Abstract
Fatigue is a subjective, unpleasant, potentially disabling symptom rooted in physiological, psychological, and behavioral causes. People living with HIV are a population highly affected by fatigue due to risk factors associated with HIV-infection, treatment, and psychosocial disease burden. People with HIV are living longer, and are facing the challenge of a longer disease trajectory. Palliative nurses with expertise in symptom management can play a crucial role in helping people with HIV to engage in health behaviors that prevent or mitigate fatigue. In this paper we present a definition and overview of fatigue, describe the problem of fatigue in people living with HIV, and present a case study that illustrates the role of the palliative nurse in helping a person with HIV to cope with fatigue.
Fatigue
Fatigue is a subjective, unpleasant, potentially disabling symptom characterized by physical and/or psychological exhaustion, experienced both acutely and chronically, that results impaired function.1,2 In states of fatigue, individuals experience an imbalance between their available physical and psychological resources and the resources required for optimal functioning.2 Fatigue has been associated with depression, chronic disease states, prolonged exertion, stress, sleep disturbance and inactivity.2 Although fatigue is often discussed in the context of physical weakness and sleepiness,2 it is a multidimensional symptom that also impacts judgment, decision-making, motivation, and awareness.3
Fatigue in People Living with HIV
Fatigue affects nearly 90% of PLWH3 and has been reported as one of the most persistent and troubling symptoms they experience. While a direct link between HIV and fatigue remains elusive,3 PLWH have an elevated risk of fatigue related to hematologic and metabolic changes that are a direct result of HIV infection and treatment, as well as psychological and psychosocial stressors that have been linked with high fatigue symptom burden2,4,5 (Figure 1).
Figure 1.
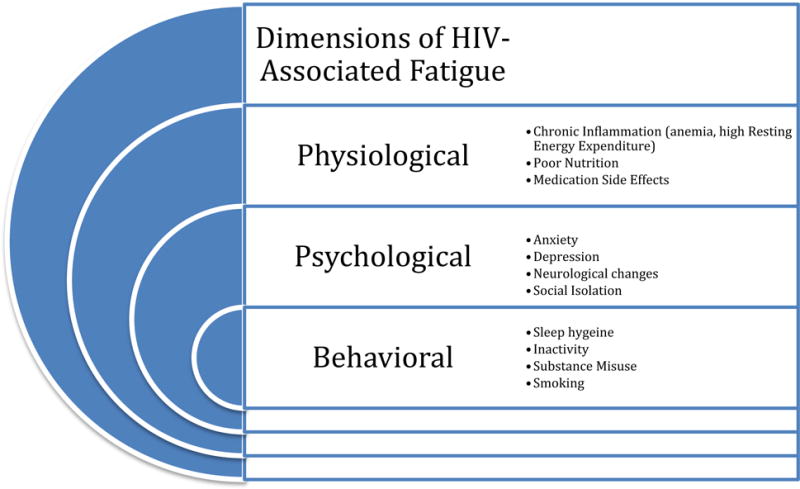
Dimensions of HIV-Associated Fatigue1,3,8–10,14,15,17,18,20,27
Physiological Factors
Anemia (generally defined as hemoglobin [Hgb] <13 in men; <12 in women] is common in PLWH, affecting more than 70% of individuals diagnosed.4,6 Anemia in PLWH is multifactorial and can be caused by chronic inflammation, medication side effects, opportunistic infections, and disrupted red blood cell production in the bone marrow.6,7 Anemia decreases the body’s ability to deliver oxygen to the tissues and results physical weakness, exhaustion, and fatigue4,8 (e.g. muscle weakness, exhaustion). Nutritional deficiencies in PLWH have been associated with fatigue, and are caused by multiple factors, including high resting energy expenditure, poor absorption of nutrients, and structural challenges related to food access. Resting energy expenditure (a.k.a resting metabolic rate) is the amount of energy (kilocalories) expended by the body while at rest. PLWH live with chronic inflammation, which causes their bodies to be expend a greater amount of energy than someone who does not have HIV.9 When energy expenditure is high, the body demands a corresponding increase in nutrients to carry out its normal function and to maintain a healthy weight. When these increased demands are not met, a person can experience profound weight loss, wasting, weakness, and fatigue.9 The problem of adequate nutrition is further exacerbated by a decreased ability to absorb nutrients due to compromised gut integrity due to intestinal inflammation.10 Furthermore, PLWH are at high risk for food insecurity, a structural challenge that can lead to an inability to access subsequently take in adequate nutrition.11 Another physiological mechanism of fatigue is related to endocrine dysfunction, including adrenal, gonadal, and thyroid dysfunction.1,3 While exact mechanisms that connect HIV to endocrine dysfunction is not well understood, current theories suggest a multifactorial cause including direct effects HIV infection on endocrine organs, and indirect effects of inflammation.12 Hormone imbalances disproportionately affect PLWH and cause fatigue through decreased production of testosterone (hypogonadism; particularly in men), adrenal insufficiency, and hypothyroidism.13,14 Fatigue has also been linked to certain HIV medications that cause poor sleep quality due to vivid dreams or insomnia (most commonly efavirenz [Sustiva]).1,15 Finally, fatigue in PLWH may be the result of co-occuring illness including opportunistic infections or comorbid conditions, particularly those that require additional pharmacological treatment.3
