Abstract.
Two dosimetric quantities [mean glandular dose (MGD) and entrance surface air kerma (ESAK)] and the diagnostic performance of phase-contrast mammography with synchrotron radiation (MSR) are compared to conventional digital mammography (DM). Seventy-one patients (age range, 41 to 82 years) underwent MSR after a DM examination if questionable or suspicious breast abnormalities were not clarified by ultrasonography. The MGD and the ESAK delivered in both examinations were evaluated and compared. Two on-site radiologists rated the images in consensus according to the Breast Imaging Reporting and Data System assessment categories, which were then correlated with the final diagnoses by means of statistical generalized linear models (GLMs). Receiver operating characteristic curves were also used to assess the diagnostic performance by comparing the area under the curve (AUC). An important MGD and ESAK reduction was observed in MSR due to the monoenergetic beam. In particular, an average 43% reduction was observed for the MGD and a reduction of more than 50% for the ESAK. GLM showed higher diagnostic accuracy, especially in terms of specificity, for MSR, confirmed by AUC analysis (). The study design implied that the population was characterized by a high prevalence of disease and that the radiologists, who read the DM images before referring the patient to MSR, could have been influenced in their assessments. Within these limitations, the use of synchrotron radiation with the phase-contrast technique applied to mammography showed an important dose reduction and a higher diagnostic accuracy compared with DM. These results could further encourage research on the translation of x-ray phase-contrast imaging into the clinics.
Keywords: x-ray phase-contrast imaging, mammography, breast cancer, synchrotron radiation, mean glandular dose, diagnostic performance
1. Introduction
X-ray beams generated by synchrotron accelerators have many properties that make them suitable for medical applications. In particular, synchrotron radiation (SR) has a high degree of spatial coherence, which allows the exploitation of phase effects. The phase shift effects induced by biological tissue can be larger than the absorption effects, so the sensitivity of x-ray imaging can be strongly enhanced. Tiny details embedded in a similar background (e.g., small malignant breast lesion surrounded by healthy glandular tissue) can be better differentiated.1–3 Several phase-sensitive x-ray imaging techniques have been developed in recent decades. The propagation-based phase-contrast imaging (PPCI) technique can be performed by placing the detector at a suitable distance from the sample; this allows the distorted wave front, generated by interaction with the sample, to evolve before being captured by the detector, leading to a strong edge enhancement.1–3
PPCI has shown great potential in the field of biomedical imaging,4 and it has been successfully applied to breast x-ray imaging.5 Mammography is one of the radiological examinations where the SR properties and PPCI technique can be highly exploited.6 The SR laminar beam geometry implies that patients are scanned through the beam providing an ideal scatter rejection without the need for antiscatter grids; moreover, an optimal energy can be selected for each patient by tuning the SR monochromatic beam, leading to a dose reduction. This latter feature is extremely important especially for the breast, which is one of the most radiosensitive organs.7 Mammography with synchrotron radiation (MSR) has the potential to reduce the mean glandular dose8 (MGD) compared with conventional digital mammography (DM). Moreover, the contrast generated by the PPCI techniques is superimposed on the absorption image thereby improving the visibility of structure borders9 (in fact, the linear attenuation coefficient of breast cancer is very similar to that of healthy glandular tissue,10 often implying further clinical investigations for an unequivocal diagnosis).
The first PPCI clinical study11 was carried out at a dedicated beamline specifically designed for medical applications12 at Elettra, the Italian synchrotron light source in Trieste. The study involved 71 female patients (age range, 41 to 82 years) who underwent MSR after reports of unresolved breast abnormalities at conventional DM and ultrasonography (US) examinations performed at the Department of Radiology of the University Hospital of Trieste (Italy).
This paper is the final report on this study and presents a comprehensive analysis of the delivered dose and of the diagnostic performance of MSR for the whole group of 71 patients, compared with DM performed at the hospital. The issues on the delivered dose have not been treated in our previous publications11–13 and, therefore, represent the first element of innovation of this paper. In particular, emphasis is given to the method for the MGD calculation, which uses the dose coefficients provided in the literature for the polychromatic spectrum14,15 (in the case of DM) and monoenergetic beams16 (in the case of MSR). A second original element of this paper is that the diagnostic performance of MSR and DM is analyzed and compared using the statistical generalized linear model17 (GLM) for multivariate analysis and evaluating the area under the curve (AUC) of the receiver operating characteristic (ROC) curve. On the contrary, the preliminary diagnostic performance comparison between MSR and DM presented in Ref. 11, which was limited to the first 47 patients who completed the study protocol, was performed by means of less refined statistical methods, requiring the introduction of possibly detrimental approximations. Finally, Ref. 13 drew a comparison between MSR and DM for the whole group of 71 patients but strictly limited to the visual assessment of image quality. It confirmed that phase-contrast MSR depicts normal structures and abnormal findings with higher image quality, but taking into account neither the dosimetric issues nor the diagnostic performance, which are indeed investigated in this paper.
2. Material and Methods
2.1. Patient Recruitment and Protocols
The local ethics committee authorized the clinical study, and written informed consent was obtained from all patients. Patients were recruited from among patients undergoing diagnostic mammography or asymptomatic women undergoing mammography (e.g., for familiarity reasons) at the Department of Radiology of the University Hospital of Trieste (Italy). None of patients were part of a screening program.
The Breast Imaging Reporting and Data System (BI-RADS)18 categories have been used in this study. BI-RADS scores represent a gradually increasing scale of severity, whereby category 1 means negative (i.e., there is nothing to comment on), category 2 applies to definitely benign findings, category 3 to probably benign findings, category 4 to suspicious abnormality, and category 5 indicates an abnormality, which is highly suspicious of malignancy.
A patient was considered for MSR when at least one of the following inclusion criteria was met: (i) negative DM results (BI-RADS category 1) but presence of a palpable mass that was not clarified at US in a heterogeneously or homogeneously dense breast, (ii) focal asymmetry of tissue density between the two breasts on DM image (BI-RADS category 3 or 4) that was not clarified at US, (iii) architectural distortion on DM image (BI-RADS category 3 or 4) that was not clarified at US, and (iv) equivocal or suspicious mass on DM image (BI-RADS category 3 or 4) that was not clarified at US. DM-US follow-up and biopsy were considered the reference standards.
