Abstract
The one-electron reduction of redox-active chemotherapeutic agents generates highly toxic radical anions and reactive oxygen intermediates (ROI). A major enzyme catalyzing this process is cytochrome P450 reductase. Because many tumor cells highly express this enzyme, redox cycling of chemotherapeutic agents in these cells may confer selective antitumor activity. Nitrofurantoin is a commonly used redox-active antibiotic that possesses antitumor activity. In the present studies we determined whether nitrofurantoin redox cycling is correlated with cytochrome P450 reductase activity and cytotoxicity in a variety of cell lines. Recombinant cytochrome P450 reductase was found to support redox cycling of nitrofurantoin and to generate superoxide anion, hydrogen peroxide, and, in the presence of redox-active iron, hydroxyl radicals. This activity was NADPH dependent and inhibitable by diphenyleneiodonium, indicating a requirement for the flavin cofactors in the reductase. Nitrofurantoin-induced redox cycling was next analyzed in different cell lines varying in cytochrome P450 reductase activity including Chinese hamster ovary cells (CHO-OR) constructed to overexpress the enzyme. Nitrofurantoin-induced hydrogen peroxide production was 16-fold greater in lysates from CHO-OR cells than from control CHO cells. A strong correlation between cytochrome P450 reductase activity and nitrofurantoin-induced redox cycling among the cell lines was found. Unexpectedly, no correlation between nitrofurantoin-induced ROI production and cytotoxicity was observed. These data indicate that nitrofurantoin-induced redox cycling and subsequent generation of ROI are not sufficient to mediate cytotoxicity and that cytochrome P450 reductase is not a determinant of sensitivity to redox-active chemotherapeutic agents.
Keywords: Cytochrome P450 reductase, Hydroxyl radicals, Redox cycling, Antitumor, Cytotoxicity, Chemotherapy
Introduction
Nitrofurantoin (N-(5-nitro-2-furfurylidene)-1-aminohydantoin) is a synthetic nitrofuran-derivative antibiotic commonly used in the treatment of urinary tract infections. Prolonged use of nitrofurantoin is associated with pulmonary fibrosis [1], hepatocellular injury [2], peripheral polyneuropathy [3], and hematological disorders [4]. Nitrofurantoin has also been proposed as a cytotoxic antitumor agent [5,6]. Both the antimicrobial and the cytotoxic effects appear to be related to reduction of the 5-nitro substitution on the molecule (see Fig. 1 for structure). Thus, in susceptible bacteria, nitroreductases convert nitrofurantoin to a highly reactive electrophilic intermediate which attacks ribosomes and inhibits protein synthesis [7]. In humans, pulmonary and hepatic toxicity have been attributed to reactive oxygen intermediates (ROI) generated during redox cycling [8,9]. This involves a one-electron transfer from donors such as NADPH to nitrofurantoin generating a nitro radical anion which, under aerobic conditions, is oxidized back to the parent compound by molecular oxygen (Fig. 1). These reactions produce superoxide, which in turn dismutates to hydrogen peroxide (H2O2) and, in the presence of redox-active metals, highly reactive hydroxyl radicals [10]. These ROI damage cellular macromolecules resulting in lipid peroxidation and protein and DNA oxidation [11].
Fig. 1. Nitrofurantoin redox cycling and ROI generation. The one-electron reduction of nitrofurantoin by enzymes such as cytochrome P450 reductase generates the nitrofurantoin radical.
In the presence of oxygen, the radical disproportionates to form superoxide anion and the parent compound. Adapted from Minchin et al. [10].
NADPH-cytochrome P450 reductase (EC 1.6.2.4) is a flavoprotein widely distributed in human tissues and tumor cells [12]. It can mediate the transfer of electrons to several enzymes including the superfamily of cytochrome P450s, cytochrome b5, and heme oxygenase, which are essential for the metabolism of xenobiotics and endogenous compounds [13]. Cytochrome P450 reductase has also been identified as a major enzyme catalyzing nitrofurantoin redox cycling [10,14]. In the present studies, we characterized nitrofurantoin redox cycling in a variety of tumor cell lines and recombinant cytochrome P450 reductase. Our results indicate that the ability of nitrofurantoin to redox cycle is directly related to cytochrome P450 reductase activity but not to tumor cytotoxicity. These findings suggest that additional mechanisms mediate the cytotoxic actions of nitrofurantoin in tumor cells.
Materials and methods
Chemicals and reagents
cDNA-expressed rat cytochrome P450 reductase was from microsomal fractions of insect cells (Supersomes; Cat. No. 456514; Lot Nos 5 and 25030; BD Gentest, Woburn, MA). Amplex red (10-acetyl-3,7-dihydroxyphenoxazine) and 2′,7′-dichlorodihydrofluorescein diacetate acetyl ester (DCFH-DA) were from Molecular Probes (Eugene, OR). Nitrofurantoin, NADPH, catalase, and all other chemicals were from Sigma–Aldrich (St. Louis, MO).
Cells and treatments
Mouse cytochrome P450 reductase-overexpressing Chinese hamster ovary cells (CHO-OR), and control cells expressing empty vector (CHO-WT) were kindly provided by Dr. Jun Yan Hong at the University of Medicine and Dentistry of New Jersey (Piscataway, NJ) [15]. Murine lung epithelial cells (MLE 15 cells) were obtained from Dr. Jacob N. Finkelstein at the University of Rochester, NY. All other cell lines were purchased from the American Type Culture Collection (Rockville, MD). CHO-WT, CHO-OR, and PC-3 cells were maintained in Ham’s F12K medium. MLE 15, RAW 264.7, C2, S-180, B16, HL-60, HT-29, and HeLa cells were maintained in Dulbecco’s modified Eagle’s medium. All medium was supplemented with 10% fetal bovine serum, penicillin (100 units/ml), and streptomycin (100 μg/ml). For CHO cells, the medium was also supplemented with 500 μg/ml hygromycin B (Invitrogen, Carlsbad, CA). Cells were incubated at 37 °C in 5% CO2 in a humidified incubator. Tissue culture reagents were from Gibco BRL (Grand Island, NY). C2 cells are a continuously grown mouse muscle cell line, while MLE 15 cells are a continuously grown type II lung cell line isolated from SV40 large tumor antigen transgenic mice [16].
To prepare lysates, cells were scraped from culture dishes in phosphate-buffered saline (PBS), washed, and centrifuged (800g, 5 min). Cell pellets were stored at – 70 °C until analysis. Before enzyme assays, cell pellets were resuspended in PBS (~107 cells/0.5 ml) and sonicated on ice using a sonic dismembrator (Artek Systems Inc., Farmingdale, NY). Homogenates were then sequentially centrifuged at 4 °C (3000g and 12,000g, for removal of cellular debris and mitochondrial fractions, respectively). The resulting supernatant fractions were used in enzyme assays. Protein concentrations were quantified using the Dc protein assay kit (Bio-Rad, Hercules, CA) with bovine serum albumin as the standard.
