Abstract
Aims
Heart failure with a preserved ejection fraction (HF‐PEF) remains a difficult clinical diagnosis. The aim of this study was to test the utility of established criteria to classify patients with HF‐PEF. We prospectively enrolled patients into one of five groups across a spectrum of cardiac disease and applied three different criteria for HF‐PEF and calculated diagnostic metrics.
Methods and results
A total of 565 patients were included in the analysis, including 170 patients with an adjudicated diagnosis of HF‐PEF, 152 patients with heart failure with reduced ejection fraction, 152 patients at risk for heart failure, and 91 age‐matched healthy controls. For the diagnosis of HF‐PEF, the positive likelihood ratios were 6.1, 6.9, and 4.8 for the Zile, European Society of Cardiology (ESC) 2007, and ESC 2016 criteria, respectively. The negative likelihood ratios were 0.58, 0.60, and 0.42 for the Zile, ESC 2007, and ESC 2016 criteria, respectively. All three criteria lacked sensitivity to detect HF‐PEF (46.5%, 44.1%, and 51.8%, respectively) but were highly specific (92.4%, 93.9%, and 89%, respectively). We further evaluated the criteria to distinguish HF‐PEF from other diagnoses after excluding heart failure with reduced ejection fraction; the results were similar.
Conclusions
In this community based cohort, the likelihood ratios of the existing criteria for HF‐PEF were not at the level necessary to be considered diagnostic. Improved criteria for the diagnosis of patients with HF‐PEF are needed.
Keywords: Heart failure, Validation, Diagnosis
Introduction
Heart failure (HF) remains a significant public health problem with high morbidity, mortality, and symptom burden. More recently, research and guidelines have focused on classifying patients based on the left ventricular ejection fraction (LVEF) creating two distinct clinical groups, HF with reduced EF (HF‐REF) or preserved EF (HF‐PEF). This has proved challenging because of the lack of a widely accepted reference standard and thus HF‐PEF remains a difficult clinical diagnosis—thus expert‐opinion derived algorithms and diagnostic criteria have been developed to aid in the diagnosis.
These criteria have not been adequately tested, and their validity remains uncertain. Additionally, many other diseases may mimic HF‐PEF because of the overlap of symptoms or signs (e.g. lung disease, hypertension, diabetes, obesity, and deconditioning) and imaging or biomarker, which results may or may not provide further clarity. Indeed, given the heterogeneity of the HF‐PEF as a syndrome, the lack of a consensus on a single criterion is not surprising. For example, the European Society of Cardiology (ESC) HF guidelines provides criteria that have evolved over time. The ESC 2007 guideline used a stepwise diagnostic algorithm that incorporated signs, symptoms, echocardiographic, ECG findings, and natriuretic peptides.1 When evaluated, in a modest‐sized cohort, the ESC 2007 criteria had a positive and negative predictive value of 81% and 80% for the diagnosis of HF‐PEF with positive and negative likelihood ratios of 5.5 and 0.3, respectively.2
The ESC further simplified this in 2016 to include relevant structural heart disease in addition to signs and symptoms typical of HF.3 Another criterion proposed by Zile et al. uses a simplified definition: the presence of clinical heart failure per Framingham criteria and an EF >50%.4 While the American Heart Association refers to this criterion, it does not provide specificity that can be applied in clinical practice. Other major guidelines do not provide specific criteria beyond an EF cutpoint.5, 6 Clinical trials have emphasized a prior HF hospitalization, symptoms, and in some cases, additional ECG, echocardiographic, or biomarker information to support the diagnosis. None of these criteria have been evaluated as to their ability to distinguish patients with HF‐PEF from other clinical entities.
Accordingly, the aim of this study was to test the utility of established criteria to classify patients with HF‐PEF within the Alberta Heart failure Aetiology and Analysis Team (HEART) study.7 In Alberta HEART, patients were prospectively enrolled into one of five groups that loosely followed the American Heart Association / American College of Cardiology (AHA/ACC) stages of HF and included clinically relevant comparators and adjudication.8 We further studied standard and advanced biomarkers and imaging as to their added discriminatory and diagnostic value.
Methods
The Alberta HEART study has been previously described.7 In brief, the cohort was recruited in Alberta, Canada, from 2010 to 2014 from a variety of different clinics and the community at large. Patients were prospectively enrolled into the study, which was approved by the Health Research Ethics Boards at the University of Alberta, University of Calgary, and Covenant Health. Written informed consent was obtained. The study is registered (clinicaltrials.gov NCT02052804).
Participants
Enrolled patients were recruited into one of five groups: Group I (At‐Risk): high risk of developing HF‐PEF and no clinically overt HF or known cardiovascular disease; Group II (At‐Risk + Symptoms): high risk of developing HF‐PEF, no clinically overt HF, and the presence of another symptomatic disease (e.g. chronic lung disease, coronary artery disease, and atrial fibrillation); Group III (HF‐PEF): clinical HF‐PEF; Group IV (HF‐REF): clinical HF‐REF; and Group V (Control): age‐matched and gender‐matched controls.
