Abstract
Mitochondrial homeostasis is essential for maintaining cellular function and survival in the central nervous system (CNS). Mitochondrial function is significantly compromised after spinal cord injury (SCI) and is associated with accumulation of high levels of calcium, increased production of free radicals, oxidative damage, and eventually mitochondrial permeability transition (mPT). The formation of the mPT pore (mPTP) and subsequent mPT state are considered to be end stage events in the decline of mitochondrial integrity, and strategies that inhibit mPT can limit mitochondrial demise. Cyclosporine A (CsA) is thought to inhibit mPT by binding to cyclophilin D and has been shown to be effective in models of CNS injury. CsA, however, also inhibits calcineurin, which is responsible for its immunosuppressive properties. In the present study, we conducted a dose-response examination of NIM811, a nonimmunosuppressive CsA analog, on recovery of function and tissue sparing in a rat model of moderate to severe SCI. The results of our experiments revealed that NIM811 (10 mg/kg) significantly improved open field locomotor performance, while the two higher doses tested (20 and 40 mg/kg) significantly improved return of reflexive bladder control and significantly decreased the rostral-caudal extent of the lesion. Taken together, these results demonstrate the ability of NIM811 to improve recovery of function in SCI and support the role of protecting mitochondrial function as a potential therapeutic target.
Keywords: : locomotor function, mitochondria, spinal cord injury
Introduction
Traumatic injury to the spinal cord results in a delayed and prolonged period of secondary injury involving several pathophysiological events.1–6 Numerous studies have demonstrated that a rapid loss of mitochondrial function after spinal cord injury (SCI) is a contributing factor to ongoing cellular damage and cell death. This includes a loss of energy production, oxidative damage,7–13 as well as formation of the mitochondrial permeability transition pore (mPTP).14 All of these mitochondrial-related events are thought to contribute to widespread necrotic and apoptotic cell death after SCI.15–21
The mPTP is associated with the inner mitochondrial membrane and plays a significant role in determining cellular fate. The Ca2+ overload, free radicals, loss of mitochondrial membrane potential, and changes in mitochondrial matrix pH can lead to permeability transition of the inner mitochondrial membrane via formation of the mPTP. Components of the pore are thought to include cyclophilin D (Cyp-D), the adenine nucleotide transporter (ANT), the adenosine triphosphate (ATP) synthase, and the voltage dependent anion channel.22–26 It has been suggested that targeting components of the mPTP has therapeutic implications as an effective cytoprotective strategy in numerous indications, including acute neurodegeneration.22,27–30
The immunosuppressant cyclosporin A (CsA) is thought to inhibit mPTP formation by binding to Cyp-D, which blocks the ability of Cyp-D to interact with ANT.22,31–35 The binding of CsA and its analogs to Cyp-D has been shown to inhibit apoptotic cell death in a number of cell types, including cells of the central nervous system.22 It is hypothesized that inhibition of mPTP formation and subsequent mPT is neuroprotective against apoptosis by inhibiting the release of pro-apoptotic molecules from the mitochondria. Interestingly, gene targeting studies provide compelling evidence that Cyp-D also plays an important role in limiting necrotic cell death.10–13 Therefore, inhibiting mitochondrial events associated with both apoptotic and necrotic cell death has significant therapeutic potential in the treatment of acute SCI.
A role for CsA as a neuroprotective agent in rat models of acute SCI is inconclusive because several studies report beneficial effects while others conclude that CsA is ineffective.36–41 Moreover, the immunosuppressive properties of CsA limit its therapeutic potential because it is difficult to distinguish whether any beneficial effects are from its actions on mPT, its immunosuppressive properties, or both.
NIM811 (N-methyl-isoleucine-cyclosporin) is a CsA analog that also binds to Cyp-D and blocks mPT in brain mitochondria at nanomolar concentrations.29,42 NIM811 was developed originally to be a less toxic replacement to CsA, but modifications made to the CsA structure eliminated the immunosuppressive properties. A second attractive property of NIM811 is that it exhibits a much lower cytotoxic profile relative to CsA.42 We have shown previously that pre-treatment with NIM811 improves mitochondrial function after SCI, while treatment at 15 min after injury reduces markers of apoptotic cell death and decreases lesion volume.43,44 Our additional studies in traumatic brain injury have also shown NIM811 efficacy in promoting mitochondrial function, tissue sparing, and functional recovery.45,46 In the present study, we examined the dose-related efficacy of repeated NIM811 treatment beginning 1 h after a moderate to severe model of SCI.
Methods
SCI
A total of 56 adult female Long-Evans rats (Harlan, Indianapolis, IN) weighing 225–250 g at the time of surgery were used in this study. Animals were maintained under environmentally controlled conditions and subjected to a 12 h/12 h light/dark cycle with food and water provided ad libitum and acclimatized to the facility for seven days before starting the experiments. All procedure and handling techniques were in strict accordance with the National Institutes of Health guidelines for the care and use of laboratory animals and approved by the Institutional Animal Care and Use Committee.
Animals were randomized into one of four treatment groups (n = 14 per group, vehicle and three doses of NIM811) and then subjected to a spinal cord contusion injury using the Infinite Horizon (IH) Impactor device (Precision Systems & Instrumentation, Lexington, KY) by surgeons blinded to the treatment conditions. Briefly, rats were anesthetized with sodium pentobarbital (Nembutal 40 mg/kg intraperitoneally, Abbott Laboratories), and the fur overlying the vertebral column shaved and a dorsal laminectomy performed at the tenth thoracic vertebra level (T10) to expose the spinal cord. The vertebral column was then stabilized by clamping the rostral T9 and caudal T11 vertebral bodies, so that the spinal cord remained in a horizontal plane.
All animals received a 200 kdyn (impact force) spinal cord contusion using the IH impactor device at the T10 level. This injury force results in a moderate to severe contusion injury in female rats in this weight range.47 Immediately after injury, the muscles and the incision site were sutured in layers, and all animals injected subcutaneously with 10 mL sterile saline and intramuscularly with cefazolin 30 mg/kg. Animals were then placed on a 37°C heating pad until they recovered fully from the anesthesia. Post-operative care included cefazolin injections twice a day for one week and the manual expression of bladders twice a day until reflexive voiding returned or the animal was euthanized.
NIM811 administration
NIM811 (a gift from Novartis) was administered orally as a microemulsion solution. A stock solution was made up the day of surgery by first mixing 100 mg of NIM811 in 0.45 L of cremaphor EL (Sigma). The resulting emulsion was then mixed with 0.4 mL of corn oil (Sigma) and brought up to 1.0 mL with ethanol (final stock concentration of 10%). Just before administration, the stock solution was diluted with saline into three separate vials to generate working concentrations of 10, 20, or 40 mg/mL. The same solution minus NIM811 was used as vehicle. The vials were coded and the working solutions or vehicle administered using oral gavage at 1, 12, and 24 h after surgery by an individual who was unaware of the vial contents.
