Abstract
Aims
There is little evidence‐based therapy existing for acute heart failure (AHF), hospitalizations are lengthy and expensive, and optimal monitoring of AHF patients during in‐hospital treatment is poorly defined. We evaluated a rapid cardiothoracic ultrasound (CaTUS) protocol, combining focused echocardiographic evaluation of cardiac filling pressures, that is, medial E/e′ and inferior vena cava index, with lung ultrasound (LUS) for guiding treatment in hospitalized AHF patients.
Methods and results
We enrolled 20 consecutive patients hospitalized for AHF, whose in‐hospital treatment was guided using the CaTUS protocol according to a pre‐specified treatment protocol targeting resolution of pulmonary congestion on LUS and lowering cardiac filling pressures. Treatment results of these 20 patients were compared with those of a standard care sample of 100 patients, enrolled previously for follow‐up purposes. The standard care sample had CaTUS performed daily for follow‐up and received standard in‐hospital treatment without ultrasound guidance. All CaTUS exams were performed by a single experienced sonographer. The CaTUS‐guided therapy resulted in significantly larger decongestion as defined by reduction in symptoms, cardiac filling pressures, natriuretic peptides, cumulative fluid loss, and resolution of pulmonary congestion (P < 0.05 for all) despite a shorter mean length of hospitalization. Congestion parameters were significantly lower also at discharge (P < 0.05 for all), without any significant difference in these parameters on admission. The treatment arm displayed better survival regarding the combined endpoint of 6 month all‐cause death or AHF re‐hospitalization (log rank P = 0.017). No significant difference in adverse events occurred between the groups.
Conclusions
The CaTUS‐guided therapy for AHF resulted in greater decongestion during shorter hospitalization without increased adverse events in this small pilot study and might be associated with a better post‐discharge prognosis.
Keywords: Echocardiography, Lung ultrasound, Cardiac filling pressures, Acute heart failure, Pulmonary congestion, Prognosis, Treatment
Introduction
The global prevalence of heart failure (HF) is growing increasingly.1 Most of the costs associated with HF are related to hospitalizations due to decompensated HF, also known as acute HF (AHF), which has become one of the leading causes for hospitalization globally.2, 3 There is to date virtually no evidence‐based therapy existing for AHF, hospitalizations are lengthy and expensive,2, 3 and post‐discharge prognosis often remains poor,4, 5 underlining the need for better evidence‐based therapy. How to optimally monitor decongestive treatment in AHF, however, is vaguely defined in the guidelines,6, 7 which might partly explain the lack of effective therapies.8, 9, 10
Acute heart failure patients represent a heterogeneous group of patients with varying underlying cardiac and cardiovascular disease and haemodynamic phenotypes.2, 3, 8 According to some authorities, tailored therapy based on individual patient characteristics could also be a way to achieve more effective therapy in AHF.8, 9, 10
Current HF guidelines recommend daily evaluation of signs and symptoms of congestion, fluid balance, vital signs, body weight, and renal function in hospitalized AHF patients, and adjustment of decongestive therapy accordingly, while natriuretic peptides are considered non‐useful for routine treatment guidance.6, 7 Of the adjunct monitoring tools, echocardiography (echo) and lung ultrasound (LUS) allow real‐time evaluation of cardiac filling pressures and pulmonary congestion, and these two modalities can be performed sequentially using the same machinery and probe.11, 12, 13, 14 Furthermore, echo can specify the underlying cardiac disease in AHF.4, 5 Echo and LUS might thus be useful for determining haemodynamic and baseline disease phenotype in AHF, as well as for monitoring and individually guiding treatment. There are, however, no studies to date on daily monitoring of AHF therapy using a combined echo and LUS protocol, to our best knowledge.
We hypothesized that individual, aggressive decongestive AHF therapy guided by a cardiothoracic ultrasound (CaTUS) protocol, combining echo‐derived cardiac filling pressures and LUS, could resolve congestion more effectively during a shorter hospitalization period and perhaps be related with an improved prognosis. The primary aim in this small non‐randomized single‐centre pilot study, hence, was to evaluate the efficacy of daily CaTUS‐guided AHF therapy in terms of improvement in various congestion parameters, that is, ultrasound‐derived cardiac filling pressures and pulmonary congestion, natriuretic peptides, symptoms, and fluid loss. The secondary aim in this study was to assess the safety and feasibility of ultrasound‐guided therapy, as well as its impact on post‐discharge prognosis.
Methods
We enrolled a population of 20 consecutive dyspnoeic patients entering our tertiary hospital emergency department (ED) and meeting the inclusion criteria between May and June 2015. These 20 patients received CaTUS‐guided treatment during their hospitalization course according to a pre‐specified treatment scheme and constituted the treatment arm in this study. The results in this treatment arm were compared with those of a population of 100 consecutive dyspnoeic ED patients enrolled previously between July 2014 and May 2015 using the exact same inclusion criteria, constituting the standard care arm in this study. Inclusion criteria consisted of dyspnoea at rest, structural heart disease on conventional echo, a baseline brain natriuretic peptide (BNP) > 100 ng/L, medial E/e′ > 15, and pulmonary congestion, that is, either bilateral B‐lines or bilateral pleural fluid, on LUS. Exclusion criteria consisted of altered mental status, chronic dialysis, mitral stenosis, pulmonary fibrosis, or a prosthetic valve in the mitral position. Structural heart disease on echo was defined as depressed systolic function, as significant (severe) valve disease, or as key structural or functional alterations indicating cardiac dysfunction. The latter structural or functional alterations mainly were related to ventricular hypertrophy, enlarged atria, or impaired diastolic tissue Doppler velocities, as is outlined for HF with preserved ejection fraction (EF) in the current guidelines.6, 7
Treatment was altered according to a pre‐specified treatment protocol, aiming at decreasing cardiac filling pressures and resolving pulmonary congestion. The primary endpoints for estimating treatment efficacy consisted of decrease in congestive parameters, that is, BNP, symptoms, pulmonary congestion, cardiac filling pressures, and cumulative fluid loss, as well as length of stay during hospitalization. The secondary endpoints consisted of adverse events, that is, symptomatic hypotension or acute kidney injury (AKI), as well as 6 month survival regarding the combined endpoint of re‐hospitalization for AHF or all‐cause mortality.
