Abstract
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disorder characterized by focal pathologic bone resorption due to excessive activity of osteoclasts (OC). Receptor activator of nuclear factor kappa B ligand (RANKL) is essential for the proliferation, differentiation, and survival of OC. Denosumab (DMab) is a humanized monoclonal antibody that binds to RANKL with high affinity and blocks its subsequent association with its receptor RANK on the surface of OC precursors.
Area Covered
The authors review the molecular and cellular mechanisms underlying therapeutic applications of DMab, provide recent highlights on pharmacology, efficacy and safety of DMab, and discuss the potential of DMab as a novel therapeutic option for the treatment of rheumatoid arthritis.
Expert opinion
Clinical results suggest that DMab is efficient both in systemic and articular bone loss in RA with limited side effects. Diminished bone erosion activity was also noted in RA patients on corticosteroids and bisphosphonates. Combination of DMab with an anti-TNF agent was not associated with increased infection rates. Collectively, these data indicate that DMab, in combination with methotrexate and possibly other conventional synthetic Disease Modifying Anti-Rheumatic Drugs (csDMARDs), is an effective, safe and cost-effective option for the treatment of RA.
1. Introduction
DMab is a human monoclonal IgG2 antibody that inhibits bone resorption by binding and inhibiting receptor activator of NF-kB ligand (RANKL), an essential cytokine for osteoclast (OC) formation, activity, and survival (1, 2). Association of DMab with RANKL will inhibit the binding of RANKL to the RANK receptor expressed on the cell surface of OC, an essential activation step for OC differentiation. The Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) trial and its Extension provide long-term information on denosumab for treating postmenopausal osteoporosis (3-5). DMab treatment for up to 8 years significantly decreased bone turnover (6), increased bone mineral density (BMD)(7), improved bone microstructure of both cortical and trabecular bone (7, 8), and reduces the risk of bone fracture and osteoporosis (3, 5, 9-12). Bone biopsies confirmed potent and sustained effects of DMab on bone quality with continuous DMab treatment for 5-8 years (3, 4). The beneficial effects of DMab, however, can be fully reversed at the tissue level within 2 years of discontinuation, indicating that the skeletal effects of DMab are directed towards regulation of bone turnover to inhibit resorption and maintain bone mineral density (BMD).
The properties of denosumab are summarized in Box 1. DMab was approved by the FDA for 1) postmenopausal women with osteoporosis at high risk for fracture; 2) fractures arising from metastastic bone cancer; and 3) prevention of skeletal-related events in patients with bone metastases from solid tumors 4) adults and skeletally mature adolescents with giant cell tumor of bone that is unresectable or where surgical resection is likely to result in severe morbidity (13-18). Elevated OC activity coupled with increased OC in bone are shared features of disorders that respond to DMab. Effective control of cancer metastasis by DMab is related to its regulation of immune cell profiles and inflammatory cytokines through the regulation of RANKL concentration (19, 20). Based on its ability to enhance bone quality and limit the progression of inflammatory bone diseases and cancer metastasis, the efficacy and safety of DMab has been examined in ongoing and completed trials. Thus, despite divergent molecular mechanisms, DMab exerts beneficial effects in cancer, inflammatory arthritis and osteoporosis that far outweigh the rare and often minor side effects including hypocalcemia and local infection (18, 21, 22). Herein we provide an update on the role of DMab in RA from the molecular, cellular and clinical perspective.
2. Denosumab - Current Indications
2.1 Profile
Denosumab (DMab) is an FDA-approved humanized monoclonal antibody to treat patients with osteoporosis (23-25) and cancer patients with bone metastasis (16, 23, 26, 27). DMab was commercialized by Amgen under two brand names, XGEVA and Prolia (Box 1). Completed and ongoing clinical trials of DMab suggest the clinical benefits of DMab outweigh its side effects (Table 1 and website: clinicaltrials.gov) (28-33). Phase III clinical trials demonstrated that DMab can (i) decrease bone turnover; (ii) reduce the risk of bone fracture in patients with osteoporosis; and (iii) increase bone mineral density with minimal side effects (23).
Table 1.
Denosumab clinical trials in RA.
| Trials | Number of subjects | Type | Concomitant Medications | Key findings in Denosumab vs. PBO | Reference |
|---|---|---|---|---|---|
| Amgen | 218 | Phase II RDBPCT | MTX | Significant decrease in erosions in hands, wrists (MRI, plain x-ray) | Cohen et al. 2008 (23) |
| Amgen | 218 | Post-hoc analysis | MTX | Significantly less metacarpal bone loss | Sharp et al. 2010 (24) |
| Amgen | 56 | Post-hoc analysis | MTX | Significant increase in hand BMD and decreased erosion | Deodhar et al. 2010 (25) |
| Amgen | 218 | Post-hoc analysis | MTX | Significant increase in spine and hip BMD and reduced sCTx-1 and PiNP levels | Dore et al. 2010 (26) |
| DRIVE | 350 | Phase II RPCT | MTX | Significant decrease in hand and wrist erosions | Takeuchi et al. 2016 (27) |
| Keio University | 160 | Cohort | TNFi | Significant decrease in erosion score | Hasegawa et al. 2016 (28) |
2.2 Side effects
Low Ca2+ and phosphate in the blood, muscle cramps, cellulitis and numbness are known DMab-associated adverse effects (21-24). Hypocalcemia is the most common side effect in DMab-treated patients (34-37). Ca2+ levels should be evaluated and low Ca2+ and Vitamin D concentration should be corrected before the initiation of DMab treatment (38, 39). It is also known that patients treated with zoledronic acid prior to DMab have a higher risk of DMab-induced hypocalcemia (p<0.05)(36). In addition to hypocalcemia, osteonecrosis of the jaw (ONJ) is a rare complication in DMab-treated RA patients (40-44). Histopathologic Analysis of bone tissue from patients who developed ONJ on DMab for bone metastases revealed a decrease number of osteoclasts with few nuclei. This morpohology is strikingly different from ONJ linked to bisphosphonates where osteoclast numbers are increased with giant, hypernucleated and detached and undergoing apoptosis (16, 45-47). Thus, a careful screening of RA patients for hypocalcemia and ONJ is recommended before DMab administration. Atypical femur Fracture is another uncommon side effect reported in RA patients on DMab. (48-50). The fractures share radiologic features with stress fractures and patients can show periosteal reactions from presumed microtrauma weeks prior to the femur fracture(s) (51). It is recommended that patients be informed about the risk of atypical femur fractures and to report new onset thigh, hip or groin pain. Rebound fractures, usually associated with rebound osteoclast activity in the absence of anti-resorptive reagents (52), have been reported in patients on DMab following withdrawal of the agent (53).
