Abstract
Aims
CORE is a continuing medical education initiative designed to support the evidence‐based management of heart failure (HF) in the primary and secondary care settings. The goal of the CORE Needs Assessment Survey is to describe current clinical practice patterns and attitudes among global stakeholders in HF care.
Methods and results
The CORE Steering Committee guided the development of survey questions to assess clinical practice, confidence, and attitudes/perceptions among cardiologists, primary care physicians, and nurses involved in HF management. In total, 346 healthcare professionals from Australia (n = 59), Austria (n = 59), Canada (n = 60), Spain (n = 58), Sweden (n = 52), and the UK (n = 58) contributed survey data. Results revealed multiple gaps over the spectrum of HF care, including diagnosis (low recognition of the signs and symptoms of HF and limited use of diagnostic tests), treatment planning (underuse of recommended agents and subtherapeutic dosing), treatment monitoring and adjustment (lack of adherence to recommendations), and long‐term management (low confidence in providing patient education). Although primary care and specialist physicians and nurses shared common unmet needs, healthcare professional‐specific clinical gaps were also identified.
Conclusions
The CORE Needs Assessment Survey provides timely data describing current clinical practices and attitudes among physicians and nurses regarding key aspects of HF care. These findings will be useful for guiding the development of interventions tailored to the specific educational needs of different provider types and designed to support the evidence‐based care of patients with HF.
Keywords: Heart failure, Clinical practice, Diagnosis, Treatment, Survey
Introduction
Heart failure (HF) is associated with frequent hospitalizations, high morbidity and mortality rates, and enormous healthcare costs.1 Moreover, given the ageing population and prolonged survival of patients with cardiovascular disease, the prevalence and public health burden of HF are expected to grow.2 Therefore, the effective diagnosis and treatment of patients with HF are essential.3, 4 However, many patients with HF are not receiving treatments and/or appropriate doses that demonstrate benefit in clinical trials and are not receiving care within a multidisciplinary framework that emphasizes effective long‐term management.5, 6, 7
Continuing medical education supports the improvement of knowledge, confidence, and clinical performance among healthcare providers (HCPs).8 CORE is an ongoing continuing medical education initiative, designed to enhance the delivery of evidence‐based care in HF by increasing prompt HF diagnosis, improving the use of appropriate interventions, improving disease management through better comorbidity competence, and increasing multidisciplinary collaboration and communication across the wider HF care team.
The current analysis of the CORE Needs Assessment Survey aims to (i) document practice and confidence gaps in HF management by HCP type and (ii) inform educational interventions for cardiologists, primary care physicians (PCPs), and nurses involved in HF management.
Methods
The CORE Needs Assessment Survey was conducted in English or the local languages as specified among primary and secondary care HCPs who manage patients with HF in six countries: Australia, Austria (German), Canada, Spain (Spanish), Sweden (Swedish), and the UK. The survey was conducted online by PCM Scientific in collaboration with Doctors.net.UK, a third‐party professional body.
Survey design
The survey was designed to assess key areas of HF knowledge and clinical practice based on the 2012 European Society of Cardiology (ESC) guidelines.3 Survey questions were developed from a literature review, preliminary needs assessment, and expert opinion and then reviewed and approved by the international and multidisciplinary CORE Steering Committee members (Appendix 1). The survey used a range of question formats to measure confidence, clinical practice patterns, and attitudes/perceptions related to the diagnosis, treatment, monitoring, long‐term management, and multidisciplinary care of patients with HF. Given variations across national HF guidelines, care was taken not to assess ideas or practices that differed by country.9, 10
Participating cardiologists and PCPs were recruited from online HCP panels (Austria, Australia, Canada, Spain, and UK) and/or telephone databases (Austria, Australia, Canada, and Sweden), and nurses were recruited via telephone. Only those who met the screening requirements were invited to complete the country‐specific online surveys. All survey respondents received a small honorarium in accordance with legal and ethical guidelines from the British Healthcare Business Intelligence Association.11
To assess self‐reported confidence in clinicians' knowledge and practice decisions, respondents were asked to rank their confidence on the following 7‐point scale: 1–2 (not at all confident), 3–5 (mid‐confidence), and 6–7 (very confident).
Statistical analysis plan
The statistical analysis includes only complete surveys; no responses were lost or missing. Data are presented as aggregate mean percentage responses among respondents from all geographic regions. The Student's t‐test and analysis of variance were used to detect differences between HCP cohorts (significance cut‐off, P < 0.005). No survey questions were excluded because of unusually high or low ‘correct’ responses rates.
The primary objective is to define global trends in HF knowledge, confidence, and practice among cardiologists, PCPs, and cardiac and primary care nurses. Secondary objectives are to detect variations across national findings; these data will be reported elsewhere.
Results
Respondents
In total, 944 HCPs replied to requests to participate (i.e. clicked on the online survey link), and 346 (37%) completed the survey. The percentage of HCPs who completed surveys varied from 32% to 75% by country: Australia (59/176; 33%), Austria (59/88; 67%), Canada (60/145; 41%), Spain (58/183; 32%), Sweden (52/106; 49%), and UK (58/77; 75%). Respondents included 65 cardiologists, 160 PCPs, 50 cardiac nurses, and 71 primary care nurses (Table 1).
Table 1.
CORE Needs Assessment respondent demographics
| Total | Cardiologist | Primary care physician | Cardiac nurse | Primary care nurse | |
|---|---|---|---|---|---|
| Australia | 59 | 12 | 26 | 8 | 13 |
| Austria | 59 | 12 | 26 | 8 | 13 |
| Canada | 60 | 10 | 29 | 8 | 13 |
| Spain | 58 | 10 | 26 | 9 | 13 |
| Sweden | 52 | 11 | 27 | 8 | 6 |
| UK | 58 | 10 | 26 | 9 | 13 |
| Total | 346 | 65 | 160 | 50 | 71 |
Physicians' attitudes and practice patterns
Key survey questions and responses among both cardiologists and PCPs are summarized in Tables 2, 3, 4.
