Abstract
The endoplasmic reticulum stress response (ERSR) is activated in a variety of neurodegenerative diseases and/or traumatic injuries. Subsequent restoration of ER homeostasis may contribute to improvement in the functional outcome of these diseases. We recently demonstrated improvements in hindlimb locomotion after thoracic spinal cord injury (SCI) and implicated oligodendrocyte survival as a potential mechanism using genetic and pharmacological inhibition of the protein kinase ribonucleic acid-like ER kinase- CCAAT/enhancer binding homologous protein (PERK-CHOP) arm of the ERSR. Here, we investigated the contribution of activating transcription factor-6 (ATF6), an ERSR signaling effector comprising the second arm of ERSR, in the pathogenesis of SCI. In contrast to what was seen after attenuation of PERK-CHOP signaling, genetic ablation of ATF6 results in modulation of ERSR and decreased survival in oligodendrocyte precursor cells against ER stress. Further, ATF6 loss delays the ERSR after SCI, potentiates PERK-ATF4-CHOP signaling and fails to improve locomotor deficits. These data suggest that deleting ATF6 levels is unlikely to be a viable therapeutic target to improve functional recovery after SCI.
Keywords: : ATF6, ER stress, oligodendrocytes, spinal cord injury
Introduction
Multiple stimuli, such as hypoxia, nutrient deprivation, viral infection, and disturbance of calcium levels, can directly or indirectly cause accumulation of unfolded or misfolded proteins in the endoplasmic reticulum (ER), triggering a stress condition that leads to an evolutionarily conserved ER stress response (ERSR). The ERSR has been proposed to be a protective mechanism that limits ER protein loading by inhibiting protein translation, facilitates protein folding through increasing the expression of ER chaperones, and removes misfolded proteins from the ER through degradation. Prolonged and unrestrained ER stress, however, can lead to the activation of proapoptotic signaling pathways.
In mammals, the ERSR includes three signal-transduction pathways initiated by three ER-resident stress-sensing proteins: protein kinase ribonucleic acid (RNA)-like ER kinase (PERK), inositol-requiring protein-1 (IRE1), and activating transcription factor-6 (ATF6). The PERK activation leads to the phosphorylation of the eukaryotic initiation factor 2α (eIF2α). While suppressing general protein translation, eIF2α phosphorylation also promotes the selective translation of some mRNAs, such as ATF4 that induces CCAAT/enhancer binding homologous protein (CHOP), a proapoptotic transcription factor. The site-specific endoribonuclease function of IRE1 mediates the specific splicing of X-box binding protein (XBP1) mRNA to generate an active form of XBP1.1
The ATF6 is encoded by two related genes, ATF6α and ATF6β, and is implicated in the transcriptional regulation of ER chaperones.2–4 The ATF6α also regulates expression of the proapoptotic transcription factor CHOP2 and XBP1 transcription factor.1 The ATF6 proteins are ubiquitously expressed transmembrane basic leucine zipper transcription factors that undergo regulated intramembrane proteolysis on accumulation of the unfolded proteins in the ER. Processing by Golgi-localized site 1 and site 2 proteases releases the cytosolic N-terminal portion of ATF6 (N-ATF6), comprising its deoxyribonucleic acid (DNA)-binding and transactivation domains, whereupon it migrates to the nucleus. Nuclear N-ATF6 then induces expression of genes containing the ER stress response elements.2–4
Recent studies demonstrated the involvement of the ERSR after a moderate contusive spinal cord injury (SCI) in rats5 and mice6 or after hemisection SCI in mice.7 Importantly, partial restoration of ER homeostasis by inhibiting the PERK-CHOP arm using genetic6 or pharmacological8 interventions showed significant improvement of hindlimb locomotion in these rodent models and indicated oligodendrocyte survival as a potential mechanism. The role of the ATF6α and IRE1 arms of the ERSR pathway in oligodendrocyte survival after SCI remains unknown, however. This study was undertaken to understand the functional contribution of ATF6α after SCI. We show that deletion of ATF6α leads to modulated ERSR and decreased survival in oligodendrocytes in response to ER stress and fails to improve locomotor deficits after SCI.
Methods
Animals
Wild type (WT) C57Bl/6 female mice (6–8 weeks) were obtained from Harlan (Indianapolis, IN). The ATF6α -/- mice on a 100% C57Bl/6 background were provided by Dr. Randal Kaufman (Sanford-Burnham Institute for Medical Research). Procedures were performed with the approval of the University of Louisville Institutional Animal Care and Use Committee and the Institutional Biosafety Committee and according to the Public Health Service Policy on Humane Care and Use of Laboratory Animals, Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Research Council, 1996).