2.3 Denosumab and Bisphosphonate: Contrasting disease mechanisms
2.3.1 Molecular Mechanism
The divergent molecular mechanisms underlying the protective effect of DMab and bisphosphonates on bone have been reviewed by Baron et al., (54). DMab prevents RANKL from binding to RANK receptor, thereby inhibiting osteoclast differentiation from osteoclast precursors. In contrast, bisphosphonates bind to calcium in bone and inhibit mature osteoclast function through induction of apoptotic pathways or blocking cytoskeletal assembly by inhibition of lipid modification of Ras, Rho, and Rac proteins.
2.3.2 Cellular distribution and action
DMab is a circulating antibody that can reach inflammatory sites. Bone penetration of DMab was demonstrated by its presence in blood vessels and the tibial cortex s (54). In contrast, bisphosphonates bind to mineral surfaces throughout the bone and agents with the strongest avidity may not reach the deeper trabecular surfaces (55). Osteoporosis clinical studies were performed to examine whether accumulation of bisphosphonates leads to a continuous decline in bone remodeling (54, 56, 57). Findings from these studies suggest that bisphosphonates accumulate in bone but the effect on resorption is not well understood (54). Distinct cellular distribution and action could explain the difference in the degree, speed and action of anti-resorptive effects between DMab and bisphosphonate (18, 31, 58). In contrast to bisphosphonate, DMab significantly reduced bone-specific alkaline phosphatase at 6 and 12 months compared with pretreatment, but had no effect on tartrate-resistant acid phosphatase 5b levels, emphasizing the effect of DMab more on bone formation than resorption rate(58). In addition, effects of DMab on the reduction of bone resorption are more reversible and profound than bisphosphonates (54). One recent study showed that neither DMab nor bisphosphonates suppress rheumatoid inflammation (58). However, DMab, but not bisphosphonates, significantly suppressed bone metabolism in a cohort of Japanese RA patients not previously treated for osteoporosis. These findings suggest distinct cellular mechanisms underlying DMab- and bisphosphonate-based RA therapy (4, 58). DMab exerts its protective effects likely through the WNT/β-catenin signaling pathway via regulating DKK-1 (25, 59). A decreased expression of DKK-1 was detected only with DMab and not bisphosphonates therapy (60). This observation may account for the distinct densitometric therapeutic duration window (larger without apparent plateau) observed with DMab therapy.
2.4 Market Potential
The sale of Xgeva and Prolia continue to show upward trends according to the market report on July 29, 2016. During the year 2015-16, Xgeva sales rose 11% to $378 million and Prolia sales increased 29% to $352 million (61). Collectively, Prolia and Xgeva earn a combined $730 million in revenue annually, which accounts for more than 16% of the market share for osteoporosis and cancer- associated bone metastasis in the United States.
Despite the fact that DMab is approved for treatment of osteoporosis and cancer patients with bone metastasis, it has not yet approved for treatment of inflammatory bone conditions such as RA and psoriatic arthritis (PsA). To reduce the medical cost of RA treatment with biologic DMARDs, several completed studies (28, 32, 33, 62, 63) evaluated the effects of DMab in combination with methotrexate in RA (discussed below). Although the molecular mechanism underlying DMab are not fully understood, DMab is a cost-effective alternative to current RA therapies with the potential to limit systemic and articular bone loss.
3. RANKL in RA Pathogenesis
3.1 Mechanism
RA is a chronic systemic inflammatory autoimmune disease characterized by bone loss that predates bone erosion on radiographs. RA has a range of extra-articular manifestations that include rheumatoid nodules/vasculitis, granulomatous skin disorders, and neutrophilic dermatoses (64). RA patients typically experience progressive joint damage associated with physical pain and functional impairment. It is now widely accepted that inflammatory bone diseases are initiated by dysregulated bone remodeling due to imbalance between bone resorption and formation. In RA, pathologic bone loss is not compensated by osteoblast-mediated repair because these pathways are inhibited as a result of synovial inflammation (65). Intriguingly, impaired osteoblast-mediated bone repair in RA is likely also inhibited by elevated DKK-1 expression, which correlates with bone erosions and systemic bone loss (66). Thus, controlling bone-resorption by OC via regulating RANKL and DKK-1 is of central importance for developing effective RA therapies.
The complex pathobiology of RA, is characterized by infiltration of the synovial membrane with cells of the innate and acquired immune system, hyperplasia of the joint lining and a progressive localized destruction of bone and cartilage mediated by fibroblastoid cells and OCs (65, 67). Given that elevated serum and tissue RANKL concentration were detected in RA patients (19, 68-71), rheumatoid bone and joint destruction in RA are likely mediated by RANKL-induced OC differentiation (Figure 1). Many cell types, including T lymphocytes, B cells and osteoblasts (OB), release RANKL during the course of RA (72, 73). Several cellular factors including PTH (74) and CXCL16 are involved in the regulation of RANKL (20). Intriguingly, the level of RANKL in RA sera is not only positively correlated with other RA-specific disease biomarkers including anti-citrullinated protein antibodies (ACPA), but also with the degree of bone erosion (19). Considering the fact that bone repair is rarely detected in RA once bone erosion is established (75), preventing initial erosion by targeting RANKL remains an effective option for therapeutic intervention.
Figure 1. Dual signals, one from RANKL and one from ITAM-bearing receptor, are required for the activation and maturation of osteoclast precursors.