Open-field locomotor activity
Open field locomotor activity was conducted using the 21 point Basso, Beattie and Bresnahan (BBB) scale,48 which is used to examine a number of conditions, including; (1) behavioral changes as a consequence of pharmacological treatments, (2) lesion severity, and (3) changes in recovery as far as 6–8 weeks post-injury.49 Animals were placed in an open field apparatus that consists of a circular molded plastic pool with a diameter of 90 cm and a wall height of 7 cm. The animals were exposed to the open field apparatus every day for 5 min over a period of five days before surgery to acclimate to the apparatus. At three and seven days after injury, and then once a week thereafter, the animals were scored in the open field for a period of 4 min by two examiners who were blinded to the treatment conditions.
Thermal hyperalgesia
The Hargreaves method, using an infrared plantar device (Harvard Apparatus), was used to test the effects of NIM811 treatment on recovery from below-level (hindpaw) thermal hyperalgesia after SCI. Rats were placed in a glass-bottomed Plexiglas chamber (unrestrained) every day for 15 min over a period of five days to allow for acclimatization to the device. On the day before surgery, animals were placed in the same Plexiglas chamber for a period of 15 min, and a moveable infrared beam was used to generate a heat source (50–52°C) on the plantar surface of the hindpaw. The latency (in seconds) to paw withdrawal was determined and used as the pre-injury baseline score.
Each animal received a total of five trials with a 3 min delay between each trial. The longest and shortest latencies were discarded, and the remaining three latencies scores averaged. A 30 sec shutoff of the thermal stimulus was observed if no paw withdrawal occurred. The SCI animals were tested at 28 and 42 days after injury and the data expressed as percent change relative to pre-injury baseline scores. The person testing the animals on this task was blinded to the treatment conditions.
Bladder function
Manual expression of bladders was conducted twice a day until micturition returned or the study end-point. The post-injury time point at which animals exhibited three consecutive days of reflexive control of bladder function was identified for each animal and recorded. A group mean was calculated for each treatment condition and the data expressed as the number of days post-injury to reach reflexive control of bladder function. The person responsible for the post-operative care of the animals was unaware of the treatment conditions.
Tissue sparing
At the study end-point, rats were deeply anesthetized intraperitoneally with 100 mg/kg sodium pentobarbital (Nembutal; Abbott Laboratories) and transcardially perfused with 100 mL of cold 0.1 M phosphate buffered saline (PBS; pH 7.4) followed by 400 mL of 4% paraformaldehyde in PBS. The laminectomy site was reexposed, and methylene blue dye was used to mark the T10 segment, which corresponds to the injury epicenter. A laminectomy was performed from T6 to L1 to expose the spinal cord.
Each spinal cord was transected caudally at the L1 spinal root and rostrally at the T6 spinal root. The 20 cm segment of spinal cord was removed from the vertebral column and fixed 1 h in 4% paraformaldehyde in PBS at 4°C and then cryoprotected for 48 h in 20% sucrose in PBS at 4°C. The rostral ends of four spinal cords (one from each treatment group) were evenly aligned and placed side by side into plastic cryomolds containing tissue-freezing medium (Triangle Biomedical Sciences, Durham, NC) and the entire mold snap-frozen in cold acetone (kept on dry ice) and stored at −80°C until sectioned on a cryostat (Microm Laborgerate, Walldorf, Germany). Serial 20 μm thick cryosections, separated by 80 μm (discarded), were mounted on 10 sequential pre-coated slides (Superfrost/Plus microscope slides, Fisher Scientific). Slides were then stored at −20°C until stained for histological analysis of the lesion epicenter and length of injury.
A modified eriochrome cyanine (EC) staining protocol for myelin was used to differentiate between white and gray matter so that the amount of spared tissue at the injury epicenter could be calculated in sections from all groups. Briefly, air-dried sections were cleared in a graded series of ethanol and then rehydrated. Sections were then placed in a solution containing 0.4% FeCl3 and 0.16% EC (Sigma) in 0.5% aqueous H2SO4 for 10 min at room temperature. Excess stain was removed from the sections by washing in running tap water for 10 min and then differentiated for 2 min in 0.5% aqueous NH4OH. The reaction was terminated by rinsing the sections in distilled water followed by dehydration in a graded series of ethanol. The sections were then cleared and coverslipped with Permount.
Stained tissue sections were subsequently examined using a Zeiss AxioPlan microscope. Myelin staining was examined under brightfield, and images corresponding to the injury length and epicenter were captured with a Zeiss AxioCam color digital camera. All images were captured at the same lighting condition and magnification. Photomicrographs of each section were viewed with SCION Image software (NIH) and the outer border of the entire section traced (tissue + lesion). Next, the lesion was outlined, followed by any areas of intact gray matter. The area of white matter spared in each section was calculated by subtracting the area of the lesion plus remaining gray matter from the area of the entire section. The percent of spared tissue at the injury epicenter as well as the amount (mm2) of white matter present throughout the rostral-caudal extent of the injury was calculated.41,50
The lesion epicenter was designated as the section containing the largest central core lesion with the least myelin-stained tissue. The lesion extent was determined as the most rostral-caudal sections in which gross morphological changes were no longer observed with EC staining.51 The area of normal appearing tissue was divided by the cross-sectional area and multiplied by 100 to obtain the percent spared tissue. An examiner who was blinded to the treatment groups conducted the histological analysis.
Statistical analyses
All data are reported as group means and standard errors of the mean for the different treatment groups (normality and homogeneity of variance were assumed). The data from the BBB open field locomotor task was analyzed using a repeated measures two-way analysis of variance (ANOVA) followed by a Tukey/Kramer post hoc test when appropriate. The Hargreaves behavioral test and recovery of bladder function were analyzed using a two-way (treatment group × days post-injury) ANOVA followed by a Tukey/Kramer post hoc test. Tissue sparing at the injury epicenter was analyzed using a one-way ANOVA with Tukey/Kramer post hoc test. Analysis of the extent of white matter sparing across the different groups was performed using a two-way ANOVA with a Bonferroni-Dunn post hoc test with corrections for multiple comparisons. In all analyses, a value of p < 0.05 was considered to be statistically significant.
Results
Injury parameters
Three variables related to the IH impactor device were examined and included applied force, spinal cord displacement, and impactor probe velocity. Animals with impact parameters more than two standard deviation (SD) units outside of the mean were not included in the study. The IH impactor uses applied force as the control variable, and the overall mean (± SD) force was 212.05 (± 12.95) kdynes with no significant difference between treatment groups (p > 0.1). The overall mean displacement distance was 1.19 mm ± .173 (SD), and no significant difference was observed between the four treatment groups (p > 0.1). Finally, the velocity at the impact site was 114 ± 6.3 (SD) mm/sec, and there was no significant difference between treatment groups (p > 0.1). All of these variables are consistent with those reported in the original study characterizing the IH impactor device.47 A total of seven animals were removed from the study because of death (five) or impact parameters more than two SD units outside of the overall mean (two), resulting in the following final number of animals per group: Vehicle n = 12, 10 mg/kg NIM811 n = 12, 20 mg/kg NIM811 n = 12, and 40 mg/kg n = 13.
