Abstract
Outcome measures used for the clinical evaluation of patients with acute heart failure differ between studies and may neither adequately address the characteristic presenting symptoms and signs nor reflect the pathophysiological processes involved. In‐hospital worsening of heart failure (WHF) is associated with poor outcomes and thus a potential endpoint conveying clinically meaningful prognostic information.
Current definitions of WHF are based on the combination of worsening symptoms and signs and the intensification of treatment during admission. Definitions vary across studies and do not fully account for baseline therapy or circumstances in which there is failure to respond to treatment. Further, there are limited data to inform healthcare professionals as to which patients are most at risk of developing in‐hospital WHF.
In this opinion piece, we review the definitions for WHF used in recent and ongoing clinical trials and propose a novel definition, which captures failure to respond to treatment as well as clinical worsening (deterioration of symptoms and signs) of the patient's condition. Such a definition, applied consistently across studies, would help clarify the characteristics of patients likely to develop in‐hospital WHF, allow comparative assessments of the effectiveness of interventions, and help guide appropriate patient management in order to improve outcomes.
Keywords: Worsening heart failure, Acute heart failure, Endpoints
Introduction
Chronic heart failure (HF) is frequently punctuated by episodes of decompensation, often necessitating hospitalization [acute HF (AHF)].1 Frequent HF‐related hospitalizations are a considerable burden for patients, carers, and healthcare providers.2, 3 Furthermore, patients hospitalized with AHF have a poor prognosis, with high rates of in‐hospital and post‐discharge mortality, and a high risk of early readmission.4
The early identification of patients with AHF at high risk of poor outcomes may be an important step in improving prognosis. To assess therapeutic interventions, robust markers of disease severity that reflect AHF pathophysiology and which are related to longer term outcomes would be beneficial.4 Biomarkers, such as N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP), are likely to be useful in this regard; high levels are associated with poor prognosis, and thus interventions guided by changes in N terminal pro brain natriuretic peptide may be helpful.5, 6, 7, 8, 9 Trials designed with sufficient power are needed to confirm the benefits of this approach.10 In addition to biomarkers, in‐hospital worsening HF (in‐hospital WHF) has been proposed as a potentially useful endpoint for identifying high‐risk patients with AHF.11
Endpoints should be consistent, reproducible, sensitive (that is, responsive to change in clinical state), and clinically meaningful, to allow comparisons across studies and be useful to physicians. However, to date, in‐hospital WHF has been defined in multiple ways, including (i) a failure to respond to standard therapy (although ‘standard therapy’ is not always well defined);12, 13 (ii) a gradual deterioration of AHF despite therapy;11 and (iii) the abrupt occurrence of WHF events, such as the development of pulmonary oedema.11, 12, 13 Table 1 shows the varying definitions of WHF used in a number of intervention trials in AHF.11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 Studies defining in‐hospital WHF as worsening symptoms and signs of HF and a need for additional intravenous (i.v.) or mechanical therapy suggest that 10–25% of patients admitted with AHF develop WHF,11, 14 although incidence rates of up to 42% have been reported.4, 14 Conversely, results from the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure (ASCEND‐HF), the largest trial of patients with AHF to date (n = 7141), showed that only approximately 5% of patients experienced in‐hospital WHF,20 which perhaps reflects the more rigorous definition used in the study [≥1 sign, symptom, or radiological evidence of new, persistent, or worsening AHF requiring addition of a new i.v. therapy (inotrope or vasodilator) or mechanical support during index hospitalization] (Table 1).
Table 1.
Comparison of in‐hospital worsening heart failure (WHF) definitions and outcomes in clinical studies
| Study | Definition | Endpoints associated with the occurrence of in‐hospital WHF |
|---|---|---|
| VERITAS12, 13 (Tezosentan) | WHF occurring during the index hospitalization was defined as development of pulmonary oedema, cardiogenic shock, or other evidence of WHF, or failure of the patient's HF condition to improve with treatment (treatment failure), requiring the initiation, re‐institution, or increase in i.v. therapy for HF and/or the implementation of mechanical circulatory or ventilator support and/or the use of ultrafiltration, haemofiltration, or haemodialysis within 7 days post‐randomization |
|
| Tel‐Aviv medical centre study14 (Standard of care) | Unresolved or recurrent signs/symptoms of HF that required an increase in or institution of i.v. HF‐specific therapy, or mechanical ventilatory or circulatory support within 7 days of admission |
|
| PROTECT pilot11 (Rolofylline) |
|
|
| Pre‐RELAX‐AHF15 (Serelaxin) | WHF signs/symptoms necessitating intensification or re‐institution of i.v. or mechanical HF treatment from admission to Day 5 |
|
| DOSE16, 21 (Furosemide) | Worsening or persistent HF requiring rescue therapy (loop diuretic, thiazide, i.v. vasoactive agents, ultrafiltration, or mechanical or respiratory support) from randomization to Day 3a |
|
| RELAX‐AHF17 (Serelaxin) | WHF signs/symptoms necessitating intensification or re‐institution of i.v. or mechanical HF treatment from admission to Day 5 |
|
| ROSE‐AHF18 (Low‐dose dopamine, low‐dose nesiritide) | Worsening or persistent HF requiring rescue therapy (i.v. vasoactive agents, ultrafiltration, or mechanical or respiratory support) |
|
| ADHERE registry19 (Standard of care) | Need for escalation of therapy (inotropic medications or i.v. vasodilator) >12 h after hospital presentation, transfer to the intensive care unit, or advanced medical therapy after the first inpatient day |
|
| ASCEND‐HF20 (Nesiritide) | Presence of ≥1 symptom or sign of new, persistent, or worsening acute HF requiring additional i.v. therapy (inotropic or vasodilator) or mechanical support during hospitalization (early WHF) |
|
ADHERE, Acute Decompensated Heart Failure National Registry; AHF, acute heart failure; ASCEND‐HF, Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure; CV, cardiovascular; DOSE, Diuretic Optimization Strategies Evaluation; HF, heart failure; i.v., intravenous; PROTECT, Effects of Rolofylline, a New Adenosine A1 Receptor Antagonist on Symptoms, Renal Function, and Outcomes in Patients with Acute Heart Failure; RELAX‐AHF, serelaxin in acute heart failure; ROSE‐AHF, Renal Optimization Strategies Evaluation in Acute Heart Failure; VERITAS, Value of Endothelin Receptor Inhibition with Tezosentan in Acute heart failure Studies; WHF, worsening heart failure.
