Abstract
Aims
The aim of this study was to evaluate the association between vitamin D deficiency and risk of heart failure in elderly patients of cardiology outpatient clinics.
Methods and results
A cross‐sectional study with an analytical approach was employed. Clinical data were collected from the elderly from August 2015 to February 2016. The dependent variable was the risk of heart failure; the independent variable was vitamin D deficiency; and intervening factors were age, gender, education, ethnicity, hypertension, diabetes mellitus, hypothyroidism, renal failure, dementia, stroke, dyslipidaemia, depression, smoking, alcoholism, obesity, andropause, and cardiac arrhythmia. To analyse the association between vitamin D deficiency and risk of heart failure, we used the bivariate logistic analysis, followed by analysis through the multivariate logistic regression model. Of the 137 elderly, the study found the following: women (75.9%); overweight (48.2%); obese (30.6%); increase in the index waist/hip (88.3%); dyslipidaemia (94.2%) and hypertension (91.2%); coronary artery disease (35.0%); and 27.7% with cardiac arrhythmia or left ventricular hypertrophy. Sixty‐five per cent of the elderly were deficient in vitamin D. The risk of heart failure was significantly associated with vitamin D deficiency [odds ratio (OR): 12.19; 95% confidence interval (CI) = 4.23–35.16; P = 0.000], male gender (OR: 15.32; 95% CI = 3.39–69.20, P = 0.000), obesity (OR: 4.17; 95% CI = 1.36–12.81; P = 0.012), and cardiac arrhythmia (OR: 3.69; 95% CI = 1.23–11.11; P = 0.020).
Conclusions
There was a high prevalence of vitamin D deficiency in the elderly, and the evidence shows a strong association between vitamin D deficiency and increased risk of heart failure in this population.
Keywords: Vitamin D, Heart failure, Risk, Elderly
Introduction
With the improvement of care in the prevention of high blood pressure (hypertension), smoking, and dyslipidaemia, coupled with technological advances in the diagnosis and treatment of cardiovascular diseases (CVDs),1 there was a decline in the mortality rate due to CVD in the elderly; however, CVD is the leading cause of morbidity and mortality in the world and the most chronic disability.2
From the point of view of cardiovascular risk, epidemiological studies have demonstrated an association between chronic and degenerative diseases such as diabetes,3, 4 CVDs, and environmental (epigenetic) conditions in the embryonic or foetal phase.5, 6, 7 From the epidemiological studies conducted in England and Wales by Barker et al., The Foetal Programming Hypothesis or ‘Baker's Hypothesis’, finally, important review addressed the various environmental, hereditary, and genetic factors involving the interaction between the parents, placenta, and embryo in the genesis of diseases of adult life, citing epigenetic factors such as nutritional deficiencies and vitamins.8
Among the unfavourable epigenetic factors in pregnant women and related to CVDs, vitamin D deficiency is an important modifiable factor with the treatment, as demonstrated in a German study, where low levels of 25‐hydroxyvitamin D were related to low birth weight, prematurity, increased perinatal mortality, and decreased glucose tolerance leading to unfavourable renal and cardiovascular outcomes in adulthood.9
Nevertheless, this fact is still relevant because the common end stage of most heart diseases is heart failure (HF), which continues to have a high prevalence, represents an important public health problem and is the biggest cause of hospitalization among the elderly, hence the importance of finding new factors and risk scores for such a condition.10
Among the independent risk factors associated with HF are advanced age, the male gender, diabetes mellitus, pulse pressure, and acute myocardial infarction, found by Chen et al., 11 who evaluated 1749 elderly in a cohort study entitled ‘Established Populations for Epidemiologic Studies of the Elderly’. Data from the Multi‐Ethnic Study of Atherosclerosis study showed that diabetes and hypertension are the risk factors responsible for the higher incidence of HF among African‐Americans. In this same study, interleukin‐6 and C‐reactive protein, as well as macroalbuminuria, were independent predictors of the HF development.12
With the progress of age, the myocardium undergoes alterations in the extracellular matrix, with increased collagen (with a higher proportion of type I collagen in relation to type III), reduced elastin content, and increased fibronectin. The balance between production and degradation of the extracellular matrix by the matrix metalloproteinases (MMPs), and by tissue inhibitors of metalloproteinases, is altered, leading to increased activity of MMPs, which degrade extracellular matrix molecules such as collagen, fibronectin, and laminin.13
The myocardial alterations described in the elderly make the ventricular relaxation slower through increased left parietal rigidity, requiring more energy for relaxation, and causing a reduction of ventricular filling speed, which prolongs left ventricular filling. This characterizes a diastolic dysfunction, which is very prevalent and an important cause of HF in the elderly, usually preceding systolic dysfunction and left ventricular hypertrophy.14
Accumulated evidence states that studies on subclinical HF in the elderly are scarce and contain methodological errors, making it impossible to use the results in early diagnosis of this population. These studies also include patients that were selected from a population with its own peculiar characteristics, which cannot be generalized, and they investigate the prevalence of individual risk factors; however, they do not develop a score for evaluating cardiovascular risk.15
The Health Aging and Body Composition (ABC) HF enables to quantify the risk of HF in the elderly through clinical, laboratory, and demographic means and is of important relevance for the development of cardiovascular prevention strategies.15 The Health ABC HF score was tested in a 5‐year cohort study including 5335 patients with mean age of 72.6 ± 5.4 years and no history of coronary artery disease and maintained independence in performing activities of daily living. The model predicted HF in 364 of the 400 patients who developed the disease over the 5‐year period and is considered a useful tool for preventing HF.16, 17
There is strong evidence in the literature relating the functions of vitamin D and cardiovascular metabolism and in this way we investigated the association between vitamin D deficiency and risk of heart failure.18
Method
This is an analytical cross‐sectional epidemiological study that was carried out in the Care Center for the Elderly and the outpatient clinic of cardiology of the Hospital das Clínicas, both of the Federal University of Pernambuco (UFPE).
The population included in this study was selected from an analysis of medical records of people of 60 years of age and over who were assisted by one of these services in the period between August 2015 and February 2016.
With a prevalence of 80% vitamin D deficiency among the elderly, the sample size was of 137 medical records, corresponding to a power of test of 95%.