Study design and choice of reference standard
Each patient was initially recruited, enrolled, and subsequently adjudicated by team members with clinical experience and expertise into a group. The adjudication process required two expert clinicians to review each case independently and blinded to each other's adjudication. Past medical details available included medical history, echocardiography, prior EF, other radiology testing, and laboratory information. Natriuretic testing is not routine for clinical purposes in our locale. No specific definition was provided to adjudicators, and the Alberta HEART specific tests (e.g. echocardiogram, cardiac magnetic resonance imaging, and blood tests) were not made available to adjudicators. After enrolment, participants underwent a research echocardiogram, cardiac magnetic resonance imaging, and blood tests—these were not used for group assignment.
Definitions
Given the breadth of criteria that have been published, we selected criteria that could be reasonably tested and provided enough detail as to their construct. We used the guideline explicitly as written without further interpretation.
ESC (2007) criteria: This uses a stepwise diagnostic construct starting with the signs and symptoms of heart failure, adding EF of >50%, and then uses a variety of electrical, biomarker, and mechanical findings to categorize patients into a binary yes/no HF‐PEF.
ESC (2016) criteria: The diagnosis of HF‐PEF requires four conditions to be satisfied including symptoms and signs typical of HF, normal, or only mildly reduced LVEF and LV not dilated and relevant structural heart disease [LV hypertrophy >95 g/m2 women, >115 g/m2 men, or left atrial enlargement with left atrial volume index (LAVI) >34 mL/m2] and/or diastolic dysfunction (e' decreased, E/e' >15) and elevated natriuretic peptides (BNP >35 pg/mL or N terminal pro‐BNP >125 pg/mL).
Zile et al.: This criterion incorporates the presence of symptoms per Framingham criteria and an EF >50%.4
Variables
Standard baseline demographics, laboratory, and other medical history were collected via direct contact with the patient and with medical record review. Transthoracic echocardiography was performed with the subjects at rest in left lateral decubitus position using commercially available Phillips iE33 ultrasound imaging system (Philips Medical Systems, Andover, MA, USA) equipped with S5‐phased or X5‐phased array transducer. All images were digitally stored for offline analysis (Xcelera, Philips Medical System, Andover, MA, USA). Standard apical four‐ and two‐chamber views were recorded with care taken to avoid foreshortening. LV volumes were measured from the apical four‐ and two‐chamber views. Left ventricular end‐systolic volume and end‐diastolic volume were calculated using Simpson's biplane method of discs.9 LVEF was subsequently derived and expressed as a percentage.
Data sources
Study data were collected and managed using REDCap electronic data capture tools hosted at the University of Alberta.10
Statistics
Data on patient characteristics at enrolment including demographic, clinical symptoms, comorbidities, and echocardiogram parameters were presented for each of the five diagnosis groups. Descriptive measures, median and interquartile range and/or mean (SD) for continuous variables, and frequency (%) for categorical variables have been estimated.
The existing criteria are primarily aimed at diagnosing HF‐PEF as ‘Yes’ or ‘No’. For the purpose of evaluating each diagnosis criterion to diagnose HF‐PEF patients, the adjudicated classification was considered as the reference standard, and thus, patients in group III were assumed as the actual HF‐PEF, while all the remaining groups were assumed to be not HF‐PEF.
Measures of performance derived from resulting 2 × 2 confusion matrix (sensitivity, specificity, positive likelihood ratio, and negative likelihood ratio) were applied. Both point estimates and the 95% confidence intervals of the measures of performance were reported. The statistical significance of the differences in accuracy among the methods was tested applying the McNemar test. Because the criteria are not model based and do not provide a probability estimate of diagnostic group membership, the area under the curve could not be computed. All statistical analysis was performed using sas version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
A total of 621 patients were enrolled in the Alberta HEART cohort between 2009 and 2014 (Figure 1 ). Based on the initial group assignment, 113, 47, 170, 141, and 91 patients were in Groups I through V, respectively. After adjudication with clinically available data, 39 patients moved between groups for a total of 115, 48, 191, 169, and 98 patients in Adjudicated Groups I through V, respectively. A total of 56 patients were not included in further analysis because of missing information on systolic (n = 49) or diastolic function (n = 7) due to poor quality echocardiographic images, leaving a total of 565 patients for the primary analyses. Patients who were excluded did not differ substantively from other patients in their adjudicated group (data not shown) and were distributed evenly across groups.
Figure 1.