Please refer to supplementary material available online at http://www.nejm.org/action/showSupplements?doi=10.1056%2FNEJMoa1005419&viewType=Popup&viewClass=Suppl.
Although definitions vary across trials, the occurrence of in‐hospital WHF consistently predicts poorer outcomes in patients with AHF and is associated with increased healthcare utilization.11, 12, 14, 15, 17, 19, 22 These studies have raised interest in the validity of in‐hospital WHF as a useful endpoint for evaluating new AHF therapies and in clinical practice.4, 11 If the concept is validated, reducing the occurrence of in‐hospital WHF might itself become a worthwhile treatment target; however, as authors, we had some scepticism as to the clinical utility of this outcome in the absence of a standard definition. Therefore, in this review, which gives our opinion rather than being data‐driven, we critically appraise the current criteria used to define in‐hospital WHF and identify areas of strength and weakness. We propose a novel, clinically relevant definition that includes both failure to respond to treatment [poorly responsive HF (PRHF)] and clinical worsening during hospital admission. We believe this new definition will help to assess the effectiveness of interventions in AHF and facilitate comparisons between them.
What is ‘in‐hospital worsening heart failure’ and how has the definition of the endpoint evolved?
One challenge with establishing a consistent definition of ‘in‐hospital WHF’ is that patients present in different ways, and thus, ‘worsening’ may vary with clinical context. Clinical trials often emphasize inclusion criteria that depend on classifications such as ‘decompensation of chronic HF’ or ‘de novo AHF’, but the emergency physician places much greater emphasis on the symptoms and signs with which the patient presents. Characterizing an AHF episode is important for both in‐hospital and post‐hospital patient management, as it determines which patients are at higher risk of poorer outcomes and, consequently, helps guide therapy. However, full characterization at admission is challenging because of multiple and overlapping clinical presentations.23, 24
The definition of any proposed new clinical endpoint should be precise and, to date, in‐hospital WHF has neither been tightly nor consistently defined.11, 14, 19 Some definitions of in‐hospital WHF are not strictly ‘worsening’. For example, if a patient simply responds slowly to i.v. diuretic, leading to an increase in the dosage, can that really be defined as worsening? Further, if a patient simply fails to respond to diuretic and remains oedematous, that is surely ‘failure to respond’ rather than ‘worsening’. Even when such concepts have been reflected as part of the inclusion criteria for a clinical trial, all is not as it seems. For example, the inclusion requirements for the Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARRESS‐HF) required that patients have worsening renal function and persistent oedema, implying that they either had WHF or HF that was failing to respond.25 However, the patients allocated to conventional therapy in the trial subsequently improved, suggesting that they were neither failing to respond nor worsening.25
In 2010, a standard definition for in‐hospital WHF was proposed by Cotter et al., incorporating both worsening symptoms and signs of HF and the need to institute or increase specific HF‐related i.v. or mechanical therapy.11 In‐hospital WHF was deemed to have occurred if the following were observed: (i) acute pulmonary congestion or oedema during the time since the previous evaluation; and/or (ii) worsening in investigator‐reported and/or patient‐reported symptoms and signs of AHF since the previous evaluation; and (iii) the events identified in (i) and/or (ii) occurred concomitantly with instituting or increasing the dose of i.v. therapy or mechanical support (ventilatory or circulatory).11 This proposal is largely consistent with endpoints used in several clinical studies (Table 1).
The growing awareness of in‐hospital WHF as a clinical endpoint has been strengthened by its inclusion in a European Society of Cardiology consensus document on clinical trial endpoints for HF.26 The document highlights that (i) WHF during the index hospitalization may be used as a component of the primary composite endpoint; (ii) in‐hospital WHF may capture important, non‐fatal events occurring prior to discharge; (iii) consistent capturing of in‐hospital WHF events across trial sites is important; and (iv) specific criteria for the diagnosis must be pre‐defined.26
In line with these recommendations, several ongoing and recently completed AHF trials include in‐hospital WHF as a prospectively defined endpoint (Table 2, 27, 28, 29, 30, 31, 32, 33, 34, 35); this is likely to contribute to its validation and increase physician familiarity with it as a clinical concept, as well as provide data on the effectiveness of interventions in preventing WHF.
Table 2.
In‐hospital worsening heart failure (WHF) definitions in ongoing and recently completed acute heart failure clinical trials
| Trial | Definition |
|---|---|
| RELAX‐AHF‐2;27 RELAX‐AHF‐EU;28 RELAX‐AHF‐ASIA29 (Data on File, Novartis Pharma AG) (Serelaxin) |
Worsening signs and/or symptoms of HF through Day 5 post‐randomization requiring intensification of i.v. therapy for HF or mechanical ventilatory, renal, or circulatory support Such treatment can include the institution or up‐titration of i.v. diuretic, i.v. nitrates, or any other i.v. medication for HF, or institution of mechanical support such as mechanical ventilation, ultrafiltration, haemodialysis, intra‐aortic balloon pump, or ventricular assist device. |
| TRUE‐AHF30, 31 (Ularitide) | Persistent or WHF requiring an intervention (initiation or intensification of i.v. therapy, circulatory or ventilatory mechanical support, surgical intervention, ultrafiltration, haemofiltration, or dialysis) within 48 h post‐start of study drug |
| BLAST‐AHF32, 33, 35 (TRV027a) | WHF requiring intensification of therapy including i.v. diuretic, i.v. nitrates, or other medications for HF, or institution of mechanical or ventilator support |
| ATOMIC‐AHF34 (Omecamtiv mecarbil) | Worsening symptoms or signs of HF necessitating initiation, reinstitution, or intensification of i.v. or mechanical HF treatment |
BLAST‐AHF, Biased Ligand of the Angiotensin Receptor Study in Acute Heart Failure; HF, heart failure; i.v., intravenous; RELAX‐AHF, serelaxin in acute heart failure; TRUE‐AHF, TRial of Ularitide's Efficacy and safety in patients with Acute Heart Failure; WHF, worsening heart failure.
