Abstract
Objectives
Intensive insulin therapy for tight glycemic control in critically ill surgical patients has been shown to reduce mortality; however, intensive insulin therapy is associated with iatrogenic hypoglycemia and increased variability of blood glucose levels. The incretin glucagon-like peptide-1 (7–36) amide is both insulinotropic and insulinomimetic and has been suggested as an adjunct to improve glycemic control in critically ill patients. We hypothesized that the addition of continuous infusion of glucagon-like peptide-1 to intensive insulin therapy would result in better glucose control, reduced requirement of exogenous insulin administration, and fewer hypoglycemic events.
Design
Prospective, randomized, double-blind, placebo-controlled clinical trial.
Setting
Surgical or burn ICU.
Patients
Eighteen patients who required intensive insulin therapy.
Interventions
A 72-hour continuous infusion of either glucagon-like peptide-1 (1.5 pmol/kg/min) or normal saline plus intensive insulin therapy.
Measurements and Main Results
The glucagon-like peptide-1 cohort (n = 9) and saline cohort (n = 9) were similar in age, Acute Physiology and Chronic Health Evaluation score, and history of diabetes. Blood glucose levels in the glucagon-like peptide-1 group were better controlled with much less variability. The coefficient of variation of blood glucose ranged from 7.2% to 30.4% in the glucagon-like peptide-1 group and from 19.8% to 56.8% in saline group. The mean blood glucose coefficient of variation for the glucagon-like peptide-1 and saline groups was 18.0% ± 2.7% and 30.3% ± 4.0% (p = 0.010), respectively. The 72-hour average insulin infusion rates were 3.37 ± 0.61 and 4.57 ± 1.18 U/hr (p = not significant). The incidents of hypoglycemia (≤ 2.78mmol/L) in both groups were low (one in the glucagon-like peptide-1 group, three in the saline group).
Conclusions
Glucagon-like peptide-1 (7–36) amide is a safe and efficacious form of adjunct therapy in patients with hyperglycemia in the surgical ICU setting. Improved stability of blood glucose is a favorable outcome, which enhances the safety of intensive insulin therapy. Larger studies of this potentially valuable therapy for glycemic control in the ICU are justified.
Keywords: GLP-1 (7–36) amide, hypoglycemia, intensive insulin therapy, surgical intensive care unit
Since the publication of the article by Van den Berghe et al in 2001 (1), attention to the benefits of strict control of glucose levels in surgical ICU (SICU) patients has increased markedly. The Leuven study enrolled greater than 1,500 patients and revealed that the mortality rate in ventilated, SICU patients was reduced 44% by the use of a continuous insulin infusion designed to maintain blood glucose levels between 4.4 and 6.1 mmol/L (80 and 110 mg/dL), as compared with conventional insulin therapy designed to maintain blood glucose levels between 10.0 and 11.9 mmol/L (180 and 214 mg/dL). The improved survival was apparent in patients whose SICU stay lasted more than 5 days, but the reduced mortality was evident for months after admission to the SICU. This and other recent studies reporting the beneficial effects of strict glucose control in high-risk patients have also emphasized the problems associated with intensive insulin therapy (IIT). Issues such as the risk of insulin-induced hypoglycemia, the added nursing work required for successful tight glycemic control (2), and the undefined duration of therapy needed to achieve long-term benefit are major obstacles to the implementation of intensive insulin infusion protocols in which the glucose target or goal is less than 6.7 mmol/L (120 mg/dL).
More recently, the results of the Normoglycemia in Intensive Care Evaluation Survival Using Glucose Algorithm Regulation (NICE-SUGAR) study drew increased attention to the risks of hypoglycemia with IIT (3). In this large randomized trial, the mortality of SICU patients in the intensive glucose control group (target level 4.5–6.0 mmol/L) was increased compared with the conventional glucose control group (target 10 mmol/L or less). Severe hypoglycemia (blood glucose ≤ 2.2 mmol/L) occurred in 6.8% of the patients in the intensive glucose control group and only 0.5% in the conventional control group. Following this report, the American Diabetes Association, the American Association of Clinical Endocrinologists, and the Endocrine Society jointly endorsed the recommendation that until more data are available, it is prudent to treat critically ill patients’ glucose levels less intensively than that required to achieve blood glucose levels of 4.5–6.0 mmol/L (3). Furthermore, these groups advised that the target value for glycemic control should be between 8.0 and 10 mmol/L to avoid hypoglycemic events (4, 5).
If an agent could be indentified which reduces the amount of insulin required for glycemic control and reduces the likelihood of hypoglycemia due to increased excursions or variability of blood glucose level, the addition of such an agent might improve the risk-benefit ratio of IIT and enhance the survival advantage of the maintenance of euglycemia in critically ill patients. glucagon-like peptide (GLP)-1 shows promise as just such an agent.
