuncture whole blood and oral swab clinical samples compared to laboratory RT-PCR169. In another system, the FilmArray Ebola system (from the company Biofire Defense) used a pouch with lyophilized reagents that were rehydrated, followed by sample dilution and injection into the reconstituted reagent pouch170; the pouch was inserted into the FilmArray instrument with results available after 1 hour170,171. Comparison of FilmArray with RT-PCR were also performed for whole blood and urine samples170. Finally, the Idylla Ebola Virus Triage Test (from the company Biocartis NV) used a RT-PCR instrument and console, and provided results in 100 min from EDTA venipuncture whole blood samples172. The reagent cartridges did not require cold chain storage172, and clinical results from the field are pending. All three systems were given Emergency Use Authorization status by the regulatory authorities, and use was still confined to facilities with moderate to high complexity168. There exist challenges to implementation in more decentralized field settings, where even greater public health impact could be achieved. Field use requires uninterrupted electricity, temperature control, and moderately trained personnel to conduct sample preparation168. In addition, without subsidies, cost of instruments and cartridges are also prohibitive for lower-resource settings (for example, the manufacturer list price for Filmarray reader is $39,500, with $129 per test kit)173.
Use Case 4. In the field with constrained budget
Use Case 4 settings represent the greatest set of constraints for POC diagnostics. These have tight budget constraints, as exemplified by field settings such as lower-level clinics and health posts, mobile health and community health outreach, and self-testing settings. A number of design choices, in cost, portability, and automation, must be made to ensure the POC device is usable in such settings (Table 2). It should be noted that, with modifications and further iterations, devices listed in other use cases could also be appropriate for this use case.
Table 2. Design considerations for different POC use cases.
Range of POC device design considerations for different settings covering material choice, reagent stability, sample pre-treatment, fluidic actuation, fluidic control, signal detection, disposal and sample type/acquisition.
|
Use Case 1 Moderate Budget, Clinic |
Use Case 2 Moderate Budget, Field |
Use Case 3 Constrained Budget, Clinic |
Use Case 4 Constrained Budget, Field |
|
|---|---|---|---|---|
| Material Choice | Glass Silicon Plastic |
Glass Silicon Plastic Flexible electronics |
Paper Plastic |
Paper Plastic |
| Reagent Stability | Short term or long-term storage | Long-term storage needed | Short term or long-term storage | Long-term storage needed |
| Sample pre-treatment | Off-chip by trained technician | None | Off-chip by minimally trained technician | None |
| Fluidic Actuation | Electrokinetic Pneumatic, Magnetic | Pneumatic Capillary |
Electrokinetic Pneumatic Magnetic |
Pneumatic Capillary |
| Fluidic Control | Machine/power Pipetting by technician Valves | Passive On-chip valves |
Machine/power (minimal) Pipetting (minimal) Valves |
Passive On-chip valves |
| Signal Detection | Fluorescent (larger machines) Colorimetric Electrochemical | Colorimetric (by eye, smart-phone, or hand-held device) | Fluorescent (small, cheap machines only) Colorimetric Electrochemical | Colorimetric (by eye, smartphone, or hand-held device – must be cheap) |
| Disposal | Not needed | Self-contained | Not needed | Self-contained |
| Sample type / acquisition | CSF, amniotic fluid, whole blood, urine, saliva, plasma, sera, swabs, tears | Whole blood, urine, saliva, swabs, tears | Whole blood, plasma, serum, urine, saliva, swabs, tears | Whole blood, urine, saliva, swabs, tears |
Following on the discussion in the previous use case on POC devices for Ebola, we note that a handheld RT-PCR device for Ebola RNA virus detection has been developed, weighing 80 g, powered by a car battery, and capable of producing results in fewer than 40 minutes174. An approach to carrying out both sample preparation and amplification-free detection of Ebola virus was developed on a hybrid optofluidic chip175. While full integration and evaluation with clinical samples are still needed, these devices show movement towards field-deployable RT-PCR detection of Ebola virus during epidemics. In addition, there are three rapid antigen detection tests for Ebola using lateral flow tests which have also received Emergency Use Authorization168 (from the companies Corgenix176, OraSure177, and SD Biosensor178). Nevertheless, developing POC diagnostics for epidemics is challenging, as illustrated by the determination by the World Health Organization in 2016 that the “Ebola situation in West Africa no longer constituted a Public Health Emergency of International Concern”, thereby halting recommendations adopted during the peak of the outbreak179.
Another recent outbreak of infectious disease which calls for a low-cost, field-ready POC test was the Zika virus, which has been linked to microcephaly in as well as Guillain-Barré syndrome in adults180. The CDC recommends RT-PCR to detect viral RNA from serum and urine collected during the first 2 weeks of symptoms181. Currently, there are no rapid POC Zika diagnostic tests that have received Emergency Use Authorization, although efforts are under way to diagnose Zika infection within 4 hours182, and to use an IgM/IgG lateral flow assay with a handheld, battery-powered reader183. Other efforts include a paper-based sensor for the detection of the Zika virus RNA genome was developed using nucleic acid sequence-based amplification, paired with a battery-powered electronic reader (manufacturing cost of $250)184. While sample preparation steps have yet to be integrated and the overall assay time is several hours, the incorporation of freeze-dried reagents brings it closer towards application in low-resource field settings. In another effort, the use of an inexpensive, POC RT-LAMP assay for Zika virus detection was also demonstrated in spiked saliva samples using disposable microfluidic chips, and a power-free, chemically heated cup for amplification185. This system could potentially be integrated with an intercalating fluorescent dye and a smartphone for signal detection and quantification, as shown by other applications185.
Paper has long been held as an inexpensive and resourceful material choice for this resource-limited, field use case. Previously, paper based diagnostics consisted of single-step reactions, as shown by lateral flow tests for infectious diseases, and exhibited long-term stability, interpretation by minimally trained users, usability with a wide range of specimens, and minimal cold chain requirements for shipping/storage186. The World Health Organization lists 28 lateral flow rapid tests as “prequalified” in vitro diagnostic products for HIV and malaria (from companies such as SD Bioline, Chembio, Alere, Trinity Biotech, bioMerieux, OraSure, ABON Biopharm, ARKRAY Healthcare, Access Bio, Premier Medical etc187). Multiplexing is also becoming increasingly prevalent for lateral flow tests. Recently, dual HIV-syphilis lateral flow tests have been developed to extend antenatal screening and treatment coverage188–190. One study used a portable, battery-powered electronic reader which displayed a numerical value based on the test line intensity189. Test performance of lateral flow tests have also been validated with clinical specimens beyond plasma and sera, as shown by testing with whole blood and oral fluids in realistic field conditions for hepatitis B191 and malaria192.
The ability to easily pattern paper into hydrophobic and hydrophilic regions sparked an increase in the use of paper due to improved fluid control193,194 and multiplexing. Paper-based POC designs show increasing structural complexity such as a three-dimensional enzymatic fuel which powered a glucose monitor requiring a low-cost multimeter195. This design builds on previous paper-based colorimetric sensors, but lowered fabrication costs and eliminated additional instrumentation and power requirements195.
To improve user interpretation of band intensities, researchers have expanded detection methods and integrated the test with electronic readers196,197. Researchers have used the flash of the smartphone as a light source and integrated image processing for simultaneous determination of pH and nitrite concentrations in water samples198. Additional developments have corrected for variability in ambient light199. To this end, many hardware additions have been developed for improved smartphone imaging platforms. One design for a compact smartphone-based fluorescence detector paired with a lateral flow test strip for avian influenza detection, and data communication via SMS to a central database with human throat swab samples200. This design used a lightweight attachment module for fluorescence excitation and collection designed to be compatible with the smartphone’s camera, and powered through connection with the smartphone’s micro-USB port200. A series of advances have been made on smartphone readers for POC tests by the Ozcan group201–203, which has led to the creation of a startup company204. Portable temperature and humidity control devices have been developed to optimize the nucleic acid and antigen-antibody binding conditions for lateral flow tests205. Efforts have also been made to build a mobile phone microscope. In one study, quantification of blood-borne filarial parasites could be performed under 2 min, which required only a glass capillary and lancet206. A custom algorithm tracked the motion of the microfilaria by quantifying the displacement of red blood cells, and the results tested in Cameroon compared to thick smear microscopy206.
There has also been increasing interest in applying lateral flow tests and paper-based diagnostic tests for non-communicable diseases. One device identified sickle-cell disorders207, with an alternate lateral flow test using monoclonal antibodies208. Another inexpensive paper-based test enabled controlled transport of red blood cells and measurement of hematocrit209. Paper sensors have also been used for enzyme quantification, such as a proof of concept quantitative estimation of α-amylase, an enzyme indicator of potential diseased states if abnormally high or low210. Micronutrient detection is another area of interest, a competitive lateral flow test, paired with a smartphone accessory, that quantified vitamin B12 levels211. An alternate design used a standalone device that provided quantitative results and wireless communication capabilities to transmit information to a near-field communication enabled phone212. A particularly innovative design for detection of non-communicable diseases paired synthetic biomarkers with paper based sensing213. The synthetic biomarkers accumulated at targeted sites where they were cleaved by local proteases and released reporters that accumulated in urine213, which could be tested on a lateral flow test and visualized by a smartphone213.
Hard plastics are a class of materials which are increasingly explored for resource-limited field use. They are transparent and can be used with a wide variety of optical detection methods, and can be manufactured in large quantities at low cost (via CNC micromilling214, injection molding, extrusion, blow molding and other methods). One plastic microfluidic chip used capillary-driven mixing to eliminate the need for external power or pumps to perform a colorimetric anemia test115. Field demonstration of hard plastic chips for HIV has been performed50,215, including with a smartphone accessory216. Simultaneous hemoglobin measurement and HIV antibody detection was also demonstrated using a disposable, injection molded, plastic microfluidic chip217. Both the colorimetric anemia test and HIV/hemoglobin detection systems leveraged smartphone integration for quantitative measurement. A low-cost, lower-power electronic device showed hematocrit measurement from whole blood via changes in impedance using disposable low-cost, electrode sensors and 50uL samples218. A fingerstick capillary tube could also be adapted to perform an anemia measurement via visual interpretation of color change219, including a demonstration of the assay with prefilled reagents stored for 8 months in local conditions in Angola219.
Thermoplastics are also attractive for applications requiring sustained periods of swings in temperature. A disposable PCR chip was developed using cyclo olefin polymer for detection of Chlamydia trachomatis and Escherichia coli O157:H7220. A syringe pump was used to cycle fluid back and forth between two regions of 95°C and 62°C for forty cycles220. Another group developed a POC device made of PMMA for detection of Zika virus185, this time via an isothermal amplification method185. The ability to couple more advanced mechanics and electronics to plastics could also augment the capabilities of the chip. A POC multiplexed RT-PCR test for hepatotoxicity was developed via integration of a microhomogenizer and paramagnetic beads into a plastic chip for automated tissue processing and RNA extraction221.