A novel biased ligand of the angiotensin‐2 type 1 receptor.
The definition of in‐hospital WHF in current and recently completed trials is still not uniform. For example, in the Serelaxin in AHF trials (RELAX‐AHF‐2, RELAX‐AHF‐EU, and RELAX‐AHF‐ASIA),27, 28, 29 in‐hospital WHF is defined as worsening signs and/or symptoms of HF between admission and Day 5 that require intensification of i.v. therapy for HF or mechanical ventilatory, renal, or circulatory support (Data on File, Novartis Pharma AG). In‐hospital WHF in the TRial of Ularitide's Efficacy and safety in patients with Acute Heart Failure (TRUE‐AHF), ularitide vs. placebo is defined differently, requiring an intervention (initiation or intensification of i.v. therapy, circulatory or ventilatory mechanical support, surgical intervention, ultrafiltration, haemofiltration, or dialysis) for persistent or worsening HF up to 48 h after the start of study drug.30, 31
What is the clinical relevance of in‐hospital worsening heart failure?
Our current ability to predict the occurrence of in‐hospital WHF is limited. WHF may be more likely in the context of poor renal function on admission, being associated with low renal perfusion pressure and marked neurohormonal activation.12 Measures indicative of worse haemodynamic function on admission and 6 h later, such as decreased cardiac power output and increased mean arterial pressure, are associated with a higher rate of in‐hospital WHF by Day 7.4 Other admission characteristics predictive of in‐hospital WHF include increased respiratory rate (which may indicate pulmonary oedema), hyponatraemia, low oxygen saturation, high troponin levels, and the need for more aggressive management, such as mechanical ventilation, i.v. diuretics, and inotropes.12, 14, 15 There is consistent evidence from several studies that in‐hospital WHF is associated with adverse prognosis—the study definitions of in‐hospital WHF used, together with key outcome data, are shown in Table 1.
Value of Endothelin Receptor Inhibition with Tezosentan in Acute Heart Failure Studies
In Value of Endothelin Receptor Inhibition with Tezosentan in Acute Heart Failure Studies (VERITAS) (n = 1347), the incidence of WHF within 7 days of admission was one of two co‐primary endpoints, combined with death. WHF could occur during the index hospitalization (in‐hospital WHF) or post‐discharge (re‐hospitalization for WHF). WHF by Day 7 during the index admission occurred in 26% of patients and, after adjustment for baseline characteristics, was associated with a mean increase in length of hospital stay (LOS) of 4.33 days (P < 0.0001) and a hazard ratio (HR) for 30‐day HF readmission or death of 2.43 (P < 0.0001; Figure 1A , 12). Any WHF within 7 days (during the index hospitalization or a re‐hospitalization) was associated with an HR for 90‐day mortality of 2.57 (P < 0.0001).12, 13 However, in‐hospital WHF was only modestly influenced by patients' baseline characteristics (such as severity of HF and end‐organ impairment) during the first day of admission, underlining the difficulty physicians encounter in early identification of patients at risk of poor outcomes. Cotter et al. suggest that current clinical assessments may not be sufficiently tailored to capture patients at risk of developing in‐hospital WHF.12
Figure 1.
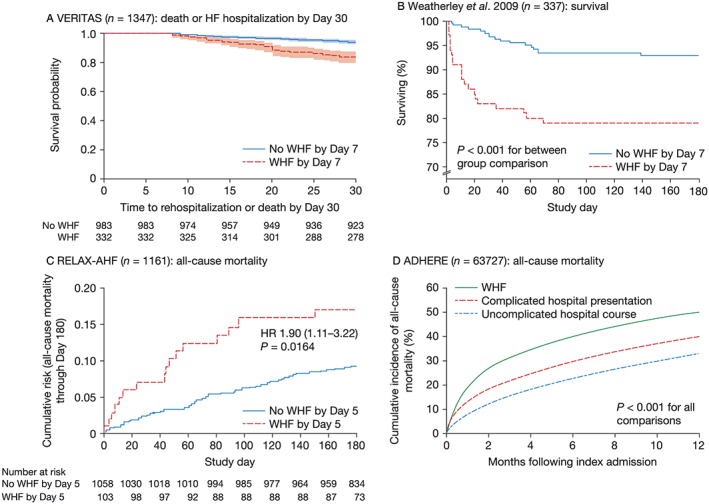
In‐hospital worsening heart failure (WHF) and reported outcomes in recent clinical trials. Kaplan–Meier estimates of (A) cumulative risk of death or heart failure (HF) hospitalization through Day 30 by occurrence of WHF through Day 7, shown with 95% confidence intervals and number of subjects at risk in VERITAS.12 (B) Survival for patients with and without WHF within 7 days of admission.14 (C) Risk of all‐cause mortality through Day 180 shown with number of subjects at risk in RELAX‐AHF.17 (D) Observed all‐cause mortality up to 1 year post‐index hospitalization in ADHERE.19 ADHERE, Acute Decompensated Heart Failure National Registry; HR, hazard ratio; RELAX‐AHF, serelaxin in acute heart failure; VERITAS, Value of Endothelin Receptor Inhibition with Tezosentan in Acute heart failure Studies. Figure (A) reproduced with permission from Cotter et al.11; Figure (B) reproduced with permission from Weatherley et al.14; Figure (C) reproduced with permission from Metra et al.17; Figure (D) reproduced with permission from DeVore et al.19
Tel‐Aviv medical centre study
Weatherley et al. assessed the association of in‐hospital WHF with prognosis over 6 month follow‐up in 337 patients admitted with AHF to a regional medical centre in Tel Aviv.14 The 29% of patients who experienced in‐hospital WHF during the early stages of admission had higher 30‐day (17.2% vs. 3.4%) and 6‐month mortality rates (21.2% vs. 7.1%; Figure 1B , 14) compared with those with no in‐hospital WHF.14 Factors associated with short‐term adverse outcomes in AHF, such as systolic blood pressure, were not predictive of in‐hospital WHF. The authors concluded that WHF is an independent predictor of adverse outcomes.14 However, caution is needed, as the results and conclusions derived from this study could be attributed to the imprecise definition used for in‐hospital WHF (Table 1). For example, if a patient did not respond to 10 mg daily of furosemide and the dosage was increased to 80 mg twice daily, then the patient would have had ‘in‐hospital WHF’. We suggest that it is important for any definition to allow for failure to respond to, or the need to augment, standard therapy; however, standard therapy should be appropriate before intensification can genuinely be regarded as an adverse event.
