Abstract
Over the last forty years, there has been tremendous progress in understanding the biological reactions of reactive oxygen species (ROS) and reactive nitrogen species (RNS). It is widely accepted that the generation of ROS and RNS is involved in physiological and pathophysiological processes. To understand the role of ROS and RNS in a variety of pathologies, the specific detection of ROS and RNS is fundamental. Unfortunately, the intracellular detection and quantitation of ROS and RNS remains a challenge. In this short review, we have focused on the mechanistic and quantitative aspects of their detection with the use of selected fluorogenic probes. The challenges, limitations and perspectives of these methods are discussed.
Keywords: Superoxide radical anion, Hydrogen peroxide, Peroxynitrite, Protein hydroperoxides, Fluorogenic probes
1. Introduction
It has been more than forty years since McCord and Fridovich discovered that erythrocuprein, known as superoxide dismutase (SOD), catalyzes the dismutation of the superoxide radical anion (O2•−) [1]. This discovery attracted the attention of the scientific community to the role of reactive molecular oxygen metabolites in biological processes. The group of reactive oxygen species (ROS) and reactive nitrogen species (RNS) consists of hydrogen peroxide (H2O2), protein and lipid peroxides (ROOH), hypochlorous acid (HOCl), peroxynitrite (ONOO−) and radical oxidants, including O2•−, hydroxyl (•OH), peroxyl (ROO•) and thiyl radicals (RS•), nitrogen dioxide (•NO2) and carbonate radical anion (CO3•−).
Since the discovery of SOD, tremendous progress in understanding the biological reactions of ROS and RNS and their physiological significance has been made, yet their intracellular detection and quantitation remain a challenge [2–4]. Several ROS-sensitive probes producing easily detectable and relatively stable products have been developed. In addition to spin trapping techniques [5,6], luminescent probes have also become widely used tools in the studies on oxidative stress.
A variety of small molecule fluorescent probes are available for detecting ROS and RNS in cells. Their rational use requires a deep understanding of the mechanism of their action. In most cases, the probe is oxidized to the corresponding fluorescent product. Determination of the reactivity pattern of the primary products of this process (i.e., probe-derived radicals) is of great importance for understanding of reaction mechanisms and proper interpretation of experimental data [2].
In this article, we discuss the mechanisms of oxidative transformation of selected fluorogenic probes: hydroethidine (detection of O2•−), Amplex® Red (detection of H2O2), and boronate-based fluorogenic probes (detection of peroxynitrite and hydroperoxides).
Hydroethidine and the detection of superoxide radical anion
Superoxide radical anion is formed by the process of one-electron reduction of molecular oxygen. It can be produced in vivo by a number of oxidases (NADPH oxidases family [7] and xanthine oxidase [8]), by the mitochondrial electron transport chain [9], or by redox cycling agents (e.g., paraquat and menadione) in the presence of electron donors [10,11]. In a protic environment, O2•− undergoes spontaneous (k = 2 × 105 M−1s−1) [12] or SOD-catalyzed dismutation (k = 1.6 × 109 M−1s−1) [13] to form O2 and H2O2. Superoxide radical anion is the primary ROS produced in vivo and a precursor of H2O2.
Two major groups of probes are used for the detection of O2•−. The first group comprises chemiluminescent probes (e.g., lucigenin, luminol and its derivative, L-012, Fig. 1A) that react with ROS to form a product in the excited state, which relaxes to ground state with the emission of photons (Fig. 1B and C). Chemiluminescent probes have been widely utilized due to their high sensitivity. The mechanism of probes oxidation involves several intermediates, including probes-derived radicals. The major limitation of their use is that the radical intermediates react with O2 leading to the formation of O2•− (Fig. 1B and C) [2,14–16]. This reactivity should be considered when interpreting chemiluminescence data. Moreover, those probes do not react directly, or react slowly with superoxide, while other biologically relevant oxidants (or reductants) can react with them, producing radical intermediates, which further complicate the system. The second major group of probes consists of fluorogenic probes: dichlorodihydrofluorescein (DCFH), dihydrorhodamine (DHR) (Fig. 2A), hydrocyanines (Fig. 2B), and hydroethidine (HE) along with its analogs hydropropidine (HPr) and MitoSOX™ Red (Fig. 2C). In the case of dichlorodihydrofluorescein and dihydrorhodamine, one has to keep in mind that the radical intermediates formed upon one-electron oxidation react rapidly with oxygen to generate O2•− (Fig. 3A and B) [17,18].
Figure 1.
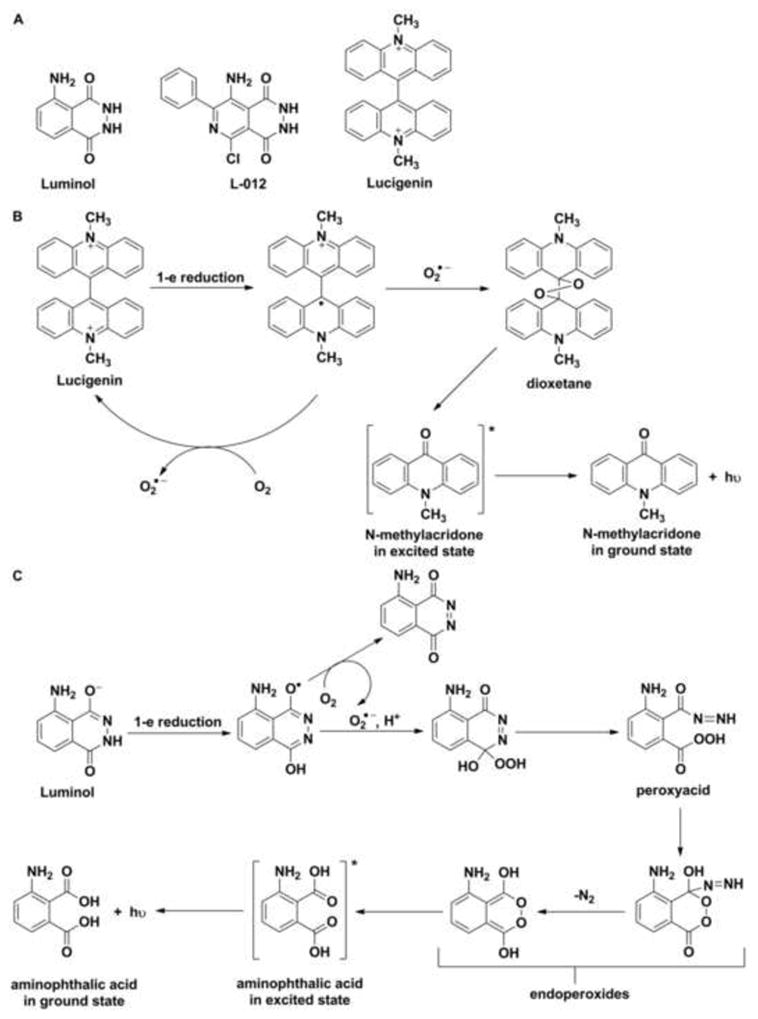
(A) Chemical structures of chemiluminescent probes for the detection of superoxide radical anion, (B) The mechanism of superoxide-dependent oxidative transformation of lucigenin, (C) The mechanism of oxidative transformation of luminol.