2.2. Mammographic Equipment
The DM examinations were performed using a Senographe DS (GE Healthcare, Chalfont St. Giles, England) system at the Department of Radiology of the University Hospital of Trieste (Italy). MSR was performed at the Elettra SYRMEP beamline, where there is a dedicated facility designed for mammography.12 The relevant characteristics of the x-ray source and the geometric setup are summarized in Table 1.
Table 1.
X-ray source and geometrical characteristic of the SYRMEP beamline at the ELETTRA synchrotron.
| X-ray source | |
|---|---|
| Type | Bending magnet |
| Source size () | |
| Horizontal angular spread | 7 mrad |
| Energy range | 8.5 to 40 keV |
| Energy range for patient exposure | 17.5 to 21 keV |
| Energy resolution |
|
| Geometric characteristics | |
| Source-to-sample distance | 30 m (26.5 m in vacuum) |
| Sample-to-detector distance | 2.0 m |
| Magnification | |
| Beam size at the patient position | |
Both cranio-caudal (CC) and medio-lateral oblique (MLO) projections were acquired.
During the MSR examinations, the patients lay prone on a movable support with the breast to be imaged hanging in pendant geometry. The breast was compressed with a system similar to the compression device used in DM to guarantee equalization and stretching of breast tissue. A planar radiographic image was acquired using a screen-film system (Kodak MIN-R 2000 Film; Carestream Health Medical Imaging & IT, Rochester, New York). Due to the laminar geometry of the beam, both the patient support and the screen-film cassette were scanned vertically through the beam (from bottom to top) during the examination. All patients were scanned in the same geometrical conditions.
The monochromatic energy level was selected in the range of 17.5 to 21 keV (with 0.5-keV steps) depending on breast thickness and glandularity according to a predefined table (Table 2).
Table 2.
Beam energy (in keV) for different breast thickness class and glandularity.
| Glandularity | ||||
|---|---|---|---|---|
| Low | Medium | High | ||
| Thickness class (cm) | 2 | 17.5 | 18 | 18 |
| 3 | 18.5 | 18.5 | 18.5 | |
| 4 | 18.5 | 19 | 19 | |
| 5 | 18.5 | 19.5 | 19.5 | |
| 6 | 19 | 20 | 20.5 | |
| 7 | 20 | 20.5 | 21 | |
Glandularity was estimated by the radiologist (i.e., low, medium, or high) before the examination, and the thickness class was determined by measuring the actual compressed breast thickness and rounding it off to the nearest integer value. Table 2 was completed with the principle of using the lowest energy (to maximize contrast) ensuring a dose comparable to or lower than DM.
The dosimeter system used during the MSR examinations consisted of two custom-made high-precision ionization chambers that evaluated the air kerma in real time. The ionization chambers were calibrated with respect to the air kerma primary standard chamber for low-energy x-rays by the Department of Ionizing Radiation Metrology of the Italian National Agency for New Technologies, Energy and Environment (ENEA).19 The whole equipment was designed to guarantee the safety of patients and operators and was strictly checked.20
2.3. Evaluations of Dosimetric Quantities
The entrance surface air kerma (ESAK) of the DM examinations was obtained from the header of the DICOM image files, together with all other parameters useful for MGD evaluation (e.g., spectrum, kVp, etc.). According to the European Guidelines,21 the MGD is estimated using the formula proposed by Dance et al.15
| (1) |
where is the ESAK at the upper surface of the breast and , , and are coefficients that take into account the breast thickness, the half value layer, the glandularity, and the x-ray spectrum.
Another approach to MGD calculation for DM was developed by Boone14,16 using a slightly different breast model. Boone used a Monte Carlo simulation program to estimate the dose either for a polychromatic spectrum14 or a monochromatic x-ray beam.16 Using the Boone’s approach, we were able to perform a dose comparison between polychromatic and monochromatic mammography free from systematic errors. The ESAK delivered during MSR is equal to the air kerma measured by the two ionization chambers of the SYRMEP dosimeter system, and the MGDs delivered to patients were calculated according Boone’s coefficients16
| (2) |
where is the measured ESAK and is a coefficient function of the monochromatic beam energy, the compressed breast thickness, and the glandularity.
The evaluated MGD values (obtained by applying the different formulas) were then organized according to the thickness class. The glandularity used to estimate the MGD varies both with the thickness class and with the patients’ age according to tables reported by Dance et al.15 Final mean values were obtained by averaging all data of MGDs and ESAK for all patients within the same thickness class. The CC and MLO projections were taken into account separately. A linear fit of all patients’ individual data was performed to investigate the dependence of ESAK upon the compressed breast thickness class. The statistical analysis of the MGDs delivered in DM and MSR was based on a paired -test applied to all patients’ individual data.
2.4. Diagnostic Performance Analysis
Two radiologists assessed the images obtained with DM and MSR in consensus. While DM images were assessed on a digital display, MSR films were not digitized but were evaluated on a view box. The readers were not informed of the patients’ final diagnoses, but they were aware of their clinical histories. The readers rated the images according to the BI-RADS assessment categories18 both for DM and MSR. Subsequently, the BI-RADS scores and the associated diagnosis were compared with the final diagnosis obtained using the findings of the reference standard (biopsy or a two-year follow-up).
The DM and MSR BI-RADS scores were compared using the GLM tools for multivariate analysis and in particular with logistic regression.17 GLMs improve the capacity and possibility of modeling data with a mathematical framework based on likelihood function, and they have been successfully applied to medical data.22 The goal of GLM is to describe the dependency relationship of possession of a dichotomous attribute (i.e., positive or negative from the reference standard methods) of independent variables (i.e., the BI-RADS scores). The BI-RADS scores can be effectively managed as ordinal statistical variables as they represent a gradually increasing scale of severity.23 The Akaike information criterion24 (AIC) was used to assess the goodness of GLM applied to the two data sets (i.e., the BI-RADS of DM and MSR). The AIC balances the goodness of fit model (as it is based on the maximum likelihood function) with the number of estimated parameters. The best-fit model has a lower AIC score.