The study included elderly patients with medical records who were present for routine cardiological evaluations, were attended by the researcher in the cardiology clinic—by referral or spontaneous demand, regardless of gender and motor restrictions—and were willing to undergo additional tests for primary prevention of aging.
The exclusion criterion was the absence of registration of all the parameters of interest in the medical record of the elderly. If the individual was considered to be in a severe state with hospitalization indication, he or she was excluded from the study.
The variables of this study: independent variable ‐ Vitamin D deficiency, qualitative, ordinal, dichotomous, corresponding to the record in a serum concentration of 25‐hydroxyvitamin D lower than 30 ng/mL and absent ≥30 ng/mL.19 The analysis of the total concentration of 25‐hydroxyvitamin D serum was performed by the chemiluminescence method by the Architect Abbott®; intervening variables are present in Figure 1 , and dependent variable the risk of HF assessed by the Health ABC HF score is shown in Figure 2 .
Figure 1.
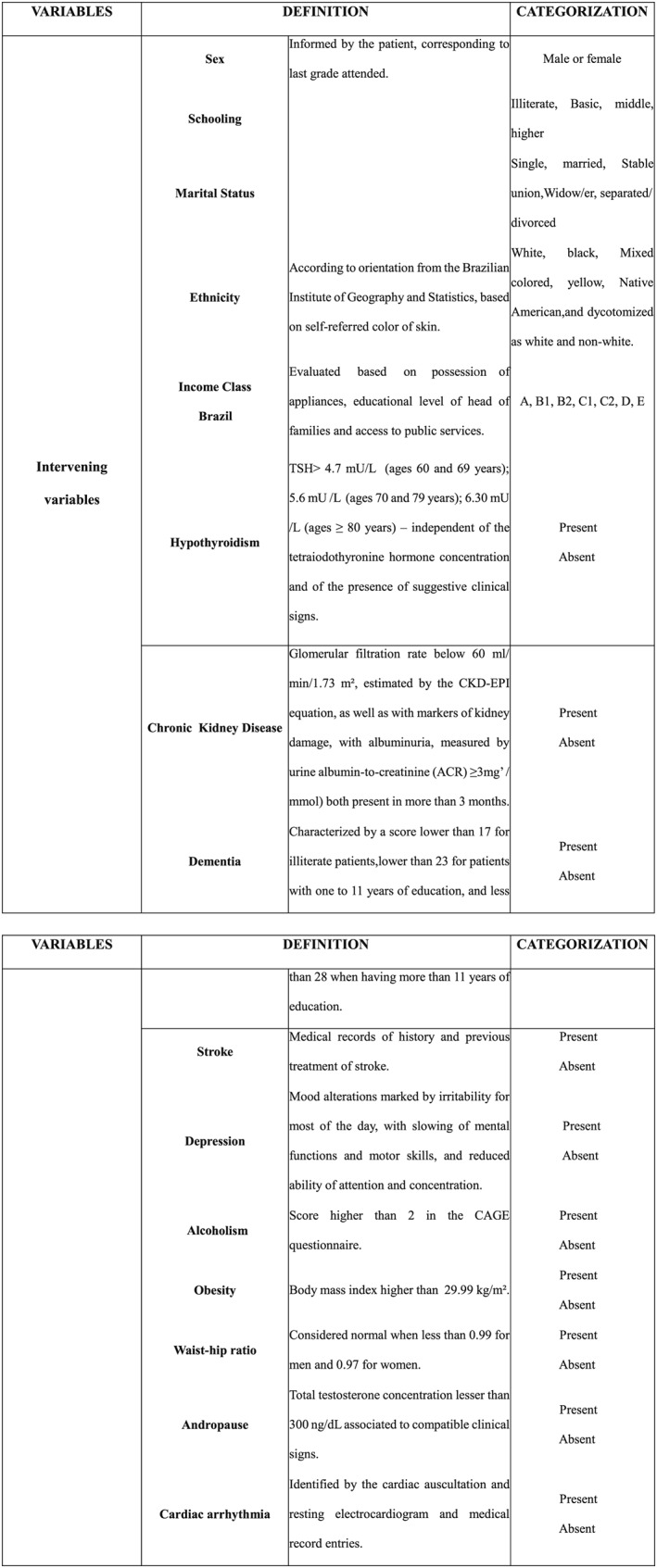
Intervening variables of the study, Hospital das Clínicas, Recife, 2015–16.
Figure 2.
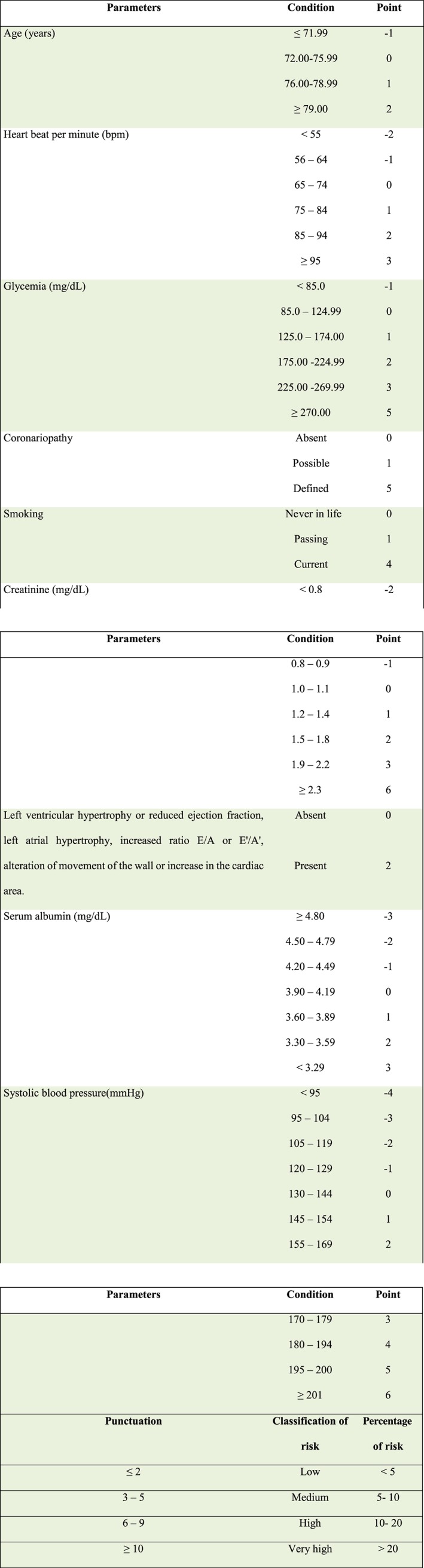
Dependent variables: punctuation attributed to each parameter of the elder, to calculate the risk for heart failure (ABC Heart Failure) and conversion of the ABC score classification and percentage of risk of heart failure.