All enrolled patients had the CaTUS protocol, alongside laboratory and clinical parameters obtained at baseline in the ED, and thereafter daily between 8 and 10 a.m. during hospitalization, including the day of discharge. Patients in the treatment arm had their treatment guided on a daily basis according to the CaTUS protocol, whereas patients in the standard care arm had CaTUS performed daily solely for follow‐up. Treating physicians of the standard care arm‐patients would have access to the results of the CaTUS protocol for ethical reasons, if they so wished. Additionally, a conventional echo exam was performed to all patients at baseline in the ED, in which precise methodology is described in Appendix A. Laboratory samples consisted of daily blood count, creatinine, electrolytes, and BNP. All ultrasound exams were performed with the Philips (Royal Philips, Amsterdam, Netherlands) CX 50® device using the cardiac (S5‐1) probe only. A written consent was obtained from all participating patients, and the study was approved by the locally appointed ethics committee. This study complies with the Declaration of Helsinki.
Cardiothoracic ultrasound protocol
The components of the CaTUS protocol are shown in Figure 1 and included a focused echo and LUS. All CaTUS measurements were performed with the patient in a supine position, with the upper body elevated at an angle of approximately 30°. The focused echo exam in the CaTUS protocol evaluating cardiac filling pressures included medial E/e′ ratio and a five‐class, scaled inferior vena cava (IVC) index, derived from IVC calibre and respiratory variation (RV). IVC index was scaled from 1 to 5, with grade 1 representing a narrow, entirely collapsing IVC; grade 2 a maximum diameter (MD) < 21 mm and RV > 50%; grade 3 an MD ≥ 21 mm or an RV < 50%, grade 4 an MD ≥ 21 mm and an RV < 50%; and grade 5 an MD ≥ 21 mm with negligible RV and dilated hepatic veins. IVC measurements were performed using M‐mode whenever feasible, 1–2 cm caudally of the first hepatic vein.
Figure 1.
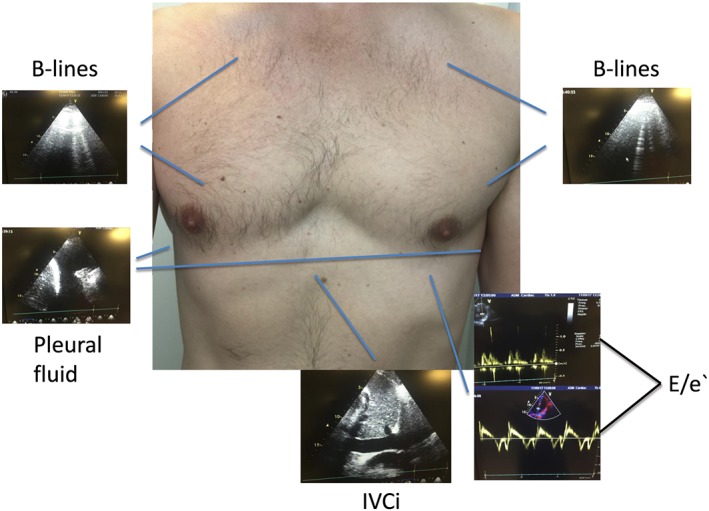
Cardiothoracic ultrasound protocol showing B‐lines on lung ultrasound as a sign of congestion, pleural fluid, a typical mitral inflow, and tissue Doppler signals used to calculate the E/e′ ratio, as well as a subcostal view of the IVC. E/e′, E/e′ ratio medially; IVC, inferior vena cava.
Lung ultrasound was performed using a rapid six‐zone scan protocol designed for daily monitoring and included evaluation of B‐lines in two regions bilaterally: the apical and mammillary regions using vertical orientation in a mid/lateral clavicular line. Additionally, pleural fluid was searched in the lower basal regions bilaterally. LUS was classified as congestive if there were three or more B‐lines in at least one region bilaterally, or >5 mm of free pleural fluid bilaterally. Decongestion on LUS was defined as resolution of both bilateral B‐lines and pleural fluid. Patients who were decongested on LUS on the day of discharge were defined as LUS responders, whereas patients discharged with residual pulmonary congestion were defined as non‐responders.
The E‐wave was recorded using pulsed wave Doppler at the tips of the opened mitral valve. If the patient was in sinus rhythm, or any other regular rhythm, three consecutive cycles at end expiration were recorded, and the average of these three E‐waves was registered. If the patient was presenting with an irregular rhythm, such as atrial fibrillation or extra‐systolia, five consecutive cycles and the average of these five E‐waves were registered. Sweep speed was adjusted to fit a proper number of cardiac cycles into one picture frame. The e′ wave was measured using tissue pulsed wave Doppler with the sample volume placed at the medial mitral annulus. The E/e′ was obtained in the four‐chamber window using minimal angulation. Gain settings were optimized to obtain a crisp, clear signal without signal aberration.