The same statistical analysis was made by dividing the patients according to the BI-RADS breast-density category: (1) almost entirely fatty ( glandular), (2) scattered fibroglandular densities ( to 50% glandular), (3) heterogeneously dense ( to 75% glandular), and (4) extremely dense ( glandular). The analysis was performed for categories 2 and 3 but not for categories 1 and 4 because the number of cases was too low (only four cases for category 1 and five for category 4).
ROC curves for MSR and DM were also evaluated using the BI-RADS scores assigned to each patient by the radiologists. This procedure is possible as the ROC curves are estimated on five operating points based on BI-RADS scores from 1 to 5 and, as noted in Ref. 25, it is an appropriate procedure for appreciating ROC curves when analyzing diagnostic mammograms. AUC of ROC curves was calculated and compared for DM and MSR.
All the statistical analysis was carried out using the statistical software R.26 ROC curve analysis was performed by means of the p-ROC package.27
3. Results
3.1. Evaluation of Dosimetric Quantities
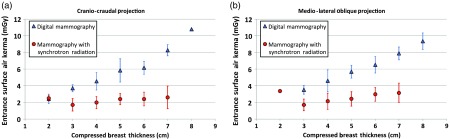
The mean ESAK values as a function of compressed breast thickness for DM and MSR (separately for CC and MLO projections) are plotted in Fig. 1.
Fig. 1.
ESAK comparison for (a) CC and (b) MLO projections: blue triangles are related to DM while red dots are referred to MSR. Error bars represent one standard deviation. Figures readapted from Ref. 28.
It should be noted that the ESAK for MSR is relatively constant for all compressed breast thicknesses whereas in DM the ESAK increases with breast thickness. Linear fits to the ESAK data for DM and MSR show that the slope of DM data is significantly different from 0 (slope for CC is and slope for MLO is ) while the slope of MSR data is not different from 0 considering a 99% confidence interval (slope for CC is and slope for MLO is ). This implies a proportional skin-dose reduction in the thick breast examinations (about 50% for 4-cm breast thickness and more than 50% for thicker breasts).
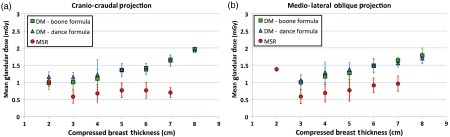
The mean values of MGDs (as a function of the compressed breast thickness) delivered in DM and in MSR examinations (for both CC and MLO projections) are shown in Fig. 2.
Fig. 2.
MGDs comparison for (a) CC and (b) MLO projections: blue triangles correspond to DM according to Dance et al.’s15 formula, green squares are attributed to DM according to Boone’s14 formula for polychromatic spectrum while red dots correspond to MSR according to Boone’s16 formula for monochromatic radiation. Error bars represent one standard deviation. Figures readapted from Ref. 28.
All the values are well below the limits suggested by the European Guidelines,21 and the MGDs for MSR are lower than those of DM (only in one case the value was comparable).
The doses calculated for DM using the two approaches (Boone’s 14 and Dance et al.’s15 formulas) show a considerable overlap on the plots, indicating that no methodological differences are introduced when evaluating the doses using Boone’s formulas. A paired -test across all the 71 patients’ dose values showed no significant difference. However, the MGDs for thin breasts evaluated with Dance et al.’s15 coefficients are possibly slightly higher than those of Boone,14 whereas the reverse is true for thicker breasts.
Significant difference () is found between the MGDs evaluated for DM and MSR when a paired -test is computed considering each of the 71 patients’ dose values. To avoid systematic error, the dose comparison between DM and MSR is done considering the Boone’s formulas for conventional mammographic spectra14 and for monochromatic beams.16
In some cases, the compression during MSR was slightly higher than in DM, with the consequence that a given patient could fall into the lower thickness class in MSR with respect to DM. That explains why in some thickness classes not all three results are available.
3.2. Diagnostic Performance Analysis
A permutation test29 among the BI-RADS scores for DM and MSR was performed, and the result shows that the BI-RADS scores for DM and MSR are significantly different in our patients group ().
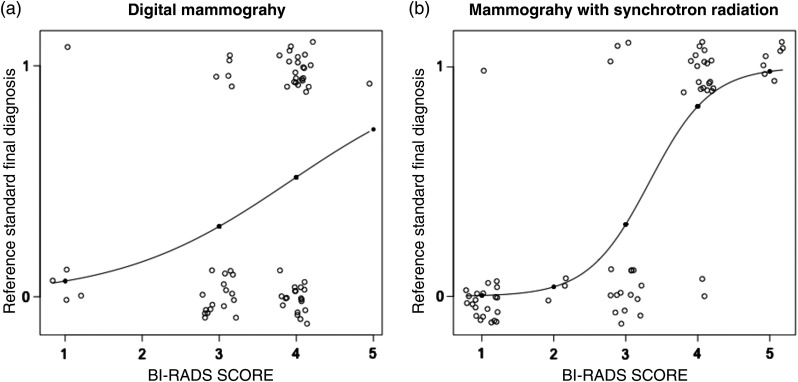
The application of GLMs for evaluating diagnostic performance of DM and MSR BI-RADS is shown in Fig. 3.
Fig. 3.
GLM applied to (a) DM and (b) MSR: the abscissa shows the BI-RADS score evaluations while the ordinate shows the final diagnosis obtained with the reference standard (0 means negative and 1 means positive). The continuous lines on the graphs are the fitted-model line while the circles represent the single patient evaluations. The circles are spread on the graph for better visualization.
Two features have to be highlighted: (i) 17 healthy patients classified as BI-RADS score 4 after DM were moved to category 1 (), category 2 (), and category 3 () after MSR and (ii) 21 positive cases classified as BI-RADS 4 after DM was reclassified as BI-RADS 5 () and BI-RADS 4 () after MSR. The fitted-model line in the MSR graph starts at BI-RADS 1 (value 0, negative reference standard) and reaches 1 (malignancy presence) at BI-RADS 5. On the contrary, for DM, the fitted-model line does not reach the value 1 for the reference standard and presents a less hanging behavior. The AIC applied to MSR shows a value of 47.02 and 91.50 for DM. The smaller value of MSR suggests that this technique has better accuracy than DM.