The instruments used for data collection were the copy of the cardiological research protocol used in the cardiology outpatient clinic of the study sites, the Mini‐Mental State Examination, the Geriatric Depression Scale, and the Health ABC scale, to calculate risk of HF.
The Health ABC HF was constructed to assess the risk of HF.15
Accordingly, most studies considered deficient levels of 25‐hydroxyvitamin D lower than 30 ng/mL as Endocrine Society National Osteoporosis Foundation, International Osteoporosis Foundation, and American Geriatric Society and when less severe deficiency of 20 ng/mL.19 Other studies with results expressed in nmol/L classified the severe deficiency in the presence of levels below 25 nmol/L, moderate of 25 to 49.9 nmol/L, insufficiency of 51 to 75 nmol/L. In this study, deficiency/insufficiency of vitamin D or vitamin D deficiency was considered, as well as the presence of levels of 25‐hydroxyvitamin D below 30 ng/mL.
The database using the Epi7 program, version 7.1.5.2, was developed by the World Health Organization. The data were analysed with the program IBM/SPSS Statistical Package for Social Sciences, version 20.0. To calculate the risk of HF, we conducted the assessment by using the ABC HF and then proceeded to scoring the risk factors of each elderly, as shown in Figure 2 .
After determining the sum of the ABC punctuation, the classification of HF risk and the risk percentage evaluation were carried out, as shown in Figure 2 . For bivariate and multivariate analyses, risk was considered normal when <5% and high when greater than or equal to 5%, thus encompassing the classifications corresponding to medium, high, and very high risk.
In the bivariate analysis, to test the association among the variables, Pearson's chi‐square test and Fisher's exact test were used when necessary. As a criterion of entry of the independent and intervening variables in the multivariate logistic analysis, the cut‐off point was P‐value ≤0.20, provided by the bivariate logistic analysis. The logistic regression model used the stepwise method to select, from all independent and intervening variables, those which had significant influence on the risk of HF. In this method, the significance analysis of the variables in the model was carried out using the Wald test. All conclusions were made using the significance level of 5%.
This research study followed the determinations of Resolution 466 of 2012 from the National Council of Health, which regulates research involving human subjects and was approved by the Ethics Committee of the UFPE under CAAE, number 47317715.6.0000.5208. Data collection was authorized through consent letters from the Care Center for the Elderly and the Direction of the Hospital das Clínicas, UFPE.
Results
The sociodemographic characteristics of 137 elderly are shown in Table 1, while the clinical characteristics are shown in Table 2.
Table 1.
Distribution of sociodemographic characteristics of the elderly—NAI (Núcleo de Atenção ao Idoso), Hospital das Clínicas, Recife, 2015–16
| Sociodemographic characteristics | Categories | Frequency | Percentage |
|---|---|---|---|
| Sex | Male | 33 | 24.1 |
| Female | 104 | 75.9 | |
| Self‐referred skin colour | White | 50 | 36.5 |
| Mixed colour | 69 | 50.4 | |
| Black | 18 | 13.1 | |
| Marital status | Single | 10 | 7.3 |
| Married or stable union | 85 | 62.0 | |
| Widow/er | 37 | 27.0 | |
| Divorced or separated | 5 | 3.6 | |
| Education of the elder | Illiterate | 11 | 8.0 |
| Basic I | 39 | 28.5 | |
| Basic II | 41 | 29.9 | |
| High school | 30 | 21.9 | |
| Higher education | 16 | 11.7 | |
| Age classes (years) | 60–69 | 64 | 46.7 |
| 70–79 | 60 | 43.8 | |
| 80+ | 13 | 9.5 | |
| Economic class, Brazil | A | 2 | 1.5 |
| B1 | 2 | 1.5 | |
| B2 | 14 | 10.2 | |
| C1 | 34 | 24.8 | |
| C2 | 61 | 44.5 | |
| D and E | 24 | 17.5 |
Table 2.
Frequency distribution of risk factors for cardiovascular disease and previous diseases of the elderly—NAI (Núcleo de Atenção ao Idoso), Hospital das Clínicas, Recife, 2015–16
| Risk factors and previous diseases | Categories | Frequency | Percentage |
|---|---|---|---|
| Smoking | Never | 89 | 65.0 |
| Passed | 44 | 32.1 | |
| Current | 4 | 2.9 | |
| Risk of alcoholism (CAGE) | Yes | 4 | 2.9 |
| No | 133 | 97.1 | |
| Hormonal and metabolic measures | |||
| Obesity | Normal weight | 29 | 21.2 |
| Overweight | 66 | 48.2 | |
| Obese | 42 | 30.6 | |
| Waist–hip ratio | Normal | 16 | 11.7 |
| Absent | 121 | 88.3 | |
| Dyslipidaemia | Present | 129 | 94.2 |
| Absent | 8 | 5.8 | |
| Andropausea | Present | 24 | 72.7 |
| Absent | 9 | 27.3 | |
| Diabetes mellitus | Present | 52 | 38.0 |
| Absent | 85 | 62.0 | |
| Hypothyroidism | Present | 8 | 5.8 |
| Absent | 129 | 94.2 | |
| Mental state | |||
| Compatible score with dementia | Present | 31 | 22.6 |
| Absent | 106 | 77.4 | |
| Depression | Normal | 94 | 68.6 |
| Moderate | 41 | 29.9 | |
| Grave | 2 | 1.5 | |
| Diagnosed conditions | |||
| Systemic hypertension | Present | 125 | 91.2 |
| Absent | 12 | 8.8 | |
| Chronic renal failure | Present | 4 | 2.9 |
| Absent | 133 | 97.1 | |
| Previous stroke | Present | 9 | 6.6 |
| Absent | 128 | 93.4 | |
| Cardiac arrhythmia | Present | 38 | 27.7 |
| Absent | 99 | 72.3 | |
| Left ventricle hypertrophy or reduction of the fraction of left ventricular ejection | Present | 38 | 27.7 |
| Absent | 99 | 72.3 | |
| Coronary artery disease | Absent | 89 | 65.0 |
| Possible/defined | 48 | 35.0 |
Calculated percentage based on 33 male elders.