GLP-1 (7–36) amide is an endocrine and paracrine incretin peptide hormone released by the L cells of the human small intestine in response to food ingestion (6). It acts by increasing glucose-mediated insulin release from pancreatic β-cell (6) and decreases hepatic glucose through mechanisms independent of its insulinotropic effect (7, 8) and through other extrapancreatic effects (9). GLP-1 potently stimulates insulin secretion in a glucose-dependent manner, that is, the higher the plasma glucose level, the more potent stimulation of endogenous insulin release (10). However, and importantly to the concept of GLP-1 as a safe adjunct to glucose control, GLP-1 has virtually no insulinotropic effects at euglycemic levels (3.9 mmol/L) (11, 12). In addition, GLP-1 reduces insulin resistance (13, 14), is an important contributor to the pathogenesis of hyperglycemia in critical illness, and is free of clinically significant adverse effects (10, 14, 15). Because of these combined attributes, the use of GLP-1 could potentially decrease the need for exogenous insulin in critically ill patients who otherwise require continuous insulin infusion to maintain optimal glycemic control and might contribute to the maintenance of stable blood glucose without regard to institute-specific or protocol-specific target levels.
We hypothesized that GLP-1 administration may be a useful adjunct treatment in the control of blood glucose because of its insulinotropic and insulinomimetic actions. To determine whether GLP-1 facilitates glycemic control in the subset of SICU patients who are hyperglycemic and require IIT, we conducted a prospective, randomized, double-blind, saline placebo-controlled trial of a 72-hour continuous infusion of GLP-1 (1.5 pmol/kg/min) in patients admitted to the SICU who required IIT for glycemic control. The primary outcomes of this trial were 1) the amount of insulin required during IIT, 2) the level of glycemic control and the degree of stability of that control, and 3) the prevalence of hypoglycemia during the infusion period. We predicted that the amount of exogenous insulin administration would be reduced with concomitant infusion of GLP-1, that the glucose profile with GLP-1 administration would be less variable compared with insulin alone, and finally that there will be fewer incidents of hypoglycemia despite concomitant infusion of insulin during GLP-1 administration.
MATERIALS, METHODS, AND PROCEDURES
IIT and GLP-1 (7–36) Amide Infusion Protocols
The IIT protocol used at Johns Hopkins Bayview Medical Center (JHBMC), designed to maintain blood glucose levels between 4.4 and 6.1 mmol/L (80–110 mg/dL), has three entry criteria: 1) an adult with significant morbidity to warrant designation as a critically ill patient; 2) a blood glucose level greater than 6.6 mmol/L; and 3) an anticipated duration of ICU stay of at least 72 hours. The IIT protocol was approved by the Pharmacy and Therapeutics Committee before its 2006 implementation at JHBMC. The IIT protocol at JHBMC is a modification of the Yale IIT protocol and has been previously described (16). It has been extensively used in our burn ICU (BICU) and SICU, where its effectiveness in reducing the rate of death and sepsis has been documented (16).
The GLP-1 (7–36) amide/saline plus IIT protocol design was a randomized, double-blind study, where each subject/patient received either an IV infusion of GLP-1 (7–36) amide or saline for a continuous 72-hour period after initiation of the IIT protocol. The GLP-1 (7–36) amide protocol for the SICU or BICU patients was approved by the Johns Hopkins Institutional Review Board. Patients who met the eligibility criteria for IIT and were between 18 and 90 years old were allowed to be enrolled. We obtained an investigator-initiated new drug application from the Food and Drug Administration (FDA IND #32,513) for GLP-1 (7–36) amide infusion. This study was registered at ClinicalTrials.gov (NCT00798590). Written informed consent was obtained from the subjects or from the subject’s next-of-kin in accordance with the Helsinki II declaration.
In the GLP-1/saline infusion protocol, coded syringes containing either GLP-1 or saline were dispensed from the research pharmacy, and the peptide was administered IV at a constant rate (1.5 pmol/kg/min) over the 72 hours, without a priming dose, using a small infusion pump (Disertronic Panomat T-10 syringe pump, Berghdorf, Switzerland). The total volume infused over the 72 hours was 3 mL. The peptide was synthesized in the Peptide-Core Laboratory at Massachusetts General Hospital, formulated for single use per patient, and was certified to be sterile and pyrogen free. The high performance liquid chromatography profile displayed a very sharp narrow peak, which was calculated to be 99% pure.
The care provider, who was blinded to the contents of the test syringe, was allowed to change the rate of the IV administered insulin infusion or stop it completely as per the approved algorithm of the IIT protocol. However, the provider could not change the rate of GLP-1 (7–36) amide/saline infusion unless hypoglycemia (blood glucose level, < 2.78 mmol/L [50 mg/dL]) was documented. If that did occur, the protocol required the rate of GLP-1/saline infusion to be reduced by half. After the double-blind code was broken, it was documented that the rate of GLP-1 infusion was never reduced and the rate of saline infusion was reduced in one volunteer who received a bolus of 50% glucose. We collected blood samples, before initiation of GLP-1/saline, at 1, 2, 4, 8 hours and every 8 hours thereafter until 72 hours, in heparinized syringes which were then placed in prechilled tubes containing EDTA, aprotinin, and dipeptidyl peptidase-4 inhibitors. Samples were centrifuged at 4°C, and plasma was stored in a −70°C freezer until assyed.