SCI
WT and ATF6α -/- mice were anesthetized by an intraperitoneal (IP) injection of 0.4 mg/g body weight Avertin (2,2,2-tribromoethanol in 0.02 mL of 1.25% 2-methyl-2-butanol in saline, Sigma). Lacri-Lube ophthalmic ointment (Allergen, Irvine, CA) was used to prevent drying of eyes and gentamicin (50 mg/kg; Boehringer Ingelheim, St. Joseph, MO) was administered subcutaneously to reduce infection. A laminectomy was performed at the T9 vertebrae, and moderate contusion injuries (50 kdyn force/400–600 μm displacement) were performed using the IH impactor9 (Infinite Horizons Inc., Lexington, KY) as described previously.10,11 Experimental controls included sham animals that received only the T9 laminectomy. Post-operation, mice had 1 mL of sterile saline administered subcutaneously, 0.1 mL of gentamicin administered subcutaneously on the day of the surgical procedure and the third and fifth day post-surgery, and 0.1 mL buprenorphine administered subcutaneously on the day of surgery and for the next two days. Animals were placed on a heating pad until full recovery from anesthesia. Post-operative care included manual expression of bladders twice a day for 7–10 days or until spontaneous voiding returned.
Behavioral assessment
Open field Basso Mouse Scale (BMS) locomotor analyses were performed before injury for each animal to determine the baseline scores and weekly after SCI for six weeks exactly as defined.8,12 All raters were trained by Dr. Basso and colleagues at the Ohio State University and were blind to the animal groups.
Isolation of mouse oligodendrocyte precursor cells (mOPCs)
Mouse cortices dissected from whole brains of WT and ATF6α-/- post-natal day 5–7 pups tissue were dissociated using the Neural Tissue Dissociation Kit (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions.13 OPC-A medium was prepared by adding 2.1 g/L NaHCO3 (Sigma-Aldrich, St. Louis, MO) to DMEM-F12 without HEPES powder (Invitrogen, Carlsbad, CA) and supplemented with N2 supplement (1%), B27 supplement (2%), penicillin/streptomycin (1%, all from Invitrogen), bovine serum albumin (BSA, 0.01%, Sigma), 40 ng/mL FGF2 (Millipore, Billerica, MA), and 20 ng/mL PDGFα (Sigma). OPCs were enriched with O4 hybridoma using magnetic cell sorting (MACS) with rat anti-mouse IgM magnetic beads (10% in MACS Buffer). The average yield was 8–10 × 106 cells/brain with a viability of 85–95%. There were 9000–15,000 cells/cm2 cells seeded on a poly-D-lysine/laminin-coated 10 cm tissue culture dish and incubated at 37°C, 5% CO2 for maintenance.
Tunicamycin treatment, MTT assay
The WT or ATF6α-/- -derived mOPCs were seeded in 96-well plates and treated with 30 nM tunicamycin (Tm), an ER-stress inducing drug. Twenty-four hours later, OPC survival was assayed by measuring the conversion of tetrazolium, MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; Sigma) to formazan at a wavelength of 570 nm.
Lactate dehydrogenase (LDH) release
Samples of culture media were collected from WT or ATF6α-/- -derived mOPCs that were cultured in 96-well plates and challenged with Tm. The LDH release was determined using Pierce™ LDH Cytotoxicity Assay Kit (Life Technologies) following manufacturer's recommendations.
Western blot analyses
Protein lysates were prepared from WT and ATF6α -/- -derived mOPCs in protein lysis solution (20 mM Tris, pH-6.8, 137 mM NaCl, 25 mM β-glycerophosphate, 2 mM NaPPi, 2 mM EDTA, 1 mM Na3VO4, 1% Triton X-100, 10% glycerol, protease inhibitor, 0.5 mM DTT, 1 mM PMSF) and quantified using the BCA Kit (Pierce, Rockland, IL). Proteins were separated on SDS-PAGE gels and transferred to nitrocellulose membrane (Whatman, Schleicher & Schuell, Piscataway, NJ). Membranes were processed as described previously8 and probed with GADD34 (Proteintech, 1:1000, Chicago, IL), GRP78 (Cell Signalling, 1/1000 dilution, Camarillo, CA), XBP1 (Santa Cruz Biotechnology, 1/500, Santa Cruz, CA), and GAPDH (Chemicon, 1/5000, Temecula, CA).
RNA extraction, reverse transcriptase polymerase chain reaction (PCR)
Total RNA was extracted from WT and ATF6α -/- -derived mOPCs treated with Tm and spinal cord tissue of sham and contused WT and ATF6α -/- mice (n = 4/group) from the injury epicenter (4 mm) using Trizol (Invitrogen) according to the manufacturer's instructions. The RNA was quantified by ultraviolet spectroscopy, and RNA integrity was confirmed on an ethidium bromide stained formaldehyde agarose gel. cDNA was synthesized with 500 ng of total RNA using the High Capacity cDNA Synthesis Kit (Applied Biosystems, Foster City, CA) in a 20 μL reaction volume. As controls, mixtures containing all components except the reverse transcriptase enzyme were prepared and treated similarly. All cDNAs and control reactions were diluted 10 × with water before using as a template for quantitative real time (qRT)-PCR.