Figure 2.
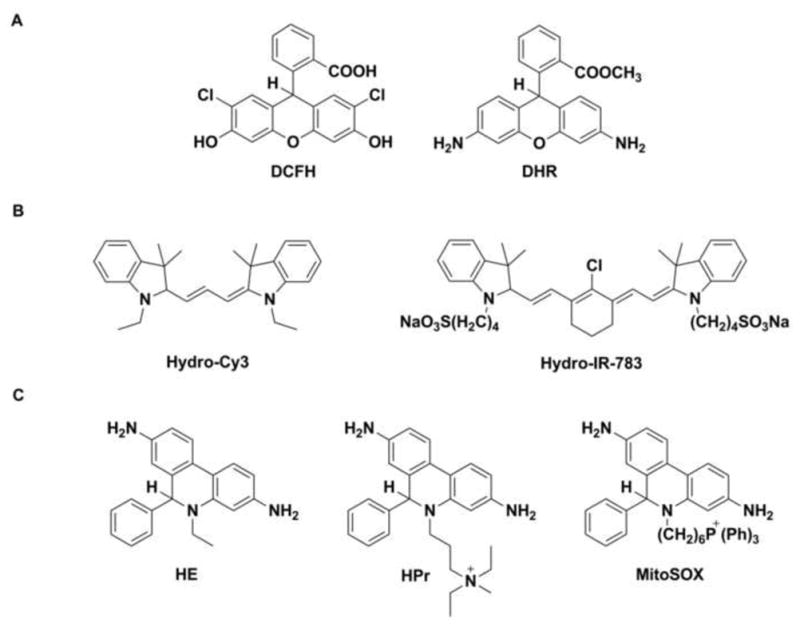
Chemical structures of fluorogenic probes for the detection of superoxide radical anion: (A) dichlorodihydrofluorescein (DCFH) and dihydrorhodamine (DHR), (B) hydrocyanines, (C) hydroethidine (HE) and its analogs.
Figure 3.
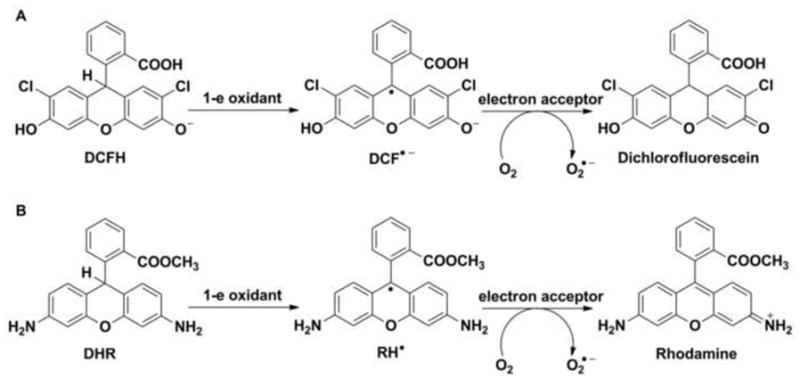
Mechanisms of one-electron oxidation of (A) dichlorodihydrofluorescein (DCFH) and (B) dihydrorhodamine (DHR).
Hydrocyanines, proposed recently as a tool for the imaging of ROS and O2•− production [19], still await better characterization of their specificity and chemical reactivity towards biologically relevant oxidants.
Hydroethidine is a cell-permeable probe that reacts with superoxide to form a unique marker product, 2-hydroxyethidium (2-OH-E+), whereas another red fluorescent product, ethidium (E+), is formed in the reaction with other cellular oxidants (Fig. 4) [20]. HE has been used for the detection of O2•− in a variety of biological systems, ranging from intracellular organelles to whole organs in live animals [21,22]. The reaction of HE and superoxide involves a radical mechanism. In the first step, hydroethidine reacts with hydroperoxyl radical HO2• or other one-electron oxidants to form a HE radical cation. Because the oxidation of HE by superoxide at pH 7.4 is rather slow (k = 6.2 ± 0.8 × 103 M−1s−1) [23], the first step of HE oxidation in cells seems more likely to be achieved by other oxidants. Importantly, the radical intermediate does not react with O2, thus the generation of superoxide by probe-derived radicals is avoided. HE radical cation combines rapidly with superoxide (k = 2 × 109 M−1s−1) to generate a specific oxidation product, 2-hydroxyethidium [24]. Although superoxide-specific (2-OH-E+) and non-specific (E+) oxidation products have slightly different fluorescent spectra, the distinction between them is difficult using currently available fluorescence techniques [25]. The formation of 2-OH-E+ has to be confirmed and quantitated with the use of other analytical techniques (e.g., HPLC with fluorescence detection or LC-MS) [26,27]. The HE–derived radical can also produce an HE-HE dimer, which can be further oxidized to the HE-E+ and E+-E+. The measurement of E+ and the dimers (HE-HE, HE-E+ and E+-E+) provides useful information about the cellular oxidation of HE [28]. It has been suggested that 2-OH-E+ formation should be considered only as a qualitative indicator of intracellular superoxide production [29–31].
Figure 4.