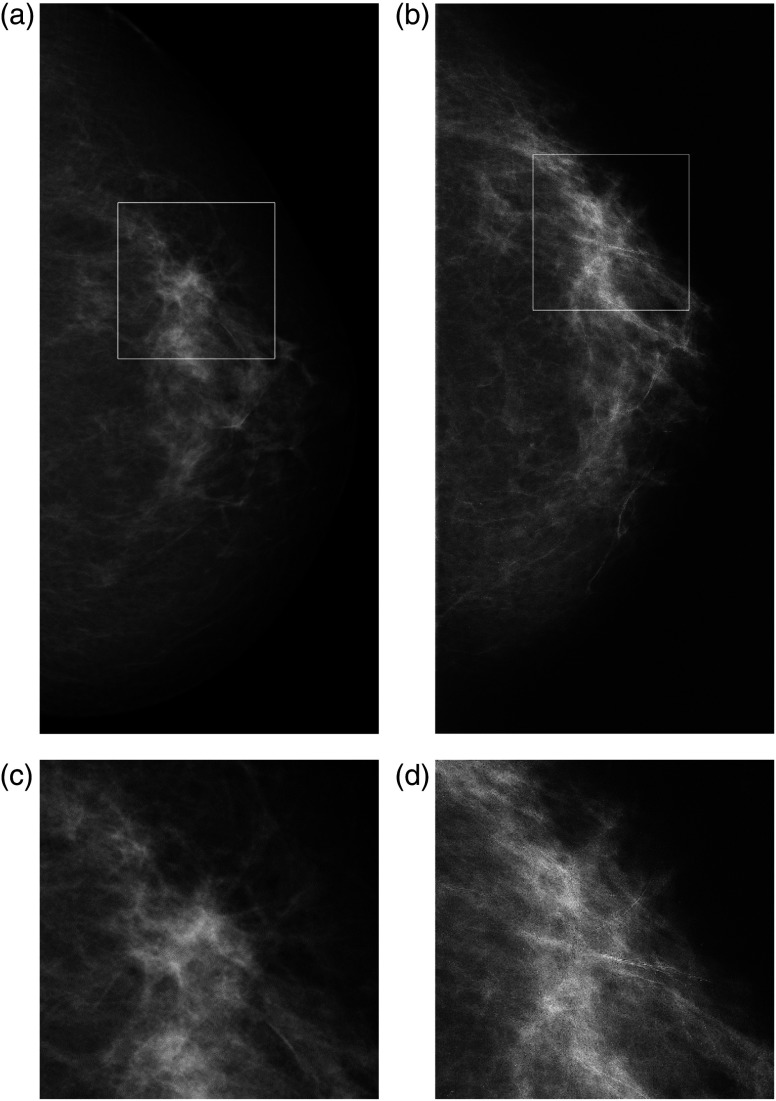
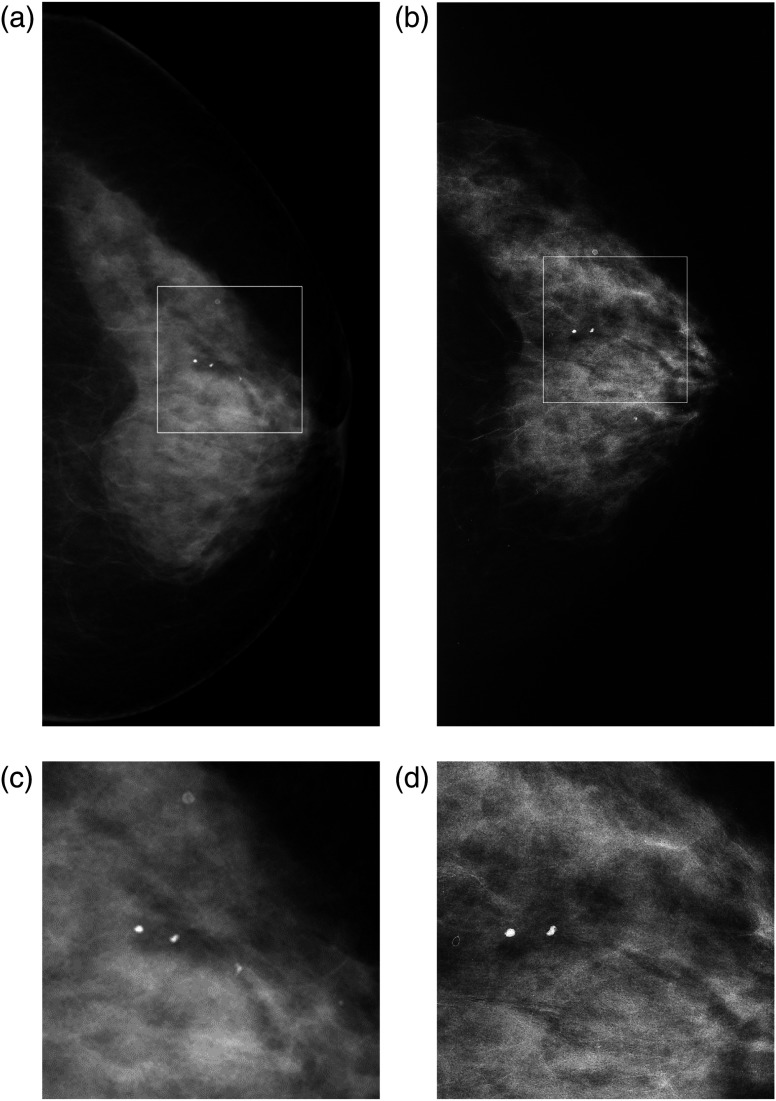
Figures 4 and 5 show two patient cases. The edge-enhancement effect, due to the phase contrast, increases visibility of the breast parenchymal structures in the MSR images. Other clinical cases are reported and discussed in previous papers.11,12,30
Fig. 4.
CC images of the left breast in 60-year-old woman. BI-RADS breast density 2: (a) DM and (b) MSR. In (a), there is a spiculated low-density lesion at the outer quadrants, which creates an architectural distortion [visible in the (c) corresponding enlarged view] that is suspicious for malignant lesion; these findings are not confirmed in image (b), where we can observe an area of heterogeneous glandular tissue, with normal parenchymal pattern [in the (d) corresponding digital zoom].