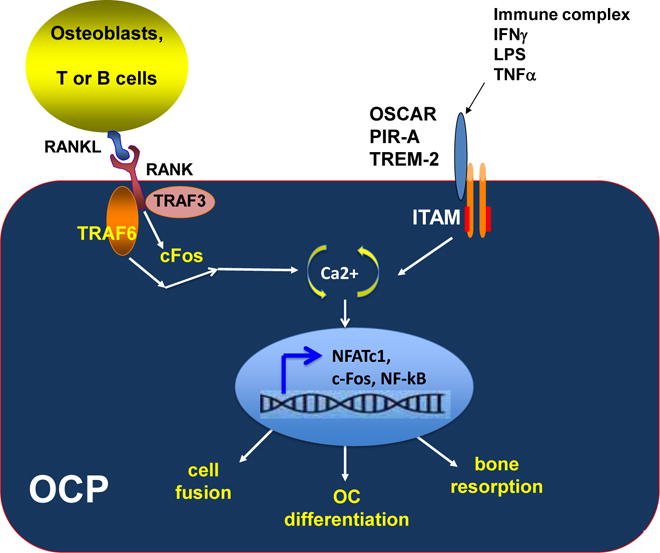
Binding of RANKL to the RANK receptor on the cell surface of osteoclasts initiates a complex signaling pathway which involves TRAF6, TRAF3 and c-Fos proteins. This activation signal, together with activation signals from ITAM-bearing receptors such as OSCAR, PIR-A and TREM-2, will modulate intracellular calcium oscillation which in turn induces NFATc1 nuclear translocation and turns on a cassette of genes related to osteoclastogenesis. It is well established that RANKL regulates osteoclastogenesis via the NFATc1/Ca2+ axis (119-122). Of note, many intermediate regulators in the RANKL-mediated osteoclastogenesis pathway are not depicted in this model for simplification purpose. Adapted from [123] with permission of John Wiley and Sons
3.2 Denosumab on osteoclast differentiation
Many proinflammatory cytokines (TNF, IL-6, M-CSF, RANKL, IL-1, IL-17, IL-15, IL-33 and DKK-1) contribute to joint inflammation and structural damage in RA, however, not all proinflammatory cytokines trigger bone loss (25, 67). Each cytokine exerts direct or indirect actions which affect immune regulation and/or osteoclastogenesis, in RA (67). Among these regulators, RANKL and M-CSF are two mediators essential for osteoclastogenesis, a process by which naïve osteoclast precursors (OCPs) differentiate into mature multinucleated osteoclasts with bone resorption activity. Dual signals, one from RANKL and a second from Immunoreceptor Tyrosine Activation Motif (ITAM)-bearing molecules including Triggering Receptor Expressed on Myeloid cells-2 (TREM)-2, and/or Osteoclast Associated Receptor (OSCAR), are required to trigger the initiation of osteoclastogenesis (76-78). As shown in Figure 2, binding of RANKL to the RANK receptor on the cell surface of osteoclasts initiates a complex signaling pathway which involves TRAF6, TRAF3 and c-Fos proteins. Binding of RANKL, together with activation signals from OSCAR and TREM-2, modulate intracellular calcium oscillation which in turn induces NFATc1 nuclear translocation and turns on a cassette of genes related to osteoclastogenesis. RANKL-dependent osteoclastogenesis is a complex pathway which involves many intermediate signaling regulators and mediators, which are not depicted in Figure 1. Without RANKL, osteoclast precursors fail to initiate the NFATc1-Ca2+ signaling cascade and differentiate into mature osteoclasts. The cellular mechanisms underlying the protective effects of DMab in RA via RANKL-mediated pathway are illustrated in Figure 2. Increased RANKL concentration promotes osteoclast formation and subsequently enhances bone-resorbing activities (Figure 2). Binding of DMab to RANKL effectively blocks RANK::RANKL association, and results in an impaired osteoclast differentiation, decreased bone erosion and joint damage (Figure 2).
Figure 2. Mechanisms of DMab action in inhibiting osteoclast differentiation.
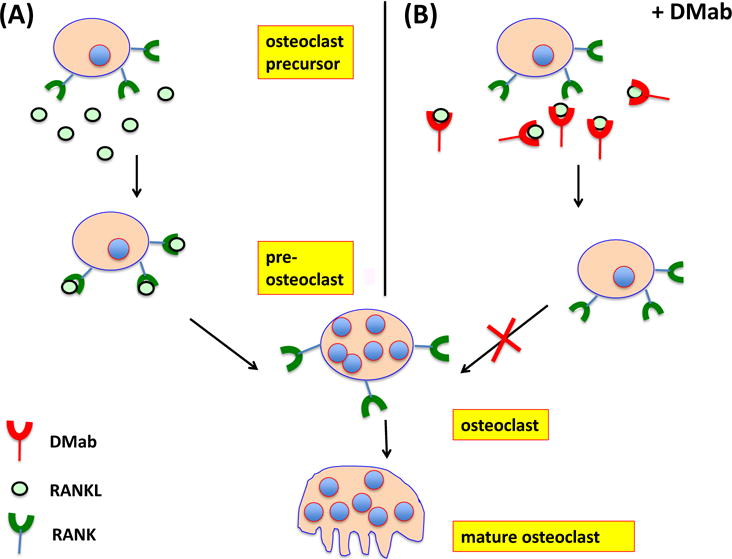
(A) Osteoclast precursors (OCPs) are present in bone marrow, circulation and inflamed joints. OCPs express RANK and c-Fms receptors on their cell surfaces. Binding of RANK to RANK ligand (RANKL) and c-FMS to M-CSF, respectively, are essential for the initiation of RANKL-dependent OC differentiation. (B) Through binding to RANKL, DMab blocks engagement with its receptor, RANK on OCPs and inhibits the subsequent activation and maturation of OCPs. Consequently, DMab decreases bone resorption and fosters an overall increase in bone mineral density (BMD).
Recently, a leucine-rich repeat-containing G-protein-coupled receptor 4 (LGR4) was identified as a second receptor for RANKL. Binding of RANKL to LGR4 induces apoptosis in mature OC providing a negative feedback loop to regulate OC survival. Studies in murine models of osteoporosis show efficacy with a fusion protein that contains the LGR4 extracellular domain (79). LGR4 competes with RANK to bind RANKL and suppresses canonical RANK signaling during OC differentiation. Injection of LGR4-extracellular domain inhibited in vitro OC differentiation in vitro and osteroporosis in 3 murine models. The finding that LGR4 expression is induced by RANKL-NFATC1 signaling may explain why mature OC undergo apoptosis in the presence of RANKL-LGR4 and provide a negative feedback signal that limits survival of mature OC. In an accompanying editorial, Zaidi and Lqbal acknowledge the therapeutic potential of LGR4 but also point out that LGR family members bind to R-spondins which regulate cell proliferation, differentiation and cell fate as well as tumor suppressors in the intestine (80). Additional studies will help determine the therapeutic utility and safety of strategies that target LGR4.