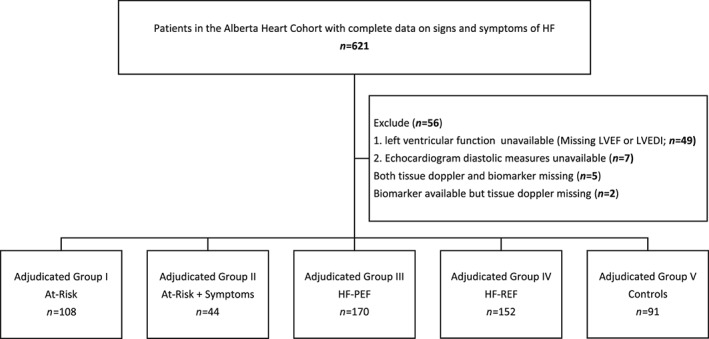
Patient distribution. HF, heart failure; HF‐PEF, heart failure with preserved ejection fraction; HF‐REF, heart failure with reduced ejection fraction; LVEF, left ventricular ejection fraction; LVEDI, left ventricular end‐diastolic index.
Baseline characteristics of the patients, by adjudicated group, are in Table 1. Notably, patients with HF‐PEF or HF‐REF had a lower haemoglobin, estimated glomerular filtration rate, and elevated natriuretic peptides compared with Groups I, II, and V. Patients with HF‐PEF were older than patients with HF‐REF.
Table 1.
Baseline characteristics of patients by the adjudicated groups (n = 565)
| Patient characteristics | I | II | III | IV | V |
|---|---|---|---|---|---|
| At‐Risk | At‐Risk + Symptoms | HF‐PEF | HF‐REF | Controls | |
| n (%) | n (%) | n (%) | n (%) | n (%) | |
| n | 108 | 44 | 170 | 152 | 91 |
| Male | 47 (44) | 36 (82) | 91 (54) | 114 (75) | 34 (37) |
| Age, median (25th–75th percentile) | 64 (57, 70) | 67 (63, 73) | 73 (63, 80) | 64 (57, 72) | 62 (53, 72) |
| Ethnicity | |||||
| Caucasian | 102(94) | 38(86) | 153(90) | 136(90) | 86(95) |
| Aboriginal | 2(5) | 5(3) | 4(3) | ||
| South Asian | 3 (3) | 2 (5) | 8 (5) | 7 (5) | 5 (6) |
| Other | 3 (3) | 2 (5) | 4 (2) | 5 (3) | |
| CCS Angina classification | |||||
| 0 | 107 (99) | 26(59) | 138 (81) | 121(80) | 91 (100) |
| ≥1 | 1 (1) | 9 (20) | 22 (13) | 23 (15) | |
| Not available | 9(21) | 10(6) | 8(5) | ||
| NYHA functional classification | |||||
| Class I | 115 (100) | 15 (34) | 42 (25) | 34 (22) | 91 (100) |
| Class II | 6 (14) | 82 (48) | 70 (46) | ||
| Class III | 2 (5) | 44 (26) | 44 (29) | ||
| Class IV | 3 (2) | ||||
| Not available | 21 (48) | 2 (1) | 1 (1) | ||
| Patient report history of HF | 0 (0) | 3 (7) | 165 (97) | 151 (99) | 0 (0) |
| Primary aetiology of HF | |||||
| Ischaemic | 3(4) | 43 (25) | 74 (49) | ||
| Non‐Ischaemic | 122 (72) | 77 (51) | |||
| Dilated NOS | 59 (35) | 52 (34) | |||
| Hypertensive | 4 (2) | 1 (1) | |||
| Myocarditis | 5 (3) | 2 (1) | |||
| Sarcoid | 1 (1) | 0 | |||
| Alcohol | 2 (1) | 5 (3) | |||
| Amyloid | 1 (1) | 1 (1) | |||
| Valvular | 5 (3) | 1 (1) | |||
| Other | 12 (7) | 7 (5) | |||
| Unknown | 33 (20) | 8 (5) | |||
| Medical comorbidity | |||||
| Atrial fibrillation | 17 (16) | 6 (14) | 84 (49) | 62 (41) | 3 (3) |
| Coronary artery disease | 5 (5) | 43 (98) | 63 (37) | 81 (53) | 0 (0) |
| Diabetes | 34 (32) | 14 (32) | 67 (39) | 57 (38) | 0 (0) |
| COPD | 10 (9) | 3 (7) | 36 (21) | 27 (18) | 1 (1) |
| Laboratory and other measurements | |||||
| Haemoglobin, g/dL, median (25th–75th percentile) | 144 (132, 151) | 148 (138, 153) | 134 (122, 145) | 139 (127, 149) | 144 (132, 150) |
| Creatinine, umol/L, median (25th–75th percentile) | 77 (69, 91) | 91 (76, 104) | 97 (76, 125) | 97 (83, 120) | 79 (65, 86) |
| eGFR, mL/min, median (25th–75th percentile) | 98 (73, 123) | 88 (69, 110) | 68 (44, 98) | 81 (58, 109) | 83 (70, 106) |
| BNP, pg/mL, median (25th–75th percentile) | 28 (15, 51) | 45 (22, 116) | 118 (59, 264) | 191 (79, 367) | 23 (13, 41) |
| NT‐proBNP, pg/mL, median (25th–75th percentile) | 59 (33, 129) | 160 (47, 323) | 561 (200, 1362) | 1032 (414, 2121) | 52 (27, 94) |
| Weight, kg, median (25th–75th percentile) | 89 (77, 101) | 87 (79, 100) | 89 (73, 101) | 88 (77, 102) | 74 (64, 84) |
| BMI, median (25th–75th percentile) | 31 (27, 35) | 28 (26, 33) | 30 (27, 35) | 29 (26, 33) | 26 (24, 30) |
BNP, B‐type natriuretic peptide; BMI, body mass index; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; HF, heart failure; HF‐PEF, heart failure with preserved ejection fraction; HF‐REF, heart failure with reduced ejection fraction; NOS; not otherwise specificed; NT‐proBNP, N terminal pro BNP; NYHA, New York Heart Association.