Psychological Factors
Anxiety and depression are common in PLWH, with anxiety affecting an more than 30%16 of PLWH. PLWH have an elevated risk for anxiety disorders due to social stigma, uncertainty regarding their disease, and issues of disclosure, and social isolation may contribute to fatigue.17 First, anxiety has been associated with higher levels of sleep disturbance among PLWH, predisposing them to fatigue due to inability to get adequate rest.17 Furthermore, medications used to treat anxiety (e.g. benzodiazepines) may induce fatigue by activating gamma-aminobutyric (GABA) receptors resulting in a sedative effect that causes drowsiness and somnolence.18
Depression is arguably the most well documented link between HIV and fatigue, affecting up to 80% of PLWH.16 Depression and depressive symptoms have been associated with low quality of life and poor adherence to HIV treatment.16,19 Depression among PLWH is rooted in biological mechanisms (decreased monoaminergic function, HIV-associated neurotoxicity) as well as psychosocial stressors (long-term disease burden, stigma).16,20 A cyclical relationship between depression and fatigue is set in motion when these biological and/or psychosocial sources of depression form a pathway to fatigue (e.g. decreased motivation and activity,16,19 excessive or deficient sleep19) which in turn exacerbate depressive symptoms, that lead to further fatigue.21 Compounding this problem are disparities in depression treatment among PLWH, particularly among highly-affected racial/ethnic minority groups.16 Without access to treatment, depression and its associated symptoms (including fatigue) persist,16 and can disrupt an individual’s ability to achieve viral suppression and a stable immune system.19 Fatigue is also seen in PLWH who are receiving treatment for both HIV and depression, as sleep disturbance and insomnia are side effects of antiretrovirals15 and antidepressants.18
Behavioral Factors
Behavioral factors that contribute to fatigue in PLWH often contain both physiological and psychosocial components. Physical activity is a widely recommended health behavior that has demonstrated potential to combat fatigue in PLWH.22 Webel et al.22 found that PLWH who engage in higher levels of physical activity reported 17% less fatigue than those who were more sedentary.22 Paradoxically, increasing physical activity may offer a solution to lower the severity of fatigue for PLWH, but fatigue symptom experience (psychological dimension), may diminish an individual’s perceived ability to be physically active.23 Researchers continue to seek out solutions to this problem through interventions designed to change behaviors that promote increased physical activity in PLWH. 24–26
One of the most prominent behavioral factors contributing to fatigue is poor sleep hygiene.27,28 Sleep disturbances are common in PLWH, affecting between 30–100% of PLWH,28 and can manifest in a lack of or poor quality of sleep.5,28,29 Inability to sleep results from physiological factors including the affect of HIV on the central nervous system,28 medication side effects (e.g. vivid dreams, neuropathy),15,29 effects of stimulant intake (e.g. nicotine, caffeine, amphetamines),28,29 or from psychological factors that impede restful sleep (e.g. anxiety and stress).17,30 In addition to inability to sleep, the aforementioned factors can diminish the quality of sleep resulting experiences of physical and mental fatigue, depression, and excessive daytime sleepiness.5,28,29 Finally, PLWH may turn to the use of substances or caffeine intake to help them sleep or to cope with fatigue or psychological symptoms,2 but studies have found that such actions often exacerbate sleep disturbances thus perpetuating fatigue rather than eliminating it.