The Effects of Rolofylline, a New Adenosine A1 Receptor Antagonist on Symptoms, Renal Function, and Outcomes in Patients with Acute Heart Failure study
The investigators in the Effects of Rolofylline, a New Adenosine A1 Receptor Antagonist on Symptoms, Renal Function, and Outcomes in Patients with Acute Heart Failure study (PROTECT pilot study) recorded prospectively the symptoms and signs of HF and diuretic administration in patients with AHF and renal impairment for 7 days following hospital admission. In a post hoc analysis, patients were categorized into three groups: (i) physician‐determined WHF (n = 29); (ii) increased i.v. diuretic therapy without physician‐determined WHF (n = 61); (iii) neither (i) nor (ii) (n = 211).11
Physician‐determined WHF was associated with slower resolution of dyspnoea (Figure 2 , 11) and longer LOS (13.8 vs. 10.5 vs. 9.3 days in Groups 1, 2, and 3; P < 0.05 for Groups 2 or 3 vs. Group 1). Death and cardiovascular (CV) or renal readmission rates at 60 days were also higher in the physician‐determined WHF group (49.7% vs. 37.3% vs. 19.5% in Groups 1, 2, and 3, respectively). The authors concluded that physician‐determined in‐hospital WHF may be an indicator of short‐term risk and lack of treatment efficacy in AHF.11 However, if failure of breathlessness to resolve rapidly leads the physician to define the patient as ‘worsening,’ it is then scarcely surprising that WHF predicts ‘slower resolution of dyspnoea’ and a longer hospitalization.
Figure 2.
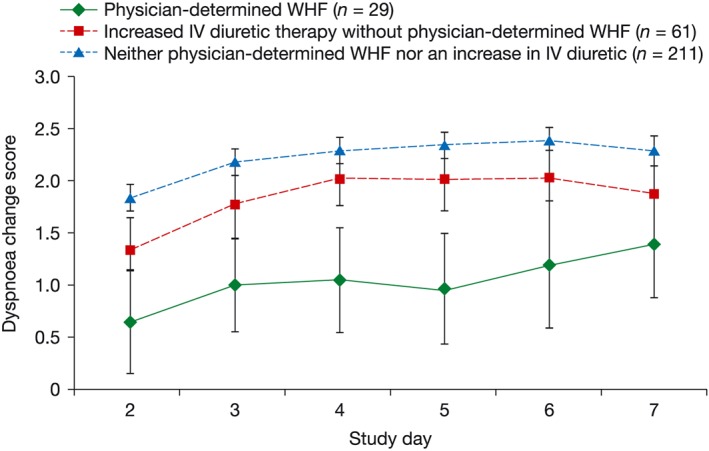
Change in dyspnoea score by study day in patients with/without in‐hospital worsening heart failure (WHF). Mean patient‐reported dyspnoea change score over time on a Likert scale, in patients with and without in‐hospital WHF.11 i.v., intravenous. Figure reproduced with permission: Cotter G et al.11. © 2009 Karger AG, Basel.
A recent post hoc analysis of data from PROTECT assessed the impact of time of WHF occurrence on patient outcomes. In total, 12.7% of patients experienced WHF, of whom 47.9% experienced WHF ‘early’ (Days 2–3) and 52.1% experienced WHF ‘late’ (Days 4–7). In‐hospital WHF was associated with a trend towards greater 60‐day mortality or CV/renal hospitalization [HR 1.26; 95% confidence interval (CI): 0.99–1.60; P = 0.063] and greater 180‐day mortality (HR 1.77; 95% CI: 1.33–2.34; P < 0.001) compared with patients who did not experience WHF. However, there was no association between the time in‐hospital WHF occurred and patient outcomes.36
The Serelaxin in Acute Heart Failure studies
In the preliminary Serelaxin in Acute Heart Failure study (Pre‐RELAX‐AHF) (n = 232), approximately 16% of patients experienced in‐hospital WHF by Day 5, which was associated with longer LOS (15.9 vs. 10.1 days; P = 0.0006), reduced number of days alive and out of hospital up to Day 60 (36.2 vs. 48.7 days; P = 0.0004), and higher 180‐day CV mortality (16.5% vs. 3.0%; P = 0.0045) compared with patients who did not experience WHF by Day 5.15 A post hoc analysis of RELAX‐AHF (n = 1161) showed that patients who developed in‐hospital WHF by Day 5 (~10% of total population) had greater 180‐day all‐cause mortality compared with patients who did not (Figure 1 C).17
Pooled analysis of the PROTECT and RELAX‐AHF studies
In a pooled analysis using data from 3691 patients in the PROTECT pilot, PROTECT, Pre‐RELAX‐AHF, and RELAX‐AHF studies, 12.4% of patients died or experienced in‐hospital WHF by Day 5. After multivariable adjustment (which excluded the patients who died by Day 5), patients with, rather than without, in‐hospital WHF had a mean increase in LOS of 5.2 days and increased risks of 180‐day all‐cause mortality and the composite endpoint of 60‐day HF re‐hospitalization/renal failure hospitalization or CV death (all P < 0.0001).37
The Acute Decompensated Heart Failure National Registry
A retrospective, observational analysis of data from the Acute Decompensated Heart Failure National Registry (ADHERE) examined outcomes for patients with in‐hospital WHF (n = 7032), defined as the need for escalation of therapy at least 12 h after hospital presentation. Patients with WHF were compared with (i) those with an uncomplicated hospital course (n = 41 334) and (ii) those with a ‘complicated hospital presentation’ in whom initiation or escalation of therapy was required within 12 h, or admission to the intensive care unit or advanced medical therapy was required on the first day (n = 15 361).19 Patients with in‐hospital WHF had a higher mortality rate (Figure 1 D19), a higher all‐cause readmission rate, and higher Medicare payments at 30 days and 1 year than patients with either an uncomplicated hospital course or a complicated presentation (all P < 0.001). Interestingly, some patients with a complicated presentation would have met the definition for in‐hospital WHF in other studies yet had better outcomes than those defined as having in‐hospital WHF in the ADHERE analysis. These data suggest that the timing of the event defining WHF may be important. However, the authors were unable to distinguish between escalation of therapy due to in‐hospital WHF and treatment of a concomitant condition, such as pneumonia, and thus, ADHERE might have captured patients with clinical presentations unrelated to HF.19
The Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure
A retrospective analysis of the ASCEND‐HF trial (n = 7141) assessed the prevalence, clinical characteristics, and outcomes of patients with in‐hospital WHF (defined as ≥1 sign or symptom of new, persistent, or worsening acute HF requiring addition of therapy or mechanical support).20 Overall, WHF occurred in 5% of patients in the study. Patients who experienced in‐hospital WHF had higher 30‐day mortality (29.7% vs. 2.5%), higher 30‐day mortality or HF re‐hospitalization (42.7% vs. 8.1%), and higher 180‐day mortality (41.5% vs. 11.3%) rates than patients who did not develop WHF (all P < 0.0001). Similar to the post hoc analysis of the PROTECT study,36 patients with WHF had poorer outcomes irrespective of whether WHF occurred ‘early’ (Days 1–3) or ‘late’ (Day 4–discharge).20
