Abstract
Stroke is a brain disease that occurs when blood flow stops that results reduced oxygen supply to neurons. Stroke occurs at any time and at any age, but increases after the age of 55. It is the second leading cause of death and the third leading cause of disability-adjusted, life-years. Recent molecular, cellular, animal models and postmortem brain studies have revealed that multiple cellular changes have been implicated, including oxidative stress/mitochondrial dysfunction, inflammatory responses, micro RNA alterations and marked changes in brain proteins. Research also revealed that stroke increases with number of modifiable factors and most strokes can be prevented and/or controlled through pharmacological or surgical interventions and lifestyle changes, as primary or secondary stroke prevention strategies. Recent research also revealed stroke is the major factor for vascular dementia (VaD) and Alzheimer’s disease (AD). This article summarizes the latest research findings on the stroke research, including causal factors, molecular links between stroke and VaD/AD and methods/approaches used to study stroke.
Introduction
Stroke is the second leading cause of death and the third leading cause of disability-adjusted, life-years worldwide [1, 2]. The risk of having a stroke increases after the age of 55 but it can occur at any age. The World Health Organization (WHO) describes a stroke as an interruption to the supply of blood to the brain, with “rapidly developing clinical signs of focal or global disruption of cerebral function, with signs lasting 24 hours or longer, or leading to death with no apparent cause other than vascular origin” [3]. Such an interruption is commonly caused by a clot blocking a blood vessel (ischemic stroke; “IS”) or by a burst blood vessel (hemorrhagic stroke; “HS”). IS is sometimes called a ‘cerebral infarction.’ A cerebral infarction, in turn caused by cerebral thrombosis or embolism, accounts for 87% of all strokes worldwide [4]. IS can be described as a lack of blood supply and oxygen availability to an area of the brain due to partial or complete obstruction of an artery leading to or within the brain. According to the TOAST diagnostic criteria for stroke, IS can be classified into five subtypes: large-vessel disease, small vessel disease, cardio-embolic stroke, stroke of another determined etiology, and stroke of undetermined etiology [5].
HS, caused by an intracerebral or subarachnoid hemorrhage, accounts for 13% of all strokes worldwide [6]. It is more frequently lethal than is IS [7]. HS is either a brain aneurism that bursts or a weakened blood vessel that leaks. Based on the origin and site of the bleeding, an HS can involve an intracerebral hemorrhage or a subarachnoid hemorrhage. Each of these hemorrhages has partially different basic pathologies, risk factors, and clinical staging, and each requires distinct management.
Apart from obstructing the brain’s blood supply, the presence of blood in the brain due to IS or HS causes swelling, leading to stroke. Patients who experience the same type of stroke can have divergent clinical symptoms. Likewise, patients with the same clinical manifestation can have dissimilar etiopathology. Thus, a stroke may be categorized as a syndrome, not as a single disease. To diagnose a stroke – and its type – accurately, modern neuroimaging of the brain, such as computed tomography or magnetic resonance imaging (MRI), is required. The purpose of this article to 1) summarize latest developments in stroke research, including prevalence and incidence, 2) causal factors, 3) links between stroke and vascular dementia (VaD) and Alzheimer’s disease (AD) and 4) methods and approaches used to study stroke,
Incidence and Prevalence
According to the WHO, stroke has affected a total of 15 million people each year, worldwide. Of these, 5 million suffer from permanent disability, and approximately 5.5 million people succumb to varying levels of stroke-related disabilities [8]. The prevalence rate of stroke is estimated to be about 400–800 per 100,000 persons [9]. For native Indians living in India, the overall age-adjusted prevalence rate for stroke is between about 84–262 per 100,000 persons in rural areas and between about 334–424 per 100,000 persons in urban areas [10], with an average prevalence rate of 90–222 per 100,000 persons [11]. The incidence of stroke is declining in many developed countries, largely as a result of improved control of high blood pressure and fewer people who smoke tobacco. However, in developing countries undergoing rapid economic growth, stroke continues to increase, especially in populations older than 65 years continues to rise by approximately nine million people per year [12].
The annual prevalence of stroke is estimated to be about 30.7 million worldwide, with 12.6 million people having moderate to severe disability following stroke. Of these, 8.9 million are from low- and middle-income countries [13]. Worldwide over the past four decades, the annual age-standardized stroke incidence rate has decreased by 1.1% in high-income countries, while it has increased by 5.3% in low- and middle-income counties [6]. Stroke accounts for about 5.7 million (16.6%) deaths each year worldwide, and 87% of these deaths typically occur in low- and middle-income counties. Sixteen million new acute strokes occur every year worldwide [14, 15]. Three million women and 2.5 million men worldwide die from stroke every year [16]. More than half of these fatalities were found in Asian countries, including India, Bangladesh, Pakistan, China, Japan, and Korea [17]. WHO estimates that by the year 2050, 80% of stroke cases worldwide would occur in low- and middle-income countries, such as India and China.
In the United States, it is estimated that since 2001*, 795,000 people have had a stroke each year. Of these, 610,000 are persons who have had their first stroke. In the United States, nearly 75% of all strokes occur in people over the age of 65 years. In the United States, stroke is the third leading cause of death, taking almost 140,000 people each year – on average, one American dies from stroke every 4 minutes. The number of people living with stroke is expected to increase by 4 million by the year 2030 [18]. Stroke mortality rates are higher for African-Americans than for whites [19].
Dementia
Dementia is a clinical disorder triggered by neurodegeneration. There are more than 100 disease conditions that can lead to dementia, the most common of which are stroke and AD. Dementia is a progressive or long-lasting condition that results in cognitive changes, such as progressively worsening cognitive skills, memory loss, changes in learning capacity, mood changes, impaired judgment, and problems in understanding language and performing routine activities, such as paying bills or preparing meals [20]. However, memory loss alone does not indicate dementia [21, 22].
In 2015, it was estimated that 47.5 million people have dementia worldwide, and the numbers are projected to rise to 75.6 million by 2030 and to 131.5 million by 2050. There are over 9.9 million new cases of dementia every year worldwide, or 1 new case every 3.2 seconds [23]. In high-income countries, 7 in 10 persons aged 70 years and older will pass away with some form of dementia [24]. Dementia has a huge economic impact. The total estimated worldwide medical cost of dementia in 2015 was $818 billion, and it will become a trillion-dollar disease by 2018 [23].
Changes in the prevalence of dementia in the elderly population is taking place the fastest in China and India, and it is occurring in elderly persons of Asian and western Pacific descent. About 4.9 million people are newly diagnosed with dementia each year, with 49% of the total in Asia, 25% in Europe, 18% in the United States, and 8% in Africa. Dementia occurs in elderly persons from low- and middle-income countries only 10% less than in elderly persons from high-income countries (e.g. United States). According to the Revised Global Burden of Disease assessment by the Institute of Health Metrics and Evaluation, dementia has dropped from 5th to 9th place of those diseases determined to be the most burdensome in people aged 60 years and over.
Dementia can be categorized in a variety of ways, such as according to the portion of the brain that is affected or whether it worsens over time (progressive dementia). Common progressive diseases or conditions that are characterized by dementia are AD, VaD, Lewy body dementia, and fronto-temporal dementia. Other diseases or conditions associated with dementia are Huntington’s disease, traumatic brain injury, Creutzfeld-Jakob disease, and Parkinson’s disease. Some dementias are reversible with treatment [25], such as those that were a response to medicine or an infection, metabolic problems and endocrine abnormalities, nutritional deficiencies, subdural hematomas, poisoning, brain tumors, anoxia, and normal pressure hydrocephalus.
Further research is needed to understand comprehensive picture of ischemic stroke, and its links with VaD and AD.
Alzheimer’s disease
AD is a multifactorial neurodegenerative disease characterized by memory loss, multiple cognitive impairments and progressive degeneration of behavioral and functional capacities [26–30]. AD accounts for more than 80% of dementia cases worldwide in people older than 65 [31]. About partial of these cases involve exclusively AD pathology; many have indication of pathologic variations linked to other dementias. AD is associated with the loss of synapses, synaptic dysfunction, mitochondrial structural and functional abnormalities, inflammatory responses, in addition to extracellular neuritic plaques and intracellular neurofibrillary tangles. These characteristics have been associated with the injury, malfunctioning, and death of neurons. There are three different types of AD: early onset AD, late-onset AD and familial AD. Early onset and familial AD is associated genetic mutations in the amyloid precursor protein (APP), presenilin 1, and presenilin 2. Late-onset AD is associated with apolipoprotein allele E4 genotype and polymorphisms/genetic variants in multiple genes. In addition, type 2 diabetes, traumatic brain injury and ischemic stroke are other contributing factors for the development of AD [32].
Vascular Dementia
Next to AD, VaD is the most seriously dementing illness, accounting for about 10% of all dementia cases. VaD is characterized by a decline in thinking skills caused by conditions that block or reduce blood flow to the brain, depriving brain cells of vital oxygen and nutrients. About 50% of persons with dementia have pathologic indications of VaD. In most cases, the infarcts exist with AD pathology [33]. VaD includes a set of varied dementing disorders owing to cerebrovascular inadequacy. Four types of VaD are: stroke-related dementia, single- and multi-infarct dementia, sub-cortical dementia, and mixed dementia. Vascular dementia may result from brain damage caused by numerous strokes or minor blood clots in heart or neck arteries that block a branch of a blood vessel in the brain [34].
Risk Factors for Stroke, Vascular Dementia and Alzheimer’s Disease
In the United States one in nine Americans over 65 years of age has AD, which is ranked as the sixth most common cause of death in the United States. By 2050, there could be as many as 7 million people age 85 years and older with AD [23]. The prevalence of VaD ranges from 1–4% in people over 65 years and seems to be higher in China and Japan than in Europe and North America [35]. The annual incidence of VaD may range from 20 to 40 per 100,000 in persons aged 60–69 years, to 200 to 700 per 100,000 in persons over age 80 years [36].
Stroke is associated with risk factors, some of which may be non-modifiable (e.g., age, sex, ethnicity and heredity) and some, modifiable (e.g., hypertension, smoking, diabetes, atrial fibrillation) [37]. The risk of stroke increases with the number of modifiable risk factors that an individual has [7]. Modifiable risk factors can be controlled through pharmacological or surgical interventions and lifestyle changes, as primary or secondary stroke prevention strategies.