Quantitative PCR analysis
The qRT-PCR was performed using he ABI 7900HT Real-time PCR instrument (Applied Biosystems). Briefly, diluted cDNAs were added to TaqMan universal PCR master mix (Applied Biosystems) and run in triplicate. Target and reference gene PCR amplification was performed in separate tubes with Assay on Demand™ primers (Applied Biosystems) as follows: ATF4 (Mm00515324_m1), CHOP (Mm01135937_g1), Claudin 11 (Mm00500915_m1), GADD34 (Mm00492555_m1), GRP78 (Mm01333323_g1), MBP (Mm00521980_1), Olig2 (Mm01210556_m1) and XBP1 (Mm00457359_m1). The RNA levels were quantified using the ΔΔCT method. Expression values obtained from triplicate runs of each cDNA sample were normalized to triplicate value for GAPDH (reference gene) from the same cDNA preparation. Transcript levels are expressed as fold changes compared with respective levels in sham controls.
Statistical analyses
A repeated measures analyses of variance (ANOVA) with fixed effects and Bonferroni post hoc t test was performed for functional assessments after injury to detect differences in BMS score and subscores between the sham and injury groups over the six week testing period. Statistical analysis of qRT-PCR data was performed using independent t test for means with equal or unequal variances or repeated measures ANOVA (one-way or two-way) followed by post hoc Tukey honest significant difference test. For all other analyses, independent t tests for means assuming equal variance were performed.
Results
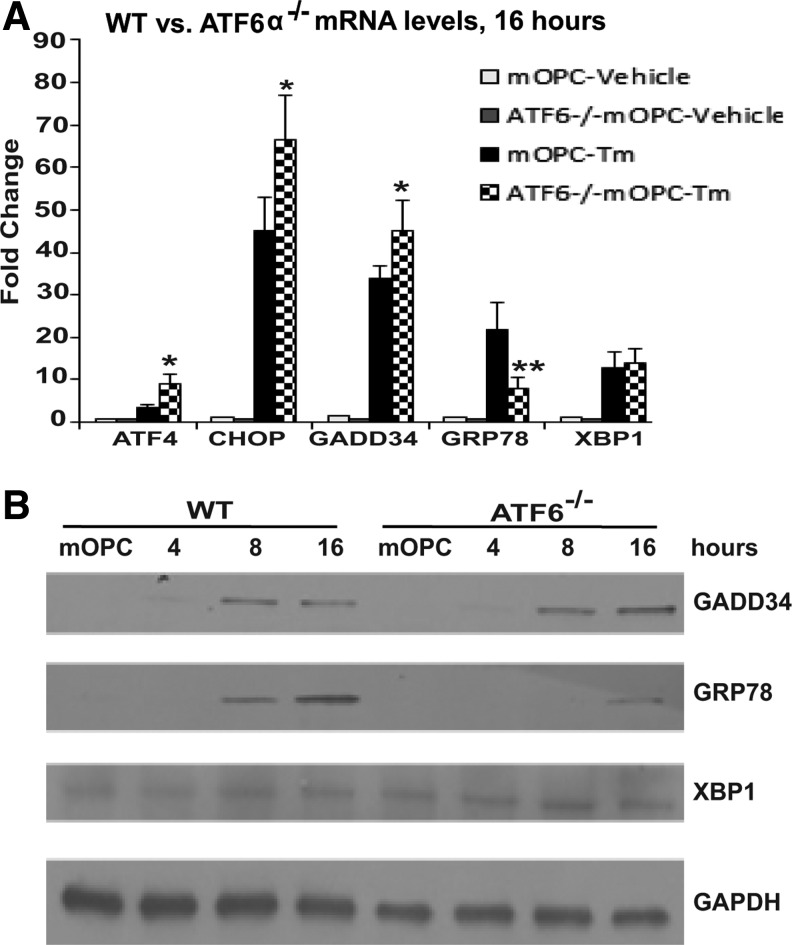
To determine the role of ATF6α specifically in mOPCs in response to ER stress, we first determined the activation of ERSR in ATF6α-/- mOPCs exposed to Tm that blocks N-glycosylation of proteins and causes ER stress. Deletion of ATF6α in mOPCs resulted in a modulated ERSR as evidenced by the significant increase in ATF4, CHOP (Fig. 1A) and GADD34 transcript levels (Fig. 1A 1B). In contrast, ATF6α-/- mOPCs demonstrated a significant decrease in the transcript levels of ER chaperone GRP78 while XBP1 levels remained unchanged (Fig. 1A,1B).