Efficacy of Vasopressin Antagonism in Heart Failure: outcome Study with Tolvaptan
A post hoc analysis of data from the Efficacy of Vasopressin Antagonism in Heart Failure: Outcome Study with Tolvaptan (EVEREST) trial (n = 3214) suggested that higher average daily loop diuretic dosage (>120 mg) during hospitalization was associated with worse clinical status, all‐cause mortality, and increased risk of in‐hospital WHF compared with patients receiving lower average daily dosages of loop diuretics (61–120 and 60 mg).38 It is possible that persistent HF requiring high‐dose diuretics reflects diuretic resistance rather than WHF.
Efficacy and safety of ularitide for the treatment of acute decompensated heart failure
The TRUE‐AHF trial assessed ularitide vs. placebo in patients with AHF (n = 2157).30, 31, 39 The primary endpoints were CV mortality and a hierarchical clinical composite endpoint, which included persistent or WHF requiring an intervention (initiation or intensification of i.v. therapy, circulatory or ventilatory mechanical support, surgical intervention, ultrafiltration, haemofiltration, or dialysis) within 48 h post‐start of study drug.30, 31, 39 Ularitide was associated with fewer in‐hospital worsening HF events at 48 h, compared with placebo (55 vs. 87; P = 0.005).39 At 15 month mean follow‐up, there was no difference in CV mortality between ularitide and placebo (HR 1.03; 95% CI: 0.85–1.25).39 There were also no statistically significant differences between ularitide and placebo in 30‐day readmissions for HF, death from any cause, or CV hospitalization at 6 months.39, 40
Future directions: towards an agreed definition of in‐hospital worsening heart failure and its implementation in clinical practice
The in‐hospital WHF definitions used in trials to date have combined several elements: slow response to treatment, failure to respond to treatment, and actual worsening despite treatment. Because of varying trial inclusion criteria, there is a lack of precision in understanding who the patients are, how severe their fluid retention was at inclusion, and how these patients responded to treatment. For example, there may well be a difference between a patient ‘failing to respond’ when they start with modest ankle oedema vs. anasarca.
For the clinical concept of in‐hospital WHF to be helpful, it must apply to a recognizable group of patients who are receiving an appropriate level of baseline therapy. We suggest that it most readily applies to patients presenting predominantly with fluid retention; those patients presenting with acute pulmonary oedema have a life‐threatening medical emergency that already requires intensive therapy, and the notion of ‘worsening’ in this circumstance is difficult to define with precision. Furthermore, it is not surprising that patients who fail to respond to treatment (or deteriorate despite treatment) are likely to spend longer in hospital and have a worse prognosis, thus leading to a circular argument. There should also be some consequence to arriving at a clinical diagnosis of in‐hospital WHF: what are the implications for therapy? Can we, and should we, do anything differently for these patients? Finally, it may be prudent to consider whether adjudication of in‐hospital WHF events in clinical trials should be blinded to help delineate those with and without events in a systematic and fair way.
We propose that in‐hospital WHF should encompass the notion of failing to respond, as well as clinical worsening and be termed ‘poorly responsive’ HF. The concept of PRHF can be applied to patients meeting the following criteria: (i) evidence of sufficiently severe fluid retention that, in the treating physician's view, the patient requires i.v. diuretic therapy and (ii) the patient is already receiving treatment with reasonable baseline therapy (e.g. i.v. furosemide 80 mg twice daily bolus or 10 mg/h infusion for at least 24 h). In patients meeting both these criteria, PRHF can then be defined as either the failure to have a clinically meaningful, or perhaps even specified (for example, 1.5 L of fluid in 24 h), net loss of fluid and/or the need (in the treating physician's opinion) for intensification of diuretic therapy, ventilatory support, or mechanical support.
Further research is needed to determine the benefits of utilizing PRHF, including robust testing in large datasets. It is also not completely clear if the previously mentioned diuretic doses are the most appropriate to define reasonable baseline therapy. Future studies should examine the possibility of using data acquired during a patient's admission to hospital to predict the likelihood that he or she will develop PRHF. The consequences if PRHF develops should also be investigated, and whether physicians can prevent PRHF or alter the patient's prognosis by managing differently those at risk. Perhaps, intensive follow‐up of these patients could mitigate the risk of future adverse events? Ultimately, future studies would need to determine whether there is a clinical benefit in diagnosing patients with PRHF.
Conclusions
Patients with in‐hospital WHF are at greater risk of adverse outcomes, including longer LOS, increased rate of re‐hospitalization, and higher mortality, compared with patients who do not experience in‐hospital WHF (Figure 3 ),11, 12, 14, 15, 17, 19, 20, 36, 37and thus, in‐hospital WHF is a clinically important endpoint in patients with AHF. Despite this, there is no universal, precise definition of in‐hospital WHF, which limits its value in defining a population at risk of poor outcomes, our ability to compare interventions, and the widespread practical application of in‐hospital WHF as an endpoint. A refined definition, which includes patients who ‘fail to respond’ as well as those with worsening symptoms and signs, is needed. We hope that the consistent application of PRHF in future clinical studies will help identify ‘at risk’ patients, forming the basis for testing management strategies directed at improving outcomes for these high‐risk patients.