3.3 DMab and immune regulation
The role of RANKL-RANK-OPG on immune regulation was reviewed by Walsh and Choi and several others (81-85). Briefly, RANKL is an essential survival factor of dendritic cells (DC) (86). RANKL secreted by T cells significantly enhanced immunity by promoting the survival and function of DCs. RANKL-expressing Th17 cells stimulate mature but non-resorptive osteoclasts to resorb bone (87, 88). In addition, RANKL secreted by memory B cells promotes bone erosion in RA (73) and OC formation in an ovariectomy model of osteoporosis (89). Lastly, RANKL was known to induce immune tolerance by promoting the differentiation of Treg cells (90-92). RANKL knock out (KO) mice are osteopetrotic, lack lymph nodes and show alterations in B and T cell maturation (93). Interestingly, in the serum transfer model, however, inflammation was equivalent in the wild type and KO mice (94).
Considering the essential role of RANKL/OPG (95-97) in the development of the immune system and the expression of these molecules by immune cells that release co-stimulatory factors for the activation of T and dendritic cells (98), it is conceivable that RANKL antagonists may influence immune regulation. This notion is further supported by data which demonstrated that DMab exhibits effects on non-skeletal systems including immune and vascular cells (54). Blockade of RANKL signaling by RANK-Fc or OPG-Fc inhibits dendritic cell-dependent T cell activation in IL-2 knockout or CD40 knockout mice, two autoimmune disease models (99, 100). The evidence outlined above support involvement of RANKL in immune regulation but there is no evidence that it significantly alters immune function at the approved doses for osteoporosis. Whether DMab directly interferes immune responses in RA remains controversial (101).
3.3.1 DMab changes the profile of cell subsets in immune and vascular systems
RANKL, RANK, osteoprotegerin are key mediators of osteoimmunology and vascular diseases (102, 103). RANKL inhibition by DMab changes immune cell profiles (104) and cell populations that are involved in bone remodeling including osteoblasts and osteocytes (54). In addition, RANKL has been known to increase vascular smooth muscle cell calcification through a RANK-BMP4-dependent pathway (105). Blocking of RANKL by DMab may affect the vascular smooth muscles and reduce calcium deposition as shown in the huRANKL mice (106). Additional studies are necessary to elucidate a possible involvement of DMab, either directly or indirectly, in immune regulation and vascular diseases.
4. Effect of DMab on bone quality
Reduced cortical bone porosity and enhanced bone mineral density (BMD) are two major effects observed following DMab treatment (11, 107, 108). Bone strength is mainly determined by the cortical components. Thus, loss of cortical bone contributes to a higher frequency of bone fractures in the elderly. DMab affects not only the thickness of the cortex but also bone strength, porosity and bone mineral density. Cortical thickness at the distal radius increased 3% following DMab treatment, compared to no significant change in the placebo group (54). Of note, cortical thinning is typically observed before the clinical onset of RA, and correlates with the risk of bone erosion (8, 109). These observations are consistent with the concept that excessive osteoclast resorption precedes radiographic evidence of bone loss and thus blocking osteoclast differentiation in early RA may have a greater impact on inhibition of structural damage. An intriguing mechanism to explain the protective effects of DMab in limiting bone loss in RA may be its action on DKK1. DKK1 levels in RA patients are higher than in healthy controls and correlate with erosion and bone loss (66). Treatment with DMab is associated with a decrease in DKK1 levels (8).
5. DMab in RA
The interplay of activated immune cells, synovial cell hyperplasia and cytokine release characteristic of RA fosters an osteoclastogenic environment fueled by TNF-α and RANKL. Indeed, the presence of local and systemic bone loss in RA patients raised the possibility that inhibition of RANKL may be an effective strategy to limit pathologic bone resorption. Several studies documented a potent effect of DMab on bone erosion and bone mineral density (BMD) in RA (Table 1). In addition, high-resolution quantitative computed tomography studies performed on RA patients revealed partial bone repair (decrease depth, width and volume of erosions) was noted after 6 months of treatment with DMab but not alendronate (110).
In the original phase II double-blind, placebo-controlled trial, 227 patients on baseline methotrexate, were randomized to receive DMab 60 mg or 180 mg every 6 months or placebo for 12 months (28). In patients on 180 mg but not 60 mg, the change in the MRI erosion score at 6 months was significantly less than placebo (mean change for 60 mg 0.13, p=0.118 and 180 mg 0.06, p=0.007). DMab treatment suppressed markers of bone turnover but no decrease in joint space narrowing was detected. Several retrospective analyses were performed on subjects in this trial. Significantly fewer patients demonstrated metacarpal bone loss in both treatment groups compared to placebo at 6 months based on assessment with digital x-ray (29). In a sub-study, bone densitometry (DEXA) of the hands in 56 patients revealed that DMab decreased erosion progression and was associated with higher BMD which declined in the placebo group (30). Lastly, significant increases in bone density were observed in the lumbar spine and hip in the DMab-treated patients compared to placebo at 6 and 12 months, despite the use of glucocorticoids or bisphosphonates (31, 111). In addition, the serum levels of type I C-telopeptide (sCTx-I) and procollagen 1N-terminal peptide (P1NP) declined significantly, regardless of baseline BMD, marker levels or concomitant bisphosphonate or glucocorticoid use in the treatment groups.
In a recent Japanese trial, DMab in patients with Rheumatoid arthritis patients on methotrexate to Validate the Inhibitory effect on bone Erosion (DRIVE), 350 RA patients on baseline methotrexate were randomized to receive DMab 60 mg every 6 months(M), every 3 months or every 2 months or placebo in a 1:1:1:1 ratio for 12 months (112). At 12 months, all doses of DMab were associated with a significant inhibition of radiographic progression assessed by the modified Sharp erosion score. Changes in this score at 12 months from baseline were 0.99 for placebo, 0.27 (p<.0.0001 compared to placebo) for Q6M, 0.14 (p=0.0036 compared to placebo) for Q3M and 0.09 (p<0.0001 compared to placebo) for Q2M. Bone mineral density was maintained in the treatment groups compared to placebo and no effect on joint space narrowing was observed. In all the studies detailed above, joint space narrowing did not decline significantly on DMab, adverse events were not increased compared to placebo and no anti-inflammatory effects were noted in the treatment groups.
Another potential niche for DMab is the treatment of osteoporosis in RA patients. For rheumatologists, this approach is of limited value since many RA patients are taking biologic DMARDs (bDMARDs) which are quite effective at limiting bone loss. Moreover, concerns regarding an increased risk of infections when these agents are taken in combination have greatly limited their use in this population. To address the efficacy and safety of DMab in combination with biologic agents in RA, a retrospective Japanese cohort trial enrolled 80 RA patients on one of the following baseline biologic bDMARDs: infliximab, adalimumab, infliximab, etanercept, abatacept or tocilizumab and randomized them in a 1:1 manner to DMab 60 mg or placebo every 6 months (33). The modified TSS erosion score was significantly less in the group that received DMab compared to placebo at 12 months without a significant increase in adverse events. This study demonstrated that DMab effectively decreased bone erosion even in patients on bDMARDs, corticosteroids or bisphosphonates without major safety signals. Radiographic analyses confirmed an improved efficacy of DMab-bDMARDs combined therapy for RA treatment (33).