FIG. 1.
Activating transcription factor-6 (ATF6) α-/- mouse oligodendrocyte precursor cells (mOPCS) are sensitive to endoplasmic reticulum (ER) stress. (A) Real-time polymerase chain reaction data show differential increase of the ER stress response (ERSR) in ATF6α-/- mOPCs exposed to tunicamycin (Tm) for 16 h. (B) Western blot depicts increase in GADD34 levels, decrease in GRP78 levels, and no changes in XBP1 levels in ATF6α-/- mOPCs exposed to Tm for 16 h. WT, wild type.
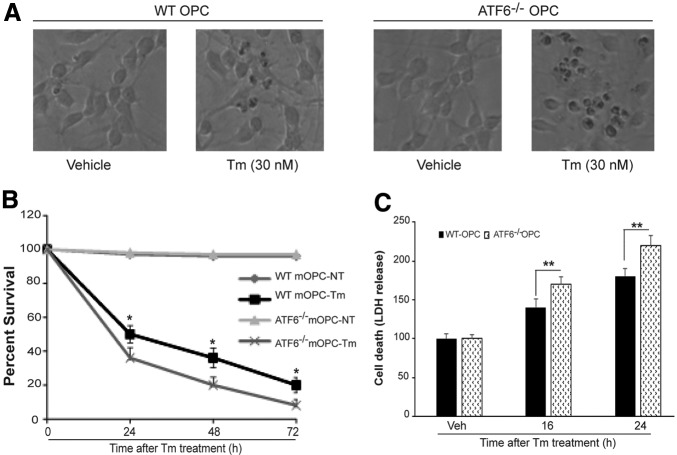
To determine the consequences of enhanced ERSR and impaired induction of ER chaperones, we compared the ability of WT and ATF6α-/- mOPCs to survive in the presence of ER stress. The Tm-induced ER stress was more toxic in ATF6α-/- mOPCs as suggested by the presence of a higher number of dead cells after 24 h exposure to the drug (Fig. 2A). Untreated WT and ATF6α-/- mOPCs showed identical growth curves (Fig. 2B). On exposure to ER stress, however, ATF6α-/- mOPCs showed a significant ∼10%–15% decrease in survival compared with WT mOPCs at all indicated time intervals (Fig. 2B). Moreover, treatment with 30 nM Tm resulting in an increased extracellular release of LDH in ATF6α-/- mOPCs further confirmed that ATF6α-/- mOPCs are more sensitive to ER stress (Fig. 2C). Similar results were obtained with thapsigargin treatment for both WT- and ATF6α-/-- derived mOPCS (data not shown). These data indicate that the deletion of ATF6α is detrimental for mOPCs.
FIG. 2.
Cytotoxic response of oligodendrocyte precursor cells (OPCs) to endoplasmic reticulum (ER) stress. Wild type (WT) and activating transcription factor-6 (ATF6α)-/- OPCs were treated with tunicamycin (Tm) as indicated. (A) Representative phase contrast micrographs depict declining density of ATF6α-/- mouse OPCs (mOPCs). (B) MTT cell survival assay shows a decreased number of viable ATF6α-/- mOPCs at all indicated time intervals. (C) Increased lactic dehydrogenase (LDH) release in ATF6α-/- mOPCs suggests enhanced cell death in response to ER stress. Data (B,C) are the mean ± standard deviation (n = 4, * p < 0.05, ** p < 0.01).