Weight changes after SCI and NIM811 treatment
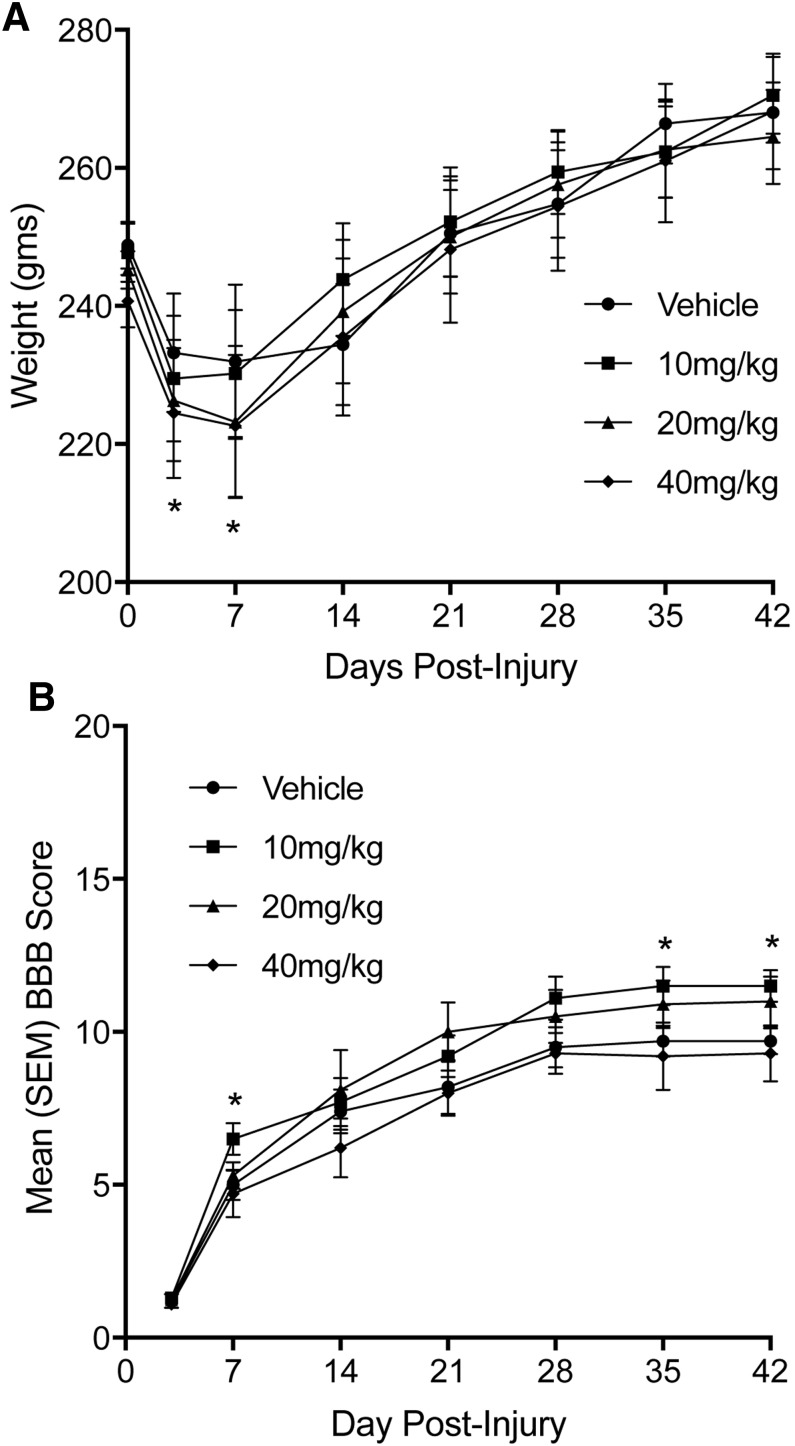
Animals were weighed daily, and a repeated measures two-way ANOVA (treatment × days) used to analyze changes in weight over the duration of the experiment. The data are presented in Figure 1A and plotted based on the animal's weights on the day of surgery and days when animals underwent open field locomotor testing. The analysis revealed a significant main effect of NIM811 treatment [F(3,45) = 13.78, p < 0.01], a significant main effect for days post-injury [F(7,315) = 237.33, p < 0.0001], and a significant treatment × days post-injury interaction [F(21,315) = 7.86, p < 0.001]. A Tukey/Kramer post hoc comparison resulted in a significant (p < 0.05) difference in weight change in the 20 mg/kg and 40 mg/kg animals compared with vehicle at days three and seven post-injury.
FIG. 1.
(A) Mean (± standard error of the mean [SEM]) body weight changes over the 42 day post-injury period. The NIM811 treatment groups (20 mg/kg and 40 mg/kg) both showed significant weight loss at days three and seven post-injury compared with vehicle treated animals. (B) Mean (±SEM) Basso, Beattie and Bresnahan (BBB) scores assessing open field locomotor activity in animals with SCI treated with vehicle or NIM811. Animals in the NIM811 treatment group of 10 mg/kg performed significantly better than vehicle treated animals at days seven, 35, and 42 post-injury. *p < 0.05.
Open field locomotor activity
Animals were tested using the BBB locomotor scale at three and seven days after injury and then once each week for a period of 42 days. The mean and standard errors of the mean for the groups over time are presented in Figure 1B. The analysis revealed a significant main effect for days post-injury [F(6,270) = 293.89, p < 0.0001], a significant main effect of NIM811 treatment [F(3,45) = 11.85, p < 0.01] and a significant treatment × days post-injury interaction [F(18,270) = 6.44, p < 0.01]. A Tukey/Kramer post hoc comparison revealed a significant (p < 0.05) increase in locomotor scores in the 10 mg/kg group compared with vehicle at seven, 35, and 42 days post-injury. Interestingly, the 20 mg/kg group scores were statistically no different from the 10 mg/kg group at 35 and 42 days post-injury. These scores, however, did not reach statistical significance when compared with vehicle treatment (p = 0.071 at 35 days and p = 0.068 at 42 days).