Values are n (%) unless otherwise stated. Medians are presented with (25th–75th percentile). Because of rounding, not all percentages equal 100. eGFR was calculated by the modified diet in renal disease formula.
Echocardiographic parameters from the Alberta HEART study echocardiogram are shown in Table 2. LAVI and left ventricular mass index were similar and higher in patients with HF‐PEF and HF‐REF when compared with patients in Groups I, II, and V. Notably, historical EFs (where available) for patients with HF‐PEF included 47, 11, and 7 patients with an EF between 45–55%, 35–44%, and <35%, respectively (Table A1).
Table 2.
Echocardiographic parameters by the adjudicated group of patients (n = 565)
| I | II | III | IV | V | |
|---|---|---|---|---|---|
| At‐Risk | At‐Risk + Symptoms | HF‐PEF | HF‐REF | Controls | |
| n (%) | n (%) | n (%) | n (%) | n (%) | |
| n | 108 | 44 | 170 | 152 | 91 |
| Measured – ejection fraction, % | 65.0 (7) | 60.7 (6.8) | 57.5 (11.1) | 36.4 (12.3) | 63.8 (5.9) |
| Left ventricular end‐diastolic volume index, mL/m2 | 47.1 (17) | 50.3 (16.5) | 52.9 (20.3) | 82.9 (30.9) | 50.1 (12.4) |
| Left ventricular mass index, g/m2 | 83.1 (23.6) | 84.5 (24.6) | 96.4 (28.4) | 118.5 (39.4) | 72.3 (20.2) |
| Left atrial volume index, mL/m2 | 27.8 (9.5) | 28.7 (6.9) | 40.4 (31.5) | 40.1 (17.5) | 25.0 (6.8) |
| E/e' average | 6.2 (4.1) | 6.4 (4.7) | 7.9 (6.3) | 9.0 (7.5) | 6.8 (3.4) |
| E/A ratio | 1.0 (0.3) | 1.1 (0.5) | 1.2 (0.9) | 1.3 (0.9) | 1.0 (0.3) |
| Deceleration time, ms | 227.1 (55.6) | 230.0 (65.3) | 240.1 (77.4) | 222.2 (81.3) | 232.8 (54.3) |
| A wave retrograde flow duration, ms | 118.8 (24.6) | 128.2 (42.2) | 125.8 (29.8) | 122.7 (31.8) | 123.8 (38.0) |
| A wave duration, ms | 131.1 (33.4) | 137.1 (28.1) | 144.0 (30.3) | 140.7 (31.8) | 137.1 (20.3) |
HF‐PEF, heart failure with preserved ejection fraction; HF‐REF, heart failure with reduced ejection fraction.
Values are means (standard deviations) unless otherwise stated.
The diagnostic metrics of the three criteria are shown in Table 3. The positive likelihood ratios were 6.1, 6.9, and 4.8 for the Zile, ESC 2007, and ESC 2016 criteria, respectively. The negative likelihood ratios were 0.58, 0.60, and 0.42 for the Zile, ESC 2007, and ESC 2016 criteria, respectively. All the criteria lacked sensitivity to detect HF‐PEF (46.5%, 44.1%, and 51.8% for Zile, ESC 2007, and ESC 2016 criteria, respectively) but were highly specific (92.4%, 93.9%, and 89% for Zile, ESC 2007, and ESC 2016 criteria, respectively). In order to further explore the diagnostic metrics of individual criteria, patients with HF‐REF (Group IV) were excluded (Table 4). The sensitivity of all three criteria improved (Zile: 46.5% to 52%; ESC2 007: 44.1% to 54%; and ESC 2016: 51.8% to 64.7%), but the specificity remained largely unchanged.