Subject Characteristics
All patients admitted to the JHBMC SICU or BICU between November 2007 and March 2010 were evaluated for study inclusion. Eighteen patients (14 men and four women) were enrolled into the study and were evaluated as having completed the study protocol. After uncoding, we discovered that nine patients received GLP-1 (seven men) and the other nine received saline (seven men). The mean age (± SEM) and body mass index (BMI) of the GLP-1 group were 58.8 ± 5.6 years and 35.4 ± 3.9 kg/m2, respectively. The corresponding values for the saline group were 67.1 ± 4.4 years (NS) and 34.1 ± 3.0 kg/m2 (NS), respectively. Both cohorts had similar Acute Physiology and Chronic Health Evaluation (APACHE) II scores and similar known histories of type 2 diabetes (Table 1).
TABLE 1.
Clinical Characteristics of the Subjects
| ID | Age | Sex | Admitting Diagnosis | Type 2 Diabetes | Body Mass Index | Acute Physiology and Chronic Health Evaluation II |
|---|---|---|---|---|---|---|
| Glucagon-like peptide-1 group | ||||||
| 1 | 61 | Male | BICU–toxic epidermal necrolysis | Yes | 35.8 | 20 |
| 2 | 57 | Male | BICU–TBSA: 25% | No | 24.5 | 30 |
| 3 | 41 | Male | MVC with multiple rib fractures | Yes | 58.6 | 16 |
| 4 | 34 | Male | Ventral hernia repair | No | 22.3 | 10 |
| 5 | 82 | Male | MVC with hemothorax | Yes | 33.5 | 24 |
| 6 | 60 | Male | MVC with pneumothorax | Yes | 33.6 | 17 |
| 7 | 86 | Female | Trauma: left subclavian artery injury and pneumothorax | No | 27.8 | 28 |
| 8 | 56 | Female | Retroperitoneal hemorrhage | No | 47.3 | 16 |
| 9 | 52 | Male | Perforated diverticulitis | No | 26.6 | 23 |
| X | 58.78 | 7 male/2 female | 4 yes/5 no | 34.44 | 20.44 | |
| SEM | 5.62 | 3.92 | 2.14 | |||
|
| ||||||
| Saline group | ||||||
| 1 | 63 | Male | Symptomatic cholelithiasis | Yes | 51.4 | 22 |
| 2 | 75 | Female | Abdominal abscess | Yes | 35 | 20 |
| 3 | 65 | Male | Gallstone pancreatitis and rupture pancreatic pseudocyst | No | 39.5 | 19 |
| 4 | 42 | Male | MVC with hepatic laceration | No | 34.4 | 25 |
| 5 | 55 | Male | Ruptured abdominal aortic aneurysm | No | 33.5 | 17 |
| 6 | 69 | Male | Necrotizing pancreatitis | Yes | 38.1 | 18 |
| 7 | 82 | Male | Gangrene of small bowel | Yes | 28.9 | 32 |
| 8 | 84 | Female | BICU–TBSA: 6% | No | 20.1 | 14 |
| 9 | 69 | Male | BICU–toxic epidermal necrolysis | Yes | 26.2 | 25 |
| X | 67.11 | 7 male/2 female | 5 yes/4 no | 34.12 | 21.33 | |
| SEM | 4.37 | 2.96 | 1.80 | |||
BICU = burn ICU, TBSA = total body surface area, MVC = motor vehicle collision.
Exclusion criteria included patients with a medical history of type 1 diabetes, malignancy, HIV/AIDS, or unexplained weight loss. Furthermore, patients were excluded if the medical staff felt they would likely not survive to complete the 72-hour infusion. Patients were excluded if they were already enlisted in other phase 1 trial or phase 2 trial. Finally, for our BICU patients, they were excluded if they had a total body surface area burn of 70% or greater, as this had been shown in our prior study to be a significant prognostic indicator of mortality (16).
Assay Measurements
Blood samples were measured, usually from an arterial catheter, with a point of care glucose meter (Roche Accucheck, Mannheim, Germany), with frequency determined by the IIT protocol, but in any event no less frequently than every 2 hours. The blood glucose levels as assessed with the point of care glucose meter were also verified, approximately every 4 hours, by the Chemistry Laboratory of JHBMC using a glucose oxidase methodology. Plasma C-peptide, glucagon, and active GLP-1 were measured with kits purchased from Mercodia (Uppsala, Sweden), Millipore (St. Charles, MO), and Alpco Diagnostics (Salem, NH), respectively, according to manufacturers’ instructions. Samples from saline and GLP-1 infusion groups were assayed simultaneously.
The initiation of insulin and GLP-1/saline infusion varied as to the actual start time, and we designated the start of GLP-1/saline infusion as time zero. We also recorded the rate of insulin infusion for 4 hours before the start of GLP-1/saline infusion. We entered all the glucose values and time of their measurements for each patient. Using a program that interpolates glucose levels for any desired interval, we derived glucose levels for each patient for every hour from −4 to 72 hours, spanning a total of 76 hours. The mean interpolated hourly glucose level for every 24-hour period was compared with the mean actual measured glucose levels obtained during each day to ensure that bias was not introduced.
Similarly, we entered the rate of insulin infusion and the time of initiation or change in the rate and derived insulin infusion rates for each patient at 4-hour intervals, from 4 hours before the start of the GLP-1/saline infusion and for every 4 hours thereafter until the end of GLP-1/saline infusion (72 hr). These glucose levels and insulin infusion rates were then averaged for each group.