Cell growth inhibition was evaluated as previously described [17]. Briefly, cells were plated at low density (2.5–10 × 104 cells/well) in six-well tissue culture plates and allowed to adhere overnight. The medium was then replaced with growth medium supplemented with increasing concentrations of nitrofurantoin. After 4–5 days, cells were removed from the plates and counted using a Z1 Coulter Particle Counter (Beckman Coulter, Hialeah, FL). Concentrations of nitrofurantoin that caused 50% growth inhibition (IC50) were then determined.
Kinetic analysis of H2O2 production
H2O2 production was measured using the Amplex red/horseradish peroxidase method [18]. Standard reaction mixes in 100 μl potassium phosphate buffer (50 mM, pH 7.4) contained 0–0.5 mM NADPH, 0–500 μM nitrofurantoin, 25 μM Amplex red, 1 unit/ml horseradish peroxidase, and 1.25 μg/ml cytochrome P450 reductase or 100 μg/ml cell protein. The fluorescent product resorufin was detected using an HTS 7000 Plus Bio Assay Reader (Perkin–Elmer Life Sciences, Shelton, CT) using a 540-nm excitation filter and a 595-nm emission filter. Increases in fluorescence intensity were measured every 2.5 min for 30 min at 37 °C. H2O2 production is formed via the one-electron reduction of nitrofurantoin by cytochrome P450 reductase, the interaction of reduced nitrofurantoin with oxygen producing the parent compound and superoxide, and the dismutation of superoxide to hydrogen peroxide. Because the last two steps proceed at very high rates [19], we assumed that the measurement of Km and Vmax reflect the one-electron enzymatic reaction.
Assays for hydroxyl radical and superoxide anion
The generation of 2-hydroxyterephthalate from terephthalate in enzyme assays was used as an indicator of hydroxyl radical production [20]. Standard reaction mixes in 0.2 ml potassium phosphate buffer (50 mM, pH 7.4) contained 6.25 μg/ml cytochrome P450 reductase or 150 μg/ml cell protein from supernatant fractions, 1 mM terephthalate, 0.5 mM NADPH. Reactions were initiated by the addition of Fe3+/EDTA (100 μM/110 μM) to the assay mixture. After incubation at 37 °C for 1 h, reactions were stopped by adding an equal volume of ice-cold methanol. 2-Hydroxyterephthalate was quantified by HPLC with fluorescence detection as previously described [20].
Superoxide anion was assayed by the formation of 2-hydroxyethidium from dihydroethidium [21]. Standard reaction mixes described above were used except that Fe3+/EDTA was omitted and dihydroethidium (40 μM) was used in place of terephthalate. 2-Hydroxyethidium was detected using a Shimadzu HPLC (Kyoto, Japan) fitted with a Luna C18 column (250 mm × 2.0 mm; Phenomenex, Torrance, CA) and a fluorescence detector with excitation and emission wavelengths set at 510 and 595 nm, respectively. The mobile phase consisted of a linear (10–40%) gradient of acetonitrile in 0.1% trifluoroacetic acid and was run at a flow rate of 0.2 ml/min in 35 min.
Assay for NADPH metabolism
NADPH metabolism was assayed in reaction mixes containing 100 μM NADPH, 7.5 μg/ml cytochrome P450 reductase, with or without 50 μM nitrofurantoin in 1 ml potassium phosphate buffer (50 mM, pH 7.4). Depletion of NADPH in reaction mixes was quantified by decreases in absorbance at 340 nm using a Lambda 20 UV/VIS spectrometer (Perkin–Elmer Life Sciences), scanning at 1 nm/s and recording at 1-nm intervals, repeating the scan in 2.5-min intervals for 25 min.
Cytochrome c reduction assay
A cytochrome c reductase (NADPH) assay kit (Sigma–Aldrich) was used to assess enzyme activity. The assay was performed in a 1.1-ml final reaction volume containing 31 μM cytochrome c in 300 mM potassium phosphate buffer (pH 7.8), 50 μl cell protein from supernatant fractions, and 0–500 μM nitrofurantoin. The reaction was initiated by the addition of 100 μl NADPH (final concentration, 100 μM). Increases in absorbance at 550 nm were monitored spectrophotometrically. Enzyme activity was expressed as units/mg protein; one unit is defined as 1 nmol cytochrome c reduced per min. In this assay, nitrofurantoin did not alter cytochrome P450 reductase-mediated reduction rates of cytochrome c.
Measurement of ROI in intact cells
Intracellular ROI were quantified using DCFH-DA in conjunction with flow cytometry as previously described [22]. In brief, cells were suspended in PBS (1 × 106/ml) and incubated with 5 μM DCFH-DA at 37 °C in a shaking water bath for 15 min. Nitrofurantoin was then added. After 3 h, cellular fluorescence was analyzed using a Cytomics FC 500 flow cytometer (Beckman Coulter, Fullerton, CA). Data were analyzed by CXP software (Beckman Coulter).
Statistical analysis
Each determination was done in duplicate or triplicate and repeated two or three times. Differences were analyzed for statistical significance using the one-way ANOVA or unpaired t test with GraphPad Instat software. p < 0.05 was considered significant.
Results
Nitrofurantoin induces redox cycling via cytochrome P450 reductase
Initially, an H2O2-generating system with cDNA-expressed rat cytochrome P450 reductase was used to characterize the ability of nitrofurantoin to redox cycle. Constitutive level of H2O2 generation without nitrofurantoin was detected in the reaction mix (Fig. 2A). The addition of nitrofurantoin increased H2O2 production in concentration-dependent manner. The Km and Vmax for nitrofurantoin in the reaction with cytochrome P450 reductase were 318.3 ± 67.9 μM and 650.7 ± 95.8 nmol H2O2/mg protein/min, respectively (Table 1). The reaction was dependent on NADPH which is utilized during the enzyme reaction (Fig. 3). Nitrofurantoin was found to increase the Vmax for NADPH utilization by cytochrome P450 reductase. With 100 μM nitrofurantoin, the Vmax for NADPH increased approximately nine fold, with no major changes in its Km (p = 0.32; Table 2). The flavoproteins inhibitor diphenyleneiodonium inhibited nitrofurantoin-induced H2O2 generation (IC50 = 0.2 μM) (Fig. 4). In contrast, no effects on this reaction were observed with potassium cyanide (0.1–30 μM), an inhibitor of respiratory oxidoreductases, dicoumarol (0.1–100 μM), an NAD(P)H: quinone oxidoreductase 1 (NQO1) inhibitor, or L-NAME (0.1–100 μM), a nitric oxide synthase inhibitor.
Fig. 2. Effects of nitrofurantoin (NFT) on ROI production by recombinant cytochrome P450 reductase (P450 OR).