In relation to metabolic changes, most seniors in this study were overweight or obese, as well as presenting with waist–hip ratio above normal, high prevalence of dyslipidaemia, and hypertension, characterizing metabolic syndrome. Among the elderly men, prevalence of andropause was 72.7%.
As for the assessment of mental status, with regard to cognitive assessment, 22.6% of seniors had test score of the Mini‐Mental State Examination compatible with dementia, and 31.4% were in the range of depression degree with moderate or severe.
For the cardiovascular evaluation, we identified 91.2% as hypertensive, 35% with definite or possible coronary artery disease, and 27.7% with cardiac arrhythmia; the same percentages have been found for left ventricular hypertrophy (Table 2).
Figure 3 presents the frequency distribution of the vitamin D status of the participants, observing also 65% of individuals with hypovitaminosis. The graph considered the presence of vitamin deficiency or vitamin insufficiency, emphasizing that among these individuals, 62% have vitamin D deficiency.
Figure 3.
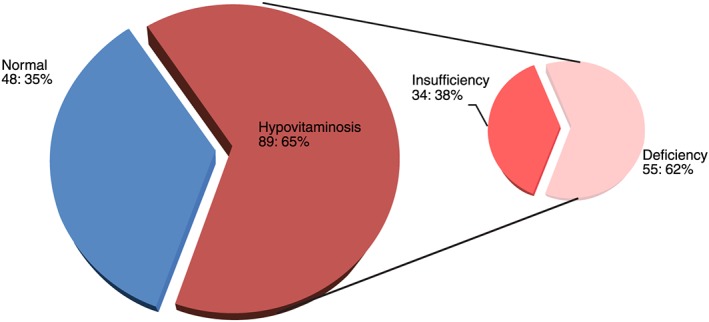
Distribution of vitamin D concentration classes for the elderly. The frequency distribution of the vitamin D condition of the participants is shown, with a percentage of 65% of these individuals with hypovitaminosis, considered as the presence of vitamin deficiency or insufficiency, among which 62% have vitamin D deficiency – NAI (Núcleo de Atenção ao Idoso), Hospital das Clínicas, Recife 2015‐16.
The results of the analysis of associations between intervening variables, as well as the independent variable, and the risk of HF are presented in Table 3. In the multivariate analysis, this risk can be explained by variables vitamin D, gender, obesity, and cardiac arrhythmia, which are considered explanatory variables.
Table 3.
Association of sociodemographic, clinical and laboratory variables with the risk of heart failure – NAI (Núcleo de Atenção ao Idoso), Hospital das Clinicas, Recife ‐2015‐16
| Variables | Categories | Heart failure risk | Bivariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|---|---|
| Extended | Normal | Relative risk (IC 95%) |
P‐value (Fisher's exact test) |
Relative risk (95% CI) |
P‐value (Wald test) |
||||
| N | % | N | % | ||||||
| Vitamin D | Poor / Insufficient | 70 | 89.7 | 19 | 32.2 | 18.42 | 0.00 | 12.19 | 0.00 |
| Enough | 8 | 10.3 | 40 | 67.8 | (7.39 – 45.89) | (4.23 – 35.16) | |||
| Sex | Male | 30 | 38.5 | 3 | 5.1 | 11.67 | 0.00 | 15.32 | 0.00 |
| Female | 48 | 61.5 | 56 | 94.9 | (3.35 – 40.63) | (3.39 – 69.21) | |||
| Education | Average / Superior | 58 | 74.4 | 33 | 55.9 | 2.28 | 0.03 | ||
| Illiterate / Elementary | 20 | 25.6 | 26 | 44.1 | (1.11 – 4.71) | ||||
| Marital status | Others | 35 | 44.9 | 17 | 28.8 | 2.01 | 0.07 | ||
| Married / Stable Union | 43 | 55.1 | 42 | 71.2 | (0.98 ‐4.17) | ||||
| Ethnicity | White | 31 | 39.7 | 19 | 32.2 | 1.39 | 0.38 | ||
| Nonwhite | 47 | 60.3 | 40 | 67.8 | (0.68 – 2.82) | ||||
| Age Group | 70 + | 49 | 62.8 | 24 | 40.7 | 2.46 | 0.01 | ||
| 60 ‐ 69 | 29 | 37.2 | 35 | 59.3 | (1.23 – 4.93) | ||||
| Class of Income Brazil | A e B | 12 | 15.4 | 6 | 10.2 | 1.61 | 0.45 | ||
| C, D e E | 66 | 84.6 | 53 | 89.8 | (0.66 – 4.56) | ||||
| Hypothyroidism | Present | 6 | 7.7 | 2 | 3.4 | 2.37 | 0.47 | ||
| Absent | 72 | 92.3 | 57 | 96.6 | (0.46 – 12.21) | ||||
| Renal insufficiency | Present | 4 | 5.1 | 0 | 0.0 | 0.13 | |||
| Absent | 74 | 94.9 | 59 | 100.0 | |||||
| Insanity | Present | 25 | 32.1 | 6 | 10.2 | 4.17 | 0.003 | ||
| Absent | 53 | 67.9 | 53 | 89.8 | (1.58 – 10.98) | ||||
| AVC | Present | 8 | 10.3 | 1 | 1.7 | 6.63 | 0.08 | ||
| Absent | 70 | 89.7 | 58 | 98.3 | (0.80 – 54.55) | ||||
| Depression | Moderate / Severe | 29 | 37.2 | 14 | 23.7 | 1.90 | 0.099 | ||
| Normal | 49 | 62.8 | 45 | 76.3 | (0.89 – 4.05) | ||||
| Alcohol consumption | Present | 3 | 3.8 | 1 | 1.7 | 2.32 | 0.63 | ||
| Absent | 75 | 96.2 | 58 | 98.3 | (0.23 – 22.89) | ||||
| Obesity | Normal/overweight | 35 | 44.9 | 7 | 11.9 | 6.05 | 0.00 | 4.18 | 0.012 |
| Obese | 43 | 55.1 | 52 | 88.1 | (2.44 – 14.97) | (1.36 – 12.81) | |||
| Waist‐hip ratio | Extended | 47 | 60.3 | 36 | 61.0 | 0.97 | 1.00 | ||
| Normal | 31 | 39.7 | 23 | 39.0 | (0.48 – 1.94) | ||||
| Andropausa | Present | 22 | 73.3 | 2 | 66.7 | 1.37 | 1.00 | ||
| Absent | 8 | 26.7 | 1 | 33.3 | (0.11 – 17.31) | ||||
| Arrhythmia heart | Present | 29 | 37.2 | 9 | 15.3 | 3.29 | 0.007 | 3.7 | 0.02 |
| Absent | 49 | 62.8 | 50 | 84.7 | (1.41 – 7.66) | (1.23 – 11.12) | |||
In this study, 78.7% of the vitamin D‐deficient elderly had increased risk of HF. In the multivariate analysis, the chance of having vitamin D deficiency for those who have risk of increased HF is 12.2 times higher than that who have normal levels of vitamin D.
