Abstract
Pathogenic autoantibodies to muscle-specific tyrosine kinase (MuSK) can be found in patients with myasthenia gravis (MG) who do not have detectable antibodies to the acetylcholine receptor (AChR). Although the autoantibody-mediated pathology is well understood, much remains to be learned about the cellular immunology that contributes to autoantibody production. To that end, our laboratory has investigated particular components associated with the cellular immunopathology of MuSK MG. First, we found that B cell tolerance defects contribute to the abnormal development of the naive repertoire, which indicates that dysregulation occurs before the production of autoantibodies. Second, both the naive and antigen-experienced memory B cell repertoire, which we examined through the application of high-throughput adaptive immune receptor repertoire sequencing (AIRR-seq), include abnormalities not found in healthy controls. This highlights a broad immune dysregulation. Third, using complementary approaches, including production of human monoclonal antibodies, we determined that circulating plasmablasts directly contribute to the production of MuSK-specific autoantibodies in patients experiencing relapse following B cell depletion therapy. These collective findings contribute to defining a mechanistic model that describes MuSK MG immunopathogenesis.
Keywords: myasthenia gravis, B cells, autoimmunity, immunopathology, autoantibodies, MuSK
Introduction
MG can be considered a prototype for peripheral autoantibody-mediated autoimmune disorders.1,2 It is now well understood that the molecular immunopathology of MG is attributed to the presence of circulating autoantibodies specifically targeting AChR, MuSK, or low-density lipoprotein receptor–related protein 4 (LRP4).3–5 The MuSK MG disease subtype, which is the focus of this report, is characterized by immunological and clinical features that are generally distinct from AChR MG. These distinguishing features of MuSK MG include IgG4 subclass involvement, favorable response to B cell depletion therapy, and a clinical course that frequently involves bulbar symptoms (Table 1). Passive transfer and active immunization studies in animals have shown that MuSK autoantibodies are pathogenic.6–8 In the AChR disease subtype, the IgG1 and IgG3 autoantibodies mediate immunopathology partly through complement-dependent mechanisms. In contrast, MuSK autoantibodies are predominantly IgG4; this subclass does not effectively activate complement.9 Thus, the immunopathology is mediated through autoantibody-dependent but complement-independent mechanical disruption of the interaction between MuSK and the postsynaptic protein LRP4 and collagen Q.10,11 Moreover, isolated Ag-specific Fabs are sufficient to induce pathology in MuSK MG, which highlights the Fc-independent pathogenic mechanism of MuSK autoantibodies that is not observed with AChR autoantibodies.11–13
Table 1.
| Feature | AChR MG | MuSK MG |
|---|---|---|
| Bulbar symptoms | Not predominant | Predominant |
| Pure ocular form | Frequent | Very rare |
| Thymus involvement | Frequent | Very rare |
| Autoantibody titer association with symptoms | Poor | Present |
| Response to pyridostigmine | Good | Medium/poor |
| Autoantibody titer decrease post-rituximab | Mild/none | Pronounced |
| Post-rituximab relapse | Frequent | Rare |
The production of autoantibodies clearly implicates a principal role for B cells in the manifestation of MG. Yet studies directed toward understanding peripheral B cell functional abnormalities in MuSK MG remain very few in number. However, the successful introduction of biological therapeutics in MG has renewed investigative interests in elucidating the details of the contributions made by B cells to the production of MuSK MG autoantibodies.14,15 In particular, several recent studies16–20 have demonstrated the benefits of B cell depletion therapy through the use of rituximab. In addition to diminished autoantibody titers and significant clinical improvement, rituximab also allowed for tapering and subsequent discontinuation of other immunotherapies in subsets of MG patients.16,19 This treatment modality has thus offered a unique opportunity to gain further insight into which specific B cell subsets produce pathogenic MG autoantibodies.
In this report, we focus on examining the contributions of B cells to the immunopathology of MuSK MG. A speculative model of autoantibody production in MuSK MG serves to navigate the spectrum of cellular immunopathology that covers early B cell development and repertoire formation through MuSK autoantibody production by specific B cell subsets. Our recent findings show that abnormally high frequencies of self-reactive naive B-cells accumulate in MuSK MG, indicating a breach in immune tolerance. Consistent with these tolerance defects, we provide evidence of a distorted naive B cell repertoire that influences the memory B cell compartment. Finally, through the study of patients receiving rituximab, we describe features of circulating memory B cells and plasmablasts that directly contribute to MuSK autoantibody-production.
B cell tolerance defects in MuSK MG
During early B cell development, immunoglobulin variable-region gene segments are stochastically recombined to generate functional antibodies that are expressed on the cell surface as B cell receptors (BCRs). This process is fundamental for generating the wide diversity of the immunoglobulin repertoire, but it also creates autoreactive B cells alongside those that make up the non-self-reactive naive repertoire. To evade the development of an immune response against self, two separate tolerance mechanisms counterselect B cells during their development.21 The first is a central tolerance checkpoint in the bone marrow between the early immature and immature B cell development stages, which removes a large population of B cells that express self-reactive BCRs.22 The second checkpoint selects against self-reactive new emigrant/transitional B cells before they enter the mature naive B cell compartment. Defects in the integrity of these tolerance mechanisms can be demonstrated through quantifying the frequency of both polyspecific and autoreactive B cells downstream of each checkpoint. A considerable number of autoimmune diseases include central and peripheral B cell checkpoints that fail to enforce tolerance,23–25 suggesting that the defect may be a fundamental requirement of autoimmunity.
Despite the detailed understanding of the autoantibody-mediated pathophysiology of MuSK MG, little is known regarding how defects in autoimmune regulation, namely tolerance, contribute to MuSK MG. In recent work, we asked whether the naive B cell repertoire in MG shows evidence of compromised tolerance due to checkpoint defects, leading to the accumulation of autoreactive B cells in the naive compartment.26 Our study included MuSK MG subjects and was designed to determine whether B cell tolerance checkpoint integrity was preserved in this form of MG. A well-described approach22,27–29 to assess the two tolerance checkpoints guiding B cell development was used. This approach was applied to measure the frequency of polyreactive and autoreactive BCRs in the naive repertoire, which are inversely correlated with the extent of negative selection, thereby providing an assessment of checkpoint integrity.
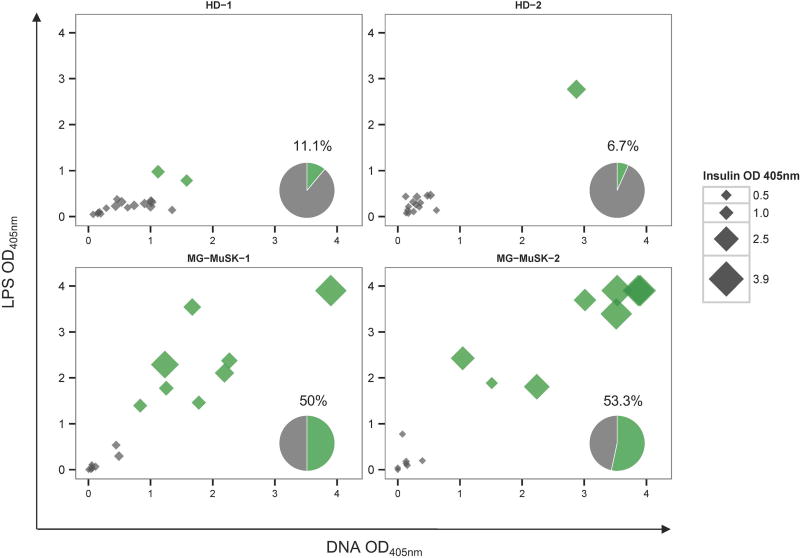
As a means to display the assay output, we show an example of data we collected in evaluating the central tolerance checkpoint in MuSK MG. The checkpoint integrity was measured by determining the frequency of new emigrant/transitional B cells that were characterized as polyreactive by displaying reactivity toward three structurally distinct antigens: double-stranded DNA (dsDNA), lipopolysaccharide (LPS), and insulin. The fraction of B cells that were positive for binding all three antigens from the healthy controls was compared to the same fraction found in the MuSK MG study subjects (Fig. 1). The median fraction of polyreactive B cells in the healthy controls was 8.9%, while in MuSK MG the fraction was 51.7%. Overall, this study26 revealed that the MuSK MG subtype harbors defects in both central and peripheral B cell tolerance checkpoints.