Non-modifiable Risk Factors
There several non-modifiable factors including, age, sex, race, ApoE4 genotype, Down syndrome, mutations in APP, PS1 and PS2 genes, and polymorphisms in BINI, CLU, ABCA7, CR1, PICALM genes. These factors are constant and risks of these factors can not avoided in developing dementia in elderly individuals.
Age and Gender
It is significant to discriminate sex and gender for the understanding of risk and defensive mechanisms of disease. Age is the significant risk factor for stroke with sudden increases in incidence rates with increasing age. The risk of life hit by a first-time stroke increases exponentially from about 30 per 100,000 individuals at 30–39 years of age, to about 2000–3000 per 100,000 at ages above 85 [38]. IS occurs predominantly in elderly adults. The incidence of stroke is estimated to be between 20 and 60 per 100,000 live births [39]. Stroke incidental rate is higher in men than in women but only at younger and middle-age groups, but this not true in the oldest age groups, incidence rates in women are about equivalent or even greater than in men [7, 19]. The differences between men and women regarding the risk of being suffering from stroke may to some extent be associated with lifestyle indicators such as smoking habits, alcohol intake and type of occupation [40].
Maximum populace with AD are diagnosed at oldness 65 or elder. The risk of emerging AD reaches 50% for persons beyond age 85. People younger than 65 can also progress the disease, though this is much odder. However age is the highest risk factor, AD is not a usual part of aging and age alone is not adequate to cause the disease [41]. It leftovers indistinct whether women have a higher risk than men to develop dementia or AD at a given age. Numerous European studies have proposed that women have a higher incidence rate of dementia or AD than men. However, studies in the United States have not revealed a difference, or the difference has diverse with age [42].
Studies show that the incidence of VaD exponentially increases with age from 65–85 years. In 2030, closely 70 million patients with dementia is expected in 65 and older peoples, without any considerable difference between men and women. Occurrence of VaD was higher in men compared to women in below 85 years of age [43]. A likely description for the increased incidence of dementia in women is that they suffer higher rates of obesity, diabetes and other conditions which increase the probability of developing AD. In a longitudinal community study of Japanese American men (Honolulu Asia Aging Study) revealed that 23%, 50% and 16% of VaD was attributed to large-vessel, small-vessel and mixed vessel disease correspondingly [44].
Race
Race has been reported to be an independent predictor of stroke severity and the subtype of stroke [45]. Stroke incidence differs across racial groups, with black persons at higher incidence rates of strokes compared to Caucasians [48].
There are more non-Hispanic whites existing with AD and other dementias than people of any added racial or ethnic group in the United States, older African-Americans and Hispanics are more likely than older whites to have AD and other dementias [48].
A review of numerous studies shown that there was evidence that missed diagnoses of AD and other dementias were more communal amongst older African-Americans and Hispanics than older whites [49]. Remarkably, they found that there was a significantly higher prevalence of AD in the rural than the urban area. The prevalence of dementia importantly varies amid dissimilar ethnic groups living in the similar country. The proportion of VaD dementia was diverse from that in Europe and other Asian countries. There was a higher prevalence of VaD in the urban than the rural area [50].
Modifiable Risk Factors
There are multiple modifiable factors that are involved with dementia, including diet, physical and mental activities, cardiovascular, hypertension, diabetes, obesity, metabolic syndrome, smoking, depression and traumatic brain injury. These factors can be controlled and prevent and/or delay dementia in elderly individuals.
Hypertension
About 50% of victims of IS have high blood pressure, making high blood pressure is the highest risk factor for stroke [51, 52]. Uncontrolled hypertension increases a person’s stroke risk by 4–6 times. Hypertension leads to atherosclerosis and hardening of the large arteries and can lead to blockage of small blood vessels in the brain. High blood pressure can also lead to HS since high blood pressure can weaken the blood vessels in the brain, causing them to balloon and burst. Several cross-sectional and longitudinal studies that have explored the relationship of hypertension to dementia and AD [53, 54]. From these studies, we have learned that hypertension can promote and exaggerate VaD, but hypertension has not been associated with AD [55].
In one study, participants who had a history of taking medications to control their hypertension were less likely to develop dementia compared to participants with hypertension who had never used an antihypertension agent [56].
A study of data from hypertension revealed that agents that lowered the blood pressure of hypertensive participants did not reduce the incidence of dementia [57].
Diabetes Mellitus
Diabetes mellitus is a main risk factor for stroke. When people with diabetes have a stroke, the effect of the stroke on them is far worse than on persons without diabetes. In people with diabetes, many bypass arteries are already affected by atherosclerosis and have impaired blood flow to the brain, so that when a person with diabetes experiences a stroke, the incidence rate of death is much higher. In one study, 65% of patients with diabetes mellitus who had a stroke passed away within hours of the stroke or they developed coronary heart disease [58].
Type 2 diabetes mellitus (T2DM) is also a risk factor for cognitive dysfunction and dementia in persons older than 65 years. T2DM has been associated with vascular diseases, ultimately leading to cognitive dysfunction and VaD [59], but recent studies have established that T2DM is also associated with AD, possibly due to T2DM accelerating AD-associated pathologies through insulin resistance. Several other mechanisms may be involved in T2DM-related cognitive dysfunction, such as increased inflammation, insulin resistance and hyperglycemia [60].
Smoking
Tobacco smoke contains 70,000 toxic chemicals, including carbon monoxide, formaldehyde, and hydrogen cyanide, each of which are known to damage brain cells structurally and/or functionally. These changes in turn increases the risk of stroke. Smoking increases the risk of stroke by three-to-four fold, and exposure to environmental smoke (e.g., a smoker in the home) increases the risk of stroke by 1.5 to two fold [61]. Smoking has also been associated with an increased risk of developing VaD and AD [62].
There is evidence that erroneous diagnoses of AD and other dementias are more common in African-Americans and Hispanics 80 years and older than in whites of this same age range [49]. Researchers have also found a significantly higher prevalence of AD in persons of any racial background, in rural areas compared to urban areas. In Europe and Asian countries, VaD was more common in urban than in rural areas [50].
Links between stroke and vascular dementia (VaD) and Alzheimer’s disease (AD)
After a few minutes or even a few seconds following IS, the IS cascade begins and can continue for hours until the disease ceases. The IS cascade involves biochemical reactions in the brain and other aerobic tissues. Dementia syndromes established after stroke were typically considered to be vascular in origin and post stroke dementia might be the significance of the effects of stroke and degenerative changes [63]. Research linking stroke and dementia has focused on shared vascular risk factors, ameliorated by lifestyle activities or medication. Aging is the most important risk factor for stroke and dementia. Dementia occurs in up to one-third of elderly patients with stroke, a subset of whom have AD rather than a pure VaD. A mixed etiology of dementia and cerebrovascular disease was thought to become more common with increasing age, although no clinical criteria for the diagnosis of dementia with cerebrovascular diseases are currently available [64]. Stroke doubles the risk for dementia (post-stroke dementia), and approximately 30% of stroke patients go on to develop cognitive dysfunction within 3 years [65, 66]. The association between stroke and dementia was also observed in patients younger than 50 years, up to 50% of whom exhibit cognitive deficits after a decade [67].
VaD and AD are important because these results from a variety of causes, including cerebrovascular dysfunction, but the evidence for their association with other neurodegenerative disorders is limited [68]. VaD, AD and stroke have common risk factors including hypertension, insulin resistance, diabetes, obesity, hyperhomocystinemia, and hyperlipidemia. Cerebrovascular disease has been suggested to contribute to AD neuropathological changes including selective brain atrophy and accumulation of abnormal proteins such as amyloid-beta [69]. Recent clinical-pathological studies have focused on cognitive impairment and increased risk of dementia in patients with cerebrovascular disease [70]. In addition, VaD is the most severe form of vascular cognitive impairment (VCI) [34], and it results from subclinical vascular brain injury and stroke. The molecular links between stoke and VaD, and stroke and AD are currently not clearly understood [71]. There are many questions raised in research linking stroke and dementia are largely unanswered. Hence, it is important to understand early events of stroke, and stroke leading to VaD and AD. Very little can be done after disease onset starts. Therefore, therapy needs to be initiated as soon as possible, with its immediate goal to normalize perfusion and to mediate any biochemical dysfunction to recover the obscurity as early as possible [72].
Methods Used in Stroke Research
Cell models of stroke
Various cell culture models have been developed to understand the underlying mechanistic features of cerebral ischemia. Primary neuronal and glial cultures from cortex, hippocampus, cerebellum, and hypothalamus of embryo or perinatal rats and mice have been used widely to study anoxic or ischemic damage. The overall objective of in vitro research on ischemia was to understand the changes specific to each cell population and how they are similar in vivo. These cell models aid the progress and testing of potential therapeutic agents designed to prevent and/or counteract specific steps in the ischemic cascade of neurodegeneration [73].
Different cellular models from rat, human, mouse, bovine and porcine brain endothelial cells have been used in reports of oxygen-glucose deprivation (OGD) experiments mimicking as accurately as possible the pathological conditions of the vascular system in the ischemic brain. OGD is widely used as an in vitro model for stroke, showing similarities with the in vivo models of brain ischemia [74]. Primary culture of human cerebro microvascular endothelial cells (HCEC) or immortalized human brain capillary endothelial cells (hCMEC/D3) were used in many cell based studies with these two human brain endothelial cells [75, 76]. Many researchers used other cellular models such as primary rat brain microvascular endothelial cells (rBMEC) [77] from adult and young rats and primary mouse brain microvascular endothelial cells (mBMEC) [78] to mimic in vivo conditions of patients. Some researchers have used primary bovine brain capillary endothelial cell (BCEC) culture to perform OGD experiments [79].
Sun et al. (2014) [80] attempted to elucidate the role of semicarbazide-sensitive amine oxidase/vascular adhesion protein 1 (SSAO/VAP-1) present in endothelial cells during ischemic stroke conditions. In order to understand changes in SSAO/VAP-1 cells, they have used an easy ischemic model using OGD as an experimental approach to the stroke development. Results showed that SSAO/VAP-1 shows a role in leukocyte adhesion, inflammation and cell damage processes under ischemic conditions and its expression increases the susceptibility of endothelial cells to OGD, and that its enzymatic activity, through specific substrate oxidation, increases vascular cell damage under these experimental conditions. Further, they have concluded that stroke-related processes in which SSAO/VAP-1 contributes, would be an interesting therapeutic target and it was useful tool for the screening of new molecules as therapeutic agents for cerebral ischemia.