Statistical Calculations
The trapezoidal rule was used to calculate the intergraded responses over 24-hour interval; the intergraded responses were decided by the time interval (i.e., 24 hr), which resulted in a mean concentration. We used repeated-measures analysis of variance to compare differences between the two groups with respect to glucose and hormone levels, as well as for rates of insulin infusion. The Wilcoxon-Mann-Whitney test was used for comparisons of the coefficient of variation (CV) for glucose levels between the two groups. Values are presented as mean ± SEM. All tests were two tailed, and a p value of less than or equal to 0.05 was considered significant.
RESULTS
Subject characteristics and demographic data are presented in Table 1. There was no significant difference in age, BMI, or level of illness severity (assessed by APACHE II scoring at admission to the ICU) between the GLP-1 group and the normal saline group. None of the patients were on renal replacement therapy during the study.
Measured glucose levels and insulin infusion rates for a representative patient in the GLP-1 group and a representative patient in the saline group are presented in Figure 1. With the start of the GLP-1 infusion, the blood glucose levels started to fall and then remained relatively stable during the 72-hour infusion period. This was accompanied by a reduction in the exogenous insulin infusion, with little or no change in the rate of the insulin infusion throughout the 72-hour infusion period. In contrast, in the patient receiving saline, the glucose level fluctuated considerably as did the changes in the insulin infusion rate. The average 72-hour blood glucose levels for the patient receiving GLP-1 and for the patient receiving saline were 6.42 ± 0.16 and 7.60 ± 0.37 mmol/L, respectively. The 72-hour average blood glucose levels do not reflect the stability of the blood glucose levels. The CV of the blood glucose levels for the patient in GLP-1 group and the patient in the saline group were 16.3% and 33.3%, respectively. The 72-hour average insulin infusion rates for these two patients receiving GLP-1 or saline were 2.85 and 8.26 U/hr.
Figure 1.
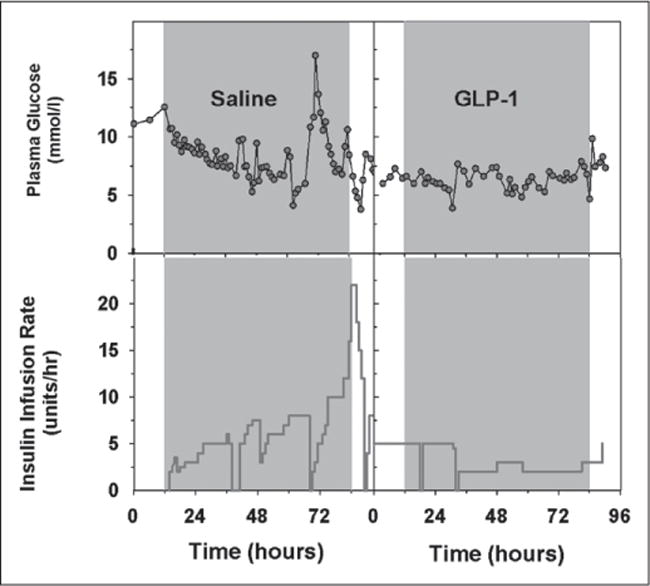
Glucose profile and insulin infusion rate during insulin infusion therapy in a patient receiving saline (left) and a patient receiving glucagon-like peptide-1 (right).
The mean 1-hour derived blood glucose profile and 4-hour insulin infusion rates for both groups are shown in Figure 2. The average blood glucose levels for the GLP-1 and saline groups for the 72-hour period were 6.61 ± 0.25 and 6.64 ± 0.26 mmol/L, respectively (p = NS). The range of blood glucose levels was from 2.72 to 13.39 mmol/L in the GLP-1 group and from 2.06 to 19.56 mmol/L in the saline group. The prevalence of hypoglycemia (≤ 2.78 mmol/L) in these two groups was relatively low: only one event (2.72 mmol/L) occurred in the GLP-1 group and three events (2.06, 2.56, and 2.72 mmol/L) occurred in the saline group.
Figure 2.
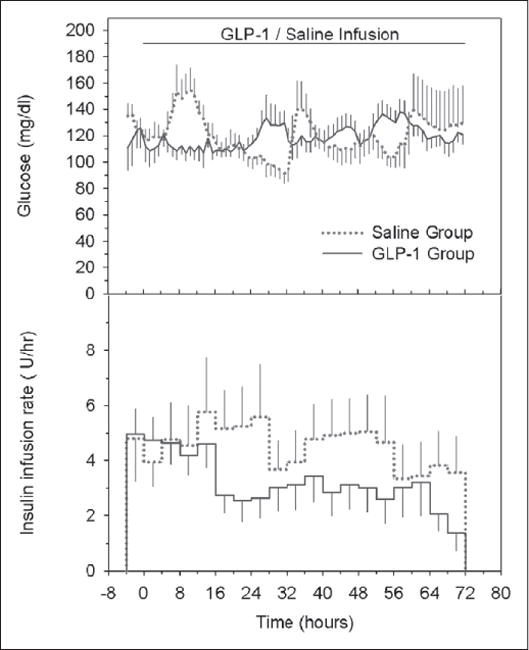
Average glucose profiles (hourly, upper) and insulin infusion rates (4-hour averages, bottom) during insulin infusion therapy for nine patients receiving saline (dotted lines) and for the nine patients receiving glucagon-like peptide (GLP)-1 (solid lines).