Bladder function
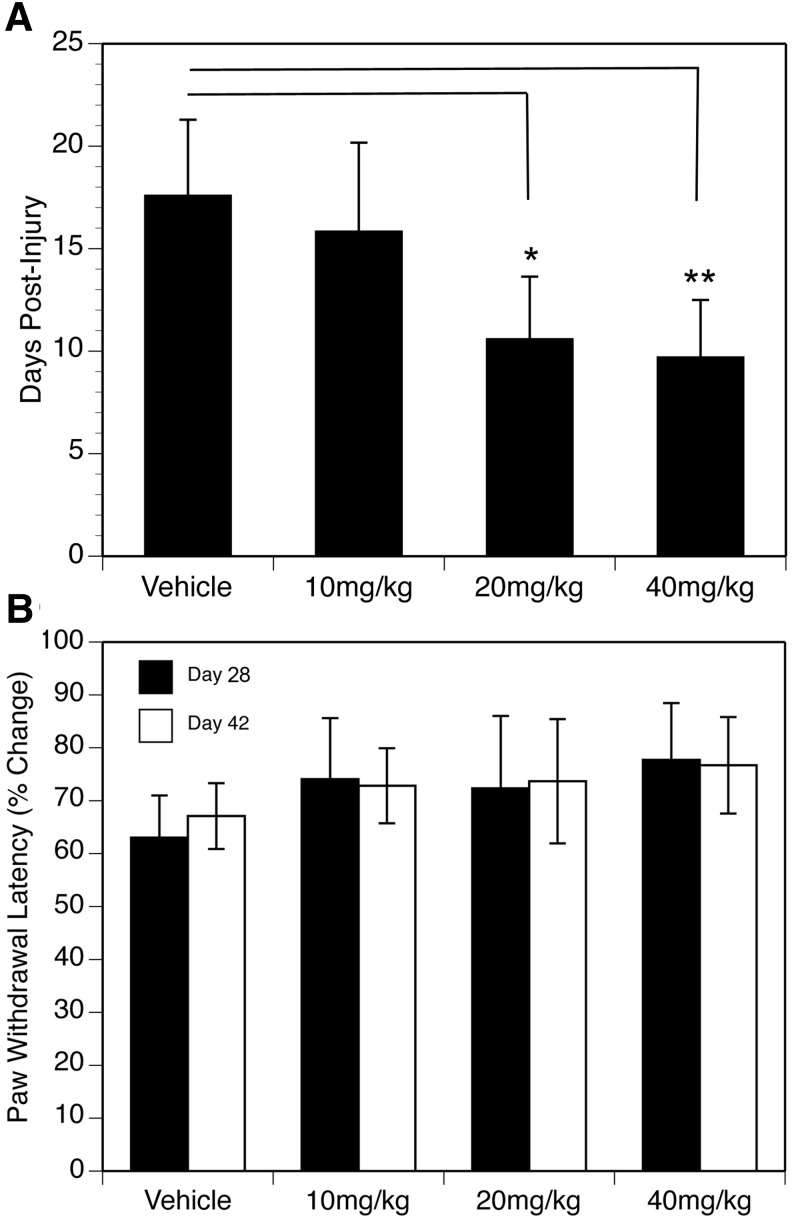
The post-injury time point at which animals exhibited three consecutive days of reflexive control of bladder function was identified for each animal in each treatment group. The data were analyzed using a one-way ANOVA, and the results are plotted in Figure 2A. The outcome of the ANOVA revealed a significant effect of NIM811 treatment [F(3,45) = 39.07, p < 0.01)], and follow-up Tukey/Kramer post hoc comparisons demonstrated a significant decrease in the number of days to reflexive bladder control in the NIM811 treatment groups of 20 mg/kg (p < 0.005) and 40 mg/kg (p < 0.001).
FIG. 2.
(A) Effect of NIM811 treatment on recovery of reflexive bladder function after spinal cord injury (SCI). Animals treated with either NIM811 20 mg/kg or 40 mg/kg took significantly fewer days (mean ± standard error of the mean [SEM]) to recover reflexive bladder control compared with vehicle and the 10 mg/kg treatment group. *p < 0.05, **p < 0.01. (B) Effect of NIM811 treatment on mean (± SEM) paw withdrawal latencies to assess thermal hyperalgesia after SCI. The data are expressed as post-injury levels of hindpaw latency withdrawal as a mean percentage of the baseline average for each animal.
Hargreaves
Hypersensitivity to noxious thermal stimuli is a common characteristic of SCI, and in our study, animals in all four groups showed evidence of thermal hyperalgesia at 28 and 42 days post-injury. The data are presented in Figure 2B, and post-injury levels of hindpaw latency withdrawal are expressed as a mean percentage of the baseline average for each animal. Although withdrawal latencies were less in all groups compared with their respective baseline latencies, a two-way ANOVA revealed no statistically significant effect of NIM811 treatment or day post-injury (p > 0.1).
Tissue histopathology
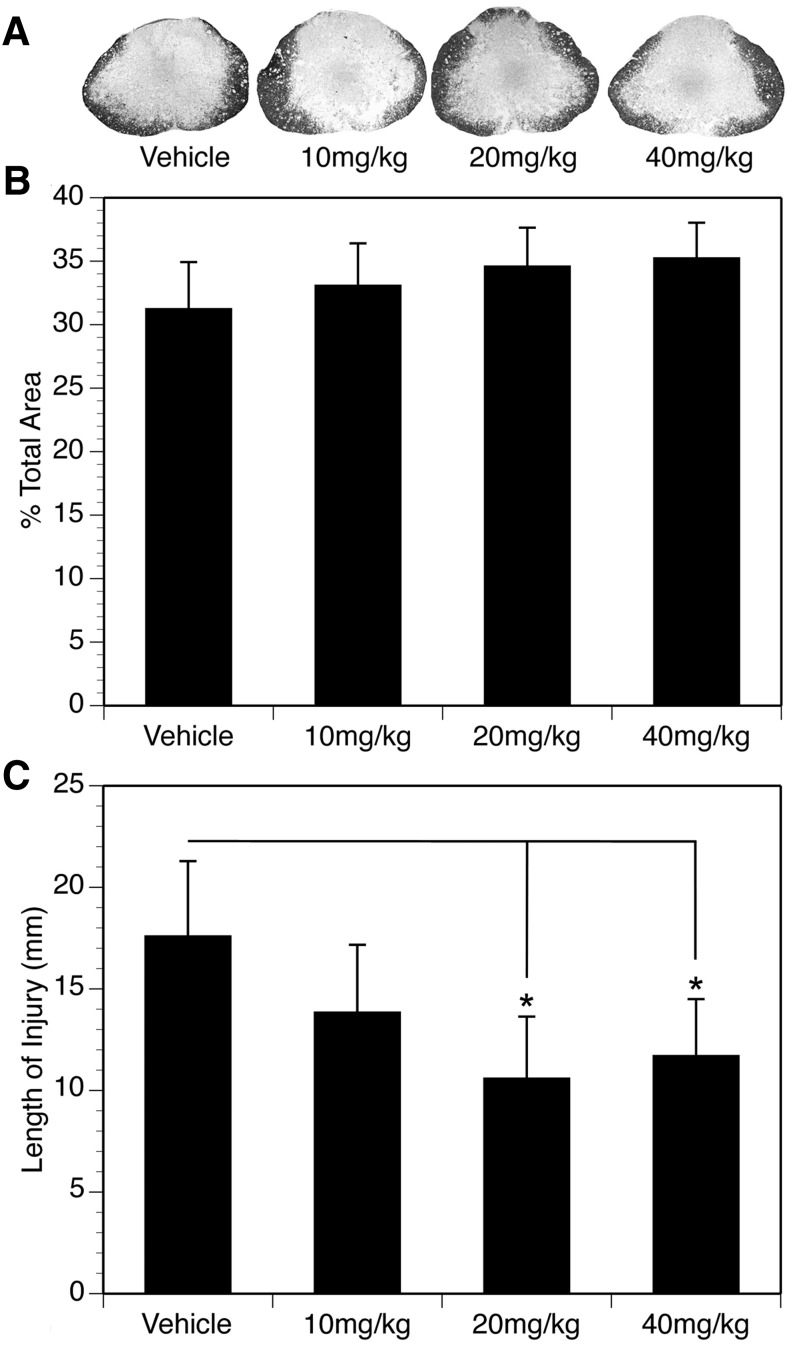
Cross sections of a 20 cm length of spinal cord were stained with EC to assess the percent of spared tissue at the injury epicenter, as well as the percent remaining white matter across the entire rostral to caudal extent of the injury. The data corresponding to the percentage of spared tissue at the injury epicenter was analyzed using a one-way ANOVA, and the results are presented in Figure 3. Histological examination revealed that the lesion epicenter of all injured rats was characterized by a peripheral, and in some cases incomplete, rim of residual tissue (Fig. 3A). While there was a slight trend toward increased tissue sparing at the injury epicenter with NIM811 treatment, the differences were not statistically significant (Fig. 3B).