Using cell culture model, Allen et al. (2010) [81] conducted research to explore the function of small GTPase RhoA and its effector Rho kinase in permeability changes mediated by OGD. They concluded that OGD concessions the structural and functional capacities of an in vitro model of human cerebral barrier through activation of RhoA/Rho kinase pathway.
A research group from Canada attempted to study the effect of neutrophils on the increase in BBB permeability associated with ischemic reperfusion injury in vitro [82]. Human brain endothelial cell line hCMEC/D3 was exposed to OGD with reoxygenation, and permeability was measured for a range of OGD exposure times (1–24 h). This study found that OGD induces reversible increases in permeability linked to nitric oxide synthesis in a human culture model of the BBB and shows that neutrophils mitigate permeability increases [82].
Singh and colleagues (2009) [83] studied the effect of glucose concentration on the cell viability of oxygen-glucose deprived PC-12 cells during re-oxygenation period. They found that glucose concentration during re-oxygenation might be one among the key factors involved in the growth and proliferation in PC-12 cells. The OGD of 6 h followed by a re-oxygenation period of 24 h with 4–6 mg/ml glucose concentration were found to be the optimum conditions to create the cerebral stroke like situations under in vitro environment using PC-12 cells [83].
Xu et al. (2000) aimed to characterize bovine cerebral endothelial cells (EC) death in relation to inducible NO synthase (iNOS) expression after OGD in vitro [84]. They showed that programmed cell death might be involved in OGD-induced bovine cerebral EC, iNOS expression contributing partially to bovine cerebral EC death. Further they have summarized that understanding the mechanism of cerebral EC death after OGD might aid in the development of therapeutic strategies to reduce secondary ischemic brain injury caused by post ischemic hypoperfusion and blood-brain barrier dysfunction.
Guo et al. (2010) conducted an experimental cellular study to determine whether the dipyridamole could ameliorate brain endothelial injury after exposure to inflammatory and metabolic insults using human brain endothelial cells [85]. They found that dipyridamole ameliorates brain endothelial injury after inflammatory and metabolic stress. They concluded that dipyridamole might possess interesting neurovascular effects in brain but how these cellular observations might relate to stroke remains to be determined.
Recently Hung et al. (2015) investigated the long-term effect of astrocytic endothelin-1 (ET-1) induction in the development of post stroke cognitive deficit in association with astrocyte derived amyloid production [86]. In this study, ET-1 overexpressing astrocytic cells showed amyloid secretion after hypoxia/ischemia insult, which activated endothelin A and endothelin B receptors in a PI3K/AKT-dependent manner, suggesting role of astrocytic ET-1 in dementia associated with stroke by astrocyte derived amyloid production.
Overall, findings from these cell culture studies significantly improved our basic understanding of mechanisms involved in stroke. Further, observations prompted to investigate in vivo studies of stroke.
Animal models of stroke
Epidemiologic surveys of human populations are essential to identify non-modifiable and modifiable risk factors of stroke, but other methods are needed to advance our understanding and development of stroke therapies [87]. In recent years, attention has been shifting to the use of transgenic mice as animal models of stroke, facilitating biochemical studies of how risk factors impact the brain, resulting in stroke. Identify a few different types of animal models of stroke.
Animal models of stroke have been used in studies of stroke-related risk factors, in particular, the modifiable risk factors of atherosclerosis [88], hypercholesterolemia (ApoE deficient) [89] and ApoB mutant mouse [90], hypertension (Ren-2 transgenic and Dahl sensitive rat) [91], and hyperhomocysteinemia (CBS and MTHFR deficient mouse) [92], and the nonmodifiable risk factor of age (spontaneously aged > 18 months and Senescence-accelerated mice) [93].
There are many confounding factors that need to be controlled when using animal models of stroke, such as species, age, sex, sample size, temperature, glucose level, blood pressure, and the anesthetic agent used to sacrifice the animal for study [94]. Numerous in vitro and in vivo models of stroke have been described over the years. A good in vivo animal model of stroke must reproduce the most significant functional, anatomical, etiology and metabolic features of human stroke pathology, and it must also permit the study of anti-ischemic drugs in situations relevant to clinical therapeutics. Major animal models of stroke can be classified into three subgroups: those that facilitate the study of focal ischemia (middle cerebral artery occlusion, single carotid artery occlusion) [95, 96], global ischemia (total body ischemia, global cerebral ischemia) [87, 97], and forebrain ischemia (bilateral common carotid occlusion, four vessels occlusion) [98, 99].
Overall, cell and models of stroke are useful and important to understand the basic mechanisms of stroke. Further research is needed to better understand early events/mechanisms of stroke.
Induction of Focal Ischemia
The induction of transient and permanent focal ischemia in animal models has been the most frequently used method to study mechanisms of IS. Most human ISs are caused by the occlusion of a middle cerebral artery (MCA), so animal models were developed to induce ischemia (transient and permanent) in this arterial territory [100]. In the induction of transient focal ischemia, blood vessels in the brain are blocked for up to 3 hours, followed by prolonged reperfusion. In permanent focal ischemia, arterial blockage continues, usually for one or more days. In both types of induced ischemia, blockage is achieved via mechanical, thermal, embolic, or chemical methods [101]. In mechanical ischemia, proximal occlusion is achieved through the intraluminal suture technique. This technique is the most frequently used method to occlude cerebral arteries in rat models of stroke since it was relatively easy to execute and it was noninvasive [102]. This method involves a poly-L-lysine–coated intraluminal suture that, yields reliably large infarcts and greatly reduced inter-animal changeability in rats [103]. Another research group aimed compare the effectiveness and reproducibility of MCA filament occlusion model in rats and mice, demonstrated that the microsurgical filament occlusion of the MCA can be more successfully performed in mice [104].
Nagai et al. (1999) studied the relative effects of chemical methods to induce ischemia, focusing on tissue plasminogen activator (tPA), streptokinase, and staphylokinase on cerebral ischemic infarction and pulmonary clot lysis in hamster models [105]. All of these drugs triggered maximal focal cerebral ischemia (FCS) and pulmonary clot analysis (PCL) rates at similar doses without α2-antiplasmin and fibrinogen depletion. Injection of 6 mg/kg human plasminogen combined with streptokinase caused a systemic fibrinolytic state with fibrinogen depletion. Thus, these thrombolytic agents caused a similar dose-related extension of FCI after MCA ligation and PCL, irrespective of the agent or systemic plasmin generation.
To learn the effects of tPA on FCI, Meng et al. (1999) used two rat models of cerebral ischemia [106]: In experiment 1, rats were subjected to focal ischemia via injection of autologous clots into the middle cerebral artery territory. In experiment 2, three groups of rats were subjected to focal ischemia via a mechanical approach in which a silicon-coated filament was used intraluminally to occlude the MCA [106]. They found that tPA is an effective chemical therapy to induce thromboembolic occlusions. They did not find any evidence of any neurotoxic effects of tPA on cerebral tissue. However, they have speculated on potentially negative effects of tPA on cerebral tissue from particular animal species and stroke models [106].
Melatonin has the neuroprotective properties comprising anti-inflammatory properties, free radical scavenging. It has testified efficacy in transient global ischemia models and a neuroprotective drug for IS [107–109]. In directive to check the efficacy of melatonin in experimental stroke model Macleod and coworkers in 2005 conducted a systemic review and meta-analysis to evaluate the evidence for a protecting effect of melatonin in animal models of focal cerebral ischemia. In this study, they have included 14 different studies concerning 432 animals. The opinion estimation for the outcome of melatonin was a 42.8% (95% CI 39.3–46.3%). Further, these findings revealed a marked efficacy of melatonin in animal models of focal cerebral ischemia, recognize significance areas for forthcoming animal research and propose melatonin as a candidate neuroprotective drug for human stroke [110].
Another chemical method to induce occlusion of arteries in animal models of stroke – in this instance a photochemical method - is a technique first described by Watson et al. (1985) [111]. This technique involves injecting an area of the brain in an animal model of stroke systemically with a photo-sensitive dye and then exposing the area with light to observe the dye. It was found to trigger the release of reactive oxygen species (ROS), which in turn induced peroxidative damage to the endothelium and to the thrombus formation [112]. Illumination of induced infarcts were used to facilitate the analysis of neurodegenerative therapies that were applied to the stroke.
Another researcher also used a photochemically induced IS in a rat model of stroke to observe the effects of therapies to reduce deficits and to improve survive following the artery occlusion [113]. Photochemical illumination of induced occlusion was also achieved with optical fibers [114]. The Tsiminis study used photochemicals to track the progress of infarcts induced by stroke. Stroke actions in real-time by optical fibers to screen variations in C57BL6 Mice were intraperitoneally injected with fluorescence concentration of the stroke-inducing dye Rose Bengal. The optical fibers facilitated the researchers in investigating to such an extent that they have suggested using optical fibers as a prognostic tool to gauge whether a stroke was successfully induced, without needing to perform labor-intensive histology or expensive magnetic resonance imaging.
The most common model of MCA occlusion uses intraluminal filament procedures. A study by Belayev et al. (1996) [103] was conducted on a rat model of stroke to evaluate a modified method of intraluminal sutures to induce occlusion of the MCA. In this modification, the sutures were uncoated, whereas in previous studies, they were coated with poly-L-lysine (a positively charged synthetic polymer of the amino acid(s) l-lysine, known to facilitate the attachment of proteins). Of the seven rats undergoing a 60-minute occlusion of the MCA induced by uncoated sutures, only three had infarcts, whereas in the group with poly-L-lysine–coated sutures, all 7 rats exhibited infarcts that were consistently larger and that exhibited greatly reduced inter-animal variability of the infarct size. Poly-L-lysine–coated intraluminal sutures also reliably yielded a reversal of the MCA.
Using a rodent stroke model (69 rats and 71 mice), Ardehali and Rondouin (2003) studied occlusion of the MCA using microsurgical intra-lumination techniques [104]. Statistical analysis of data from surgical procedures confirmed a success rate of occlusion that was significantly lower in the rats than in the mice, similar to statistical analysis of data from autopsies of the rats and mice that underwent microsurgical intralumination occlusion. The study also demonstrated that occlusion of the MCA using microsurgical filaments could be performed more successfully in the mice, suggesting that mice are more suitable than rats to study the molecular basis of stroke and to determine preclinical assessment of neuroprotective agents against stroke. Although occlusion of the MCA with microsurgical filaments were found to be more successfully performed in mice, the procedure was more easily performed in rats [115].