The safety of combining DMAb with a biologic agent in RA patients for treatment of osteoporosis was further examined in a Medicare administrative database that included 5814 patients, 1354 exposed to DMab and 4460 to zolendronic acid (113). A subgroup analysis was also performed on 463 patients in each group matched on infection risk score and several demographic variables. The crude rate of hospitalized infection in the two RA cohorts was not significantly different in patients on DMab compared to zolendronic acid in both the main and subgroup analyses. These results must be interpreted with the understanding that the time on DMab was variable, the infections were limited to hospitalized patients and the hospitalizations were not confirmed. Nevertheless, these data provide the first confirmation that combination of DMab with bDMARDs to treat concomitant osteoporosis does not increase infection risk leading to hospitalization.
6. Expert Opinion
Evidence from 2 phase II trials and one randomized observational trial indicate that DMab inhibits focal and systemic bone loss in RA. These findings are particularly relevant in RA because many of the risk factors associated with systemic bone loss are present including a high prevalence of post-menopausal females, concomitant glucocorticoid use, systemic osteoclast activation and limited functional mobility. For those patients who do require additional treatment for osteoporosis on bDMARDs, DMab may be a good option based on administrative data showing no increase in adverse events in patients on a combination of a bDMARD and DMab. It is important to point out, however, that while DMab does inhibit bone erosion, it does not appear to have a major impact on pathologic cartilage resorption and it does not demonstrate anti-inflammatory properties so its actions are limited and require co-treatment.
Given these findings, is there a role for DMab in RA? This is not a trivial question particularly given that the first pivotal trial was published 8 years ago and this approach has not been formally examined in a phase III trial. The landscape of RA therapy is changing rapidly, however, with the entry of biosimilars, novel biologic agents and the JAK inhibitors. The rapid uptake and high penetration of bDMARDs, particularly in the U.S. market, has been accompanied by unprecedented price inflation that will be only partially offset by biosimilars due to high development costs. These inflated drug costs are limiting the use of bDMARDs and this problem is expected to increase as we move towards a system focused on population health and cost savings. These trends, coupled with studies that indicate conventional synthetic (cs) DMARDs are appropriate and effective therapy, either alone or in combination, provide new opportunities for regimens that include DMab as a cost savings alternative.
Combination regimens of csDMARDs or bDMARDs with DMab may be appropriate in several different settings. First, methotrexate monotherapy is effective in about 30 percent of patients, some of whom will develop erosive changes on this treatment which could be ameliorated with DMab (114). Second, some patients with early RA present with baseline erosions and the traditional approach has been to initiate a bDMARD in combination with methotrexate (115). Addition of DMab in place of a biologic is significantly less expensive and may provide a similar outcome. Third, many patients cannot afford biologics and a significant percentage may be intolerant, have comorbid diseases that prohibit the use of a bDMARD (multiple sclerosis, congestive heart failure) or fear self-injection. Fourth, several studies have documented subclinical synovitis on imaging studies and histopathology in patients in remission and progressive joint damage has been reported (116, 117). These patients would benefit from an additional agent that targets erosions, particularly given that strategies are under study to taper bDMARDs in patients with remission (118). The addition of DMab will limit structural damage that may arise when the bDMARD is withdrawn. It is anticipated that development of surrogate markers of radiographic damage and treatment stratification biomarkers which can classify responders from non-responders prior to treatment may also allow for improved treatment assignment in relation to csDMARDs and bDMARDs which may facilitate early regimens that include DMab.
Phase III trials are required to discern the magnitude of the inhibitory effect on bone erosions and help to establish an optimal dose. In the initial study by Cohen et al. (28), bone erosions on MRI were not significantly inhibited by 60 mg but 180 mg was effective and in the Takeuchi study, shorter dosing intervals demonstrated greater inhibition of structural damage. The role of DMab in RA patients with osteoporosis is also not well understood. The ability of DMab to reduce cortical porosity may be distinctly advantageous in a disease characterized by focused resorption of cortical bone and this may explain why it was effective even in patients taking bisphosphonates. The ability to prevent fractures in RA patients with multiple risk factors, particularly glucocorticoids also deserves further study.
DMab, an antibody to RANKL, limits osteoclast proliferation, activation and survival. It is approved in the US for the treatment of postmenopausal osteoporosis and bone metastases associated with solid tumors. Despite demonstrated efficacy for limitation of focal and systemic bone loss in RA, this agent has not been widely adopted for treatment of rheumatoid joint disease or concomitant osteoporosis. Changing market dynamics coupled with that appreciation csDMARDs are effective for some patients, either alone or in combination with other csDMARDs along with the impetus to taper bDMARDs in patients who reach sustained remission, hold promise that DMab may emerge as an important therapy to limit local erosions and maintain BMD. Additional studies will be required to establish optimal treatment regimens and confirm long-term safety for combination bDMARD and DMab regimens. Future DMab-based RA therapy may not only targeting inflammation but also improve cortical bone porosity and BMD at early stages of RA and lead to better treatment outcomes.
Table 2.
Potential therapeutic value of Denosumab for treatment of RA
| 1 | Early response to csDMARD* |
| 2 | Early RA with baseline erosions |
| 3 | Unable to take bDMARDs** (cost, intolerance, comorbidity) |
| 4 | In clinical remission with synovitis on imaging |
| 5 | In clinical remission and withdrawal of bDMARDs** |
csDMARD : conventional synthetic DMARD
bDMARDs : biological DMARD
Drug Summary Box.
| Drug name | Denosumab |
| Phase | Pre-Registration |
| Indication | Rheumatoid Arthritis |
| Pharmacology description | RANKL antagonist |
| Route of administration | Subcutaneous |
| Pivotal trial(s) | [23-28] |
Acknowledgments
The authors are funded by National Institutes of Health grant R01 AR069000-01.