All CaTUS examinations were performed by a single sonographer with over 5 years of experience in both LUS and echo in daily practice. As this was a single‐centre, single‐operator study, LUS classification, as well as echocardiographic filling pressure measurements (E/e′ and IVC grading), was validated on a separate subset of 20 patients with experienced blinded validators (one validator for LUS and another for filling pressures), being reported in the results section.
Cardiothoracic ultrasound‐guided treatment in the treatment arm
According to the pre‐specified treatment protocol, the primary treatment targets within the treatment arm in priority order were (1) resolution of pulmonary congestion on LUS as defined earlier (resolution of both bilateral B‐lines and pleural fluid), (2) an E/e′ of <15, and (3) an IVC grade ≤ 2.
Decongestive pharmacologic therapy consisted mainly of loop diuretics and vasodilators. In case of diuretic resistance, early combination with thiazide diuretics or mineralocorticoid receptor antagonists at diuretic doses, and if necessary also ultrafiltration, was considered. Mineralocorticoid receptor antagonists were considered earlier in cases of hypokalaemia. Other pharmacologic or procedural evidence‐based HF therapies, such as devices, beta‐blockers, and angiotensin‐converting enzyme inhibitors, were administered per usual protocol during hospitalization.
Pre‐specified adverse events, causing discontinuation of decongestive therapy, consisted of hypotension or signs of hypoperfusion in combination with symptoms or AKI. In case of such adverse events, decongestive treatment was halted before treatment targets 2 or 3 were achieved, as long as the primary treatment target, that is, resolution of pulmonary congestion, was achieved. AKI was defined as a rise in creatinine of >26.5 μmol/mL or a rise in creatinine >1.5 times within 48 h as originally defined by the Acute Kidney Injury Network criteria. Hypotension was defined as a systolic arterial blood pressure of <100 mmHg or a mean arterial blood pressure < 65 mmHg, while hypoperfusion was defined as two or more cold extremities from the wrist or ankle distally.
Statistical analysis
SPSS version 23 was used for statistical analysis. Continuous variables were presented as mean values including standard deviation or median values including interquartile range as was appropriate. Categorical variables were presented as counts and percentages. Differences between two groups were determined by unpaired t‐test or Mann–Whitney U‐test for continuous variables and Pearson chi‐square for grouping variables. Differences in survival between groups were analysed with the log‐rank test and graphically displayed with Kaplan–Meier survival curve. Univariate analysis by Cox proportional hazards model was performed to assess the association between each group.
Results
Baseline characteristics of patients in the treatment arm as compared with those of the follow‐up arm can be seen in Table 1. Forty‐five percent of the patients had an EF < 40%, 23% had an EF between 40% and 50%, and 32% had an EF > 50%. There were no significant differences in any of these baseline parameters on admission between the groups except for IVC index, which was higher in the treatment arm, indicating higher right‐sided filling pressures in this group.
Table 1.
Baseline characteristics in the treatment arm compared with those in the standard care arm
| All | Treatment arm | Standard care arm | P | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age (years) | 76.0 (SD 10.6) | 75.3 (SD 9.65) | 76.2 (SD 10.8) | 0.736 |
| Male gender | 50.0% | 50.0% | 50.0% | 1.000 |
| Diabetes | 45.0% | 50.0% | 44.0% | 0.622 |
| Hypertension | 85.8% | 85.0% | 90.0% | 0.434 |
| Coronary artery disease | 45.8% | 35.0% | 48.0% | 0.287 |
| Previous HF | 60.8% | 55.0% | 62.0% | 0.558 |
| Pulmonary disease | 2.5% | 5.0% | 2.0% | 0.433 |
| Clinical parameters | ||||
| Systolic BP (mmHg) | 145 (SD 30.7) | 144 (SD 18.6) | 145 (SD 32.5) | 0.892 |
| Pulse rate (/min) | 84.5 (SD 22.6) | 86.2 (SD 26.8) | 84.1 (SD 21.9) | 0.723 |
| Sinus rhythm | 47.5% | 35.0% | 50.0% | 0.220 |
| Bundle branch block | 38.3% | 30.0% | 40.0% | 0.383 |
| Dyspnoea VAS score (0–10) | 6.15 (SD 2.53) | 5.89 (SD 2.02) | 6.20 (SD 2.62) | 0.633 |
| Rales on auscultation | 30.0% | 30.0% | 30.0% | 1.000 |
| Obstruction on auscultation | 20.0% | 10.0% | 18.0% | 0.381 |
| Respiratory rate (/min) | 22.6 (SD 5.67) | 20.7 (SD 3.61) | 23.0 (SD 5.93) | 0.111 |
| Respiratory support | 39.2% | 35.0% | 40.0% | 0.796 |
| Echo parameters | ||||
| Left ventricular EF (%) | 42.34 | 42.6 (SD 14.2) | 42.3 (SD 16.5) | 0.945 |
| E/e′ | 20.67 | 20.8 (SD 4.05) | 20.6 (SD 4.21) | 0.859 |
| e′ | 5.79 | 6.29 (SD 1.25) | 5.69 (SD 1.58) | 0.113 |
| Significant valve disease | 56.7% | 55.0% | 57.0% | 0.869 |
| Estimated SPaP (mmHg) | 66.7 (SD 18.7) | 64.1 (SD 17.9) | 67.4 (SD 18.9) | 0.558 |
| IVCi | 3.28 (SD 0.65) | 3.74 (SD 0.57) | 3.20 (SD 0.63) | <0.001 |
| RV dysfunction | 30.0% | 45.0% | 27.0% | 0.109 |
| Laboratory | ||||
| BNP (ng/L) | 696 (342–1497) | 543 (296–900) | 715 (365–1676) | 0.072 |
| eGFR (mL/min/1.73 m2) | 57.2 (SD 25.3) | 61.7 (SD 24.2) | 56.3 (SD 25.5) | 0.391 |
| Haemoglobin | 120 (SD 20.7) | 119 (SD 20.1) | 120 (SD 20.9) | 0.822 |
BNP, brain natriuretic peptide; BP, blood pressure; E/e′, medial E to e′ ratio; EF, ejection fraction; eGFR, estimated glomerular filtration rate; HF, heart failure; IVCi, inferior vena cava index (scale 1–5); RV, right ventricle; SD, standard deviation; SPaP, systolic pulmonary artery pressure; VAS, visual analogue scale. Bold means statistically significant.