In the multivariate analysis, the chance of increased risk for HF in the men of this group is 15.3 higher than that in women. The chances of HF for obese individuals present in this multivariate analysis are four times of those who are not obese. Those who have heart arrhythmia have a 3.6 higher chance of HF than those who never had such a condition.
Discussion
Although vitamin D deficiency is well documented in the elderly living in northern countries, a high percentage (65%) of vitamin D‐deficient elderly investigated in this study was identified. It is worth noting that this study, conducted in Recife, northeastern Brazil, where the tropical climate predominates most of the time, showed a prevalence of vitamin D deficiency in the elderly similar to the data from the third National Health and Nutrition Examination Survey.20 The prevalence of hypovitaminosis D in the present study was also lower than that reported for the French population, mainly for those above 60 years of age (80.3%),21 as well as for populations in northern India, near the Himalayas, where the prevalence of vitamin D deficiency was 83% and is associated with chronic stable angina.22 Consideration was given to the greater latitude of these countries, where winters are longer and people use clothes that cover almost the entire body, in contrast to the regions close to the equator, where there is predominantly sunny weather the entire year.
Important studies have addressed vitamin D deficiency associated with HF, such as the prospective study LURIC conducted on 3299 patients with CVD and which found high prevalence of myocardial dysfunction identified by the coronary angiography and ventriculography. These changes were associated with severe deficiency of vitamin D with risk of death three to five times higher than that with HF and sudden death over a period of 7 years. Calcidiol and calcitriol were both (P < 0.001) inversely related to deficit of left ventricular function, and also low calcidiol and calcitriol levels were associated with higher New York Heart Association class.23
The increased risk of HF in this study was present in more than half of the elderly and was significantly associated with vitamin D deficiency (increasing by 12.2 times the risk of HF) and may be due to inflammatory mechanisms, aside from the mechanisms already mentioned in this study regarding left ventricular hypertrophy. However, adequate concentrations of vitamin D can suppress the inflammatory response through inhibition of the prostaglandin and cyclooxygenase, overstimulation of anti‐inflammatory cytokines, reduction of cytokines induced by expression of adhesion molecules, reduction of extracellular matrix metalloproteinases, and inhibition of the release of the pro‐inflammatory factor NF‐kappa beta, which is associated with endothelial dysfunction.24 Calcitriol stimulates the tissue inhibitors of metalloproteinases, preventing excessive degradation of the extracellular matrix through the MMPs, particularly MMP‐2 and MMP‐9, which consequently lead to ventricular progressive remodelling, dilatation, and HF.25 Another inflammatory mechanism through which vitamin D deficiency acts, by increasing the risk of HF, is through the decrease of Treg cells and the increase of Th17,26 as well as through their polymorphisms VDR‐type BSMI receiver, which were related to left ventricular hypertrophy.27
In multivariate analysis, in addition to vitamin D deficiency, the risk of HF was only associated with male gender, obesity, and cardiac arrhythmias.
The high risk of HF presented by men in this work, which was confirmed after multivariate analysis, is consistent with the literature,28 also noting that this variable was significantly associated with vitamin D deficiency in relation to women. Of equal importance in this context of higher risk of HF in men is the strong association of andropause with vitamin D deficiency, which can justify this high risk, considering the positive relationship between vitamin D and testosterone levels, as reported in the literature.29 Especially important is the protective effect that testosterone plays on the myocardium, reducing the risk of heart disease and mortality.30 Thus, male seniors in this study are significantly at increased risk of vitamin D deficiency, HF, and higher prevalence of andropause.
In the evaluation of the elderly in this study, the association between HF and obesity proved to be significant in both bivariate and multivariate analyses, revealing concordance with literature data,31 with obesity being considered a risk factor for HF,32 as well as significantly associated with vitamin D deficiency, which also increased the risk for HF. However, studies have shown that although obesity increases the risk for HF, it does not confer increased risk of mortality in patients already with HF, yet it may provide a better prognosis, thus characterizing the obesity paradox.33
With reference to the analysis of the association between cardiac arrhythmia and increased risk of HF in the elderly, the present study showed a strong association in both bivariate and multivariate analyses, findings that they reported in the literature, where supraventricular tachyarrhythmia has a high prevalence in HF, especially Atrial Fibrillation (AF), which is the most common treatable arrhythmia, worsening the long‐term prognosis34 and characterized by the highest prevalence with aging and increasing the risk of HF and mortality.35
Regarding ventricular arrhythmias, considered independent markers of sudden cardiac death, they are associated with the degree of left ventricular dysfunction.36 They are also independent predictive factors, along with HF, of higher hospital mortality in octogenarian elderly submitted to percutaneous coronary intervention after suffering acute myocardial infarction.37
The results presented in this study show that in older people, disabilities/vitamin D insufficiency was strongly associated with increased risk of HF.