FIG. 3.
(A) Representative spinal cord injury (SCI) sections showing the percent total area of tissue sparing at the injury epicenter in vehicle and NIM811 treated animals. (B) Mean (± standard error of the mean [SEM]) percent total area of spared tissue at the injury epicenter in the vehicle and NIM811 treatment groups. (C) Effects of NIM811 on the rostral-caudal length (mm) of the lesion at 42 days post-SCI. Both the 20 mg/kg and 40 mg/kg doses of NIM811 significantly reduced the mean (± SEM) length of the lesion compared with vehicle, but not the 10 mg/kg treatment group. *p < 0.05.
A one-way ANOVA was also used to assess possible effects of NIM811 treatment on the overall rostral-caudal length of the lesion (Fig. 3C). There was evidence of cavitation and vacuolization for several millimeters rostral-caudal to the injury epicenter. The ANOVA showed a significant effect of NIM811 treatment [F(3,45) = 33.21, p < 0.01)], and Tukey/Kramer post hoc comparisons revealed a significant decrease (p < 0.05) in the length of the lesion in the NIM811 treatment groups of 20 and 40 mg/kg compared with vehicle control. The rostral-caudal extent of the lesion was ∼30% less than vehicle in both of these treatment groups. The length of the lesion in the NIM811 treatment group of 10 mg/kg was 18.23% less than vehicle, but this was not statistically significant (Tukey/Kramer, p = 0.077).
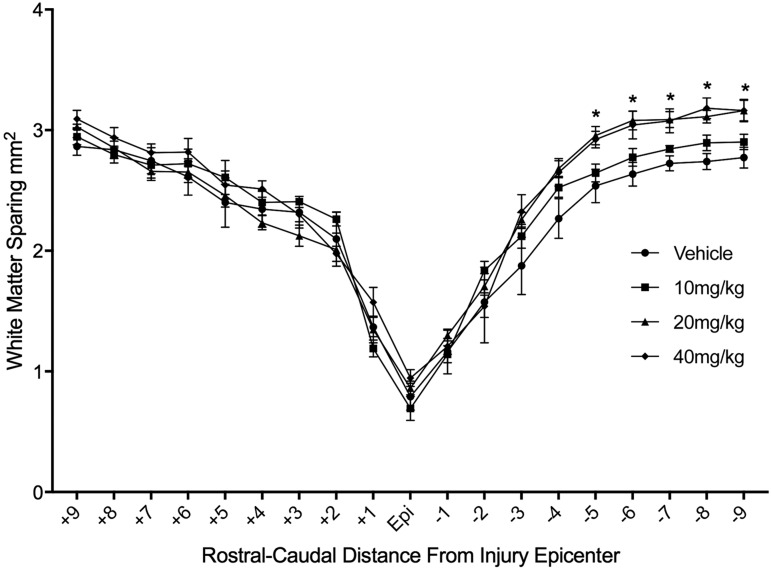
We also examined the effect of NIM811 treatment on white matter sparing across the entire 20 mm spinal cord segment. A two-way ANOVA demonstrated a significant effect of NIM811 treatment [F(3,855) = 13.01, p < 0.01], as well as a significant effect of distance [F(18,855) = 254.2 p < 0.0001)]. Bonferroni-Dunn post hoc tests with corrections for multiple comparisons revealed a significant (p < 0.05) increase in white matter tissue sparing extending 5–9 mm caudal to the injury epicenter in animals treated with NIM811 doses of 20 mg and 40 mg (Fig. 4).
FIG. 4.
Histological assessment of white matter sparing in evenly spaced tissue sections throughout the rostral-caudal extent of the lesion. There was a significant increase (p < 0.05) in white matter sparing (mm2) in sections caudal to the injury epicenter in the NIM811 doses of 20 and 40 mg/kg compared with vehicle and the NIM811 dose of 10 mg/kg.
Discussion
In the present study, we examined the dose-response efficacy of the CsA analog NIM811 on functional recovery and tissue sparing in a rat model of moderate to severe SCI. The results demonstrate that post-injury NIM811 treatment improves locomotor behavior at a dose of 10 mg/kg and improves bladder control and white matter tissue sparing at doses of 20 and 40 mg/kg. Interestingly, only the 10 mg/kg NIM811 treatment group showed statistically significant improvement in locomotor behavior, while improvements in bladder function and white matter tissue sparing were observed only in animals treated with NIM81120 mg/kg and 40 mg/kg.
The 21-point BBB scale was used to assess open field locomotor behavior, and significant improvements in the scores of the NIM811 10 mg/kg treatment group were observed at days seven, 35, and 42 post-injury. Specifically, the NIM811 10 mg/kg treatment group received mean scores of 11 and 12 on days 35 and 42 post-injury, while the vehicle treatment group scored nearly two points less. Animals scoring 11 and 12 on the BBB scale exhibit frequent to consistent weight supported plantar stepping with occasional to frequent coordination of forelimbs and hindlimbs.49 In contrast, vehicle treated animals received mean scores of nine and 10 on days 35 and 42, which corresponds to plantar placement of the hindlimb with weight support in stance only, or occasional weight supported plantar stepping without any forelimb/hindlimb coordination. This observation suggests that although there was only a 1.8–2.0 improvement in locomotor behavior in the NIM811 10 mg/kg treatment group, the recovery is functionally significant in terms of weight supported coordinated stepping.