Fig. 5.
CC images of left breast in 67-year-old woman. BI-RADS breast density 4: (a) DM image and (b) MSR. In (a) in the para-areolar external area, a small oval-shaped lesion is visible with ill-defined and partially spiculated contours with architectural distortion, suspicious for malignant lesion [enhanced in the (c) corresponding digital zoom]; in the corresponding MSR image, the lesion presents benign findings and appears as a normal parenchymal area [see also the (d) corresponding digital zoom].
Patients were then classified according to BI-RADS breast density: 4 patients in category 1, 37 patients in category 2, 25 patients in category 3, and 5 patients in category 4. The BI-RADS categories 1 and 4 were not further analyzed as the data were insufficient.
Figure 6 shows the results of the analysis applied to BI-RADS density categories 2 and 3. MSR has very relevant results for both categories: while the images obtained at DM appear to be of difficult diagnostic evaluation (especially in heterogeneously dense breasts where the fitted-model line shows a flat behavior), MSR shows a good step-like fitting for category 2 (AIC values 17.50 for MSR and 49.37 for DM) and a higher diagnostic accuracy for category 3 (AIC values 27.84 for MSR and 36.56 for DM).
Fig. 6.
(a) Patients classified as BI-RADS density category 2 and (b) patients with BI-RADS density category 3. The abscissa shows the BI-RADS score evaluations while the ordinate shows the final diagnosis obtained with the reference standard (0 means negative and 1 means positive). The continuous lines on the graphs are the fitted-model line while the circles represent the single patient evaluations. The circles are spread on the graph for better visualization.
The ROC curve comparison for the two techniques is shown in Fig. 7. Table 3 reports the AUC values. DeLong et al.’s32 test was used to test the difference between the two ROC curves: the low -value () confirms the significant difference of the AUC between the two techniques.
Fig. 7.
ROC curves for MSR in solid-black line and for DM in dashed-red line. The dots refer to the data points used for building the curves.
Table 3.
AUC of the ROC curves: values, confidence interval, and qualitative interpretation according to Swets.31
| AUC | 99% Confidence interval | Interpretation | |
|---|---|---|---|
| DM | 0.76 | 0.52 to 0.81 | Fairly accurate |
| MSR | 0.96 | 0.86 to 1.00 | Highly accurate |
4. Discussion
The ESAK in DM increases with breast thickness, and for thicker breasts (i.e., thicker than 4 cm), selection of the spectral parameters leads to higher ESAK and ensures a good image quality. On the contrary, during MSR examinations the ESAK is almost constant for all thickness classes with a reduction of more than 50% for thick breast examinations (i.e., thicker than 4 cm). This dose reduction is due to the monochromatic beam, whose energy is tuned according to each patient’s breast thickness and glandularity (Table 2). A major decrease in the MGD (on average 43%) delivered during MSR examinations compared to DM was also found. The monochromatic SR beam is free from the low-energy components that are present in the conventional x-ray spectra. The SR beam energy was in the 17.5- to 21-keV range, where the relative weight of the photoelectric effect (mostly responsible for dose deposition) and Compton interactions change importantly even for a 0.5-keV energy step. Increasing the energy of the monochromatic beam, the relative weight of the Compton effect increases; thus, in general, the delivered doses decrease.30 In DM examinations, the low-energy component of the conventional x-ray spectrum is completely absorbed and does not contribute to the image but only to the delivered dose. Hence, the MGDs delivered in DM are higher than in MSR. It is worth noticing that a similar dose reduction would be observed also in the case of MSR operated in contact mode, i.e., with the detector in contact with the sample, as opposed to PPCI modality. However, the edge-enhancement typical of phase contrast would be lost, with a negative impact on the image quality.
The reduction of both ESAK and MGD with the monochromatic beam, keeping a relatively high image quality, could be further improved using digital detectors instead of screen-film systems.30 However, it is essential that the spatial resolution of the digital detector should be high enough to preserve the edge enhancement due to PPCI. In fact, in this study, the screen-film system was preferred to digital detectors, since it could ensure the necessary spatial resolution for detecting phase-contrast effects.11
The MGDs calculated according to Boone14 and Dance et al.15 are quite similar, albeit the use of Dance et al.’s15 coefficients leads to slightly higher MGD for thin breasts compared to Boone.14 This is probably due to the different skin layer model.
The diagnostic performances of the two techniques were investigated using different statistical analysis tools (i.e., GLM and AUC-ROC). The results point to a pre-eminence of MSR over DM. Despite the use of a screen-film system during MSR (instead of a digital system as used in DM), MSR shows better diagnostic accuracy than DM. In breast-density category 2 (i.e., scattered areas of fibroglandular tissue), all the healthy patients classified as BI-RADS 4 at DM were subsequently classified as BI-RADS 1, 2, or 3 at MSR with an MGD reduction of 35%. For the heterogeneously dense breast patients (i.e., breast-density category 3) classified as BI-RADS 4 or 5 at MSR, an average MGD reduction of 45% was reported compared to DM. This is a relevant result as malignancies in dense breasts are more difficult to identify and often require further clinical examinations.33 Therefore, for this density category, MSR allows for an improved diagnostic performance with a lower MGD if compared with DM.
The AUC-ROC analysis quantifies the significant difference between the two techniques: no overlap of the AUC-ROC values in 99% confidence interval and according to DeLong et al.’s32 test. The relatively low AUC of the DM ROC curve is due to the inclusion criteria of the study. Only those patients with questionable or suspicious abnormalities identified at DM and not clarified by US were enrolled. To further increase diagnostic performance, it is possible (in principle) to increase the MGD delivered to patients in MSR. This can be accomplished by decreasing the beam energy if screen-film systems are used or by increasing the exposure if a digital detector system is used.