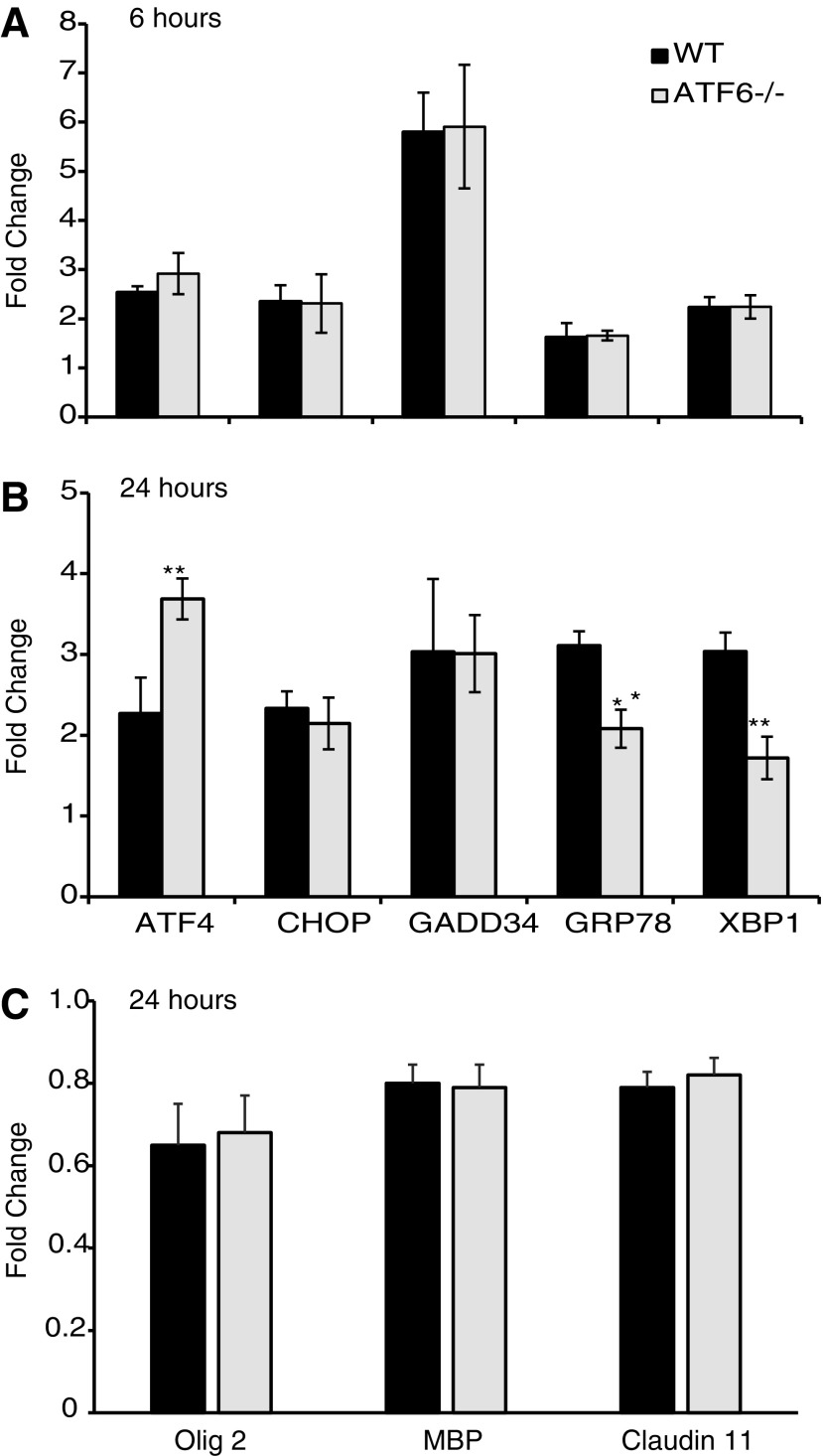
Functional recovery after midthoracic contusive SCI is mainly from white matter sparing because motor neuronal loss does not correlate with functional status.14 To determine the contribution of ATF6α to SCI pathogenesis, basal levels of ERSR effectors in ATF6α-/- mice were compared with WT mice. The average Ct values of 3.40 versus 3.872 for ATF4, 8.03 versus 8.44 for CHOP, 4.68 versus 5.12 for GRP78 and 5.28 versus 5.78 for XBP1 in ATF6α-/-and WT mice, respectively, indicated identical basal ERSR in both groups. Six hours post-SCI, there was an identical upregulation of ERSR genes in WT and ATF6α-/- null mice, respectively (Fig. 3A). At 24 h post-SCI, however, ATF6α-/- mice demonstrated a significant decrease in the expression of GRP78 and XBP1 (Fig. 3B) and increase in ATF4 transcript levels. The elevated CHOP and GADD34 levels remained similar in both genotypes. This modulated ERSR in ATF6α-/- mice resulted in no difference in oligodendrocyte- (Olig 2, MBP, Claudin 11) (Fig. 3C), neuron- (NSE and Map 2a,b, data not shown), and astrocyte-specific (GFAP and glutamine synthetase, data not shown) transcript levels.
FIG. 3.
Endoplasmic reticulum stress response (ERSR) in contused wild type (WT) and activating transcription factor-6 (ATF6α) -/- mice. Total ribonucleic acid (RNA) extracted from injury epicenter of WT and ATF6α-/- mice at 6 h and 24 h-post spinal cord injury show differential effects on ERSR genes (A, B). At 24 h post-SCI, there is no difference in Olig2, MBP, and Claudin11 mRNA levels between the two genotypes (C). Data are the mean ± standard deviation (n = 4, ** p < 0.01).