Findings from these studies are useful and improved basic understanding of focal ischemia. Further research is needed to identify precise causal factors of focal ischemia.
Postmortem Brain Studies in Stroke
Postmortem human brain tissue was being used for quantifying cellular and molecular markers of neural courses with the area of improved understanding the variations in the brain caused by neurological diseases [116]. The accurate molecular mechanisms complex in ischemia tempted brain injury endure poorly unstated. The pathophysiology of stroke injury was highly complex, involving interactions among multiple cell types and signal systems [117]. IS, still missing an active neuroprotective therapy, lasts to be a major socioeconomic problem through the world. The contribution of peripheral organs through bidirectional communications with the brain following an ischemic stroke has been highlighted [118].
Studies of postmortem brain tissue have previously established their effectiveness in drug finding. The majority of the animal models used that were phylogenetically separated from humans loads of years before [119]. Hence, human post-mortem brain tissues are needed to exclude model-specific objects and to classify stroke-specific ‘target space’ including dissimilar pathological activities and brain cell kinds. When studying human postmortem brains, we need quality control parameters to assess its quality which might reduce the heterogeneity among samples. However, the results of these techniques are affected by other factors also [120]. New research techniques such as gene expression profiling and proteomics using post mortem brain tissues were providing exciting new avenues for research on human subjects.
Inflammation is a hallmark of stroke pathology and these inflammatory biomarkers (tumor necrosis factor, interleukin 1 and interleukin 6) control tissue injury in experimental stroke and were therefore potential targets in future stroke therapy. Tumor necrosis factor (TNF), the only cytokine that has been considered by immunohistochemical methods in post-mortem human stroke tissue [121]. Previous research findings supported the hypothesis that tumor necrosis factor-alpha (TNF-α) might be involved both in the acute proliferation of inflammatory developments and cell demise and perhaps in the more late reconstruct processes of human IS [122, 123].
Several reports have shown that T-cells can promote ischemic damage, the signaling intermediates but the mechanisms which involved in it were not yet known [124, 125]. In 2011, Chaitanya and coworkers addressed the role of cytotoxic T-cell (CTL) derived granzyme-b (Gra-b) in neuronal demise related with ischemic damage using human post-mortem stroke samples. The study indicated that Gra-b released by CTLs could mediate neuronal death by inhibiting anti-apoptotic molecules and by activating pro-apoptotic molecules in the stroke brain. These results suggested that increased leukocyte infiltration and elevated Gra-b levels in the post-stroke brain could persuade contact-dependent and autonomous post-ischemic neuronal death to worse stroke injury. Further they have concluded that future therapeutic targets for stroke might be enhanced by effectively inhibiting several cytotoxic proteases especially Gra-b to mitigate the pathology of cerebral ischemia [126].
Cuadrado et al. (2010) used autopsy brains from 6 patients (3 males, 3 females), who had total anterior circulation infarcts and documented occlusion of the MCA and the brains of 3 patients who died because of non-inflammatory and non-neurological disease were used as controls to compare and determine the protein expression pattern within the different areas of the ischemic brain [127]. Dihydropyrimidinaserelated protein 2 (DRP-2), vesicle-fusing ATPase (NSF), and Rho dissociation inhibitor 1 (RhoGDI1) were determined the cellular localization in brain parenchyma. Further, these results contributed to understand the progressions that follow cerebral ischemia and the recognized proteins might be beneficial targets or biologic markers for defining the analysis and prediction of stroke.
First quantitative clinical proteomics study in the area of ischemic stroke was done in the year 2013 using an iTRAQ-2D-LC–MS/MS based quantitative proteomic profiling approach. The proteins related to reactive gliosis such as Vimentin (VIM), Glial fibrillary acidic protein (GFAP) and anti-inflammatory response proteins Annexin (ANXA1, ANXA2) showed an increasing trend. The elevation of ferritin proteins (FTL, FTH1) might indicate an iron-mediated oxidative imbalance aggravating the mitochondrial failure and neurotoxicity. Deregulated proteins could be useful as probable therapeutic targets or biomarkers for ischemic stroke [128].
Experiments have revealed that cerebral ischemia induced using the MCA occlusion method alters the receptor expression in cerebral arteries [129, 130]. In order to examine the hypothesis was valid in humans by defining if cerebral ischemia upregulates receptors in the MCA, Vikman and Edvinsson conducted a gene expression profiling in the human MCA after cerebral ischemia. ELK3, LY64, Metallothionin IG, POU3F4, Actin a2, RhoA and smoothelin were regulated the same way when checking array expression with real-time PCR. Gene expression studies in the human MCA leading to the ischemic region was alike to that seen after MCA occlusion in rats. Further, they found new genes that support the dynamic changes that occur in the MCA distributing to the ischemic region [131]. A research group from UK conducted microarray RNA expression analysis of cerebral white matter lesions (WML) reveals changes in multiple functional pathways. They identified 8 major pathways in which multiple genes showed changed RNA transcription amongst 502 genes that were differentially expressed in WML compared to controls. In WML, 409 genes were altered involving the same pathways. Identified these number of genes that might be relevant to the pathogenesis of WML associated with aging [132].
Mitsios and coworkers demonstrated a microarray study of gene and protein regulation in human and rat brain following MCA occlusion. For this study they have obtained 12 patients human brain tissue samples who died from acute IS. Their findings confirmed previous studies reporting that gene expression screening can detect identified and unidentified transcriptional structures of stroke and highpoint the significance of research by human brain tissue in the search for unique therapeutic agents [133].
Very recently Frugier et al. (2016) [134] studied for the first time the expression and cellular localization of three autophagic markers such as microtubule-associated protein 1 light chain 3 (LC3), sequestosome 1 (SQSTM1) and Beclin 1 (BECN1) using post-mortem brain tissue from patients with a history of stroke. They observed that observed an increase in staining in LC3, SQSTM1 and the improved presence of autophagic vesicles after stroke. Further, they have concluded that there was still controversy over whether autophagy was of benefit or detrimental to the neurological outcome after stroke, the confirmation of the existence of altered autophagy in human samples will ultimately aid in the translation of compounds targeting autophagy into the clinical sphere as potential therapeutics to combat the cell death associated with stroke.
These postmortem brain studies have improved our understanding of stroke and its molecular links to vascular dementia. Further research is still needed to understand causes of stroke, leading to vascular dementia.
Conclusions and Future Directions
Stroke is the second leading cause of death and the third leading cause of disability-adjusted, life-years worldwide. The risk of having a stroke increases with age of 55 but it can occur at any age. Stroke as an interruption to the supply of blood to the brain, with developing clinical signs of disruption of cerebral function leading to death with no apparent cause other than vascular origin. Cerebral abnormalities in stroke, particularly ischemic stroke may lead to biochemical dysfunction in the brain, ultimately leading to VaD and AD. Recent research revealed that stroke can be controlled by modifiable risk factors, including diet, cardiovascular, hypertension, smoking, diabetes, obesity, metabolic syndrome, depression and traumatic brain injury. Animal models have been used to study modifiable factors of stroke, including atherosclerosis, hypercholesterolemia, and hypertension. Multiple approaches have been developed to develop biomarkers, including circulatory miRNAs, blood based protein markers, coagulation and thrombosis biomarkers. Among these, circulatory miRNAs are reported to be promising peripheral biomarkers in stroke and stroke linked VaD and AD.
Although so much research has been done in ischemic stroke and its molecular and cellular links with VaD, 1) we still do not have complete understanding of causal factors of stroke and its molecular basis of pathogenesis, 2) and we still do not have complete understanding of genetic basis of ischemic stroke leading to vascular dementia and AD. Further research is needed to answer these important questions.
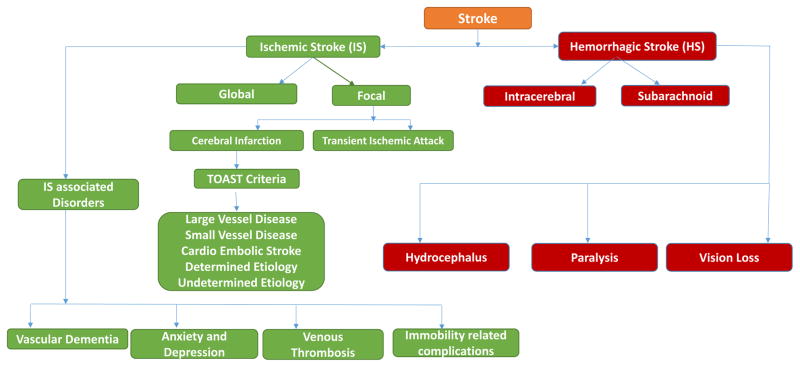
Figure 1.
Schematic representation of clinical subtypes of ischemic and hemorrhagic stroke and its associated disorders. Ischemic stroke is further classified into five types such as large-vessel disease, small vessel disease, cardio-embolic stroke, stroke of other determined aetiology and stroke of undetermined aetiology. Hemorrhagic stroke is classified into two types: intracerebral and subarachnoid.
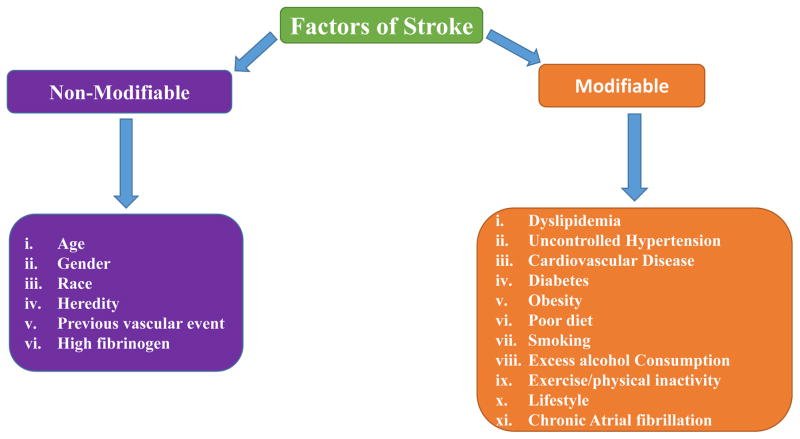
Figure 2.
List of modifiable and non-modifiable risk factors associated with ischemic stroke. The stroke is pathologically heterogeneous and the risk factor profiles leading to different types of stroke.
