Abstract
Reactive oxygen species have long been implicated in the pathophysiology of acute liver injury. However, the translation of these findings to the clinic and the development of therapeutic agents have been slow mainly due to the poor mechanistic understanding of the pathophysiology and the many indirect approaches used to characterize the role of oxidant stress in liver injury. The current review discusses in depth the sources of reactive oxygen, the oxidants involved and the impact of this oxidant stress in the mechanism of cell death in 3 different clinically relevant acute liver injury models.
Keywords: oxidant stress, acute liver injury, acetaminophen hepatotoxicity, obstructive cholestasis, ischemia
1. Introduction
Generation of oxygen free radicals is a necessary by-product of an aerobic existence and the liver has substantial anti-oxidant defenses, such as the tri-peptide glutathione to prevent damaging effects of these radicals during normal physiology. This was clear in early experiments, where evaluation of glutathione disulfide efflux into bile demonstrated an extremely high resistance of the liver against intracellular reactive oxygen formation (even with impaired detoxification systems) [1]. However, in pathophysiological conditions, either due to metabolism of drugs such as acetaminophen, obstruction of the bile duct or conditions of ischemia, the balance between generation of free radicals and their capacity to detoxify them can be shifted such that oxidative stress can negatively influence cellular homeostasis and organelle function. The current information on the role of free radicals in various clinically relevant acute liver injury models will be examined here.
2. Oxidative and nitrosative stress in acetaminophen hepatotoxicity
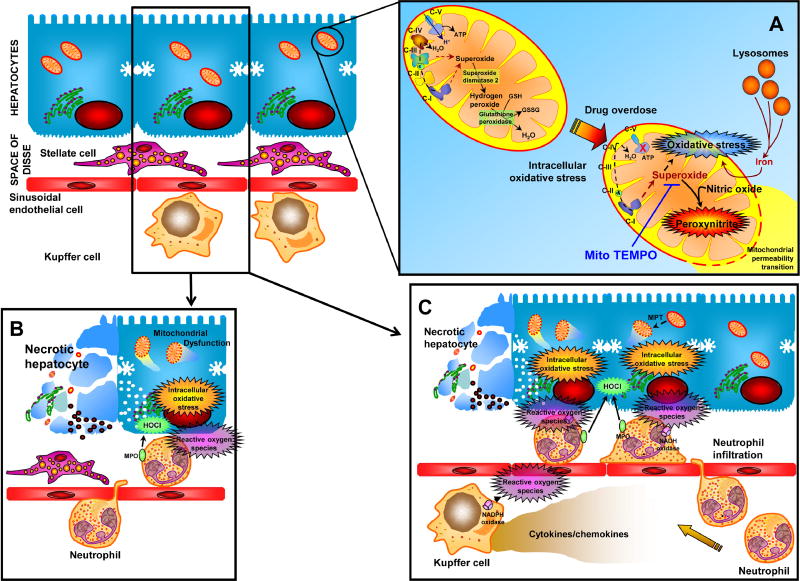
Acetaminophen overdose is the most common cause of acute liver failure in the United States [2] and intense investigation of the mechanism of hepatotoxicity over the last few decades have established the role of oxygen free radicals in the etiopathogenesis and resulted in the development of the only clinically available antidote, N-acetylcysteine. Initially it was thought that the source of free radicals in APAP-induced liver injury was the cytochrome P450 system, which can release superoxide during drug metabolism [3]. However, later studies both in rodent models [4] as well as in isolated hepatocytes in vitro [5] provided evidence that this was not the case. Though highly sensitive detection methods have recently demonstrated hydrogen peroxide generation during the APAP metabolism phase, it is unlikely that these low levels play a role in hepatocyte necrosis [6]. Extensive research into the source of oxygen free radicals after APAP overdose has now established that mitochondrial dysfunction and generation of free radicals such as superoxide are pivotal for APAP-induced hepatocyte necrosis [7]. This mitochondrial dysfunction is initiated by formation of adducts between the reactive APAP metabolite N-acetyl-p-benzoquinone imine (NAPQI) and mitochondrial proteins, especially components of the electron transport chain such as ATP synthase [8]. In addition, activity of complex I, which has been characterized as a source of mitochondrial free radicals in a number of contexts [6], was increased after acetaminophen overdose and this correlated with the extent of liver injury [9] (Figure 