Table 2.
Heart failure diagnosis practices among physicians and nurses
| Cardiologists (n = 65) | PCPs (n = 160) | Cardiac nurses (n = 50) | Primary care nurses (n = 71) | |
|---|---|---|---|---|
| Diagnosis | ||||
| Symptoms and signs: In your clinical practice, which of the following indicators do you routinely assess or consider as specific or non‐specific markers for HF? | ||||
| Abdominal bloating (%) | 53.8 | 41.9 | 58.0 | 43.7 |
| Ankle swelling (%) | 96.9 | 98.8 | 98.0 | 93.0 |
| Anorexia (%) | 58.5 | 44.4 | 44.0 | 40.8 |
| Breathlessness on exertion (%) | 98.5 | 98.8 | 100 | 97.2 |
| Chest pain (%) | 67.7 | 80.6* | 94.0 | 93.0 |
| Chronic cough (%) | 64.6 | 84.4* | 84.0 | 76.1 |
| Confusion (%) | 36.9 | 35.6 | 52.0 | 43.7 |
| Displaced apex beat (%) | 76.9* | 57.5 | 54.0 | 53.5 |
| Dizziness (%) | 58.5 | 60.0 | 74.0 | 73.2 |
| Elevated jugular venous pressure (%) | 95.4* | 84.4 | 88.0** | 69.0 |
| Fatigue (%) | 95.4* | 87.5 | 90.0 | 87.3 |
| Forgetfulness (%) | 13.8 | 21.9 | 34.0 | 28.2 |
| Heart murmur (%) | 87.7 | 78.8 | 70.0 | 64.8 |
| Nocturia (%) | 58.5 | 53.8 | 54.0 | 54.1 |
| Orthopnoea (%) | 96.9 | 93.8 | 88.0 | 78.9 |
| Palpitations (%) | 75.4 | 65.6 | 84.0 | 78.9 |
| Paroxysmal nocturnal dyspnoea (%) | 96.9 | 95.0 | 86.0 | 74.6 |
| Pulmonary crackles (%) | 96.9 | 97.5 | 84.0 | 74.6 |
| Reduced exercise tolerance (%) | 96.9 | 96.3 | 88.0 | 90.1 |
| Sputum (%) | 30.8 | 40.6 | 56.0 | 45.1 |
| Tachycardia (%) | 95.4* | 81.3 | 94.0 | 85.9 |
| Third heart sound (%) | 90.8* | 73.1 | 62.0 | 49.3 |
| Essential diagnostic tests: When assessing patients for suspected HF, which of the following do you carry out or refer the patient for? | ||||
| Assessment of medical history, comorbidities, and current medications (%) | 83.1 | 75.6 | 78.0 | 77.5 |
| Assessment of signs and symptoms (%) | 83.1 | 76.3 | 76.0 | 80.3 |
| Chest X‐ray (%) | 67.7 | 75.6 | 48.0 | 59.2 |
| Coronary angiography (%) | 56.9* | 33.8 | 42.0 | 38.0 |
| CT angiography (%) | 38.5 | 28.1 | 40.0 | 25.4 |
| Echocardiogram (%) | 81.5 | 75.0 | 64.0 | 39.4 |
| Electrocardiogram (%) | 80.0 | 71.9 | 70.0 | 60.6 |
| Fasting glucose and lipid level (%) | 67.7 | 70.6 | 72.0 | 71.8 |
| Lab tests for renal function, electrolytes, and to detect comorbidity (%) | 75.4 | 81.3 | 72.0 | 69.0 |
| Liver function tests (%) | 69.2 | 71.3 | 64.0 | 69.0 |
| Renal function and electrolytes (%) | 73.8 | 78.8 | 68.0 | 67.6 |
| Serum natriuretic peptides (%) | 70.8 | 59.4 | 58.0 | 47.9 |
| Spirometry (%) | 44.6 | 41.9 | 48.0 | 63.4 |
| Thyroid function tests (%) | 66.2 | 65.6 | 46.0 | 63.4 |
CT, computed tomography; HF, heart failure; PCP, primary care physician.
Significant difference (P < 0.005) between physician groups.
Significant difference (P < 0.005) between nurse groups.
Table 3.
Heart failure treatment practices among physicians
| Cardiologists (n = 65) | PCPs (n = 160) | Cardiac nurses (n = 50) | Primary care nurses (n = 71) | |
|---|---|---|---|---|
| Treatment | ||||
| Physician prescribing habits: Which of the following medications/classes do you routinely prescribe with regard to your patients with HF? | ||||
| ACE inhibitors (%) | 99.4 | 100* | ||
| ARBs (%) | 93.1 | 89.2 | ||
| Beta‐blockers (%) | 96.9 | 98.5* | ||
| Digoxin (%) | 56.9 | 58.5 | ||
| Diuretics (%) | 100* | 93.8 | ||
| Ivabradine (%) | 17.5 | 57.0 | ||
| MRAs (%) | 94 | 88 | ||
| Nitrates (%) | 51.2 | 41.5 | ||
| Dosing practices: For each of the following medication classes, please indicate the medication you use most often and the mean total initiation and target daily doses that you prescribe of that specific medication. | ||||
| ACE inhibitors | ||||
| Ramipril (%) | 63 | 34 | ||
| Average starting dose (mg/day) (ESC recommendation: 2.5 mg/day) | 4.3 | 4.6 | ||
| Mean target dose (mg/day) (ESC recommendation: 10 mg/day) | 10.8 | 10.3 | ||
| ARBs | ||||
| Candesartan (%) | 54 | 22.5 | ||
| Average starting dose (mg/day) (ESC recommendation: 4–8 mg/day) | 6.9 | 11 | ||
| Mean target dose (mg/day) (ESC recommendation: 32 mg/day) | 27.4 | 27.1 | ||
| Losartan (%) | — | 27 | ||
| Average starting dose (mg/day) (ESC recommendation: 50 mg/day) | — | 39.4 | ||
| Mean target dose (mg/day) (ESC recommendation: 150 mg/day) | — | 76.1 | ||
| MRAs | ||||
| Eplenerone (%) | 22 | — | ||
| Average starting dose (mg/day) (ESC recommendation: 25 mg/day) | 24.1 | — | ||
| Mean target dose (mg/day) (ESC recommendation: 50 mg/day) | 39.3 | — | ||
| Spironolactone (%) | 15 | 11 | ||
| Average starting dose (mg/day) (ESC recommendation: 25 mg/day) | 22.5 | 37.5 | ||
| Mean target dose (mg/day) (ESC recommendation: 25–50 mg/day) | 40 | 80.6 | ||
ACE, angiotensin‐converting enzyme; ARBs, angiotensin receptor blockers; ESC, European Society of Cardiology; HF, heart failure; MRAs, mineralocorticoid receptor agonists; PCP, primary care physician.