Values are expressed as mean ± SD except for BNP expressed as median (25th–75th interquartile percentile). Categorical variables are expressed as number of cases (%).
Within the whole 120 patient study population, LUS responders, that is, patients achieving the primary treatment target of pulmonary decongestion on LUS as evaluated on the day of discharge, experienced a significantly better post‐discharge prognosis regarding both 6 month all‐cause mortality, as well as the composite endpoint of 6 month all‐cause mortality or hospitalization for AHF (Figure 2 ) compared with non‐responders, that is, patients discharged with residual pulmonary congestion on LUS. In comparing these two groups at baseline, LUS responders had a lower baseline BNP with no other significant differences in on‐admission parameters between LUS responders and non‐responders (Table 2).
Figure 2.
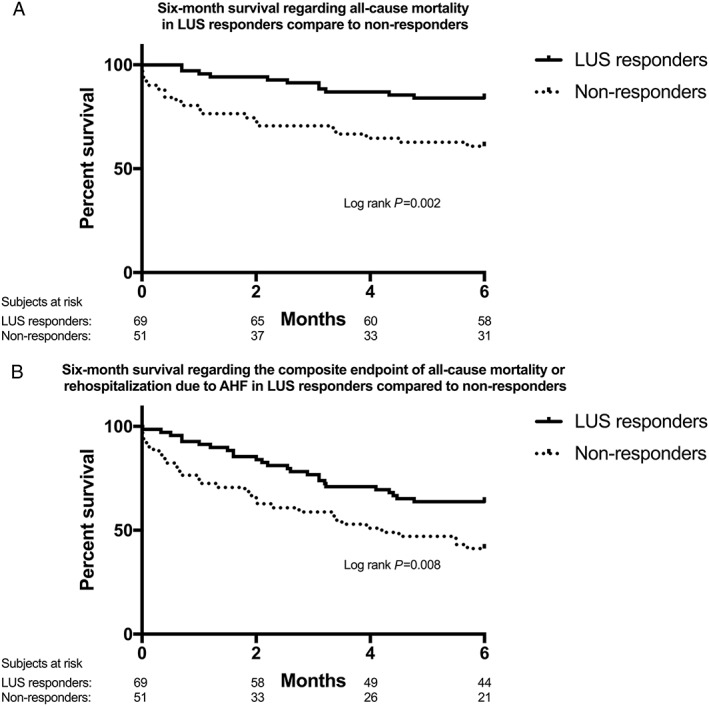
Six‐month survival regarding (A) all‐cause mortality and (B) the composite endpoint of all‐cause mortality or hospitalization for AHF in LUS responders, that is, patients who experienced resolution of pulmonary congestion on lung ultrasound, compared with that in non‐responders. AHF, acute heart failure; LUS, lung ultrasound.
Table 2.
Baseline characteristics in the lung ultrasound responders, that is, patients who achieved pulmonary decongestion, compared with those in non‐responders
| All | LUS responders (n = 69) | Non‐responders (n = 51) | P | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age (years) | 76.0 (SD 10.6) | 75.0 (SD 11.5) | 77.4 (SD 9.07) | 0.228 |
| Male gender | 50.0% | 49.3% | 51.0% | 1.000 |
| Diabetes | 45.0% | 47.8% | 41.2% | 0.571 |
| Hypertension | 83.3% | 79.7% | 88.2% | 0.322 |
| Coronary artery disease | 45.8% | 44.9% | 47.1% | 0.854 |
| Previous HF | 60.8% | 53.6% | 70.6% | 0.088 |
| Pulmonary disease | 2.5% | 2.9% | 2.0% | 1.000 |
| Clinical parameters | ||||
| Systolic BP (mmHg) | 145 (SD 30.7) | 148 (SD 29.8) | 140 (SD 31.3) | 0.138 |
| Pulse rate (/min) | 84.5 (SD 22.6) | 84.7 (SD 23.5) | 84.1 (SD 21.7) | 0.881 |
| Sinus rhythm | 47.5% | 42.0% | 54.9% | 0.197 |
| Bundle branch block | 38.3% | 42.0% | 33.3% | 0.345 |
| Dyspnoea VAS score (0–10) | 6.15 (SD 2.53) | 6.18 (SD 2.53) | 6.10 (SD 2.55) | 0.847 |
| Rales on auscultation | 30.0% | 33.3% | 25.5% | 0.422 |
| Obstruction on auscultation | 16.7% | 18.8% | 13.7% | 0.621 |
| Respiratory rate (/min) | 22.6 (SD 5.67) | 22.6 (SD 5.48) | 22.6 (SD 5.98) | 0.985 |
| Respiratory support | 39.2% | 42.0% | 36.0% | 0.571 |
| Echo parameters | ||||
| Left ventricular EF (%) | 42.3 (SD 16.1) | 42.9 (SD 16.2) | 41.5 (SD 16.1) | 0.628 |
| E/e′ | 20.7 (SD 4.17) | 20.5 (SD 3.89) | 20.9 (SD 4.56) | 0.573 |
| e′ | 5.79 (SD 1.55) | 5.90 (SD 1.45) | 5.63 (SD 1.68) | 0.339 |
| Significant valve disease | 56.7% | 55.1% | 58.8% | 0.713 |
| Estimated SPaP (mmHg) | 66.7 (SD 18.7) | 63.5 (SD 17.9) | 71.4 (SD 18.9) | 0.077 |
| IVCi | 3.28 (SD 0.65) | 3.32 (SD 0.65) | 3.22 (SD 0.64) | 0.391 |
| RV dysfunction | 30.0% | 30.4% | 29.4% | 1.000 |
| Laboratory | ||||
| BNP (ng/L) | 696 (342–1497) | 602 (328–962) | 942 (373–1782) | 0.027 |
| eGFR (mL/min/1.73 m2) | 57.2 (SD 25.3) | 59.3 (SD 24.6) | 54.2 (SD 26.1) | 0.279 |
| Haemoglobin | 120 (SD 20.7) | 119 (SD 20.1) | 120 (SD 20.9) | 0.822 |