It is not clear at this time why the higher doses of NIM811 were not statistically significant in promoting significant recovery in locomotor behavior. The doses and treatment time points were based on the pharmacokinetics of NIM811 and our previous studies.43,44 For example, we showed previously that effective concentrations of NIM811 are present in the injured spinal cord at 24 h after a single systemic injection of 40 mg/kg.43 In addition, treatment with NIM811 20 mg/kg (oral gavage) improved tissue sparing at seven days after SCI,44 which would be consistent with our current findings. It should be noted that the BBB scores of animals receiving the NIM811 20 mg/kg dose were statistically no different from the 10 mg/kg dose scores at 35 and 42 days post-injury and close to reaching statistical significance compared with vehicle (p = 0.071 and p = 0.068, respectively). Therefore, while 10 mg/kg was the only dose of NIM811 to show a statistically significant improvement on the BBB scale, the near significant effect of the 20 mg/kg dose is compelling.
One observation that was unique to the two higher doses was the significant decrease in body weight at three and seven days after injury compared with vehicle. It is not clear at this time whether this early significant weight loss was detrimental to subsequent locomotor recovery or contributed to the higher variability of the BBB scores in the 20 and 40 mg/kg dose treatment groups. Finally, as pointed out above, the broad range of scores obtained on the BBB (9–12) encompasses very different levels of locomotor functional recovery. Therefore, future studies utilizing kinematic or gait analysis may be more revealing in explaining the apparent disconnect between dose and functional recovery.
Bladder function is a priority for persons with SCI because of the risk for urinary tract infections and dysfunction of the circuitry mediating bladder activity. In our study, we found that animals receiving the two higher doses NIM811 recovered reflexive bladder voiding 7–8 days sooner than vehicle treated animals. The SCIs at T10 disrupt descending pathways from micturition brainstem nuclei, while T1 injuries appear to spare these descending influences.52 It is interesting to point out that the two doses of NIM811 that improved bladder function also increased white matter tissue sparing in terms of the rostral-caudal length of the lesion. In the rat, the micturition reflex center is located in the medial portion of the dorsolateral pons, which sends projections to motoneurons controlling bladder function in the sacral intermediolateral cell columns.53 These pontine projections descend predominately in the ipsilateral lateral funiculus of the spinal cord and terminate bilaterally in sacral regions controlling reflexive micturition. Although tracing studies were not conducted in these animals, it could be suggested that the increase in white matter tissue NIM811 20 and 40 mg/kg treatments resulted in a partial sparing of descending projections from these micturition brainstem nuclei. In addition, it is possible that the NIM811 treatment impacted local lumbosacral circuits controlling bladder function below the lesion.54
At no time did we observe statistically significant improvement to nociceptive stimuli as determined using hindpaw withdrawal as an indicator of thermal hyperalgesia. All groups of animals were tested at one day before surgery to obtain baseline paw withdrawal latencies and then again at days 28 and 42 after injury. It has been well documented that thermal hyperalgesia can persists for weeks after injury,55,56 and our data are consistent with these previous findings. While there was a tendency of the NIM811 treatment groups to show some improvement compared with vehicle, the differences were not statistically different. Given this outcome, it appears that the change in lesion length in the 20 and 40 mg/kg treatment groups was insufficient to affect responses to nociceptive stimuli despite the significant improvement in reflexive bladder function.
Conclusion
This study demonstrates that post-injury treatment with NIM811 significantly improves recovery of locomotor function (10 mg/kg NIM811) and promotes recovery of reflexive bladder function while also limiting the rostral-caudal extent of the lesion (20 and 40 mg/kg NIM811). Taken together, it could be argued that the most effect dose of NIM811 would fall between the 10–20 mg/kg dose range. This is based on the statistically significant effects of the 20 mg/kg dose on promoting reflexive bladder function and reducing the lesion extent, as well as the near significant effect on recovery of locomotor activity. The mechanism by which NIM811 treatment impacted recovery of function may be explained, in part, by our previous studies showing the positive effects of NIM811 on improving mitochondrial function in both SCI and traumatic brain injury.43–46 Interestingly, we have previously demonstrated that brain and spinal cord mitochondria responses to mPTP modulation are significantly different, which may contribute to these findings.57 Finally, it is possible that the dose specific effect of NIM811 on recovery of locomotor behavior versus bladder function and injury length may be related to the therapeutic window of NIM811.
Future studies examining the potential of NIM811 in the treatment of SCI should focus on determining the appropriate pre-clinical therapeutic window, as well as whether treatment should continue beyond 24 h. In addition, it might be relevant to determine whether mitochondria in supraspinal centers regulating locomotor recovery are more sensitive to NIM811 treatment relative to local circuits that may regulate recovery of bladder function.54 Regardless, given that mPT is thought to be an end stage event for mitochondrial survival, strategies that inhibit mitochondrial demise by inhibiting mPTP formation may prove beneficial in the treatment of those with SCI.