Significant difference (P < 0.005) between physician groups.
Table 4.
Heart failure monitoring and management practices among physicians and nurses
| Cardiologists (n = 65) | PCPs (n = 160) | Cardiac nurses (n = 50) | Primary care nurses (n = 71) | |
|---|---|---|---|---|
| Monitoring | ||||
| Involvement in medication monitoring: At which of the following stages, if any, are you involved in monitoring patients taking pharmacotherapy? | ||||
| Initiation (%) | 86.2* | 71.9 | 68.0 | 52.1 |
| After each dose increment (%) | 55.4 | 63.8 | 66.0** | 35.2 |
| At end of medication (%) | 53.8 | 56.9 | 44.0** | 19.7 |
| None (%) | 1.5 | 0.6 | 4.0 | 19.7** |
| Long‐term management | ||||
| Screening for other conditions: Which of the following cardiovascular and non‐cardiovascular conditions do you routinely screen for specifically with regard to your HF patients? | ||||
| Angina (%) | 87.7 | 91.3 | 90.0 | 78.9 |
| Anaemia (%) | 84.6 | 83.8 | 62.0 | 46.5 |
| Asthma (%) | 49.2 | 46.9 | 38.0 | 46.5 |
| Cancer (%) | 12.3 | 16.9 | 8.0 | 12.7 |
| Cognitive impairment (%) | 24.6 | 38.1* | 44.0 | 31.0 |
| Cachexia (%) | 46.2 | 36.3 | 30.0 | 31.0 |
| COPD (%) | 66.2 | 60.0 | 54.0 | 56.3 |
| Diabetes mellitus (%) | 84.6 | 85.0 | 64.0 | 59.2 |
| Depression and anxiety (%) | 32.3 | 52.5* | 56.0 | 46.5 |
| Hypertension (%) | 96.4 | 96.9 | 92.0 | 94.4 |
| Hyperuricaemia and gout (%) | 33.8 | 44.4 | 24.0 | 25.4 |
| Obesity (%) | 63.1 | 73.8 | 56.0 | 70.4 |
| Renal dysfunction (%) | 87.7 | 93.8 | 75.0 | 66.2 |
| Thyroid disease (%) | 64.6 | 78.1* | 42.0 | 47.9 |
| Recognizing end of life benefits: Of the following cases, which do you consider indicates that the patient might benefit from end‐of‐life care? | ||||
| Cardiac cachexia (%) | 87.7 | 80.6 | 36.0 | 45.1 |
| Clinically judged to be close to the end of life (%) | 87.7 | 90.6 | 92.0 | 91.5 |
| Communication barrier (%) | 16.9 | 10.0 | 12.0 | 16.9 |
| Continued high levels of serum natriuretic peptides (BNP/NT‐proBNP) (%) | 23.1 | 16.3 | 10.0 | 19.7 |
| Dependence in most activities of daily living (%) | 61.5 | 58.1 | 50.0 | 53.5 |
| Frequent admission to hospital or other serious episodes of decompensation, despite exhausting all treatment options (%) | 76.9 | 80.0 | 70.0 | 70.4 |
| Low serum albumin (%) | 35.4* | 21.3 | 10.0 | 9.9 |
| Low serum sodium (%) | 44.6* | 14.4 | 12.0 | 9.9 |
| Symptoms at rest, and heart transplantation and mechanical circulatory support ruled out (%) | 83.1 | 72.5 | 66.0 | 54.9 |
| Treatment failure at tertiary centre (%) | 60.0 | 77.5* | 60.0 | 71.8 |
| Very poor quality of life (%) | 83.1 | 82.5 | 68.0 | 85.9** |
BNP/NT‐proBNP, brain natriuretic peptide/N‐terminal pro‐brain natriuretic peptide; COPD, chronic obstructive pulmonary disease; PCP, primary care physician.
Significant difference (P < 0.005) between physician groups.
Significant difference (P < 0.005) between nurse groups.
Diagnosis
Among 22 symptoms and signs of HF, just 11 (50%) were routinely assessed and considered to be HF markers by ≥90% of physicians. In particular, fewer than 50% of cardiologists and PCPs routinely assessed or considered the following as markers of HF: forgetfulness, confusion, and sputum (Table 2).
When asked about diagnostic tests, many PCPs did not consider echocardiography (34%), ECG (39%), or laboratory tests (59%) to be essential for HF diagnosis. Approximately one‐third of PCPs (32%) reported that performing or providing a referral for echocardiography was not relevant to their role in patient management (Table 2).