The CVs for the blood glucose levels for the 72-hour infusion period in all nine patients in the GLP-1 group ranged from 7.2% to 30.4%; for all nine patients in the saline group, the CVs of the blood glucose levels ranged from 19.8% to 56.8%. The mean measured blood glucose CV and the calculated 1-hour blood glucose CV for the 72-hour infusion period for the GLP-1 infusion group were 19.8% ± 2.7% and 18.0% ± 2.7%, respectively. The corresponding values for the saline group were 30.4% ± 3.2% and 30.3% ± 4.0%. The mean glucose CVs of the two groups were statistically significantly different (p = 0.010, for both actual and derived), which indicates the significantly lower degree of glucose variation in the GLP-1 infusion group.
The 72-hour average insulin infusion rates for the GLP-1 and saline groups were 3.37 ± 0.61 and 4.57 ± 1.18 U/hr (p = NS). The insulin infusion rate in the GLP-1 group decreased 18% ± 7.3% in the 12- to 24-hour period and 38% ± 16.6% in the 24- to 36-hour period, compared with the 0- to 12-hour value. These changes in insulin infusion requirements contrasted with increases of 19% ± 27.7% and 25% ± 37.2% in the saline group, but these differences between the groups were not statistically significant.
Plasma C-peptide levels and glucagon levels are shown for GLP-1 and saline infusion groups in Figure 3. We compared the time course of changes in C-peptide levels of the diabetic and the nondiabetic subjects in the GLP-1 and saline groups and found that they were similar and not statistically significantly different from each other during the 72 hours of treatment. Therefore, the responses for each group were combined, and the time course of changes in plasma levels of C-peptide and glucagon are presented in Figure 3. Basal C-peptide levels were not significantly different between the two groups and neither were the time courses of their responses during treatment. The C-peptide area under the curve (AUC0–72 hr) for the GLP-1 and the saline groups were 588 ± 208 and 281 ± 105 pmol/L, respectively. C-peptide levels rose somewhat in the final 24 hours of GLP-1 infusion but remained essentially unchanged despite significant fluctuations in blood glucose levels in the saline infusion group. Plasma glucagon levels were not significantly different between the two groups at the beginning of saline or GLP-1 infusion but decreased during GLP-1 infusion. The glucagon AUC0–72 hr for the GLP-1 and saline groups were 32 ± 11 and 68 ± 26 pmol/L. The basal GLP-1 levels were approximately 8 pmol/L and did not change during the saline infusion. In contrast, and as expected, the GLP-1 levels increased during the GLP-1 infusion reaching a level of 56 pmol/L by day 1.
Figure 3.
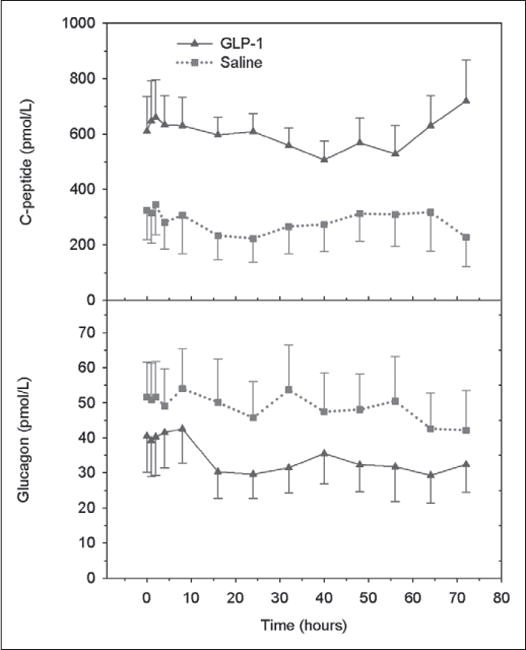
Plasma C-peptide levels (upper) and glucagon levels (lower) in patients receiving insulin infusion therapy who also received a 72-hour infusion of glucagon-like peptide (GLP)-1 (solid lines) or saline (dotted lines).
We recorded the use of vasopressors required during the 72-hour GLP-1/saline infusion interval. In the GLP-1 group, three of the nine patients required the use of vasopressors during the infusion period, whereas in the saline group, four of the nine patients required vasopressors. Of note, after the GLP-1/saline infusion administration (72 hr), more patients in the saline group (n = 4) required vasopressors before discharge from the ICU than in the GLP-1 group (n = 2). Also of interest, more patients in the saline group (n = 4) required antiarrhythmic medications during the 72-hour infusion period than patients in the GLP-1 group (n = 2). None of these differences were statistically significant, however.