BNP, brain natriuretic peptide; BP, blood pressure; E/e′, medial E to e′ ratio; EF, ejection fraction; eGFR, estimated glomerular filtration rate; HF, heart failure; IVCi, inferior vena cava index (scale 1–5); LUS, lung ultrasound; RV, right ventricle; SD, standard deviation; SPaP, systolic pulmonary artery pressure; VAS, visual analogue scale. Bold means statistically significant.
Values are expressed as mean ± SD except for BNP expressed as median (25th–75th interquartile percentile). Categorical variables are expressed as number of cases (%).
As can be seen in Table 3, patients in the treatment arm had a significantly greater improvement in all decongestive parameters during treatment, including echocardiographic parameters, pulmonary congestion on LUS, natriuretic peptides, fluid loss, and symptoms compared with those in the standard care arm. Patients in the treatment arm also experienced non‐significantly less adverse events, with no adverse events associated with hypotension or hypoperfusion, and displayed a significantly shorter mean hospitalization, lasting less than 4 days on average (Table 3).
Table 3.
Treatment‐related parameters in the treatment arm compared with those in the standard care arm
| Treatment arm | Standard care arm | P | |
|---|---|---|---|
| n = 20 | n = 100 | ||
| During hospitalization | |||
| Decrease in E/e′ | 6.48 (SD 2.92) | 2.62 (SD 4.67) | 0.001 |
| Decrease in IVCi (1–5) | 1.79 (SD 1.02) | 0.39 (SD 0.82) | <0.001 |
| % decrease in BNP (ng/L) | 35.9 (SD 26.3) | 16.6 (SD 61.1) | 0.029 |
| LOH (days) | 3.74 (SD 2.02) | 6.85 (SD 4.22) | 0.002 |
| Cumulative fluid loss (mL) | 5447 (SD 5364) | 3072 (SD 3059) | <0.001 |
| Decrease in eGFR (mL/min/1.73 m2) | 3.47 (SD 8.64) | 4.41 (SD 13.8) | 0.778 |
| On the day of discharge | |||
| Final E/e′ | 14.4 (SD 3.14) | 18.0 (SD 5.63) | 0.007 |
| Final IVCi (1–5) | 1.21 (SD 0.91) | 1.82 (SD 0.76) | 0.005 |
| Final BNP (ng/L) | 249 (172–408) | 426 (242–1015) | 0.011 |
| Pulmonary decongested on LUS | 80.0% | 53.0% | 0.039 |
| E/e′ <15 | 60.0% | 35.0% | 0.020 |
| Pulmonary decongestion or E/e′ < 15 | 95.0% | 63.0% | 0.007 |
| Asymptomatic at discharge | 95.0% | 72.0% | 0.036 |
| Final eGFR (mL/min/1.73 m2) | 58.3 (SD 24.2) | 51.9 (SD 23.8) | 0.287 |
| Adverse events | |||
| Acute kidney injury | 15.0% | 21.0% | 0.617 |
| Symptomatic hypotension | 0% | 4.0% | 0.378 |
BNP, brain natriuretic peptide; E/e′, medial E to e′ ratio; eGFR, estimated glomerular filtration rate; IVCi, inferior vena cava index (scale 1–5); LOH, length of hospitalization; LUS, lung ultrasound; SD, standard deviation. Bold means statistically significant.
Values are expressed as mean ± SD except for BNP expressed as median (25th–75th interquartile percentile). Categorical variables are expressed as number of cases (%).
Patients in the treatment arm also presented a significantly lower 6 month event rate regarding the combined endpoint of all‐cause mortality and re‐hospitalization due to AHF (Figure 3 , log rank P = 0.017). This difference was mainly driven by a significantly larger difference in re‐hospitalization (log rank P = 0.005), while there was only a non‐significant trend towards a lower all‐cause mortality (log rank P = 0.488).
Figure 3.