Figure 1.
The fidelity of the B cell tolerance checkpoints is compromised in patients with MuSK MG. A representative example of data analysis used to determine central B cell tolerance checkpoint fidelity is shown. The integrity of the tolerance checkpoint was examined using a well-established assay, which operates by quantifying the fraction of naive B cell subsets that are polyreactive. Recombinant antibodies, representing the BCR, were generated from single new emigrant/transitional (CD19+ CD21lo CD10+ IgMhi CD27−) B cells (which are downstream of the central B cell tolerance checkpoint) derived from two MuSK MG patients (MG-MuSK-1, MG-MuSK-2) and two healthy controls (HD-1 and HD-2). The antibodies were then tested for reactivity with a set of three structurally distinct antigens (dsDNA, lipopolysaccharide (LPS), and insulin) by enzyme-linked immunosorbent assay (ELISA). The consolidated three-dimensional plots summarize the reactivity of each antibody from the subjects toward each antigen in the ELISA. The absorbance values for LPS (y-axis), dsDNA (x-axis), and insulin (data point size) are plotted together. Green symbols indicate antibodies that were positive for binding all three antigens (defined as polyreactive), and gray symbols indicate those that were not positive for all three antigens. The values and pie charts shown in the bottom right corner of each graph indicate the fraction of antibodies that were polyreactive (in green). Adapted from Ref. 26.
Defective B cell tolerance may be a fundamental contributor to autoimmunity in MuSK MG. It is interesting to speculate that dysfunctional tolerance is among the requirements needed for the disease to develop. The population of self-reactive naive B cells that accumulate due to defective tolerance checkpoints in MuSK MG may indirectly contribute to the production of autoantibodies by serving as precursors for autoantibody-producing B cells. Conceivable approaches for testing such a hypothesis could include extensively testing the reactivity of the naive repertoire to MuSK and applying the tolerance assay, described above, to germline precursors of antigen-experienced, somatically hypermutated human MuSK autoantibody–producing B cells. Given the pronounced clinical improvement commonly observed with rituximab-mediated B cell depletion in MuSK MG, it is reasonable to question the ability of rituximab to restore checkpoint fidelity in MuSK MG, although this remains to be tested. It has been shown that B cell depletion in type 1 diabetes, where B cell tolerance is defective, does not result in B cell tolerance checkpoint fidelity restoration.30 Consequently, in MuSK MG, it is anticipated that B cell depletion with rituximab would fail to correct elevated frequencies of polyreactive/autoreactive naive B cells. If these polyreactive/autoreactive naive B cells are indeed precursors of MuSK autoantibody producers, one could assume that the durability of MG treatment strategies that eliminate B cells may be not be long-lasting. The failure to reset tolerance checkpoint defects and eliminate the self-reactive pool may contribute to relapses, thus underlining the need for biomarkers serving as relapse predictors.
The circulating B cell repertoire in MuSK MG is distorted
High-throughput adaptive immune receptor repertoire sequencing (AIRR-seq) allows for the comprehensive evaluation of BCR repertoire properties in health and disease. It provides the depth necessary to adequately depict large populations, such as the circulating peripheral repertoire, which includes up to 1 × 1011 B cells in humans.31 An increasing compendium of AIRR-seq studies have shown altered repertoires in a number of diseases, including celiac disease,32 IgG4-related disease,33 systemic lupus erythematosus,34 and multiple sclerosis.35
We recently applied this approach to compare the broad repertoire features of both the naive and memory B cell compartments in MuSK MG patients with those of healthy donors (HDs).36 We reasoned that a disease driven by autoantibodies may have conspicuous abnormalities present in the circulating B cell repertoire. We focused on determining whether the MuSK MG repertoires included abnormal clonal expansions, skewed immunoglobulin gene usage (including the H, κ, and λ V(D)J loci), and distinctive antigen-binding region properties. We found that large, dominant clonal lineages were not readily apparent in the memory compartment of MuSK MG repertoires. However, several repertoire-scale characteristics consistent with dysregulation of B cell development and selection were evident.
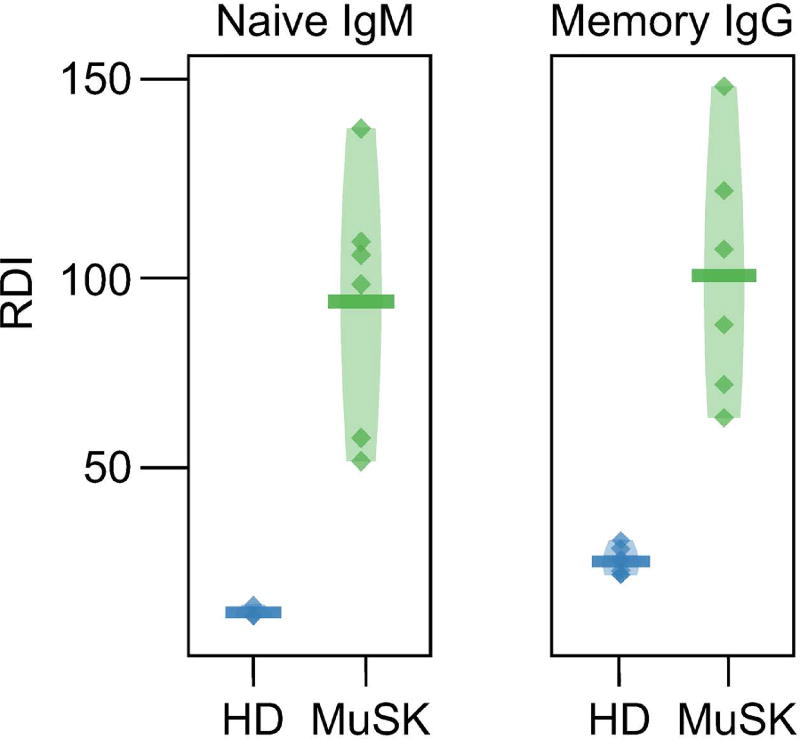
The most prominent repertoire abnormality involved the biased use of variable (V) region gene segments. We observed significant shifts in the relative abundance and variability of each V gene family between MuSK MG and healthy donors.36 In the naive compartment of MuSK MG subjects, V-family biases were observed in the form of decreased usage of IGHV3 as well as an increase in IGHV1 and IGHV4 usage. The same V-family biases observed in naive MG repertoires were apparent in the class-switched memory populations, particularly in the case of the IgG isotype. The IGHV usage biases in the naive compartment of MuSK MG subjects were also associated with an increase in the variability of IGHV usage across different MuSK MG patients. To quantify this effect, we compared V-family variability using the repertoire dissimilarity index (RDI).37 The RDI scores the aggregate difference in gene usage between any pair of subjects (within the HD or MuSK MG cohort), providing a measure of how dissimilar two gene usage distributions are from each other. MuSK MG repertoires display considerably higher RDIs and more individual RDI variability within both the naive and memory compartments compared with HD repertoires, suggesting that B cell developmental abnormalities in MG may be partially patient specific (Fig. 2). Furthermore, the most pronounced difference was observed within the naive compartment, where the naive HD repertoires show remarkable consistency in IGHV usage in contrast to MuSK MG repertoires. Overall, these results show that the naive MuSK MG repertoire is abnormally formed and appears to propagate deformations into the postgerminal center memory compartment for which it is a precursor.