Acknowledgments
This work was supported by PHS grant U01-NS066915, P30 NS051220, and the Craig H. Neilsen Foundation.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Hall E.D., and Springer J.E. (2004). Neuroprotection and acute spinal cord injury: a reappraisal. NeuroRx 1, 80–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Popovich P.G., Wei P., and Stokes B.T. (1997). Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J. Comp. Neurol. 377, 443–464 [DOI] [PubMed] [Google Scholar]
- 3.Blight A.R., and Zimber M.P. (2001). Acute spinal cord injury: pharmacotherapy and drug development perspectives. Curr. Opin. Investig. Drugs 2, 801–808 [PubMed] [Google Scholar]
- 4.Tator C., and Fehlings M. (1991). Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J. Neurosurg. 75, 15–26 [DOI] [PubMed] [Google Scholar]
- 5.Young W. (1993). Secondary injury mechanisms in acute spinal cord injury. J. Emerg. Med. 11, Suppl 1, 13–22 [PubMed] [Google Scholar]
- 6.Braughler J.M., and Hall E.D. (1989). Central nervous system trauma and stroke, I. Biochemical considerations for oxygen radical formation and lipid peroxidation. Free Radic. Biol. Med. 6, 289–301 [DOI] [PubMed] [Google Scholar]
- 7.Sullivan P.G., Krishnamurthy S., Patel S.P., Pandya J.D., and Rabchevsky A.G. (2007). Temporal characterization of mitochondrial bioenergetics after spinal cord injury. J. Neurotrauma 24, 991–999 [DOI] [PubMed] [Google Scholar]
- 8.McEwen M.L., Sullivan P.G., Rabchevsky A.G., and Springer J.E. (2011). Targeting mitochondrial function for the treatment of acute spinal cord injury. Neurotherapeutics 8, 168–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Visavadiya N.P., Patel S.P., VanRooyen J.L., Sullivan P.G., and Rabchevsky A.G. (2016). Cellular and subcellular oxidative stress parameters following severe spinal cord injury. Redox Biol. 8, 59–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schinzel A.C., Takeuchi O., Huang Z., Fisher J.K., Zhou Z., Rubens J., Hetz C., Danial N.N., Moskowitz M.A., and Korsmeyer S.J. (2005). Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc. Natl. Acad. Sci. U. S. A. 102, 12005–12010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakagawa T., Shimizu S., Watanabe T., Yamaguchi O., Otsu K., Yamagata H., Inohara H., Kubo T,. and Tsujimoto Y. (2005). Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 434, 652–658 [DOI] [PubMed] [Google Scholar]
- 12.Basso E., Fante L., Fowlkes J., Petronilli V., Forte M.A., and Bernardi P. (2005). Properties of the permeability transition pore in mitochondria devoid of Cyclophilin D. J. Biol. Chem. 280, 18558–18561 [DOI] [PubMed] [Google Scholar]
- 13.Baines C.P., Kaiser R.A., Purcell N.H., Blair N.S., Osinska H., Hambleton M.A., Brunskill E.W., Sayen M.R., Gottlieb R.A., Dorn G.W., Robbins J., and Molkentin J.D. (2005). Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434, 658–662 [DOI] [PubMed] [Google Scholar]
- 14.Sullivan P.G., Rabchevsky A.G., Waldmeier P.C., and Springer J.E. (2005). Mitochondrial permeability transition in CNS trauma: cause or effect of neuronal cell death? J. Neurosci. Res. 79, 231–239 [DOI] [PubMed] [Google Scholar]
- 15.Brustovetsky N., Brustovetsky T., Jemmerson R., and Dubinsky J.M. (2002). Calcium-induced cytochrome c release from CNS mitochondria is associated with the permeability transition and rupture of the outer membrane. J. Neurochem 80, 207–218 [DOI] [PubMed] [Google Scholar]
- 16.Cande C., Cohen I., Daugas E., Ravagnan L., Larochette N., Zamzami N., and Kroemer G. (2002). Apoptosis-inducing factor (AIF): a novel caspase-independent death effector released from mitochondria. Biochimie 84, 215–222 [DOI] [PubMed] [Google Scholar]
- 17.Du C., Fang M., Li Y., Li L., and Wang X. (2000). Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 102, 33–42 [DOI] [PubMed] [Google Scholar]
- 18.Li L.Y., Luo X., and Wang X. (2001). Endonuclease G is an apoptotic DNase when released from mitochondria. Nature 412, 95–99 [DOI] [PubMed] [Google Scholar]
- 19.Liu X., Kim C.N., Yang J., Jemmerson R., and Wang X. (1996). Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86, 147–157 [DOI] [PubMed] [Google Scholar]
- 20.van Loo G., Schotte P., van Gurp M., Demol H., Hoorelbeke B., Gevaert K., Rodriguez I., Ruiz-Carrillo A., Vandekerckhove J., Declercq W., Beyaert R., and Vandenabeele P. (2001). Endonuclease G: a mitochondrial protein released in apoptosis and involved in caspase-independent DNA degradation. Cell Death Differ. 8, 1136–1142 [DOI] [PubMed] [Google Scholar]
- 21.Petronilli V., Penzo D., Scorrano L., Bernardi P., and Di Lisa F. (2001). The mitochondrial permeability transition, release of cytochrome c and cell death. Correlation with the duration of pore openings in situ. J. Biol. Chem. 276, 12030–12034 [DOI] [PubMed] [Google Scholar]
- 22.Waldmeier P.C., Zimmermann K., Qian T., Tintelnot-Blomley M., and Lemasters J.J. (2003). Cyclophilin D as a drug target. Curr. Med. Chem. 10, 1485–1506 [DOI] [PubMed] [Google Scholar]
- 23.Szabo I., and Zoratti M. (1992). The mitochondrial megachannel is the permeability transition pore. J. Bioenerg. Biomembr. 24, 111–117 [DOI] [PubMed] [Google Scholar]
- 24.Reed J.C., and Kroemer G. (2000). Mechanisms of mitochondrial membrane permeabilization. Cell Death Differ. 7, 1145. [DOI] [PubMed] [Google Scholar]
- 25.Halestrap A.P., McStay G.P., and Clarke S.J. (2002). The permeability transition pore complex: another view. Biochimie 84, 153–166 [DOI] [PubMed] [Google Scholar]
- 26.Bernardi P., Colonna R., Costantini P., Eriksson O., Fontaine E., Ichas F., Massari S., Nicolli A., Petronilli V., and Scorrano L. (1998). The mitochondrial permeability transition. Biofactors 8, 273–281 [DOI] [PubMed] [Google Scholar]
- 27.Friberg H., Connern C., Halestrap A.P., and Wieloch T. (1999). Differences in the activation of the mitochondrial permeability transition among brain regions in the rat correlate with selective vulnerability. J. Neurochem. 72, 2488–2497 [DOI] [PubMed] [Google Scholar]
- 28.Friberg H., and Wieloch T. (2002). Mitochondrial permeability transition in acute neurodegeneration. Biochimie 84, 241–250 [DOI] [PubMed] [Google Scholar]
- 29.Hansson M.J., Mattiasson G., Mansson R., Karlsson J., Keep M.F., Waldmeier P., Ruegg U.T., Dumont J.M., Besseghir K., and Elmer E. (2004). The nonimmunosuppressive cyclosporin analogs NIM811 and UNIL025 display nanomolar potencies on permeability transition in brain-derived mitochondria. J. Bioenerg. Biomembr. 36, 407–413 [DOI] [PubMed] [Google Scholar]
- 30.Hansson M.J., Persson T., Friberg H., Keep M.F., Rees A., Wieloch T., and Elmer E. (2003). Powerful cyclosporin inhibition of calcium-induced permeability transition in brain mitochondria. Brain Res. 960, 99–111 [DOI] [PubMed] [Google Scholar]