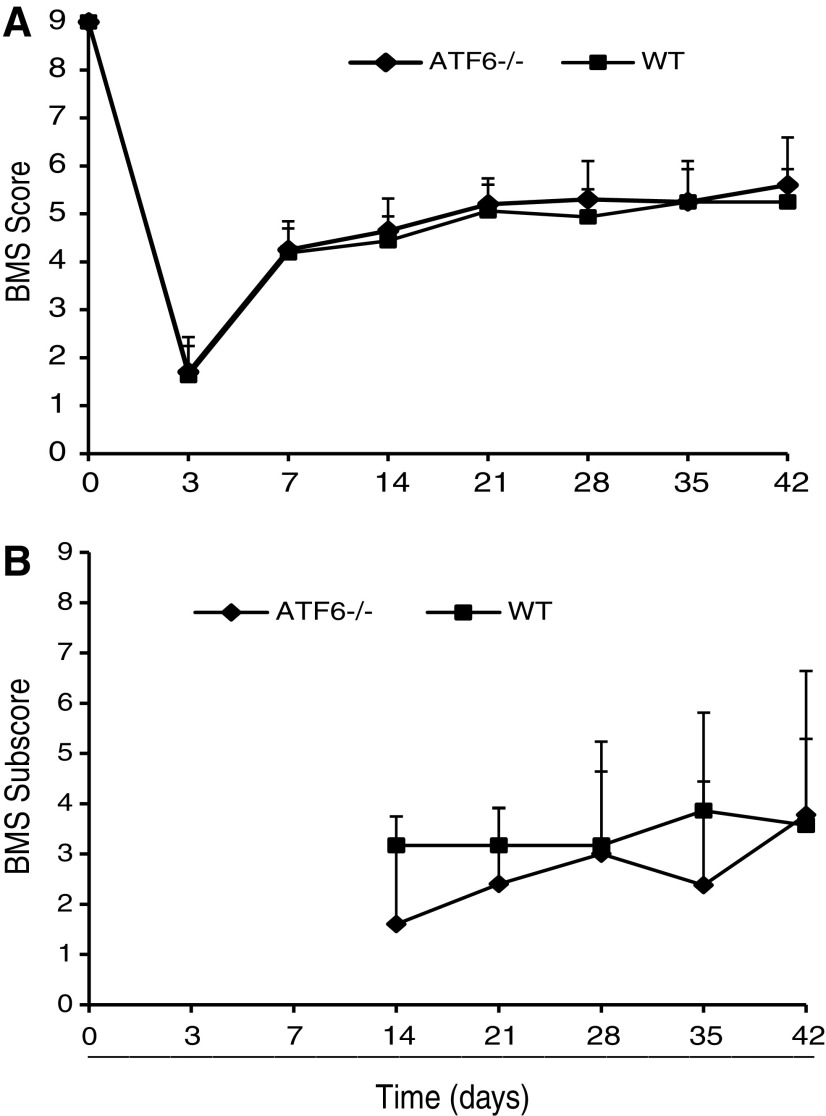
To understand the consequences of modulated ERSR in ATF6α-/- mice after SCI, we evaluated the role of ATF6α in functional recovery post-SCI. Comparison of the BMS scores between moderately contused WT (n = 9) and ATF6α-/- (n = 11) mice revealed no differences (Fig. 4A). The average BMS score for WT and ATF6α-/- animals was 4.19 ± 0.65 and 4.25 ± 0.44 at week one and 5.25 ± 0.68 and 5.6 ± 0.99 at week six, respectively. Analysis of the BMS subscore also did not show any improvement in the stepping characteristics between WT and ATF6α-/- mice (Fig. 4B). Altogether, these data indicate that deletion of ATF6α potentiates the PERK-ATF4-CHOP arm of ERSR after thoracic SCI and fails to improve locomotor recovery.
FIG. 4.
Locomotor assessment in activating transcription factor-6 (ATF6) α-/- mice after spinal cord injury (SCI). (A) Basso Mouse Scale (BMS) locomotor analyses performed weekly after moderate SCI did not reveal any functional recovery in ATF6α-/– mice (solid diamonds) compared with wild type (WT) mice (solid squares). (B) Analysis of BMS subscores post-SCI showed no significant differences in stepping characteristics between the two groups.
Discussion
Effective clinical therapy after SCI remains elusive despite numerous research endeavors and technological advances. This is perhaps because of simultaneous activation of multiple pathophysiological mechanisms after SCI that include inflammation, hypoxia, excitotoxicity, ischemia, Ca2+ dysregulation, and demyelination.15 What is needed to optimize neuroprotection is to take a more fundamental biological approach that targets multiple neuropathological mechanisms in all cell types. The ERSR is one such potential target that has proven effective in the acute treatment of those with SCI.