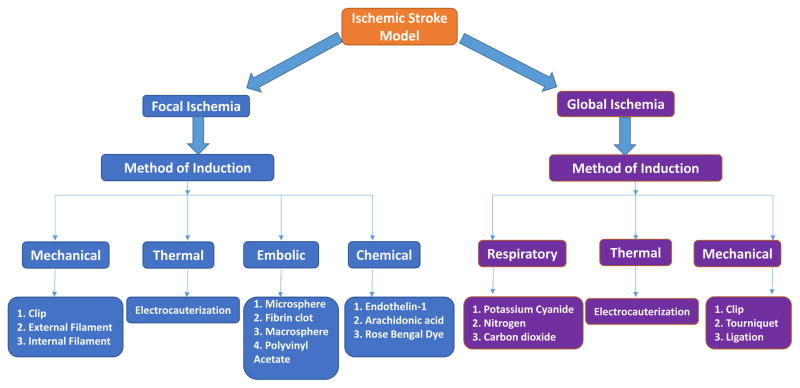
Figure 3.
Different type of models of cerebral ischemia and their method of induction. Animal models of stroke have been used in studies of stroke-related risk factors. A good in vivo animal model of stroke must reproduce the most significant metabolic features of human stroke pathology.
Acknowledgments
Work presented in this article is supported by NIH grants – AG042178 and AG47812 and Garrison Family Foundation.
References
- 1.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O’Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA, 3rd, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De León FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basáñez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabé E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan- Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng AT, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere-Derry J, Danaei G, Davis A, De Leo D, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais DC, Dharmaratne SD, Dherani M, Diaz-Torne C, Dolk H, Dorsey ER, Driscoll T, Duber H, Ebel B, Edmond K, Elbaz A, Ali SE, Erskine H, Erwin PJ, Espindola P, Ewoigbokhan SE, Farzadfar F, Feigin V, Felson DT, Ferrari A, Ferri CP, Fèvre EM, Finucane MM, Flaxman S, Flood L, Foreman K, Forouzanfar MH, Fowkes FG, Fransen M, Freeman MK, Gabbe BJ, Gabriel SE, Gakidou E, Ganatra HA, Garcia B, Gaspari F, Gillum RF, Gmel G, Gonzalez-Medina D, Gosselin R, Grainger R, Grant B, Groeger J, Guillemin F, Gunnell D, Gupta R, Haagsma J, Hagan H, Halasa YA, Hall W, Haring D, Haro JM, Harrison JE, Havmoeller R, Hay RJ, Higashi H, Hill C, Hoen B, Hoffman H, Hotez PJ, Hoy D, Huang JJ, Ibeanusi SE, Jacobsen KH, James SL, Jarvis D, Jasrasaria R, Jayaraman S, Johns N, Jonas JB, Karthikeyan G, Kassebaum N, Kawakami N, Keren A, Khoo JP, King CH, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Laden F, Lalloo R, Laslett LL, Lathlean T, Leasher JL, Lee YY, Leigh J, Levinson D, Lim SS, Limb E, Lin JK, Lipnick M, Lipshultz SE, Liu W, Loane M, Ohno SL, Lyons R, Mabweijano J, MacIntyre MF, Malekzadeh R, Mallinger L, Manivannan S, Marcenes W, March L, Margolis DJ, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGill N, McGrath J, Medina-Mora ME, Meltzer M, Mensah GA, Merriman TR, Meyer AC, Miglioli V, Miller M, Miller TR, Mitchell PB, Mock C, Mocumbi AO, Moffitt TE, Mokdad AA, Monasta L, Montico M, Moradi-Lakeh M, Moran A, Morawska L, Mori R, Murdoch ME, Mwaniki MK, Naidoo K, Nair MN, Naldi L, Narayan KM, Nelson PK, Nelson RG, Nevitt MC, Newton CR, Nolte S, Norman P, Norman R, O’Donnell M, O’Hanlon S, Olives C, Omer SB, Ortblad K, Osborne R, Ozgediz D, Page A, Pahari B, Pandian JD, Rivero AP, Patten SB, Pearce N, Padilla RP, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Phillips MR, Pierce K, Pion S, Polanczyk GV, Polinder S, Pope CA, 3rd, Popova S, Porrini E, Pourmalek F, Prince M, Pullan RL, Ramaiah KD, Ranganathan D, Razavi H, Regan M, Rehm JT, Rein DB, Remuzzi G, Richardson K, Rivara FP, Roberts T, Robinson C, De Leòn FR, Ronfani L, Room R, Rosenfeld LC, Rushton L, Sacco RL, Saha S, Sampson U, Sanchez-Riera L, Sanman E, Schwebel DC, Scott JG, Segui-Gomez M, Shahraz S, Shepard DS, Shin H, Shivakoti R, Singh D, Singh GM, Singh JA, Singleton J, Sleet DA, Sliwa K, Smith E, Smith JL, Stapelberg NJ, Steer A, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Syed S, Tamburlini G, Tavakkoli M, Taylor HR, Taylor JA, Taylor WJ, Thomas B, Thomson WM, Thurston GD, Tleyjeh IM, Tonelli M, Towbin JA, Truelsen T, Tsilimbaris MK, Ubeda C, Undurraga EA, van der Werf MJ, van Os J, Vavilala MS, Venketasubramanian N, Wang M, Wang W, Watt K, Weatherall DJ, Weinstock MA, Weintraub R, Weisskopf MG, Weissman MM, White RA, Whiteford H, Wiebe N, Wiersma ST, Wilkinson JD, Williams HC, Williams SR, Witt E, Wolfe F, Woolf AD, Wulf S, Yeh PH, Zaidi AK, Zheng ZJ, Zonies D, Lopez AD, AlMazroa MA, Memish ZA. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 3.WHO MONICA Project Principal Investigators. The World Health Organization MONICA Project (monitoring trends and determinants in cardiovascular disease): a major international collaboration. J Clin Epidemiol. 1988;41:105–114. doi: 10.1016/0895-4356(88)90084-4. [DOI] [PubMed] [Google Scholar]
- 4.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 5.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE., 3rd Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 6.Feigin V, Lawes C, Bennet D, Barker Cello S, Parag V. Worldwide stroke incidence and early case fatality in 56 population based studies: a systematic review. Lancet Neurolo. 2009;8:355– 369. doi: 10.1016/S1474-4422(09)70025-0. [DOI] [PubMed] [Google Scholar]
- 7.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 8.WHO. The atlas of heart disease and stroke. 2011 Retrieved 19.10.2011 from http://www.who.int/cardiovascular_diseases/resources/atlas/en/
- 9.Banarjee TK, Roy MK, Bhoi KK. Is stroke increasing in India- preventive measures that need to be implemented. J Indian Med Assoc. 2005;103:162–166. [PubMed] [Google Scholar]
- 10.Gupta R, Joshi P, Mohan V, Reddy S, Yusuf S. Epidemiology and causation of coronary heart disease and stroke in India. Heart. 2008;94:16–26. doi: 10.1136/hrt.2007.132951. [DOI] [PubMed] [Google Scholar]
- 11.Dalal P, Bhattacharjee M, Vairale J, Bhat P. UN millennium development goals: can we halt the stroke epidemic in India? Ann Indian Acad Neurol. 2007;10:130–136. [Google Scholar]
- 12.Mukherjee D, Chirag PG. Epidemiology and the Global Burden of Stroke. World Neurosurg. 2011;76:S85–S90. doi: 10.1016/j.wneu.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 13.Fisher M, Norrving B. 1st Global Conference on Healthy Lifestyles and Non communicable diseases Control; Moscow. April 28–29.2011. [Google Scholar]
- 14.Sridharan SE, Unnikrishnan JP, Sukumaran S, Sylaja PN, Nayak SD, Sarma PS, Radhakrishnan K. Incidence, types, risk factors, and outcome of stroke in a developing country: the Trivandrum Stroke Registry. Stroke. 2009;40:1212–8. doi: 10.1161/STROKEAHA.108.531293. [DOI] [PubMed] [Google Scholar]
- 15.Strong K, Mathers C, Bonita R. Preventing stroke: saving lives around the world. Lancet Neurol. 2007;6:182–187. doi: 10.1016/S1474-4422(07)70031-5. [DOI] [PubMed] [Google Scholar]
- 16.Towfighi AI, Ovbiagele B, Saver JL. Therapeutic milestone: stroke declines from the second to the third leading organ- and disease-specific cause of death in the United States. Stroke. 2010;41:499–503. doi: 10.1161/STROKEAHA.109.571828. [DOI] [PubMed] [Google Scholar]
- 17.Banerjee I, Gupta V, Ahmed T, Faizaan M, Agarwal P, Ganesh S. Inflammatory system gene polymorphism and the risk of stroke: a case-control study in an Indian population. Brain Res Bull. 2008;75:158–65. doi: 10.1016/j.brainresbull.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ. American Heart Association Advocacy Coordinating Committee; Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Arteriosclerosis; Thrombosis and Vascular Biology; Council on Cardiopulmonary; Critical Care; Perioperative and Resuscitation; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease; Council on Cardiovascular Surgery and Anesthesia, and Interdisciplinary Council on Quality of Care and Outcomes Research. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–44. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 19.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 20.American Psychiatric Association. Diagnostic and statistical manual of mental disorders, (4th ed., text revision) Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 21.Bozoki A, Giordani B, Heidebrink JL, Berent S, Foster NL. Mild cognitive impairments predict dementia in nondemented elderly patients with memory loss. Arch Neurol. 2001;58:411–6. doi: 10.1001/archneur.58.3.411. [DOI] [PubMed] [Google Scholar]
- 22.Steinberg M, Sheppard JM, Tschanz JT, Norton MC, Steffens DC, Breitner JC, Lyketsos The incidence of mental and behavioral disturbances in dementia: the Cache County Study. J Neuropsychiatry Clin Neurosci. 2003;15:340–345. doi: 10.1176/jnp.15.3.340. [DOI] [PubMed] [Google Scholar]
- 23.Alzheimer’s Disease International. World Alzheimer Report (2015) London: Alzheimer’s Disease International; 2015. [Google Scholar]
- 24.World Health Organization. Dementia: A public health priority. Geneva: World Health Organization; 2012. [Google Scholar]
- 25.Piccini C, Bracco L, Amaducci L. Treatable and reversible dementias: an update. J Neurol Sci. 1998;153:172–81. doi: 10.1016/s0022-510x(97)00289-x. [DOI] [PubMed] [Google Scholar]
- 26.Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–66. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 27.Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430:631–9. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LaFerla FM, Green KN, Oddo S. Intracellular amyloid-beta in Alzheimer’s disease. Nat Rev Neurosci. 2007;8:499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- 29.Reddy PH, Manczak M, Mao P, Calkins MJ, Reddy AP, Shirendeb U. Amyloid-beta and mitochondria in aging and Alzheimer’s disease: implications for synaptic damage and cognitive decline. J Alzheimers Dis. 2010;20:S499–512. doi: 10.3233/JAD-2010-100504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anand R, Gill KD, Mahdi AA. Therapeutics of Alzheimer’s disease: Past, present and future. Neuropharmacology. 2014;76:27–50. doi: 10.1016/j.neuropharm.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 31.Reitz C, Mayeux R. Alzheimer disease: epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem Pharmacol. 2014;88:640–651. doi: 10.1016/j.bcp.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duthey B. Background paper 6.11. [Accessed May 15, 2014];Alzheimer disease and other dementias. 2013 Available from: http://www.who.int/medicines/areas/priority_medicines/BP6_11Alzheimer.pdf.