Fundamental Advances
Fluid handling methods
Researchers fabricated miniaturized 3-layer PDMS valves that could be multiplexed and of particular interest to optofluidic device222. Optically-driven photodeformable polymers could be manipulated to exert control over a variety of fluids223, which could represent a new tool in microfluidic design223, although feasibility of actuating one component without affecting others in a high density chip would still need to be demonstrated. Valves based on magnetic adhesives have been developed for mixing reagents, requiring no power224. A wax-based valve that was thermally actuated and could be used repeatedly was demonstrated on lateral flow assays to increase flexibility and complexity225. Diaphragm valves in a plastic lab-on-a-disk platform could be automated with relatively simple components226. A chip where reagent droplets were mixed electrostatically was demonstrated227. A microfluidic device allowing for control of shear rates and gradients in a fluid stream allowed for monitoring of whole blood hemostasis and platelet function228. An electrochemical sensor system that monitored liquid flow in porous materials without affecting real flow in paper samples was built, showing the ability to characterize wettability signatures for paper samples of different geometry or composition229. Finally, novel designs for capillary pumps were introduced to minimize bubble entrapment and variability in metered volumes230.
Towards sample processing for clinical specimens, a plasma separation device (SIMBAS)231 was developed to increase the volume of plasma that can be obtained and to allow for flexible handling of the plasma aliquots232. A self-contained sample preparation technique integrated with LAMP has been demonstrated233. A nested PCR device for detection of bacterial pathogen, with integrated sample preparation from serum samples, has been shown234, although the serum must first be isolated. A one-step method for purification and concentration of DNA ono a lateral flow device using chitosan polysaccharide in porous membranes was developed235, as well as an alternate to one-step purification method of nucleic acids from tissue lysate236. Finally, a platform for extraction of nucleic acids from clinical specimens using paramagnetic particles, an oil-water interface, and a small magnet in a microfluidic channel was demonstrated, showing the ability to isolate influenza RNA from clinical nasopharyngeal swab samples237. Further, ultrasensitive detection of nucleic acids was demonstrated based on concentration of the target in chitosan-functionalized nylon membranes as a charge-switch matrix238. Further development of sample-processing techniques and integration with other microfluidic modules will be crucial to realize the vision of a sample-to-answer POC device which performs sophisticated diagnostic tests.
Detection platforms
There has been continued work in label-free sensing platforms for detecting multiple targets from clinical specimens. A novel HIV-viral load detection system used a CMOS chip platform integrated with a USB stick (for mobile or desktop devices) that detects small changes in pH generated by on chip reactions239. Bendable microposts have been used to monitor stiffness of blood clots over time240. An electrochemical steric-hindrance hybridization assay (eSHHA) for multiplexed detection of proteins was demonstrated, with demonstration on whole blood241. Advances in microcantilever-based detection have continued, based on their potential for label-free and sensitive detection242. For instance, a polymeric microfluidic cantilever was developed for detection of waterborne pathogens and DNA243, and achieved fast transport of the analyte to the detection region. Another example used a functionalized cantilever to detect and analyze Listeria, a food-borne pathogen244. Furthermore, drug resistance could be detected by introducing antibiotics into the chip and observing for changes in resonance frequency244.
There have also been efforts to integrate microfluidics with plasmonic sensors, which are a label-free detection method by monitoring interactions among electromagnetic waves and free electrons in metals (including surface plasmon resonance and surface-enhanced Raman scattering245)142. They are small, low-cost, can be integrated into a microarray, and are potentially very sensitive in detecting biomolecular interactions and binding events246. A nanoplasmonic electrical field-enhanced resonating device was demonstrated in a portable reader format with a disposable fluidic chip, using spectral measurements to monitor capture events247. A commercial effort was also made to build a plasmonics-based sensor248. Other demonstrations included detection of microbial pathogens, detection of bacteria in urine samples, and monitoring of therapeutic response103,104,113,146,147.
There have also been developments in surface acoustic waves (SAW) for POC diagnostics. Advantages include simple fabrication, biocompatibility, fast fluid actuation, contact and label-free manipulation, and compatibility249. SAW could be used to isolate circulating tumor cells from blood without large disturbances to the cells250. A SAW sensor detected cardiac troponin I from plasma and whole blood251. A SAW sensor was also developed to quantify the effects of anticoagulants in POC settings252. Commercial efforts (such as OJ-Bio) are developing SAW-based POC tests for C-Reactive Protein, a biomarker for inflammatory disease, as well as targets for HIV, periodontal disease, and flu253.
Wearable POC devices
Concepts are being tested for integrating POC sensors into new form factors. An electrochemical sensor to monitor zinc levels in sweat was built in the form of a temporary tattoo, based on voltammetry from a bismuth/Nafion working electrode124. While electrochemical measurements still required benchtop instruments, additional miniaturization and integration of electronics could be developed into a consumer wearable device124.
Integration of soft microfluidics, structured adhesive surfaces, and mechanical buckling has also been proposed to form flexible and conformal electronic devices to collect electrophysiological data254. Carbon nanotube networks that are stretchable have been developed as wearable strain sensors255.
Highly sophisticated analytical methods
Highly sophisticated techniques, such as mass spectrometry and DNA sequencing, are currently too technically complex to be developed into field-use systems. Nevertheless, some of these systems can be used in certain POC settings (such as Use Case 1), and with an eye towards the future, important developments are being made. For mass spectrometry, developments in ambient ionization and instrument miniaturization present new opportunities for rapid and unbiased analysis of targets256. Paper spray ionization is a potentially simple and robust method for analyzing clinical analytes from biological fluids256,257, with a demonstration of detecting 8 illicit drugs from whole blood256,258. Solid-phase extraction coupled to blade spray ionization were shown to detect cocaine and diazepam in urine259. For DNA sequencing, rapid progress in recent years has led to progressively smaller devices which make sequencing at POC settings steps closer to reality. Currently, the MiniSeq system from Illumina is the smallest of the company’s fleet of sequencers; with dimensions of 18 in × 18.9 in × 20.4 in and weight of 99lbs, it is small enough to fit on top of a laboratory bench140. This system features onboard analysis to make it easy-to-use. In another example, the MinION device from the company Oxford Nanopore Technologies is a pocket-sized sequencer, with an array of nanopores to conduct sequencing139. There have also been announcements to introduce other platforms (such as the SmidgION and PromethION)139 which could be appropriate for some of the use cases described in this Review.
4. Case Studies: From Basic Technology to Use-Case Demonstration
Use Case 1: Blood gas analyzers in a relatively high-budget and well-equipped POC setting
Blood-gas testing, which is useful for diagnosis of respiratory disturbances and acid-base imbalances in the blood involved in life-threatening conditions, is traditionally performed by skilled technicians in a centralized laboratory. As such, these tests provide historical values, rather than real-time measurements, of a patient’s blood gas levels. By contrast, POC testing for blood gases at the point of need, including intensive care units, emergency departments, operating rooms, and during labor and delivery, would help direct immediate care and could potentially improve health outcomes.
Building on the technologies of blood gas testing machines in centralized labs, a number of companies are now offering arterial blood gas analysis systems for POC testing as benchtop portable form factors260. As one example, Siemens Healthineers offers different blood gas systems to choose from depending on sample size, specimen type (whole blood, pleural fluid, or dialysate fluid), and throughput with additional assays (co-oximetry, electrolytes, and metabolites). The systems can be operated in well-equipped POC settings in U.S. and Europe, and provide test results in approximately 60 seconds, such that tests can be carried out by nurses and doctors for rapid measurements of blood gases, electrolytes, and metabolites. The systems can be integrated with a bar-code scanner and support wireless connectivity, to further enhance clinical utility and improve the workflow of blood gas testing at the hospitals. One of the systems, RAPIDPoint® 500 Blood Gas System, has received CE Mark approval199 and in 2013, clearance from the Food and Drug Administration261 to offer pleural fluid pH testing (Fig. 3). Opportunities to improve the device include lowering of cost of operation, expansion of test menu, reduction of sample size, reduction in maintenance, and expansion of data connectivity to include wireless and open systems262.
Figure 3. Representative POC diagnostic devices.

Examples of POC technology from industry and academia that feature integrated device designs that align with Use Cases 1 to 4: (1) RAPIDPoint® 500 Blood Gas System from the company Siemens, Photo courtesy of Siemens Healthineers. ©2016 Siemens Healthcare Diagnostics Inc. Used with permission. (2) BioStampRC® system from the company MC10, Copyright 2016 MC10, Inc. Used with permission; (3) GeneXpert system (four-module configuration) from the company Cepheid, Photo courtesy of Cepheid Inc.; (4) VPAD technology (electronics-enabled paper-based diagnostic test & sliding strip nucleic-acid testing device) from the company Diagnostics For All and the mChip smartphone dongle system from our lab. Reprinted from Biosensors and Bioelectronics, Vol. 78, Lee, S.; Aranyosi, A.; Wong, M. D.; Hong, J. H.; Lowe, J.; Chan, C.; Garlock, D.; Shaw, S.; Beattie, P. D.; Kratochvil, Z., Flexible opto-electronics enabled microfluidics systems with cloud connectivity for point-of-care micronutrient analysis, pp. 290-299 (ref212), Copyright 2016, with permission from Elsevier. Reproduced from Connelly, J. T.; Rolland, J. P.; Whitesides, G. M. Anal Chem 2015, 87, 7595-7601. (ref161) Copyright 2015 American Chemical Society. From Laksanasopin, T.; Guo, T. W.; Nayak, S.; Sridhara, A. A.; Xie, S.; Olowookere, O. O.; Cadinu, P.; Meng, F.; Chee, N. H.; Kim, J. Science translational medicine 2015, 7, 273re271-273re271. (ref216) Reprinted with permission from AAAS.
Use Case 2: Sophisticated wearable sensors for the consumer
In an effort to expanding wearable technologies to health applications, the Rogers group has developed ultrathin, flexible electronics. The first prototypes of skin-mounted devices were shown in 2011263. The ultrathin electronics are as thin as a temporary tattoo, as stretchy as skin, and permeable to air and moisture264. The patch is embedded with sensors that can continuously monitor a person’s movements, heartbeat, and temperature. With a smartphone or tablet connectivity via Bluetooth to the cloud, a doctor can continuously monitor a detailed record of that person’s physical activity and vital signs.
Various materials, mechanical designs, and manufacturing techniques have been further developed since then by the Rogers group and others to integrate with the bending and stretching surfaces of human body265. Potential applications of the flexible sensors include monitoring health and wellness, studying disease states, improving surgical procedures, and establishing human-machine interfaces266. One of the concerns of flexible sensors is battery life. In response, developments were made on stretchable lithium ion batteries with integrated wireless recharging system267. Other materials and assembly approaches have been explored to engineer the electronic devices which can be integrated with the human body based on the area coverage, mechanical properties and geometry. For continuous monitoring of blood flow268, this device provided heat to the skin surface and then measures the direction and amount of the heat as it moves, unlike the conventional non-invasive technique for monitoring blood flow. The quantitative blood flow rates are determined by combining information from thermal sensors with models that account for the fluid dynamics of blood flow.
These skin-wearable sensors are commercialized by a company cofounded by Rogers called MC10. The stretchable sensor platform was beta-tested by the U.S. Air Force in September 20151. The company has partnered with a cosmetics company to develop a skin sensor that monitors UV exposure over time, and a biopharmaceutical company to develop a sensor for patients with neurological disorders. The company has also involved clinical partners from universities and pharmaceutical companies to test the platform, starting with a product called Biostamp Research Connect™ (BioStampRC®, Fig. 3). This product is commercially available in the U.S. and Canada, and can help academic researchers achieve more accurate and streamlined results in their physiological studies. Future iterations of the product will likely move into medical applications and applications for the general consumer.