In terms of diagnostic confidence, cardiologists reported high levels of confidence (≥70% scoring 6–7) in identifying a number of aspects of HF diagnosis (Figure 1 ); they were relatively less confident in diagnosing HF with preserved ejection fraction (HF‐PEF) and providing patient education. Although PCPs were most confident in identifying atrial fibrillation, <70% scored 6–7 for any other aspect of diagnosis. Notably, PCPs were least confident in the following aspects: diagnosing HF‐PEF, diagnosing HF with reduced ejection fraction (HF‐REF), and providing patient education relevant to HF diagnosis and management.
Figure 1.
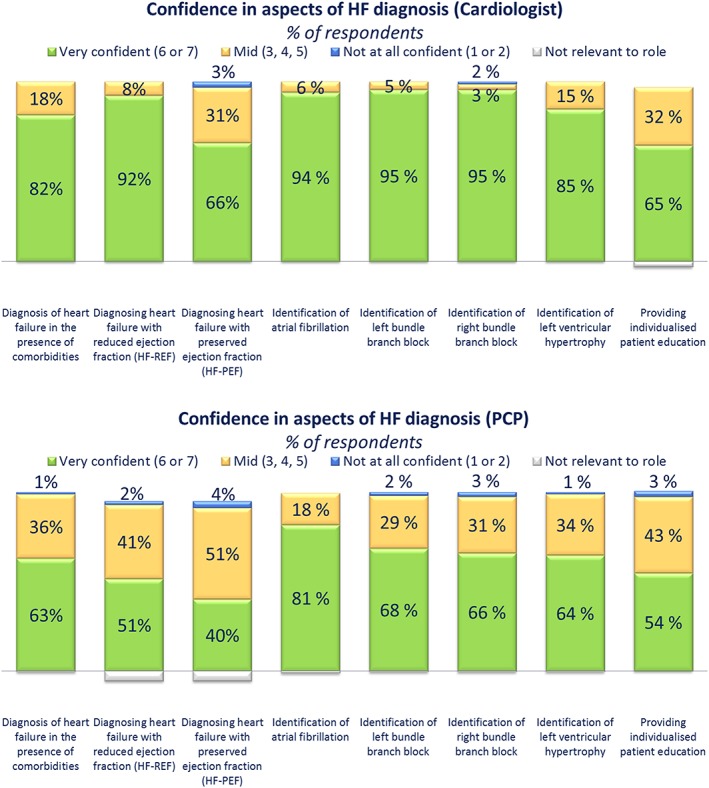
Confidence gaps in heart failure (HF) diagnosis among physicians. Cardiologists and primary care physicians (PCPs) were asked: ‘How confident are you in carrying out each of the following aspects of HF diagnosis on a scale of 1 to 7, where 1 is not at all confident and 7 is very confident?’
Treatment
Physicians reported high rates of using diuretics (94–100%), angiotensin‐converting enzyme (ACE) inhibitors (99–100%), and beta‐blockers (97–99%) in patients with HF (Table 3). The use of mineralocorticoid receptor antagonists (MRAs) was slightly lower (88–94%). Although prescribing rates for indicated agents were high, dosing was often inconsistent with guideline recommendations.
Physicians were generally less confident in aspects of non‐pharmacological interventions compared with pharmacological treatments. Of note, when asked about identifying patients suitable for cardiac resynchronization therapy, implantable cardiac defibrillator, left ventricular assist devices, or heart transplant, 38–82% of cardiologists and 9–14% of PCPs described themselves as ‘very confident’.
Monitoring
The survey results revealed that among physicians, a lower proportion (54–64%) reported being involved in patient monitoring after each dose increment or at the end of medication compared with during treatment initiation (71.9–86.2%; Table 4).
Regarding medication‐specific monitoring, only 76% of cardiologists and 68% of PCPs reported serial monitoring of serum electrolytes in patients prescribed MRAs. In addition, <70% of physicians reported monitoring blood chemistry (creatinine and potassium) 1–2 weeks after initiating ACE inhibitors and 1–2 weeks after final dose titration. Only 58% of cardiologists and 57% of PCPs reported monitoring blood chemistry 1–2 weeks after initiating or dose‐titrating diuretic therapy.
Long‐term management
More than 85% of cardiologists and PCPs routinely screened HF patients for angina and hypertension. By comparison, <60% reported screening HF patients for other comorbidities including asthma, cancer, cognitive impairment, cachexia, depression/anxiety, or gout (Table 4). In both physician groups, few respondents reported being ‘very confident’ in managing HF in the presence of a number of other commonly co‐occurring conditions: for PCPs, this was particularly evident in patients with cancer, cerebrovascular disease, Alzheimer's disease or dementia, and cachexia (23–34%, ‘very confident’); for cardiologists, this was true for anxiety, cancer, Alzheimer's disease or dementia, cachexia, and depression (18–35%, ‘very confident’).
Regarding end‐of‐life (EOL) care, the majority of physicians acknowledged the potential benefits of EOL care among HF patients who are clinically judged to be close to the end of life (88–92%), who have a very poor quality of life (83%), and who undergo frequent hospital admissions (77–80%; Table 4). Many cardiologists and PCPs (13–23%) reported they were not involved in the management of HF patients during EOL care.
Physicians reported substantial gaps related to HF patient education, with a large portion of clinicians (25–56%) ‘rarely’ or ‘never’ discussing advanced care, the role of the caretaker, psychological health, prognosis, self‐care, sexual activity, and travel/leisure with their patients. Relative to cardiologists, PCPs were more likely to discuss HF aetiology with their patients.
Neither primary care nor specialist HCPs were confident in the referral process; PCPs expressed difficulty in following guidelines and local procedures for this (47% and 49% ‘very confident’, respectively), and just 29% of cardiologists were ‘very confident’ transferring patients to primary care (see Supporting Information, Figure S1 ). PCPs expressed uncertainty around referring HF patients to secondary care, as well as low expectations that adequate handover information will be supplied when HF patients are transferred back into primary care. By comparison, cardiologists reported a lack of confidence that patients would be managed effectively in primary care, with 58% citing the lack of sufficient primary care resources as a cause for concern (see Supporting Information, Figure S1 ).