1). Generation of mitochondrial superoxide was significantly elevated within mitochondria in primary mouse hepatocytes as well as the metabolically competent human liver HepaRG cells [10, 11] an effect also seen in mitochondria isolated from mice treated with APAP [9]. The critical role of mitochondrial oxidant stress was further confirmed by the protection of the mitochondrial targeted anti-oxidant Mito-Tempo against APAP-induced hepatotoxicity, an effect not seen with Tempo, the same anti-oxidant lacking the mitochondrial targeting moiety [12]. Importance of mitochondrial superoxide in APAP-induced liver injury is illustrated by the exacerbation of liver injury in APAP-treated mice with a partial deficiency of manganese superoxide dismutase (MnSOD), the mitochondrial enzyme responsible for scavenging superoxide [13, 14]. This also suggests that hydrogen peroxide formation is benign in the context of APAP-induced liver injury, since deficiency of MnSOD would reduce H2O2 generation from superoxide, but that did not protect. Clues towards the pathophysiological relevance of superoxide formation came from studies demonstrating selective generation of nitrotyrosine protein adducts within mitochondria after APAP overdose, accompanied by oxidative damage to mitochondrial DNA [15]. MnSOD was also shown to be nitrated and inactivated in mice after APAP overdose [16]. This implies that reaction of superoxide with nitric oxide within mitochondria, resulting in generation of the highly reactive peroxynitrite species is the molecular basis of initiation of mitochondrial dysfunction and subsequent downstream events [6] (Figure 1). This is supported by the protection against APAP-induced liver injury by resveratrol [17], which can function as a direct scavenger of peroxynitrite [18]. Hence, it is now established that mitochondrial superoxide and peroxynitrite generation are the primary molecules responsible for oxidative stress during APAP overdose. An additional initiator of mitochondrial ROS generation could be lysosomal iron, which is released from disrupted lysosomes and taken up by mitochondria [19] through the calcium uniporter, generating hydroxyl radicals [20]. Though early studies had implicated lipid peroxidation in the process [21], this was later established to be due to the vitamin E-deficient diet the animals were subjected to, with very limited lipid peroxidation in animals on a normal diet [22]. In addition, there was no protection seen by vitamin E administration against APAP-induced liver injury in animals on a normal diet [22], ruling out a relevant role for lipid peroxidation in liver injury. Thus, while increased generation of free radicals due to dysfunction of the mitochondrial electron transport chain is important for APAP-induced liver injury, its biological effect is amplified due to the parallel deterioration in anti-oxidant status as seen by the depletion of glutathione in the cytosol and in mitochondria and the inactivation of MnSOD. A hint towards the clinical relevance of this is provided by the protection against liver injury seen with a mitochondrial targeted SOD mimetic such as Mito-Tempo. In addition, the beneficial effects of treatment with N-acetylcysteine even when administered after the metabolism phase suggests that the newly synthesized GSH scavenges peroxynitrite and reactive oxygen species in mice and in humans (reviewed in [6]).
Figure 1.