Assessment of Fatigue
Fatigue assessment is a crucial component of an HIV care plan that allows clinicians to understand patient-specific circumstances and conditions that lead to fatigue symptom experiences. As explained in a review by Barroso and Voss,1 accurate assessments of fatigue are challenging due to limitations in how fatigue is measured. Measures may be unidimensional, only assessing certain aspects of fatigue (e.g. frequency, intensity, or functionality). For example, the widely-used Fatigue Severity Scale (FSS)31 primarily addresses the impact of fatigue on functionality without regard to intensity (despite its name). Furthermore, scales may have primarily been tested and validated some populations (e.g. cancer patients) but may not capture specific aspects of people living with other illnesses. Even though patients may primarily be focused on the physical dimension of fatigue, failure to assess fatigue as a multidimensional symptom results in a limited ability to adequately address fatigue. Barroso and Lynn32 addressed these limitations by creating the HIV-related Fatigue scale, a validated fatigue measure for assessment of the intensity of fatigue, responsiveness to fatigue circumstances, and fatigue-related functional impairment in PLWH. The scale is ideal for determining how fatigue is affecting activities of daily living as well as mental and social functioning.
Proactive patient involvement in fatigue assessment through daily recording (e.g. journaling) can provide invaluable information for clinician understanding of symptoms, including fatigue. Mobile health (aka mHealth) technology has made it possible for people to record their symptoms in real time through smartphone applications and short message service (SMS).33 While much of the mHealth research in PLWH has been focused on medication adherence,34 it may prove to be an innovative and useful way to track and manage symptoms, and research into the uses of mobile technology in PLWH is ongoing.
Management of Fatigue
Once clinicians have been able to assess and measure fatigue they are faced with the challenge of determining the best course of action to treat and help patients manage fatigue. Clinical and pharmacological options include supplements to address hormone imbalances and anemia (when blood loss is not indicated) and to improve nutritional deficiencies that cause fatigue, as well as stimulant medications to directly treat fatigue symptoms.1 While these measures been effective in alleviating fatigue symptoms in PLWH, little is known about how effective they are long term1. Non-pharmacological interventions, particularly engagement in health behaviors (stress reduction, exercise),22 have also been effective in alleviating fatigue symptoms and have strong potential to be effective long term solutions for addressing fatigue in chronic illness. Furthermore, Webel et al.27 found that a diet and exercise self-management intervention resulted in improved sleep hygiene in PLWH. Palliative nurses can play a pivotal role both as healthcare team members and directly with patients to recommend supplementation and nutritional consultation, educate patients on how to integrate health behaviors that will decrease inflammation, promote daytime energy through activity and rest planning as well as healthy sleep patterns, ultimately improving fatigue symptoms.
The following case study illustrates the potential role of palliative care integration in helping an individual living with HIV and suffering from fatigue.