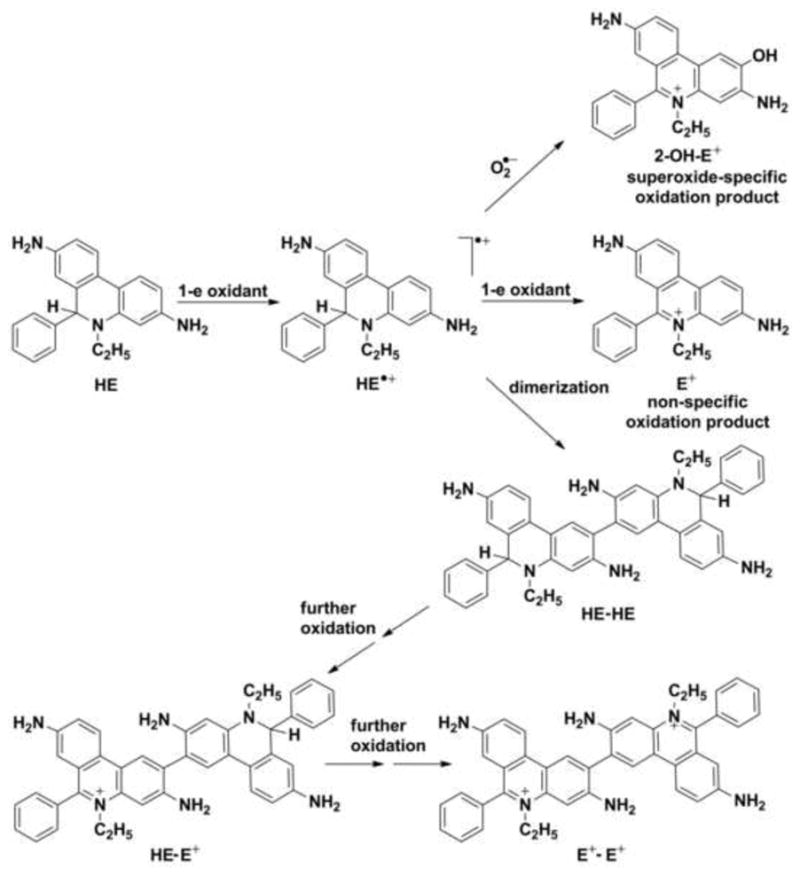
Proposed radical mechanism of the oxidative transformation of hydroethidine (HE).
Hydroethidine analogs (e.g., hydropropidine and MitoSOX™ Red, Fig. 2C) can be used for the detection of superoxide with the same limitations as assigned to HE [28]. Hydropropidine, a water-soluble analog of HE, possesses a highly localized positive charge that prevents its cellular uptake, making this probe a convenient tool for measurements of extracellular superoxide. It seems possible that in the presence of horseradish peroxidase, (HRP) the sensitivity of detection for extracellular superoxide with hydropropidine can be significantly increased. Recently, we have shown that the addition of HRP to HE and xanthine/xanthine oxidase system dramatically enhances 2-OH-E+ formation [31].
The requirement of HPLC separation for 2-OH-E+ from other HE oxidation products is the major limitation of superoxide detection in cells. The rational design and synthesis of new hydroethidine analogs is needed to make real-time monitoring and/or imaging of superoxide in biological systems possible [32].
Amplex Red and the detection of hydrogen peroxide
Hydrogen peroxide can be generated in vivo, directly by an enzymatic two-electron reduction of O2 or from dismutation of O2•− [13,33]. H2O2 can be produced by several enzymes, such as L-amino acid oxidase, urate oxidase, glycolate oxidase and monoamine oxidase [34,35]. H2O2 is assumed to be an important signaling molecule regulating enzymes such as protein kinases and phosphatases [36–38].
Hydrogen peroxide is a neutral molecule that can diffuse through lipid membranes. It can slowly react with thiols, which can lead to the formation of sulfenic acids or sulfoxides [39]. High H2O2 toxicity is ascribed to •OH formed through the Fenton reaction. To protect cells from H2O2 toxicity, the iron pool in cells is tightly controlled, and cells possess endogenous enzymatic H2O2 scavengers: peroxiredoxins, glutathione peroxidases, and catalase [40–42].
Currently, there are very few methods that yield reliable quantitative data on H2O2 production in cells. One method is the Amplex® Red (AR, 10-acetyl-3 7-dihydroxyphenoxazine) assay, which is based on the enzymatic oxidation of a resorufin derivative [43]. Amplex® Red is a colorless and non-fluorescent compound that upon oxidation by H2O2, in the presence of HRP, is transformed into a highly fluorescent resorufin [43]. The mechanism of its oxidation involves a multi-step reaction (Fig. 5). In the first step Amplex® Red undergoes one-electron oxidation to form an AR-derived radical, which subsequently can be oxidized by H2O2/HRP. AR-derived radicals can also undergo dismutation to form the secondary transient product. Further de-N-acetylation of that product leads to the formation of resorufin [44]. The Amplex® Red assay is assumed to be highly selective toward H2O2. However, our results indicate that AR can also be oxidized by other oxidants [45]. Moreover, the traces of resorufin present in the sample are able to photosensitize Amplex® Red when exposed to visible light (e.g., excitation light during monitoring of Amplex® Red oxidation). The rate of photooxidation is increased when HRP is present [46,47]. Therefore, real-time, fluorescence-based monitoring of Amplex® Red oxidation should not be used for quantitative analysis of H2O2 formation. Additionally, NADH and reduced glutathione in the presence of HRP can interfere with the measurement [4,48]. Our results indicate that the addition of peroxynitrite can lead to the oxidation of Amplex® Red [44]. The mechanism of this reaction involves the formation of oxidizing radicals (i.e., CO3•− and •NO2) from the decomposition of peroxynitrite. Pulse radiolysis experiments have indicated that those radicals are able to oxidize Amplex® Red. HRP can significantly increase the yield of resorufin from Amplex® Red oxidation by peroxynitrite [44].
Figure 5.
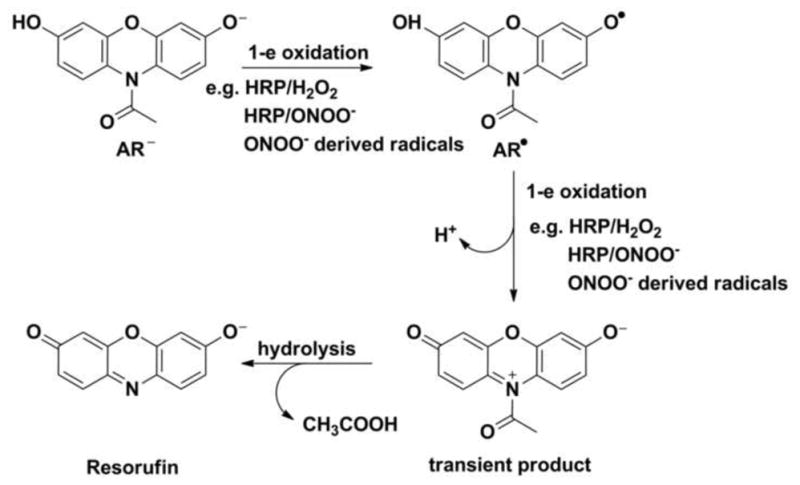
Mechanism of the oxidative transformation of Amplex® Red (AR) in its deprotonated form under basic conditions.