Hepatic oxidative stress can occur in a number of contexts: (A) In etiology of drug-induced liver injury as in acetaminophen overdose, enhanced mitochondrial generation of superoxide, probably through respiratory chain complex I initiates oxidative stress and generation of peroxynitrite by reaction with nitric oxide. This ultimately leads to activation of the mitochondrial permeability transition and hepatic necrosis, which can be prevented by mitochondrial targeted superoxide scavengers such as Mito-TEMPO. (B) In conditions such as obstructive cholestasis, the oxidative stress is mainly from infiltrating neutrophils generating hypochlorous acid, which ultimately induces mitochondria dysfunction. (C) In ischemia-reperfusion injury, however, both Kupffer cells and neutrophils are involved, which are primed by release of cytokines such as TNF-α. (modified from ref. 50)
3. Reactive Oxygen and Obstructive Cholestasis
Cholestasis is a disease process that involves reduction in bile flow and hepatic accumulation of bile acids. Cholestasis can be caused by drugs, endogenous compounds (e.g. estrogens) or in its most severe form obstruction of the bile duct by gallstones or pancreatic tumors. Early studies using rodent hepatocytes assumed that cholestatic liver injury is mainly caused by direct toxicity of hydrophobic bile acids, which induce a mitochondrial oxidant stress and apoptosis (reviewed in [23]. However, this hypothesis has been questioned for rodent hepatocytes when it was shown that the levels of toxic bile acids such as glycochenodeoxycholic acids and others remained several orders of magnitude below cytotoxically relevant concentrations after bile duct ligation in mice [24]. In contrast, the predominant bile acids in rodents, which include tauro-cholic acid and tauro-muricholic acid, are not cytotoxic and promote inflammatory gene expression in hepatocytes instead of cell death [24–26]. These cytokines and chemokines, together with the initial release of cleaved osteopontin [27], are responsible for hepatic neutrophil recruitment and an inflammatory injury in areas where bile leaks back into parenchyma [28, 29]. The focal necrosis caused by neutrophil cytotoxicity involves evidence for an extensive neutrophil-derived oxidant stress generated by hypochlorous acid formation as indicated by staining for chlorotyrosine adducts on intracellular proteins [28, 29] (Figure 1). Inhibitors of NADPH oxidase eliminated chlorotyrosine staining and consequently prevented hepatocellular necrosis [30] and glutathione peroxidase deficiency enhanced liver injury [31] in a galactosamine/endotoxin shock model. These data support the hypothesis that neutrophil-derived extracellular oxidants generated in close proximity diffuse into the hepatocytes and induce a direct intracellular oxidant stress in target cells [32]. However, this may not be sufficient to directly cause cell death. Experiments where hepatocytes were exposure to moderate levels of t-butylhydroperoxide in vitro, cell death was caused by mitochondrial dysfunction and a mitochondrial oxidant stress, which triggered the formation of mitochondrial membrane permeability transition pores (MPTP) and collapse of the membrane potential [33, 34]. Similar to APAP toxicity, the mitochondrial translocation of lysosomal iron provides a second hit together with the oxidants to facilitate the MPTP opening [35]. This insight into sources and the mechanism of oxidant stress-mediated liver injury during obstructive cholestasis suggests that therapeutic targets for this disease could include inhibitors of critical adhesion molecules responsible for neutrophil recruitment to the liver, inhibitors of NADPH oxidase that prevent reactive oxygen formation by neutrophils, intracellular antioxidants that scavenge mitochondria-derived oxidants and iron chelators. In contrast, lipid-soluble antioxidants would be expected to be ineffective.
4. Reactive Oxygen and Hepatic Ischemia