Recent studies demonstrated the crucial role of the PERK-CHOP arm of the ERSR in the pathogenesis after SCI.5–8,16 Specifically, ablation of the proapoptotic transcription factor CHOP resulted in significant improvement in hindlimb locomotion that correlated with increased white matter sparing and decrease in oligodendrocyte apoptosis.6 Pharmacological intervention into the PERK/CHOP arm of the ERSR with salubrinal, an inhibitor of the PP1 complex that dephosphorylates peIF2α, similarly demonstrated enhanced recovery in locomotor function and coincided with increased oligodendrocyte sparing.8 Collectively, these studies suggested that (1) oligodendrocytes are uniquely sensitive to ERSR, and (2) restoration of ER homeostasis after SCI could be a therapeutically viable approach.
To further extend studies on the role of the other arms of the ERSR on oligodendrocyte survival after SCI, we took advantage of the ATF6α null mice. Unlike our previous studies, OPCs derived from ATF6α-/- mice demonstrated an enhanced ERSR and a decrease in survival in response to ER stress. Consistent with these data, ATF6α-/- mouse embryonic fibroblasts (MEFs) showed reduced viability when exposed to ER stress17,18 as did primary brain endothelial cells (unpublished data). The sensitivity of oligodendrocytes to ER stress mediated apoptosis is because of their highly developed ER serving the need to produce vast amounts of lipids and proteins for myelin synthesis.19–21
SCI-associated dysregulation of intracellular Ca2+ homeostasis is one of the triggers of oligodendrocyte ER stress.22 Because oligodendrocytes are highly sensitive to oxidative stress, such mechanism may contribute to their demise in traumatized SCI. Supporting this, after contusive thoracic SCI, oligodendrocytes are the predominant cell type undergoing apoptosis.23,24 Given that ATF6α is needed both in cells and animals for induction of protein folding, processing, and degradation capacity of the ER, it is possible that ATF6α deletion compromises the highly developed secretory pathway in oligodendrocytes during conditions of ER stress. Consistent with this suggestion, ATF6α-/- MEFs were shown to be defective in secretion in response to ER stress.17
As in cultured cells, CHOP expression remains unchanged in ATF6α-/- mice compared with WT mice with SCI whereas ATF4 transcripts levels were increased in ATF6α-/- mice. Expression of the negative regulator of eIF2α, GADD34, was also similar in both genotypes after SCI. Only the expression of GRP78 and XBP1 was reduced in the ATF6α-/- after SCI. In addition, ATF6α deletion was not able to protect oligodendrocytes, as evident by similar cell-specific transcript levels. These data are in contrast to our earlier studies of CHOP null mice6 and salubrinal-treated mice8 wherein a complete attenuation of the ERSR was observed.
Finally, ATF6α deletion resulted in potentiating the PERK-ATF4-CHOP arm of ERSR after SCI both in vitro and in vivo. These data would suggest worsening of the SCI functional outcome. Mice with genetic ablation of ATF6α after thoracic SCI, however, did not show any changes in functional recovery. We speculate that effects of ATF6α deletions are either too small or compensated by ATF6β for its loss in vivo after SCI. As white matter sparing and consequently oligodendrocyte protection are critical to functional recovery after thoracic contusive SCI, enhanced OPC/oligodendrocyte death of ATF6α-/- mOPCs correlates with lack of functional improvement after SCI. The PERK-ATF4-CHOP pathways remain the most attractive ERSR target for therapeutic intervention after SCI.
Acknowledgments
We thank Kariena Andres for maintenance of transgenic mice and animal perfusions, Christine Yarberry for help with surgical procedures, Darlene A. Burke for statistical analyses, Johnny Morehouse and Jason Beare for BMS analyses, and Allison Metz for culturing of mouse oligodendrocyte precursor cells. This work was supported by RR15576, GM103507, NS045734, Commonwealth of Kentucky Challenge for Excellence, Kentucky Spinal Cord and Head Injury Reseach Trust (SRW, MH).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Yoshida H., Matsui T., Yamamoto A., Okada T., and Mori K. (2001). XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107, 881–891 [DOI] [PubMed] [Google Scholar]
- 2.Wang Y., Shen J., Arenzana N., Tirasophon W., Kaufman R.J., and Prywes R. (2000). Activation of ATF6 and an ATF6 DNA binding site by the endoplasmic reticulum stress response. J. Biol. Chem. 275, 27013–27020 [DOI] [PubMed] [Google Scholar]
- 3.Yoshida H., Haze K., Yanagi H., Yura T., and Mori K. (1998). Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins. Involvement of basic leucine zipper transcription factors. J. Biol. Chem. 273, 33741–33749 [DOI] [PubMed] [Google Scholar]