Boronate probes and the detection of peroxynitrite and hydroperoxides
Boronate-based probes have recently emerged as a versatile tool for detecting ROS and RNS. It has been proposed that boronate-derivatives of fluorescent dyes can be used as convenient and selective probes for H2O2. The first reported fluorogenic boronate probe for H2O2 was p-dihydroxyborylbenzyloxycarbonyl derivative of 7-aminocoumarin. The mechanism of detection was based on the oxidative deprotection of the fluorescent aminocoumarin reporter (Fig. 6A) [49]. In 2004, Chang et al. described the synthesis of a diboronate derivative of fluorescein PF1 (Fig. 6B) [50]. During the next decade many boronate-based fluorogenic probes were designed and synthesized. Generally, the mechanism of action of boronate probes is based on oxidative transformation of non- or weakly fluorescent boronates into strongly fluorescent products [49–51].
Figure 6.
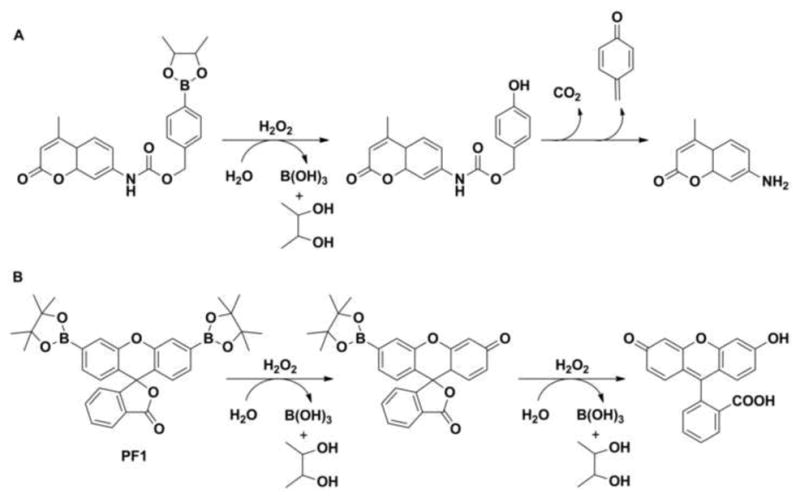
The reaction of hydrogen peroxide with (A) p-dihydroxyborylbenzyloxycarbonyl derivative of 7-aminocoumarin and (B) diboronate derivative of fluorescein (PF1).
Reactivity towards oxidants
The oxidation of arylboronates in alkaline solutions of H2O2 was reported for the first time over 80 years ago [52]. However, at physiological pH, the reaction between boronate and H2O2 is slow (k ~ 1–2 M−1s−1) [53]. Thus, it has been postulated that in biological systems oxidation of boronate probes by H2O2 seems rather unlikely [54].
Several other oxidants can also convert boronates into corresponding phenols. That reaction is typical for hypohalous ions and nucleophilic peroxy oxidants (Fig. 7) [53,55,56]. A few years ago, we demonstrated that boronates react rapidly with hypochlorite and peroxynitrite anions [51,53] (Fig. 7). We also showed that boronates can be oxidized by selected organic hydroperoxides [57,58].
Figure 7.
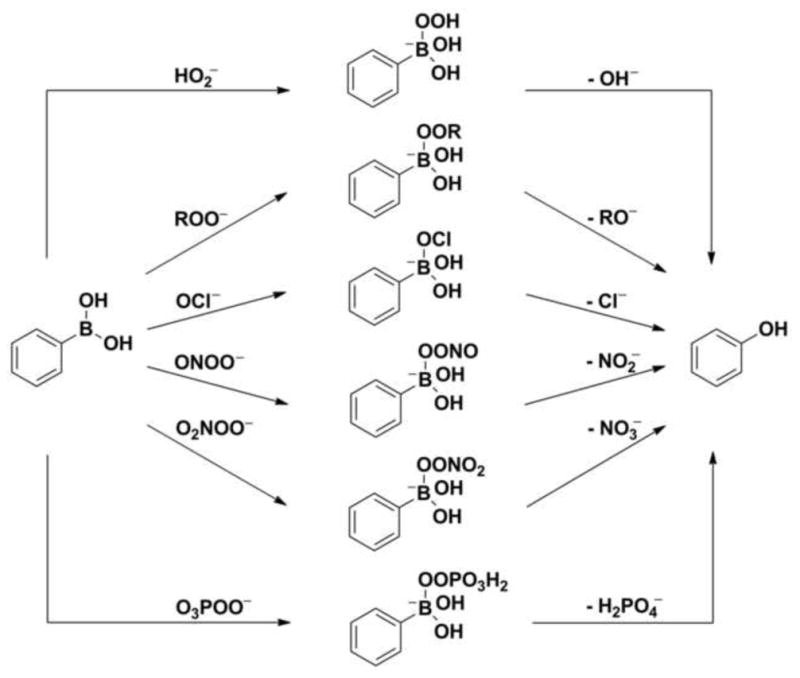
Proposed mechanisms of oxidation of boronate probes by various oxidants.
The mechanism of peroxynitrite-derived oxidation
Peroxynitrite (ONOO−) is a primary product of the reaction between nitric oxide (•NO) and O2•− (k ~ 1010 M−1s−1) [59]. We have recently demonstrated that peroxynitrite is also produced in the reaction of O2 with HNO (k = 1.8 × 104 M−1s−1) [60]. Although peroxynitrite possesses a relatively short half-life in biological systems, it is able to diffuse through biological membranes, and it can diffuse up to two cell diameters distance [61]. ONOO− is a relatively strong non-radical oxidant. Under physiological conditions, it exists in acid-base equilibrium with its protonated form, peroxynitrous acid (ONOOH, pKa = 6.8). Peroxynitrite can directly oxidize low molecular weight thiols, as well as sulfhydryls groups in proteins. This compound can also react with CO2 (high concentration in vivo, k = 4.6 × 104 M−1s−1) [62]. The product of this reaction is unstable and decomposes up to 35% into •NO2 (nitrating radical) and CO3•− (strong one-electron oxidant). In the absence of scavengers, homolysis of ONOOH, considered as a minor pathway in biology, yields •OH and •NO2 radicals [63]. Formation of such radicals leads to the oxidation and nitration of relevant targets, such as amino acids (tyrosine, phenylalanine and histidine), sugars (deoxyribose) and lipids [59,64].