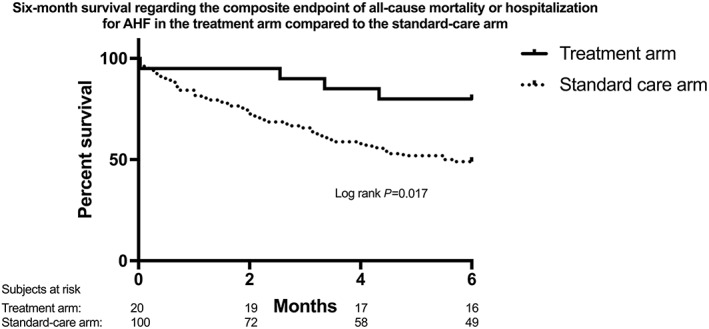
Six‐month survival regarding the composite endpoint of all‐cause death or hospitalization for AHF in the treatment arm, receiving cardiothoracic ultrasound‐guided therapy, as compared with that in the standard care arm. AHF, acute heart failure.
The CaTUS exam was rapid to perform, lasting <5 min in all patients during the first 30 time‐measured exams. Regarding qualitative validations of LUS and focused echo, inter‐observer agreement for congestion on LUS was 100%, and thus the κ coefficient was 1.0. For IVC index, the mean inter‐observer coefficient of variation was 4.37%, and for E/e′ 9.99%.
Discussion
This study was designed to test the CaTUS protocol for guiding decongestive AHF treatment in a small pilot sample, constituting the treatment arm. The results within this population were compared with those of a larger population, enrolled earlier for follow‐up purposes, who were treated per standard, daily‐basis protocol during their hospitalization by their treating physicians. Treatment in this standard care arm was considered to reflect standard in‐hospital treatment for AHF in a tertiary care hospital, with the exception that, for ethical reasons, treating physicians would have access to results of the CaTUS exams if they so wished.
As such, the groups were not balanced, and the treatment arm was considered too small for prognostic purposes. Nevertheless, patients in the treatment arm experienced substantially greater decongestion as estimated by every congestive parameter during a significantly shorter mean hospitalization, and perhaps most importantly, no safety issues associated with CaTUS‐guided treatment occurred. Patients in the treatment arm further showed a signal towards improved prognosis, mainly driven by a reduction in re‐hospitalizations.
In the standard care arm, almost half of the patients were discharged with residual pulmonary congestion and over one‐fourth with dyspnoea at rest. These rather poor results are in line with those of previous studies, underlining current difficulty of achieving adequate decongestion during AHF hospitalizations, even though residual congestion is known to have a detrimental impact on prognosis,4, 15, 16, 17 as was the case in this study as well.
Factors explaining the difficulty of achieving adequate decongestion during in‐hospital AHF treatment are poorly understood but may include excessive intravenous fluid and sodium load during lengthier hospitalizations18 and diuretic resistance or inadequate diuretic dosing,19 resulting in failure to achieve a negative sodium and fluid balance. Some patients also seem to suffer from vasoconstriction and fluid misdistribution rather than pure volume overload, probably requiring an individualized, more vasodilative treatment approach.8, 9, 20 Decongestive therapy may also be halted owing to assumed adverse events, mainly kidney injury, which may theoretically occur as a result of excess diuretic therapy.21 In association with AHF, however, AKI may also occur owing to increased systemic venous congestion and hypervolaemia,22 in which case fluid removal might conversely improve kidney function.23 Perhaps most importantly, AKI in combination with adequate decongestion does not seem to impair prognosis, thus representing a sort of ‘benign’ AKI partly owing to haemo‐concentration, whereas the very opposite is true in case of AKI in combination with persistent congestion.23, 24 This underlines the importance of haemodynamic monitoring when treating AHF, especially in the case of renal failure.
In chronic HF, guidance of decongestive therapy by pulmonary artery pressure was associated with a significant reduction in hospitalizations in the outpatient CHAMPION trial,25 but in AHF, no such positive trials on treatment guidance exist. In the ESCAPE trial, AHF treatment guided by pulmonary artery catheter was non‐useful compared with conventional therapy.26 It is noteworthy, however, that physicians treating ESCAPE trial patients had central venous pressure measurements and echocardiograms available on a daily basis in both groups, although their role for guiding treatment was not clearly reported. The study setting in this study was quite close to that seen in the ESCAPE trial, but the benefits of echo compared with those of pulmonary artery catheter include the lack of adverse events and feasibility also outside an intensive care unit setting.
Cardiac filling pressures, considered the driving force behind pulmonary congestion, have shown potential to decrease rapidly following aggressive AHF treatment initiation,27, 28 while pulmonary decongestion can be assessed in real time with LUS.14 Echo‐derived filling pressures and LUS have also displayed prognostic significance prior to discharge, making them potential treatment targets during AHF hospitalizations.16, 17, 29 As patients discharged with pulmonary congestion are known to carry a poor prognosis,16, 17 resolution of pulmonary congestion constituted our primary treatment target in our treatment protocol. In our own previous study analysing chronologic improvement in E/e′, IVC index, and pulmonary congestion on LUS, we found E/e′ to precede pulmonary decongestion among treatment‐responsive patients, which took place with a mean E/e′ value of 17.2.30 E/e′ thereafter continued declining towards the end of hospitalization, reaching a mean discharge value of 16.2, still considered abnormal, while IVC index declined later towards end of hospitalization. Thus, the two latter treatment targets, that is, close‐to‐normal left‐sided cardiac filling pressures and a non‐plethoric IVC, represent attempts to achieve more thorough decongestion prior to discharge, after resolution of pulmonary decongestion. Although our pre‐specified treatment protocol included an order to halt therapy in case of adverse events associated with over‐aggressive treatment, this did not become an issue in this study, as no such events occurred in the treatment arm.