Figure 3.
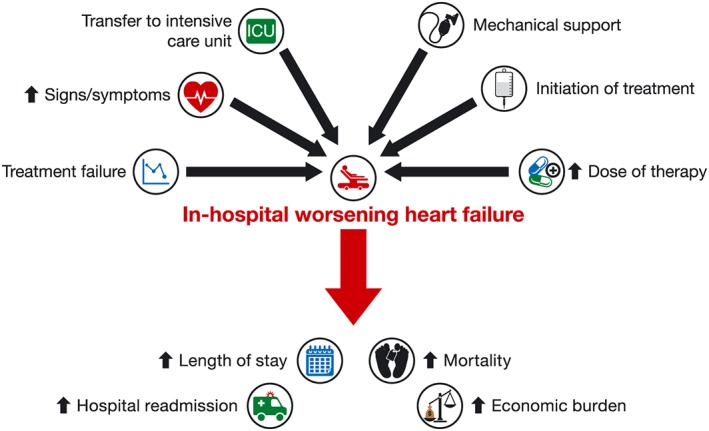
Irrespective of definition, in‐hospital worsening heart failure (WHF) is associated with poor outcomes for patients with heart failure. The definition of in‐hospital WHF varies across studies but is consistently associated with adverse outcomes including longer length of stay, increased rate of re‐hospitalization, higher mortality rate, and greater economic burden.11, 12, 14, 15, 17, 19, 37
Conflict of interest
A.C. has received financial support for travel to meetings from Servier and has accepted fees for service on behalf of his department from Novartis. M.C. is an employee of Novartis Pharmaceuticals UK Limited. T.M. has received honoraria for speaking (Novartis, Vifor, ZS Pharma) and has received an unrestricted educational grant from Novartis. I.S. has received fees from Novartis for participation in advisory boards and educational events.
Funding
The writing/editorial support and open access publication charges for this article were funded by Novartis Pharma AG, Basel, Switzerland. The sponsor reviewed the initial draft and subsequent versions of the manuscript for own data accuracy and for proprietary evaluation.
Acknowledgements
The authors were assisted in the preparation of the manuscript by Jane Murphy (CircleScience, an Ashfield company, part of UDG Healthcare plc), who was funded by Novartis Pharma AG, Basel, Switzerland. A.C. had full access to all the data in this article and takes responsibility for the integrity of the data and accuracy of the data analysis.
Clark, A. L. , Cherif, M. , McDonagh, T. A. , and Squire, I. B. (2018) In‐hospital worsening heart failure: a clinically relevant endpoint?. ESC Heart Failure, 5: 9–18. doi: 10.1002/ehf2.12195.
References
- 1. Gheorghiade M, De LL, Fonarow GC, Filippatos G, Metra M, Francis GS. Pathophysiologic targets in the early phase of acute heart failure syndromes. Am J Cardiol 2005; 96: 11G–17G. [DOI] [PubMed] [Google Scholar]
- 2. Kristensen SL, Kober L, Jhund PS, Solomon SD, Kjekshus J, McKelvie RS, Zile MR, Granger CB, Wikstrand J, Komajda M, Carson PE, Pfeffer MA, Swedberg K, Wedel H, Yusuf S, McMurray JJ. International geographic variation in event rates in trials of heart failure with preserved and reduced ejection fraction. Circulation 2015; 131: 43–53. [DOI] [PubMed] [Google Scholar]
- 3. Neumann T, Biermann J, Erbel R, Neumann A, Wasem J, Ertl G, Dietz R. Heart failure: the commonest reason for hospital admission in Germany: medical and economic perspectives. Dtsch Arztebl Int 2009; 106: 269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Torre‐Amione G, Milo‐Cotter O, Kaluski E, Perchenet L, Kobrin I, Frey A, Rund MM, Weatherley BD, Cotter G. Early worsening heart failure in patients admitted for acute heart failure: time course, hemodynamic predictors, and outcome. J Card Fail 2009; 15: 639–644. [DOI] [PubMed] [Google Scholar]
- 5. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012; 33: 1787–1847. [DOI] [PubMed] [Google Scholar]
- 6. Ewald B, Ewald D, Thakkinstian A, Attia J. Meta‐analysis of B type natriuretic peptide and N‐terminal pro B natriuretic peptide in the diagnosis of clinical heart failure and population screening for left ventricular systolic dysfunction. Intern Med J 2008; 38: 101–113. [DOI] [PubMed] [Google Scholar]
- 7. Zaphiriou A, Robb S, Murray‐Thomas T, Mendez G, Fox K, McDonagh T, Hardman SM, Dargie HJ, Cowie MR. The diagnostic accuracy of plasma BNP and NTproBNP in patients referred from primary care with suspected heart failure: results of the UK natriuretic peptide study. Eur J Heart Fail 2005; 7: 537–541. [DOI] [PubMed] [Google Scholar]
- 8. Fuat A, Murphy JJ, Hungin AP, Curry J, Mehrzad AA, Hetherington A, Johnston JI, Smellie WS, Duffy V, Cawley P. The diagnostic accuracy and utility of a B‐type natriuretic peptide test in a community population of patients with suspected heart failure. Br J Gen Pract 2006; 56: 327–333. [PMC free article] [PubMed] [Google Scholar]
- 9. Srinivas P, Manjunath CN, Banu S, Ravindranath KS. Prognostic significance of a multimarker strategy of biomarkers in acute heart failure. J Clin Diagn Res 2014; 8: MC01–MC06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Richards AM, Troughton RW. Use of natriuretic peptides to guide and monitor heart failure therapy. Clin Chem 2012; 58: 62–71. [DOI] [PubMed] [Google Scholar]
- 11. Cotter G, Metra M, Weatherley BD, Dittrich HC, Massie BM, Ponikowski P, Bloomfield DM, O'Connor CM. Physician‐determined worsening heart failure: a novel definition for early worsening heart failure in patients hospitalized for acute heart failure – association with signs and symptoms, hospitalization duration, and 60‐day outcomes. Cardiology 2010; 115: 29–36. [DOI] [PubMed] [Google Scholar]
- 12. Cotter G, Metra M, Davison BA, Senger S, Bourge RC, Cleland JG, Jondeau G, Krum H, O'Connor CM, Parker JD, Torre‐Amione G, van Veldhuisen DJ, Milo O, Kobrin I, Rainisio M, McMurray JJ, Teerlink JR. Worsening heart failure, a critical event during hospital admission for acute heart failure: results from the VERITAS study. Eur J Heart Fail 2014; 16: 1362–1371. [DOI] [PubMed] [Google Scholar]
- 13. McMurray JJ, Teerlink JR, Cotter G, Bourge RC, Cleland JG, Jondeau G, Krum H, Metra M, O'Connor CM, Parker JD, Torre‐Amione G, van Veldhuisen DJ, Lewsey J, Frey A, Rainisio M, Kobrin I. Effects of tezosentan on symptoms and clinical outcomes in patients with acute heart failure: the VERITAS randomized controlled trials. JAMA 2007; 298: 2009–2019. [DOI] [PubMed] [Google Scholar]