Hepatic ischemia is a common clinical problem, which can be caused by elective surgery (Pringle Maneuver during tumor resection, liver transplantation), hemorrhagic shock and drug overdose–induced hypotension episodes. Re-establishing blood flow and adequate oxygen supply is vital to prevent ischemia necrosis, however, it was recognized several decades ago that reperfusion also causes damage, termed reperfusion injury [36]. The early studies hypothesized that activation of the intracellular enzyme xanthine oxidase is the main source of reactive oxygen species (e.g., superoxide and hydrogen peroxide) during reperfusion resulting in lipid peroxidation, which causes cell death [37]. However, this hypothesis was questioned because direct evidence for a relevant intracellular stress was missing and it could be demonstrated that the liver was still able to detoxify orders of magnitude more superoxide and hydrogen peroxide than was generated during reperfusion [38]. On the other hand, it was recognized that initially Kupffer cells and later neutrophils contribute to a vascular oxidant stress in close proximity to the stressed hepatocytes, which causes cell death [39–41]. The priming for reactive oxygen formation for both Kupffer cells and neutrophils and the recruitment of neutrophils into the liver is caused by cytokines, especially TNF-α, CXC chemokines and complement factors [42–44] (Figure 1). These detailed studies on the oxidant stress during reperfusion have been performed in both rats [41] and mice [45]. Interestingly, lipid peroxidation was considered the main mechanism by which oxidant stress causes cell death during hepatic ischemia-reperfusion and other liver disease processes [46]. However, many lipid peroxidation parameters are elevated only 2-to-3-times above baseline despite severe liver reperfusion injury [47]. In contrast, it was shown that it requires a very severe oxidant stress by t-butyl hydroperoxide to cause sufficient lipid peroxidation (50-times above baseline) that can kill hepatocytes [47]. In fact, even excessive intracellular oxidant stress caused by the herbicide diquat, which is a redox-cycling agent that produces superoxide, can be effectively detoxified in hepatocytes with very little damage [38]. The well-developed antioxidant defense system, which includes superoxide dismutases, glutathione peroxidase, thioredoxins, peroxyredoxin and glutaredoxins together with glutathione in the cytosol and mitochondria, the iron chelator ferritin in the cytosol and vitamin E in membranes prevents lipid peroxidation unless there is an excessive oxidant stress and a serious compromise of the antioxidant system [48]. In fact, ischemic preconditioning, an occasionally used intervention during elective surgery (tumor resections), produces a sublethal oxidant stress, which is thought to protect against ischemia-reperfusion injury by strengthening the antioxidant defense system in the liver [49]. Even a lethal oxidant stress rarely causes direct destruction of the cell but more frequently triggers disturbances of the intracellular homeostasis, which results in the formation of a secondary hit, e.g. a mitochondrial oxidant stress, that promotes signaling mechanisms of apoptosis or programmed necrosis [50].
5. Perspectives
Oxidant stress has been implicated in a number of liver disease processes. However, therapeutic interventions that specifically target reactive oxygen species have shown limited clinically efficacy. Part of the reason can be that some cell or animal models used to study the oxidant stress in a pathophysiology may not accurately mimic the human disease. In addition, the oxidant stress or antioxidants used may have been insufficiently characterized. As the discussion of several well studied examples showed, it is important to identify the source and the exact nature of the reactive oxygen species that may be generated. These events can be time-sensitive and dose-dependent and may not be limited to a single source. Furthermore, the specificity of any intervention against reactive oxygen needs to be established and preferably multiple interventions need to be tested. As an oxidant stress can be the cause of cell dysfunction and cell death but also a consequence of the injury, it is vital to establish causality through time course studies and specific interventions. Most importantly, translational studies using biomarkers need to evaluate the relevance of the mechanisms studied in experimental models for humans. Considering these important aspects will enhance the chances that interventions discovered in cell and animal models will be successfully translated to the human pathophysiology.