Eventually, patients in the treatment arm seemed to be more thoroughly decongested by every parameter after receiving CaTUS‐guided therapy as compared with patients who received standard care. Post‐discharge prognosis in the treatment arm was also significantly better despite a low number of patients in this arm, lowering the statistical power of the survival analysis. Concurrently, though, this small population size simultaneously requires these intriguing results to be interpreted with caution. Nevertheless, CaTUS‐guided therapy with our treating protocol was feasible and seemed safe, and no larger practical issues occurred while using it. Future randomized trials with bigger balanced populations are needed in order to define whether individualized ultrasound‐guided treatment could improve prognosis and shorten hospitalizations.
Study limitations
This was a small pilot study with unequal populations, with the two non‐randomized populations enrolled during sequential time periods. Nevertheless, these two populations were not convenience samples but sequential patients entering the ED, thus diminishing the chance of selection bias. This study was a single‐operator, single‐centre study, although the CaTUS measurements were blindly validated with high agreement. Exact cumulative doses of medications used could also not be archived, and hence we could not report prognostically important dose‐related measures such as cumulative use of diuretics and diuretic resistance.
Conclusions
In this small pilot trial, CaTUS seemed safe and feasible for guiding AHF treatment. Ultrasound‐guided AHF treatment might be associated with improved total decongestion, shorter hospitalizations, and improved prognosis, which need to be verified in future trials.
Conflict of interest
None declared.
Appendix A. Conventional echo
All patients enrolled in the study had a comprehensive echocardiography performed by experienced operators in order to evaluate for structural heart disease. Left ventricular ejection fraction was measured using Simpsons bi‐plane method of discs. Right ventricle dysfunction was defined as either tricuspid annular plane systolic excursion < 16 mm or a fractional area change < 35%. The presence of significant valve disease was reported as evaluated by the echocardiographer. E‐waves, A‐waves, and tissue Doppler imaging velocities were recorded as described earlier concerning the cardiothoracic ultrasound protocol. Mitral stenosis as an exclusion criterion was defined as a mitral inflow pressure half‐time > 70 ms or a mitral inflow mean gradient of >5 mmHg. The presence of structural heart disease was defined as described in the guidelines.
Öhman, J. , Harjola, V.‐P. , Karjalainen, P. , and Lassus, J. (2018) Focused echocardiography and lung ultrasound protocol for guiding treatment in acute heart failure. ESC Heart Failure, 5: 120–128. doi: 10.1002/ehf2.12208.
References
- 1. van Riet EE , Hoes AW, Wagenaar KP, Limburg A, Landman MA, Rutten FH. Epidemiology of heart failure: the prevalence of heart failure and ventricular dysfunction in older adults over time. A systematic review. Eur J Heart Fail 2016. Mar; 18: 242–252. [DOI] [PubMed] [Google Scholar]
- 2. Adams KF Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M, Horton DP; ADHERE Scientific Advisory Committee and Investigators. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J 2005; 149: 209–216. [DOI] [PubMed] [Google Scholar]
- 3. Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CS, Sato N, Shah AN, Gheorghiade M. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 2014; 63: 1123–1133. [DOI] [PubMed] [Google Scholar]
- 4. Greene SJ, Fonarow GC, Vaduganathan M, Khan SS, Butler J, Gheorghiade M. The vulnerable phase after hospitalization for heart failure. Nat Rev Cardiol 2015; 12: 220–229. [DOI] [PubMed] [Google Scholar]
- 5. Ambrosy AP, Cerbin LP, Armstrong PW, Butler J, Coles A, DeVore AD, Dunlap ME, Ezekowitz JA, Felker GM, Fudim M, Greene SJ, Hernandez AF, O'Connor CM, Schulte P, Starling RC, Teerlink JR, Voors AA, Mentz RJ. Body weight change during and after hospitalization for acute heart failure: patient characteristics, markers of congestion, and outcomes: findings from the ASCEND‐HF Trial. JACC Heart Fail 2017; 5: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure. Circulation 2013; 128: 240–327. [DOI] [PubMed] [Google Scholar]
- 7. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P ; Authors/Task Force Members. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 8. Weintraub NL, Collins SP, Pang PS, Levy PD, Anderson AS, Arslanian‐Engoren C, Gibler WB, McCord JK, Parshall MB, Francis GS, Gheorghiade M; American Heart Association Council on Clinical Cardiology and Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Acute heart failure syndromes: emergency department presentation, treatment, and disposition: current approaches and future aims. A scientific statement from the American Heart Association. Circulation 2010; 122: 1975–1996. [DOI] [PubMed] [Google Scholar]
- 9. Martens P, Nijst P, Mullens W. Current approach to decongestive therapy in acute heart failure. Curr Heart Fail Rep 2015. Dec; 12: 367–378. [DOI] [PubMed] [Google Scholar]
- 10. Gheorghiade M1, Follath F, Ponikowski P, Barsuk JH, Blair JE, Cleland JG, Dickstein K, Drazner MH, Fonarow GC, Jaarsma T, Jondeau G, Sendon JL, Mebazaa A, Metra M, Nieminen M, Pang PS, Seferovic P, Stevenson LW, van Veldhuisen DJ , Zannad F, Anker SD, Rhodes A, McMurray JJ, Filippatos G; European Society of Cardiology; European Society of Intensive Care Medicine. Assessing and grading congestion in acute heart failure: a scientific statement from the acute heart failure committee of the Heart Failure Association of the European Society of Cardiology and endorsed by the European Society of Intensive Care Medicine. Eur J Heart Fail 2010. May; 12: 423–433. [DOI] [PubMed] [Google Scholar]