Table 3.
Diagnostic criteria among 565 eligible patients
| I | II | III | IVF | V | |
|---|---|---|---|---|---|
| At‐Risk | At‐Risk + Symptoms | HF‐PEF | HF‐REF | Controls | |
| n (%) | n (%) | n (%) | n (%) | n (%) | |
| n | 108 | 44 | 170 | 152 | 91 |
| Zile criteria | |||||
| A. Signs and symptoms (Framingham criteria) | 11 (10) | 10 (23) | 97 (57) | 75 (49) | 1 (1) |
| B. Ejection fraction >50% | 106 (98) | 42 (96) | 135 (79) | 22 (15) | 89 (98) |
| Meet criteria (A and B) | 10 (9) | 9 (21) | 79 (47) | 10 (7) | 1 (1) |
| ESC 2007 criteria | |||||
| A. Signs and symptoms | 54 (50) | 29 (66) | 150 (88) | 135 (89) | 6(7) |
| B. Ejection fraction > 50% and LVEDVI <97 mL/m2 | 105 (97) | 42 (96) | 133 (78) | 22 (15) | 89 (98) |
| C. Evidence of diastolic dysfunction | 14 (13) | 11 (25) | 107 (63) | 103 (68) | 2 (2) |
| Meet criteria (A and B and C) | 5 (5) | 9 (21) | 74 (44) | 10 (7) | 1 (1) |
| ESC 2016 criteria | |||||
| A. Signs and symptoms | 63 (58) | 34 (77) | 164 (97) | 145 (95) | 9 (10) |
| B. Ejection fraction ≥50% | 106 (98) | 42 (96) | 136 (80) | 22 (15) | 89 (98) |
| C. Elevated natriuretic peptides (BNP >35 pg/mL or NT‐BNP >125 pg/mL) | 38 (35) | 25 (57) | 146 (86) | 129 (85) | 25 (28) |
| D. Structural/functional alteration | 57 (53) | 26 (59) | 132 (78) | 121 (80) | 43 (47) |
| Meet criteria (A and B and C and D) | 19 (18) | 12 (28) | 88 (52) | 10 (7) | 2 (2) |
ESC, European Society of Cardiology; HF‐PEF, heart failure with preserved ejection fraction; HF‐REF, heart failure with reduced ejection fraction; LVEDI, left ventricular end‐diastolic volume index; NT‐proBNP, N terminal pro BNP.
Table 4.
Number of patients classified as heart failure with preserved ejection fraction according to different criteria
| Adjudicated diagnosis | Criteria | |||||
|---|---|---|---|---|---|---|
| Zile 2001 | ESC 2007 | ESC 2016 | ||||
| HF‐PEF (Group III) | No HF‐PEF (Group I, II, IV, or V) | HF‐PEF (Group III) | No HF‐PEF (Group I, II, IV, or V) | HF‐PEF (Group III) | No HF‐PEF (Group I, II, IV, or V) | |
| HF‐PEF | 79 | 91 | 74 | 96 | 88 | 82 |
| No HF‐PEF | 30 | 365 | 25 | 370 | 43 | 352 |
| LR+ | 6.1 | 6.9 | 4.8 | |||
| LR− | 0.58 | 0.60 | 0.42 | |||
| Sensitivity | 46.5% (39.0–54.0) | 43.5% (36.0–51.3) | 51.8% (44.0–59.5) | |||
| Specificity | 92.4% (89.8–95.0) | 93.7% (91.3–96.1) | 89.1% (85.6–92.0) | |||
ESC, European Society of Cardiology; HF‐PEF, heart failure with preserved ejection fraction; HF‐REF, heart failure with reduced ejection fraction; LR, likelihood ratio.
In a comparison between criteria, there was no difference in sensitivity between either of the ESC criteria and the Zile criteria in overall comparisons (Table 4) or those not including patients with HF‐REF (Table 5). There was greater specificity of the Zile criteria compared with the ESC 2016 criteria for both the above comparisons reaching borderline statistical significance (P = 0.047 and P = 0.033).
Table 5.