Use Case 3: Diagnostics for drug-resistant tuberculosis in a clinic
Tuberculosis (TB) remained one of the top 10 causes of death worldwide in 2015269. The GeneXpert system from the company Cepheid is a cartridge-based, automated real-time PCR instrument. The system was originally developed for anthrax detection for a self-contained, fully integrated and automated platform270. The disposable cartridge, pre-loaded with reagents for the assay, can automatically perform the entire process of nucleic acid analysis from unprocessed clinical specimens. The nucleic acids were extracted from samples by ultrasonic lysis, followed by real-time PCR and fluorescent readout of multiple targets and control271. Once the cartridge is inserted in the GeneXpert system, the instrument identifies the pathogen-specific barcode on the cartridge and start the assay on that particular protocol.
The platform developed for TB in the Xpert MTB/RIF assay was validated272 and implemented in the field271. The goal of this assay development is to overcome the sensitivity of TB diagnosis in sputum smear-negative and extrapulmonary TB as well as identify drug resistant TB. Five different hybridization probes are used in the same reaction to span the entire region of rpoB gene273. The work on TB motivated Cepheid to expand their multiplexing capabilities to detect a range of different pathogens at a time, as the system is now available in a one, two, four, 16, 48, or 80-module configuration, with each module independently runs one cartridge at a time24 (Fig. 3). First validated with spiked sputum with known numbers of M.tuberculosis and archived frozen specimens274, an evaluation was performed in four countries275 and in district and sub-district health facilities in countries with high TB burden276. In 2010, the World Health Organization recommended the use of Xpert MTB/RIF271,277 to support a global roll-out of the platform in 145 countries272. While the test was significantly more expensive than conventional screening tests for TB, outside parties subsidized the purchases to achieve substantial cost reductions (up to 84%, relative to prices in high-income countries) for use in 116 high-burden developing countries271. In terms of usability, a large-scale evaluation found that GeneXpert MTB/RIF was successfully implemented at the district and sub-district health facilities in countries with high TB burden276. In terms of health outcomes, one study found the Xpert assay reduced the time to start treatment from 56 days to 5 days for patients with smear-negative TB, and decreased the rates of untreated smear-negative culture-positive TB from 39% to 15%.
Use Case 4: Diagnostics for global health in the field
Our group has developed a low-cost, disposable, plastic microfluidic cartridge (“mChip”) to run immunoassays in global health settings. Initially, the technology integrated fluid handling, signal amplification, and plastic manufacturing, and was commercialized into a startup company which is now part of OPKO Diagnostics278. We collaborated with OPKO Diagnostics and industrial design partners50, including in-country interviews of end users early on in the design process, to build an automated device for multiplexed HIV and syphilis detection, showing laboratory-quality results in sub-Saharan African on clinical blood samples in 15 to 20 minutes50,215,216. A recent form factor was a smartphone accessory216 that operated on disposable low-cost microfluidic cassettes (about $1.50 in material cost per chip) which consisted only of plastic and chemical reagents. Recently, we have also demonstrated integration of two different classes of diagnostic tests into this single platform, showing simultaneous measurement of hemoglobin concentration and HIV antibody detection217, and detection of Lyme disease279.
POC molecular diagnostics tests, for detecting DNA and RNA from pathogens, are difficult to miniaturize, and especially so for resource-limited settings where they must be low-cost and simple-to-use. Multiple research groups are developing paper-based diagnostic tests to perform molecular diagnostic tests280–283. Diagnostics For All is a non-profit company developing paper-based diagnostic technologies from the Whitesides lab201. The paper-based technology from this company has been evaluated in the field with clinical specimens for a variety of applications41. For their first prototype of a complex molecular diagnostics test, they demonstrated integrated sample preparation, isothermal DNA amplification and detection steps into a single paper-based device161. The device combined various materials (magnetic sheet, thin-film lubricant, and commercial filter paper) in a manner that allowed for serial operations of cell lysis, DNA isolation and purification, LAMP amplification, and detection, as carried out on a single sliding-strip device161 (Fig. 3). During the amplification step at 65° C, a seal prevented evaporation. Using a hand-held UV source and smartphone camera as detector, this prototype measured as few as five cells of E. coli. As shown by the success of traditional POC tests for pregnancy, glucose, and HIV, the low cost and simplicity of paper-based systems will continue to be appealing for POC settings with constrained infrastructure and budget, especially with advances in enhancing sensitivity and precision.
5. Conclusion and Future Directions
Since the early 1990’s, developments in POC diagnostics have been buoyed by several trends, including miniaturization of techniques in analytical chemistry and genomics284, large-scale integration of fluid handling capabilities with an analogy to microelectronics285, and global health applications67,286. In recent years, the most influential force for POC diagnostics has been the rise of consumer electronics and connected devices. In today’s connected age, the POC ecosystem is more diverse than ever before (Fig. 1), an unsurprising development for a field which could some day fundamentally revolutionize how health care is delivered throughout the world.
As POC diagnostic devices are becoming increasingly integrated, and tested with clinical specimens, we have analyzed recent developments in the field through the lens of different use-case scenarios, which impose different design constraints on all aspects of the POC diagnostics device, from material choice to signal detection (Table 2). Unlike previous classifications of use-case scenarios, we propose a framework which decouples the infrastructure available to run the test, from the budget allocated to purchase the POC device. This decoupling presents use cases which pose distinct constraints for researchers (Fig. 2), including different design constraints for use in a clinic or in the field which would depend on the budget available. This framework presents the constraints across a more diverse set of POC settings than just global health. In addition, it brings the relevant criteria up to date to contemporary settings. For example, the acronym “ASSURED” has been widely used287 for development of POC diagnostic devices, especially for global health applications. While initially useful for researchers, its introduction in the early 2000’s came before the age of smartphones and widespread data connectivity. The letter “E”, standing for “Equipment-free”, did not take into account the recent explosive rise of consumer electronics, and availability of hardware components with increasing capabilities at falling prices.
What is the future of POC diagnostic technologies? In the short term, numerous challenges – technological and non-technological – will persist before a large set of new POC devices will be introduced to the market. In the long term, the field is on track to deliver POC diagnostic devices across all appropriate decentralized settings – the remaining technological hurdles are addressable, and the social benefits too rich for governments, clinicians, insurers, and consumers to ignore. In fact, with some of the most important social trends of today (autonomous drones, space colonization), the end-use scenarios, which are already vast, have become only more intriguing and relevant to tomorrow’s world. The rapid progress of non-traditional components in the new POC ecosystem – such as consumer electronics, connectivity, and data analytics – has infused POC diagnostics with new energy, perspectives, and risk capital. The path between where the field stands today and the long-vision – perhaps an inevitable one – of widespread use of POC diagnostic devices will likely involve the marriage of this new energy with the traditional scientific advances in analytical chemistry and microfluidics.
Acknowledgments
N.R.B thanks the Columbia Irving Institute for Clinical and Translational Research TL1 Precision Medicine Predoctoral Funding (Grant: 1TL1TR00187501) for support of this work. We thank members of the Sia Lab (N. Tejavibulya, R. Chaves, F. Marcogliese, D. Colburn, P. Anandakumaran, R. Field, S. Arumugam, L. Cusenza, A. Mohan, M. Januzi and E. Chen) for collaboration and discussion.
Biographies
Samiksha Nayak received her B.S. in Chemical-Biological Engineering from the Massachusetts Institute of Technology in 2010 and M.S. in Biomedical Engineering from Columbia University in 2014. She is currently a graduate student in the Department of Biomedical Engineering at Columbia University. Her research focuses on development of point-of-care diagnostic devices for global health and consumer applications.
Nicole R. Blumenfeld received her B.S. in Bioengineering from the University of Pennsylvania in 2013. She is currently a graduate student in the Department of Biomedical Engineering at Columbia University. Her research focuses on the development of low-cost, fully-integrated, miniaturized devices for genomic analysis and precision medicine.
Tassaneewan Laksanasopin received her Ph.D. in Biomedical Engineering from the Columbia University in 2015. She is currently a faculty member in Biological Engineering Program at King Mongkut’s University of Technology Thonburi, Thailand. She specializes in microfluidics, point-of-care diagnostics, field testing of new healthcare technologies (pre-clinical trial), and healthcare IoT.
Samuel K. Sia is a Professor in the Department of Biomedical Engineering at Columbia University. His lab focuses on using microfluidics for global health diagnostics and for 3D tissue biology. He obtained his B.S. in Biochemistry at the University of Alberta, Ph.D. in Biophysics at Harvard University, and postdoctoral fellowship in Chemistry at Harvard University. He was named one of the world’s top young innovators by MIT Technology Review (2010), and is an elected fellow of AIMBE (American Institute for Medical and Biological Engineering). He is a founder of Claros Diagnostics, a venture capital-backed company developing diagnostics products which was acquired by OPKO Health, and Harlem Biospace, a life-science incubator in New York City.
References
- 1.Christiansen, C.; Grossman, J. H.; Hwang, J.; New York: McGraw-Hill, 2008.