- 33.Fernando MS, Ince PG. MRC Cognitive Function and Ageing Neuropathology Study Group. Vascular pathologies and cognition in a population-based cohort of elderly people. J Neurol Sci. 2004;226:13–7. doi: 10.1016/j.jns.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S. American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:2672–713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rizzi L, Rosset I, Roriz-Cruz M. Global Epidemiology of Dementia: Alzheimer’s and Vascular Types. BioMed Research International. 2014;2014:908915. doi: 10.1155/2014/908915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brayne C, Richardson K, Matthews FE, Fleming J, Hunter S, Xuereb JH, Paykel E, Mukaetova-Ladinska EB, Huppert FA, O’Sullivan A, Dening T. Cambridge City Over-75s Cohort Cc75c Study Neuropathology Collaboration. Neuropathological correlates of dementia in over-80-year-old brain donors from the population-based Cambridge city over-75s cohort (CC75C) study. J Alzheimers Dis. 2009;18:645–58. doi: 10.3233/JAD-2009-1182. [DOI] [PubMed] [Google Scholar]
- 37.Goldstein LB, Adams R, Becker K, Furberg CD, Gorelick PB, Hademenos G, Hill M, Howard G, Howard VJ, Jacobs B, Levine SR, Mosca L, Sacco RL, Sherman DG, Wolf PA, del Zoppo GJ. Primary prevention of ischemic stroke: A statement for healthcare professionals from the Stroke Council of the American Heart Association. Stroke. 2001;32:280–99. doi: 10.1161/01.str.32.1.280. [DOI] [PubMed] [Google Scholar]
- 38.Fagius J, Aquilonius SM. Neurologi. 4. Stockholm: Liber AB; 2006. pp. 258–76. [Google Scholar]
- 39.Lee J, Croen LA, Lindan C, Nash KB, Yoshida CK, Ferriero DM, Barkovich AJ, Wu YW. Predictors of outcome in perinatal arterial stroke: a population-based study. Ann Neurol. 2005;58:303–8. doi: 10.1002/ana.20557. [DOI] [PubMed] [Google Scholar]
- 40.Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke. 1998;29:159–165. doi: 10.1161/01.str.29.1.159. [DOI] [PubMed] [Google Scholar]
- 41.Kukull WA, Higdon R, Bowen JD, McCormick WC, Teri L, Schellenberg GD, van Belle G, Jolley L, Larson EB. Dementia and Alzheimer disease incidence: a prospective cohort study. Arch Neurol. 2002;59:1737–46. doi: 10.1001/archneur.59.11.1737. [DOI] [PubMed] [Google Scholar]
- 42.Mielke MM, Vemuri P, Rocca WA. Clinical epidemiology of Alzheimer’s disease: assessing sex and gender differences. Clin Epidemiol. 2014;6:37–48. doi: 10.2147/CLEP.S37929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dong MJ, Peng B, Lin XT, Zhao J, Zhou YR, Wang RH. The prevalence of dementia in the People’s Republic of China: a systematic analysis of 1980–2004 studies. Age Ageing. 2007;36:619–624. doi: 10.1093/ageing/afm128. [DOI] [PubMed] [Google Scholar]
- 44.Launer LJ, Ross GW, Petrovitch H, Masaki K, Foley D, White LR, Havlik RJ. Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiol Aging. 2000;21:49–55. doi: 10.1016/s0197-4580(00)00096-8. [DOI] [PubMed] [Google Scholar]
- 45.Kirshner HS. Differentiating ischemic stroke subtypes: risk factors and secondary prevention. J Neurol Sci. 2009;279:1–8. doi: 10.1016/j.jns.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 46.Bao DQ, Mori TA, Burke V, Puddey IB, Beilin LJ. Effects of dietary fish and weight reduction on ambulatory blood pressure in overweight hypertensives. Hypertension. 1998;32:710–7. doi: 10.1161/01.hyp.32.4.710. [DOI] [PubMed] [Google Scholar]
- 47.Hackam DG, Spence JD. Combining multiple approaches for the secondary prevention of vascular events after stroke: a quantitative modeling study. Stroke. 2007;38:1881–5. doi: 10.1161/STROKEAHA.106.475525. [DOI] [PubMed] [Google Scholar]
- 48.Dilworth-Anderson P, Hendrie HC, Manly JJ, Khachaturian AS, Fazio S. Social, Behavioral and Diversity Research Workgroup of the Alzheimer’s Association. Diagnosis and assessment of Alzheimer’s disease in diverse populations. Alzheimers Dement. 2008;4:305–9. doi: 10.1016/j.jalz.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 49.Clark PC, Kutner NG, Goldstein FC, Peterson-Hazen S, Garner V, Zhang R, Bowles T. Impediments to timely diagnosis of Alzheimer’s disease in African Americans. J Am Geriatr Soc. 2005;53:2012–2017. doi: 10.1111/j.1532-5415.2005.53569.x. [DOI] [PubMed] [Google Scholar]
- 50.Xu WL, Qiu CX, Wahlin A, Winblad B, Fratiglioni L. Diabetes mellitus and risk of dementia in the Kungsholmen project: a 6-year follow-up study. Neurology. 2004;63:1181–1186. doi: 10.1212/01.wnl.0000140291.86406.d1. [DOI] [PubMed] [Google Scholar]
- 51.Dickinson CJ. Strokes and their relationship to hypertension. Curr Opin Nephrol Hypertens. 2003;12:91–6. doi: 10.1097/00041552-200301000-00015. [DOI] [PubMed] [Google Scholar]
- 52.National Stroke Association. [accessed June 28, 2012];2012 Available from http://www.stroke.org/site/PageServer?pagename=aamer.
- 53.Staessen JA, Richart T, Birkenhäger WH. Less atherosclerosis and lower blood pressure for a meaningful life perspective with more brain. Hypertension. 2007;49:389–400. doi: 10.1161/01.HYP.0000258151.00728.d8. [DOI] [PubMed] [Google Scholar]
- 54.Nagai M, Hoshide S, Kario K. Hypertension and dementia. Am J Hypertens. 2010;23:116–24. doi: 10.1038/ajh.2009.212. [DOI] [PubMed] [Google Scholar]
- 55.Ninomiya T, Ohara T, Hirakawa Y, Yoshida D, Doi Y, Hata J, Kanba S, Iwaki T, Kiyohara Y. Midlife and late-life blood pressure and dementia in Japanese elderly: the Hisayama study. Hypertension. 2011;58:22–8. doi: 10.1161/HYPERTENSIONAHA.110.163055. [DOI] [PubMed] [Google Scholar]
- 56.Haag MD, Hofman A, Koudstaal PJ, Breteler MM, Stricker BH. Duration of antihypertensive drug use and risk of dementia: A prospective cohort study. Neurology. 2009 May 19;72(20):1727–34. doi: 10.1212/01.wnl.0000345062.86148.3f. [DOI] [PubMed] [Google Scholar]
- 57.McGuinness B, Todd S, Passmore AP, Bullock R. Systematic review: Blood pressure lowering in patients without prior cerebrovascular disease for prevention of cognitive impairment and dementia. J Neurol Neurosurg Psychiatry. 2008;79:4–5. doi: 10.1136/jnnp.2007.118505. [DOI] [PubMed] [Google Scholar]
- 58.Air EL, Kissela BM. Diabetes, the metabolic syndrome, and ischemic stroke: epidemiology and possible mechanisms. Diabetes Care. 2007;30:3131–3140. doi: 10.2337/dc06-1537. [DOI] [PubMed] [Google Scholar]
- 59.Kodl CT, Seaquist ER. Cognitive dysfunction and diabetes mellitus. Endocr Rev. 2008;29:494–511. doi: 10.1210/er.2007-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Umegaki H. Impaired glycemia and Alzheimer’s disease. Neurobiol Aging. 2014;35:e21. doi: 10.1016/j.neurobiolaging.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 61.Shah RS, Cole JW. Smoking and stroke: the more you smoke the more you stroke. Expert Rev Cardiovasc Ther. 2010;8:917–932. doi: 10.1586/erc.10.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reitz C, Tang MX, Manly J, Mayeux R, Luchsinger JA. Hypertension and the risk of mild cognitive impairment. Arch Neurol. 2007;64:1734–40. doi: 10.1001/archneur.64.12.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hénon H, Pasquier F, Durieu I, Godefroy O, Lucas C, Lebert F, Leys D. Preexisting dementia in stroke patients. Baseline frequency, associated factors, and outcome. Stroke. 1997;28:2429–2436. doi: 10.1161/01.str.28.12.2429. [DOI] [PubMed] [Google Scholar]
- 64.Selnes OA, Vinters HV. Vascular cognitive impairment. Nat Clin Pract Neurol. 2006;2:538–547. doi: 10.1038/ncpneuro0294. [DOI] [PubMed] [Google Scholar]
- 65.Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol. 2009;8:1006–18. doi: 10.1016/S1474-4422(09)70236-4. [DOI] [PubMed] [Google Scholar]
- 66.Allan LM, Rowan EN, Firbank MJ, Thomas AJ, Parry SW, Polvikoski TM, O’Brien JT, Kalaria RN. Long term incidence of dementia, predictors of mortality and pathological diagnosis in older stroke survivors. Brain. 2011;134:3716–3727. doi: 10.1093/brain/awr273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schaapsmeerders P, Maaijwee NA, van Dijk EJ, Rutten-Jacobs LC, Arntz RM, Schoonderwaldt HC, Dorresteijn LD, Kessels RP, de Leeuw FE. Long-term cognitive impairment after first-ever ischemic stroke in young adults. Stroke. 2013;44:1621–1628. doi: 10.1161/STROKEAHA.111.000792. [DOI] [PubMed] [Google Scholar]
- 68.Toledo JB, Arnold SE, Raible K, Brettschneider J, Xie SX, Grossman M, Monsell SE, Kukull WA, Trojanowski JQ. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer’s Coordinating Centre. Brain. 2013;136:2697–2706. doi: 10.1093/brain/awt188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kalaria RN, Akinyemi R, Ihara M. Does vascular pathology contribute to Alzheimer changes? J Neurol Sci. 2012;322:141–7. doi: 10.1016/j.jns.2012.07.032. [DOI] [PubMed] [Google Scholar]