(A) Nitrofurantoin-induced H2O2 production. Enzyme reactions were run in absence or presence of increasing concentrations of nitrofurantoin. Accumulation of H2O2 in the assay mixes was quantified using the Amplex red/horseradish peroxidase reaction (n = 3 ± SE). (B and C) Nitrofurantoin-induced hydroxyl radical and superoxide production. Reactions were run in the absence or presence of 25 or 100 μM NFT. Hydroxyl radicals in enzyme assays were quantified by the formation of 2-hydroxyterephthalate (2-OH TPT) derived from terephthalate (n = 2 ± SD). In some assays, catalase (400 U/ml) or DMSO (70 mM) was added to prevent hydroxyl radical formation. Note that the generation of hydroxyl radicals was dependent on the presence of redox-active iron. Superoxide in the enzyme assays was quantified as the formation of 2-hydroxyethidium derived from dihydroethidium. In some of the assays, SOD (350 U/ml) was added to inhibit superoxide formation. C shows HPLC tracings of 2-hydroxyethidium formation. One of two representative experiments is shown. All enzyme reaction mixes contained 0.5 mM NADPH. **p < 0.01 compared to rat P450 OR without NFT, # p < 0.05 and ## p < 0.01 compared to rat P450 OR with 100 μM NFT.
Table 1.
Kinetic parameters of nitrofurantoin-stimulated H2O2 generation with CYP 450 reductase and microsome-containing fractions from different cell types
| Species | Origin | Km (μM)* | Vmax (nmol H2O2/mg protein/min) | |
|---|---|---|---|---|
| CYP 450 OR | Supersomes | 318.3 ± 67.9 | 650.7 ± 95.8 | |
| Hamster | CHO-WT | Ovary | 150.9 ± 3.9 | 2.1 ± 0.4 |
| CHO-OR | Ovary | 175.9 ± 21.3 | 32.9 ± 4.8 | |
| Mouse | MLE 15 | Lung | 298.1 ± 27.3 | 12.1 ± 1.0 |
| RAW 264.7 | Macrophage | 289.5 ± 42.2 | 6.7 ± 0.6 | |
| C2 | Muscle | 193.8 ± 7.6 | 3.5 ± 0.2 | |
| S-180 | Sarcoma | 214.4 ± 18.8 | 4.4 ± 0.3 | |
| B16 | Melanoma | 207.6 ± 20.3 | 6.2 ± 0.4 | |
| Human | HL-60 | Leukemia | 200.0 ± 26.1 | 1.8 ± 0.1 |
| HT-29 | Colon | 645.2 ± 88.5 | 13.6 ± 1.4 | |
| HeLa | Cervix | 240.3 ± 16.6 | 5.7 ± 0.3 | |
| PC-3 | Prostate | 162.8 ± 13.0 | 3.3 ± 0.4 |
Using increasing concentrations of nitrofurantoin, reaction rates were measured in the linear phase in standard reaction mixes in the presence of NADPH (500 μM). Km and Vmax values were obtained using Michaelis–Menton kinetics. In cell lysates, each point represents the mean of three independent determinations ± SE. With CYP 450 OR, each point represents the mean of two independent determinations ± SD.
Fig. 3. Nitrofurantoin-stimulated NADPH depletion by recombinant cytochrome P450 reductase.
Reaction mixes containing 0.1 mM NADPH were analyzed in 1-ml spectrophotometer cuvettes. Absorbance spectra were recorded at 2.5-min intervals. (Top) Nitrofurantoin-stimulated NADPH depletion; 50 μM nitrofurantoin was added directly to the cuvette. (Bottom) NADPH metabolism in the enzyme assay in the absence of nitrofurantoin.
Table 2.
Effects of nitrofurantoin on the kinetic constants for NADPH with recombinant CYP 450 reductase and microsome-containing fractions from different cell types
| Species | Treatments | Km (μM)* | Vmax (nmol H2O2/mg protein/min) | |
|---|---|---|---|---|
| CYP450 OR | - | 28.3 ± 5.5 | 7.8 ± 1.3 | |
| +NFT | 19.2 ± 2.3 | 71.4 ± 13.7 | ||
| Hamster | CHO-WT | - | 28.9 ± 7.9 | 0.2 ± 0.0 |
| +NFT | 42.2 ± 12.3 | 0.8 ± 0.2 | ||
| CHO-OR | - | 30.7 ± 8.9 | 0.6 ± 0.1 | |
| +NFT | 290.3 ± 75.2 | 24.0 ± 6.2 | ||
| Mouse | MLE 15 | - | 19.5 ± 5.2 | 0.2 ± 0.0 |
| +NFT | 33.7 ± 1.5 | 2.3 ± 0.2 | ||
| RAW 264.7 | - | 19.7 ± 3.0 | 0.1 ± 0.0 | |
| +NFT | 18.2 ± 2.7 | 1.7 ± 0.3 | ||
| C2 | - | 13.9 ± 3.3 | 0.2 ± 0.0 | |
| +NFT | 24.9 ± 2.0 | 1.0 ± 0.0 | ||
| S-180 | - | 6.6 ± 1.0 | 0.2 ± 0.0 | |
| +NFT | 19.5 ± 2.1 | 1.3 ± 0.2 | ||
| B16 | - | 27.6 ± 5.2 | 0.4 ± 0.1 | |
| +NFT | 28.3 ± 3.5 | 2.0 ± 0.3 | ||
| Human | HL-60 | - | 8.3 ± 1.5 | 0.1 ± 0.0 |
| +NFT | 13.2 ± 2.6 | 0.5 ± 0.0 | ||
| HT-29 | - | ND** | ND | |
| +NFT | 19.6 ± 6.4 | 2.1 ± 0.2 | ||
| HeLa | - | 8.2 ± 1.5 | 0.2 ± 0.0 | |
| +NFT | 23.3 ± 1.1 | 1.7 ± 0.1 | ||
| PC-3 | - | 3.6 ± 0.5 | 0.1 ± 0.0 | |
| +NFT | 58.0 ± 5.8 | 1.3 ± 0.1 |
Using increasing concentrations of NADPH, reaction rates were measured in the linear phase in the absence and presence of nitrofurantoin (NFT; 100 μM). Km and Vmax values were obtained using Michaelis–Menton kinetics. In cell lysates, each point represents the mean of three independent determinations ± SE. With CYP 450 OR, each point represents the mean of two independent determinations ± SD.
The data obtained do not fit Michaelis–Menton kinetics.
Fig. 4. Effects of inhibitors on nitrofurantoin-induced H2O2 production via recombinant cytochrome P450 reductase.
H2O2 formation was assayed in reaction mixes containing 0.5 mM NADPH, 100 μM nitrofurantoin, and increasing concentration of diphenyleneiodonium (DPI), potassium cyanide (KCN), dicoumarol, or L -NAME. Control rate is 93.4 ± 11.1 nmol H2O2/mg protein/min; n = 3 ± SE. **p < 0.01 compared to control.
Nitrofurantoin also stimulated superoxide anion and hydroxyl radical production supported by cytochrome P450 reductase (Figs. 2B and 2C). As noted for H2O2, constitutive levels of these ROI were detectable in control reactions. Increases in superoxide anion (p < 0.05) and hydroxyl radical (p < 0.01) generation in the presence of nitrofurantoin were time and concentration dependent (Figs. 2B and 2C). Whereas superoxide dismutase (SOD) inhibited the formation of superoxide (p < 0.05) (Fig. 2C), hydroxyl radicals were abolished by catalase (p < 0.01) and by DMSO (p < 0.05) (Fig. 2B). Production of hydroxyl radicals was dependent on iron, a finding consistent with the ability of nitrofurantoin to generate H2O2 during redox cycling, and its subsequent conversion to hydroxyl radicals via the Fenton reaction [23]. As observed with H2O2 production, diphenyleneiodonium also inhibited cytochrome-P450-reductase-mediated production of superoxide and hydroxyl radicals (data not shown).