- 2.Sia SK, Kricka LJ. Lab on a Chip. 2008;8:1982–1983. doi: 10.1039/b817915h. [DOI] [PubMed] [Google Scholar]
- 3.Chin CD, Linder V, Sia SK. Lab on a Chip. 2012;12:2118–2134. doi: 10.1039/c2lc21204h. [DOI] [PubMed] [Google Scholar]
- 4.Slomovic S, Pardee K, Collins JJ. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:14429–14435. doi: 10.1073/pnas.1508521112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Groff K, Brown J, Clippinger AJ. Biotechnology advances. 2015;33:1787–1798. doi: 10.1016/j.biotechadv.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Xiang D, Shigdar S, Qiao G, Wang T, Kouzani AZ, Zhou SF, Kong L, Li Y, Pu C, Duan W. Theranostics. 2015;5:23–42. doi: 10.7150/thno.10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun H, Zu Y. Molecules. 2015;20:11959–11980. doi: 10.3390/molecules200711959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barhona F, Bardliving C, Phifer A, Bruno J, Batt C. Ind Biotechnol. 2013;9:42–49. [Google Scholar]
- 9.Najafabadi ME, Khayamian T, Hashemian Z. Journal of pharmaceutical and biomedical analysis. 2015;107:244–250. doi: 10.1016/j.jpba.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, Shen Z, Xiang Y, Lu Y. ACS Sensors. 2016;1:1091–1096. [Google Scholar]
- 11.Sormanni P, Aprile FA, Vendruscolo M. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:9902–9907. doi: 10.1073/pnas.1422401112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pluckthun A. Annual review of pharmacology and toxicology. 2015;55:489–511. doi: 10.1146/annurev-pharmtox-010611-134654. [DOI] [PubMed] [Google Scholar]
- 13.Skrlec K, Strukelj B, Berlec A. Trends in biotechnology. 2015;33:408–418. doi: 10.1016/j.tibtech.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 14.Hassanzadeh-Ghassabeh G, Devoogdt N, De Pauw P, Vincke C, Muyldermans S. Nanomedicine. 2013;8:1013–1026. doi: 10.2217/nnm.13.86. [DOI] [PubMed] [Google Scholar]
- 15.Steeland S, Vandenbroucke RE, Libert C. Drug discovery today. 2016;21:1076–1113. doi: 10.1016/j.drudis.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Sherwood LJ, Hayhurst A. PLoS One. 2013;8:e61232. doi: 10.1371/journal.pone.0061232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pereira SS, Moreira-Dill LS, Morais MS, Prado ND, Barros ML, Koishi AC, Mazarrotto GA, Goncalves GM, Zuliani JP, Calderon LA, Soares AM, Pereira da Silva LH, Duarte dos Santos CN, Fernandes CF, Stabeli RG. PLoS One. 2014;9:e108067. doi: 10.1371/journal.pone.0108067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J, Cheng F, Li J, Zhu JJ, Lu Y. Nano today. 2016;11:309–329. doi: 10.1016/j.nantod.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petryayeva E, Algar WR. Rsc Advances. 2015;5:22256–22282. [Google Scholar]
- 20.Tanke HJ, Zuiderwijk M, Wiesmeijer KC, Breedveld RN, Abrams WR, Claudia J, Fat EMTK, Corstjens PL. SPIE BiOS. International Society for Optics and Photonics; 2014. pp 89470P-89470P-89415. [Google Scholar]
- 21.Nash MA, Waitumbi JN, Hoffman AS, Yager P, Stayton PS. ACS nano. 2012;6:6776–6785. doi: 10.1021/nn3015008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tian T, Wei X, Jia S, Zhang R, Li J, Zhu Z, Zhang H, Ma Y, Lin Z, Yang CJ. Biosensors and Bioelectronics. 2016;77:537–542. doi: 10.1016/j.bios.2015.09.049. [DOI] [PubMed] [Google Scholar]
- 23.Ham JY, Jung J, Hwang B-G, Kim W-J, Kim Y-S, Kim E-J, Cho M-Y, Hwang M-S, Won DI, Suh JS. Annals of laboratory medicine. 2015;35:50–56. doi: 10.3343/alm.2015.35.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yen C-W, de Puig H, Tam JO, Gómez-Márquez J, Bosch I, Hamad-Schifferli K, Gehrke L. Lab on a Chip. 2015;15:1638–1641. doi: 10.1039/c5lc00055f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui F, Rhee M, Singh A, Tripathi A. Annual review of biomedical engineering. 2015;17:267–286. doi: 10.1146/annurev-bioeng-071114-040538. [DOI] [PubMed] [Google Scholar]
- 26.Cheung SF, Cheng SK, Kamei DT. Journal of laboratory automation. 2015;20:316–333. doi: 10.1177/2211068215577197. [DOI] [PubMed] [Google Scholar]
- 27.Li CG, Lee CY, Lee K, Jung H. Biomed Microdevices. 2013;15:17–25. doi: 10.1007/s10544-012-9683-2. [DOI] [PubMed] [Google Scholar]
- 28.Bandodkar AJ, Wang J. Trends in biotechnology. 2014;32:363–371. doi: 10.1016/j.tibtech.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Wang S, Chinnasamy T, Lifson MA, Inci F, Demirci U. Trends in biotechnology. 2016;34:909–921. doi: 10.1016/j.tibtech.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vilela D, Romeo A, Sanchez S. Lab Chip. 2016;16:402–408. doi: 10.1039/c5lc90136g. [DOI] [PubMed] [Google Scholar]
- 31.Sackmann EK, Fulton AL, Beebe DJ. Nature. 2014;507:181–189. doi: 10.1038/nature13118. [DOI] [PubMed] [Google Scholar]
- 32.Vashist SK, Luppa PB, Yeo LY, Ozcan A, Luong JH. Trends in biotechnology. 2015;33:692–705. doi: 10.1016/j.tibtech.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Hitzbleck M, Delamarche E. Chem Soc Rev. 2013;42:8494–8516. doi: 10.1039/c3cs60118h. [DOI] [PubMed] [Google Scholar]
- 34.Sharma S, Zapatero-Rodriguez J, Estrela P, O’Kennedy R. Biosensors. 2015;5:577–601. doi: 10.3390/bios5030577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y, Xuan J, Song Y, Qi W, He B, Wang P, Qin L. ACS Nano. 2016;10:1640–1647. doi: 10.1021/acsnano.5b07357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peeling RW, McNerney R. Expert Review of Molecular Diagnostics. 2014;14:525–534. doi: 10.1586/14737159.2014.915748. [DOI] [PubMed] [Google Scholar]
- 37.Song Y, Huang Y-Y, Liu X, Zhang X, Ferrari M, Qin L. Trends in biotechnology. 2014;32:132–139. doi: 10.1016/j.tibtech.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tay A, Pavesi A, Yazdi SR, Lim CT, Warkiani ME. Biotechnology advances. 2016;34:404–421. doi: 10.1016/j.biotechadv.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.King KR, Grazette LP, Paltoo DN, McDevitt JT, Sia SK, Barrett PM, Apple FS, Gurbel PA, Weissleder R, Leeds H. JACC: Basic to Translational Science. 2016;1:73–86. doi: 10.1016/j.jacbts.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schroeder LF, LaBarre P, Weigl BH, Amukele T. Point of Care. 2016;15:93–95. [Google Scholar]
- 41.Kumar AA, Hennek JW, Smith BS, Kumar S, Beattie P, Jain S, Rolland JP, Stossel TP, Chunda-Liyoka C, Whitesides GM. Angew Chem Int Edit. 2015;54:5835–5852. doi: 10.1002/anie.201411741. [DOI] [PubMed] [Google Scholar]
- 42.Bond MM, Richards-Kortum RR. American journal of clinical pathology. 2015;144:885–894. doi: 10.1309/AJCP1L7DKMPCHPEH. [DOI] [PubMed] [Google Scholar]
- 43.Sia SK, Owens MP. Nat Biotechnol. 2015;33:1224–1228. doi: 10.1038/nbt.3422. [DOI] [PubMed] [Google Scholar]
- 44.Ozcan A. Lab on a chip. 2014;14:3187–3194. doi: 10.1039/c4lc00010b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lan T, Zhang JJ, Lu Y. Biotechnology advances. 2016;34:331–341. doi: 10.1016/j.biotechadv.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moore CL, Copel JA. New England Journal of Medicine. 2011;364:749–757. doi: 10.1056/NEJMra0909487. [DOI] [PubMed] [Google Scholar]
- 47.Vashist SK, Luong JH. Biotechnology advances. 2016;34:137–138. doi: 10.1016/j.biotechadv.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 48.Sanner TA, Roland LK, Braa K. Health Policy and Technology. 2012;1:155–164. [Google Scholar]
- 49.Labrique AB, Vasudevan L, Kochi E, Fabricant R, Mehl G. Global health, science and practice. 2013;1:160–171. doi: 10.9745/GHSP-D-13-00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chin CD, Cheung YK, Laksanasopin T, Modena MM, Chin SY, Sridhara AA, Steinmiller D, Linder V, Mushingantahe J, Umviligihozo G. Clinical chemistry. 2013;59:629–640. doi: 10.1373/clinchem.2012.199596. [DOI] [PubMed] [Google Scholar]
- 51.Defense, B., 2016.
- 52.Seiner DR, Colburn HA, Baird C, Bartholomew RA, Straub T, Victry K, Hutchison JR, Valentine N, Bruckner-Lea CJ. Journal of applied microbiology. 2013;114:992–1000. doi: 10.1111/jam.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Priye A, Wong S, Bi Y, Carpio M, Chang J, Coen M, Cope D, Harris J, Johnson J, Keller A. Analytical chemistry. 2016;88:4651–4660. doi: 10.1021/acs.analchem.5b04153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fukuba T, Aoki Y, Fukuzawa N, Yamamoto T, Kyo M, Fujii T. Lab on a Chip. 2011;11:3508–3515. doi: 10.1039/c1lc20523d. [DOI] [PubMed] [Google Scholar]
- 55.Kim J, Jensen EC, Stockton AM, Mathies RA. Analytical chemistry. 2013;85:7682–7688. doi: 10.1021/ac303767m. [DOI] [PubMed] [Google Scholar]
- 56.Cable ML, Stockton AM, Mora MF, Willis PA. Analytical chemistry. 2012;85:1124–1131. doi: 10.1021/ac3030202. [DOI] [PubMed] [Google Scholar]
- 57.Eisenstein M. Nat Biotechnol. 2015;33:1013–1014. doi: 10.1038/nbt1015-1013a. [DOI] [PubMed] [Google Scholar]
- 58.Sheridan C. Nat Biotechnol. 2015;33:322–322. doi: 10.1038/nbt0115-5. [DOI] [PubMed] [Google Scholar]
- 59.Vayena E, Salathé M, Madoff LC, Brownstein JS. PLoS Comput Biol. 2015;11:e1003904. doi: 10.1371/journal.pcbi.1003904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yom-Tov E, Borsa D, Hayward AC, McKendry RA, Cox IJ. Journal of medical Internet research. 2015;17 doi: 10.2196/jmir.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yom-Tov E, Borsa D, Cox IJ, McKendry RA. Journal of medical Internet research. 2014;16:e154. doi: 10.2196/jmir.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lazer D, Kennedy R, King G, Vespignani A. Science. 2014;343:1203–1205. doi: 10.1126/science.1248506. [DOI] [PubMed] [Google Scholar]
- 63.Marr B. LinkedIn Pulse. 2015 [Google Scholar]
- 64.McRae MP, Simmons G, Wong J, McDevitt JT. Accounts of Chemical Research. 2016;49:1359–1368. doi: 10.1021/acs.accounts.6b00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mavandadi S, Feng S, Yu F, Dimitrov S, Yu R, Ozcan A. GAMES FOR HEALTH: Research, Development, and Clinical Applications. 2012;1:373–376. doi: 10.1089/g4h.2012.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cohen JK. Becker’s Health IT & CIO Review. Becker’s Hospital Review; 2016. [Google Scholar]
- 67.Chin CD, Linder V, Sia SK. Lab on a Chip. 2007;7:41–57. doi: 10.1039/b611455e. [DOI] [PubMed] [Google Scholar]
- 68.Boppart SA, Richards-Kortum R. Science translational medicine. 2014;6:253rv252–253rv252. doi: 10.1126/scitranslmed.3009725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Malone B. Innovatin, Expansion Evident at AACC Clinical Lab Expo in Los Angeles. AACC; Washington, D.C.: 2012. [Google Scholar]
- 70.Software, O. Laboratory Point-of_Care Testing: A Future Outlook. 2015 [Google Scholar]
- 71.Weinert K, Gyorda P, Rifai N, Evans S, Kaufman HW, Eby CS, Ashwood ER, Moore N. Clinical chemistry. 2015;61:1320–1327. doi: 10.1373/clinchem.2015.248112. [DOI] [PubMed] [Google Scholar]
- 72.Futrell K. Laboratory Point-of-Care Testing: A Future Outlook; POCT Progression & the Importance of Connectivity. Orchard Software; Carmel, Indiana: 2015. [Google Scholar]
- 73.Fellner S, Hentze S, Kempin U, Richter E, Rocktäschel J, Langer B. 2015 doi: 10.1016/j.plabm.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Buhr S. TechCrunch. TechCrunch; 2015. [Google Scholar]
- 75.Pager.