Case Study
Sam is a 41-year-old Caucasian man living with HIV receiving care at an urban university-based infectious disease clinic specializing in the care of PLWH. He was diagnosed one year ago and presents to his regular clinic appointment with his brother whom he has lived with since being diagnosed. He has attended all scheduled appointments and adhered to his antiretroviral medication regimen. His appearance is unkempt and he is tearful, stating “I cannot keep going like this, I am so tired all the time I can’t do anything anymore.” His brother adds that he “doesn’t do anything anymore, he never eats- just snacks, drinks coffee, and smokes cigarettes” and “sleeps most of the day and is up all night on his computer”. He denies alcohol use, is a 20 pack-year smoker, and occasionally takes a psychostimulant medication (dextroamphetamine/amphetamine; Adderall®) that he buys from a friend to “get some energy to go out” but that this is rare. His labs indicate an undetectable HIV-1 RNA viral load and normal CD4+ T-lymphocyte count. All laboratory values including hemoglobin and hematocrit, basic metabolic panel, thyroid stimulating hormone (TSH), and testosterone levels were within normal limits. Sam reports that he has not visited with his case manager because he “doesn’t need her”. His provider has requested a palliative care consultation for symptom management. Upon review, the palliative care nurse notes he reported 8 out of 10 on a basic fatigue assessment and a Beck Depression Inventory35 score of 20 out of a possible 63 at this visit.
Sam’s fatigue is likely due to multiple factors including but not limited to physiological factors such as HIV medication side effects and poor nutrition; psychological factors including depression and anxiety; and behavioral factors including substance misuse, poor sleep hygiene, smoking inactivity, and excessive caffeine. The normal hemoglobin and hematocrit, testosterone, and TSH levels rule out a hematologic or hormonal cause, and the self-reported substance use (e.g. marijuana, amphetamines, caffeine, nicotine) provide insight into Sam’s difficulty achieving restful sleep. The palliative care nurse wants to gather more information about Sam’s fatigue that has not been captured from the basic rating scale. Sam completes the HIV-related Fatigue Scale which indicates poor responsiveness to fatigue circumstances, including high disruption to daily activities and high levels of social and mental functioning. Based on these findings, the palliative care nurse develops the following care plan.
Symptom Management Plan
-
-
Provide Sam with encouragement, emphasizing his strengths including his adherence to medication and achievement of an undetectable viral load; also focusing on reinforcing that there are potential solutions to the problem of fatigue.
-
-
Educate Sam on the benefits of working with case management emphasizing that case managers help to coordinate patient care, keep patients connected within the healthcare setting, and help patients solve unexpected problems. Ensure that Sam knows that having a case manager does not suggest that he is not independent, but that the case manager is someone he can work with on health-related matters including venues of care and healthcare financing.
-
-
Educate Sam on sleep hygiene, emphasizing that rest periods are important, but sleeping throughout the day will make it difficult to sleep at night and perpetuates the cycle of fatigue. The palliative care nurse can leverage resources from the American Sleep Association at https://www.sleepassociation.org.
-
-
Sam has some high-risk behaviors that likely have a direct relationship to fatigue, particularly his use of amphetamines, nicotine, and caffeine. In a judgment-free manner, express understanding of his desire to take substances that make him feel energized, but remind him that while caffeine, nicotine, and amphetamines may appear to provide energy, they actually drain the body’s resources more quickly, interfere with sleep, and can contribute more to fatigue than they prevent. The palliative care nurse can suggest stopping all caffeine intake after noon, and encourage Sam to stop taking amphetamines due to their high addiction potential and adverse psychological impact when not taken as prescribed.
-
-
Sam’s diet, particularly the snacking behaviors and lack of intake of nutritious food, is of particular concern both to his overall health and may be a major contributor to his fatigue. The palliative care nurse can provide Sam with resources such as the Veteran’s Association website on nutrition for people with HIV/AIDS (www.hiv.va.gov/patient/daily/diet/index.asp), and review the information with him. The palliative care nurse should help Sam with meal planning emphasizing preparation of foods in advance and including food choices that Sam likes that contain adequate amounts of calories, protein, fruits, vegetables, and water.
-
-
Work with Sam on a plan to increase physical activity, beginning with lower intensity activities (e.g. walking) and encouraging incremental increases in intensity (e.g. calisthenics) as tolerated. Emphasize that engaging in any form of exercise is better than none.