Boronates react rapidly, directly and stoichiometrically with ONOO− (k ~ 106 M−1s−1) to form corresponding phenols (80–90% yield) [53]. We showed that the remaining 10–20% can be ascribed to minor products derived from radical intermediates [53,57,60,65].
The reaction mechanism for peroxynitrite-derived oxidation of boronates has been studied and described in detail (Fig. 8) [57,60,65]. The formation of anionic adduct of peroxynitrite to the boronate moiety is the first reaction step. There are two pathways of subsequent transformation of the adduct. The first pathway (80–90%) proceeds via heterolytic cleavage of the O-O bond in the anionic adduct and results in the formation of the major phenolic product. The second, minor pathway involves homolytic cleavage (~ 10–20%) of the peroxide bond and results in the formation of a RB(OH)2O•− radical anion, which undergoes further spontaneous fragmentation with the formation of phenyl radicals [57,60,65]. As the minor free-radical pathway is specific for the reaction of boronates with peroxynitrite, the formation of phenyl radical-derived minor products may be used to support its identification (Fig. 8) [60]. During the last few years, the formation of such peroxynitrite specific products was shown for simple arylboronate compounds [57], mitochondria targeted isomeric arylboronates [65] as well as for fluorogenic boronate probe coumarin 7-boronic acid (CBA) [69]. We have demonstrated that such products are of diagnostic value and can be used as a “peroxynitrite fingerprint” [60,66].
Figure 8.
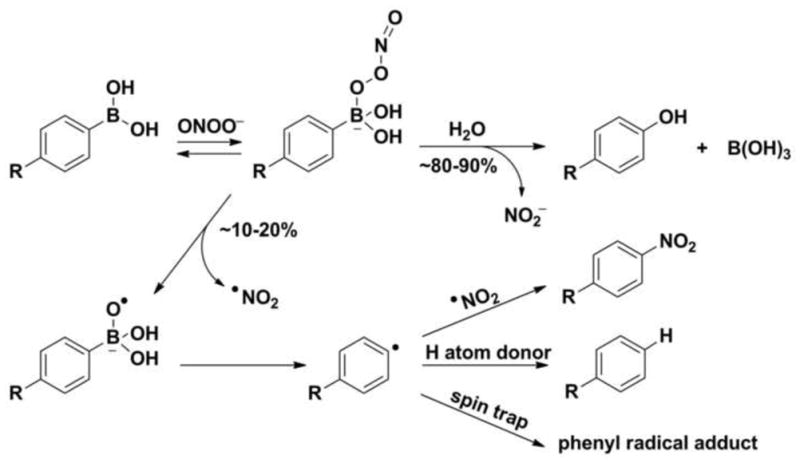
Mechanism of the reaction between peroxynitrite and boronic probes.
Detection of peroxynitrite in cell culture studies
The usefulness of boronate probes for monitoring peroxynitrite generation in cellular systems was first shown in a study performed on activated macrophages, RAW 264.7 [67]. Recently [54], a boronate-based bioluminescent probe Peroxy Caged Luciferin-1 (PCL-1, Fig. 9), previously reported as a selective probe for hydrogen peroxide [68], was shown to detect peroxynitrite in macrophage cells [54]. After reaction of PCL-1 with an oxidant, luciferin is released, and in the presence of ATP and luciferase, luciferin is oxidized producing a bioluminescence signal.
Figure 9.
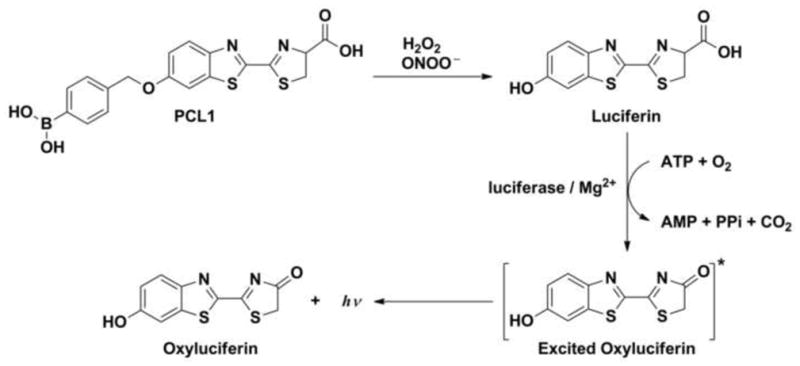
Mechanism of the detection of hydrogen peroxide or peroxynitrite by Peroxy Caged Luciferin-1 (PCL-1).
Detection of protein hydroperoxides with the use of boronate probes
Boronate probes can also be used to detect other oxidants in a complex biological system. Recently, we have shown that CBA is oxidized to the highly fluorescent 7-hydroxycoumarin by amino acid and protein hydroperoxides [58]. Based on this observation, we have developed a fluorometric real-time assay to detect protein hydroperoxides (Fig. 10) generated in cells. The use of CBA probe is a convenient way to detect and determine protein hydroperoxides, superior to other assays i.e., FOX or iodometric assays (both FOX and iodometric assays possess several limitations, as discussed elsewhere [69]).
Figure 10.
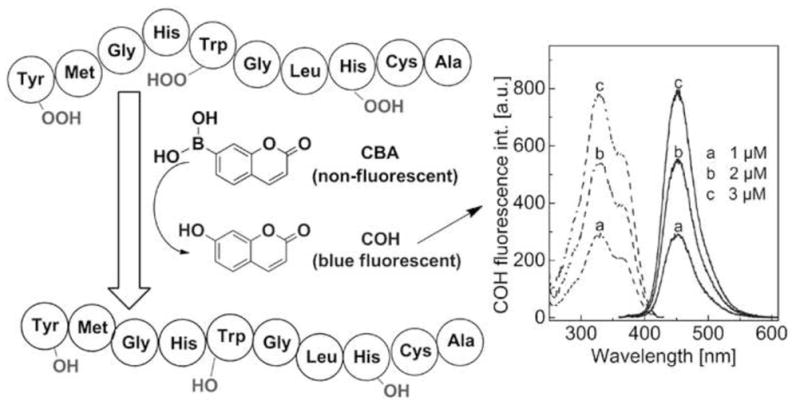
Fluorometric, real-time assay for protein hydroperoxides based on the CBA probe.