The phase-contrast approach adopted in this study is based on the spatial coherence of the SR.1 The main characteristic of PPCI relies in the edge enhancement, which can provide the radiologists with a better visualization of the lesion contour, thus allowing a more effective diagnosis. In fact, the same approach can be applied to digital breast tomosynthesis by replacing the screen-film system with a high-resolution digital detector.34 More generally, PPCI can be applied to breast computed tomography, and actually, the application of the phase-contrast technique to tomographic breast imaging by means of digital detectors is under investigation in a few synchrotron facilities,35–38 including Elettra.37,38 An important advantage brought by digital PPCI is that the phase information can be decoupled from absorption by applying a phase-retrieval algorithm and, thus, increasing the contrast resolution.
Alongside this research with SR, a number of techniques are under development that aim to translate phase-contrast mammography away from the synchrotrons and eventually to the clinical environment.5 Although a PPCI x-ray mammography system based on a practical molybdenum x-ray tube (focal spot size 0.1 to 0.3 mm) has been successfully tested,39 the low degree of spatial coherence of conventional sources typically allows only a limited edge-enhancement effect. On the other hand, microfocus x-ray tubes allow strong edge enhancement in PPCI but may lack sufficient power for practical use.40 A possible solution has been recently proposed by operating a microfocus source at relatively high voltage (120 kVp) to reduce the breast absorption and consequently the dose and the exposure time.41
In general, two different approaches to the translation of phase contrast away from the synchrotrons can be outlined. The first goes through the development of new coherent x-ray sources.1 Examples include tabletop synchrotron light sources,42 where a high energy () electron beam is circulated hitting a wire or rod target, and electron-impact liquid-metal-jet hard x-ray sources, where a conventional energy ( to 100 keV) electron beam is focused on a jet of liquid metal, achieving a brilliance at least 1 order of magnitude higher than solid-anode microfocus x-ray tubes.43,44 In this context, very promising is also the development of Thomson-scattering sources45,46 that could lead to a possible transition to clinical practice, provided that a large field of view and sufficient flux are obtained. The second approach relies upon using different phase-contrast techniques. For example, the first mastectomy studies with conventional x-ray tube were performed by grating-based techniques,47 and recent results confirmed the potential of this approach.48–50 Promising results were also obtained with the so-called “edge illumination technique,” which combines good imaging quality with clinically acceptable doses,51–53 and has been successfully applied to tomosynthesis.54 Of course, researchers are simultaneously investigating both these approaches, combining innovative and more compact x-ray sources with different phase-contrast techniques, with the aim of finding the perfect combination that could make phase-contrast imaging available in the clinics.
5. Limitations
Our study had two main limitations. First, only those patients with questionable or suspicious abnormalities identified at DM and not clarified by US were included in the study. Focus on these problematic cases was recommended by the ethics committee, with the idea that the patients undergoing this clinical study could take advantage of the additional MSR examination. This restriction implied that the study population was characterized by a high prevalence of disease, which limited our ability to assess diagnostic performance. The second limitation is that the BI-RADS scores were assigned in consensus between two on-site radiologists who, according to the study design, read the DM images before referring the patient to MSR. As a result, they could have been biased in their assessments. Finally, for the same reason, the locations of the lesions were known prior to the MSR examinations, and this might have constituted an additional bias that facilitated improved diagnosis.
6. Conclusions
This work reports the conclusive analysis of the first clinical study of phase-contrast MSR. A total of 71 patients with a palpable breast mass or with questionable or suspicious breast abnormalities identified at combined DM and US underwent MSR. The delivered doses and the diagnostic performance of MSR were evaluated and compared with DM.
The application of tunable monochromatic beams allowed the acquisition of high-quality images13 with a substantial reduction of both ESAK and MGD. Due to the radiosensitive nature of the breast, achieving a dose reduction in mammography is extremely important to prevent the risk of inducing secondary cancers through exposure of the breast to radiation. The statistical analysis demonstrated that phase-contrast MSR has better diagnostic accuracy than DM in this group of patients.
On one hand, the peculiarity of the patients’ sample, which is characterized by a high disease prevalence, can be regarded as a limitation; on the other hand, the fact that phase-contrast MSR outperforms DM in these questionable or suspicious cases (where an accurate diagnosis is more difficult) suggests that phase-contrast mammography could be particularly helpful in problematic cases reducing the level of reader experience required for achieving an accurate diagnosis. This assumption will be investigated in further studies. Despite the limitations inherent in this clinical study, the significance of all the statistical analyses suggest that phase-contrast MSR combines both low-dose values and an accurate diagnostic performance, encouraging research on the translation of x-ray phase-contrast imaging into the clinics.
Acknowledgments
The authors duly recognize E. Castelli, retired professors of the University of Trieste, as a pioneer of this work. The authors thank the radiologists, D. Sanabor and E. Quaia, and the radiographers, R. Perabò and L. Venanzi, who enthusiastically cooperated during the mammographic examinations at the SYRMEP beam line. M. Borelli and L. Torelli are kindly acknowledged for the contribution to the statistical analysis discussion. The collaboration of both the radioprotection staff of Elettra and the Department of Radiology of Cattinara University Hospital is gratefully acknowledged. For this study, a grant was received from the Fondazione CRTrieste (Trieste, Italy).
Biography
Biographies for the authors are not available.
Disclosures
All authors discussed the results and contributed to the final version of the paper. The authors declare no competing financial interests.
Dedication
This work is dedicated to the memory of Ludovico Dalla Palma, professor of radiology, who recently passed away. He was a master of teaching and a reference for the Italian and international radiology community, that he served through leading positions in many European societies.
We will never forget him, his passion and his enthusiasm for radiology, and his vision of the interdisciplinary team in medical imaging. He pioneered this work that is the result of the collaboration between medical doctors and physicists.