Figure 2.
Immunoglobulin variable-region gene segment usage is skewed in MuSK MG repertoires. Heavy-chain V gene family usage variability for the naive and memory B cell compartments. Usage variability is represented by the repertoire dissimilarity index (RDI) for naive (Naive-IgM) and memory (Memory-IgG) V families (IGHV1 thru IGHV5) among healthy donors (HDs) and MuSK MG patients. Each point indicates a pairwise RDI score (y-axis) for two subjects, with the mean score for each set of subjects marked by a horizontal bar and a density estimate indicated by the shaded region. RDI scores are grouped by status comparisons for HD in blue and MuSK-MG (MuSK) in green. Adapted from Ref. 36.
These findings fit well with the defects we observed in the fidelity of MuSK MG B cell tolerance mechanisms. Given that properly functioning tolerance checkpoints affect counterselection of a considerable fraction of developing B cells,21 it is reasonable to conclude that unique BCR repertoire characteristics would be conspicuous in a naive B cell compartment that is shaped without proper tolerance regulation. Consequently, we suggest that these repertoire abnormalities are consistent with, and may be reflective of, inadequate counterselection during B cell development in MuSK MG.
MuSK autoantibody production: lessons from B cell depletion therapy
The specific B cell subsets that produce MuSK MG autoantibodies have been inadequately defined. We studied such subsets in MuSK MG patients treated with rituximab who relapsed after having achieved sustained remission.38 Because of the pronounced clinical improvement commonly observed with CD20 depletion in MuSK MG, immunosuppressive agents, such as steroids, mycophenolate mofetil, azathioprine, and other broad-acting agents, can be tapered and often completely withdrawn.16,19,39 Once the confounding effects of such therapeutics are absent, the reemergence and evolution of the B cell compartment can be investigated and associated with clinical status. The postdepletion period leading up to relapse may reflect mechanisms transpiring in the early stages of the disease.
It is recognized that B cell populations that do not express CD20, such as long-lived plasma cells situated in the bone marrow, produce a considerable portion of circulating immunoglobulin.40 Given the absence of CD20 on their surface, they are neither directly (by cell lysis) nor indirectly (by depletion of their CD20+ progenitors) affected by rituximab. This is supported by unchanged immunization-induced and plasma cell–dependent antibody titers to microbial antigens, such as tetanus, varicella, and pneumococcus, following B cell depletion.16,41,42 Accordingly, the markedly diminished MuSK autoantibody titer, as early as 3 months after rituximab-mediated CD20+ B cell depletion,16 suggests that long-lived plasma cells are unlikely candidates for MuSK autoantibody production. Rather, short-lived antibody-secreting cells, such as plasmablasts, are more viable candidates. As only a small fraction of these cells expresses CD20, the effectiveness of rituximab in MuSK MG may depend upon depleting a pool of plasmablast-progenitor CD20+ memory B cells.43,44 One alternative version of this model takes into consideration that a fraction of plasmablasts43 may be CD20+; consequently, the autoantibody-expressing plasmablast fraction may be directly depleted by rituximab.
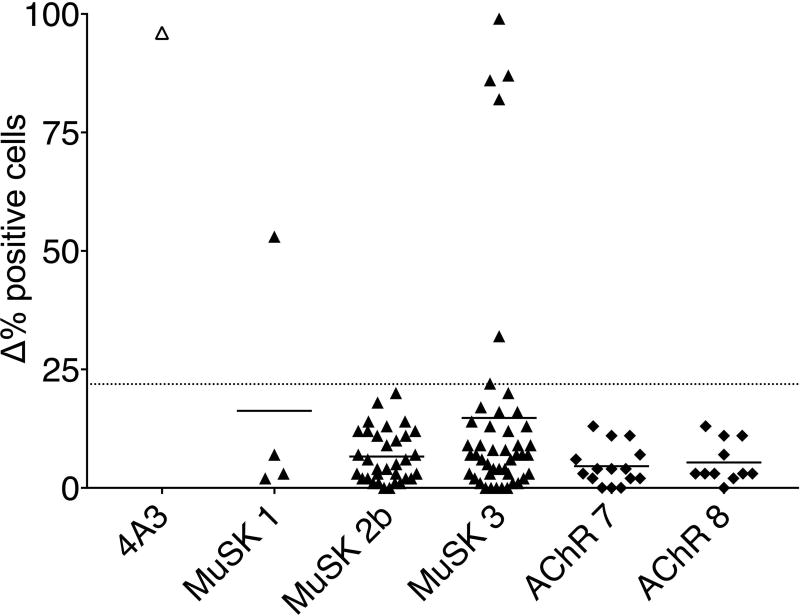
We were able to support this hypothesis through our recent observations of MuSK MG patients who experienced a post-rituximab relapse.38 To investigate the details of autoantibody production and thereby refine the mechanisms of cellular immunopathology in MuSK MG, we tested whether antigen-specific memory B cells and autoantibody-expressing plasmablasts emerge during disease relapse. We first isolated CD27+ B cells from post-rituximab relapse MuSK MG patients and controls and stimulated them with CpG, IL-21, IL-6, and CD40L to induce differentiation into antibody-producing cells. We showed that the CD27+ B cell population derived from MuSK MG relapse patients, but not those of the controls, were capable of MuSK autoantibody production upon stimulation. Since CD27+ B cells can include both memory and short-lived antibody-secreting cells (plasmablasts), we next focused exclusively on the plasmablasts in order to determine whether they contributed to autoantibody production. Employing multi-chromatic flow cytometry, we found mildly elevated plasmablast populations (defined as CD19+CD27hiCD38hi cells34) in MuSK MG patients experiencing disease exacerbation/relapse. From these populations, single plasmablasts were sorted into individual wells in 96-well plates. Antibody heavy- and light-chain variable regions were then amplified and cloned so that monoclonal recombinant antibodies (mAb), each representing a single plasmablast, could be expressed. The evaluation of binding to MuSK-, clustered AChR- and GFP-transfected human embryonic kidney cells showed that a considerable number (seven) of the 82 MuSK plasmablast-derived mAbs, but none of the 26 control (AChR-MG) plasmablast-derived mAbs, bound specifically to MuSK (Fig. 3).
Figure 3.
Summary of MuSK cell-based assay data performed with plasmablast-derived monoclonal antibodies (mAbs). Results are presented as Δ% positive (for MuSK binding) cells on the y-axis. Δ% positive cells = (% frequency of positive MuSK–GFP–transfected cells/% frequency of MuSK–GFP–transfected cells) – (% frequency of positive GFP–transfected cells/% frequency of GFP–transfected cells). Bars represent means, and dots represent individual mAb values. Testing of all samples was performed in duplicate. Plasmablast-derived mAb from MuSK MG post-rituximab relapse patients MuSK 1 (n = 4), MuSK 2b (n = 33), MuSK 3 (n = 45) and AChR control patients AChR 7 (n = 15) and AChR 8 (n = 11) are shown. The 4A3 mAb is a humanized, murine hybridoma–derived MuSK-specific mAb that was used as a positive control. The dotted line represents the Δ% positive cells cutoff (control mean + 4SD = 21.9). Adapted from Ref. 38.