- 76.Hamel MB, Cortez NG, Cohen IG, Kesselheim AS. The New England journal of medicine. 2014;371:372–379. doi: 10.1056/NEJMhle1403384. [DOI] [PubMed] [Google Scholar]
- 77.Halvorson GC. Health care will not reform itself: A user’s guide to refocusing and reforming American health care. CRC Press; 2009. [Google Scholar]
- 78.National Institutes of Health. 2010.
- 79.Crocker JB, Lee-Lewandrowski E, Lewandrowski N, Baron J, Gregory K, Lewandrowski K. Am J Clin Pathol. 2014;142:640–646. doi: 10.1309/AJCPYK1KV2KBCDDL. [DOI] [PubMed] [Google Scholar]
- 80.Bloss CS, Wineinger NE, Peters M, Boeldt DL, Ariniello L, Kim JY, Sheard J, Komatireddy R, Barrett P, Topol EJ. PeerJ. 2016;4:e1554. doi: 10.7717/peerj.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Borry P, van Hellemondt RE, Sprumont D, Jales CFD, Rial-Sebbag E, Spranger TM, Curren L, Kaye J, Nys H, Howard H. European Journal of Human Genetics. 2012;20:715–721. doi: 10.1038/ejhg.2011.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bloss CS, Schork NJ, Topol EJ. New England Journal of Medicine. 2011;364:524–534. doi: 10.1056/NEJMoa1011893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kidd BA, Hoffman G, Zimmerman N, Li L, Morgan JW, Glowe PK, Botwin GJ, Parekh S, Babic N, Doust MW. The Journal of clinical investigation. 2016;126:1734–1744. doi: 10.1172/JCI86318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nelson B. Cancer cytopathology. 2016;124:7–8. doi: 10.1002/cncy.21758. [DOI] [PubMed] [Google Scholar]
- 85.Chokshi DA. The Lancet. 2015;385:2037. [Google Scholar]
- 86.Organization, W.H. Geneva: WHO.
- 87.Pai NP, Vadnais C, Denkinger C, Engel N, Pai M. PLoS medicine. 2012;9:e1001306. doi: 10.1371/journal.pmed.1001306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kratz A, McKenzie-McKie C, Guo TW, Sia SK. Point of Care. 2015;14:124–126. [Google Scholar]
- 89.Loo J, Kwok H, Leung C, Wu S, Law I, Cheung Y, Cheung Y, Chin M, Kwan P, Hui M. Biosensors and Bioelectronics. 2016 doi: 10.1016/j.bios.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 90.Yang J, Selvaganapathy PR, Gould TJ, Dwivedi DJ, Liu D, Fox-Robichaud AE, Liaw PC. Lab on a Chip. 2015;15:3925–3933. doi: 10.1039/c5lc00681c. [DOI] [PubMed] [Google Scholar]
- 91.Prieto JL, Su HW, Hou HW, Vera MP, Levy BD, Baron RM, Han J, Voldman J. Lab on a Chip. 2016 doi: 10.1039/c6lc00940a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li Y, Xuan J, Song Y, Wang P, Qin L. Lab on a Chip. 2015;15:3300–3306. doi: 10.1039/c5lc00529a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hou HW, Bhattacharyya RP, Hung DT, Han J. Lab on a Chip. 2015;15:2297–2307. doi: 10.1039/c5lc00311c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Convert L, Lebel R, Gascon S, Fontaine R, Pratte JF, Charette P, Aimez V, Lecomte R. Journal of Nuclear Medicine. 2016 doi: 10.2967/jnumed.115.162768. jnumed. 115.162768. [DOI] [PubMed] [Google Scholar]
- 95.Chaemsaithong P, Romero R, Korzeniewski SJ, Dong Z, Yeo L, Hassan SS, Kim YM, Yoon BH, Chaiworapongsa T. The Journal of Maternal-Fetal & Neonatal Medicine. 2015;28:1510–1519. doi: 10.3109/14767058.2014.961417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Papadimitriou KI, Wang C, Rogers ML, Gowers SA, Leong CL, Boutelle MG, Drakakis EM. Frontiers in human neuroscience. 2016;10 doi: 10.3389/fnhum.2016.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Eigl S, Prattes J, Lackner M, Willinger B, Spiess B, Reinwald M, Selitsch B, Meilinger M, Neumeister P, Reischies F. Critical care. 2015;19:1. doi: 10.1186/s13054-015-0905-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Romey M, Jovanoviĉ L, Bevier W, Markova K, Strasma P, Zisser H. Journal of diabetes science and technology. 2012;6:1260–1266. doi: 10.1177/193229681200600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pandya H, Park K, Chen W, Goodell L, Foran D, Desai J. 2016 [Google Scholar]
- 100.Kanwar SS, Dunlay CJ, Simeone DM, Nagrath S. Lab on a Chip. 2014;14:1891–1900. doi: 10.1039/c4lc00136b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xie Y, Zhi X, Su H, Wang K, Yan Z, He N, Zhang J, Chen D, Cui D. Nanoscale research letters. 2015;10:1. doi: 10.1186/s11671-015-1153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shamsi MH, Choi K, Ng AH, Wheeler AR. Lab on a Chip. 2014;14:547–554. doi: 10.1039/c3lc51063h. [DOI] [PubMed] [Google Scholar]
- 103.Rampazzi S, Danese G, Leporati F, Marabelli F. IEEE Transactions on Instrumentation and Measurement. 2016;65:317–327. [Google Scholar]
- 104.Schröder U-C, Bokeloh F, O’Sullivan M, Glaser U, Wolf K, Pfister W, Popp J, Ducrée J, Neugebauer U. Biomicrofluidics. 2015;9:044118. doi: 10.1063/1.4928070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jain A, van der Meer AD, Papa A-L, Barrile R, Lai A, Schlechter BL, Otieno MA, Louden CS, Hamilton GA, Michelson AD. Biomedical microdevices. 2016;18:73. doi: 10.1007/s10544-016-0095-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Choi J, Kang M, Jung JH. Scientific reports. 2015;5 doi: 10.1038/srep15983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yeom E, Park JH, Kang YJ, Lee SJ. Scientific reports. 2016;6 doi: 10.1038/srep24994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yu ZTF, Guan H, Cheung MK, McHugh WM, Cornell TT, Shanley TP, Kurabayashi K, Fu J. Scientific reports. 2015;5 doi: 10.1038/srep11339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pholwat S, Liu J, Stroup S, Gratz J, Banu S, Rahman SM, Ferdous SS, Foongladda S, Boonlert D, Ogarkov O. MBio. 2015;6:e02273–02214. doi: 10.1128/mBio.02273-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wu Y, Xue P, Hui KM, Kang Y. Biosensors and Bioelectronics. 2014;52:180–187. doi: 10.1016/j.bios.2013.08.039. [DOI] [PubMed] [Google Scholar]
- 111.Sarioglu AF, Aceto N, Kojic N, Donaldson MC, Zeinali M, Hamza B, Engstrom A, Zhu H, Sundaresan TK, Miyamoto DT. Nature methods. 2015;12:685–691. doi: 10.1038/nmeth.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Das J, Ivanov I, Montermini L, Rak J, Sargent EH, Kelley SO. Nature chemistry. 2015;7:569–575. doi: 10.1038/nchem.2270. [DOI] [PubMed] [Google Scholar]
- 113.Berger AG, Restaino SM, White IM. Analytica Chimica Acta. 2016 doi: 10.1016/j.aca.2016.10.035. [DOI] [PubMed] [Google Scholar]
- 114.Chen J, Gorman M, O’Reilly B, Chen Y. Data in brief. 2016;6:847–852. doi: 10.1016/j.dib.2016.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Plevniak K, Campbell M, Myers T, Hodges A, He M. Biomicrofluidics. 2016;10:054113. doi: 10.1063/1.4964499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wire, B.; BusinessWire: Dublin, 2016.
- 117.Jina A, Tierney MJ, Tamada JA, McGill S, Desai S, Chua B, Chang A, Christiansen M. Journal of diabetes science and technology. 2014;8:483–487. doi: 10.1177/1932296814526191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lee SJ, Yoon HS, Xuan X, Park JY, Paik S-J, Allen MG. Sensors and Actuators B: Chemical. 2016;222:1144–1151. [Google Scholar]
- 119.Skoog SA, Miller PR, Boehm RD, Sumant AV, Polsky R, Narayan RJ. Diamond and Related Materials. 2015;54:39–46. [Google Scholar]
- 120.Munje RD, Muthukumar S, Prasad S. Sensors and Actuators B: Chemical. 2017;238:482–490. [Google Scholar]
- 121.Zhang J, Xiang Y, Wang M, Basu A, Lu Y. Angewandte Chemie International Edition. 2016;55:732–736. doi: 10.1002/anie.201507563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gu C, Lan T, Shi H, Lu Y. Analytical chemistry. 2015;87:7676–7682. doi: 10.1021/acs.analchem.5b01085. [DOI] [PubMed] [Google Scholar]
- 123.Gao W, Emaminejad S, Nyein HYY, Challa S, Chen K, Peck A, Fahad HM, Ota H, Shiraki H, Kiriya D. Nature. 2016;529:509–514. doi: 10.1038/nature16521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kim J, de Araujo WR, Samek IA, Bandodkar AJ, Jia W, Brunetti B, Paixão TR, Wang J. Electrochemistry Communications. 2015;51:41–45. [Google Scholar]
- 125.Kim J, Imani S, de Araujo WR, Warchall J, Valdés-Ramírez G, Paixão TR, Mercier PP, Wang J. Biosensors and Bioelectronics. 2015;74:1061–1068. doi: 10.1016/j.bios.2015.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kim J, Valdés-Ramírez G, Bandodkar AJ, Jia W, Martinez AG, Ramírez J, Mercier P, Wang J. Analyst. 2014;139:1632–1636. doi: 10.1039/c3an02359a. [DOI] [PubMed] [Google Scholar]
- 127.Kassal P, Kim J, Kumar R, de Araujo WR, Steinberg IM, Steinberg MD, Wang J. Electrochemistry Communications. 2015;56:6–10. [Google Scholar]
- 128.Webb RC, Ma Y, Krishnan S, Li Y, Yoon S, Guo X, Feng X, Shi Y, Seidel M, Cho NH. Science advances. 2015;1:e1500701. doi: 10.1126/sciadv.1500701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cho S, San Park T, Nahapetian TG, Yoon J-Y. Biosensors and Bioelectronics. 2015;74:601–611. doi: 10.1016/j.bios.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 130.Baday M, Calamak S, Durmus NG, Davis RW, Steinmetz LM, Demirci U. Small. 2015 doi: 10.1002/smll.201501845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hassan U, Reddy B, Jr, Damhorst G, Sonoiki O, Ghonge T, Yang C, Bashir R. Technology. 2015;3:201–213. doi: 10.1142/S2339547815500090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Beynon C, Erk AG, Potzy A, Mohr S, Popp E. Scandinavian journal of trauma, resuscitation and emergency medicine. 2015;23:1. doi: 10.1186/s13049-015-0139-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cui X, Hu J, Choi JR, Huang Y, Wang X, Lu TJ, Xu F. Analytica Chimica Acta. 2016;935:207–212. doi: 10.1016/j.aca.2016.07.046. [DOI] [PubMed] [Google Scholar]