Number of patients classified as heart failure with preserved ejection fraction according to different criteria, excluding patients with heart failure with reduced ejection fraction
| Adjudicated diagnosis | Criteria | |||||
|---|---|---|---|---|---|---|
| Zile 2001 | ESC 2007 | ESC 2016 | ||||
| HF‐PEF (Group III) | Not HF‐PEF (Group I, II, or V) | HF‐PEF (Group III) | Not HF‐PEF (Group I, II, or V) | HF‐PEF (Group III) | Not HF‐PEF (Group I, II, or V) | |
| HF‐PEF | 79 | 73 | 74 | 64 | 88 | 48 |
| Not HF‐PEF | 20 | 221 | 15 | 226 | 33 | 205 |
| LR+ | 6.3 | 8.6 | 4.7 | |||
| LR− | 0.43 | 0.43 | 0.25 | |||
| Sensitivity | 52.0% (44.0–59.9) | 53.6% (44.9–62.2) | 64.7% (56.7–72.7) | |||
| Specificity | 91.7% (88.2–95.2) | 93.8% (89.9–96.5) | 86.1% (81.7–90.5) | |||
ESC, European Society of Cardiology; HF‐PEF, heart failure with preserved ejection fraction; HF‐REF, heart failure with reduced ejection fraction; LR, likelihood ratio.
Clinical outcomes
All‐cause mortality occurred in 10 patients in 1 year and 57 patients over the median follow‐up of 1355 days (25th–75th percentile 854–1774) giving an overall annualized event rate of 2.9/100 patient years. The annualized event rate was 0.7, 1.8, 4.0, 4.6, and 0.0 for Groups I through V, respectively. Event rates including for (cardiovascular) hospitalizations are shown in Table 6.
Table 6.
Clinical outcomes (n = 565)
| I | II | III | IV HF‐REF | V | |
|---|---|---|---|---|---|
| At‐Risk | At‐Risk + Symptoms | HF‐PEF | HF‐REF | Controls | |
| n (%) | n (%) | n (%) | n (%) | n (%) | |
| n | 108 | 44 | 170 | 152 | 91 |
| Mortality [total, n (%)] | 2 (2) | 3 (7) | 24 (14) | 28 (18) | 0 (0) |
| Annualized event rate per 100 patient years | 0.7 | 1.8 | 4.0 | 4.6 | 0 |
| Total hospitalization [total, n (%)] | 27 (25) | 25 (57) | 111 (65) | 92 (61) | 21 (23) |
| Annualized event rate per 100 patient years | 9.2 | 14.8 | 18.6 | 15.1 | 6.8 |
| CV hospitalization [total, n (%)] | 7 (6) | 13 (30) | 53 (31) | 57 (38) | 3 (3) |
| Annualized event rate per 100 patient years | 2.4 | 7.7 | 8.9 | 9.4 | 1.0 |
CV, cardiovascular; HF‐PEF, heart failure with preserved ejection fraction; HF‐REF, heart failure with reduced ejection fraction.
Discussion
The Alberta HEART cohort provides an opportunity to evaluate the performance of various HF diagnostic criteria against clinical judgement. Our study has an important finding with implications for those in clinical practice and the research community. The overall likelihood ratios of the existing criteria are reasonable but not to the level generally accepted as definitively diagnostic for positive or negative likelihood ratio (>10 and <0.1).11 The overall sensitivity of all three criteria is poor—highlighting the clinical challenges in this area attempting to screen for disease earlier. Many of the patients in our cohort had marginally elevated natriuretic peptides or echocardiographic criteria for HF‐PEF despite their adjudication by two experts into a non‐HF‐PEF group, highlighting the common nature of these findings in non‐HF populations.
Because of the lack of a reference standard and evolving clinical and published data in the field, we chose to use experienced clinicians as the adjudicators as this is a preferred method when no reference standard exists. Indeed, there were patients in both Groups III and IV who had a historical EF cut above or below 45%, which may have occurred quite some time before enrolment, and thus, we chose to use the Alberta HEART study echocardiogram for decisions about testing the existing criteria. This may introduce a small degree of bias (in both directions) but is unlikely to obviate the poor sensitivity and high specificity seen in the existing criteria.