In the analysis of the results, only four patients were considered to have chronic kidney disease (CKD), with a glomerular filtration rate below 60 mL/min/1.73 m2, estimated by the CKD Epidemiology Collaboration equation, as well as with markers of kidney damage, with albuminuria (measured by urine albumin to creatinine ≥ 3 mg/mmol) present in more than 3 months,38 all included in the high‐risk group of HF. CKD, considered a risk factor for the development of vitamin D deficiency/insufficiency, is associated with increased morbimortality, mainly for CVDs, which are more prevalent in chronic kidney patients than in patients with normal renal function.39 In patients with HF, there was a prevalence of CKD of up to 29.6%,40 and renal insufficiency was considered a poor prognostic factor in these patients.41 It was higher in patients with HF with reduced ejection fraction.42
From a pioneering study by Dong et al.,43 it was reported that the expression of cyclooxygenase‐2 in the renal artery is elevated in oophorectomy‐induced oestrogen‐deficient rats, which in turn results in increased thromboxane‐prostanoid receptor expression, which reduces bioavailability of nitric oxide, leading to compromised endothelium‐dependent renovascular relaxation. Thus, chronic administration of calcitriol restores vascular function by normalizing the endothelial expression of cyclooxygenase‐2 and thromboxane‐prostanoid receptors, thus avoiding the reduction of nitric oxide production, which is induced by the activation of the thromboxane‐prostanoid receptor.
Hocher et al.,44 reviewing the effect of vitamin D on cardiovascular risk in post‐menopausal women, report that double‐blind randomized clinical trials with placebo are needed in order to provide definitive proof of the effects of vitamin D supplementation on cardiovascular risk in this population, emphasizing that these should answer the following questions: What is the ideal dose of calcitriol to reduce cardiovascular risk, taking into account the concentration–mortality curve of vitamin D in the form of U, according to meta‐analyzes of association45? If native vitamin D has the same effect as calcitriol, what consequences in the long run will interfere with renal and cardiovascular functions and mortality?
In secondary hyperparathyroidism, parathyroid hormone (PTH) is one of several ‘uremic factors’ implicated in the pathogenesis of cardiac abnormalities present in patients with chronic renal disease.46 PTH receptors are present in cardiomyocytes and endothelial cells, and PTH probably plays a role in cardiac remodelling, and therefore on the morphology and function of this organ. Left ventricular hypertrophy, dilatation, and dysfunction are common in patients with CKD, and about 50% and 70% of them present this alteration before and during dialysis, respectively.47
Loncar et al. evaluated secondary hyperparathyroidism and its prognostic impact in all‐cause mortality in elderly patients with HF, finding that hyperparathyroidism was highly prevalent in elderly men with this syndrome and was associated with lower survival. Patients with insufficiency cardiac and secondary hyperparathyroidism had more severe myocardial dysfunction compared with those with normal serum PTH, and determination of serum PTH levels provided additional value to NT‐proBNP for risk stratification in these patients.48 In a recent prospective study, correcting vitamin D deficiency in HD patients in associated with better secondary hyperparathyroidism control with lower doses of vitamin D analogues, as well as an improvement in inflammatory status.49
All patients in this study underwent a Doppler echocardiography; and left ventricular hypertrophy changes and reduced left ventricular function seen by this method were associated with vitamin D deficiency, demonstrating the importance of this examination, because the changes of the structure and cardiac function in elderly are usually subclinical, and these often precede the development of HF.17
Based on the evidence presented in this study, which is supported by the literature, the high percentage of elderly individuals with vitamin D deficiency and its consequences for increased risk of HF suggest a need of dosage recommendations for this vitamin, especially in primary healthcare services. The facility of quantifying vitamin D, the low cost of its supplementation, and the possibility of preventing and treating CVDs point to the need for more studies on the supplementation with vitamin D in prospective cohort, so that the conduct of supplementation is implanted with a solid base of evidence.
Among the limitations of this study, it is important to highlight its transversal nature, which prevents the establishment of causal relationships.
Conclusions
The risk of HF was present in more than half of the elderly and was strongly associated with vitamin D deficiency, with male gender and obesity characterized as risk factors for developing HF and cardiac arrhythmia, conferring a worse prognosis in failure heart, as well as being an independent marker of sudden cardiac death and being associated with more severe left ventricular dysfunction.
This research study proved that there is association between vitamin D deficiency and increased risk of HF in the elderly assisted in the UFPE cardiology clinics.
Conflict of interest
None declared.
Author contributions
Catarina Porto: Author and researcher of the master's degree in gerontology, responsible for data collection, interpretation of results, conclusion, and literature review.
Vanessa de Lima Silva: Co‐supervisor of the master's degree. Responsible for the methodology of the study.
Brivaldo Markman Filho: Responsible for the interpretation of data and results.
Vera Magalhães da Silveira: Responsible for the interpretation of the results.
João Soares Brito da Luz: Responsible for blood sample collection and performing the laboratory tests.
Porto, C. M. , Silva, V. D. L. , da Luz, J. S. B. , Filho, B. M. , and da Silveira, V. M. (2018) Association between vitamin D deficiency and heart failure risk in the elderly. ESC Heart Failure, 5: 63–74. doi: 10.1002/ehf2.12198.