References
- 1.Rigon L., “X-ray imaging with coherent sources,” in Comprehensive Biomedical Physics, Brahme A., Ed., Vol. 2, pp. 193–220, Amsterdam, Elsevier: (2014). [Google Scholar]
- 2.Nugent K. A., “Coherent methods in the X-ray sciences,” Adv. Phys. 59(4), 1–99 (2010). 10.1080/00018730903270926 [DOI] [Google Scholar]
- 3.Zhou S. A., Brahme A., “Development of phase-contrast x-ray imaging techniques and potential medical applications,” Phys. Med. 24, 129–148 (2008). 10.1016/j.ejmp.2008.05.006 [DOI] [PubMed] [Google Scholar]
- 4.Bravin A., Coan P., Suortti P., “X-ray phase-contrast imaging: form pre-clinical applications towards clinics,” Phys. Med. Biol. 58(1), R1–R35 (2013). 10.1088/0031-9155/58/1/R1 [DOI] [PubMed] [Google Scholar]
- 5.Coan P., Bravin A., Tromba G., “Phase-contrast x-ray imaging of the breast: recent developments towards clinics,” J. Phys. D Appl. Phys. 46(49), 494007 (2013). 10.1088/0022-3727/46/49/494007 [DOI] [Google Scholar]
- 6.Longo R., “Current studies and future perspective of synchrotron radiation imaging trials in human patients,” Nucl. Instrum. Methods Phys. Res. Sect. A 809, 13–22 (2016). 10.1016/j.nima.2015.10.110 [DOI] [Google Scholar]
- 7.ICRP 2007, “The 2007 recommendations of the International Commission on Radiological Protection,” Elsevier, Oxford: (2007). [Google Scholar]
- 8.Dance D. R., Sechopoulos I., “Dosimetry in x-ray-based breast imaging,” Phys. Med. Biol. 61, R271–R304 (2016). 10.1088/0031-9155/61/19/R271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arfelli F., et al. , “Low-dose phase contrast x-ray medical imaging,” Phys. Med. Biol. 43(10), 2845–2852 (1998). 10.1088/0031-9155/43/10/013 [DOI] [PubMed] [Google Scholar]
- 10.Chen R. C., et al. , “Measurement of the linear attenuation coefficients of breast tissues by synchrotron radiation computed tomography,” Phys. Med. Biol. 55(17), 4993–5005 (2010). 10.1088/0031-9155/55/17/008 [DOI] [PubMed] [Google Scholar]
- 11.Castelli E., et al. , “Mammography with synchrotron radiation: first clinical experience with phase-detection technique,” Radiology 259(3), 684–694 (2011). 10.1148/radiol.11100745 [DOI] [PubMed] [Google Scholar]
- 12.Dreossi D., et al. , “The mammography project at the SYRMEP beamline,” Eur. J. Radiol. 68(3), S58–S62 (2008). 10.1016/j.ejrad.2008.04.038 [DOI] [PubMed] [Google Scholar]
- 13.Longo R., et al. , “Clinical study in phase-contrast mammography: image-quality analysis,” Phil. Trans. R. Soc. A Math. Phys. Eng. Sci. 372, 20130025 (2014). 10.1098/rsta.2013.0025 [DOI] [PubMed] [Google Scholar]
- 14.Boone J. M., “Glandular breast dose for monoenergetic and high-energy x-ray beams: Monte Carlo assessment,” Radiology 213, 23–37 (1999). 10.1148/radiology.213.1.r99oc3923 [DOI] [PubMed] [Google Scholar]
- 15.Dance D. R., et al. , “Additional factors for the estimation of mean glandular breast dose using the UK mammography dosimetry protocol,” Phys. Med. Biol. 45(11), 3225–3240 (2000). 10.1088/0031-9155/45/11/308 [DOI] [PubMed] [Google Scholar]
- 16.Boone J. M., “Normalized glandular dose (DgN) coefficients for arbitrary x-ray spectra in mammography: computer-fit values of Monte Carlo derived data,” Med. Phys. 29(5), 869–875 (2002). 10.1118/1.1472499 [DOI] [PubMed] [Google Scholar]
- 17.Annette J. D., An Introduction to Generalized Linear Models, 2nd ed., Chapman & Hall/CRC Press LLC, Boca Raton, Florida: (2002). [Google Scholar]
- 18.American College of Radiology, Breast Imaging Reporting and Data System (BI-RADS), 4th ed., American College of Radiology, Reston, Virginia: (2003). [Google Scholar]
- 19.Burns D. T., Toni M. P., Bovi M., “Comparison of the air-kerma standards of the ENEA-INMRI and the BIPM in the low-energy x-ray range,” Rapport BIPM-99/11 (2002).
- 20.Longo R., et al. , “Phase contrast mammography with synchrotron radiation: physical aspects of the clinical trial,” Proc. SPIE 6510, 65100T (2007). 10.1117/12.708403 [DOI] [Google Scholar]
- 21.Perry N., et al. , European Guidelines for Quality Assurance in Breast Cancer Screening and Diagnosis, 4th ed., Office for the Official Publications of the European Communities, Luxembourg: (2006). [Google Scholar]
- 22.Lindsey J. K., Jones B., “Choosing among generalized linear model applied to medical data,” Stat. Med. 17, 59–68 (1998). 10.1002/(ISSN)1097-0258 [DOI] [PubMed] [Google Scholar]
- 23.O’Connell A. A., “Logistic regression models for ordinal response variables,” Sage Publications, Series/Number 07–146 (2006).