-
-
Present Sam with options for smoking cessation, being careful not to appear judgmental or intolerant of smoking behavior. The palliative care nurse should emphasize Sam’s ability to take steps to reduce and eventually quit smoking, highlighting that each step he takes to cut back on smoking can improve energy levels and help him get restful sleep. Furthermore, the palliative care nurse can refer Sam to the AIDS.gov smoking cessation website that offers help to PLWH to quit smoking including social support (e.g. via internet and phone support groups), smartphone app recommendations, and smoking cessation information materials.
-
-
Refer Sam to a local support group for PLWH if available which may help him to find others he relates to and prevent social isolation related to HIV and his symptoms. If no such support group exists in the area, web-based resources such as Poz (www.poz.com) and The Body (www.thebody.com) contain informational and social support resources that Sam can explore to learn more about HIV and hear the stories of others living with HIV.
-
-
Encourage Sam to keep a fatigue diary or use a symptom tracking smartphone app (e.g. trakmyhealth-www.trakmyhealthapps.com) that will help him identify time points and specific contexts associated with his fatigue, will be an invaluable resource in future clinical interactions, and will provide a tangible source from which Sam can identify improvements and setbacks with his health.
-
-
Set up weekly phone calls for one month to provide Sam with support in the proposed changes.
Three-month Follow-up
At Sam’s next appointment, his appearance is much improved. He has been in regular contact with a case manager who has connected him with a local HIV support group that meets two nights each week and does a group walk every weekend. In addition to support group meetings he has started volunteering three days each week answering phones at the organization’s central office. He reports that he and his brother have started cooking meals for the week each Sunday and they are working together on eating healthy. His fatigue journal helped him realize that he was sleeping primarily during the mid-afternoon, often when he feels overwhelmed and frustrated by his current life situation. He states that while he often needs to rest, he does something to busy himself so he does not fall asleep during the day and so he can sleep through the night. His Beck Depression Inventory35 and HRFS32 scores have both decreased and he states that he feels like he is doing something that helps himself and others by being involved with the support network. Finally, he states that he is working on not drinking caffeine after noon and has is using the web resources to help him cut down on cigarette smoking, learn more about how he can better his health, and connect with other people living with HIV.
Summary
In this paper we have provided an overview of fatigue in PLWH, and have illustrated potential nursing interventions to help a person living with HIV who is experiencing fatigue. Sam’s palliative care nurse addressed his fatigue holistically, providing interventions and referrals consistent with physiological, psychological, and behavioral factors that cause fatigue. We believe that thorough assessment with validated measures and interventions designed to address the multiple dimensions of fatigue will yield the best results when helping patients to reduce fatigue. We are still learning about the implications of long-term infection with HIV, but in this paper have demonstrated the potentially disabling effects of fatigue. Palliative care nurses will continue to play an increasingly pivotal role in the care of PLWH as this population ages. Expertise in symptom management will be crucial to helping PLWH to achieve high quality of life and optimal health as they age.
Key Implications for Palliative Care Nurses.
-
-
People living with HIV are at high risk for fatigue due to cardiovascular and metabolic changes that occur as a result of HIV infection and treatment.
-
-
Fatigue is a multidimensional symptom that includes physical sensation changes, changes in thought processes and awareness, and is can impact behavioral and social function.
-
-
Palliative care nurses with expertise in symptom management can advocate for people living with HIV by facilitating necessary clinical assessments and referrals, and can help people with HIV address physiological, psychological, and behavioral factors that cause fatigue through holistic care planning with patients.
References
- 1.Barroso J, Voss JG. Fatigue in HIV and AIDS: an analysis of evidence. Journal of the Association of Nurses in AIDS Care. 2013;24(1):S5–S14. doi: 10.1016/j.jana.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Medicine USNLo. Medical Encyclopedia. Fatigue. 2016 https://medlineplus.gov/ency/article/003088.htm. Accessed 12/1/2016, 2016.