Nurses' attitudes and practice patterns
Diagnosis
Nurses, particularly primary care nurses, perceived many aspects of diagnosis as ‘not relevant’ to their role. In total, <70% of primary care nurses assessed the following signs and symptoms of HF: third heart sound, excess sputum, nocturia, heart murmur, forgetfulness, elevated jugular pressure, displaced apex beat, confusion, anorexia, and abdominal bloating (Table 2). Additionally, 50% of cardiac nurses and 58% of primary care nurses considered echocardiography to be outside of their scope of practice. Regarding diagnostic confidence, of all aspects of diagnosis (Figure 2 ), primary care nurses were least confident in diagnosing HF‐REF and HF‐PEF (15%, ‘very confident’). However, it is noteworthy that 37–38% of primary care nurses considered diagnosing HF‐REF or HF‐PEF to be not relevant to their role. Cardiac nurses were also least confident in diagnosing HF‐REF (38%, ‘very confident’) and HF‐PEF (30%, ‘very confident’) relative to the other aspects of diagnosis (identification of atrial fibrillation, left bundle branch block, right bundle branch block, and left ventricular hypertrophy and providing individualized patient education).
Figure 2.
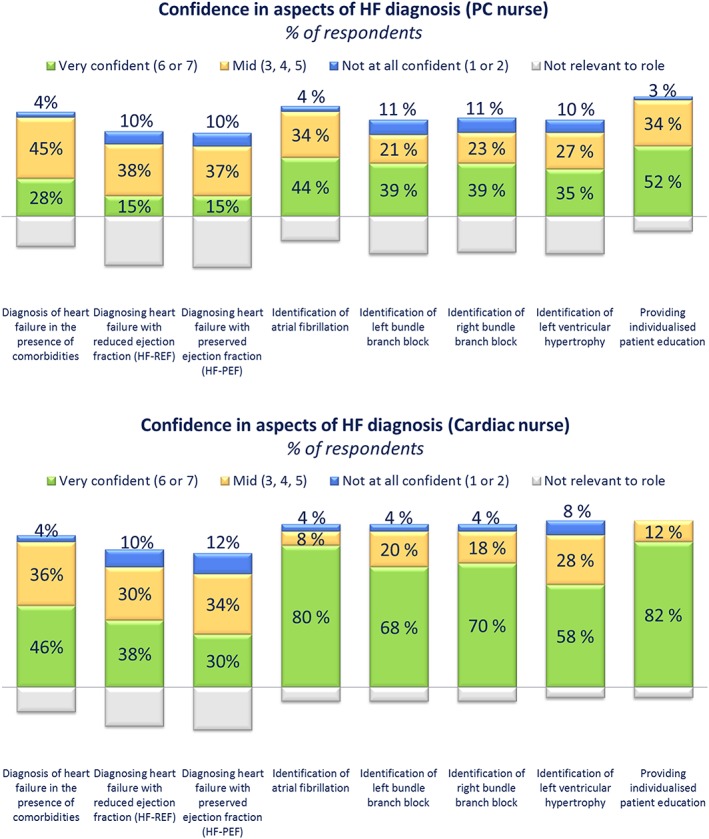
Confidence gaps in heart failure (HF) diagnosis among nurses. Cardiac and primary care (PC) nurses were asked: ‘How confident are you in carrying out each of the following aspects of HF diagnosis on a scale of 1 to 7, where 1 is not at all confident and 7 is very confident?’
Monitoring
In general, nurses' involvement with patient monitoring was lower than that of physicians at all stages of HF management (Table 4). However, cardiac nurses were more involved than primary care nurses, particularly after each dose increment (66% vs. 35%; P < 0.005) and at the end of medication (44% vs. 20%; P < 0.005). Furthermore, cardiac nurses were significantly less likely than primary care nurses to report not being involved in patient monitoring at all (4% vs. 20%; P < 0.005).
Long‐term management
Compared with physicians, nurses were less likely than physicians to recognize several clinical features that indicate that an HF patient might benefit from EOL care, including cardiac cachexia, low serum albumin and sodium, and having symptoms at rest (Table 4). In addition, a substantial proportion of nurses (20% cardiac nurses and 30% primary care nurses) reported that they were not involved in the management of HF patients during EOL care (see Supporting Information, Table S1 ).
Notable differences between primary and secondary care
Substantial differences in practice and knowledge are anticipated between primary care HCPs and those who possess specialist training in cardiology. Nonetheless, it is of some interest to understand where appreciable differences exist between the two groups. The most notable differences are summarized in Supporting Information, Tables S2 and S3 .
Initial observations of differences between countries
Although a full analysis of national differences is reserved for future analyses, a primary review of these data highlighted some interesting differences between countries, which we plan to expand upon in future work.
With respect to diagnostic tests, there was variation in the use of several key diagnostic tests. The use of natriuretic peptides was lowest in Australia (44%) and highest in the UK (71%); spirometry use was lowest in the UK (38%) and highest in Canada (58%), and the use of chest X‐ray was lowest in Sweden (42%) and highest in Australia and the UK (76%; data on file). We suspect this reflects heterogeneity of test availability and access in each of the countries, as well as some differences between national guidelines. For example, at the time of issuing the survey, natriuretic peptide testing was not a routine practice in Australian guidelines and has only recently been considered preferable (an essential when echocardiogram is unavailable).
With respect to prescribing habits, cardiologists from the UK and Canada generally prescribed most medication classes less frequently than cardiologists from all other countries. Notably, angiotensin receptor blockers, diuretics, and MRAs were routinely prescribed by <80% of cardiologists in the UK and Canada, whereas this was 100% by cardiologists in all other countries. Of note, beta‐blockers were routinely prescribed by 90% of cardiologists in Canada, compared with 100% in all other countries (data on file). Again, without further analysis, we can only attribute this to differences in medication availability at this point.