Global profiling of oxidants
Recently, we proposed a “global profiling approach” [67] with the use of a set of selected fluorogenic probes for simultaneous real-time monitoring of oxidants formation in living cells. Such an approach seems to be an ideal tool for complete characterization of cellular ROS and RNS generation under oxidative stress conditions. Hydroethidine or hydropropidine [23] can be used to monitor superoxide and/or other oxidants formation. Boronate probes can be used to detect peroxynitrite, and the Amplex Red/HRP assay can be used to detect both H2O2 and peroxynitrite [67,70]. As it has been emphasized, the proper identification of the oxidant is not possible without simultaneous HPLC-based measurements verifying the identities of the monitored fluorescent species [67,70].
With a better understanding of the redox chemistry of the probes and the recent developments in rapid HPLC analyses of 2-hydroxyethidium and hydroxycoumarin [67], rigorous high throughput profiling of various reactive oxygen and nitrogen species in biological systems seems closer than ever to being a reality.
Acknowledgments
The support from grant coordinated by JCET, No. POIG.01.01.02-00-069/09 (supported by the European Union from the resources of the European Regional Development Fund under the Innovative Economy Programme) is acknowledged. RM was supported by a grant from the Foundation for Polish Science (FNP) within the “Homing Plus” program supported by the European Union within European Regional Development Fund, through the Innovative Economy Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–55. [PubMed] [Google Scholar]
- 2.Wardman P. Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: progress, pitfalls, and prospects. Free Radic Biol Med. 2007;43:995–1022. doi: 10.1016/j.freeradbiomed.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 3.Kalyanaraman B, Darley-Usmar V, Davies KJA, Dennery PA, Forman HJ, Grisham MB, et al. Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radic Biol Med. 2012;52:1–6. doi: 10.1016/j.freeradbiomed.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winterbourn CC. The challenges of using fluorescent probes to detect and quantify specific reactive oxygen species in living cells. Biochim Biophys Acta. 2014;1840:730–8. doi: 10.1016/j.bbagen.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Hardy M, Poulhés F, Rizzato E, Rockenbauer A, Banaszak K, Karoui H, et al. Mitochondria-targeted spin traps: synthesis, superoxide spin trapping, and mitochondrial uptake. Chem Res Toxicol. 2014;27:1155–65. doi: 10.1021/tx500032e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abbas K, Hardy M, Poulhès F, Karoui H, Tordo P, Ouari O, et al. Detection of superoxide production in stimulated and unstimulated living cells using new cyclic nitrone spin traps. Free Radic Biol Med. 2014;71:281–90. doi: 10.1016/j.freeradbiomed.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 7.Bedard K, Krause K-H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 8.Fridovich I. Quantitative aspects of the production of superoxide anion radical by milk xanthine oxidase. J Biol Chem. 1970;245:4053–7. [PubMed] [Google Scholar]
- 9.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim KA, Lee JY, Park KS, Kim MJ, Chung JH. Mechanism of menadione-induced cytotoxicity in rat platelets. Toxicol Appl Pharmacol. 1996;138:12–9. doi: 10.1006/taap.1996.0092. [DOI] [PubMed] [Google Scholar]
- 11.Shimada H, Hirai K-I, Simamura E, Pan J. Mitochondrial NADH–quinone oxidoreductase of the outer membrane is responsible for paraquat cytotoxicity in rat livers. Arch Biochem Biophys. 1998;351:75–81. doi: 10.1006/abbi.1997.0557. [DOI] [PubMed] [Google Scholar]
- 12.Bielski BHJ, Cabelli DE, Arudi RL, Ross AB. Reactivity of HO2/O2− radicals in aqueous solution. J Phys Chem Ref Data. 1985;14:1041–100. [Google Scholar]
- 13.Forman HJ, Fridovich I. Superoxide dismutase: a comparison of rate constants. Arch Biochem Biophys. 1973;158:396–400. doi: 10.1016/0003-9861(73)90636-x. [DOI] [PubMed] [Google Scholar]
- 14.Vásquez-Vivar J, Hogg N, Pritchard KA, Martasek P, Kalyanaraman B. Superoxide anion formation from lucigenin: an electron spin resonance spin-trapping study. FEBS Lett. 1997;403:127–30. doi: 10.1016/s0014-5793(97)00036-7. [DOI] [PubMed] [Google Scholar]
- 15.Wardman P, Burkitt MJM, Patel KBK, Lawrence A, Jones CM, Everett SA, et al. Pitfalls in the use of common luminescent probes for oxidative and nitrosative stress. J Fluoresc. 2002;12:65–8. [Google Scholar]
- 16.Zielonka J, Lambeth JD, Kalyanaraman B. On the use of L-012, a luminol-based chemiluminescent probe, for detecting superoxide and identifying inhibitors of NADPH oxidase: a reevaluation. Free Radic Biol Med. 2013;65:1310–4. doi: 10.1016/j.freeradbiomed.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonini MG, Rota C, Tomasi A, Mason RP. The oxidation of 2′,7′-dichlorofluorescein to reactive oxygen species: a self-fulfilling prophesy? Free Radic Biol Med. 2006;40:968–75. doi: 10.1016/j.freeradbiomed.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 18.Folkes LK, Patel KB, Wardman P, Wrona M. Kinetics of reaction of nitrogen dioxide with dihydrorhodamine and the reaction of the dihydrorhodamine radical with oxygen: implications for quantifying peroxynitrite formation in cells. Arch Biochem Biophys. 2009;484:122–6. doi: 10.1016/j.abb.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Kundu K, Knight SF, Willett N, Lee S, Taylor WR, Murthy N. Hydrocyanines: a class of fluorescent sensors that can image reactive oxygen species in cell culture, tissue, and in vivo. Angew Chemie - Int Ed. 2009;48:299–303. doi: 10.1002/anie.200804851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao H, Kalivendi S, Zhang H, Joseph J, Nithipatikom K, Vásquez-Vivar J, et al. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic Biol Med. 2003;34:1359–68. doi: 10.1016/s0891-5849(03)00142-4. [DOI] [PubMed] [Google Scholar]
- 21.Fink B, Laude K, McCann L, Doughan A, Harrison DG, Dikalov S. Detection of intracellular superoxide formation in endothelial cells and intact tissues using dihydroethidium and an HPLC-based assay. Am J Physiol Cell Physiol. 2004;287:C895–902. doi: 10.1152/ajpcell.00028.2004. [DOI] [PubMed] [Google Scholar]