What should go into criteria for HF‐PEF? Based on the current criteria, there is much room for improvement. In part, that improvement may come from identifying sub‐groups within the heterogeneous syndrome based on phenotypical variants using latent class analysis or phenomapping.12, 13 This may provide unique insight based on clinical events and several traits rather than indistinct symptoms and EF alone. Any future criteria should include consideration of comorbid diseases (e.g. lung disease, frailty/deconditioning, obesity, renal disease, and anaemia) and could be further enhanced by imaging techniques specific to HF‐PEF (e.g. LAVI) or biomarkers representing the mechanistic pathways. Critically, in diagnosing HF‐PEF, clinicians must avoid an overreliance on existing imaging markers that have not validated or are inconsistent across the spectrum of disease (such as E/e'). The changing nature of EF over time, as well as imaging quality, is also important to consider. Provocative testing may also be of value and has been proposed in order to confirm the cause of symptoms and are likely to improve the overall diagnostic accuracy of criteria.14
The clinical outcomes in the group recruited in Alberta HEART from the community mirror those in some but not all cohorts. For example, in the TOPCAT trial,15 the annualized mortality rate was 4.4%, I‐PRESERVE16 was 5.2%, whereas in Alberta HEART, the group with HF‐PEF, also recruited as an outpatient but without enriching criteria, was 4%. Because our patients were drawn from an outpatient community, rather than an inhospital or recently hospitalized cohort, this may reflect a lower risk than those from cohorts recruited in hospital.17
Strengths and limitations
Some strengths and limitations deserve consideration. First, the Alberta HEART cohort was predominantly Caucasian, and there are known differences in reference ranges between ethnicities.18 However, the subtle variations are unlikely to substantially alter the performance metrics of the diagnostic criteria. Second, this was a stable outpatient cohort. Patients during or with a recent hospitalization, especially in Groups 3 or 4, may have elevated natriuretic peptides, which may alter their inclusion by the ESC 2007 or ESC 2016 criteria improving the overall sensitivity of these criteria, but are unlikely to change the specificity. Third, the lack of a reference standard in this area meant that we used clinical HF experts to adjudicate each case in duplicate to assign them to a group. The lack of a reference standard in diagnostic tests is not a new issue and has been well described elsewhere, including methodologic considerations that are applicable to issue surrounding HF‐PEF.19 Invasive haemodynamics, while of interest, remain sparsely used in practice and are unlikely to be used to distinguish causes of dyspnoea in patients with suspected HF‐PEF—hence, our choice of guideline—endorsed or literature‐based criteria. Despite the availability of modestly sized cohorts demonstrating that invasive testing may be of value in patients with exertional dyspnoea and suspected HF‐PEF, this is neither established nor recommended by any of the major guidelines explicitly. As such, this was not included in this study as a reference standard. Finally, because of the prospective enrolment and purposeful oversampling of patients with HF‐PEF and HF‐REF, the sensitivity and specificity may be distorted, hence our reporting and emphasis of likelihood ratios.
Conclusions
We identified patients across a spectrum of disease and applied available criteria for the diagnosis of HF‐PEF. In this community based cohort, the overall likelihood ratios of the existing criteria were reasonable but not to the level generally accepted as diagnostic for use in clinical practice. Improved criteria for the diagnosis of patients with HF‐PEF are needed.
Conflicts of interest
None declared.
Funding
Funding was provided by an Alberta Innovates – Health Solutions Interdisciplinary Team Grant to Alberta Heart Failure Etiology and Analysis Research Team (Alberta HEART), grant no. AHFMR ITG 200801018.
Acknowledgements
We are grateful for the contribution of the patients, the project team, and specifically the study coordinators.
Table A1.
Historical echocardiographic parameters by the adjudicated group of patients (n = 565)
| I | II | III | IV | V | |
|---|---|---|---|---|---|
| At‐Risk | At‐Risk + Symptoms | HF‐PEF | HF‐REF | Controls | |
| n | 108 | 44 | 170 | 152 | 91 |
| Ejection fraction, n (%) | |||||
| >55 | 12 (11) | 15 (34) | 96 (57) | 6 (4) | 1 (1) |
| 45–55 | 4 (4) | 3 (7) | 47 (28) | 14 (9) | |
| 35–44 | 2 (5) | 11 (7) | 32 (21) | ||
| <35 | 7 (4) | 96 (63) | |||
| Unavailable | 92 (85) | 24 (55) | 9 (5) | 4 (3) | 90 (99) |
HF‐PEF, heart failure with preserved ejection fraction; HF‐REF, heart failure with reduced ejection fraction.
Ezekowitz, J. A. , McAlister, F. A. , Howlett, J. , Alemayehu, W. , Paterson, I. , Belenkie, I. , Oudit, G. Y. , Kaul, P. , Dyck, J. R. , Anderson, T. , and on behalf of the Alberta HEART Investigators (2018) A prospective evaluation of the established criteria for heart failure with preserved ejection fraction using the Alberta HEART cohort. ESC Heart Failure, 5: 19–26. doi: 10.1002/ehf2.12200.