- 134.Weng X, Zhao W, Neethirajan S, Duffield T. Journal of nanobiotechnology. 2015;13:1. doi: 10.1186/s12951-015-0076-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Narang J, Malhotra N, Singhal C, Mathur A, Chakraborty D, Anil A, Ingle A, Pundir CS. Biosensors and Bioelectronics. 2016 doi: 10.1016/j.bios.2016.08.043. [DOI] [PubMed] [Google Scholar]
- 136.Lee J-R, Choi J, Shultz TO, Wang SX. Analytical Chemistry. 2016;88:7457–7461. doi: 10.1021/acs.analchem.6b01688. [DOI] [PubMed] [Google Scholar]
- 137.Peters KL, Corbin I, Kaufman LM, Zreibe K, Blanes L, McCord BR. Analytical Methods. 2015;7:63–70. [Google Scholar]
- 138.Gao R, Ko J, Cha K, Jeon JH, Rhie G-E, Choi J, Choo J. Biosensors and Bioelectronics. 2015;72:230–236. doi: 10.1016/j.bios.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 139.Long KD, Yu H, Cunningham BT. SPIE Sensing Technology+ Applications. International Society for Optics and Photonics; 2015. pp 94820J-94820J-94828. [Google Scholar]
- 140.Wang L-J, Chang Y-C, Ge X, Osmanson AT, Du D, Lin Y, Li L. ACS Sensors. 2016;1:366–373. [Google Scholar]
- 141.Yu H, Tan Y, Cunningham BT. Analytical chemistry. 2014;86:8805–8813. doi: 10.1021/ac502080t. [DOI] [PubMed] [Google Scholar]
- 142.Wang L-J, Chang Y-C, Sun R, Li L. Biosensors and Bioelectronics. 2017;87:686–692. doi: 10.1016/j.bios.2016.09.021. [DOI] [PubMed] [Google Scholar]
- 143.Berg B, Cortazar B, Tseng D, Ozkan H, Feng S, Wei Q, Chan RY-L, Burbano J, Farooqui Q, Lewinski M. ACS nano. 2015;9:7857–7866. doi: 10.1021/acsnano.5b03203. [DOI] [PubMed] [Google Scholar]
- 144.Sanjay ST, Dou M, Sun J, Li X. Scientific Reports. 2016;6 doi: 10.1038/srep30474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li Y, Xuan J, Song Y, Qi W, He B, Wang P, Qin L. ACS nano. 2015;10:1640–1647. doi: 10.1021/acsnano.5b07357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tokel O, Yildiz UH, Inci F, Durmus NG, Ekiz OO, Turker B, Cetin C, Rao S, Sridhar K, Natarajan N. Scientific reports. 2015;5:9152. doi: 10.1038/srep09152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chung HJ, Pellegrini KL, Chung J, Wanigasuriya K, Jayawardene I, Lee K, Lee H, Vaidya VS, Weissleder R. PloS one. 2015;10:e0133417. doi: 10.1371/journal.pone.0133417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lin R, Skandarajah A, Gerver RE, Neira HD, Fletcher DA, Herr AE. Lab on a Chip. 2015;15:1488–1496. doi: 10.1039/c4lc01370k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Banoth E, Kasula VK, Jagannadh VK, Gorthi SS. Journal of biophotonics. 2015 doi: 10.1002/jbio.201500118. [DOI] [PubMed] [Google Scholar]
- 150.Wei Q, Luo W, Chiang S, Kappel T, Mejia C, Tseng D, Chan RYL, Yan E, Qi H, Shabbir F. ACS nano. 2014;8:12725–12733. doi: 10.1021/nn505821y. [DOI] [PubMed] [Google Scholar]
- 151.Barbosa AI, Gehlot P, Sidapra K, Edwards AD, Reis NM. Biosensors and Bioelectronics. 2015;70:5–14. doi: 10.1016/j.bios.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 152.Phaneuf CR, Mangadu B, Piccini ME, Singh AK, Koh C-Y. Biosensors. 2016;6:49. doi: 10.3390/bios6040049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nie S, Henley WH, Miller SE, Zhang H, Mayer KM, Dennis PJ, Oblath EA, Alarie JP, Wu Y, Oppenheim FG. Lab on a Chip. 2014;14:1087–1098. doi: 10.1039/c3lc51303c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.UNITAID. HIV/AIDS diagnostics technology landscape. 5th. UNITAID; Geneva, Switzerland: 2015. [Google Scholar]
- 155.Watkins NN, Hassan U, Damhorst G, Ni H, Vaid A, Rodriguez W, Bashir R. Science translational medicine. 2013;5:214ra170–214ra170. doi: 10.1126/scitranslmed.3006870. [DOI] [PubMed] [Google Scholar]
- 156.Coulter J, Fulwyler M, Steinkamp J. Google Patents. 1973 [Google Scholar]
- 157.Wang S, Tasoglu S, Chen PZ, Chen M, Akbas R, Wach S, Ozdemir CI, Gurkan UA, Giguel FF, Kuritzkes DR. Scientific reports. 2014;4 doi: 10.1038/srep03796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Park KS, Huang C-H, Lee K, Yoo Y-E, Castro CM, Weissleder R, Lee H. Science Advances. 2016;2:e1600300. doi: 10.1126/sciadv.1600300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Strohmeier O, Keil S, Kanat B, Patel P, Niedrig M, Weidmann M, Hufert F, Drexler J, Zengerle R, von Stetten F. RSC Advances. 2015;5:32144–32150. [Google Scholar]
- 160.Hsieh K, Ferguson BS, Eisenstein M, Plaxco KW, Soh HT. Accounts of chemical research. 2015;48:911–920. doi: 10.1021/ar500456w. [DOI] [PubMed] [Google Scholar]
- 161.Connelly JT, Rolland JP, Whitesides GM. Analytical chemistry. 2015;87:7595–7601. doi: 10.1021/acs.analchem.5b00411. [DOI] [PubMed] [Google Scholar]
- 162.Liu M, Hui CY, Zhang Q, Gu J, Kannan B, Jahanshahi-Anbuhi S, Filipe CD, Brennan JD, Li Y. Angewandte Chemie (International ed in English) 2016;55:2709–2713. doi: 10.1002/anie.201509389. [DOI] [PubMed] [Google Scholar]
- 163.Choi JR, Hu J, Tang R, Gong Y, Feng S, Ren H, Wen T, Li X, Abas WABW, Pingguan-Murphy B. Lab on a Chip. 2016;16:611–621. doi: 10.1039/c5lc01388g. [DOI] [PubMed] [Google Scholar]
- 164.Cook J, Aydin-Schmidt B, González IJ, Bell D, Edlund E, Nassor MH, Msellem M, Ali A, Abass AK, Mårtensson A. Malaria journal. 2015;14:1. doi: 10.1186/s12936-015-0573-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Damhorst GL, Duarte-Guevara C, Chen W, Ghonge T, Cunningham BT, Bashir R. Engineering. 2015;1:324–335. doi: 10.15302/J-ENG-2015072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Safavieh M, Ahmed MU, Sokullu E, Ng A, Braescu L, Zourob M. Analyst. 2014;139:482–487. doi: 10.1039/c3an01859h. [DOI] [PubMed] [Google Scholar]
- 167.Song J, Liu C, Bais S, Mauk MG, Bau HH, Greenberg RM. PLoS Negl Trop Dis. 2015;9:e0004318. doi: 10.1371/journal.pntd.0004318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Broadhurst MJ, Brooks TJ, Pollock NR. Clinical Microbiology Reviews. 2016;29:773–793. doi: 10.1128/CMR.00003-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Semper AE, Broadhurst MJ, Richards J, Foster GM, Simpson AJ, Logue CH, Kelly JD, Miller A, Brooks TJ, Murray M, Pollock NR. PLoS medicine. 2016;13:e1001980. doi: 10.1371/journal.pmed.1001980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Southern TR, Racsa LD, Albariño CG, Fey PD, Hinrichs SH, Murphy CN, Herrera VL, Sambol AR, Hill CE, Ryan EL. Journal of clinical microbiology. 2015;53:2956–2960. doi: 10.1128/JCM.01317-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Weller SA, Bailey D, Matthews S, Lumley S, Sweed A, Ready D, Eltringham G, Richards J, Vipond R, Lukaszewski R. Journal of clinical microbiology. 2016;54:114–119. doi: 10.1128/JCM.02287-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Administration, U.S.F.a. D., 2016.
- 173.Leski TA, Ansumana R, Taitt CR, Lamin JM, Bangura U, Lahai J, Mbayo G, Kanneh MB, Bawo B, Bockarie AS, Scullion M, Phillips CL, Horner CP, Jacobsen KH, Stenger DA. J Clin Microbiol. 2015;53:2368–2370. doi: 10.1128/JCM.00527-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ahrberg CD, Manz A, Neužil P. Analytical Chemistry. 2016;88:4803–4807. doi: 10.1021/acs.analchem.6b00278. [DOI] [PubMed] [Google Scholar]
- 175.Cai H, Parks J, Wall T, Stott M, Stambaugh A, Alfson K, Griffiths A, Mathies R, Carrion R, Patterson J. Scientific reports. 2015;5 doi: 10.1038/srep14494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Broadhurst MJ, Kelly JD, Miller A, Semper A, Bailey D, Groppelli E, Simpson A, Brooks T, Hula S, Nyoni W. The Lancet. 2015;386:867–874. doi: 10.1016/S0140-6736(15)61042-X. [DOI] [PubMed] [Google Scholar]
- 177.Administration, U. S. F. a. D., 2016.
- 178.Organization, W. H. Emergency use assessment and listing for Ebola virus disease IVDs, public report, product: SD Q Line Ebola Zaire Ag. World Health Organization; Geneva, Switzerland: 2015. [Google Scholar]
- 179.Organization, W. H.; World Health Organization: Geneva, Switzerland, 2016.
- 180.Speer SD, Pierson TC. Science. 2016;353:750–751. doi: 10.1126/science.aah6187. [DOI] [PubMed] [Google Scholar]
- 181.Prevention, C. f. D. C.; CDC: Atlanta, Georgia, 2016.
- 182.Abbasi J. JAMA. 2016;316:1249–1249. doi: 10.1001/jama.2016.12760. [DOI] [PubMed] [Google Scholar]
- 183.Inc, C. D. S.; Chembio: Medford, NY, 2016.
- 184.Pardee K, Green AA, Takahashi MK, Braff D, Lambert G, Lee JW, Ferrante T, Ma D, Donghia N, Fan M. Cell. 2016;165:1255–1266. doi: 10.1016/j.cell.2016.04.059. [DOI] [PubMed] [Google Scholar]
- 185.Song J, Mauk MG, Hackett BA, Cherry S, Bau HH, Liu C. Analytical Chemistry. 2016;88:7289–7294. doi: 10.1021/acs.analchem.6b01632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sharma S, Zapatero-Rodríguez J, Estrela P, O’Kennedy R. Biosensors. 2015;5:577–601. doi: 10.3390/bios5030577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Organization, W. H.; WHO: Geneva, Switzerland, 2016.