- 14. Weatherley BD, Milo‐Cotter O, Felker GM, Uriel N, Kaluski E, Vered Z, O'Connor CM, Adams KF, Cotter G. Early worsening heart failure in patients admitted with acute heart failure – a new outcome measure associated with long‐term prognosis? Fundam Clin Pharmacol 2009; 23: 633–639. [DOI] [PubMed] [Google Scholar]
- 15. Metra M, Teerlink JR, Felker GM, Greenberg BH, Filippatos G, Ponikowski P, Teichman SL, Unemori E, Voors AA, Weatherley BD, Cotter G. Dyspnoea and worsening heart failure in patients with acute heart failure: results from the Pre‐RELAX‐AHF study. Eur J Heart Fail 2010; 12: 1130–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, LeWinter MM, Deswal A, Rouleau JL, Ofili EO, Anstrom KJ, Hernandez AF, McNulty SE, Velazquez EJ, Kfoury AG, Chen HH, Givertz MM, Semigran MJ, Bart BA, Mascette AM, Braunwald E, O'Connor CM, NHLBI Heart Failure Clinical Research Network . Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 2011; 364: 797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Metra M, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Ponikowski P, Unemori E, Voors AA, Adams KF Jr, Dorobantu MI, Grinfeld L, Jondeau G, Marmor A, Masip J, Pang PS, Werdan K, Prescott MF, Edwards C, Teichman SL, Trapani A, Bush CA, Saini R, Schumacher C, Severin T, Teerlink JR. Effect of serelaxin on cardiac, renal, and hepatic biomarkers in the Relaxin in Acute Heart Failure (RELAX‐AHF) development program: correlation with outcomes. J Am Coll Cardiol 2013; 61: 196–206. [DOI] [PubMed] [Google Scholar]
- 18. Chen HH, Anstrom KJ, Givertz MM, Stevenson LW, Semigran MJ, Goldsmith SR, Bart BA, Bull DA, Stehlik J, LeWinter MM, Konstam MA, Huggins GS, Rouleau JL, O'Meara E, Tang WH, Starling RC, Butler J, Deswal A, Felker GM, O'Connor CM, Bonita RE, Margulies KB, Cappola TP, Ofili EO, Mann DL, Davila‐Roman VG, McNaulty SE, Borlaug BA, Velazquez EJ, Lee KL, Shah MR, Hernandez AF, Braunwald E, Redfield MM. Low‐dose dopamine or low‐dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA 2013; 310: 2533–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. DeVore AD, Hammill BG, Sharma PP, Qualls LG, Mentz RJ, Waltman JK, Fonarow GC, Curtis LH, Hernandez AF. In‐hospital worsening heart failure and associations with mortality, readmission, and healthcare utilization. J Am Heart Assoc 2014; 3: e001088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kelly JP, Mentz RJ, Hasselblad V, Ezekowitz JA, Armstrong PW, Zannad F, Felker GM, Califf RM, O'Connor CM, Hernandez AF. Worsening heart failure during hospitalization for acute heart failure: insights from the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure (ASCEND‐HF). Am Heart J 2015; 170: 298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brisco MA, Zile MR, Hanberg JS, Wilson FP, Parikh CR, Coca SG, Tang WH, Testani JM. Relevance of changes in serum creatinine during a heart failure trial of decongestive strategies: insights from the DOSE Trial. J Card Fail 2016; 22: 753–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Teerlink JR, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Ponikowski P, Unemori E, Voors AA, Adams KF Jr, Dorobantu MI, Grinfeld LR, Jondeau G, Marmor A, Masip J, Pang PS, Werdan K, Teichman SL, Trapani A, Bush CA, Saini R, Schumacher C, Severin TM, Metra M. Serelaxin, recombinant human relaxin‐2, for treatment of acute heart failure (RELAX‐AHF): a randomised, placebo‐controlled trial. Lancet 2013; 381: 29–39. [DOI] [PubMed] [Google Scholar]
- 23. Dickstein K, Cohen‐Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole‐Wilson PA, Stromberg A, van Veldhuisen DJ, Atar D, Hoes AW, Keren A, Mebazaa A, Nieminen M, Priori SG, Swedberg K. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur Heart J 2008; 29: 2388–2442. [DOI] [PubMed] [Google Scholar]
- 24. Gheorghiade M, Zannad F, Sopko G, Klein L, Pina IL, Konstam MA, Massie BM, Roland E, Targum S, Collins SP, Filippatos G, Tavazzi L. Acute heart failure syndromes: current state and framework for future research. Circulation 2005; 112: 3958–3968. [DOI] [PubMed] [Google Scholar]
- 25. Bart BA, Goldsmith SR, Lee KL, Givertz MM, O'Connor CM, Bull DA, Redfield MM, Deswal A, Rouleau JL, LeWinter MM, Ofili EO, Stevenson LW, Semigran MJ, Felker GM, Chen HH, Hernandez AF, Anstrom KJ, McNulty SE, Velazquez EJ, Ibarra JC, Mascette AM, Braunwald E. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 2012; 367: 2296–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zannad F, Garcia AA, Anker SD, Armstrong PW, Calvo G, Cleland JG, Cohn JN, Dickstein K, Domanski MJ, Ekman I, Filippatos GS, Gheorghiade M, Hernandez AF, Jaarsma T, Koglin J, Konstam M, Kupfer S, Maggioni AP, Mebazaa A, Metra M, Nowack C, Pieske B, Pina IL, Pocock SJ, Ponikowski P, Rosano G, Ruilope LM, Ruschitzka F, Severin T, Solomon S, Stein K, Stockbridge NL, Stough WG, Swedberg K, Tavazzi L, Voors AA, Wasserman SM, Woehrle H, Zalewski A, McMurray JJ. Clinical outcome endpoints in heart failure trials: a European Society of Cardiology Heart Failure Association consensus document. Eur J Heart Fail 2013; 15: 1082–1094. [DOI] [PubMed] [Google Scholar]
- 27. Clinicaltrials.gov. NCT01870778 . Efficacy, safety and tolerability of serelaxin when added to standard therapy in AHF (RELAX‐AHF‐2). https://www.clinicaltrials.gov/ct2/show/NCT01870778?term=NCT01870778&rank=1 (5 May 2017).