- 22.Georgiou CD, Papapostolou I, Patsoukis N, Tsegenidis T, Sideris T. An ultrasensitive fluorescent assay for the in vivo quantification of superoxide radical in organisms. Anal Biochem. 2005;347:144–51. doi: 10.1016/j.ab.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 23.Michalski R, Zielonka J, Hardy M, Joseph J, Kalyanaraman B. Hydropropidine: a novel, cell-impermeant fluorogenic probe for detecting extracellular superoxide. Free Radic Biol Med. 2013;54:135–47. doi: 10.1016/j.freeradbiomed.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zielonka J, Sarna T, Roberts JE, Wishart JF, Kalyanaraman B. Pulse radiolysis and steady-state analyses of the reaction between hydroethidine and superoxide and other oxidants. Arch Biochem Biophys. 2006;456:39–47. doi: 10.1016/j.abb.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao H, Joseph J, Fales HM, Sokoloski EA, Levine RL, Vasquez-Vivar J, et al. Detection and characterization of the product of hydroethidine and intracellular superoxide by HPLC and limitations of fluorescence. Proc Natl Acad Sci USA. 2005;102:5727–32. doi: 10.1073/pnas.0501719102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zielonka J, Vasquez-Vivar J, Kalyanaraman B. Detection of 2-hydroxyethidium in cellular systems: a unique marker product of superoxide and hydroethidine. Nat Protoc. 2008;3:8–21. doi: 10.1038/nprot.2007.473. [DOI] [PubMed] [Google Scholar]
- 27.Zielonka J, Hardy M, Kalyanaraman B. HPLC study of oxidation products of hydroethidine in chemical and biological systems: ramifications in superoxide measurements. Free Radic Biol Med. 2009;46:329–38. doi: 10.1016/j.freeradbiomed.2008.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalyanaraman B, Dranka BP, Hardy M, Michalski R, Zielonka J. HPLC-based monitoring of products formed from hydroethidine-based fluorogenic probes - the ultimate approach for intra- and extracellular superoxide detection. Biochim Biophys Acta. 2014;1840:739–44. doi: 10.1016/j.bbagen.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zielonka J, Srinivasan S, Hardy M, Ouari O, Lopez M, Vasquez-Vivar J, et al. Cytochrome c-mediated oxidation of hydroethidine and mito-hydroethidine in mitochondria: identification of homo- and heterodimers. Free Radic Biol Med. 2008;44:835–46. doi: 10.1016/j.freeradbiomed.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zielonka J, Kalyanaraman B. Hydroethidine- and MitoSOX-derived red fluorescence is not a reliable indicator of intracellular superoxide formation: another inconvenient truth. Free Radic Biol Med. 2010;48:983–1001. doi: 10.1016/j.freeradbiomed.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michalski R, Michalowski B, Sikora A, Zielonka J, Kalyanaraman B. On the use of fluorescence lifetime imaging and dihydroethidium to detect superoxide in intact animals and ex vivo tissues: a reassessment. Free Radic Biol Med. 2014;67:278–84. doi: 10.1016/j.freeradbiomed.2013.10.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cairns AG, Senn HM, Murphy MP, Hartley RC. Expanding the palette of phenanthridinium cations. Chem - A Eur J. 2014;20:3742–51. doi: 10.1002/chem.201304241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marklund S. Spectrophotometric study of spontaneous disproportionation of superoxide anion radical and sensitive direct assay for superoxide dismutase. J Biol Chem. 1976;251:7504–7. [PubMed] [Google Scholar]
- 34.Boveris A, Oshino N, Chance B. The cellular production of hydrogen peroxide. Biochem J. 1972;128:617–30. doi: 10.1042/bj1280617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1005–28. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- 36.Adler V, Yin Z, Tew KD, Ronai Z. Role of redox potential and reactive oxygen species in stress signaling. Oncogene. 1999;18:6104–11. doi: 10.1038/sj.onc.1203128. [DOI] [PubMed] [Google Scholar]
- 37.Meng T-C, Fukada T, Tonks NK. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol Cell. 2002;9:387–99. doi: 10.1016/s1097-2765(02)00445-8. [DOI] [PubMed] [Google Scholar]
- 38.Veal EA, Day AM, Morgan BA. Hydrogen peroxide sensing and signaling. Mol Cell. 2007;26:1–14. doi: 10.1016/j.molcel.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 39.Winterbourn CC, Metodiewa D. Reactivity of biologically important thiol compounds with superoxide and hydrogen peroxide. Free Radic Biol Med. 1999;27:322–8. doi: 10.1016/s0891-5849(99)00051-9. [DOI] [PubMed] [Google Scholar]
- 40.Mueller S, Riedel HD, Stremmel W. Direct evidence for catalase as the predominant H2O2 -removing enzyme in human erythrocytes. Blood. 1997;90:4973–8. [PubMed] [Google Scholar]
- 41.Winterbourn CC, Hampton MB. Thiol chemistry and specificity in redox signaling. Free Radic Biol Med. 2008;45:549–61. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 42.Mitozo PA, de Souza LF, Loch-Neckel G, Flesch S, Maris AF, Figueiredo CP, et al. A study of the relative importance of the peroxiredoxin-, catalase-, and glutathione-dependent systems in neural peroxide metabolism. Free Radic Biol Med. 2011;51:69–77. doi: 10.1016/j.freeradbiomed.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 43.Zhou M, Diwu Z, Panchuk-Voloshina N, Haugland RP. A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal Biochem. 1997;253:162–8. doi: 10.1006/abio.1997.2391. [DOI] [PubMed] [Google Scholar]
- 44.Debski D, Zielonka J, Michalowski B, Jakubowska M, Debowska K, Adamus J, et al. Mechanism of oxidative conversion of Amplex Red to resorufin: pulse radiolysis and enzymatic studies. 2015 doi: 10.1016/j.freeradbiomed.2016.03.027. Manuscript in preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Debski D, Michalowski B, Adamus J, Wojciechowski J, Zielonka J, Kalyanaraman B, et al. Direct characterization of transient species formed upon one-electron oxidation of Amplex Red. Free Radic Biol Med. 2013;65:S88–9. [Google Scholar]