References
- 1. Bovet P, Paccaud F. Cardiovascular disease and the changing face of global public health: a focus on low and middle income countries. Public Health Rev 2013; 33: 397–415. [Google Scholar]
- 2. Smith SC Jr, Collins A, Ferrari R, Holmes DR Jr, Logstrup S, McGhie DV, Ralston J, Sacco RL, Stam H, Taubert K, Wood DA, Zoghbi WA. Our time: a call to save preventable death from cardiovascular disease (heart disease and stroke). Glob Heart 2013; 7: 297–305. [DOI] [PubMed] [Google Scholar]
- 3. Hales CN, Barker DJ. The thrifty phenotype hypothesis: type 2 diabetes. Br Med Bull 2001; 60: 5. [DOI] [PubMed] [Google Scholar]
- 4. Gillman MW. Prenatal famine and developmental origins of type 2 diabetes. Lancet Diabetes Endocrinol 2015; 3: 751–752. [DOI] [PubMed] [Google Scholar]
- 5. Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 1986; 1: 1077–1081. [DOI] [PubMed] [Google Scholar]
- 6. Osmond C, Barker DJP, Winter PD, Fall CHD, Simmonds SJ. Early growth and death from cardiovascular disease in women. BMJ 1993; 307: 1519–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Olsen J. David Barker (1938–2013)—a giant in reproductive epidemiology. Acta Obstet Gynecol Scand 2014; 93: 1077–1080. [DOI] [PubMed] [Google Scholar]
- 8. Reichetzeder C, Dwi Putra SE, Li J, Hocher B. Developmental origins of disease—crisis precipitates change. Cell Physiol Biochem 2016; 39: 919–938. [DOI] [PubMed] [Google Scholar]
- 9. Reichetzeder C, Chen H, Föller M, Slowinski T, Li J, Chen YP, Lang F, Hocher B. Maternal vitamin D deficiency and fetal programming—lessons learned from humans and mice. Kidney Blood Press Res 2014; 39: 315–329. [DOI] [PubMed] [Google Scholar]
- 10. Schmidt MI, Duncan BB, Chor D, Azevedo e Silva G, Menezes AM, Monteiro CA, Barreto SM. Chronic non‐communicable diseases in Brazil: burden and current challenges. Lancet 2011; 377: 1949–1961. [DOI] [PubMed] [Google Scholar]
- 11. Chen YT, Vaccarino V, Williams CS, Butler J, Berkman LF, Krumholz HM. Risk factors for heart failure in the elderly: a prospective community‐based study. Am J Med 1999; 106: 605. [DOI] [PubMed] [Google Scholar]
- 12. Bahrami H. Novel metabolic risk factors for incident heart failure and their relationship with obesity: the MESA (Multi‐Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol 2008; 51: 1775–1783. [DOI] [PubMed] [Google Scholar]
- 13. Chiao YA, Ramirez TA, Zamilpa R, Okoronkwo SM, Dai Q, Zhang J, Jin YF, Lindsey ML. Matrix metalloproteinase‐9 suppression attenuates myocardial fibrosis and diastolic dysfunction in ageing mice. Cardiovasc Res 2012; 96: 444–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Strait B, Lakatta EG. Aging‐associated cardiovascular changes and their relationship to heart failure. Heart Fail Clin 2012; 8: 143–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Butler J, Kalogeropoulos A, Georgiopoulou V, Belue R, Rodondi N, Garcia M, Bauer DC, Satterfield S, Smith AL, Vaccarino V, Newman AB, Harris TB, Wilson PW, Kritchevsky SB. Incident heart failure prediction in the elderly: the health ABC heart failure score. Circ Heart Fail 2008; 1: 125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kalogeropoulos A, Psaty BM, Vasan RS, Georgiopoulou V, Smith AL, Smith NL, Kritchevsky SB, Wilson PW, Newman AB, Harris TB, Butler J. Validation of the health ABC heart failure model for incident heart failure risk prediction: the cardiovascular health study. Circ Heart Fail 2010; 3: 495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kalogeropoulos AP, Georgiopoulou VV, de Filippi CR, Gottdiener JS, Butler J. Echocardiography, natriuretic peptides, and risk for incident heart failure in older adults: the cardiovascular health study. JACC Cardiovasc Imaging 2012; 5: 131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pareja‐Galeano H, Alis R, Sanchis‐Gomar F, Lucia A, Emanuele E. Vitamin D, precocious acute myocardial infarction, and exceptional longevity. Int J Cardiol 2015; 199: 405–406. [DOI] [PubMed] [Google Scholar]
- 19. Holick MF, Binkley NC, Bischoff‐Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metabol 2011; 96: 1911–1930. [DOI] [PubMed] [Google Scholar]
- 20. Martins D, Wolf M, Pan D, Zadshir A, Tareen N, Thadhani R, Felsenfeld A, Levine B, Mehrotra R, Norris K. Prevalence of cardiovascular risk factors and the serum levels of 25‐hydroxyvitamin D in the United States: data from the third National Health and Nutrition Examination Survey. Arch Int Med 2007; 167: 1159–1165. [DOI] [PubMed] [Google Scholar]
- 21. Souberbielle JC, Massart C, Brailly‐Tabard S, Cavalier E, Chanson P. Prevalence and determinants of vitamin D deficiency in healthy French adults: the VARIETE study. Endocrine 2016; 53: 543–550. [DOI] [PubMed] [Google Scholar]
- 22. Raina AH, Allai MS, Shah ZA, Changal KH, Raina MA, Bhat FA. Association of low levels of vitamin D with chronic stable angina: a prospective case–control study. N Am J Med Sci 2016; 8: 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pilz S, März W, Wellnitz B, Seelhorst U, Fahrleitner‐Pammer A, Dimai HP, Boehm BO, Dobnig H. Association of vitamin D deficiency with heart failure and sudden cardiac death in a large cross‐sectional study of patients referred for coronary angiography. J Clin Endocrinol Metab 2008; 93: 3927–3935. [DOI] [PubMed] [Google Scholar]
- 24. Kunadian V, Ford GA, Bawamia B, Qiu W, Manson JE. Vitamin D deficiency and coronary artery disease: a review of the evidence. Am Heart J 2014; 167: 283–291. [DOI] [PubMed] [Google Scholar]
- 25. Polyakova V, Loeffler I, Hein S, Miyagawa S, Piotrowska I, Dammer S, Risteli J, Schaper J, Kostin S. Fibrosis in end stage human heart failure: severe changes in collagens metabolism and MMP/TIMP profiles. Int J Cardiol 2011; 151: 18–33. [DOI] [PubMed] [Google Scholar]