Finally, with respect to patient referral practices, all countries displayed a moderate degree of confidence with following local procedures, following local guidelines and identifying patients who require referral to secondary care (average confidence level 5.5 out of 7), with the exception of Austria. Austria displayed notably lower confidence (average confidence 3.5 out of 7; data on file).
Discussion
To date, multiple studies have identified gaps and challenges in modern HF care focused on specific provider types and geographic regions.5, 6, 7, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 The CORE Needs Assessment Survey adds to the evidence base by describing practice patterns across primary care and specialist physicians and nurses using the same instrument. By including HCPs from multiple continents, the CORE Needs Assessment Survey provides a more global insight on HF practice trends. Findings from the survey will be useful for tailoring educational interventions to address knowledge and practice gaps in the following key areas: HF diagnosis, treatment planning, treatment monitoring and adaptation, long‐term management, and multidisciplinary care in HF.
Addressing gaps in heart failure diagnosis
The accurate and timely diagnosis of HF is critical for guiding appropriate treatment,3, 4 yet diagnostic tests are underutilized by many clinicians, as evidenced in the Study on Heart failure Awareness and Perception Europe and EuroHeart Failure Survey.5, 6 In primary care, reluctance to use certain tests, including echocardiography, may be due to lack of knowledge about how to interpret results.18
In the present study, clinicians reported considering just half of all symptoms and signs of HF. Therefore, there is a need to raise awareness about the importance of assessing less specific symptoms and signs (e.g. confusion or dizziness). Case studies are an ideal format for reinforcing best practices around the diagnostic assessment of patients with suspected HF.
Among diagnostic tests for HF, PCPs and nurses reported low confidence in carrying out and interpreting the results of echocardiography, and nurses were unlikely to carry out or refer patients for echocardiography, ECG, and laboratory tests. It should be noted that, depending on local procedures, such activities may lie outside of the professional remit of some nurses, although, regardless of this, nurses and PCPs would benefit from tailored education that reviews when to order these tests and how to interpret the results.
Only cardiologists were confident in their diagnostic skills related to HF‐REF, and all HCP cohorts were least confident in diagnosing HF‐PEF. Educational interventions designed to address gaps in HF diagnosis should focus on all HF subtypes to reinforce baseline knowledge on HF‐PEF and improve knowledge and confidence around the diagnosis of HF‐REF.
Many PCPs and primary care nurses—and, to a lesser extent, cardiac nurses—expressed a lack of confidence in providing patient education at the time of HF diagnosis. Others reported that HF patient education was not part of their role as a member of the HF care team. However, the ESC guidelines emphasize the critical role of patient education is supporting optimal patient outcomes.3 To enhance confidence around patient education, PCPs, primary care nurses, and cardiac nurses would benefit from a checklist of key patient education topics, as well as practical tools for improving patient/HCP communication.
Addressing gaps in treatment planning
In the Study on Heart failure Awareness and Perception Europe and EuroHeart Failure studies, effective first‐line medications were underutilized and/or prescribed at subtherapeutic doses in many patients with HF.5, 6 The CORE survey provides a current snapshot of prescribing practices on a global level and suggests that cardiologists and PCPs appeared to prescribe diuretics, MRAs, and other common HF medications at rates and/or doses that are inconsistent with guideline recommendations. Although the underlying reasons driving these prescribing patterns are unclear, these findings support the need for education reinforcing evidence‐based dosing, titration, indications, and sequencing of HF medications, with an emphasis on diuretics, MRAs, ACE inhibitors, angiotensin receptor blockers, and beta‐blockers. Clinicians would benefit from detailed guidance on identifying appropriate HF patients for non‐pharmacological interventions.
Both cardiac and primary care nurses infrequently engaged with patients about pharmacological treatment, highlighting the presence of barriers in this aspect of patient education. Nurses may benefit from an educational intervention that models effective patient education around basic HF treatment options.
Addressing gaps in treatment monitoring and adaptation
Poor patient monitoring is a widespread barrier to better HF care: in the UK, only half of NHS organizations had institutional protocols that met minimum requirements regarding patient monitoring13 and across Europe, only seven of 26 countries surveyed reported having organized protocols for HF patient monitoring in more than 30% of their hospitals.14
In the present study, medication monitoring emerged as an area with a high degree of variability in knowledge and confidence across respondent cohorts. These findings support an educational strategy that provides context around monitoring requirements from a treatment‐pathway perspective, for example, what tests should be carried out and when for patients receiving an ACE inhibitor. This provides a practical addition to reviewing abstract concepts related to blood pressure, heart rhythm, and serum marker monitoring. Of note, all HCP groups tended to monitor patients less frequently after each dose increment and at the end of treatment, with a substantial minority of primary care nurses reporting that they were not involved in patient monitoring at any stage. These findings highlight the need for a ‘best practice’ section that includes a checklist for each HCP group to raise awareness of the importance of medication monitoring at each HF treatment stage.
Respondents demonstrated variable levels of confidence related to the management of patients with cardiac and non‐cardiac comorbidities, highlighting the importance of reviewing all comorbid conditions, with an emphasis placed on common pitfalls and how to resolve them. This will be particularly important for building clinical skills related to HF management in patients with conditions that were rated universally low in confidence (i.e. cancer, cerebrovascular disease, Alzheimer's disease, and cachexia.)
Addressing gaps in long‐term heart failure management
More than half of HF readmissions may be preventable with better discharge planning, patient education, and follow‐up care, yet these components of long‐term HF management are often lacking.15, 16
The CORE needs assessment evaluated a spectrum of best practices related to long‐term HF management, including emerging options for long‐term monitoring (e.g. telemonitoring and home‐based care), patient education, transitions of care, and EOL care.