- 24.Akaike H., “Information theory and an extension of the maximum likelihood principle,” in 2nd Int. Symp. on Information Theory, Petrov B. N., Csaki F., Eds., Akadémia Kiado, Budapest, Hungary: (1973). [Google Scholar]
- 25.Yulei J., Charles E. M., “BI-RADS data should not be used to estimate ROC curves,” Radiology 256(1), 29–31 (2010). 10.1148/radiol.10091394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.R Core Team, “R: a language and environment for statistical computing,” R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org/ (2014). [Google Scholar]
- 27.Tobias S., et al. , “ROCR: visualizing classifier performance in R. Bioinformatics,” 21(20), 3940–3941 (2005). [DOI] [PubMed] [Google Scholar]
- 28.Fedon C., et al. , “Phase-contrast mammography with synchrotron radiation: dosimetric results,” Eur. Congr. Radiol. (2014). 10.1594/ecr2014/C-1253 [DOI] [Google Scholar]
- 29.Pesarin F., Salmaso L., Permutation Tests for Complex Data: Theory, Applications and Software, John Wiley & Sons Ltd., Chichester, United Kingdom: (2010). [Google Scholar]
- 30.Quai E., et al. , “First application of computed radiology to mammography with synchrotron radiation,” Radiol. Med. 118(1), 89–100 (2013). 10.1007/s11547-012-0847-1 [DOI] [PubMed] [Google Scholar]
- 31.Swets J. A., “Measuring the accuracy of diagnostic system,” Science 240(4857), 1285–1293 (1998). 10.1126/science.3287615 [DOI] [PubMed] [Google Scholar]
- 32.DeLong E. R., DeLong D. M., Clarke-Pearson D. L., “Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach,” Biometrics 44, 837–845 (1988). 10.2307/2531595 [DOI] [PubMed] [Google Scholar]
- 33.Boyd N. F., et al. , “Mammographic density and the risk and detection of breast cancer,” N. Engl. J. Med. 356(6), 227–236 (2007). 10.1056/NEJMoa062790 [DOI] [PubMed] [Google Scholar]
- 34.Szafraniec M. B., et al. , “Synchrotron based planar imaging and digital tomosynthesis of breast and biopsy phantoms using a CMOS active pixel sensor,” Phys. Med. 31(2), 192–198 (2015). 10.1016/j.ejmp.2014.11.003 [DOI] [PubMed] [Google Scholar]
- 35.Zhao Y., et al. , “High-resolution, low-dose phase contrast X-ray tomography for 3D diagnosis of human breast cancers,” Proc. Natl. Acad. Sci. U. S. A. 109, 18290–18294 (2012). 10.1073/pnas.1204460109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nesterets Y. I., et al. , “A feasibility study of X-ray phase-contrast mammographic tomography at the Imaging and Medical beamline of the Australian Synchrotron,” J. Synchrotron Radiat. 22, 1509–1523 (2015). 10.1107/S160057751501766X [DOI] [PubMed] [Google Scholar]
- 37.Longo R., et al. , “Towards breast tomography with synchrotron radiation at Elettra: first images,” Phys. Med. Biol. 61, 1634–1649 (2016). 10.1088/0031-9155/61/4/1634 [DOI] [PubMed] [Google Scholar]
- 38.Pacilè S., et al. , “Clinical application of low-dose phase contrast breast CT: methods for the optimization of the reconstruction workflow,” Biomed. Opt. Exp. 6(8), 3099–3112 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanaka T., et al. , “The first trial of phase contrast imaging for digital full-field mammography using a practical molybdenum x-ray tube,” Invest. Radiol. 40, 385–396 (2005). 10.1097/01.rli.0000165575.43381.48 [DOI] [PubMed] [Google Scholar]
- 40.Wilkins S. W., et al. , “Phase-contrast imaging using polychromatic hard x-rays,” Nature 384, 335–338 (1996). 10.1038/384335a0 [DOI] [Google Scholar]
- 41.Ghani M. U., et al. , “Detectability comparison between a high energy x-ray phase sensitive and mammography systems in imaging phantoms with varying glandular- adipose ratios,” Phys. Med. Biol. 62, 3523–3538 (2017). 10.1088/1361-6560/aa644b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamada N., et al. , “Tabletop synchrotron light source,” in Comprehensive Biomedical Physics, Brahme A., Ed., Vol. 8, pp. 43–65, Elsevier, Amsterdam: (2014). [Google Scholar]
- 43.Tuohimaa T., Otendal M., Hertz H. M., “Phase-contrast x-ray imaging with a liquid-metal-jet-anode microfocus source,” Appl. Phys. Lett. 91, 074104 (2007). 10.1063/1.2769760 [DOI] [Google Scholar]
- 44.Hertz H. M., et al. , “Electron-impact liquid-metal-jet hard x-ray sources,” in Comprehensive Biomedical Physics, Brahme A., Ed., Vol. 8, pp. 91–109, Elsevier, Amsterdam: (2014). [Google Scholar]
- 45.Oliva P., et al. , “Quantitative evaluation of single-shot inline phase contrast imaging using an inverse Compton x-ray source,” Appl. Phys. Lett. 97, 134104 (2010). 10.1063/1.3491430 [DOI] [Google Scholar]
- 46.De Caro L., et al. , “A theoretical study on phase-contrast mammography with Thomson-scattering x-ray sources,” Med. Phys. 36(10), 4644–4653 (2009). 10.1118/1.3213086 [DOI] [PubMed] [Google Scholar]
- 47.Stampanoni M., et al. , “The first analysis and clinical evaluation of native breast tissue using differential phase-contrast mammography,” Invest. Radiol. 46(12), 801–806 (2011). 10.1097/RLI.0b013e31822a585f [DOI] [PubMed] [Google Scholar]
- 48.Wang Z., et al. , “Non-invasive classifications of microcalcifications with phase-contrast X-ray mammography,” Nat. Commun. 5, 3797 (2014). 10.1038/ncomms4797 [DOI] [PubMed] [Google Scholar]
- 49.Grandl S., et al. , “Improved visualization of breast cancer features in multifocal carcinoma using phase-contrast and dark-field mammography: an ex vivo study,” Eur. Radiol. 25(12), 3659–3668 (2015). 10.1007/s00330-015-3773-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scherer K., et al. , “Toward clinically compatible phase-contrast mammography,” PLoS One 10(6), e0130776 (2015). 10.1371/journal.pone.0130776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Olivo A., et al. , “Low-dose phase contrast mammography with conventional x-ray sources,” Med. Phys. 40, 090701 (2013). 10.1118/1.4817480 [DOI] [PubMed] [Google Scholar]
- 52.Longo M., et al. , “A simplified edge illumination set-up for quantitative phase contrast mammography with synchrotron radiation at clinical doses,” Phys. Med. Biol. 60(3), N21–N34 (2015). 10.1088/0031-9155/60/3/N21 [DOI] [PubMed] [Google Scholar]
- 53.Diemoz P. C., et al. , “A method for high-energy, low-dose mammography using edge illumination x-ray phase-contrast imaging,” Phys. Med. Biol. 61(24), 8750–8761 (2016). 10.1088/1361-6560/61/24/8750 [DOI] [PubMed] [Google Scholar]
- 54.Szafraniec M. B., et al. , “Proof-of-concept demonstration of edge-illumination x-ray phase contrast imaging combined with tomosynthesis,” Phys. Med. Biol. 59(5), N1–N10 (2014). 10.1088/0031-9155/59/5/N1 [DOI] [PubMed] [Google Scholar]