In our next series of experiments, we compared nitrofurantoin redox cycling in CHO cells with stable expression of cytochrome P450 reductase (CHO-OR) and wild-type cells (CHO-WT). CHO-OR cells express 30-fold more cytochrome P450 reductase activity, as measured by cytochrome c reduction, than CHO-WT cells [15]. In the absence of nitrofurantoin, constitutive levels of H2O2 were generated by both cell types. CHO-OR cells produced approximately 2- to 3-fold more H2O2 than CHO-WT cells (p = 0.01; Figs. 5A and 5B). Nitrofurantoin caused a concentration-dependent increase in H2O2 production in enzyme preparations from both cell types (Figs. 5A and 5B). Significantly greater activity was detected in CHO-OR cells overexpressing cytochrome P450 reductase. The nitrofurantoin-stimulated Vmax for H2O2 production in lysates from cytochrome-P450-reductase-overexpressing cells was approximately 16-fold greater than that in wild-type cells (32.9 ± 4.8 nmol H2O2/mg protein/min vs 2.1 ± 0.4 nmol H2O2/mg protein/min, respectively). No major differences in the Km values for nitrofurantoin in the two cell types were observed (p = 0.36; Table 1). In contrast to the kinetic constants for recombinant cytochrome P450 reductase, nitrofurantoin increased both the Km for NADPH and the Vmax for the generation of H2O2 in the CHO cells. Thus, in the presence of 100 μM nitrofurantoin, the Km for NADPH increased about 1.5-fold in CHO-WT cells and more than 9-fold in CHO-OR cells whereas the Vmax increased 4-fold in CHO-WT cells and 40-fold in CHO-OR cells (Table 2).
Fig. 5. Effects of nitrofurantoin on ROI production by CHO-WT and CHO-OR cells.
(A and B) Nitrofurantoin-induced H2O2 production with lysates from CHO-WT cells (A) and CHO-OR cells (B). H2O2 production in the assay mixes was quantified in absence or presence of nitrofurantoin every 2.5 min for 30 min (n = 3 ± SE). Constitutive production of hydrogen peroxide was 8 nmol/30 min/mg protein for CHO-WT cells and 21 nmol/30 min/mg protein for CHO-OR cells. (C and D) Generation of hydroxyl radicals and superoxide anion in reaction mixes stimulated by nitrofurantoin. Reactions were run in the absence or presence of 25 or 100 μM nitrofurantoin (NFT). Hydroxyl radicals in enzyme assays were quantified by the formation of 2-hydroxyterephthalate (2-OH TPT) (n = 2 ± SD). In some assays, catalase (400 U/ml) or DMSO (70 mM) was added to prevent hydroxyl radical formation. Superoxide in the enzyme assays was quantified by the formation of 2-hydroxyethidium from dihydroethidium. In some of the assays, SOD (350 U/ml) was added to prevent superoxide formation. D shows HPLC tracings of 2-hydroxyethidium formation. One of two representative experiments is shown. *p < 0.05 and ***p < 0.001 comparing CHO-WT with CHO-OR cells.
Constitutive production of superoxide anion and hydroxyl radicals was also detected in CHO cells (Figs. 5C and 5D). CHO-OR cells produced greater amounts of hydroxyl radicals than CHO-WT cells (p < 0.05). No differences in basal levels of superoxide anion were evident in the two cell types (p = 0.34), possibly because of increased SOD in lysates of CHO-OR cells compared to CHO-WT cells (data not shown). Whereas only a relatively small increase in the production of hydroxyl radicals and superoxide anion was noted in CHO-WT cells treated with nitrofurantoin, CHO-OR cells produced significantly greater amounts of these ROI (p < 0.05, Figs. 5C and 5D). As observed with recombinant cytochrome P450 reductase, hydroxyl radical formation in these cells was dependent on iron and prevented by catalase and DMSO (p < 0.05 for both CHO-WT and CHO-OR cells) (Fig. 5C). Moreover, SOD inhibited superoxide anion production in the cells (p < 0.05; Fig. 5D).
Cytochrome P450 reductase and redox cycling of nitrofurantoin in different cell types
We next determined whether nitrofurantoin-induced H2O2 generation was correlated with cytochrome P450 reductase activity in various cell types. These results are shown in Table 3. A mouse-lung-derived epithelial cell line, MLE 15, was found to contain the highest NADPH-cytochrome c reductase activity and a human prostate cell line, PC-3, exhibited the lowest activity (17.5 ± 1.0 units/mg protein vs 2.5 ± 0.4 units/mg protein, respectively). Table 1 compares nitrofurantoin-induced redox cycling in these cells. The Km for nitrofurantoin varied from 162.8 ± 13.0 μM in human PC-3 prostate cells to 645.2 ± 88.5 μM in human HT-29 colon tumor cells. The Vmax for nitrofurantoin-stimulated H2O2 generation varied eight-fold among the cells; the highest activity was detected in HT-29 cells (Vmax = 13.6 ± 1.4 nmol H2O2/mg protein/min). As observed with the CHO cells, in all cells except RAW 264.7 macrophages and B16 melanoma, nitrofurantoin increased the Km and Vmax values for NADPH. In the macrophages and melanoma, nitrofurantoin-induced increase in H2O2 generation was due to increases in Vmax of the enzyme without significant alterations in the Km (Table 2).
Table 3.
Cytochrome c reductase in microsome-containing fractions from different cell types and ability of nitrofurantoin to inhibit cell growth
| Species | Cell type | Origin | Cytochrome c reductase activity (units/mg protein)* | Growth inhibition IC50 (μM)** |
|---|---|---|---|---|
| Hamster | CHO-WT | Ovary | 2.9 ± 0.6 | 53 ± 1 |
| CHO-OR | Ovary | 87.1 ± 5.9 | 42 ± 3 | |
| Mouse | MLE 15 | Lung | 17.5 ± 1.0 | 55 ± 4 |
| RAW 264.7 | Macrophage | 13.5 ± 1.5 | 51 ± 2 | |
| C2 | Muscle | 6.1 ± 0.2 | 80 ± 12 | |
| S-180 | Sarcoma | 7.4 ± 1.0 | 41 ± 4 | |
| B16 | Melanoma | 9.6 ± 0.5 | 33 ± 4 | |
| Human | HL-60 | Leukemia | 3.4 ± 0.6 | 14 ± 1 |
| HT-29 | Colon | 8.4 ± 1.7 | 35 ± 1 | |
| HeLa | Cervix | 8.9 ± 1.4 | 41 ± 2 | |
| PC-3 | Prostate | 2.5 ± 0.4 | 19 ± 3 |
One unit is defined as the reduction of 1 nmol of oxidized cytochrome c in the presence of 100 μM NADPH per minute at pH 7.8 at 25 °C. Values for the calculations were taken in the linear phase of the reactions. Each point represents the mean of three independent determinations ± SE.