Of the seven MuSK-specific mAbs we identified, six were derived from the same patient’s plasmablast compartment. Of these six, three were individual members of a clone, while the remaining three were unique clones. With these early-stage findings in mind, we reasoned that the MuSK-specific plasmablast response was polyclonal/oligoclonal in that patient. Given that these mAbs were derived from several different clones, a continuum of binding strengths is not unexpected. Indeed, the plasmablast-derived mAbs displayed a range of MuSK-binding capacities. With some behaving as strong binders, others appeared to be relatively weaker while remaining positive compared with the AChR-derived controls, all of which were negative. This range of binding strengths is in agreement with other studies of human monoclonal recombinant immunoglobulins derived from patients with autoantibody-mediated diseases. This includes pemphigus,45,46 neuromyelitis optica,47 and AChR MG,48 all of which include mAbs reported to exhibit a range of binding capacities. What is important to consider in addition to the binding strength of these MuSK-specific mAbs, which was measured with an in vitro assay, is understanding the relationship between their binding strength and their pathogenic capacity. It would be presumptuous to assume that those with lower binding capacity may not be pathogenic, given that human-derived MuSK mAbs have not yet been studied. This gap in our knowledge highlights the value of these mAbs as tools for improving our understanding of MuSK MG pathology. We anticipate that their role in pathology will be evaluated using in vitro models of AChR-mediated signaling activity and broader effects through in vivo studies with rodent models. In addition, they will be useful in furthering our understanding of how the structural characteristics of MuSK mAbs of the IgG4 subclass play a role in their antigen recognition and pathogenicity. In particular, an interesting feature of secreted IgG4 antibodies48 is their ability to engage in “Fab-arm exchange,” where a monospecific IgG4 antibody may swap a heavy- and light-chain pair with another IgG4 to become bispecific.49 The biological consequences of Fab-arm exchange are not yet fully understood. It has recently been shown that Fab-arm–exchanged autoantibodies are present in MuSK MG.12 Their presence in patients further highlights that our MuSK-specific mAbs, which display a range of binding capacities, may play important roles in MuSK MG pathology. This is because one arm of the autoantibody may be swapped out for another with a different specificity (perhaps also targeting the neuromuscular junction), producing a bivalent autoantibody with an augmented binding capacity compared with the parent MuSK monovalent mAb.
In summary, these collective results suggest that oligoclonal-circulating plasmablasts contribute to MuSK autoantibody production. Furthermore, these cells appear to emerge in the reconstituted B cell repertoire of patients experiencing relapse after B cell depletion therapy. To the best of our knowledge, these represent the first reported human-derived MuSK-specific mAbs,38 and the results provide a more granular understanding of the mechanisms governing MuSK autoantibody production.
A speculative model of MuSK MG immunopathology
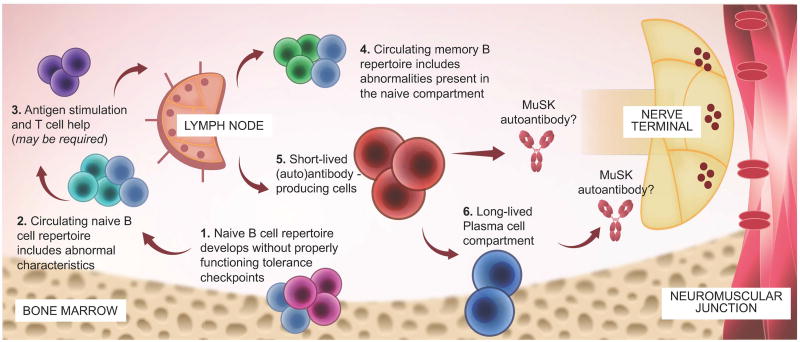
Taken together, the findings detailed above support a speculative model describing the role of B cell subsets in MuSK MG immunopathology (Fig. 4). In brief, the model suggests that pathology begins with the accumulation of autoreactive naive B cells due to defective central and peripheral tolerance checkpoints.26 The memory B cell compartment repertoire reflects abnormalities of the deformed naive repertoire.36 In addition, we postulate that this deformed memory repertoire harbors MuSK-specific B cells. The memory B cell pool can then supply the antibody-secreting B cell pool, which comprises a spectrum of mainly CD20− short-lived (plasmablasts) and long-lived antibody secretors (plasma cells). Our more recent study points toward the role of such peripheral blood plasmablasts in MuSK autoantibody production.38 Apart from our recent laboratory-based findings, this model is supported by clinical observations in different diseases associated with IgG4 autoantibodies, including MuSK MG, pemphigus, thrombotic thrombocytopenic purpura, and membranous nephropathy. Such observations include the rather rapid decrease of disease-specific autoantibody titers occurring after B cell depletion with rituximab.16,39,50–52 On the basis of our proposed model, this decrease could be attributed to the depletion of CD20+ memory B cells and the consequent ablation of the continuous supply of antibody-secreting cells. The terminated supply would affect a decrease in the population of short-lived antibody producers. Alternatively, a fraction of plasmablasts may be CD20+,43 the consequence of which is their direct depletion, which contributes to decreased titers and a favorable clinical response.
Figure 4.
Speculative mechanistic hypothesis for the production of MuSK MG autoantibodies. The proposed mechanistic path to autoantibody production in MuSK MG begins with naive B cells (steps 1 and 2). These cells develop in the presence of defective B cell tolerance checkpoints, which results in a naive population that includes an abnormally high population of self-reactive cells and a skewed repertoire (indicated by light blue cells). Activation and differentiation of the naive compartment to effector B cell populations (memory and antibody-secreting) may require antigen exposure and help from T cells (step 3), but direct evidence of such in MuSK MG is required. The memory B cell compartment (step 4) includes a skewed repertoire closely resembling that of the abnormal naive compartment (indicated by light blue cells). Circulating short-lived plasmablasts and bone marrow–inhabiting plasma cells (steps 5 and 6) may contribute to MuSK MG autoantibody production. However, observations following B cell depletion therapy with rituximab in MuSK MG suggests that plasma cells may not play a major role. B cell depletion therapy eliminates CD20+ memory and naive B cells but does not directly eliminate plasmablasts or plasma cells, the majority of which do not express appreciable levels of CD20. After CD20-targeted depletion, serum autoantibody titers markedly diminish in MuSK MG, suggesting that long-lived plasma cells are unlikely candidates for autoantibody production. Rather, short-lived plasmablasts are more viable candidates. As only a small fraction of these cells express CD20, the effectiveness of B cell depletion therapy may depend upon depletion of a pool of plasmablast-progenitor CD20+ memory B cells. Conversely, autoantibody titers that remain elevated following CD20 targeted depletion may be the product of longer-lived plasma cells.