- 188.Yin Y-P, Ngige E, Anyaike C, Ijaodola G, Oyelade TA, Vaz RG, Newman LM, Chen X-S. International Journal of Gynecology & Obstetrics. 2015;130:S22–S26. doi: 10.1016/j.ijgo.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 189.Leon S, Ramos L, Vargas S, Kojima N, Perez D, Caceres C, Klausner J. Journal of clinical microbiology. 2016;54:492–494. doi: 10.1128/JCM.03152-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Bristow CC, Severe L, Pape JW, Javanbakht M, Lee S-J, Comulada WS, Klausner JD. BMC Infectious Diseases. 2016;16:302. doi: 10.1186/s12879-016-1574-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Njai HF, Shimakawa Y, Sanneh B, Ferguson L, Ndow G, Mendy M, Sow A, Lo G, Toure-Kane C, Tanaka J. Journal of clinical microbiology. 2015;53:1156–1163. doi: 10.1128/JCM.02980-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Djimde AA, Maiga H, Sagara I, Traore K, Niangaly H, Bamadio A, Grivoyannis A, Tekete M, Traore OB, Kodio A. Journal of Parasitology and Vector Biology. 2016;8:1–9. [Google Scholar]
- 193.Martinez AW, Phillips ST, Butte MJ, Whitesides GM. Angewandte Chemie International Edition. 2007;46:1318–1320. doi: 10.1002/anie.200603817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Han KN, Choi JS, Kwon J. Scientific reports. 2016;6 doi: 10.1038/srep25710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Fischer C, Fraiwan A, Choi S. Biosensors and Bioelectronics. 2016;79:193–197. doi: 10.1016/j.bios.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 196.Cate DM, Adkins JA, Mettakoonpitak J, Henry CS. Analytical chemistry. 2014;87:19–41. doi: 10.1021/ac503968p. [DOI] [PubMed] [Google Scholar]
- 197.Tobjörk D, Österbacka R. Advanced Materials. 2011;23:1935–1961. doi: 10.1002/adma.201004692. [DOI] [PubMed] [Google Scholar]
- 198.Lopez-Ruiz N, Curto VF, Erenas MM, Benito-Lopez F, Diamond D, Palma AJ, Capitan-Vallvey LF. Analytical chemistry. 2014;86:9554–9562. doi: 10.1021/ac5019205. [DOI] [PubMed] [Google Scholar]
- 199.Shen L, Hagen JA, Papautsky I. Lab on a Chip. 2012;12:4240–4243. doi: 10.1039/c2lc40741h. [DOI] [PubMed] [Google Scholar]
- 200.Yeo S-J, Choi K, Cuc BT, Hong NN, Bao DT, Ngoc NM, Le MQ, Hang NLK, Thach NC, Mallik SK. Theranostics. 2016;6:231. doi: 10.7150/thno.14023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Coskun AF, Nagi R, Sadeghi K, Phillips S, Ozcan A. Lab on a Chip. 2013;13:4231–4238. doi: 10.1039/c3lc50785h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Coskun AF, Wong J, Khodadadi D, Nagi R, Tey A, Ozcan A. Lab on a Chip. 2013;13:636–640. doi: 10.1039/c2lc41152k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wei Q, Nagi R, Sadeghi K, Feng S, Yan E, Ki SJ, Caire R, Tseng D, Ozcan A. ACS nano. 2014;8:1121–1129. doi: 10.1021/nn406571t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Cellmic. 2016.
- 205.Choi JR, Hu J, Feng S, Abas WABW, Pingguan-Murphy B, Xu F. Biosensors and Bioelectronics. 2016;79:98–107. doi: 10.1016/j.bios.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 206.D’Ambrosio MV, Bakalar M, Bennuru S, Reber C, Skandarajah A, Nilsson L, Switz N, Kamgno J, Pion S, Boussinesq M. Science translational medicine. 2015;7:286re284–286re284. doi: 10.1126/scitranslmed.aaa3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kanter J, Telen MJ, Hoppe C, Roberts CL, Kim JS, Yang X. BMC medicine. 2015;13:225. doi: 10.1186/s12916-015-0473-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Quinn CT, Paniagua MC, DiNello RK, Panchal A, Geisberg M. British Journal of Haematology. 2016 doi: 10.1111/bjh.14298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Berry SB, Fernandes SC, Rajaratnam A, DeChiara NS, Mace CR. Lab on a Chip. 2016;16:3689–3694. doi: 10.1039/c6lc00895j. [DOI] [PubMed] [Google Scholar]
- 210.Dutta S, Mandal N, Bandyopadhyay D. Biosensors and Bioelectronics. 2016;78:447–453. doi: 10.1016/j.bios.2015.11.075. [DOI] [PubMed] [Google Scholar]
- 211.Lee S, O’Dell D, Hohenstein J, Colt S, Mehta S, Erickson D. Scientific Reports. 2016;6 doi: 10.1038/srep28237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lee S, Aranyosi A, Wong MD, Hong JH, Lowe J, Chan C, Garlock D, Shaw S, Beattie PD, Kratochvil Z. Biosensors and Bioelectronics. 2016;78:290–299. doi: 10.1016/j.bios.2015.11.060. [DOI] [PubMed] [Google Scholar]
- 213.Warren AD, Kwong GA, Wood DK, Lin KY, Bhatia SN. Proceedings of the National Academy of Sciences. 2014;111:3671–3676. doi: 10.1073/pnas.1314651111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Guckenberger DJ, de Groot TE, Wan AM, Beebe DJ, Young EW. Lab on a Chip. 2015;15:2364–2378. doi: 10.1039/c5lc00234f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Chin CD, Laksanasopin T, Cheung YK, Steinmiller D, Linder V, Parsa H, Wang J, Moore H, Rouse R, Umviligihozo G. Nature medicine. 2011;17:1015–1019. doi: 10.1038/nm.2408. [DOI] [PubMed] [Google Scholar]
- 216.Laksanasopin T, Guo TW, Nayak S, Sridhara AA, Xie S, Olowookere OO, Cadinu P, Meng F, Chee NH, Kim J. Science translational medicine. 2015;7:273re271–273re271. doi: 10.1126/scitranslmed.aaa0056. [DOI] [PubMed] [Google Scholar]
- 217.Guo T, Patnaik R, Kuhlmann K, Rai AJ, Sia SK. Lab on a Chip. 2015;15:3514–3520. doi: 10.1039/c5lc00609k. [DOI] [PubMed] [Google Scholar]
- 218.Punter-Villagrasa J, Cid J, Páez-Avilés C, Rodríguez-Villarreal I, Juanola-Feliu E, Colomer-Farrarons J, Miribel-Català PL. Sensors. 2015;15:4564–4577. doi: 10.3390/s150204564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.McGann PT, Tyburski EA, De Oliveira V, Santos B, Ware RE, Lam WA. American journal of hematology. 2015;90:1122–1127. doi: 10.1002/ajh.24180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Fernández-Carballo BL, McGuiness I, McBeth C, Kalashnikov M, Borrós S, Sharon A, Sauer-Budge AF. Biomedical microdevices. 2016;18:1–10. doi: 10.1007/s10544-016-0060-4. [DOI] [PubMed] [Google Scholar]
- 221.Lim GS, Chang JS, Lei Z, Wu R, Wang Z, Cui K, Wong S. Lab on a Chip. 2015;15:4032–4043. doi: 10.1039/c5lc00798d. [DOI] [PubMed] [Google Scholar]
- 222.Araci IE, Quake SR. Lab on a Chip. 2012;12:2803–2806. doi: 10.1039/c2lc40258k. [DOI] [PubMed] [Google Scholar]
- 223.Lv J-A, Liu Y, Wei J, Chen E, Qin L, Yu Y. Nature. 2016;537:179–184. doi: 10.1038/nature19344. [DOI] [PubMed] [Google Scholar]
- 224.Harper JC, Andrews JM, Ben C, Hunt AC, Murton JK, Carson BD, Bachand GD, Lovchik JA, Arndt WD, Finley MR. Lab on a Chip. 2016;16:4142–4151. doi: 10.1039/c6lc00858e. [DOI] [PubMed] [Google Scholar]
- 225.Phillips EA, Shen R, Zhao S, Linnes JC. Lab on a Chip. 2016;16:4230–4236. doi: 10.1039/c6lc00945j. [DOI] [PubMed] [Google Scholar]
- 226.Kim T-H, Sunkara V, Park J, Kim C-J, Woo H-K, Cho Y-K. Lab on a Chip. 2016;16:3741–3749. doi: 10.1039/c6lc00629a. [DOI] [PubMed] [Google Scholar]
- 227.Norian H, Field RM, Kymissis I, Shepard KL. Lab on a Chip. 2014;14:4076–4084. doi: 10.1039/c4lc00443d. [DOI] [PubMed] [Google Scholar]
- 228.Jain A, Graveline A, Waterhouse A, Vernet A, Flaumenhaft R, Ingber DE. Nature communications. 2016;7 doi: 10.1038/ncomms10176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Bathany C, Han J-R, Abi-Samra K, Takayama S, Cho Y-K. Biosensors and Bioelectronics. 2015;70:115–121. doi: 10.1016/j.bios.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 230.Safavieh R, Tamayol A, Juncker D. Microfluidics and Nanofluidics. 2015;18:357–366. [Google Scholar]
- 231.Dimov IK, Basabe-Desmonts L, Garcia-Cordero JL, Ross BM, Ricco AJ, Lee LP. Lab on a Chip. 2011;11:845–850. doi: 10.1039/c0lc00403k. [DOI] [PubMed] [Google Scholar]
- 232.Samborski A, Jankowski P, Węgrzyn J, Michalski JA, Pawłowska S, Jakieła S, Garstecki P. Engineering in Life Sciences. 2015;15:333–339. [Google Scholar]
- 233.Creecy A, Russ PK, Solinas F, Wright DW, Haselton FR. PloS one. 2015;10:e0130260. doi: 10.1371/journal.pone.0130260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Czilwik G, Messinger T, Strohmeier O, Wadle S, von Stetten F, Paust N, Roth G, Zengerle R, Saarinen P, Niittymäki J. Lab on a Chip. 2015;15:3749–3759. doi: 10.1039/c5lc00591d. [DOI] [PubMed] [Google Scholar]
- 235.Byrnes S, Bishop J, Lafleur L, Buser J, Lutz B, Yager P. Lab on a Chip. 2015;15:2647–2659. doi: 10.1039/c5lc00317b. [DOI] [PubMed] [Google Scholar]
- 236.Berry SM, Alarid ET, Beebe DJ. Lab on a chip. 2011;11:1747–1753. doi: 10.1039/c1lc00004g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Cui FR, Wang J, Opal SM, Tripathi A. PloS one. 2016;11:e0149522. doi: 10.1371/journal.pone.0149522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Schlappi TS, McCalla SE, Schoepp NG, Ismagilov RF. Analytical Chemistry. 2016;88:7647–7653. doi: 10.1021/acs.analchem.6b01485. [DOI] [PubMed] [Google Scholar]