IC50, concentration of nitrofurantoin inhibiting cell growth by 50%.
Interestingly, in all of the cell lines except HT-29 cells, redox cycling of nitrofurantoin was found to directly correlate with cytochrome P450 reductase activity (Fig. 6, top). In HT-29 cells, significantly more nitrofurantoin redox activity was detected relative to cytochrome P450 reductase activity. Because cytochrome P450 reductase is a microsomal enzyme [24,25], we next analyzed nitrofurantoin redox cycling in microsomal and cytosolic fractions of HT-29 cells. For comparison, we also analyzed MLE 15 cells. As expected, in MLE 15 cells, the majority of nitrofurantoin-stimulated H2O2 generation appeared in the microsomal fraction of the cells (p = 0.001) (Table 4). In contrast, the cytosolic fraction of HT-29 cells contained significantly more nitrofurantoin-stimulated H2O2 generation activity than the microsomal fraction (p = 0.005). These data indicate that HT-29 cells contain high activity of additional NADPH oxidoreductases in the cytosolic fraction capable of redox cycling nitrofurantoin.
Fig. 6. Cytochrome P450 reductase activity, nitrofurantoin (NFT)-induced redox cycling, and cytotoxicity in different cell lines.
(Top) Correlation between cellular cytochrome P450 reductase activity and nitrofurantoin-induced H2O2 production. Cytochrome P450 reductase activity was measured using a cytochrome c reduction assay. The Vmax for nitrofurantoin-induced H2O2 generation (Table 1) was used to compare nitrofurantoin-induced redox cycling with cellular cytochrome P450 reductase activity. The inset shows the same data with CHO-OR cells omitted. Note the positive correlation between cytochrome P450 reductase activity and nitrofurantoin-induced redox cycling. (Bottom) Lack of correlation between nitrofurantoin-induced redox cycling and cellular cytotoxicity. Redox cycling capacity of the cell lines was plotted against the concentration of nitrofurantoin inhibiting growth by 50% (IC50) in each cell line (Table 3). (●PC-3, ○CHO-WT, ▼HL-60, △C2, ■S-180, □ HT-29, ◆HeLa, ◇ B16, ▲RAW 264.7, ▽MLE 15, ⬢CHO-OR).
Table 4.
Kinetic parameters for nitrofurantoin in different fractions of HT 29 and MLE 15 cells
| Km (μM)* | Vmax (nmol H2O2/mg protein/min) | ||
|---|---|---|---|
| Cytosol | HT-29 | ND** | 13.8 ± 0.7 |
| MLE 15 | 297.5 ± 0.7 | 3.4 ± 0.8 | |
| Microsomal | HT-29 | 233.2 ± 7.4 | 6.9 ± 0.2 |
| MLE 15 | 228.0 ± 54.3 | 13.6 ± 0.9 |
Using increasing concentrations of nitrofurantoin, reaction rates were measured in the linear phase in standard reaction mixes in the presence of NADPH (500 μM). Km and Vmax values were obtained using Michaelis–Menton kinetics. Each point represents the mean of three independent determinations ± SE.
The data obtained do not fit Michaelis–Menton kinetics.
Effects of nitrofurantoin on intracellular ROI production and cell growth inhibition
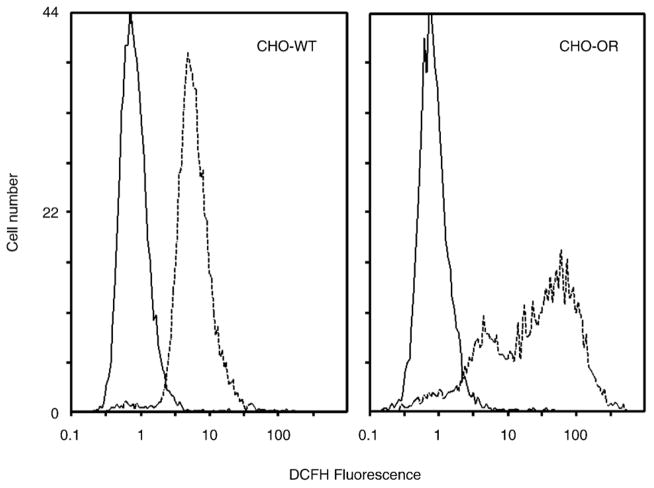
Because cell lysates were found to redox cycle nitrofurantoin, we next determined whether this was associated with ROI production in intact cells. For these studies we used techniques in flow cytometry in conjunction with the ROI-sensitive probe, DCFH-DA, to evaluate intracellular H2O2 production. We found that nitrofurantoin readily induced H2O2 in intact CHO cells. In CHO-WT cells, nitrofurantoin increased intracellular H2O2 production approximately 7-fold (p < 0.001; Fig. 7, left). In contrast, in CHO-OR cells, nitrofurantoin was significantly more effective in increasing intracellular H2O2 production. Thus, after nitrofurantoin treatment, a subpopulation of extremely bright CHO-OR cells (approximately 75% of the total number of cells analyzed) increased H2O2 production approximately 60-fold (p = 0.0016; Fig. 7, right). The remaining cells in a less bright subpopulation also increased H2O2 production approximately 5-fold (p < 0.001). At the present time, the origin of the subpopulation heterogeneity in CHO-OR cells with respect to H2O2 production is not known. However, these data are consistent with the increased abililty of CHO-OR cells to redox cycle nitrofurantoin. Interestingly, despite marked differences in nitrofurantoin-induced redox cycling and intracellular H2O2 production in CHO-WT and CHO-OR cells, their responses to nitrofurantoin-induced cytotoxicity, as measured by growth inhibition, were similar (IC50 = 53 ± 1 μM vs 42 ± 3 μM for CHO-WT and CHO-OR cells, respectively) (Fig. 8, left and Table 3). We also found that the different cell types varied approximately 5- to 6-fold in their sensitivity to nitrofurantoin-induced growth inhibition (IC50 = 14–80 μM). Moreover, no correlation was observed between nitrofurantoin redox cycling and growth inhibition (Fig. 6, bottom). For example, although similar cytochrome P450 reductase activity was detected in C2 and S-180 cells (6.1 ± 0.2 units/mg protein vs 7.4 ± 1.0 units/mg protein; p > 0.05) and the ability of the two cell types to redox cycle nitrofurantoin was similar (Vmax = 3.5 ± 0.2 nmol H2O2/mg protein/min vs 4.4 ± 0.3 nmol H2O2/mg protein/min for C2 and S-180 cells, respectively; p > 0.05), S-180 cells were significantly more sensitive to nitrofurantoin-induced cytotoxicity (p < 0.05, Fig. 8, right and Table 3). Furthermore, PC-3 cells, which display significantly less nitrofurantoin-induced redox cycling activity than MLE 15 (p < 0.001) or HT-29 cells (p < 0.05), were 2- to 3-fold more sensitive to nitrofurantoin-induced growth inhibition (p < 0.001 comparing PC-3 with MLE 15 cells and p < 0.01 comparing PC-3 with HT-29 cells; compare Tables 1 and 3).