DISCUSSION
Our study demonstrates that the addition of a continuous 72-hour IV administration of GLP-1 (7–36) amide during a standardized IIT protocol in hyperglycemic surgical patients who require intensive care results in greater glycemic stability. Large glycemic variation has been shown to be an independent predictor of increased mortality independent of glucose value (17, 18), and we have shown that the glycemic variation in the GLP-1 infusion group is significantly less than the saline infusion control group, by a factor of nearly two. Our incidence of hypoglycemia in this study was so small (three episodes in the saline group; one episode in the GLP-1 infusion group) that we were unable to assess the effect of GLP-1 on this outcome with confidence. Furthermore, better glycemic control with GLP-1 may also have had a beneficial effect on patient’s hemodynamics as there was a trend for less vasopressors and antiarrhythmic medications in the GLP-1 group even after the discontinuation of therapy. Our data show a trend of decreased exogenous insulin infusion need in the GLP-1 infusion group; however, this failed to reach statistical significance. It is likely that GLP-1 infusion increased endogenous insulin secretion in our subjects, as mean C-peptide levels for the entire infusion period were higher in the GLP-1 infusion group. Plasma glucagon levels were lower in the GLP-1 infusion group, consistent with the known glucagonostatic effects of this peptide.
The increased stability of glycemic control seen in our study was impressive and is likely due to a combination of enhanced suppression of endogenous glucose production by GLP-1 in addition to modest insulinotropic and glucagonostatic effects (7, 8). The finding of more stable glycemic control in the absence of dramatic effects on insulin requirements is similar to the study by Müssig et al (19). They found comparable glycemic control in type 2 diabetic subjects treated with GLP-1 infusion (3.6 nmol/kg/min) plus insulin compared with subjects treated with insulin alone (19). These findings suggest that the extrapancreatic effects of GLP-1 (7–36) amide, or its primary metabolite GLP-1 (9–36) amide, are equally important glucoregulatory effects as the insulinotropic actions of GLP-1 (7, 8).
The dramatic benefits of “tight” glucose control in some studies, and the lack of benefit in others, have resulted in ongoing confusion and an inconsistent standard of care for critically ill patients. The most impressive benefits of strict maintenance of euglycemia have been seen in surgical patient populations (where the risk of sepsis is dominant) or in patients with heart failure. In these groups, maintenance of euglycemia has been shown to decrease mortality by about half. In other patient populations, a more modest benefit of glycemic control is attenuated by an increased risk of hypoglycemia. Consequently, insulin therapy has been recommended to be used less intensively (20, 21). Therefore, strategies that enhance the ability to achieve and maintain euglycemia while at the same time reducing the risk of hypoglycemia are likely to prove beneficial in all critical care patients (22).
The major advantages of using the naturally occurring incretin, GLP-1, in the ICU, in contrast to other incretinmimetic agents, is its short half life (2.5 min), as well as its glucose dependency for insulinotropic effects. With termination of GLP-1 infusion, the insulinotropic property quickly disappears, and at glucose levels of approximately 3.89 mmol/L, it is virtually devoid of any insulinotropic action. Thus, the potency and safety characteristics are excellent attributes for the use of GLP-1 in the ICU. In a randomized, crossover study of hyperglycemic surgical patients, GLP-1 infusion significantly lowered plasma glucose levels to euglycemia over the course of 8 hours (23). Furthermore, in our randomized trial of GLP-1 infusion versus placebo in postinfarct heart failure patients, blood glucose levels were better controlled and cardiac performance was enhanced in patients receiving GLP-1 (24). This significant increase in cardiac function was noted to persist out to 120 days after cessation of GLP-1 therapy. Also, in GLP-1-treated patients, there was a trend toward a survival benefit and a reduced hospital length of stay (24).
Sourij et al (25) reported that GLP-1 administered alone in eight type 2 diabetic patients controlled glucose levels more efficiently than insulin following a standardized test meal. The improvements noted were lower glucose levels, faster achievement of the target range, reduced prevalence of hypoglycemia in the GLP-1 arm, and lower plasma insulin levels in the GLP-1 arm, confirming insulinomimetic effects of GLP-1 per se. There are relatively few other studies (19, 26, 27) where GLP-1 has been administered to critically ill surgical patients. The results of all these studies have recently been reviewed by Kovalaske and Gandhi (28), who noted that in all of these pilot studies of GLP-1 administration in critically ill patients, the results were promising and suggest a potential method to effect a reduced exogenous insulin need for the regulation of hyperglycemia in critically ill patients. Pinelli et al (29) performed a meta-analysis of the reported safety and efficacy of exogenous GLP-1 therapy for hyperglycemia in critically ill patients in studies published until September 2011. Only seven of the 2015 potentially relevant articles met their eligibility criteria, and they report that in the eligible studies, relative to insulin or placebo therapy, GLP-1 therapy effectively lowered blood glucose levels. These studies all support the use of GLP-1 for the regulation of hyperglycemia in critically ill patients.