Highlights.
The current status of the role of oxygen free radicals in models of acute liver injury is discussed
Mitochondrial-derived superoxide and peroxynitrite are critical mediators of acetaminophen hepatotoxicity
Mitochondrial free radical generation facilitated by lysosomal iron functions as a second hit after neutrophil mediated hypochlorite formation in obstructive cholestasis
Cytokine mediated priming is important for neutrophil and Kupffer cell mediated oxidative stress in hepatic ischemia-reperfusion injury
Acknowledgments
Work in the authors’ laboratory was supported by the National Institutes of Health grants R01 DK070195 and R01 AA12916, and by grants from the National Institute of General Medical Sciences (P20 GM103549 and P30 GM118247) of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
special interest (•)
outstanding interest (••)
- 1.Jaeschke H, Benzick AE. Pathophysiological consequences of enhanced intracellular superoxide formation in isolated perfused rat liver. Chem Biol Interact. 1992;84(1):55–68. doi: 10.1016/0009-2797(92)90120-a. [DOI] [PubMed] [Google Scholar]
- 2.Budnitz DS, Lovegrove MC, Crosby AE. Emergency department visits for overdoses of acetaminophen-containing products. Am J Prev Med. 2011;40(6):585–92. doi: 10.1016/j.amepre.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 3.Wendel A, Feuerstein S. Drug-induced lipid peroxidation in mice--I. Modulation by monooxygenase activity, glutathione and selenium status. Biochem Pharmacol. 1981;30(18):2513–20. doi: 10.1016/0006-2952(81)90576-1. [DOI] [PubMed] [Google Scholar]
- 4.Smith CV, Jaeschke H. Effect of acetaminophen on hepatic content and biliary efflux of glutathione disulfide in mice. Chem Biol Interact. 1989;70(3–4):241–8. doi: 10.1016/0009-2797(89)90047-1. [DOI] [PubMed] [Google Scholar]
- 5.Bajt ML, et al. Acetaminophen-induced oxidant stress and cell injury in cultured mouse hepatocytes: protection by N-acetyl cysteine. Toxicol Sci. 2004;80(2):343–9. doi: 10.1093/toxsci/kfh151. [DOI] [PubMed] [Google Scholar]
- 6*.Du K, Ramachandran A, Jaeschke H. Oxidative stress during acetaminophen hepatotoxicity: Sources, pathophysiological role and therapeutic potential. Redox Biol. 2016;10:148–156. doi: 10.1016/j.redox.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaeschke H, McGill MR, Ramachandran A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug Metab Rev. 2012;44(1):88–106. doi: 10.3109/03602532.2011.602688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qiu Y, Benet LZ, Burlingame AL. Identification of the hepatic protein targets of reactive metabolites of acetaminophen in vivo in mice using two-dimensional gel electrophoresis and mass spectrometry. J Biol Chem. 1998;273(28):17940–53. doi: 10.1074/jbc.273.28.17940. [DOI] [PubMed] [Google Scholar]
- 9.Du K, et al. Editor's Highlight: Metformin Protects Against Acetaminophen Hepatotoxicity by Attenuation of Mitochondrial Oxidant Stress and Dysfunction. Toxicol Sci. 2016;154(2):214–226. doi: 10.1093/toxsci/kfw158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan HM, et al. The oxygen tension modulates acetaminophen-induced mitochondrial oxidant stress and cell injury in cultured hepatocytes. Toxicol Sci. 2010;117(2):515–23. doi: 10.1093/toxsci/kfq208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGill MR, et al. HepaRG cells: a human model to study mechanisms of acetaminophen hepatotoxicity. Hepatology. 2011;53(3):974–82. doi: 10.1002/hep.24132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12**.Du K, Farhood A, Jaeschke H. Mitochondria-targeted antioxidant Mito-Tempo rotects against acetaminophen hepatotoxicity. Arch Toxicol. 2017;91(2):761–773. doi: 10.1007/s00204-016-1692-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujimoto K, et al. Sensitivity of liver injury in heterozygous Sod2 knockout mice treated with troglitazone or acetaminophen. Toxicol Pathol. 2009;37(2):193–200. doi: 10.1177/0192623308329282. [DOI] [PubMed] [Google Scholar]