Footnotes
Declaration of Interest:
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
- 1.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93(2):165–76. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 2.Lacey DL, Tan HL, Lu J, Kaufman S, Van G, Qiu W, et al. Osteoprotegerin ligand modulates murine osteoclast survival in vitro and in vivo. The American journal of pathology. 2000;157(2):435–48. doi: 10.1016/S0002-9440(10)64556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3*.Papapoulos S, Lippuner K, Roux C, Lin CJ, Kendler DL, Lewiecki EM, et al. The effect of 8 or 5 years of denosumab treatment in postmenopausal women with osteoporosis: results from the FREEDOM Extension study. Osteoporos Int. 2015;26(12):2773–83. doi: 10.1007/s00198-015-3234-7. The extension study of this pivotal trial demonstrated that DMab maintained bone quality and decreased fracture risk in osteoporosis patients on 5-8 years of continuous treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torring O. Effects of denosumab on bone density, mass and strength in women with postmenopausal osteoporosis. Ther Adv Musculoskelet Dis. 2015;7(3):88–102. doi: 10.1177/1759720X15579189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adami S, Libanati C, Boonen S, Cummings SR, Ho PR, Wang A, et al. Denosumab treatment in postmenopausal women with osteoporosis does not interfere with fracture-healing: results from the FREEDOM trial. J Bone Joint Surg Am. 2012;94(23):2113–9. doi: 10.2106/JBJS.K.00774. [DOI] [PubMed] [Google Scholar]
- 6.Takeuchi T, Tanaka Y, Ishiguro N, Yamanaka H, Yoneda T, Ohira T, et al. Response to: ‘Denosumab, cortical bone and bone erosion in rheumatoid arthritis’ by Rossini et al. Ann Rheum Dis. 2016;75(10):e71. doi: 10.1136/annrheumdis-2016-210027. [DOI] [PubMed] [Google Scholar]
- 7.Rossini M, Adami G, Viapiana O, Idolazzi L, Gatti D. Denosumab, cortical bone and bone erosions in rheumatoid arthritis. Ann Rheum Dis. 2016;75(10):e70. doi: 10.1136/annrheumdis-2016-210022. [DOI] [PubMed] [Google Scholar]
- 8*.Rossini M, Adami G, Viapiana O, Idolazzi L, Gatti D. Denosumab, cortical bone and bone erosions in rheumatoid arthritis. Ann Rheum Dis. 2016 doi: 10.1136/annrheumdis-2016-210022. Interesting discussion ot the potential effect of DMab on cortical bone loss in RA. [DOI] [PubMed] [Google Scholar]
- 9.Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361(8):756–65. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 10.Diedhiou D, Cuny T, Sarr A, Norou Diop S, Klein M, Weryha G. Efficacy and safety of denosumab for the treatment of osteoporosis: A systematic review. Ann Endocrinol (Paris) 2015;76(6):650–7. doi: 10.1016/j.ando.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Zebaze RM, Libanati C, McClung MR, Zanchetta JR, Kendler DL, Hoiseth A, et al. Denosumab Reduces Cortical Porosity of the Proximal Femoral Shaft in Postmenopausal Women with Osteoporosis. J Bone Miner Res. 2016 doi: 10.1002/jbmr.2855. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Z, Chen C, Zhang J, Ji X, Liu L, Zhang G, et al. Safety of denosumab in postmenopausal women with osteoporosis or low bone mineral density: a meta-analysis. Int J Clin Exp Pathol. 2014;7(5):2113–22. [PMC free article] [PubMed] [Google Scholar]
- 13.Cleeland CS, Body JJ, Stopeck A, von Moos R, Fallowfield L, Mathias SD, et al. Pain outcomes in patients with advanced breast cancer and bone metastases: results from a randomized, double-blind study of denosumab and zoledronic acid. Cancer. 2013;119(4):832–8. doi: 10.1002/cncr.27789. [DOI] [PubMed] [Google Scholar]
- 14.Martin M, Bell R, Bourgeois H, Brufsky A, Diel I, Eniu A, et al. Bone-related complications and quality of life in advanced breast cancer: results from a randomized phase III trial of denosumab versus zoledronic acid. Clin Cancer Res. 2012;18(17):4841–9. doi: 10.1158/1078-0432.CCR-11-3310. [DOI] [PubMed] [Google Scholar]
- 15.Stopeck A. Denosumab findings in metastatic breast cancer. Clin Adv Hematol Oncol. 2010;8(3):159–60. [PubMed] [Google Scholar]
- 16.Stopeck AT, Fizazi K, Body JJ, Brown JE, Carducci M, Diel I, et al. Safety of long-term denosumab therapy: results from the open label extension phase of two phase 3 studies in patients with metastatic breast and prostate cancer. Support Care Cancer. 2016;24(1):447–55. doi: 10.1007/s00520-015-2904-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stopeck AT, Lipton A, Body JJ, Steger GG, Tonkin K, de Boer RH, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28(35):5132–9. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Yang KH, Wanyan P, Tian JH. Comparison of the efficacy and safety of denosumab versus bisphosphonates in breast cancer and bone metastases treatment: A meta-analysis of randomized controlled trials. Oncology letters. 2014;7(6):1997–2002. doi: 10.3892/ol.2014.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hensvold AH, Joshua V, Li W, Larkin M, Qureshi F, Israelsson L, et al. Serum RANKL levels associate with anti- citrullinated protein antibodies in early untreated rheumatoid arthritis and are modulated following methotrexate. Arthritis Res Ther. 2015;17:239. doi: 10.1186/s13075-015-0760-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li CH, Xu LL, Zhao JX, Sun L, Yao ZQ, Deng XL, et al. CXCL16 upregulates RANKL expression in rheumatoid arthritis synovial fibroblasts through the JAK2/STAT3 and p38/MAPK signaling pathway. Inflamm Res. 2016;65(3):193–202. doi: 10.1007/s00011-015-0905-y. [DOI] [PubMed] [Google Scholar]
- 21.Hoyer-Kuhn H, Franklin J, Allo G, Kron M, Netzer C, Eysel P, et al. Safety and efficacy of denosumab in children with osteogenesis imperfecta - a first prospective trial. J Musculoskelet Neuronal Interact. 2016;16(1):24–32. [PMC free article] [PubMed] [Google Scholar]