- 11. Ang SH. Lung ultrasound in the management of acute decompensated heart failure. Curr Cardiol Rev 2012; 8: 123–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Beigel R, Cercek B, Arsanjani R, Siegel RJ. Echocardiography in the use of noninvasive hemodynamic monitoring. J Crit Care 2014; 29: 184–188. [DOI] [PubMed] [Google Scholar]
- 13. Papadimitriou L, Georgiopoulou VV, Kort S, Butler J, Kalogeropoulos AP. Echocardiography in acute heart failure: current perspectives. J Card Fail 2016. Jan; 22: 82–94. [DOI] [PubMed] [Google Scholar]
- 14. Strnad M, Prosen G, Borovnik Lesjak V. Bedside lung ultrasound for monitoring the effectiveness of prehospital treatment with continuous positive airway pressure in acute decompensated heart failure. Eur J Emerg Med 2016; 1: 50–55. [DOI] [PubMed] [Google Scholar]
- 15. Lala A, Mcnulty SE, Mentz RJ, Dunlay SM, Vader JM, AbouEzzeddine OF, DeVore AD, Khazanie P, Redfield MM, Goldsmith SR, Bart BA, Anstrom KJ, Felker GM, Hernandez AF, Stevenson LW. Relief and recurrence of congestion during and after hospitalization for acute heart failure: insights from Diuretic Optimization Strategy Evaluation in Acute Decompensated Heart Failure (DOSE‐AHF) and Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARESS‐HF). Circ Heart Fail 2015; 4: 741–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Coiro S, Rossignol P, Ambrosio G, Carluccio E, Alunni G, Murrone A, Tritto I, Zannad F, Girerd N. Prognostic value of residual pulmonary congestion at discharge assessed by lung ultrasound imaging in heart failure. Eur J Heart Fail 2015; 11: 1172–1181. [DOI] [PubMed] [Google Scholar]
- 17. Gargani L, Pang PS, Frassi F, Miglioranza MH, Dini FL, Landi P, Picano E. Persistent pulmonary congestion before discharge predicts rehospitalization in heart failure: a lung ultrasound study. Cardiovasc Ultrasound 2015; 4: 13–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tafreshi J, Hoang TM, Grigorian T, Pai AD, Tafreshi AR, Pai RG. Impact of iatrogenic, excessive, nondietary sodium administration in patients with acute heart failure exacerbation on hospital length of stay. Pharmacotherapy 2011. Jan; 31: 58–61. [DOI] [PubMed] [Google Scholar]
- 19. Bonow RO, Mann DL, ZIpes DP, Libby P. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. Vol 10. ch 28, p. 557 [Google Scholar]
- 20. Colombo PC, Doran AC, Onat D, Wong KY, Ahmad M, Sabbah HN, Demmer RT. Venous congestion, endothelial and neurohormonal activation in acute decompensated heart failure: cause or effect? Curr Heart Fail Rep 2015; 12: 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu X, Zhang W, Ren H, Chen X, Xie J, Chen N. Diuretics associated acute kidney injury: clinical and pathological analysis. Ren Fail 2014; 36: 1051–1055. [DOI] [PubMed] [Google Scholar]
- 22. Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, Young JB, Tang WH. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol 2009; 53: 589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Damman K, Testani JM. The kidney in heart failure: an update. Eur Heart J 2015; 36: 1437–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Testani JM, Chen J, McCauley BD, Kimmel SE, Shannon RP. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation 2010; 122: 265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Abraham WT, Stevenson LW, Bourge RC, Lindenfeld JA, Bauman JG, Adamson PB; CHAMPION Trial Study Group. Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: complete follow‐up results from the CHAMPION randomised trial. Lancet 2016; 387: 453–461. [DOI] [PubMed] [Google Scholar]
- 26. Binanay C, Califf RM, Hasselblad V, O'Connor CM, Shah MR, Sopko G, Stevenson LW, Francis GS, Leier CV, Miller LW; ESCAPE Investigators and ESCAPE Study Coordinators. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA 2005; 294: 1625–1633. [DOI] [PubMed] [Google Scholar]
- 27. Harada K, Yamamoto T, Okumura T, Shigekiyo M, Terada N, Okada A, Kawata A, Iima T, Harada T, Fujisawa K, Kageyama N, Saito A, Yamamoto H, Fujinaga H. Intravenous nicorandil for treatment of the urgent phase acute heart failure syndromes: a randomized, controlled trial. Eur Heart J Acute Cardiovasc Care 2016; 16 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 28. Ponikowski P, Mitrovic V, Ruda M, Fernandez A, Voors AA, Vishnevsky A, Cotter G, Milo O, Laessing U, Zhang Y, Dahlke M, Zymlinski R, Metra M. A randomized, double‐blind, placebo‐controlled, multicentre study to assess haemodynamic effects of serelaxin in patients with acute heart failure. Eur Heart J 2014; 35: 431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gackowski A, Isnard R, Golmard JL, Pousset F, Carayon A, Montalescot G, Hulot JS, Thomas D, Piwowarska W, Komajda M. Comparison of echocardiography and plasma B‐type natriuretic peptide for monitoring the response to treatment in acute heart failure. Eur Heart J 2004; 25: 1788–1796. [DOI] [PubMed] [Google Scholar]
- 30. Öhman J, Harjola VP, Karjalainen P, Lassus J. Assessment of early treatment response by rapid cardiothoracic ultrasound in acute heart failure: cardiac filling pressures, pulmonary congestion and mortality. Eur Heart J Acute Cardiovasc Care. 10.1177/2048872617708974 [DOI] [PubMed] [Google Scholar]