Fig. 7. Intracellular ROI production in CHO-WT and CHO-OR cells.
DCFH-DA was used to quantify intracellular ROI generation. Cells were incubated with 5 μM DCFH-DA at 37 °C for 3 h in the absence (solid lines) or presence (dashed lines) of 500 μM nitrofurantoin. Cellular fluorescence was analyzed by flow cytometry. One of three representative experiments is shown. The mean fluorescence of CHO-WT control peak is 1.01 ± 0.04 (n = 3 ± SE) and 7.25 ± 0.28 after nitrofurantoin treatment. For CHO-OR cells, the mean fluorescence of the control peak is 1.13 ± 0.03, 5.01 ± 0.21 for subpeak 1, and 66.40 ± 4.49 for subpeak 2 after nitrofurantoin treatment.
Fig. 8. Growth inhibition by nitrofurantoin.
Cells were seeded in six-well plates and allowed to adhere overnight. The medium was then replaced with growth medium supplemented with increasing concentrations of nitrofurantoin. Cell growth was assayed after 4–5 days. (Left) Comparison of the growth inhibitory activity of nitrofurantoin in CHO-WT and CHO-OR cells. (Right) Comparison of the growth inhibitory activity of nitrofurantoin in C2 and S-180 cells.
Discussion
Several enzymes in mammalian cells that mediate one-electron reduction of nitroaromatic compounds to reactive electrophilic intermediates have been identified including cytochrome P450 reductase [14,26], NADH:lipoamide oxidoreductase [27], NAD(P)H:quinone oxidoreductase (NQO1) [28], thioredoxin reductase [29], nitric oxide synthase [30], xanthine oxidase [31], and ferredoxin: NADP+reductase [32]. Oxidation of reactive electrophilic intermediates back to the parent compound generates toxic ROI. It is well recognized that nitrofurantoin can undergo a one-electron reduction; however, the enzymatic activity mediating this reaction is not well characterized. Previous studies have demonstrated that microsomal fractions from rat lung and liver can reduce nitrofurantoin, suggesting that this process is mediated by cytochrome P450 reductase [33]. This is supported by the observation that inhibitors of this enzyme suppress reduction of nitrofurantoin in rat hepatocytes [14]. Using purified rat liver cytochrome P450 reductase, it has also been demonstrated that nitrofurantoin undergoes a one-electron reduction to the nitro aromatic anion radical and is reoxidized under aerobic condition [34]. Our data show that redox cycling of nitrofurantoin by a recombinant cytochrome P450 reductase, or lysates from a variety of cell types, generates superoxide anion, hydrogen peroxide, and, in the presence of redox-active iron, hydroxyl radicals. This redox cycling reaction requires NADPH and is inhibited by diphenyleneiodonium, demonstrating that it is dependent on the flavin cofactors in the cytochrome P450 reductase.
ROI are known to be cytotoxic and their production during nitrofurantoin redox cycling has the potential to contribute to tissue injury. To examine the relationship between nitrofurantoin-induced ROI production during redox cycling and cytotoxicity, we used cell lines varying in their cytochrome P450 reductase activity. We found that there was a direct correlation between the nitrofurantoin redox cycling and the activity of cytochrome P450 reductase in the cells. This was most evident in CHO cells constructed to overexpress cytochrome P450 reductase in which nitrofurantoin redox cycling was more than 15 times greater than that in wild-type cells. The overexpressing cells also produced approximately six-fold more intracellular ROI following nitrofurantoin treatment. Unexpectedly, no major differences between the two cell types with respect to nitrofurantoin-induced cytotoxicity as measured by growth inhibition were evident. These data suggest that cytotoxicity is independent of nitrofurantoin redox cycling and ROI production. This is supported by similar findings in several cell lines. For example, S-180 cells were two times more sensitive to growth inhibition by nitrofurantoin than C2 cells, despite that the cells displayed similar nitrofurantoin redox cycling activity. Furthermore, although MLE 15 cells displayed approximately four-fold more nitrofurantoin redox cycling activity than PC-3 cells, they were three-fold less sensitive to nitrofurantoin-induced cytotoxicity. These data provide support for the idea that nitrofurantoin redox cycling and subsequent production of ROI does not mediate cytotoxicity. Thus, levels of cytochrome P450 reductase activity in tumor cells may not be predictive for a higher level of sensitivity against nitrofurantoin toxicity.
At the present time, the mechanisms underlying nitrofurantoin toxicity is not known. Among the ROI generated during nitrofurantoin-induced redox cycling, hydroxyl radicals are the most damaging [35,36]. Cytotoxicity may, in part, be related to the ability of nitrofurantoin to generate hydroxyl radicals in each cell line, a process that depends on the availability of redox-active metals such as iron and copper. It is possible that the different cell lines vary in their metal content and that this limits nitrofurantoin-induced cytotoxicity. In this regard, Staubli and Boelsterli [37] and Sturm et al. [38] reported that nitrofurantoin increases the intracellular labile iron pool, a factor that may control redox cycling. Alternatively, intracellular levels of antioxidants that detoxify and protect against ROI-induced damage may be important in regulating nitrofurantoin-induced cytotoxicity. In this regard, superoxide dismutase, catalase, and antioxidants including alpha-tocopherol, ascorbic acid, and dimethyl sulfoxide have been reported to block nitrofurantoin-induced cytotoxicity in a rat lung explant model [8]. Metabolism of nitrofurantoin to reactive metabolites is another factor that may regulate cytotoxicity. Electrophilic intermediates formed as a result of redox cycling of nitrofurantoin have been reported to bind glutathione and cellular macromolecules [33]. The generation of these metabolites depends on localized oxygen tension which can control bioreduction of nitrofurantoin in target tissues [10]. Reduction of nitrofurantoin to its amine and further derivatization may also generate cytotoxic metabolites [34,39]. Additional studies are needed to characterize metabolism of nitrofurantoin in the various cell types under different conditions and to determine its role in cytotoxicity.
Although redox cycling of nitrofurantoin is directly correlated with cytochrome P450 reductase activity in almost all the cell lines, it is likely that additional oxidoreductases in the cells redox cycle this antibiotic. This is supported by our findings of significant nitrofurantoin redox cycling in the cytosol of HT-29 cells which does not contain cytochrome P450 reductase activity. Low levels of nitrofurantoin redox cycling activity were also evident in cytosolic fractions of MLE 15 cells, suggesting that additional enzymes also redox cycle nitrofurantoin in these cells. In this regard, our laboratory has recently demonstrated paraquat redox cycling by thioredoxin reductase in cytosolic fractions of MLE 15 cells [40]. Studies to identify the NADPH oxidoreductases that are important in redox cycling nitrofurantoin in the cytosolic fraction of cells are in progress. It should be noted that we also cannot exclude the possibility that, in addition to cytochrome P450 reductase, other oxidoreductases mediate redox cycling of nitrofurantoin in the microsomal fraction of cells.