- 22.Langdahl BL, Teglbjaerg CS, Ho PR, Chapurlat R, Czerwinski E, Kendler DL, et al. A 24-month study evaluating the efficacy and safety of denosumab for the treatment of men with low bone mineral density: results from the ADAMO trial. J Clin Endocrinol Metab. 2015;100(4):1335–42. doi: 10.1210/jc.2014-4079. [DOI] [PubMed] [Google Scholar]
- 23.Castellano D, Sepulveda JM, Garcia-Escobar I, Rodriguez-Antolin A, Sundlov A, Cortes-Funes H. The role of RANK-ligand inhibition in cancer: the story of denosumab. Oncologist. 2011;16(2):136–45. doi: 10.1634/theoncologist.2010-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller PD. Denosumab: anti-RANKL antibody. Curr Osteoporos Rep. 2009;7(1):18–22. doi: 10.1007/s11914-009-0004-5. [DOI] [PubMed] [Google Scholar]
- 25.Gatti D, Viapiana O, Fracassi E, Idolazzi L, Dartizio C, Povino MR, et al. Sclerostin and DKK1 in postmenopausal osteoporosis treated with denosumab. J Bone Miner Res. 2012;27(11):2259–63. doi: 10.1002/jbmr.1681. [DOI] [PubMed] [Google Scholar]
- 26.Brown JE, Coleman RE. Denosumab in patients with cancer-a surgical strike against the osteoclast. Nat Rev Clin Oncol. 2012;9(2):110–8. doi: 10.1038/nrclinonc.2011.197. [DOI] [PubMed] [Google Scholar]
- 27.Brown-Glaberman U, Stopeck AT. Impact of denosumab on bone mass in cancer patients. Clin Pharmacol. 2013;5:117–29. doi: 10.2147/CPAA.S30330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.**Cohen SB, Dore RK, Lane NE, Ory PA, Peterfy CG, Sharp JT, et al. Denosumab treatment effects on structural damage, bone mineral density, and bone turnover in rheumatoid arthritis: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, phase II clinical trial. Arthritis Rheum. 2008;58(5):1299–309. doi: 10.1002/art.23417. Phase II trial that was the first study to demonstate that 180 mg of DMab decreased bone erosions on MRI in RA patients. [DOI] [PubMed] [Google Scholar]
- 29.*Sharp JT, Tsuji W, Ory P, Harper-Barek C, Wang H, Newmark R. Denosumab prevents metacarpal shaft cortical bone loss in patients with erosive rheumatoid arthritis. Arthritis Care Res (Hoboken) 2010;62(4):537–44. doi: 10.1002/acr.20172. Substudy of the Cohen trial (reference 28) that reported decreased cortical bone loss in the hands in RA patients. [DOI] [PubMed] [Google Scholar]
- 30.*Deodhar A, Dore RK, Mandel D, Schechtman J, Shergy W, Trapp R, et al. Denosumab-mediated increase in hand bone mineral density associated with decreased progression of bone erosion in rheumatoid arthritis patients. Arthritis Care Res (Hoboken) 2010;62(4):569–74. doi: 10.1002/acr.20004. A second substudy of the Cohen trial that demonstrated decreased systemic bone loss in RA patients on DMab. [DOI] [PubMed] [Google Scholar]
- 31.**Dore RK, Cohen SB, Lane NE, Palmer W, Shergy W, Zhou L, et al. Effects of denosumab on bone mineral density and bone turnover in patients with rheumatoid arthritis receiving concurrent glucocorticoids or bisphosphonates. Ann Rheum Dis. 2010;69(5):872–5. doi: 10.1136/ard.2009.112920. Third substudy of the Cohen trials where efficacy on BMD and bone turnover was observed in RA patients despite the use of glucocorticoids or bisphosphonates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.**Takeuchi T, Tanaka Y, Ishiguro N, Yamanaka H, Yoneda T, Ohira T, et al. Effect of denosumab on Japanese patients with rheumatoid arthritis: a dose-response study of AMG 162 (Denosumab) in patients with RheumatoId arthritis on methotrexate to Validate inhibitory effect on bone Erosion (DRIVE)-a 12-month, multicentre, randomised, double-blind, placebo-controlled, phase II clinical trial. Ann Rheum Dis. 2016;75(6):983–90. doi: 10.1136/annrheumdis-2015-208052. The second Phase II trial in RA demonstrating the effiacy of DMab in limiting bone erosion and systemic bone loss in RA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33**.Hasegawa T, Kaneko Y, Izumi K, Takeuchi T. Efficacy of denosumab combined with bDMARDs on radiographic progression in rheumatoid arthritis. Joint Bone Spine. 2016 doi: 10.1016/j.jbspin.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 34.Laskowski LK, Goldfarb DS, Howland MA, Kavcsak K, Lugassy DM, Smith SW. A RANKL Wrinkle: Denosumab-Induced Hypocalcemia. J Med Toxicol. 2016 doi: 10.1007/s13181-016-0543-y. In this single centre, retrospective cohort study 40 RA patients treated with denosumab plus bDMARDs were compared to40 patients treated with bDMARDs. The increase in the modified Sharp score was significantly lower in the combination group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yerram P, Kansagra S, Abdelghany O. Incidence of hypocalcemia in patients receiving denosumab for prevention of skeletal-related events in bone metastasis. J Oncol Pharm Pract. 2016 doi: 10.1177/1078155216628325. [DOI] [PubMed] [Google Scholar]
- 36.Okada N, Kawazoe K, Teraoka K, Kujime T, Abe M, Shinohara Y, et al. Identification of the risk factors associated with hypocalcemia induced by denosumab. Biol Pharm Bull. 2013;36(10):1622–6. doi: 10.1248/bpb.b13-00496. [DOI] [PubMed] [Google Scholar]
- 37.Body JJ, Bone HG, de Boer RH, Stopeck A, Van Poznak C, Damiao R, et al. Hypocalcaemia in patients with metastatic bone disease treated with denosumab. Eur J Cancer. 2015;51(13):1812–21. doi: 10.1016/j.ejca.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 38.Prolia. (FDA) FaDA. 2010 [Google Scholar]
- 39.XGEVA. (FDA) FaDA. 2013 [Available from: http://www.fda.gov/safety/medwatch/safetyinformation/ucm303740.htm http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm356528.htm.