- 28. Clinicaltrials.gov. NCT02064868 . Effect of serelaxin versus standard of care in acute heart failure (AHF) patients (RELAX‐AHF‐EU). https://www.clinicaltrials.gov/ct2/show/NCT02064868?term=NCT02064868&rank=1 (5 May 2017).
- 29. Clinicaltrials.gov. NCT02007720 . Efficacy, safety and tolerability of serelaxin when added to standard therapy in AHF (RELAX‐AHF‐ASIA). https://www.clinicaltrials.gov/ct2/show/NCT02007720?term=NCT02007720&rank=1 (5 May 2017).
- 30. Clinicaltrials.gov. NCT01661634 . Efficacy and safety of ularitide for the treatment of acute decompensated heart failure (TRUE‐AHF). https://www.clinicaltrials.gov/ct2/show/NCT01661634?term=NCT01661634&rank=1 (5 May 2017).
- 31. TRUE‐AHF brochure. http://www.cardiorentis.com/de‐wAssets/docs/ressouces/TRUE‐AHF_brochure_050314.pdf (5 May 2017).
- 32. Felker GM, Butler J, Collins SP, Cotter G, Davison BA, Ezekowitz JA, Filippatos G, Levy PD, Metra M, Ponikowski P, Soergel DG, Teerlink JR, Violin JD, Voors AA, Pang PS. Heart failure therapeutics on the basis of a biased ligand of the angiotensin‐2 type 1 receptor. Rationale and design of the BLAST‐AHF study (Biased Ligand of the Angiotensin Receptor Study in Acute Heart Failure). JACC Heart Fail 2015; 3: 193–201. [DOI] [PubMed] [Google Scholar]
- 33. Cooper LB, DeVore AD, Michael FG. The impact of worsening heart failure in the United States. Heart Fail Clin 2015; 11: 603–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Teerlink JR, Felker GM, McMurray JJ, Ponikowski P, Metra M, Filippatos GS, Ezekowitz JA, Dickstein K, Cleland JG, Kim JB, Lei L, Knusel B, Wolff AA, Malik FI, Wasserman SM. Acute Treatment with Omecamtiv Mecarbil to Increase Contractility in Acute Heart Failure: The ATOMIC‐AHF Study. J Am Coll Cardiol 2016; 67: 1444–1455. [DOI] [PubMed] [Google Scholar]
- 35. Clinicaltrials.gov. NCT01966601 . A study to explore the efficacy of TRV027 in patients hospitalized for acute decompensated heart failure (BLAST‐AHF). https://www.clinicaltrials.gov/ct2/show/NCT01966601?term=NCT01966601&rank=1 (5 May 2017).
- 36. Mentz RJ, Metra M, Cotter G, Milo O, McKendry C, Chiswell K, Davison BA, Cleland JG, Bloomfield DM, Dittrich HC, Fiuzat M, Ponikowski P, Givertz MM, Voors AA, Teerlink JR, O'Connor CM. Early vs. late worsening heart failure during acute heart failure hospitalization: insights from the PROTECT trial. Eur J Heart Fail 2015; 17: 697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Davison BA, Metra M, Cotter G, Massie BM, Cleland JG, Dittrich HC, Edwards C, Filippatos G, Givertz MM, Greenberg B, Ponikowski P, Voors AA, O'Connor CM, Teerlink JR. Worsening heart failure following admission for acute heart failure: a pooled analysis of the PROTECT and RELAX‐AHF studies. JACC Heart Fail 2015; 3: 395–403. [DOI] [PubMed] [Google Scholar]
- 38. Mecklai A, Subacius H, Katz S. Diuretic resistance and clinical outcomes in patients hospitalized for worsening heart failure: insights from the EVEREST (Efficacy of Vasopressin Antagoism in Heart Failure: outcome Study with Tolvaptan) trial. J Card Fail 2013; 19: S33–S34 (Abstract 094). [Google Scholar]
- 39. TRUE‐AHF: Ularitide improves congestion, does not reduce CV death in patients with acute HF. Highlights from the AHA Scientific Sessions. http://www.healio.com/cardiology/hf‐transplantation/news/online/%7B5eb87eec‐ca58‐4481‐b3de‐7700d92a8198%7D/true‐ahf‐ularitide‐improves‐congestion‐does‐not‐reduce‐cv‐death‐in‐patients‐with‐acute‐hf (5 May 2017).
- 40. Packer M. Short‐ and long‐term effect of immediate vasodilator therapy in acutely decompensated heart failure: results of the TRUE‐AHF trial. LBCT.01 – Big trials for big questions. Late breaking oral presentation at the Scientific Sessions of the American Heart Association, New Orleans, Louisiana, USA, 12–16 November 2016. http://professional.heart.org/idc/groups/ahamah‐public/@wcm/@sop/@scon/documents/downloadable/ucm_489869.pdf (5 May 2017).