Using recombinant cytochrome P450 reductase, we found that increased nitrofurantoin redox cycling was due to an increase in Vmax for NADPH with little change in affinity of the enzyme for the pyridine nucleotide. Moreover, in all cell types except RAW 264.7 and B16 cells, nitrofurantoin redox cycling increased the Vmax but appeared to decrease the enzyme affinity for NADPH. The changes in reaction kinetics for NADPH in RAW 264.7 and B16 cells were similar to the recombinant cytochrome P450 reductase. Increases in Vmax were not surprising because we are measuring redox cycling in nitrofurantoin-sensitive redox enzymes. However, apparent changes in affinity of NADPH in enzyme assays are most likely due to differences in the kinetic characteristics of various constitutive enzymes in cell lysates that utilize NADPH as a one-electron donor for redox cycling in the absence of nitrofurantoin and the specific characteristics of the enzyme(s) activated by the drug. The mechanisms underlying the distinct reaction kinetics for NADPH in RAW 264.7 and B16 cells are not apparent. It will be of interest to characterize the enzymes in these and the other cell lines that are important in nitrofurantoin redox cycling.
In summary, our data show that nitrofurantoin readily redox cycles with recombinant cytochrome P450 reductase and lysates of many different cell types. In the cells, ROI generated by nitrofurantoin redox cycling directly correlates with cytochrome P450 reductase activity. However, it does not correlate with the cytotoxicity of nitrofurantoin. Thus, in these cells, ROI-induced oxidative stress alone does not mediate the toxicity of nitrofurantoin. In humans and other animals, nitrofurantoin is known to induce toxicity in many tissues including lung, liver, peripheral nerves and blood. Further studies to determine the role of redox cycling in nitrofurantoin-induced toxicity in these tissues and to identify alternative sites of action of this drug in tumor cells are needed.
Acknowledgments
This work was supported in part by National Institutes of Health Grants CA100994, CA093798, ES004738, GM034310, and ES005022 and by the National Institutes of Health CounterACT Program through the National Institute of Arthritis and Musculoskeletal and Skin Diseases (Award No. U54AR055073). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the federal government.
Abbreviations
- ROI
reactive oxygen intermediates
- CHO
Chinese hamster ovary
- MLE
murine lung epithelial
- PBS
phosphate-buffered saline
- SOD
superoxide dismutase
- DMSO
dimethyl sulfoxide
References
- 1.Witten CM. Pulmonary toxicity of nitrofurantoin. Arch Phys Med Rehabil. 1989;70:55–57. [PubMed] [Google Scholar]
- 2.Paiva LA, Wright PJ, Koff RS. Long-term hepatic memory for hypersensitivity to nitrofurantoin. Am J Gastroenterol. 1992;87:891–893. [PubMed] [Google Scholar]
- 3.Jacknowitz AI, Le Frock JL, Prince RA. Nitrofurantoin polyneuropathy: report of two cases. Am J Hosp Pharm. 1977;34:759–762. [PubMed] [Google Scholar]
- 4.Gait JE. Hemolytic reactions to nitrofurantoin in patients with glucose-6-phosphate dehydrogenase deficiency: theory and practice. Dicp. 1990;24:1210–1213. doi: 10.1177/106002809002401213. [DOI] [PubMed] [Google Scholar]
- 5.Kamat AM, Lamm DL. Antitumor activity of common antibiotics against superficial bladder cancer. Urology. 2004;63:457–460. doi: 10.1016/j.urology.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 6.Oscier D, Bramble J, Hodges E, Wright D. Regression of mucosa-associated lymphoid tissue lymphoma of the bladder after antibiotic therapy. J Clin Oncol. 2002;20:882. doi: 10.1200/JCO.2002.20.3.882. [DOI] [PubMed] [Google Scholar]
- 7.McOsker CC, Fitzpatrick PM. Nitrofurantoin: mechanism of action and implications for resistance development in common uropathogens. J Antimicrob Chemother. 1994;33(Suppl A):23–30. doi: 10.1093/jac/33.suppl_a.23. [DOI] [PubMed] [Google Scholar]
- 8.Martin WJ., 2nd Nitrofurantoin: evidence for the oxidant injury of lung parenchymal cells. Am Rev Respir Dis. 1983;127:482–486. doi: 10.1164/arrd.1983.127.4.482. [DOI] [PubMed] [Google Scholar]
- 9.Rossi L, Silva JM, McGirr LG, O’Brien PJ. Nitrofurantoin-mediated oxidative stress cytotoxicity in isolated rat hepatocytes. Biochem Pharmacol. 1988;37:3109–3117. doi: 10.1016/0006-2952(88)90308-5. [DOI] [PubMed] [Google Scholar]
- 10.Minchin RF, Ho PC, Boyd MR. Reductive metabolism of nitrofurantoin by rat lung and liver in vitro. Biochem Pharmacol. 1986;35:575–580. doi: 10.1016/0006-2952(86)90350-3. [DOI] [PubMed] [Google Scholar]
- 11.Neuzil J, Gebicki JM, Stocker R. Radical-induced chain oxidation of proteins and its inhibition by chain-breaking antioxidants. Biochem J. 1993;293(Pt 3):601–606. doi: 10.1042/bj2930601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall PM, Stupans I, Burgess W, Birkett DJ, McManus ME. Immunohistochemical localization of NADPH-cytochrome P450 reductase in human tissues. Carcinogenesis. 1989;10:521–530. doi: 10.1093/carcin/10.3.521. [DOI] [PubMed] [Google Scholar]
- 13.Shimada T, Mernaugh RL, Guengerich FP. Interactions of mammalian cytochrome P450, NADPH-cytochrome P450 reductase, and cytochrome b(5) enzymes. Arch Biochem Biophys. 2005;435:207–216. doi: 10.1016/j.abb.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Pourahmad J, Khan S, O’Brien PJ. Lysosomal oxidative stress cytotoxicity induced by nitrofurantoin redox cycling in hepatocytes. Adv Exp Med Biol. 2001;500:261–265. doi: 10.1007/978-1-4615-0667-6_41. [DOI] [PubMed] [Google Scholar]
- 15.Han JF, Wang SL, He XY, Liu CY, Hong JY. Effect of genetic variation on human cytochrome p450 reductase-mediated paraquat cytotoxicity. Toxicol Sci. 2006;91:42–48. doi: 10.1093/toxsci/kfj139. [DOI] [PubMed] [Google Scholar]