- 70.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 71.Honig LS, Tang MX, Albert S, Costa R, Luchsinger J, Manly J, Stern Y, Mayeux R. Stroke and the risk of Alzheimer disease. Arch Neurol. 2003;60:1707–1712. doi: 10.1001/archneur.60.12.1707. [DOI] [PubMed] [Google Scholar]
- 72.Doyle KP, Simon RP, Stenzel-Poore MP. Mechanisms of ischemic brain damage. Neuropharmacology. 2008;55:310–318. doi: 10.1016/j.neuropharm.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Honegger P, Pardo B. Aggregating brain cell cultures: investigation of stroke related brain damage. ALTEX. 2007;24:32–34. [PubMed] [Google Scholar]
- 74.Tasca CI, Dal-Cim T, Cimarosti H. In vitro oxygen-glucose deprivation to study ischemic cell death. Methods Mol Biol. 2015;1254:197–210. doi: 10.1007/978-1-4939-2152-2_15. [DOI] [PubMed] [Google Scholar]
- 75.Stanimirovic D, Shapiro A, Wong J, Hutchison J, Durkin J. The induction of ICAM-1 in human cerebromicrovascular endothelial cells (HCEC) by ischemia-like conditions promotes enhanced neutrophil/HCEC adhesion. J Neuroimmunol. 1997;76:193–205. doi: 10.1016/s0165-5728(97)00057-x. [DOI] [PubMed] [Google Scholar]
- 76.Zhu D, Wang Y, Singh I, Bell RD, Deane R, Zhong Z, Sagare A, Winkler EA, Zlokovic BV. Protein S controls hypoxic/ischemic blood-brain barrier disruption through the TAM receptor Tyro3 and sphingosine 1-phosphate receptor. Blood. 2010;115:4963–4972. doi: 10.1182/blood-2010-01-262386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hiu T, Nakagawa S, Hayashi K, Kitagawa N, Tsutsumi K, Kawakubo J, Honda M, Suyama K, Nagata I, Niwa M. Tissue plasminogen activator enhances the hypoxia/reoxygenation-induced impairment of the blood-brain barrier in a primary culture of rat brain endothelial cells. Cell Mol Neurobiol. 2008;28:1139–1146. doi: 10.1007/s10571-008-9294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yin KJ, Chen SD, Lee JM, Xu J, Hsu CY. ATM gene regulates oxygen-glucose deprivation-induced nuclear factor-kappaB DNA-binding activity and downstream apoptotic cascade in mouse cerebrovascular endothelial cells. Stroke. 2002;33:2471–2477. doi: 10.1161/01.str.0000030316.79601.03. [DOI] [PubMed] [Google Scholar]
- 79.Culot M, Mysiorek C, Renftel M, Roussel BD, Hommet Y, Vivien D, Cecchelli R, Fenart L, Berezowski V, Dehouck MP, Lundquist S. Cerebrovascular protection as a possible mechanism for the protective effects of NXY-059 in preclinical models: an in vitro study. Brain Res. 2009;1294:144–152. doi: 10.1016/j.brainres.2009.07.035. [DOI] [PubMed] [Google Scholar]
- 80.Sun P, Solé M, Unzeta M. Involvement of SSAO/VAP-1 in oxygen-glucose deprivation-mediated damage using the endothelial hSSAO/VAP-1-expressing cells as experimental model of cerebral ischemia. Cerebrovasc Dis. 2014;37:171–180. doi: 10.1159/000357660. [DOI] [PubMed] [Google Scholar]
- 81.Allen C, Srivastava K, Bayraktutan U. Small GTPase RhoA and its effector rho kinase mediate oxygen glucose deprivation-evoked in vitro cerebral barrier dysfunction. Stroke. 2010;41:2056–63. doi: 10.1161/STROKEAHA.109.574939. [DOI] [PubMed] [Google Scholar]
- 82.Cowan KM, Easton AS. Neutrophils block permeability increases induced by oxygen glucose deprivation in a culture model of the human blood-brain barrier. Brain Res. 2010;1332:20–31. doi: 10.1016/j.brainres.2010.03.066. [DOI] [PubMed] [Google Scholar]
- 83.Singh G, Siddiqui MA, Khanna VK, Kashyap MP, Yadav S, Gupta YK, Pant KK, Pant AB. Oxygen glucose deprivation model of cerebral stroke in PC-12 cells: glucose as a limiting factor. Toxicol Mech Methods. 2009;19:154–60. doi: 10.1080/15376510802355216. [DOI] [PubMed] [Google Scholar]
- 84.Xu J, He L, Ahmed SH, Chen SW, Goldberg MP, Beckman JS, Hsu CY. Oxygen-glucose deprivation induces inducible nitric oxide synthase and nitrotyrosine expression in cerebral endothelial cells. Stroke. 2000;31:1744–1751. doi: 10.1161/01.str.31.7.1744. [DOI] [PubMed] [Google Scholar]
- 85.Guo S, Stins M, Ning M, Lo EH. Amelioration of inflammation and cytotoxicity by dipyridamole in brain endothelial cells. Cerebrovasc Dis. 2010;30:290–296. doi: 10.1159/000319072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hung VK, Yeung PK, Lai AK, Ho MC, Lo AC, Chan KC, Wu EX, Chung SS, Cheung CW, Chung SK. Selective astrocytic endothelin-1 overexpression contributes to dementia associated with ischemic stroke by exaggerating astrocyte-derived amyloid secretion. J Cereb Blood Flow Metab. 2015;35:1687–1696. doi: 10.1038/jcbfm.2015.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bacigaluppi M, Comi G, Hermann DM. Animal models of ischemic stroke. Part two: modeling cerebral ischemia. Open Neurol J. 2010;4:34–38. doi: 10.2174/1874205X01004020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Humphries SE, Morgan L. Genetic risk factors for stroke and carotid atherosclerosis: insights into pathophysiology from candidate gene approaches. Lancet Neurol. 2004;3:227–235. doi: 10.1016/S1474-4422(04)00708-2. [DOI] [PubMed] [Google Scholar]
- 89.Willner EL, Tow B, Buhman KK, Wilson M, Sanan DA, Rudel LL, Farese RV., Jr Deficiency of acyl CoA:cholesterol acyltransferase 2 prevents atherosclerosis in apolipoprotein E-deficient mice. Proc Natl Acad Sci U S A. 2003;100:1262–1267. doi: 10.1073/pnas.0336398100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Whitworth CE, Fleming S, Cumming AD, Morton JJ, Burns NJ, Williams BC, Mullins JJ. Spontaneous development of malignant phase hypertension in transgenic Ren-2 rats. Kidney Intl. 1994;46:1528–1532. doi: 10.1038/ki.1994.437. [DOI] [PubMed] [Google Scholar]
- 91.Chen Z, Karaplis AC, Ackerman SL, Pogribny IP, Melnyk S, Lussier-Cacan S, Chen MF, Pai A, John SW, Smith RS, Bottiglieri T, Bagley P, Selhub J, Rudnicki MA, James SJ, Rozen R. Mice deficient in methylenetetrahydrofolate reductase exhibit hyperhomocysteinemia and decreased methylation capacity, with neuropathology and aortic lipid deposition. Hum Mol Genet. 2001;10:433–443. doi: 10.1093/hmg/10.5.433. [DOI] [PubMed] [Google Scholar]
- 92.Takeda T, Matsushita T, Kurozumi M, Takemura K, Higuchi K, Hosokawa M. Pathobiology of the senescence-accelerated mouse (SAM) Expergerontology. 1997;32:117–127. doi: 10.1016/s0531-5565(96)00068-x. [DOI] [PubMed] [Google Scholar]
- 93.Gupta YK, Sharma M, Briyal S. Antinociceptive effect of trans-resveratrol in rats: Involvement of an opioidergic mechanism. Methods Find Exp Clin Pharmacol. 2004;26:667–672. doi: 10.1358/mf.2004.26.9.872563. [DOI] [PubMed] [Google Scholar]
- 94.Howells DW, Porritt MJ, Rewell SS, O’Collins V, Sena ES, van der Worp HB, Traystman RJ, Macleod MR. Different strokes for different folks: the rich diversity of animal models of focal cerebral ischemia. J Cereb Blood Flow Metab. 2010;30:1412–1431. doi: 10.1038/jcbfm.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Braeuninger S, Kleinschnitz C, Nieswandt B, Stoll G. Focal cerebral ischemia. Methods Mol Biol. 2012;788:29–42. doi: 10.1007/978-1-61779-307-3_3. [DOI] [PubMed] [Google Scholar]
- 96.Traystman RJ. Animal models of focal and global cerebral ischemia. ILAR J. 2003;44:85–95. doi: 10.1093/ilar.44.2.85. [DOI] [PubMed] [Google Scholar]
- 97.Smith ML, Bendek G, Dahlgren N, Rosen I, Wieloch T, Siesjo BK. Models for studying long-term recovery following forebrain ischemia in the rat. 2. A 2-vessel occlusion model. Acta Physiol Scand. 1984;69:385–401. doi: 10.1111/j.1600-0404.1984.tb07822.x. [DOI] [PubMed] [Google Scholar]
- 98.Seal JB, Buchh BN, Marks JD. New variability in cerebrovascular anatomy determines severity of hippocampal injury following forebrain ischemia in the Mongolian gerbil. Brain Res. 2006;1073–1074:451–459. doi: 10.1016/j.brainres.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 99.del Zoppo GJ, Poeck K, Pessin MS, Wolpert SM, Furlan AJ, Ferbert A, Alberts MJ, Zivin JA, Wechsler L, Busse O. Recombinant tissue plasminogen activator in acute thrombotic and embolic stroke. Ann Neurol. 1992;32:78–86. doi: 10.1002/ana.410320113. [DOI] [PubMed] [Google Scholar]
- 100.O’Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006;59:467–477. doi: 10.1002/ana.20741. [DOI] [PubMed] [Google Scholar]