- 14.Ramachandran A, et al. The impact of partial manganese superoxide dismutase (SOD2)-deficiency on mitochondrial oxidant stress, DNA fragmentation and liver injury during acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 2011;251(3):226–33. doi: 10.1016/j.taap.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cover C, et al. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2005;315(2):879–87. doi: 10.1124/jpet.105.088898. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal R, et al. Acetaminophen-induced hepatotoxicity in mice occurs with inhibition of activity and nitration of mitochondrial manganese superoxide dismutase. J Pharmacol Exp Ther. 2011;337(1):110–6. doi: 10.1124/jpet.110.176321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du K, et al. Resveratrol prevents protein nitration and release of endonucleases from mitochondria during acetaminophen hepatotoxicity. Food Chem Toxicol. 2015;81:62–70. doi: 10.1016/j.fct.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holthoff JH, et al. Resveratrol, a dietary polyphenolic phytoalexin, is a functional scavenger of peroxynitrite. Biochem Pharmacol. 2010;80(8):1260–5. doi: 10.1016/j.bcp.2010.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kon K, et al. Lysosomal iron mobilization and induction of the mitochondrial permeability transition in acetaminophen-induced toxicity to mouse hepatocytes. Toxicol Sci. 2010;117(1):101–8. doi: 10.1093/toxsci/kfq175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu J, et al. Translocation of iron from lysosomes to mitochondria during acetaminophen-induced hepatocellular injury: Protection by starch-desferal and minocycline. Free Radic Biol Med. 2016;97:418–426. doi: 10.1016/j.freeradbiomed.2016.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wendel A, Feuerstein S, Konz KH. Acute paracetamol intoxication of starved mice leads to lipid peroxidation in vivo. Biochem Pharmacol. 1979;28(13):2051–5. doi: 10.1016/0006-2952(79)90223-5. [DOI] [PubMed] [Google Scholar]
- 22.Knight TR, et al. Role of lipid peroxidation as a mechanism of liver injury after acetaminophen overdose in mice. Toxicol Sci. 2003;76(1):229–36. doi: 10.1093/toxsci/kfg220. [DOI] [PubMed] [Google Scholar]
- 23.Copple BL, Jaeschke H, Klaassen CD. Oxidative stress and the pathogenesis of cholestasis. Semin Liver Dis. 2010;30(2):195–204. doi: 10.1055/s-0030-1253228. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, et al. Effect of bile duct ligation on bile acid composition in mouse serum and liver. Liver Int. 2012;32(1):58–69. doi: 10.1111/j.1478-3231.2011.02662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25*.Allen K, Jaeschke H, Copple BL. Bile acids induce inflammatory genes in hepatocytes: a novel mechanism of inflammation during obstructive cholestasis. Am J Pathol. 2011;178(1):175–86. doi: 10.1016/j.ajpath.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cai SY, et al. Bile acids initiate cholestatic liver injury by triggering a hepatocyte-specific inflammatory response. JCI Insight. 2017;2(5):e90780. doi: 10.1172/jci.insight.90780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang M, et al. Osteopontin is an initial mediator of inflammation and liver injury during obstructive cholestasis after bile duct ligation in mice. Toxicol Lett. 2014;224(2):186–95. doi: 10.1016/j.toxlet.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gujral JS, et al. Neutrophils aggravate acute liver injury during obstructive cholestasis in bile duct-ligated mice. Hepatology. 2003;38(2):355–63. doi: 10.1053/jhep.2003.50341. [DOI] [PubMed] [Google Scholar]
- 29.Gujral JS, et al. Functional importance of ICAM-1 in the mechanism of neutrophil-induced liver injury in bile duct-ligated mice. Am J Physiol Gastrointest Liver Physiol. 2004;286(3):G499–507. doi: 10.1152/ajpgi.00318.2003. [DOI] [PubMed] [Google Scholar]
- 30.Gujral JS, et al. NADPH oxidase-derived oxidant stress is critical for neutrophil cytotoxicity during endotoxemia. Am J Physiol Gastrointest Liver Physiol. 2004;287(1):G243–52. doi: 10.1152/ajpgi.00287.2003. [DOI] [PubMed] [Google Scholar]
- 31.Jaeschke H, et al. Glutathione peroxidase-deficient mice are more susceptible to neutrophil-mediated hepatic parenchymal cell injury during endotoxemia: importance of an intracellular oxidant stress. Hepatology. 1999;29(2):443–50. doi: 10.1002/hep.510290222. [DOI] [PubMed] [Google Scholar]