There are some limitations to our study. First, only a small number of patients were enrolled in this study, due to restricted eligibility for enrollment. Second, some patients in the GLP-1 infusion group responded dramatically, with a striking reduction in their insulin requirement, whereas other patients showed little benefit. This finding implies that some patients with ample endogenous insulin secretory capacity are ideal candidates for GLP-1 therapy, whereas other patients might be less likely to benefit. In addition, we were unable to assess the extrapancreatic effects of GLP-1 in our subjects, so as to assess the insulinomimetic actions of GLP-1 separate from its insulinotropic affects. With the small size of our study, we are unable to identify characteristics which might be used prospectively to identify those who would likely benefit from GLP-1 infusion. Third, we were limited by FDA mandate that the dose of IV administered GLP-1 could not exceed 1.5 pmol/kg/min, so we were unable to study a higher dose. Fourth, again by FDA mandate, the duration of GLP-1 infusion was limited to 72 hours. Another limitation of the study is that we did not control for the routes or quantity of nutritional support. We also did not measure many variables which potentially would elucidate possible mechanisms of action, such as levels of catecholamines and glucocorticoids. Furthermore, we were able to maintain the blood glucose levels in the desired range of 4.44–6.11 mmol/L in only a minority of patients in either group. However, although the mean 72-hour glucose levels were nearly identical in the two groups, the variation in the blood glucose profile of the GLP-1 group was significantly reduced by half compared with the saline infusion group.
Despite these limitations, our results strongly support the concept that GLP-1 is a safe and useful form of adjunct therapy in the patient with hyperglycemia in the ICU setting and add to a growing body of literature which supports the use of GLP-1 for acute glycemic control in hospitalized patients. Further and larger studies of this potentially valuable therapy would appear well justified.
Acknowledgments
We are grateful for the willingness of the subjects and their families who were enrolled in the study. We thank the nursing staff of the JHBMC SICU and BICU with assisting in the implementation of the protocol. We thank Melissa Scudder for her assistance in the preparation of the manuscript.
Dr. Gibson received support for research from The Society of Critical Care Medicine and Jahnigan Award of the American Geriatrics Society. Dr. Shannon consulted for Merck, Pfizer, and AstraZeneca/Bristol-Myers Squibb and is a board member with ABIM. He lectured for IRSW, BOMC Institute for Heart care Improvement (visiting professor). He has a patent with Ventrigen, LCC (founder). His institution received grant support from Pfizer and Astra-Zeneca/Bristol-Myers Squibb. Dr. Andersen is employed by Johns Hopkins University and the National Institutes of Health and consulted for Z-Medica (member scientific advisory board). He is a board member with the Association for Academic Surgery Foundation and lectured at scientific meetings and medical centers. He receives royalties from McGraw Hill (member, editorial board, textbook). Dr. Elahi consulted for Merck.
Footnotes
Drs. Galiatsatos, Gibson, Rabiee, Carlson, Egan, and Elahi assisted in the conduct of the study. Drs. Gibson, Shannon, Andersen, and Elahi designed the study. Dr. Elahi wrote the first draft of the article. All other authors contributed to the writing of the article.
The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 2.Aragon D. Evaluation of nursing work effort and perceptions about blood glucose testing in tight glycemic control. Am J Crit Care. 2006;15:370–377. [PubMed] [Google Scholar]
- 3.Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 4.American Diabetes Association, Endocrinologists AAoC. Joint Statement from American Diabetes Association and American Association of Clinical Endocrinologists on the NICE-SUGAR Study on intensive versus conventional glucose control in critically ill patients. 2009 Available at: http://www.diabetes.org/for-media/pr-NICE_SUGAR-study.jsp Accessed April 6, 2009.
- 5.The Endocrine Society. The Endocrine Society suggests tailored approach to glycemic control in response to the NICE-SUGAR Study published this week in the New England Journal of Medicine. 2009 Available at: http://www.endo-society.org/media/press/2008/Society-SuggestsTailoredApproachtoGlycemicControlinResponsetotheNICE-SUGAR.cfm Accessed April 7, 2009.
- 6.Frezza EE, Wachtel MS, Chiriva-Internati M. The multiple faces of glucagon-like peptide-1-obesity, appetite, and stress: What is next? A review Dig Dis Sci. 2007;52:643–649. doi: 10.1007/s10620-006-9096-2. [DOI] [PubMed] [Google Scholar]
- 7.Elahi D, Egan JM, Shannon RP, et al. GLP-1 (9–36) amide, cleavage product of GLP-1 (7–36) amide, is a glucoregulatory peptide. Obesity (Silver Spring) 2008;16:1501–1509. doi: 10.1038/oby.2008.229. [DOI] [PubMed] [Google Scholar]
- 8.Prigeon RL, Quddusi S, Paty B, et al. Suppression of glucose production by GLP-1 independent of islet hormones: A novel extrapancreatic effect. Am J Physiol Endocrinol Metab. 2003;285:E701–E707. doi: 10.1152/ajpendo.00024.2003. [DOI] [PubMed] [Google Scholar]