- 101.Koizumi J, Yoshida Y, Nakazawa T, Ooneda G. Experimental studies of ischemic brain edema, I: a new experimental model of cerebral embolism in rats in which recirculation can be introduced in the ischemic area. Jpn J Stroke. 1986;8:1–8. [Google Scholar]
- 102.Belayev L, Alonso OF, Busto R, Zhao W, Ginsberg MD. Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model. Stroke. 1996;27:1616–1622. doi: 10.1161/01.str.27.9.1616. [DOI] [PubMed] [Google Scholar]
- 103.Ardehali MR, Rondouin G. Microsurgical intraluminal middle cerebral artery occlusion model in rodents. Acta Neurol Scand. 2003;107:267–275. doi: 10.1034/j.1600-0404.2003.00010.x. [DOI] [PubMed] [Google Scholar]
- 104.Nagai N, Vanlinthout I, Collen D. Comparative effects of tissue plasminogen activator, streptokinase, and staphylokinase on cerebral ischemic infarction and pulmonary clot lysis in hamster models. Circulation. 1999;100:2541–2546. doi: 10.1161/01.cir.100.25.2541. [DOI] [PubMed] [Google Scholar]
- 105.Meng W, Wang X, Asahi M, Kano T, Asahi K, Ackerman RH, Lo EH. Effects of tissue type plasminogen activator in embolic versus mechanical models of focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 1999;19:1316–1321. doi: 10.1097/00004647-199912000-00004. [DOI] [PubMed] [Google Scholar]
- 106.Zhang J, Guo J, Xing SH, Gu SL, Dai TJ. The protective effect of melatonin on global cerebral ischemia reperfusion injury in gerbils. Yao Xue Xue Bao. 2002;37:329–333. [PubMed] [Google Scholar]
- 107.Cuzzocrea S, Reiter RJ. Pharmacological actions of melatonin in acute and chronic inflammation. Curr Top Med Chem. 2002;2:153–165. doi: 10.2174/1568026023394425. [DOI] [PubMed] [Google Scholar]
- 108.Reiter RJ, Sainz RM, Lopez-Burillo S, Mayo JC, Manchester LC, Tan DX. Melatonin ameliorates neurologic damage and neurophysiologic deficits in experimental models of stroke. Ann N Y Acad Sci. 2003;993:35–47. doi: 10.1111/j.1749-6632.2003.tb07509.x. [DOI] [PubMed] [Google Scholar]
- 109.Macleod MR, O’Collins T, Horky LL, Howells DW, Donnan GA. Systematic review and meta-analysis of the efficacy of melatonin in experimental stroke. J Pineal Res. 2005;38:35–41. doi: 10.1111/j.1600-079X.2004.00172.x. [DOI] [PubMed] [Google Scholar]
- 110.Watson BD, Dietrich WD, Busto R, Wachtel MS, Ginsberg MD. Induction of reproducible brain infarction by photochemically initiated thrombosis. Ann Neurol. 1985;17:497–504. doi: 10.1002/ana.410170513. [DOI] [PubMed] [Google Scholar]
- 111.Lozano JD, Abulafia DP, Danton GH, Watson BD, Dietrich WD. Characterization of a thromboembolic photochemical model of repeated stroke in mice. J Neurosci Methods. 2007;162:244–254. doi: 10.1016/j.jneumeth.2007.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schmidt A, Hoppen M, Strecker JK, Diederich K, Schabitz WR, Schilling M, Minnerup J. Photochemically induced ischemic stroke in rats. Exp Transl Stroke Med. 2012;4:13. doi: 10.1186/2040-7378-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tsiminis G, Klarić TS, Schartner EP, Warren-Smith SC, Lewis MD, Koblar SA, Monro TM. Generating and measuring photochemical changes inside the brain using optical fibers: exploring stroke. Biomed Opt Express. 2014;5:3975–3980. doi: 10.1364/BOE.5.003975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Güzel A, Rölz R, Nikkhah G, Kahlert UD, Maciaczyk J. A microsurgical procedure for middle cerebral artery occlusion by intraluminal monofilament insertion technique in the rat: a special emphasis on the methodology. Exp Transl Stroke Med. 2014;6:6. doi: 10.1186/2040-7378-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schmitt A, Parlapani E, Bauer M, Heinsen H, Falkai P. Is brain banking of psychiatric cases valuable for neurobiological research? Clinics. 2008;63:255–266. doi: 10.1590/s1807-59322008000200015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Taoufik E, Probert L. Ischemic neuronal damage. Curr Pharm Des. 2008;14:3565–3573. doi: 10.2174/138161208786848748. [DOI] [PubMed] [Google Scholar]
- 117.Iadecola C, Anrather J. Stroke research at a crossroad: asking the brain for directions. Nat Neurosci. 2011;14:1363–1368. doi: 10.1038/nn.2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Marian AJ. Modeling human disease phenotype in model organisms: “It’s only a model!”. Circ Res. 2011;109:356–359. doi: 10.1161/CIRCRESAHA.111.249409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Grünblatt E, Monoranu CM, Apfelbacher M, Keller D, Michel TM, Alafuzoff I, Ferrer I, Al-Saraj S, Keyvani K, Schmitt A, Falkai P, Schittenhelm J, McLean C, Halliday GM, Harper C, Deckert J, Roggendorf W, Riederer P. Tryptophan is a marker of human postmortem brain tissue quality. J Neurochem. 2009;110:1400–1408. doi: 10.1111/j.1471-4159.2009.06233.x. [DOI] [PubMed] [Google Scholar]
- 120.Lambertsen KL, Biber K, Finsen B. Inflammatory cytokines in experimental and human stroke. J Cereb Blood Flow Metab. 2012;32:1677–1698. doi: 10.1038/jcbfm.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dziewulska D, Mossakowski MJ. Cellular expression of tumor necrosis factor a and its receptors in human ischemic stroke. Clin Neuropathol. 2003;22:35–40. [PubMed] [Google Scholar]
- 122.Sairanen T, Carpén O, Karjalainen-Lindsberg ML, Paetau A, Turpeinen U, Kaste M, Lindsberg PJ. Evolution of cerebral tumor necrosis factor-alpha production during human ischemic stroke. Stroke. 2001;32:1750–1758. doi: 10.1161/01.str.32.8.1750. [DOI] [PubMed] [Google Scholar]
- 123.Yilmaz G, Arumugam TV, Stokes KY, Granger DN. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation. 2006;113:2105–2112. doi: 10.1161/CIRCULATIONAHA.105.593046. [DOI] [PubMed] [Google Scholar]
- 124.Hurn PD, Subramanian S, Parker SM, Afentoulis ME, Kaler LJ, Vandenbark AA, Offner H. T- and B-cell-deficient mice with experimental stroke have reduced lesion size and inflammation. J Cereb Blood Flow Metab. 2007;27:1798–1805. doi: 10.1038/sj.jcbfm.9600482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chaitanya GV, Eeka P, Munker R, Alexander JS, Babu PP. Role of cytotoxic protease granzyme-b in neuronal degeneration during human stroke. Brain Pathol. 2011;21:16–30. doi: 10.1111/j.1750-3639.2010.00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cuadrado E, Rosell A, Colomé N, Hernández-Guillamon M, García-Berrocoso T, Ribo M, Alcazar A, Ortega-Aznar A, Salinas M, Canals F, Montaner J. The proteome of human brain after ischemic stroke. J Neuropathol Exp Neurol. 2010;69:1105–1115. doi: 10.1097/NEN.0b013e3181f8c539. [DOI] [PubMed] [Google Scholar]
- 127.Datta A, Akatsu H, Heese K, Sze SK. Quantitative clinical proteomic study of autopsied human infarcted brain specimens to elucidate the deregulated pathways in ischemic stroke pathology. J Proteomics. 2013;91:556–568. doi: 10.1016/j.jprot.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 128.Stenman E, Malmsjo M, Uddman E, Gido G, Wieloch T, Edvinsson L. Cerebral ischemia upregulates vascular endothelin ET(B) receptors in rat (2002) Stroke. 33:2311–2316. doi: 10.1161/01.str.0000028183.04277.32. [DOI] [PubMed] [Google Scholar]
- 129.Hansen-Schwartz J, Hoel NL, Zhou M, Xu CB, Svendgaard NA, Edvinsson L. Subarachnoid hemorrhage enhances endothelin receptor expression and function in rat cerebral arteries. Neurosurgery. 2003;52:1188–1195. [PubMed] [Google Scholar]
- 130.Vikman P, Edvinsson L. Gene expression profiling in the human middle cerebral artery after cerebral ischemia. Eur J Neurol. 2006;13:1324–1332. doi: 10.1111/j.1468-1331.2006.01496.x. [DOI] [PubMed] [Google Scholar]
- 131.Simpson JE, Hosny O, Wharton SB, Heath PR, Holden H, Fernando MS, Matthews F, Forster G, O’Brien JT, Barber R, Kalaria RN, Brayne C, Shaw PJ, Lewis CE, Ince PG. Medical Research Council Cognitive Function and Ageing Study Neuropathology Group. Microarray RNA expression analysis of cerebral white matter lesions reveals changes in multiple functional pathways. Stroke. 2009;40:369–375. doi: 10.1161/STROKEAHA.108.529214. [DOI] [PubMed] [Google Scholar]
- 132.Mitsios N, Saka M, Krupinski J, Pennucci R, Sanfeliu C, Wang Q, Rubio F, Gaffney J, Kumar P, Kumar S, Sullivan M, Slevin M. A microarray study of gene and protein regulation in human and rat brain following middle cerebral artery occlusion. BMC Neurosci. 2007;8:93. doi: 10.1186/1471-2202-8-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Frugier T, Taylor JM, McLean C, Bye N, Beart PM, Devenish RJ, Crack PJ. Evidence for the recruitment of autophagic vesicles in human brain after stroke. Neurochem Int. 2016;96:62–68. doi: 10.1016/j.neuint.2016.02.016. [DOI] [PubMed] [Google Scholar]