Clinicians reported rarely or never discussing several topics with their patients (e.g. advanced care, the role of the caretaker, psychological health, prognosis, self‐care, sexual activity, and travel/leisure), while other topics were discussed at the time of HF diagnosis only (e.g. HF aetiology). These findings indicate an ongoing need to build skills related to effective patient education across a range of topics throughout the HF treatment continuum.
Regardless of provider type, multiple barriers appeared to contribute to poor transitions of care for patients with HF. PCPs and primary care nurses reported low confidence related to following guidelines, procedures, and identifying patients who require referring to secondary care. Similarly, confidence was low among cardiologists and cardiac nurses regarding the level of care offered to patients moving back into primary care. To address these, HCPs may benefit from practical advice about implementing guidelines around care transitions, while interactive exercises may help to build skills around recognizing which patients are most likely to benefit from being moved into secondary care.
The majority of HCPs failed to identify many of the clinical signs that indicate that a patient may benefit from EOL care, suggesting missed opportunities in clinical practice. To address this gap, all HCP cohorts would benefit from exercises that explore the clinical signs of end‐stage HF and highlight the rationale for EOL care.
Study limitations
Limitations associated with the design of the CORE Needs Assessment Survey include the lack of a focus group for preliminary testing and instrument validation, the online format of administration, the potential for selection bias among respondents, and the relatively small number of respondents from widely disparate health systems. Practice patterns were measured against the 2012 ESC HF guidelines, which have since been updated.4 Further, the CORE Needs Assessment Survey was not designed to detect institutional or systematic barriers to optimal HF care. Where discrepancies between guideline recommendations and clinical practice were identified, the extent to which health system protocols, local standards of care, legal restrictions, time constraints, and other issues contributed to these differences is not clear.
Clinical implications
In summary, the CORE Needs Assessment Survey identified multiple knowledge and confidence gaps that are shared across members of the HF care team, as well as those that are unique to cardiologists, PCPs, cardiac nurses, and primary care nurses involved in the management of patients with HF. These data can inform future educational initiatives aimed to support improved patient outcomes through enhanced delivery of evidence‐based HF care.
Conflict of Interest
Professor Kenneth Dickstein reports honoraria and/or research support from the following device companies: Medtronic, Boston Scientific St Jude, Biotronik, and Sorin and the following pharmaceutical companies: Merck, Novartis, Amgen, Boehringer Ingelheim, Astra Zeneca, Pfizer, Bayer, GSK, Roche, Sanofi, Abbott, Otsuka, Leo, Servier, and Bristol Meyers Squibb from outside the submitted work. He is currently a member of the following steering committees: ATMOSPHERE (Novartis), ELIXA (Sanofi), CRT Survey II (ESC), and CORE (PCM Healthcare). He is also a DSMB member of ADAPT (Medtronic) and RELAX (Novartis).
Dr Ahmet Fuat reports personal fees from Servier, personal fees from Novartis, personal fees from Roche, personal fees from Roche Diagnostics, and personal fees from Alere, outside the submitted work.
Dr Jonathan Howlett reports speaker fees from Novartis, Servier, Medtronic, Bayer Canada, and AstraZeneca.
Dr Gerhard Poelzl reports speaker fees from Novartis and is the medical director of a collaborative heart failure disease management programme in Austria.
Dr Josep Comin‐Colet has nothing to disclose.
Funding
CORE is supported by funding from Novartis Pharma AG. All educational content and materials are created by the CORE Steering Committee in collaboration with PCM Scientific, the medical education company acting as secretariat. The financial supporter has had no involvement in the creation or development of the educational content.
Supporting information
Table S1. Nurse and physician interaction with MDT members during EOL care
Table S2. Heart failure practice differences among physicians
Table S3. Heart failure practice differences among nurses
Figure S1. Confidence gaps in referral practices
Acknowledgements
This report is based on the expert opinion of the CORE Steering Committee. Editorial assistance was provided by Anne Jacobson of CPD Content Group LLC and Dr Sean Delaney of PCM Healthcare Ltd, professional medical education companies, in drafting this manuscript. The authors have had full control of the final content and the decision to publish.
Appendix A. CORE Steering Committee
-
1
Dr Josep Comin‐Colet (Spain)
-
2
Mrs Amanda Crundall‐Goode (UK)
-
3
Prof Kenneth Dickstein (Norway)
-
4
Prof Ahmet Fuat (UK)
-
5
Dr Joe Gallagher (Ireland)
-
6
Dr Jonathan Howlett (Canada)
-
7
Dr Gerhard Pölzl (Austria)
-
8
Prof Simon Stewart (Australia)
-
9
Prof Anna Stromberg Strömberg (Sweden)
Howlett, J. , Comin‐Colet, J. , Dickstein, K. , Fuat, A. , Pölzl, G. , and Delaney, S. (2018) Clinical practices and attitudes regarding the diagnosis and management of heart failure: findings from the CORE Needs Assessment Survey. ESC Heart Failure, 5: 172–183. doi: 10.1002/ehf2.12205.