The model mechanism we propose for describing the post-rituximab MuSK autoantibody titer decrease is well supported by observations in other IgG4 autoantibody–related conditions. These conditions could be viewed as a disease group that shares common pathogenic characteristics. Among those, pemphigus is a well-characterized and extensively studied autoimmune mucocutaneous blistering disease with an immunopathological profile that is in many ways parallel to that of MuSK MG. First, pemphigus pathology is mediated by autoantibodies against desmosome proteins desmoglein (DSG)-1 and DSG3.53 These autoantibodies are predominately of the IgG4 subclass (and secondarily of the IgG1 subclass as in MuSK MG) and correlate with disease activity.50,54–59 Second, pemphigus responds well to B cell depletion with rituximab and does so in a manner that is quite similar to MuSK MG.50,56–58,60 Importantly, rituximab induces a significant decline in serum anti-DSG1 and anti-DSG3 antibodies along with clinical improvement.50,56,60,61 In addition to pemphigus, analogous clinical and serological responses to rituximab have been reported in thrombotic thrombocytopenic purpura (TTP) and membranous nephropathy.51,52 In TTP, rituximab induces a significant decrease of the predominantly IgG4, pathogenic disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13- (ADAMTS13-) autoantibodies, accompanied by recovery in ADAMTS13 activity and clinical improvement.51,62 Similarly, in membranous nephropathy, the PLA2R antibodies, which are primarily of the IgG4 subclass,63 decrease with rituximab therapy.52,64 Collectively, these data provide evidence that rituximab affects a decrease of IgG4 autoantibody titers in a variety of similar autoimmune disorders. It could therefore be inferred that our proposed model, which features short-lived plasmablasts in MuSK MG cellular pathogenesis, is similar to those that describe other IgG4 autoantibody-mediated diseases, such as pemphigus, TTP, and membranous nephropathy.
Clinical relapses after achieving remission with rituximab and associated biomarkers are interesting and relevant points of comparison between MuSK MG and pemphigus or membranous nephropathy. Since pemphigus has been studied more extensively than MuSK MG, and given that the two conditions are analogous, as described above, clinical and serological observations in pemphigus could inform anticipated responses to rituximab in MuSK MG. Our group has been following a rituximab-treated MuSK MG cohort for almost 10 years.19,38,39 Our experience is comparable with that of other centers (Table 2). Some larger series16,39 did not report relapses with a median follow-up that was approximately 2–4 years. In contrast to these series, isolated patients that have relapsed have been reported with similar follow-up periods.19,65,66 Moreover, we recently reported three such relapses.38 In pemphigus, clinical relapses post-rituximab vary from a reported low of 0–20% (intensive rituximab dosing combined with IVIg and 10 years of follow-up)57 to 50–80% (occurring on average after 1.5 years with standard rituximab dosing).57,58 Relapsing pemphigus patients had either increased or persistently high levels of pemphigus autoantibodies.56–58 Furthermore, in membranous nephropathy, an increase or reemergence of PLA2R antibodies predicted clinical relapse.64 In our recent report,38 all MuSK MG patients experiencing relapse had measurable MuSK autoantibodies. Given that MuSK MG autoantibodies show correlations with clinical fluctuations,9,59 they could provide a useful post-rituximab relapse biomarker as in the case of pemphigus and membranous nephropathy.67
Table 2.
Summary of the available literature for the use of rituximab in MuSK MG.
| Study | N | Protocol | Follow up (months) |
MGFA-PIS at the end of follow up |
Relapse (n) |
|---|---|---|---|---|---|
| Hain74 | 1 | 375 mg/m2 weeks 1, 2, 3, and 4 and months 2, 3, 4, and 5 | 12 | I | 0 |
| Lin75 | 1 | 375 mg/m2 weeks 1, 2, 3, and 4 | 18 | U | 0 |
| Thakre76 | 1 | 375 mg/m2 weeks 1, 2, 3, and 4 (cycle); cycle Q6M | 13 | CSR | 0 |
| Baek77 | 1 | 375 mg/m2 weeks 1, 2, 3, and 4 (cycle); cycle Q6M 1×, 375 mg/m2 Q10W for 6 months | 18 | CSR | 0 |
| Illa18 | 3 | 375 mg/m2 weeks 1, 2, 3, and 4; Q2M | 9 | MM | 0 |
| Lebrun 200917 | 3 | 375 mg/m2 weeks 1, 2, 3, and 4; Q2M for 6 months | 12–25 | CSR, MM | 0 |
| Zebardast20 | 4 | 375 mg/m2 weeks 1, 2, 3, 4, 5, and 6 (1 cycle); repeat after 1 year (weeks 1, 2, 3, 4, and 5) | 13–27 | CSR, PR, MM | 0 |
| Maddison78 | 3 | 375 mg/m2 weeks 1, 2, 3, and 4; Q1M for 3 months | 4–18 | PR, I | 0 |
| Blum65 | 3 | 500 mg/m2 w1, 3 or 500 mg/m2 weeks 1, 2, 3, and 4 or 500 mg/m2 week 1 (last two patients relapsed) | 12–47 | CSR, PR | 2 |
| Stein79 | 2 | 1000 mg/m2 months 1 and 2 or 375/m2 weeks 1, 2, 3, and 4 | 14–18 | PR | 0 |
| Burusnukul80 | 2 | 375/m2 weeks 1, 2, 3, and 4 | 21–24 | CSR | 0 |
| Nowak19 | 8 | 375 mg/m2 weeks 1, 2, 3, and 4 (cycle); cycle Q6M | 12–30 | CSR, PR, MM, I | 2 |
| Guptill66 | 6 | 375 mg/m2 weeks 1, 2, 3, and 4, then Q1M for 1–2 months. Repeat in the case of relapse | 2–40 | MM, PR, I | 1 |
| Diaz–Manera16 | 6 | 375 mg/m2 weeks 1, 2, 3, and 4, Q2M for 2 months, retreat if symptomatic | 6–60 | CSR, PR, MM | 0 |
| Catzola81 | 1 | 375 mg/m2 weeks 1, 2, 3, and 4 and 3 months | 20 | MM | 0 |
| Keung39 | 9 | 375 mg/m2 weeks 1, 2, 3, and 4 (cycle), cycle Q6M stop at 2–5 cycles | 26–72 | CSR, MM | 0 |
| Yi82 | 1 | 1000 mg/m2 × 6 in 8 weeks | 34 | W | 0 |
| Guptill14 | 3 | Not available | 13–36 | MM, I | 0 |
| Govindarajan83 | 1 | 375 mg/m2 weeks 1, 3, 13, and 23 | 15 | CSR | 0 |
| Stathopoulos38 | 4 | 375 mg/m2 weeks 1, 2, 3, abd 4, repeat in 6 months (cycle). Then repeat in the case of relapse. | 25–96 | CSR, E | 3 |
CSR, complete stable remission; I, improved; MGFA, MG Foundation of America clinical classification; MM, minimal manifestations; PIS, postintervention status classification; PR, pharmacologic remission; Q1M, Every 1 month; Q2M, every 2 monthsnths; Q6M, every 6 months; Q10W, every 10 weeks; U, unchanged; W, worse
Our proposed model postulates that autoreactive naive B cells differentiate into memory B cells with the help of MuSK-specific T cells. Further, the distorted memory B cell compartment likely includes MuSK-specific memory B cells that differentiate to MuSK autoantibody–secreting cells. Given the analogies of MuSK MG and pemphigus, our model is supported by studies that have explored antigen-specific B and T cell repertories in pemphigus. Specifically, circulating DSG1- and DSG3-specific-IgG+ B cells were examined before and after rituximab. B cells were stained with fluorescently labeled recombinant DSG1 or DSG3, and their frequency was determined by flow cytometry. Clinical and serological response correlated with disappearance of circulating DSG-specific IgG+ B cells, whereas relapsing patients showed high levels of the same.58,60 Moreover, peripheral blood immunophenotyping in these responding patients showed an increase of CD19+ CD27− naive B cells and a decrease in CD19+ CD27+ memory B cells. In patients with long-lasting remissions, circulating DSG-specific B cells could not be detected. In addition to B cell studies, the T cell compartment has also been explored in pemphigus.61 Analysis of DSG3-reactive T cell subsets showed that, while rituximab did not alter the overall pool of peripheral T cells, DSG3-specific CD4+ T cells declined significantly. One potential explanation for this effect on T cells is the removal of CD20+ B cells that function as antigen-presenting cells (APCs). Moreover, the decrease of DSG3-specific CD4+ T cells preceded the decline in DSG3 serum autoantibodies. The presence of antigen-specific cells (T and B) that parallel disease activity in pemphigus supports the existence of pathogenic, MuSK-specific memory B cells and T helper cells, given the similarities between the two disorders.