- 4.Yoshida H., Okada T., Haze K., Yanagi H., Yura T., Negishi M., and Mori K. (2000). ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol. Cell. Biol. 20, 6755–6767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Penas C., Guzmán M.S., Verdú E., Forés J., Navarro X., and Casas C. (2007). Spinal cord injury induces endoplasmic reticulum stress with different cell-type dependent response. J. Neurochem. 102, 1242–1255 [DOI] [PubMed] [Google Scholar]
- 6.Ohri S.S., Maddie M.A., Zhao Y., Qiu M.S., Hetman M., and Whittemore S.R. (2011). Attenuating the endoplasmic reticulum stress response improves functional recovery after spinal cord injury. Glia 59, 1489–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valenzuela V., Collyer E., Armentano D., Parsons G.B., Court F.A., and Hetz C. (2012). Activation of the unfolded protein response enhances motor recovery after spinal cord injury. Cell Death Dis. 3: e272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohri S.S., Hetman M., and Whittemore S.R. (2013). Restoring endoplasmic reticulum homeostasis improves functional recovery after spinal cord injury. Neurobiol. Dis. 58, 29–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheff S.W., Rabchevsky A.G., Fugaccia I., Main J.A., and Lumpp J.E., Jr. (2003). Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J. Neurotrauma 20, 179–193 [DOI] [PubMed] [Google Scholar]
- 10.Benton R.L., Maddie M.A., Minnillo D.R., Hagg T., and Whittemore S.R. (2008). Griffonia simplicifolia isolectin B4 identifies a specific subpopulation of angiogenic blood vessels following contusive spinal cord injury in the adult mouse. J. Comp. Neurol. 507, 1031–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han S., Arnold S.A., Sithu S.D., Mahoney E.T., Geralds J.T., Tran P., Benton R.L., Maddie M.A., D'Souza S.E., Whittemore S.R., and Hagg T. (2010). Rescuing vasculature with intravenous angiopoietin-1 and αvβ3 integrin peptide is protective after spinal cord injury. Brain 133, 1026–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Basso D.M., Fisher L.C., Anderson A.J., Jakeman L.B., McTigue D.M., and Popovich P.G. (2006). Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J. Neurotrauma 23, 635–659 [DOI] [PubMed] [Google Scholar]
- 13.Dincman T.A., Beare J.E., Ohri S.S., and Whittemore S.R. (2012). Isolation of cortical mouse oligodendrocyte precursor cells. J. Neurosci. Methods 30, 219–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magnuson D.S., Trinder T.C., Zhang Y.P., Burke D., Morassutti D.J., and Shields C.B. (1999). Comparing deficits following excitotoxic and contusion injuries in the thoracic and lumbar spinal cord of the adult rat. Exp. Neurol. 156, 191–204 [DOI] [PubMed] [Google Scholar]
- 15.Rowland J.W., Hawryluk G.W., Kwon B., and Fehlings M.G. (2008). Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg. Focus 25, E2. [DOI] [PubMed] [Google Scholar]
- 16.Ohri S.S., Maddie M.A., Zhang Y., Shields C.B., Hetman M., and Whittemore S.R. (2012). Deletion of the pro-apoptotic endoplasmic reticulum stress response effector CHOP does not result in improved locomotor function after severe contusive spinal cord injury. J. Neurotrauma 29, 579–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamamoto K., Sato T., Matsui T., Sato M., Okada T., Yoshida H., Harada A., and Mori K. (2007). Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6α and XBP1. Dev. Cell 13, 365–376 [DOI] [PubMed] [Google Scholar]
- 18.Wu J., Rutkowski D.T., Dubois M., Swathirajan J., Saunders T., Wang J., Song B., Yau G.D., and Kaufman R.J. (2007). ATF6α optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev. Cell 13, 351–364 [DOI] [PubMed] [Google Scholar]
- 19.D'Antonio M., Feltri M.L., and Wrabetz L. (2009). Myelin under stress. J. Neurosci. Res. 87, 3241–3249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin W., and Popko B. (2009). Endoplasmic reticulum stress in disorders of myelinating cells. Nat. Neurosci. 12, 379–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monk K.R., Voas M.G., Franzini-Armstrong C., Hakkinen I.S., and Talbot W.S. (2013). Mutation of sec63 in zebrafish causes defects in myelinated axons and liver pathology. Dis. Model. Mech. 6, 135–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McTigue D.M., and Tripathi R.B. (2008). The life, death, and replacement of oligodendrocytes in the adult CNS. J. Neurochem. 107, 1–19 [DOI] [PubMed] [Google Scholar]
- 23.Li G.L., Brodin G., Farroque M., Funa K., Holtz A., Wang W.L., and Olsson Y. (1996). Apoptosis and expression of Bcl-2 after compression trauma to rat spinal cord. J. Neuropathol. Exp. Neurol. 55, 280–289 [DOI] [PubMed] [Google Scholar]
- 24.Casha S., Yu W.R., and Fehlings M.G. (2001). Oligodendroglial apoptosis occurs along degenerating axons and is associated with FAS and p75 expression following spinal cord injury in the rat. Neuroscience 103, 203–218 [DOI] [PubMed] [Google Scholar]