- 3.Barroso J, Leserman J, Harmon JL, Hammill B, Pence BW. Fatigue in HIV-Infected people: a three-year observational study. Journal of pain and symptom management. 2015;50(1):69–79. doi: 10.1016/j.jpainsymman.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Redig AJ, Berliner N. Pathogenesis and clinical implications of HIV-related anemia in 2013. ASH Education Program Book. 2013;2013(1):377–381. doi: 10.1182/asheducation-2013.1.377. [DOI] [PubMed] [Google Scholar]
- 5.Wibbeler T, Reichelt D, Husstedt I-W, Evers S. Sleepiness and sleep quality in patients with HIV infection. Journal of psychosomatic research. 2012;72(6):439–442. doi: 10.1016/j.jpsychores.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Martí-Carvajal AJ, Solà I, Peña-Martí GE, Comunián-Carrasco G. Treatment for anemia in people with AIDS. The Cochrane Library. 2011 doi: 10.1002/14651858.CD004776.pub3. [DOI] [PubMed] [Google Scholar]
- 7.Martin C, Poudel-Tandukar K, Poudel KC. HIV Symptom Burden and Anemia among HIV-Positive Individuals: Cross-Sectional Results of a Community-Based Positive Living with HIV (POLH) Study in Nepal. PLps ONE. 2014;9(12):e116263. doi: 10.1371/journal.pone.0116263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meidani M, Rezaei F, Maracy MR, Avijgan M, Tayeri K. Prevalence, severity, and related factors of anemia in HIV/AIDS patients. Journal of Research in Medical Sciences. 2012;17(2) [PMC free article] [PubMed] [Google Scholar]
- 9.Mittelsteadt AL, Hileman CO, Harris SR, Payne KM, Gripshover BM, McComsey GA. Effects of HIV and antiretroviral therapy on resting energy expenditure in adult HIV-infected women—a matched, prospective, cross-sectional study. Journal of the Academy of Nutrition and Dietetics. 2013;113(8):1037–1043. doi: 10.1016/j.jand.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Webel A, Sattar A, Funderburg N, et al. Alcohol and dietary factors associate with gut integrity and inflammation in HIV-infected adults. HIV medicine. 2016 doi: 10.1111/hiv.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palar K, Kushel M, Frongillo EA, et al. Food insecurity is longitudinally associated with depressive symptoms among homeless and marginally-housed individuals living with HIV. AIDS and Behavior. 2015;19(8):1527–1534. doi: 10.1007/s10461-014-0922-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tripathy SK, Agrawala RK, Baliarsinha AK. Endocrine alterations in HIV-infected patients. Indian journal of endocrinology and metabolism. 2015;19(1):143. doi: 10.4103/2230-8210.146870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barroso J, Hammill BG, Leserman J, Salahuddin N, Harmon JL, Pence BW. Physiological and psychosocial factors that predict HIV-related fatigue. AIDS and Behavior. 2010;14(6):1415–1427. doi: 10.1007/s10461-010-9691-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barroso J, Harmon JL, Madison JL, Pence BW. Intensity, chronicity, circumstances, and consequences of HIV-related fatigue: A longitudinal study. Clinical nursing research. 2013:1054773813492998. doi: 10.1177/1054773813492998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kenedi CA, Goforth HW. A systematic review of the psychiatric side-effects of efavirenz. AIDS and Behavior. 2011;15(8):1803–1818. doi: 10.1007/s10461-011-9939-5. [DOI] [PubMed] [Google Scholar]
- 16.Bengtson AM, Pence BW, Crane HM, et al. Disparities in depressive symptoms and antidepressant treatment by gender and race/ethnicity among people living with HIV in the United States. PLoS One. 2016;11(8):e0160738. doi: 10.1371/journal.pone.0160738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leyro TM, Babson KA, Bonn-Miller MO. Anxiety sensitivity in relation to sleep quality among HIV-infected individuals. The Journal of the Association of Nurses in AIDS Care: JANAC. 2013;25(6):638–645. doi: 10.1016/j.jana.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zlott DA, Byrne M. Mechanisms by which pharmacologic agents may contribute to fatigue. PM&R. 2010;2(5):451–455. doi: 10.1016/j.pmrj.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 19.Dalmida SG, Holstad MM, Fox R, Delaney AM. Depressive symptoms and fatigue as mediators of relationship between poor sleep factors and medication adherence in HIV-positive women. Journal of Research in Nursing. 2015;20(6):499–514. [Google Scholar]