Additional data supporting our MuSK MG model, in the context of B cell depletion, is found in the field of IgG4-related disease (IgG4-RD). IgG4-RD is a multiorgan immune-mediated disorder characterized by IgG4 lymphoplasmacytic infiltration of affected tissues and fibrosis.68,69 Many patients show elevated serum IgG4,69 but its role is unknown. In contrast to MuSK MG, there is no known antigenic target. Nevertheless, IgG4-RD activity correlates with the presence of peripheral, clonally expanded IgG4 plasmablasts.70 Moreover, IgG4-RD responds to B cell depletion with rituximab.71 Rituximab decreases total serum IgG4 antibodies, but also reduces IgG4-expressing plasma cells in tissue.71 Interestingly, peripheral blood plasmablasts decline during remission and rise before clinical relapse70 and are thus a potentially useful biomarker for disease monitoring during rituximab therapy.72 The post-rituximab disappearance of CD20− plasmablasts and plasma cells suggests that these populations are short-lived and continuously supplied by a CD20+ memory pool that is depleted by rituximab.
In conclusion, the mechanistic model that we have developed to describe the contributions of B cells to MuSK MG immunopathology spans early B cell development through MuSK autoantibody production. To date, we have collected highly relevant data in support of this model. Specifically, we identified a self-reactive naive B repertoire in MuSK MG patients that develops due to defective central and peripheral checkpoints. Moreover, AIRR-seq of the B cell repertoire revealed that the antigen-experienced B compartment includes abnormalities present in the naive compartment, which is shaped by defective tolerance checkpoints. Finally, we have recently identified MuSK-producing plasmablasts from the re-emergent repertoire of patients experiencing post-rituximab relapse. We believe that these findings advance the mechanistic understanding of MuSK MG immunopathology. Moreover, our model is supported by clinical, serological, and immunophenotypical observations with rituximab therapy in other IgG4-mediated autoimmune disorders. However, certain antigen-specific components of this model, in particular the presence of MuSK-specific cells within the memory B cell and T cell compartments, await validation by further study.
Acknowledgments
This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health through a grant to K.C.O. under Award Number R01AI114780 and was supported, in part, by a pilot research award to K.C.O. from Conquer Myasthenia Gravis. R.J.N. is supported, in part, by the National Institute of Neurological Disorders and Stroke (NINDS) of the National Institutes of Health under Award Number U01NS084495. J.A.V.H. is supported, in part, by National Institutes of Health Award Number R01AI104739. The authors thank Karen Boss for editorial assistance.
Competing interests
K.C.O. has received personal compensation in the past year from Genentech for educational activities and from Proclara Biosciences and Editas Medicine for consulting services. R.J.N. reports support through an investigator-initiated trial agreement from Genentech for placebo/drug for the currently underway clinical trial (www.clincial trials.gov, NCT02110706).
References
- 1.Yi JS, et al. B cells in the pathophysiology of myasthenia gravis. Muscle Nerve. 2017 doi: 10.1002/mus.25973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phillips WD, Vincent A. Pathogenesis of myasthenia gravis: update on disease types, models, and mechanisms. F1000Res. 2016;5 doi: 10.12688/f1000research.8206.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patrick J, Lindstrom J. Autoimmune response to acetylcholine receptor. Science. 1973;180:871–872. doi: 10.1126/science.180.4088.871. [DOI] [PubMed] [Google Scholar]
- 4.Hoch W, et al. Auto-antibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. Nat Med. 2001;7:365–368. doi: 10.1038/85520. [DOI] [PubMed] [Google Scholar]
- 5.Higuchi O, et al. Autoantibodies to low-density lipoprotein receptor-related protein 4 in myasthenia gravis. Ann Neurol. 2011;69:418–422. doi: 10.1002/ana.22312. [DOI] [PubMed] [Google Scholar]
- 6.Klooster R, et al. Muscle-specific kinase myasthenia gravis IgG4 autoantibodies cause severe neuromuscular junction dysfunction in mice. Brain. 2012;135:1081–1101. doi: 10.1093/brain/aws025. [DOI] [PubMed] [Google Scholar]
- 7.Viegas S, et al. Passive and active immunization models of MuSK-Ab positive myasthenia: electrophysiological evidence for pre and postsynaptic defects. Exp Neurol. 2012;234:506–512. doi: 10.1016/j.expneurol.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 8.Shigemoto K, et al. Induction of myasthenia by immunization against muscle-specific kinase. J Clin Invest. 2006;116:1016–1024. doi: 10.1172/JCI21545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niks EH, et al. Clinical fluctuations in MuSK myasthenia gravis are related to antigen-specific IgG4 instead of IgG1. J Neuroimmunol. 2008;195:151–156. doi: 10.1016/j.jneuroim.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Ohno K, Otsuka K, Ito M. Roles of collagen Q in MuSK antibody-positive myasthenia gravis. Chem Biol Interact. 2016;259:266–270. doi: 10.1016/j.cbi.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 11.Koneczny I, et al. MuSK myasthenia gravis IgG4 disrupts the interaction of LRP4 with MuSK but both IgG4 and IgG1–3 can disperse preformed agrin-independent AChR clusters. PLoS One. 2013;8:e80695. doi: 10.1371/journal.pone.0080695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koneczny I, et al. IgG4 autoantibodies against muscle-specific kinase undergo Fab-arm exchange in myasthenia gravis patients. J Autoimmun. 2017;77:104–115. doi: 10.1016/j.jaut.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Drachman DB, et al. Myasthenic antibodies cross-link acetylcholine receptors to accelerate degradation. N Engl J Med. 1978;298:1116–1122. doi: 10.1056/NEJM197805182982004. [DOI] [PubMed] [Google Scholar]
- 14.Guptill JT, et al. Characterization of B cells in muscle-specific kinase antibody myasthenia gravis. Neurol Neuroimmunol Neuroinflamm. 2015;2:e77. doi: 10.1212/NXI.0000000000000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kohler S, et al. Disturbed B cell subpopulations and increased plasma cells in myasthenia gravis patients. J Neuroimmunol. 2013;264:114–119. doi: 10.1016/j.jneuroim.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Diaz-Manera J, et al. Long-lasting treatment effect of rituximab in MuSK myasthenia. Neurology. 2012;78:189–193. doi: 10.1212/WNL.0b013e3182407982. [DOI] [PubMed] [Google Scholar]
- 17.Lebrun C, et al. Successful treatment of refractory generalized myasthenia gravis with rituximab. Eur J Neurol. 2009;16:246–250. doi: 10.1111/j.1468-1331.2008.02399.x. [DOI] [PubMed] [Google Scholar]