- 31.Bernardi P. (1996). The permeability transition pore. Control points of a cyclosporin A-sensitive mitochondrial channel involved in cell death. Biochim. Biophys. Acta 1275, 5–9 [DOI] [PubMed] [Google Scholar]
- 32.Broekemeier K.M., and Pfeiffer D.R. (1995). Inhibition of the mitochondrial permeability transition by cyclosporin A during long time frame experiments: relationship between pore opening and the activity of mitochondrial phospholipases. Biochemistry 34, 16440–16449 [DOI] [PubMed] [Google Scholar]
- 33.Brustovetsky N., and Dubinsky J.M. (2000). Limitations of cyclosporin A inhibition of the permeability transition in CNS mitochondria. J. Neurosci. 20, 8229–8237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Halestrap A.P., Connern C.P., Griffiths E.J., and Kerr P.M. (1997). Cyclosporin A binding to mitochondrial cyclophilin inhibits the permeability transition pore and protects hearts from ischaemia/reperfusion injury. Mol. Cell. Biochem. 174, 167–172 [PubMed] [Google Scholar]
- 35.Scorrano L., Nicolli A., Basso E., Petronilli V., and Bernardi P. (1997). Two modes of activation of the permeability transition pore: the role of mitochondrial cyclophilin. Mol. Cell. Biochem. 174, 181–184 [PubMed] [Google Scholar]
- 36.Ibarra A., Guizar-Sahagun G., Correa D., Kretschmer R., Grijalva I., Flores-Murrieta F.J., Castaneda-Hernandez G., Odor A., Lopez R.M., Franco-Bourland R., Espitia A.L., Salgado-Ceballos H., and Madrazo I. (1996). Alteration of cyclosporin-A pharmacokinetics after experimental spinal cord injury. J. Neurotrauma 13, 267–272 [DOI] [PubMed] [Google Scholar]
- 37.Diaz-Ruiz A., Rios C., Duarte I., Correa D., Guizar-Sahagun G., Grijalva I., Madrazo I., and Ibarra A. (2000). Lipid peroxidation inhibition in spinal cord injury: cyclosporin-A vs methylprednisolone. Neuroreport 11, 1765–1767 [DOI] [PubMed] [Google Scholar]
- 38.Diaz-Ruiz A., Rios C., Duarte I., Correa D., Guizar-Sahagun G., Grijalva I. and Ibarra A. (1999). Cyclosporin-A inhibits lipid peroxidation after spinal cord injury in rats. Neurosci. Lett. 266, 61–64 [DOI] [PubMed] [Google Scholar]
- 39.Ibarra A., Correa D., Willms K., Merchant M.T., Guizar-Sahagun G., Grijalva I., and Madrazo I. (2003). Effects of cyclosporin-A on immune response, tissue protection and motor function of rats subjected to spinal cord injury. Brain Res. 979, 165–178 [DOI] [PubMed] [Google Scholar]
- 40.Ibarra A., Reyes J., Martinez S., Correa D., Guizar-Sahagun G., Grijalva I., Castaneda-Hernandez G., Flores-Murrieta F.J., Franco-Bourland R., and Madrazo I. (1996). Use of cyclosporin-A in experimental spinal cord injury: design of a dosing strategy to maintain therapeutic levels. J. Neurotrauma 13, 569–572 [DOI] [PubMed] [Google Scholar]
- 41.Rabchevsky A.G., Fugaccia I., Sullivan P.G., and Scheff S.W. (2001). Cyclosporin A treatment following spinal cord injury to the rat: behavioral effects and stereological assessment of tissue sparing. J. Neurotrauma 18, 513–522 [DOI] [PubMed] [Google Scholar]
- 42.Waldmeier P.C., Feldtrauer J.J., Qian T., and Lemasters J.J. (2002). Inhibition of the mitochondrial permeability transition by the nonimmunosuppressive cyclosporin derivative NIM811. Mol. Pharmacol. 62, 22–29 [DOI] [PubMed] [Google Scholar]
- 43.McEwen M.L., Sullivan P.G., and Springer J.E. (2007). Pretreatment with the cyclosporin derivative, NIM811, improves the function of synaptic mitochondria following spinal cord contusion in rats. J. Neurotrauma 24, 613–624 [DOI] [PubMed] [Google Scholar]
- 44.Ravikumar R., McEwen M.L., and Springer J.E. (2007). Post-treatment with the cyclosporin derivative, NIM811, reduced indices of cell death and increased the volume of spared tissue in the acute period following spinal cord contusion. J. Neurotrauma 24, 1618–1630 [DOI] [PubMed] [Google Scholar]
- 45.Mbye L.H., Singh I.N., Sullivan P.G., Springer J.E., and Hall E.D. (2008). Attenuation of acute mitochondrial dysfunction after traumatic brain injury in mice by NIM811, a non-immunosuppressive cyclosporin A analog. Exp. Neurol. 209, 243–253 [DOI] [PubMed] [Google Scholar]
- 46.Readnower R.D., Pandya J.D., McEwen M.L., Pauly J.R., Springer J.E., and Sullivan P.G. (2011). Post-injury administration of the mitochondrial permeability transition pore inhibitor, NIM811, is neuroprotective and improves cognition after traumatic brain injury in rats. J. Neurotrauma 28, 1845–1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scheff S.W., Rabchevsky A.G., Fugaccia I., Main J.A., and Lumpp J.E., Jr (2003). Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J. Neurotrauma 20, 179–193 [DOI] [PubMed] [Google Scholar]
- 48.Scheff S.W., and Sullivan P.G. (1999). Cyclosporin A significantly ameliorates cortical damage following experimental traumatic brain injury in rodents. J. Neurotrauma 16, 783–792 [DOI] [PubMed] [Google Scholar]
- 49.Basso D.M., Beattie M.S., and Bresnahan J.C. (1995). A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 12, 1–21 [DOI] [PubMed] [Google Scholar]
- 50.Wrathall J.R., Choiniere D., and Teng Y.D. (1994). Dose-dependent reduction of tissue loss and functional impairment after spinal cord trauma with the AMPA/kainate antagonist NBQX. J. Neurosci. 14, 6598–6607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Springer J.E., Rao R.R., Lim H.R., Cho S.I., Moon G.J., Lee H.Y., Park E.J., Noh J.S., and Gwag B.J. (2010). The functional and neuroprotective actions of Neu2000, a dual-acting pharmacological agent, in the treatment of acute spinal cord injury. J. Neurotrauma 27, 139–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.David B.T., and Steward O. (2010). Deficits in bladder function following spinal cord injury vary depending on the level of the injury. Exp. Neurol. 226, 128–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Loewy A.D., Saper C.B., and Baker R.P. (1979). Descending projections from the pontine micturition center. Brain Res. 172, 533–538 [DOI] [PubMed] [Google Scholar]
- 54.Hou S., Carson D.M., Wu D., Klaw M.C., Houle J.D., and Tom V.J. (2016). Dopamine is produced in the rat spinal cord and regulates micturition reflex after spinal cord injury. Exp. Neurol. 285, 136–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Christensen M.D., and Hulsebosch C.E. (1997). Chronic central pain after spinal cord injury. J. Neurotrauma 14, 517–537 [DOI] [PubMed] [Google Scholar]
- 56.Hulsebosch C.E., Hains B.C., Crown E.D., and Carlton S.M. (2009). Mechanisms of chronic central neuropathic pain after spinal cord injury. Brain Res. Rev. 60, 202–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sullivan P.G., Rabchevsky A.G., Keller J.N., Lovell M., Sodhi A., Hart R.P., and Scheff S.W. (2004). Intrinsic differences in brain and spinal cord mitochondria: Implication for therapeutic interventions. J. Comp. Neurol. 474, 524–534 [DOI] [PubMed] [Google Scholar]