- 20.Nanni MG, Caruso R, Mitchell AJ, Meggiolaro E, Grassi L. Depression in HIV infected patients: a review. Current psychiatry reports. 2015;17(1):1–11. doi: 10.1007/s11920-014-0530-4. [DOI] [PubMed] [Google Scholar]
- 21.Voss JG, Portillo CJ, Holzemer WL, Dodd MJ. Symptom cluster of fatigue and depression in HIV/AIDS. Journal of prevention & intervention in the community. 2007;33(1–2):19–34. doi: 10.1300/J005v33n01_03. [DOI] [PubMed] [Google Scholar]
- 22.Webel AR, Perazzo J, Decker M, Horvat-Davey C, Sattar A, Voss J. Physical activity is associated with reduced fatigue in adults living with HIV/AIDS. Journal of Advanced Nursing. 2016;72(12):3104–3112. doi: 10.1111/jan.13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tiesinga LJ, Dassen TW, Halfens RJ. Fatigue: a summary of the definitions, dimensions, and indicators. International Journal of Nursing Terminologies and Classifications. 1996;7(2):51–62. doi: 10.1111/j.1744-618x.1996.tb00293.x. [DOI] [PubMed] [Google Scholar]
- 24.Jaggers JR, Sneed JM, Lobelo RF, et al. Results of a nine month home-based physical activity intervention for people living with HIV. International Journal of Clinical Trials. 2016;3(3):106–119. [Google Scholar]
- 25.Webel AR, Barkley J, Longenecker CT, Mittelsteadt A, Gripshover B, Salata RA. A cross-sectional description of age and gender differences in exercise patterns in adults living with HIV. Journal of the Association of Nurses in AIDS Care. 2015;26(2):176–186. doi: 10.1016/j.jana.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Webel AR, Moore SM, Hanson JE, Salata RA. The Rationale, Design, and Initial Efficacy of SystemCHANGE™-HIV: A Systems-Based Intervention to Improve Physical Activity in People Living with HIV. Journal of AIDS & clinical research. 2013;4(3) doi: 10.4172/2155-6113.1000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Webel AR, Moore SM, Hanson JE, Patel SR, Schmotzer B, Salata RA. Improving sleep hygiene behavior in adults living with HIV/AIDS: a randomized control pilot study of the SystemCHANGE TM–HIV intervention. Applied Nursing Research. 2013;26(2):85–91. doi: 10.1016/j.apnr.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phillips KD, Gunther ME. Sleep Medicine. Springer; 2015. Sleep and HIV Disease; pp. 167–179. [Google Scholar]
- 29.Wu J, Wu H, Lu C, Guo L, Li P. Self-reported sleep disturbances in HIV-infected people: a meta-analysis of prevalence and moderators. Sleep medicine. 2015;16(8):901–907. doi: 10.1016/j.sleep.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 30.Kemppainen JK, Wantland D, Voss J, et al. Self-care behaviors and activities for managing HIV-related anxiety. Journal of the Association of Nurses in AIDS Care. 2012;23(2):111–123. doi: 10.1016/j.jana.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale: application to patients with multiple sclerosis and systemic lupus erythematosus. Archives of neurology. 1989;46(10):1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 32.Barroso J, Lynn MR. Psychometric properties of the HIV-related fatigue scale. Journal of the Association of Nurses in AIDS Care. 2002;13(1):66–75. doi: 10.1016/S1055-3290(06)60242-2. [DOI] [PubMed] [Google Scholar]
- 33.Fox S, Duggan M. Tracking for health. Pew Research Center’s Internet & American Life Project; 2013. [Google Scholar]
- 34.Thirumurthy H, Lester RT. M-health for health behaviour change in resource-limited settings: applications to HIV care and beyond. Bulletin of the World Health Organization. 2012;90(5):390–392. doi: 10.2471/BLT.11.099317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Erbauch J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;562:53–63. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]