- 18.Illa I, et al. Sustained response to Rituximab in anti-AChR and anti-MuSK positive Myasthenia Gravis patients. J Neuroimmunol. 2008;201–202:90–94. doi: 10.1016/j.jneuroim.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 19.Nowak RJ, et al. Response of patients with refractory myasthenia gravis to rituximab: a retrospective study. Ther Adv Neurol Disord. 2011;4:259–266. doi: 10.1177/1756285611411503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zebardast N, et al. Rituximab in the management of refractory myasthenia gravis. Muscle Nerve. 2010;41:375–378. doi: 10.1002/mus.21521. [DOI] [PubMed] [Google Scholar]
- 21.Meffre E, Wardemann H. B-cell tolerance checkpoints in health and autoimmunity. Curr Opin Immunol. 2008;20:632–638. doi: 10.1016/j.coi.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Wardemann H, et al. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 23.Samuels J, et al. Impaired early B cell tolerance in patients with rheumatoid arthritis. J Exp Med. 2005;201:1659–1667. doi: 10.1084/jem.20042321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yurasov S, et al. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J Exp Med. 2005;201:703–711. doi: 10.1084/jem.20042251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kinnunen T, et al. Specific peripheral B cell tolerance defects in patients with multiple sclerosis. J Clin Invest. 2013;123:2737–2741. doi: 10.1172/JCI68775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee JY, et al. Compromised fidelity of B-cell tolerance checkpoints in AChR and MuSK myasthenia gravis. Ann Clin Transl Neurol. 2016;3:443–454. doi: 10.1002/acn3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herve M, et al. CD40 ligand and MHC class II expression are essential for human peripheral B cell tolerance. J Exp Med. 2007;204:1583–1593. doi: 10.1084/jem.20062287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Isnardi I, et al. IRAK-4- and MyD88-dependent pathways are essential for the removal of developing autoreactive B cells in humans. Immunity. 2008;29:746–757. doi: 10.1016/j.immuni.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sauer AV, et al. Defective B cell tolerance in adenosine deaminase deficiency is corrected by gene therapy. J Clin Invest. 2012;122:2141–2152. doi: 10.1172/JCI61788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chamberlain N, et al. Rituximab does not reset defective early B cell tolerance checkpoints. J Clin Invest. 2016;126:282–287. doi: 10.1172/JCI83840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glanville J, et al. Precise determination of the diversity of a combinatorial antibody library gives insight into the human immunoglobulin repertoire. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:20216–20221. doi: 10.1073/pnas.0909775106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roy B, et al. High-Throughput Single-Cell Analysis of B Cell Receptor Usage among Autoantigen-Specific Plasma Cells in Celiac Disease. J Immunol. 2017;199:782–791. doi: 10.4049/jimmunol.1700169. [DOI] [PubMed] [Google Scholar]
- 33.Maillette de Buy Wenniger LJ, et al. Immunoglobulin G4+ clones identified by next-generation sequencing dominate the B cell receptor repertoire in immunoglobulin G4 associated cholangitis. Hepatology. 2013;57:2390–2398. doi: 10.1002/hep.26232. [DOI] [PubMed] [Google Scholar]
- 34.Tipton CM, et al. Diversity, cellular origin and autoreactivity of antibody-secreting cell population expansions in acute systemic lupus erythematosus. Nat Immunol. 2015;16:755–765. doi: 10.1038/ni.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palanichamy A, et al. Immunoglobulin class-switched B cells form an active immune axis between CNS and periphery in multiple sclerosis. Sci Transl Med. 2014;6:248ra106. doi: 10.1126/scitranslmed.3008930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vander Heiden JA, et al. Dysregulation of B Cell Repertoire Formation in Myasthenia Gravis Patients Revealed through Deep Sequencing. J Immunol. 2017;198:1460–1473. doi: 10.4049/jimmunol.1601415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rubelt F, et al. Individual heritable differences result in unique cell lymphocyte receptor repertoires of naive and antigen-experienced cells. Nat Commun. 2016;7:11112. doi: 10.1038/ncomms11112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stathopoulos P, et al. Autoantibody-producing plasmablasts after B cell depletion identified in muscle-specific kinase myasthenia gravis. JCI Insight. 2017;2:e94263–e94275. doi: 10.1172/jci.insight.94263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keung B, et al. Long-term benefit of rituximab in MuSK autoantibody myasthenia gravis patients. J Neurol Neurosurg Psychiatry. 2013;84:1407–1409. doi: 10.1136/jnnp-2012-303664. [DOI] [PubMed] [Google Scholar]
- 40.Manz RA, et al. Maintenance of serum antibody levels. Annu Rev Immunol. 2005;23:367–386. doi: 10.1146/annurev.immunol.23.021704.115723. [DOI] [PubMed] [Google Scholar]
- 41.Cambridge G, et al. Serologic changes following B lymphocyte depletion therapy for rheumatoid arthritis. Arthritis Rheum. 2003;48:2146–2154. doi: 10.1002/art.11181. [DOI] [PubMed] [Google Scholar]
- 42.Hall RP, 3rd, et al. Association of serum B-cell activating factor level and proportion of memory and transitional B cells with clinical response after rituximab treatment of bullous pemphigoid patients. J Invest Dermatol. 2013;133:2786–2788. doi: 10.1038/jid.2013.236. [DOI] [PubMed] [Google Scholar]
- 43.Quach TD, et al. Distinctions among Circulating Antibody-Secreting Cell Populations, Including B-1 Cells, in Human Adult Peripheral Blood. J Immunol. 2016;196:1060–1069. doi: 10.4049/jimmunol.1501843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lanzillotta M, Della-Torre E, Stone JH. Roles of Plasmablasts and B Cells in IgG4-Related Disease: Implications for Therapy and Early Treatment Outcomes. Curr Top Microbiol Immunol. 2017;401:85–92. doi: 10.1007/82_2016_58. [DOI] [PubMed] [Google Scholar]
- 45.Ishii K, et al. Isolation of pathogenic monoclonal anti-desmoglein 1 human antibodies by phage display of pemphigus foliaceus autoantibodies. J Invest Dermatol. 2008;128:939–948. doi: 10.1038/sj.jid.5701132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Payne AS, et al. Genetic and functional characterization of human pemphigus vulgaris monoclonal autoantibodies isolated by phage display. J Clin Invest. 2005;115:888–899. doi: 10.1172/JCI24185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bennett JL, et al. Intrathecal pathogenic anti-aquaporin-4 antibodies in early neuromyelitis optica. Ann Neurol. 2009;66:617–629. doi: 10.1002/ana.21802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Graus YF, et al. Human anti-nicotinic acetylcholine receptor recombinant Fab fragments isolated from thymus-derived phage display libraries from myasthenia gravis patients reflect predominant specificities in serum and block the action of pathogenic serum antibodies. J Immunol. 1997;158:1919–1929. [PubMed] [Google Scholar]
- 49.van der Neut Kolfschoten M, et al. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science. 2007;317:1554–1557. doi: 10.1126/science.1144603. [DOI] [PubMed] [Google Scholar]