- 9.Abu-Hamdah R, Rabiee A, Meneilly GS, et al. Clinical review: The extrapancreatic effects of glucagon-like peptide-1 and related peptides. J Clin Endocrinol Metab. 2009;94:1843–1852. doi: 10.1210/jc.2008-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nauck MA, Heimesaat MM, Behle K, et al. Effects of glucagon-like peptide 1 on counterregulatory hormone responses, cognitive functions, and insulin secretion during hyperinsulinemie, stepped hypoglycemic clamp experiments in healthy volunteers. J Clin Endocrinol Metab. 2002;87:1239–1246. doi: 10.1210/jcem.87.3.8355. [DOI] [PubMed] [Google Scholar]
- 11.Kieffer TJ, Habener JF. The glucagon-like peptides. Endocr Rev. 1999;20:876–913. doi: 10.1210/edrv.20.6.0385. [DOI] [PubMed] [Google Scholar]
- 12.Elahi D, McAloon-Dyke M, Fukagawa NK, et al. The insulinotropic actions of glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (7–37) in normal and diabetic subjects. Regul Pept. 1994;51:63–74. doi: 10.1016/0167-0115(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 13.Zander M, Madsbad S, Madsen JL, et al. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: A parallel-group study. Lancet. 2002;359:824–830. doi: 10.1016/S0140-6736(02)07952-7. [DOI] [PubMed] [Google Scholar]
- 14.Meneilly GS, Greig N, Tildesley H, et al. Effects of 3 months of continuous subcutaneous administration of glucagon-like peptide 1 in elderly patients with type 2 diabetes. Diabetes Care. 2003;26:2835–2841. doi: 10.2337/diacare.26.10.2835. [DOI] [PubMed] [Google Scholar]
- 15.Nikolaidis LA, Elahi D, Hentosz T, et al. Recombinant glucagon-like peptide-1 increases myocardial glucose uptake and improves left ventricular performance in conscious dogs with pacing-induced dilated cardiomyopathy. Circulation. 2004;110:955–961. doi: 10.1161/01.CIR.0000139339.85840.DD. [DOI] [PubMed] [Google Scholar]
- 16.Gibson BR, Galiatsatos P, Rabiee A, et al. Intensive insulin therapy confers a similar survival benefit in the burn intensive care unit to the surgical intensive care unit. Surgery. 2009;146:922–930. doi: 10.1016/j.surg.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 17.Egi M, Bellomo R, Stachowski E, et al. Hypoglycemia and outcome in critically ill patients. Mayo Clin Proc. 2010;85:217–224. doi: 10.4065/mcp.2009.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyfroidt G, Keenan DM, Wang X, et al. Dynamic characteristics of blood glucose time series during the course of critical illness: Effects of intensive insulin therapy and relative association with mortality. Crit Care Med. 2010;38:1021–1029. doi: 10.1097/CCM.0b013e3181cf710e. [DOI] [PubMed] [Google Scholar]
- 19.Müssig K, Oncü A, Lindauer P, et al. Effects of intravenous glucagonlike peptide-1 on glucose control and hemodynamics after coronary artery bypass surgery in patients with type 2 diabetes. Am J Cardiol. 2008;102:646–647. doi: 10.1016/j.amjcard.2008.06.029. [DOI] [PubMed] [Google Scholar]
- 20.Wiener RS, Wiener DC, Larson RJ. Benefits and risks of tight glucose control in critically ill adults: A meta-analysis. JAMA. 2008;300:933–944. doi: 10.1001/jama.300.8.933. [DOI] [PubMed] [Google Scholar]
- 21.Marik PE, Preiser JC. Toward understanding tight glycemic control in the ICU: A systematic review and metaanalysis. Chest. 2010;137:544–551. doi: 10.1378/chest.09-1737. [DOI] [PubMed] [Google Scholar]
- 22.Van den Berghe G. Intensive insulin therapy in the ICU-reconciling the evidence. Nat Rev Endocrinol. 2012;8:374–378. doi: 10.1038/nrendo.2012.14. [DOI] [PubMed] [Google Scholar]
- 23.Meier JJ, Weyhe D, Michaely M, et al. Intravenous glucagon-like peptide 1 normalizes blood glucose after major surgery in patients with type 2 diabetes. Crit Care Med. 2004;32:848–851. doi: 10.1097/01.ccm.0000114811.60629.b5. [DOI] [PubMed] [Google Scholar]
- 24.Nikolaidis LA, Mankad S, Sokos GG, et al. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004;109:962–965. doi: 10.1161/01.CIR.0000120505.91348.58. [DOI] [PubMed] [Google Scholar]
- 25.Sourij H, Schmölzer I, Kettler-Schmut E, et al. Efficacy of a continuous GLP-1 infusion compared with a structured insulin infusion protocol to reach normoglycemia in nonfasted type 2 diabetic patients: A clinical pilot trial. Diabetes Care. 2009;32:1669–1671. doi: 10.2337/dc09-0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deane AM, Chapman MJ, Fraser RJ, et al. The effect of exogenous glucagon-like peptide-1 on the glycaemic response to small intestinal nutrient in the critically ill: A randomised double-blind placebo-controlled cross over study. Crit Care. 2009;13:R67. doi: 10.1186/cc7874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sokos GG, Bolukoglu H, German J, et al. Effect of glucagon-like peptide-1 (GLP-1) on glycemic control and left ventricular function in patients undergoing coronary artery bypass grafting. Am J Cardiol. 2007;100:824–829. doi: 10.1016/j.amjcard.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 28.Kovalaske MA, Gandhi GY. Incretins in the ICU: Is insulin on its way out? Crit Care. 2009;13:161. doi: 10.1186/cc7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pinelli NR, Jones MC, Monday LM, et al. Exogenous glucagon-like peptide-1 for hyperglycemia in critically ill patients. Ann Pharmacother. 2012;46:124–129. doi: 10.1345/aph.1Q417. [DOI] [PubMed] [Google Scholar]