- 239.Gurrala R, Lang Z, Shepherd L, Davidson D, Harrison E, McClure M, Kaye S, Toumazou C, Cooke G. Scientific Reports. 2016;6 doi: 10.1038/srep36000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Judith RM, Fisher JK, Spero RC, Fiser BL, Turner A, Oberhardt B, Taylor R, Falvo MR, Superfine R. Lab on a Chip. 2015;15:1385–1393. doi: 10.1039/c4lc01478b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Mahshid SS, Camiré Sb, Ricci F, Vallée-Bélisle A. Journal of the American Chemical Society. 2015;137:15596–15599. doi: 10.1021/jacs.5b04942. [DOI] [PubMed] [Google Scholar]
- 242.Liu D, Shu W. Current Organic Chemistry. 2011;15:477–485. [Google Scholar]
- 243.Bridle H, Wang W, Gavriilidou D, Amalou F, Hand DP, Shu W. Sensors and Actuators A: Physical. 2016 [Google Scholar]
- 244.Etayash H, Khan M, Kaur K, Thundat T. Nature Communications. 2016;7 doi: 10.1038/ncomms12947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Tokel O, Inci F, Demirci U. Chemical reviews. 2014;114:5728–5752. doi: 10.1021/cr4000623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Niu L, Zhang N, Liu H, Zhou X, Knoll W. Biomicrofluidics. 2015;9:052611. doi: 10.1063/1.4929579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Inci F, Filippini C, Baday M, Ozen MO, Calamak S, Durmus NG, Wang S, Hanhauser E, Hobbs KS, Juillard F. Proceedings of the National Academy of Sciences. 2015;112:E4354–E4363. doi: 10.1073/pnas.1510824112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Freifeld CC, Brownstein JS, Menone CM, Bao W, Filice R, Kass-Hout T, Dasgupta N. Drug safety. 2014;37:343–350. doi: 10.1007/s40264-014-0155-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Ding X, Li P, Lin S-CS, Stratton ZS, Nama N, Guo F, Slotcavage D, Mao X, Shi J, Costanzo F. Lab on a Chip. 2013;13:3626–3649. doi: 10.1039/c3lc50361e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Li P, Mao Z, Peng Z, Zhou L, Chen Y, Huang P-H, Truica CI, Drabick JJ, El-Deiry WS, Dao M. Proceedings of the National Academy of Sciences. 2015;112:4970–4975. doi: 10.1073/pnas.1504484112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Lee W, Jung J, Hahn YK, Kim SK, Lee Y, Lee J, Lee T-H, Park J-Y, Seo H, Lee JN. Analyst. 2013;138:2558–2566. doi: 10.1039/c3an00182b. [DOI] [PubMed] [Google Scholar]
- 252.Moll J, Meyer DSS, Hils B, Dornuf F, Meyer DSI, Singer OC, Ferreirós N, Labocha S, Bluecher A, Harder S. International journal of clinical pharmacology and therapeutics. 2016;54:177–184. doi: 10.5414/CP202492. [DOI] [PubMed] [Google Scholar]
- 253.Ginsberg J, Mohebbi MH, Patel RS, Brammer L, Smolinski MS, Brilliant L. Nature. 2009;457:1012–1014. doi: 10.1038/nature07634. [DOI] [PubMed] [Google Scholar]
- 254.Xu S, Zhang Y, Jia L, Mathewson KE, Jang K-I, Kim J, Fu H, Huang X, Chava P, Wang R. Science. 2014;344:70–74. doi: 10.1126/science.1250169. [DOI] [PubMed] [Google Scholar]
- 255.Park SJ, Kim J, Chu M, Khine M. Advanced Materials Technologies. 2016 [Google Scholar]
- 256.Ferreira CR, Yannell KE, Jarmusch AK, Pirro V, Ouyang Z, Cooks RG. Clinical chemistry. 2016;62:99–110. doi: 10.1373/clinchem.2014.237164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Yang Q, Wang H, Maas JD, Chappell WJ, Manicke NE, Cooks RG, Ouyang Z. International journal of mass spectrometry. 2012;312:201–207. doi: 10.1016/j.ijms.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Espy RD, Teunissen SF, Manicke NE, Ren Y, Ouyang Z, van Asten A, Cooks RG. Analytical chemistry. 2014;86:7712–7718. doi: 10.1021/ac5016408. [DOI] [PubMed] [Google Scholar]
- 259.Gómez‐Ríos GA, Pawliszyn J. Angewandte Chemie International Edition. 2014;53:14503–14507. doi: 10.1002/anie.201407057. [DOI] [PubMed] [Google Scholar]
- 260.Luukkonen AA, Lehto TM, Hedberg PS, Vaskivuo TE. Clinical Chemistry and Laboratory Medicine (CCLM) 2016;54:585–594. doi: 10.1515/cclm-2015-0592. [DOI] [PubMed] [Google Scholar]
- 261.Chemistry, A. A. f. C.; AACC, 2013.
- 262.Cashin-Garbutt, A., 2015.
- 263.Kim DH, Lu NS, Ma R, Kim YS, Kim RH, Wang SD, Wu J, Won SM, Tao H, Islam A, Yu KJ, Kim TI, Chowdhury R, Ying M, Xu LZ, Li M, Chung HJ, Keum H, McCormick M, Liu P, Zhang YW, Omenetto FG, Huang YG, Coleman T, Rogers JA. Science. 2011;333:838–843. doi: 10.1126/science.1206157. [DOI] [PubMed] [Google Scholar]
- 264.Chandler DL. Ieee Pulse. 2016;7:9–12. doi: 10.1109/MPUL.2015.2498463. [DOI] [PubMed] [Google Scholar]
- 265.Song Y, Wang Y, Qi W, Li Y, Xuan J, Wang P, Qin L. Lab on a Chip. 2016;16:2955–2962. doi: 10.1039/c6lc00561f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Kim DH, Ghaffari R, Lu N, Rogers JA. Annual review of biomedical engineering. 2012;14:113–128. doi: 10.1146/annurev-bioeng-071811-150018. [DOI] [PubMed] [Google Scholar]
- 267.Xu S, Zhang Y, Cho J, Lee J, Huang X, Jia L, Fan JA, Su Y, Su J, Zhang H, Cheng H, Lu B, Yu C, Chuang C, Kim TI, Song T, Shigeta K, Kang S, Dagdeviren C, Petrov I, Braun PV, Huang Y, Paik U, Rogers JA. Nat Commun. 2013;4:1543. doi: 10.1038/ncomms2553. [DOI] [PubMed] [Google Scholar]
- 268.Webb RC, Ma Y, Krishnan S, Li Y, Yoon S, Guo X, Feng X, Shi Y, Seidel M, Cho NH, Kurniawan J, Ahad J, Sheth N, Kim J, Taylor JGt, Darlington T, Chang K, Huang W, Ayers J, Gruebele A, Pielak RM, Slepian MJ, Huang Y, Gorbach AM, Rogers JA. Sci Adv. 2015;1:e1500701. doi: 10.1126/sciadv.1500701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.World Health Organization. Global Tuberculosis Report 2016. World Health Organization; Geneva: 2016. [Google Scholar]
- 270.Ulrich MP, Christensen DR, Coyne SR, Craw PD, Henchal EA, Sakai SH, Swenson D, Tholath J, Tsai J, Weir AF, Norwood DA. Journal of applied microbiology. 2006;100:1011–1016. doi: 10.1111/j.1365-2672.2006.02810.x. [DOI] [PubMed] [Google Scholar]
- 271.Lawn SD, Nicol MP. Future microbiology. 2011;6:1067–1082. doi: 10.2217/fmb.11.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Weyer K, Mirzayev F, Migliori GB, Van Gemert W, D’Ambrosio L, Zignol M, Floyd K, Centis R, Cirillo DM, Tortoli E, Gilpin C, Iragena JD, Falzon D, Raviglione M. Eur Respir J. 2013;42:252–271. doi: 10.1183/09031936.00157212. [DOI] [PubMed] [Google Scholar]
- 273.Piatek AS, Telenti A, Murray MR, El-Hajj H, Jacobs WR, Jr, Kramer FR, Alland D. Antimicrobial agents and chemotherapy. 2000;44:103–110. doi: 10.1128/aac.44.1.103-110.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Helb D, Jones M, Story E, Boehme C, Wallace E, Ho K, Kop J, Owens MR, Rodgers R, Banada P, Safi H, Blakemore R, Lan NT, Jones-Lopez EC, Levi M, Burday M, Ayakaka I, Mugerwa RD, McMillan B, Winn-Deen E, Christel L, Dailey P, Perkins MD, Persing DH, Alland D. J Clin Microbiol. 2010;48:229–237. doi: 10.1128/JCM.01463-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, Allen J, Tahirli R, Blakemore R, Rustomjee R, Milovic A, Jones M, O’Brien SM, Persing DH, Ruesch-Gerdes S, Gotuzzo E, Rodrigues C, Alland D, Perkins MD. The New England journal of medicine. 2010;363:1005–1015. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Boehme CC, Nicol MP, Nabeta P, Michael JS, Gotuzzo E, Tahirli R, Gler MT, Blakemore R, Worodria W, Gray C, Huang L, Caceres T, Mehdiyev R, Raymond L, Whitelaw A, Sagadevan K, Alexander H, Albert H, Cobelens F, Cox H, Alland D, Perkins MD. Lancet. 2011;377:1495–1505. doi: 10.1016/S0140-6736(11)60438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.World Health Organization. World Health Organization Geneva, 2011.
- 278.Linder V. Analyst. 2007;132:1186–1192. doi: 10.1039/b706347d. [DOI] [PubMed] [Google Scholar]
- 279.Nayak S, Sridhara A, Melo R, Richer L, Chee NH, Kim J, Linder V, Steinmiller D, Sia SK, Gomes-Solecki M. Scientific Reports. 2016;6 doi: 10.1038/srep35069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Lafleur LK, Bishop JD, Heiniger EK, Gallagher RP, Wheeler MD, Kauffman P, Zhang XH, Kline EC, Buser JR, Kumar S, Byrnes SA, Vermeulen NMJ, Scarr NK, Belousov Y, Mahoney W, Toley BJ, Ladd PD, Lutz BR, Yager P. Lab on a Chip. 2016;16:3777–3787. doi: 10.1039/c6lc00677a. [DOI] [PubMed] [Google Scholar]
- 281.Pardee K, Green AA, Takahashi MK, Braff D, Lambert G, Lee JW, Ferrante T, Ma D, Donghia N, Fan M, Daringer NM, Bosch I, Dudley DM, O’Connor DH, Gehrke L, Collins JJ. Cell. 2016;165:1255–1266. doi: 10.1016/j.cell.2016.04.059. [DOI] [PubMed] [Google Scholar]
- 282.Rodriguez NM, Linnes JC, Fan A, Ellenson CK, Pollock NR, Klapperich CM. Analytical Chemistry. 2015;87:7872–7879. doi: 10.1021/acs.analchem.5b01594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Choi JR, Hu J, Tang RH, Gong Y, Feng SS, Ren H, Wen T, Li XJ, Abas WAW, Pingguan-Murphy B, Xu F. Lab on a Chip. 2016;16:611–621. doi: 10.1039/c5lc01388g. [DOI] [PubMed] [Google Scholar]
- 284.Whitesides GM. Nature. 2006;442:368–373. doi: 10.1038/nature05058. [DOI] [PubMed] [Google Scholar]
- 285.Hong JW, Quake SR. Nat Biotechnol. 2003;21:1179–1183. doi: 10.1038/nbt871. [DOI] [PubMed] [Google Scholar]
- 286.Yager P, Edwards T, Fu E, Helton K, Nelson K, Tam MR, Weigl BH. Nature. 2006;442:412–418. doi: 10.1038/nature05064. [DOI] [PubMed] [Google Scholar]
- 287.Mabey D, Peeling RW, Ustianowski A, Perkins MD. Nature Reviews Microbiology. 2004;2:231–240. doi: 10.1038/nrmicro841. [DOI] [PubMed] [Google Scholar]