References
- 1. Guha K, McDonagh T. Heart failure epidemiology: European perspective. Current cardiology reviews 2013; 9: 123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McMurray JJV, Stewart S. The burden of heart failure. Eur Heart J Supplements 2002; 4(suppl D): D50–D58. [Google Scholar]
- 3. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012; 33: 1787–1847. [DOI] [PubMed] [Google Scholar]
- 4. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. Authors/Task Force M, Document R. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 5. Remme WJ, McMurray JJ, Hobbs FD, Cohen‐Solal A, Lopez‐Sendon J, Boccanelli A, Zannad F, Rauch B, Keukelaar K, Macarie C, Ruzyllo W, Cline C, Group SS . Awareness and perception of heart failure among European cardiologists, internists, geriatricians, and primary care physicians. Eur Heart J 2008; 29: 1739–1752. [DOI] [PubMed] [Google Scholar]
- 6. Komajda M, Follath F, Swedberg K, Cleland J, Aguilar JC, Cohen‐Solal A, Dietz R, Gavazzi A, Van Gilst WH, Hobbs R, Korewicki J, Madeira HC, Moiseyev VS, Preda I, Widimsky J, Freemantle N, Eastaugh J, Mason J. The EuroHeart Failure Survey programme—a survey on the quality of care among patients with heart failure in Europe. Part 2: treatment. Eur Heart J 2003; 24: 464–474. [DOI] [PubMed] [Google Scholar]
- 7. Calvert MJ, Shankar A, McManus RJ, Ryan R, Freemantle N. Evaluation of the management of heart failure in primary care. Fam Pract 2009; 26: 145–153. [DOI] [PubMed] [Google Scholar]
- 8. Cervero RM, Gaines JK. The impact of CME on physician performance and patient health outcomes: an updated synthesis of systematic reviews. The Journal of continuing education in the health professions 2015; 35: 131–138. [DOI] [PubMed] [Google Scholar]
- 9. Maggioni AP, Dahlstrom U, Filippatos G, Chioncel O, Crespo Leiro M, Drozdz J, Fruhwald F, Gullestad L, Logeart D, Fabbri G, Urso R, Metra M, Parissis J, Persson H, Ponikowski P, Rauchhaus M, Voors AA, Nielsen OW, Zannad F, Tavazzi L. Heart Failure Association of the European Society of C. EURObservational Research Programme: regional differences and 1‐year follow‐up results of the Heart Failure Pilot Survey (ESC‐HF Pilot). Eur J Heart Fail 2013; 15: 808–817. [DOI] [PubMed] [Google Scholar]
- 10. Seferovic PM, Stoerk S, Filippatos G, Mareev V, Kavoliuniene A, Ristic AD, Ponikowski P, McMurray J, Maggioni A, Ruschitzka F, van Veldhuisen DJ, Coats A, Piepoli M, McDonagh T, Riley J, Hoes A, Pieske B, Dobric M, Papp Z, Mebazaa A, Parissis J, Ben Gal T, Vinereanu D, Brito D, Altenberger J, Gatzov P, Milinkovic I, Hradec J, Trochu JN, Amir O, Moura B, Lainscak M, Comin J, Wikstrom G, Anker S. Committee of National Heart Failure Societies or Working Groups of the Heart Failure Association of the European Society of C. Organization of heart failure management in European Society of Cardiology member countries: survey of the Heart Failure Association of the European Society of Cardiology in collaboration with the Heart Failure National Societies/Working Groups. Eur J Heart Fail 2013; 15: 947–959. [DOI] [PubMed] [Google Scholar]
- 11. British Healthcare Business Intelligence Association (BHBIA) . Legal and ethical guidelines for healthcare market research: your essential guide 2016. Available online at: http://www.bhbia.org.uk/guidelines/legalandethicalguidelines.aspx. Accessed August 10, 2016.
- 12. Cline CM, Boman K, Holst M, Erhardt LR. Swedish Society of Cardiology Working Group for Heart F. The management of heart failure in Sweden. Eur J Heart Fail 2002; 4: 373–376. [DOI] [PubMed] [Google Scholar]
- 13. Sutherland K. Bridging the quality gap: heart failure. The Health Foundation—Inspiring Improvement. 2010. [Google Scholar]
- 14. Jaarsma T, Stromberg A, De Geest S, Fridlund B, Heikkila J, Martensson J, Moons P, Scholteop Reimer W, Smith K, Stewart S, Thompson DR. Heart failure management programmes in Europe. Eur J Cardiovasc Nurs 2006; 5: 197–205. [DOI] [PubMed] [Google Scholar]
- 15. Paul S. Hospital discharge education for patients with heart failure: what really works and what is the evidence? Crit Care Nurse 2008; 28: 66–82. [PubMed] [Google Scholar]
- 16. Adib‐Hajbaghery M, Maghaminejad F, Abbasi A. The role of continuous care in reducing readmission for patients with heart failure. J Caring Sci 2013; 2: 255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Close H, Hancock H, Mason JM, Murphy JJ, Fuat A, de Belder M, Hungin AP. “It's Somebody else's responsibility”—perceptions of general practitioners, heart failure nurses, care home staff, and residents towards heart failure diagnosis and management for older people in long‐term care: a qualitative interview study. BMC Geriatr 2013; 13: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hancock HC, Close H, Fuat A, Murphy JJ, Hungin AP, Mason JM. Barriers to accurate diagnosis and effective management of heart failure have not changed in the past 10 years: a qualitative study and national survey. BMJ Open 2014; 4: e003866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McAlister FA, Stewart S, Ferrua S, McMurray JJ. Multidisciplinary strategies for the management of heart failure patients at high risk for admission: a systematic review of randomized trials. J Am Coll Cardiol 2004; 44: 810–819. [DOI] [PubMed] [Google Scholar]
- 20. Heart Chest & Scotland Stroke. Review of Specialist Heart Failure Nurse Services. 2013. [Google Scholar]
- 21. British Heart Foundation . Heart failure nurse services in England: executive summary. August 2008.
- 22. Blue L, Lang E, McMurray JJ, Davie AP, McDonagh TA, Murdoch DR, Petrie MC, Connolly E, Norrie J, Round CE, Ford I, Morrison CE. Randomised controlled trial of specialist nurse intervention in heart failure. BMJ 2001; 323: 715–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Erhardt L, Komajda M, Hobbs FD, Soler‐Soler J. Cardiologists' awareness and perceptions of guidelines for chronic heart failure. The ADDress your Heart survey. Eur J Heart Fail 2008; 10: 1020–1025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Nurse and physician interaction with MDT members during EOL care
Table S2. Heart failure practice differences among physicians
Table S3. Heart failure practice differences among nurses
Figure S1. Confidence gaps in referral practices