- 32**.Jaeschke H. Mechanisms of Liver Injury. II. Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia-reperfusion and other acute inflammatory conditions. Am J Physiol Gastrointest Liver Physiol. 2006;290(6):G1083–8. doi: 10.1152/ajpgi.00568.2005. [DOI] [PubMed] [Google Scholar]
- 33.Nieminen AL, et al. Contribution of the mitochondrial permeability transition to lethal injury after exposure of hepatocytes to t-butylhydroperoxide. Biochem J. 1995;307(Pt 1):99–106. doi: 10.1042/bj3070099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34**.Lemasters JJ, Nieminen AL. Mitochondrial oxygen radical formation during reductive and oxidative stress to intact hepatocytes. Biosci Rep. 1997;17(3):281–91. doi: 10.1023/a:1027332611839. [DOI] [PubMed] [Google Scholar]
- 35.Uchiyama A, et al. Translocation of iron from lysosomes into mitochondria is a key event during oxidative stress-induced hepatocellular injury. Hepatology. 2008;48(5):1644–54. doi: 10.1002/hep.22498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Granger DN, Kvietys PR. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 2015;6:524–51. doi: 10.1016/j.redox.2015.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshikawa T, et al. Role of active oxygen species and lipid peroxidation in liver injury induced by ischemia-reperfusion. Nihon Shokakibyo Gakkai Zasshi. 1990;87(2):199–205. [PubMed] [Google Scholar]
- 38.Jaeschke H, Smith CV, Mitchell JR. Reactive oxygen species during ischemia-reflow injury in isolated perfused rat liver. J Clin Invest. 1988;81(4):1240–6. doi: 10.1172/JCI113441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jaeschke H, Farhood A. Neutrophil and Kupffer cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. Am J Physiol. 1991;260(3 Pt 1):G355–62. doi: 10.1152/ajpgi.1991.260.3.G355. [DOI] [PubMed] [Google Scholar]
- 40.Jaeschke H, et al. Functional inactivation of neutrophils with a Mac-1 (CD11b/CD18) monoclonal antibody protects against ischemia-reperfusion injury in rat liver. Hepatology. 1993;17(5):915–23. [PubMed] [Google Scholar]
- 41*.Hasegawa T, et al. Generation of hypochlorite-modified proteins by neutrophils during ischemia-reperfusion injury in rat liver: attenuation by ischemic preconditioning. Am J Physiol Gastrointest Liver Physiol. 2005;289(4):G760–7. doi: 10.1152/ajpgi.00141.2005. [DOI] [PubMed] [Google Scholar]
- 42.Jaeschke H, et al. Complement activates Kupffer cells and neutrophils during reperfusion after hepatic ischemia. Am J Physiol. 1993;264(4 Pt 1):G801–9. doi: 10.1152/ajpgi.1993.264.4.G801. [DOI] [PubMed] [Google Scholar]
- 43.Lentsch AB, et al. Chemokine involvement in hepatic ischemia/reperfusion injury in mice: roles for macrophage inflammatory protein-2 and KC. Hepatology. 1998;27(4):1172–7. doi: 10.1002/hep.510270440. [DOI] [PubMed] [Google Scholar]
- 44.Colletti LM, et al. Role of tumor necrosis factor-alpha in the pathophysiologic alterations after hepatic ischemia/reperfusion injury in the rat. J Clin Invest. 1990;85(6):1936–43. doi: 10.1172/JCI114656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hasegawa T, et al. Reduced inflammatory response and increased microcirculatory disturbances during hepatic ischemia-reperfusion injury in steatotic livers of ob/ob mice. Am J Physiol Gastrointest Liver Physiol. 2007;292(5):G1385–95. doi: 10.1152/ajpgi.00246.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poli G, Albano E, Dianzani MU. The role of lipid peroxidation in liver damage. Chem Phys Lipids. 1987;45(2–4):117–42. doi: 10.1016/0009-3084(87)90063-6. [DOI] [PubMed] [Google Scholar]
- 47.Mathews WR, et al. Lipid peroxidation as molecular mechanism of liver cell injury during reperfusion after ischemia. Free Radic Biol Med. 1994;16(6):763–70. doi: 10.1016/0891-5849(94)90191-0. [DOI] [PubMed] [Google Scholar]
- 48.Jaeschke H, Ramachandran A. Cellular Antioxidant Defense Mechanisms. In: McQueen CA, editor. Comprehensive Toxicology. Elsevier; 2017. [Google Scholar]
- 49.Rudiger HA, Graf R, Clavien PA. Sub-lethal oxidative stress triggers the protective effects of ischemic preconditioning in the mouse liver. J Hepatol. 2003;39(6):972–7. doi: 10.1016/s0168-8278(03)00415-x. [DOI] [PubMed] [Google Scholar]
- 50.Jaeschke H, Ramachandran A. Reactive oxygen species in the normal and acutely injured liver. J Hepatol. 2011;55(1):227–8. doi: 10.1016/j.jhep.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]