References
- 1. Paulus WJ, Tschöpe C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite‐Moreira AF, Borbély A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 2007; 28: 2539–2550. [DOI] [PubMed] [Google Scholar]
- 2. Shuai X‐X, Chen Y‐Y, Lu Y‐X, Su G‐H, Wang Y‐H, Zhao H‐L, Han J. Diagnosis of heart failure with preserved ejection fraction: which parameters and diagnostic strategies are more valuable? Eur J Heart Fail 2014; 13: 737–745. [DOI] [PubMed] [Google Scholar]
- 3. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola V‐P, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2016; 37: 2129–2200.27206819 [Google Scholar]
- 4. Zile MR. New concepts in diastolic dysfunction and diastolic heart failure: part I: diagnosis, prognosis, and measurements of diastolic function. Circulation 2002; 105: 1387–1393. [DOI] [PubMed] [Google Scholar]
- 5. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJV, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WHW, Tsai EJ, Wilkoff BL, American College of Cardiology Foundation, American Heart Association Task Force on Practice Guidelines . 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62: e147–e239. [DOI] [PubMed] [Google Scholar]
- 6. McKelvie RS, Moe GW, Ezekowitz JA, Heckman GA, Costigan J, Ducharme A, Estrella‐Holder E, Giannetti N, Grzeslo A, Harkness K, Howlett JG, Kouz S, Leblanc K, Mann E, Nigam A, O'Meara E, Rajda M, Steinhart B, Swiggum E, Le VV, Zieroth S, Arnold JMO, Ashton T, D'Astous M, Dorian P, Haddad H, Isaac DL, Leblanc M‐H, Liu P, Rao V, Ross HJ, Sussex B. The 2012 Canadian cardiovascular society heart failure management guidelines update: focus on acute and chronic heart failure. Canadian Journal of Cardiology 2013; 29:168–181. [DOI] [PubMed] [Google Scholar]
- 7. Ezekowitz JA, Becher H, Belenkie I, Clark AM, Duff HJ, Friedrich MG, Haykowsky MJ, Howlett JG, Kassiri Z, Kaul P, Kim DH, Knudtson ML, Light PE, Lopaschuk GD, McAlister FA, Noga ML, Oudit GY, Paterson DI, Quan H, Schulz R, Thompson RB, Weeks SG, Anderson TJ, Dyck JRB. The Alberta Heart Failure Etiology and Analysis Research Team (HEART) study. BMC Cardiovasc Disord 2014; 14: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hunt SA, Baker DW, Chin MH, Cinquegrani MP, Feldman AM, Francis GS, Ganiats TG, Goldstein S, Jessup ML, Noble RJ, Packer M, Silver MA, Stevenson LW, Gibbons RJ, Antman EM, Alpert JS, Faxon DP, Fuster V, Gregoratos G, Jacobs AK, Hiratzka LF, Russell RO, Smith SC. American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1995 Guidelines for the Evaluation and Management of Heart Failure), International Society for Heart and Lung Transplantation, Heart Failure Society of America. ACC/AHA Guidelines for the Evaluation and Management of Chronic Heart Failure in the Adult: Executive Summary A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1995 Guidelines for the Evaluation and Management of Heart Failure): Developed in Collaboration With the International Society for Heart and Lung Transplantation; Endorsed by the Heart Failure Society of America. Circulation 2001; 104: 2996–3007. [DOI] [PubMed] [Google Scholar]
- 9. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt J‐U. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015; 28: 1–39. e14. [DOI] [PubMed] [Google Scholar]
- 10. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jaeschke R, Guyatt GH, Sackett DL. Users' guides to the medical literature. III. How to use an article about a diagnostic test. B. What are the results and will they help me in caring for my patients? The Evidence‐Based Medicine Working Group. JAMA 1994; 271: 703–707. [DOI] [PubMed] [Google Scholar]
- 12. Shah SJ, Katz DH, Selvaraj S, Burke MA, Yancy CW, Gheorghiade M, Bonow RO, Huang C‐C, Deo RC. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation 2015; 131: 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kao DP, Lewsey JD, Anand IS, Massie BM, Zile MR, Carson PE, McKelvie RS, Komajda M, McMurray JJ, Lindenfeld J. Characterization of subgroups of heart failure patients with preserved ejection fraction with possible implications for prognosis and treatment response. Eur J Heart Fail 2015; 17: 925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Erdei T, Smiseth OA, Marino P, Fraser AG. A systematic review of diastolic stress tests in heart failure with preserved ejection fraction, with proposals from the EU‐FP7 MEDIA study group. Eur J Heart Fail 2014; 16: 1345–1361. [DOI] [PubMed] [Google Scholar]
- 15. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O'Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014; 370: 1383–1392. [DOI] [PubMed] [Google Scholar]
- 16. Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A, I‐PRESERVE Investigators . Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 2008; 359: 2456–2467. [DOI] [PubMed] [Google Scholar]
- 17. Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population‐based study. N Engl J Med 2006; 355: 260–269. [DOI] [PubMed] [Google Scholar]
- 18. Echocardiographic Normal Ranges Meta‐Analysis of the Left Heart Collaboration . Ethnic‐specific normative reference values for echocardiographic LA and LV size, LV mass, and systolic function: the EchoNoRMAL Study. JACC Cardiovasc Imaging 2015; 8: 656–665. [DOI] [PubMed] [Google Scholar]
- 19. Reitsma JB, Rutjes AWS, Khan KS, Coomarasamy A, Bossuyt PM. A review of solutions for diagnostic accuracy studies with an imperfect or missing reference standard. J Clin Epidemiol 2009; 62: 797–806. [DOI] [PubMed] [Google Scholar]