- 16.Wikenheiser KA, Vorbroker DK, Rice WR, Clark JC, Bachurski CJ, Oie HK, Whitsett JA. Production of immortalized distal respiratory epithelial cell lines from surfactant protein C/simian virus 40 large tumor antigen transgenic mice. Proc Natl Acad Sci USA. 1993;90:11029–11033. doi: 10.1073/pnas.90.23.11029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mariano TM, Vetrano AM, Gentile SL, Heck DE, Whittemore MS, Guillon CD, Jabin I, Rapp RD, Heindel ND, Laskin JD. Cell-impermeant pyridinium derivatives of psoralens as inhibitors of keratinocyte growth. Biochem Pharmacol. 2002;63:31–39. doi: 10.1016/s0006-2952(01)00855-3. [DOI] [PubMed] [Google Scholar]
- 18.Zhou M, Diwu Z, Panchuk-Voloshina N, Haugland RP. A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal Biochem. 1997;253:162–168. doi: 10.1006/abio.1997.2391. [DOI] [PubMed] [Google Scholar]
- 19.Tocher JH, Edwards DI. Electrochemical characteristics of nitroheterocyclic compounds of biological interest. V. Measurement and comparison of nitro radical lifetimes. Int J Radiat Biol. 1990;57:45–53. doi: 10.1080/09553009014550331. [DOI] [PubMed] [Google Scholar]
- 20.Mishin VM, Thomas PE. Characterization of hydroxyl radical formation by microsomal enzymes using a water-soluble trap, terephthalate. Biochem Pharmacol. 2004;68:747–752. doi: 10.1016/j.bcp.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Zhao H, Joseph J, Fales HM, Sokoloski EA, Levine RL, Vasquez-Vivar J, Kalyanaraman B. Detection and characterization of the product of hydroethidine and intracellular superoxide by HPLC and limitations of fluorescence. Proc Natl Acad Sci USA. 2005;102:5727–5732. doi: 10.1073/pnas.0501719102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heck DE, Laskin DL, Gardner CR, Laskin JD. Epidermal growth factor suppresses nitric oxide and hydrogen peroxide production by keratinocytes. Potential role for nitric oxide in the regulation of wound healing. J Biol Chem. 1992;267:21277–21280. [PubMed] [Google Scholar]
- 23.Wardman P, Candeias LP. Fenton chemistry: an introduction. Radiat Res. 1996;145:523–531. [PubMed] [Google Scholar]
- 24.Williams CH, Jr, Kamin H. Microsomal triphosphopyridine nucleotide-cytochrome c reductase of liver. J Biol Chem. 1962;237:587–595. [PubMed] [Google Scholar]
- 25.Phillips AH, Langdon RG. Hepatic triphosphopyridine nucleotide-cytochrome c reductase: isolation, characterization, and kinetic studies. J Biol Chem. 1962;237:2652–2660. [PubMed] [Google Scholar]
- 26.Lemaire P, Livingstone DR. Inhibition studies on the involvement of flavoprotein reductases in menadione- and nitrofurantoin-stimulated oxyradical production by hepatic microsomes of flounder (Platichthys flesus) J Biochem Toxicol. 1994;9:87–95. doi: 10.1002/jbt.2570090206. [DOI] [PubMed] [Google Scholar]
- 27.Sreider CM, Grinblat L, Stoppani AO. Catalysis of nitrofuran redox-cycling and superoxide anion production by heart lipoamide dehydrogenase. Biochem Pharmacol. 1990;40:1849–1857. doi: 10.1016/0006-2952(90)90366-s. [DOI] [PubMed] [Google Scholar]
- 28.Hasspieler BM, Haffner GD, Adeli K. Roles of DT diaphorase in the genotoxicity of nitroaromatic compounds in human and fish cell lines. J Toxicol Environ Health. 1997;52:137–148. doi: 10.1080/00984109708984057. [DOI] [PubMed] [Google Scholar]
- 29.Cenas N, Prast S, Nivinskas H, Sarlauskas J, Arner ES. Interactions of nitroaromatic compounds with the mammalian selenoprotein thioredoxin reductase and the relation to induction of apoptosis in human cancer cells. J Biol Chem. 2006;281:5593–5603. doi: 10.1074/jbc.M511972200. [DOI] [PubMed] [Google Scholar]
- 30.Boelsterli UA, Ho HK, Zhou S, Leow KY. Bioactivation and hepatotoxicity of nitroaromatic drugs. Curr Drug Metab. 2006;7:715–727. doi: 10.2174/138920006778520606. [DOI] [PubMed] [Google Scholar]
- 31.Washburn PC, Di Giulio RT. Nitrofurantoin-stimulated superoxide production by channel catfish (Ictalurus punctatus) hepatic microsomal and soluble fractions. Toxicol Appl Pharmacol. 1988;95:363–377. doi: 10.1016/0041-008x(88)90355-9. [DOI] [PubMed] [Google Scholar]
- 32.Miskiniene V, Dickancaite E, Nemeikaite A, Cenas N. Nitroaromatic betulin derivatives as redox cycling agents. Biochem Mol Biol Int. 1997;42:391–397. doi: 10.1080/15216549700202791. [DOI] [PubMed] [Google Scholar]
- 33.Boyd MR, Stiko AW, Sasame HA. Metabolic activation of nitrofurantoin-possible implications for carcinogenesis. Biochem Pharmacol. 1979;28:601–606. doi: 10.1016/0006-2952(79)90142-4. [DOI] [PubMed] [Google Scholar]
- 34.Holtzman JL, Crankshaw DL, Peterson FJ, Polnaszek CF. The kinetics of the aerobic reduction of nitrofurantoin by NADPH-cytochrome P-450 (c) reductase. Mol Pharmacol. 1981;20:669–673. [PubMed] [Google Scholar]
- 35.Kehrer JP. The Haber-Weiss reaction and mechanisms of toxicity. Toxicology. 2000;149:43–50. doi: 10.1016/s0300-483x(00)00231-6. [DOI] [PubMed] [Google Scholar]
- 36.Welch KD, Davis TZ, Van Eden ME, Aust SD. Deleterious iron-mediated oxidation of biomolecules. Free Radic Biol Med. 2002;32:577–583. doi: 10.1016/s0891-5849(02)00760-8. [DOI] [PubMed] [Google Scholar]
- 37.Staubli A, Boelsterli UA. The labile iron pool in hepatocytes: prooxidant-induced increase in free iron precedes oxidative cell injury. Am J Physiol. 1998;274:G1031–G1037. doi: 10.1152/ajpgi.1998.274.6.G1031. [DOI] [PubMed] [Google Scholar]
- 38.Sturm B, Twaroch T, Knapitsch B, Czingraber S, Ternes N, Goldenberg H, Scheiber-Mojdehkar B. Differential response of iron metabolism to oxidative stress generated by antimycin A and nitrofurantoin. Biochimie. 2006;88:575–581. doi: 10.1016/j.biochi.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 39.Silva JM, Khan S, O’Brien PJ. Molecular mechanisms of nitrofurantoin-induced hepatocyte toxicity in aerobic versus hypoxic conditions. Arch Biochem Biophys. 1993;305:362–369. doi: 10.1006/abbi.1993.1433. [DOI] [PubMed] [Google Scholar]
- 40.Gray JP, Heck DE, Mishin V, Smith PJ, Hong JY, Thiruchelvam M, Cory-Slechta DA, Laskin DL, Laskin JD. Paraquat increases cyanide-insensitive respiration in murine lung epithelial cells by activating an NAD(P)H:paraquat oxidoreductase: identification of the enzyme as thioredoxin reductase. J Biol Chem. 2007;282:7939–7949. doi: 10.1074/jbc.M611817200. [DOI] [PubMed] [Google Scholar]