- 46.Zhao B, Ranguelova K, Jiang J, Mason RP. Studies on the photosensitized reduction of resorufin and implications for the detection of oxidative stress with Amplex Red. Free Radic Biol Med. 2011;51:153–9. doi: 10.1016/j.freeradbiomed.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao B, Summers FA, Mason RP. Photooxidation of Amplex Red to resorufin: implications of exposing the Amplex Red assay to light. Free Radic Biol Med. 2012;53:1080–7. doi: 10.1016/j.freeradbiomed.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Votyakova TV, Reynolds IJ. Detection of hydrogen peroxide with Amplex Red: interference by NADH and reduced glutathione auto-oxidation. Arch Biochem Biophys. 2004;431:138–44. doi: 10.1016/j.abb.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 49.Lo L, Chu C. Development of highly selective and sensitive probes for hydrogen peroxide. Chem Commun. 2003:2728–9. doi: 10.1039/b309393j. [DOI] [PubMed] [Google Scholar]
- 50.Chang MCY, Pralle A, Isacoff EY, Chang CJ. A selective, cell-permeable optical probe for hydrogen peroxide in living cells. J Am Chem Soc. 2004;126:15392–3. doi: 10.1021/ja0441716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zielonka J, Sikora A, Joseph J, Kalyanaraman B. Peroxynitrite is the major species formed from different flux ratios of co-generated nitric oxide and superoxide: direct reaction with boronate-based fluorescent probe. J Biol Chem. 2010;285:14210–6. doi: 10.1074/jbc.M110.110080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ainley AD, Challenger F. CCLXXX.-Studies of the boron-carbon linkage. Part I. The oxidation and nitration of phenylboric acid. J Chem Soc. 1930;1:2171–80. [Google Scholar]
- 53.Sikora A, Zielonka J, Lopez M, Joseph J, Kalyanaraman B. Direct oxidation of boronates by peroxynitrite: mechanism and implications in fluorescence imaging of peroxynitrite. Free Radic Biol Med. 2009;47:1401–7. doi: 10.1016/j.freeradbiomed.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sieracki NA, Gantner BN, Mao M, Horner JH, Ye RD, Malik AB, et al. Bioluminescent detection of peroxynitrite with a boronic acid-caged luciferin. Free Radic Biol Med. 2013;61:40–50. doi: 10.1016/j.freeradbiomed.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zielonka J, Sikora A, Hardy M, Joseph J, Dranka BP, Kalyanaraman B. Boronate probes as diagnostic tools for real time monitoring of peroxynitrite and hydroperoxides. Chem Res Toxicol. 2012;25:1793–9. doi: 10.1021/tx300164j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.LaButti JN, Gates KS. Biologically relevant chemical properties of peroxymonophosphate (=O3POOH) Bioorg Med Chem Lett. 2009;19:218–21. doi: 10.1016/j.bmcl.2008.10.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sikora A, Zielonka J, Lopez M, Dybala-Defratyka A, Joseph J, Marcinek A, et al. Reaction between peroxynitrite and boronates: EPR spin-trapping, HPLC analyses, and quantum mechanical study of the free radical pathway. Chem Res Toxicol. 2011;24:687–97. doi: 10.1021/tx100439a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Michalski R, Zielonka J, Gapys E, Marcinek A, Joseph J, Kalyanaraman B. Real-time measurements of amino acid and protein hydroperoxides using coumarin boronic acid. J Biol Chem. 2014;289:22536–53. doi: 10.1074/jbc.M114.553727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beckman JSJ, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA. 1990;87:1620–4. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smulik R, Debski D, Zielonka J, Michałowski B, Adamus J, Marcinek A, et al. Nitroxyl (HNO) reacts with molecular oxygen and forms peroxynitrite at physiological pH: biological implications. J Biol Chem. 2014;289:35570–81. doi: 10.1074/jbc.M114.597740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Denicola A, Souza JM, Radi R. Diffusion of peroxynitrite across erythrocyte membranes. Proc Natl Acad Sci. 1998;95:3566–71. doi: 10.1073/pnas.95.7.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Denicola A, Freeman BA, Trujillo M, Radi R. Peroxynitrite reaction with carbon dioxide/bicarbonate: kinetics and influence on peroxynitrite-mediated oxidations. Arch Biochem Biophys. 1996;333:49–58. doi: 10.1006/abbi.1996.0363. [DOI] [PubMed] [Google Scholar]
- 63.Radi R. Peroxynitrite reactions and diffusion in biology. Chem Res Toxicol. 1998;11:720–1. doi: 10.1021/tx980096z. [DOI] [PubMed] [Google Scholar]
- 64.Ramezanian MS, Padmaja S, Koppenol WH. Nitration and hydroxylation of phenolic compounds by peroxynitrite. Chem Res Toxicol. 1996;9:232–40. doi: 10.1021/tx950135w. [DOI] [PubMed] [Google Scholar]
- 65.Sikora A, Zielonka J, Adamus J, Debski D, Dybala-Defratyka A, Michalowski B, et al. Reaction between peroxynitrite and triphenylphosphonium-substituted arylboronic acid isomers: identification of diagnostic marker products and biological implications. Chem Res Toxicol. 2013;26:856–67. doi: 10.1021/tx300499c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zielonka J, Sikora A, Adamus J, Kalyanaraman B. Detection and differentiation between peroxynitrite and hydroperoxides using mitochondria-targeted arylboronic Acid. Methods Mol Biol. 2015;1264:171–81. doi: 10.1007/978-1-4939-2257-4_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zielonka J, Zielonka M, Sikora A, Adamus J, Joseph J, Hardy M, et al. Global profiling of reactive oxygen and nitrogen species in biological systems: high-throughput real-time analyses. J Biol Chem. 2012;287:2984–95. doi: 10.1074/jbc.M111.309062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van de Bittner GC, Dubikovskaya EA, Bertozzi CR, Chang CJ. In vivo imaging of hydrogen peroxide production in a murine tumor model with a chemoselective bioluminescent reporter. Proc Natl Acad Sci USA. 2010;107:21316–21. doi: 10.1073/pnas.1012864107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bou R, Codony R, Tres A, Decker EA, Guardiola F. Determination of hydroperoxides in foods and biological samples by the ferrous oxidation-xylenol orange method: a review of the factors that influence the method’s performance. Anal Biochem. 2008;377:1–15. doi: 10.1016/j.ab.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 70.Zielonka J, Joseph J, Sikora A, Kalyanaraman B. Real-time monitoring of reactive oxygen and nitrogen species in a multiwell plate using the diagnostic marker products of specific probes. Methods Enzymol. 2013;526:145–57. doi: 10.1016/B978-0-12-405883-5.00009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