- 50.Joly P, et al. A single cycle of rituximab for the treatment of severe pemphigus. N Engl J Med. 2007;357:545–552. doi: 10.1056/NEJMoa067752. [DOI] [PubMed] [Google Scholar]
- 51.Scully M, et al. A phase 2 study of the safety and efficacy of rituximab with plasma exchange in acute acquired thrombotic thrombocytopenic purpura. Blood. 2011;118:1746–1753. doi: 10.1182/blood-2011-03-341131. [DOI] [PubMed] [Google Scholar]
- 52.Beck LH, Jr, et al. Rituximab-induced depletion of anti-PLA2R autoantibodies predicts response in membranous nephropathy. J Am Soc Nephrol. 2011;22:1543–1550. doi: 10.1681/ASN.2010111125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kasperkiewicz M, et al. Pemphigus. Nat Rev Dis Primers. 2017;3:17026. doi: 10.1038/nrdp.2017.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rock B, et al. The pathogenic effect of IgG4 autoantibodies in endemic pemphigus foliaceus (fogo selvagem) N Engl J Med. 1989;320:1463–1469. doi: 10.1056/NEJM198906013202206. [DOI] [PubMed] [Google Scholar]
- 55.Futei Y, et al. Predominant IgG4 subclass in autoantibodies of pemphigus vulgaris and foliaceus. J Dermatol Sci. 2001;26:55–61. doi: 10.1016/s0923-1811(00)00158-4. [DOI] [PubMed] [Google Scholar]
- 56.Ahmed AR, et al. Treatment of pemphigus vulgaris with rituximab and intravenous immune globulin. N Engl J Med. 2006;355:1772–1779. doi: 10.1056/NEJMoa062930. [DOI] [PubMed] [Google Scholar]
- 57.Ahmed AR, Kaveri S, Spigelman Z. Long-Term Remissions in Recalcitrant Pemphigus Vulgaris. N Engl J Med. 2015;373:2693–2694. doi: 10.1056/NEJMc1508234. [DOI] [PubMed] [Google Scholar]
- 58.Colliou N, et al. Long-term remissions of severe pemphigus after rituximab therapy are associated with prolonged failure of desmoglein B cell response. Sci Transl Med. 2013;5:175ra130. doi: 10.1126/scitranslmed.3005166. [DOI] [PubMed] [Google Scholar]
- 59.Bartoccioni E, et al. Anti-MuSK antibodies: correlation with myasthenia gravis severity. Neurology. 2006;67:505–507. doi: 10.1212/01.wnl.0000228225.23349.5d. [DOI] [PubMed] [Google Scholar]
- 60.Joly P, et al. First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux 3): a prospective, multicentre, parallel-group, open-label randomised trial. Lancet. 2017;389:2031–2040. doi: 10.1016/S0140-6736(17)30070-3. [DOI] [PubMed] [Google Scholar]
- 61.Eming R, et al. Rituximab exerts a dual effect in pemphigus vulgaris. J Invest Dermatol. 2008;128:2850–2858. doi: 10.1038/jid.2008.172. [DOI] [PubMed] [Google Scholar]
- 62.Froissart A, et al. Efficacy and safety of first-line rituximab in severe, acquired thrombotic thrombocytopenic purpura with a suboptimal response to plasma exchange. Experience of the French Thrombotic Microangiopathies Reference Center. Crit Care Med. 2012;40:104–111. doi: 10.1097/CCM.0b013e31822e9d66. [DOI] [PubMed] [Google Scholar]
- 63.Beck LH, Jr, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ruggenenti P, et al. Anti-Phospholipase A2 Receptor Antibody Titer Predicts Post-Rituximab Outcome of Membranous Nephropathy. J Am Soc Nephrol. 2015;26:2545–2558. doi: 10.1681/ASN.2014070640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blum S, et al. Use and monitoring of low dose rituximab in myasthenia gravis. J Neurol Neurosurg Psychiatry. 2011;82:659–663. doi: 10.1136/jnnp.2010.220475. [DOI] [PubMed] [Google Scholar]
- 66.Guptill JT, Sanders DB, Evoli A. Anti-MuSK antibody myasthenia gravis: clinical findings and response to treatment in two large cohorts. Muscle Nerve. 2011;44:36–40. doi: 10.1002/mus.22006. [DOI] [PubMed] [Google Scholar]
- 67.Abasq C, et al. ELISA testing of anti-desmoglein 1 and 3 antibodies in the management of pemphigus. Arch Dermatol. 2009;145:529–535. doi: 10.1001/archdermatol.2009.9. [DOI] [PubMed] [Google Scholar]
- 68.Deshpande V, et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 2012;25:1181–1192. doi: 10.1038/modpathol.2012.72. [DOI] [PubMed] [Google Scholar]
- 69.Kamisawa T, et al. IgG4-related disease. Lancet. 2015;385:1460–1471. doi: 10.1016/S0140-6736(14)60720-0. [DOI] [PubMed] [Google Scholar]
- 70.Mattoo H, et al. De novo oligoclonal expansions of circulating plasmablasts in active and relapsing IgG4-related disease. J Allergy Clin Immunol. 2014;134:679–687. doi: 10.1016/j.jaci.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Della-Torre E, et al. B-cell depletion attenuates serological biomarkers of fibrosis and myofibroblast activation in IgG4-related disease. Ann Rheum Dis. 2015;74:2236–2243. doi: 10.1136/annrheumdis-2014-205799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wallace ZS, et al. Plasmablasts as a biomarker for IgG4-related disease, independent of serum IgG4 concentrations. Ann Rheum Dis. 2015;74:190–195. doi: 10.1136/annrheumdis-2014-205233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gilhus NE. Myasthenia Gravis. N Engl J Med. 2016;375:2570–2581. doi: 10.1056/NEJMra1602678. [DOI] [PubMed] [Google Scholar]
- 74.Hain B, et al. Successful treatment of MuSK antibody-positive myasthenia gravis with rituximab. Muscle Nerve. 2006;33:575–580. doi: 10.1002/mus.20479. [DOI] [PubMed] [Google Scholar]
- 75.Lin PT, et al. High-dose cyclophosphamide in refractory myasthenia gravis with MuSK antibodies. Muscle Nerve. 2006;33:433–435. doi: 10.1002/mus.20411. [DOI] [PubMed] [Google Scholar]
- 76.Thakre M, Inshasi J, Marashi M. Rituximab in refractory MuSK antibody myasthenia gravis. J Neurol. 2007;254:968–969. doi: 10.1007/s00415-006-0442-2. [DOI] [PubMed] [Google Scholar]
- 77.Baek WS, Bashey A, Sheean GL. Complete remission induced by rituximab in refractory, seronegative, muscle-specific, kinase-positive myasthenia gravis. J Neurol Neurosurg Psychiatry. 2007;78:771. doi: 10.1136/jnnp.2006.093567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maddison P, et al. The use of rituximab in myasthenia gravis and Lambert-Eaton myasthenic syndrome. J Neurol Neurosurg Psychiatry. 2011;82:671–673. doi: 10.1136/jnnp.2009.197632. [DOI] [PubMed] [Google Scholar]
- 79.Stein B, Bird SJ. Rituximab in the treatment of MuSK antibody-positive myasthenia gravis. J Clin Neuromuscul Dis. 2011;12:163–164. doi: 10.1097/CND.0b013e3181df2b3e. [DOI] [PubMed] [Google Scholar]
- 80.Burusnukul P, Brennan TD, Cupler EJ. Prolonged improvement after rituximab: two cases of resistant muscle-specific receptor tyrosine kinase + myasthenia gravis. J Clin Neuromuscul Dis. 2010;12:85–87. doi: 10.1097/CND.0b013e3181fcc109. [DOI] [PubMed] [Google Scholar]
- 81.Catzola V, et al. Changes in regulatory T cells after rituximab in two patients with refractory myasthenia gravis. J Neurol. 2013;260:2163–2165. doi: 10.1007/s00415-013-6987-y. [DOI] [PubMed] [Google Scholar]
- 82.Yi JS, et al. Prolonged B-cell depletion in MuSK myasthenia gravis following rituximab treatment. Muscle Nerve. 2013;48:992–993. doi: 10.1002/mus.24063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Govindarajan R, et al. Selective response to rituximab in a young child with MuSK-associated myasthenia gravis. Neuromuscul Disord. 2015;25:651–652. doi: 10.1016/j.nmd.2015.03.014. [DOI] [PubMed] [Google Scholar]