- 26. Ma YH, Zhou YL, Yue CY, Zhang GH, Deng L, Xie GH, Xu WP, Shen LS. Vitamin D deficiency contributes to the reduction and impaired function of naïve CD45RA+ regulatory T cell in chronic heart failure. J Immunol Res 2015; 2015: 547697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Santoro D, Giorgia G, Angela A, Riccardo I, Guido B, Vincenzo S, Michele B, Daniela C. Vitamin D receptor gene polymorphism and left ventricular hypertrophy in chronic kidney disease. Forum Nutr 2014; 6: 1029–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. American Heart Association . Heart Disease and Stroke Statistics 2009 Update. Dallas: AHA; 2009. [Google Scholar]
- 29. Nimptsch K, Platz EA, Willett WC, Giovannucci E. Association between plasma 25‐OH vitamin D and testosterone levels in men. Clin Endocrinol (Oxf) 2012; 77: 106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Morgentaler A, Miner MM, Caliber M, Guay AT, Khera M, Traish AM. Testosterone therapy and cardiovascular risk: advances and controversies. Mayo Clin Proc 2015; 90: 224–251. [DOI] [PubMed] [Google Scholar]
- 31. Patel SA, Ali MK, Alam D, Yan LL, Levitt NS, Bernabe‐Ortiz A, Checkley W, Wu Y, Irazola V, Gutierrez L, Rubinstein A, Shivashankar R, Li X, Miranda JJ, Chowdhury MA, Siddiquee AT, Gaziano TA, Kadir MM, Prabhakaran D. Obesity and its relation with diabetes and hypertension: a cross‐sectional study across 4 geographical regions. Glob Heart 2016; 11: 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Ramachandran SV. Obesity and the risk of heart failure. N Engl J Med 2002; 347: 305–313. [DOI] [PubMed] [Google Scholar]
- 33. Piepoli MF, Corrà U, Veglia F, Bonomi A, Salvioni E, Cattadori G, Metra M, Lombardi C, Sinagra G, Limongelli G, Raimondo R, Re F, Magrì D, Belardinelli R, Parati G, Minà C, Scardovi AB, Guazzi M, Cicoira M, Scrutinio D, Di Lenarda A, Bussotti M, Frigerio M, Correale M, Villani GQ, Paolillo S, Passino C, Agostoni P. Exercise tolerance can explain the obesity paradox in patients with systolic heart failure: data from the MECKI Score Research Group. Eur J Heart Fail 2016; 18: 545–553. [DOI] [PubMed] [Google Scholar]
- 34. Jessup M, Abraham WT, Casey DE, Feldman AM, Francis GS, Ganiats TG, Konstam MA, Mancini DM, Rahko PS, Silver MA, Stevenson LW, Yancy CW. Focused update: ACCF/AHA guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines developed in collaboration with the International Society for Heart and Lung Transplantation. J Am Coll Cardiol 2009; 53: 1343–1382. [DOI] [PubMed] [Google Scholar]
- 35. Patel NJ, Deshmukh A, Pant S, Singh V, Patel N, Arora S, Shah N, Chothani A, Savani GT, Mehta K, Parikh V, Rathod A, Badheka AO, Lafferty J, Kowalski M, Mehta JL, Mitrani RD, Viles‐Gonzalez JF, Paydak H. Contemporary trends of hospitalization for atrial fibrillation in the United States, 2000 through 2010: implications for healthcare planning. Circulation 2014; 129: 2371–2379. [DOI] [PubMed] [Google Scholar]
- 36. Singh BN. Significance and control of cardiac arrhythmias in patients with congestive cardiac failure. Heart Fail Rev 2002; 7: 285–300. [DOI] [PubMed] [Google Scholar]
- 37. Ipek G, Kurmus O, Koseoglu C, Onuk T, Gungor B, Kirbas O, Karatas MB, Keskin M, Betul Borklu E, Hayiroglu MI, Tanik O, Oz A, Bolca O. Predictors of in‐hospital mortality in octogenarian patients who underwent primary percutaneous coronary intervention after ST segment elevated myocardial infarction. Geriatr Gerontol Int 2016; 18: 120–173. [DOI] [PubMed] [Google Scholar]
- 38. KDIGO . 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3: 1–150. [DOI] [PubMed] [Google Scholar]
- 39. Nigwekar SU, Thadhani R. Vitamin D receptor activation: cardiovascular and renal implications. Kidney Int 2013; 3: 427–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Reis FJ, Fernandes AM, Bitencourt AG, Neves FB, Kuwano AY, França VH, Macedo CR, da Cruz CG, Sahade V, Aras R Jr. Prevalence of anemia and renal insufficiency in non‐hospitalized patients with heart failure. Arq Bras Cardiol 2009; 93: 268–274. [DOI] [PubMed] [Google Scholar]
- 41. Feola M, Lombardo E, Taglieri C, Piccolo S, Vado A. Plasma BNP and renal failure as prognostic factors of mid‐term clinical outcome in congestive heart failure patients. Int J Cardiol 2011; 149: 114–115. [DOI] [PubMed] [Google Scholar]
- 42. Villacorta H, Saenz‐Tello BF, Santos EB, Steffen R, Wiefels C, Lima LC, Sales AL, Soares P, Mesquita ET. Renal dysfunction and anemia in patients with heart failure with reduced versus normal ejection fraction. Arq Bras Cardiol 2010; 94: 357–363. [DOI] [PubMed] [Google Scholar]
- 43. Dong J, Wong SL, Lau CW, Liu J, Wang YX, Dan He Z, Fai Ng C, Yu Chen Z, Yao X, Xu A, Ni X, Wang H, Huang Y. Calcitriol restores the renovascular function in estrogen‐deficient mice by decreasing regulation of cyclooxygenase‐2 and the thromboxane‐prostanoid receptor. Kidney Int 2013; 84: 54–63. [DOI] [PubMed] [Google Scholar]
- 44. Hocher B, Reichetzeder C. Vitamin D and cardiovascular risk in postmenopausal women: how to translate preclinical evidence into benefit for patients. Kidney Int 2013; 84: 9–11. [DOI] [PubMed] [Google Scholar]
- 45. Melamed ML, Michos ED, Post W, Astor B. 25‐Hydroxyvitamin D and mortality risk in the general population. Arch Intern Med 2008; 168: 1629–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Neves CL, Custódio MR, Neves KR, Moysés e Vanda Jorgetti RMA. Secondary hyperparathyroidism and cardiovascular disease in chronic kidney disease. J Bras Nefrol 2008; 30: 18–22. [Google Scholar]
- 47. Parfrey PS, Foley RN, Harnett JD, Kent GM, Murray DC, Barre PE. Outcome and risk factors for left ventricular disorders in chronic uremia. Nephrol Dial Transplant 1996; 11: 1277–1285. [PubMed] [Google Scholar]
- 48. Loncar G, Bozic B, Cvetinovic N. Secondary hyperparathyroidism prevalence and prognostic role in elderly males with heart failure. J Endocrinol Invest 2017; 40: 297. [DOI] [PubMed] [Google Scholar]
- 49. Ojeda López R, Esquivias de Motta E, Carmona A, García Montemayor V, Berdud I, Martín Malo A, Aljama García P. Correction of 25‐OH‐vitamin D deficiency improves control of secondary hyperparathyroidism an reduces the inflammation in stable haemodialysis patients. Nefrología 2017. pii: S0211‐6995(17)30131‐5. 10.1016/j.nefro.2017.05.008 [DOI] [PubMed] [Google Scholar]