- 40.de Souza Povoa RC, Marliere DA, da Silveira HM, Pires FR. Denosumab-related osteonecrosis of the jaws: successful management with a conservative surgical approach. Spec Care Dentist. 2016;36(4):231–6. doi: 10.1111/scd.12168. [DOI] [PubMed] [Google Scholar]
- 41.Kyrgidis A, Toulis KA. Denosumab-related osteonecrosis of the jaws. Osteoporos Int. 2011;22(1):369–70. doi: 10.1007/s00198-010-1177-6. [DOI] [PubMed] [Google Scholar]
- 42.Malan J, Ettinger K, Naumann E, Beirne OR. The relationship of denosumab pharmacology and osteonecrosis of the jaws. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114(6):671–6. doi: 10.1016/j.oooo.2012.08.439. [DOI] [PubMed] [Google Scholar]
- 43.O’Halloran M, Boyd NM, Smith A. Denosumab and osteonecrosis of the jaws - the pharmacology, pathogenesis and a report of two cases. Aust Dent J. 2014;59(4):516–9. doi: 10.1111/adj.12217. [DOI] [PubMed] [Google Scholar]
- 44.Pichardo SE, van Merkesteyn JP. Evaluation of a surgical treatment of denosumab-related osteonecrosis of the jaws. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016 doi: 10.1016/j.oooo.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 45.Sivolella S, Lumachi F, Stellini E, Favero L. Denosumab and anti-angiogenetic drug-related osteonecrosis of the jaw: an uncommon but potentially severe disease. Anticancer Res. 2013;33(5):1793–7. [PubMed] [Google Scholar]
- 46.Matsushita Y, Hayashida S, Morishita K, Sakamoto H, Naruse T, Sakamoto Y, et al. Denosumab-associated osteonecrosis of the jaw affects osteoclast formation and differentiation: Pathological features of two cases. Mol Clin Oncol. 2016;4(2):191–4. doi: 10.3892/mco.2015.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Di Nisio C, Zizzari VL, Zara S, Falconi M, Teti G, Tete G, et al. RANK/RANKL/OPG signaling pathways in necrotic jaw bone from bisphosphonate-treated subjects. Eur J Histochem. 2015;59(1):2455. doi: 10.4081/ejh.2015.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Selga J, Nunez JH, Minguell J, Lalanza M, Garrido M. Simultaneous bilateral atypical femoral fracture in a patient receiving denosumab: case report and literature review. Osteoporos Int. 2016;27(2):827–32. doi: 10.1007/s00198-015-3355-z. [DOI] [PubMed] [Google Scholar]
- 49.Edwards BJ, Sun M, West DP, Guindani M, Lin YH, Lu H, et al. Incidence of Atypical Femur Fractures in Cancer Patients: The MD Anderson Cancer Center Experience. J Bone Miner Res. 2016;31(8):1569–76. doi: 10.1002/jbmr.2818. [DOI] [PubMed] [Google Scholar]
- 50.Edwards BJ, Gradishar WJ, Smith ME, Pacheco JA, Holbrook J, McKoy JM, et al. Elevated incidence of fractures in women with invasive breast cancer. Osteoporos Int. 2016;27(2):499–507. doi: 10.1007/s00198-015-3246-3. [DOI] [PubMed] [Google Scholar]
- 51.Villiers J, Clark DW, Jeswani T, Webster S, Hepburn AL. An atraumatic femoral fracture in a patient with rheumatoid arthritis and osteoporosis treated with denosumab. Case Rep Rheumatol. 2013;2013:249872. doi: 10.1155/2013/249872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Teixeira MZ. Antiresorptive drugs (bisphosphonates), atypical fractures and rebound effect: new evidence of similitude. Homeopathy. 2012;101(4):231–42. doi: 10.1016/j.homp.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 53.Popp AW, Zysset PK, Lippuner K. Rebound-associated vertebral fractures after discontinuation of denosumab-from clinic and biomechanics. Osteoporos Int. 2016;27(5):1917–21. doi: 10.1007/s00198-015-3458-6. [DOI] [PubMed] [Google Scholar]
- 54.Baron R, Ferrari S, Russell RG. Denosumab and bisphosphonates: different mechanisms of action and effects. Bone. 2011;48(4):677–92. doi: 10.1016/j.bone.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 55.Rodan G, Reszka A, Golub E, Rizzoli R. Bone safety of long-term bisphosphonate treatment. Curr Med Res Opin. 2004;20(8):1291–300. doi: 10.1185/030079904125004475. [DOI] [PubMed] [Google Scholar]
- 56.Tonino RP, Meunier PJ, Emkey R, Rodriguez-Portales JA, Menkes CJ, Wasnich RD, et al. Skeletal benefits of alendronate: 7-year treatment of postmenopausal osteoporotic women. Phase III Osteoporosis Treatment Study Group. J Clin Endocrinol Metab. 2000;85(9):3109–15. doi: 10.1210/jcem.85.9.6777. [DOI] [PubMed] [Google Scholar]
- 57.Bone HG, Hosking D, Devogelaer JP, Tucci JR, Emkey RD, Tonino RP, et al. Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004;350(12):1189–99. doi: 10.1056/NEJMoa030897. [DOI] [PubMed] [Google Scholar]
- 58.Kinoshita H, Miyakoshi N, Kashiwagura T, Kasukawa Y, Sugimura Y, Shimada Y. Comparison of the efficacy of denosumab and bisphosphonates for treating secondary osteoporosis in patients with rheumatoid arthritis. Mod Rheumatol. 2016:1–5. doi: 10.1080/14397595.2016.1232776. [DOI] [PubMed] [Google Scholar]
- 59.Adami G, Orsolini G, Adami S, Viapiana O, Idolazzi L, Gatti D, et al. Effects of TNF Inhibitors on Parathyroid Hormone and Wnt Signaling Antagonists in Rheumatoid Arthritis. Calcif Tissue Int. 2016 doi: 10.1007/s00223-016-0161-3. [DOI] [PubMed] [Google Scholar]
- 60.Rossini M, Gatti D, Adami S. Involvement of WNT/beta-catenin signaling in the treatment of osteoporosis. Calcif Tissue Int. 2013;93(2):121–32. doi: 10.1007/s00223-013-9749-z. [DOI] [PubMed] [Google Scholar]
- 61.Street_Journal_Market_Report AW. [Available from: http://www.fool.com/investing/general/2014/02/03/xgeva-and-prolia-setting-the-pace-for-amgen-inc.aspx http://investors.amgen.com/phoenix.zhtml?c=61656&p=irol-newsArticle&ID=1653300 http://marketrealist.com/2016/07/xgeva-nplate-sensipar-key-growth-drivers-amgens-revenues-2q16/
- 62.Hazlewood GS, Barnabe C, Tomlinson G, Marshall D, Devoe D, Bombardier C. Methotrexate monotherapy and methotrexate combination therapy with traditional and biologic disease modifying antirheumatic drugs for rheumatoid arthritis: abridged Cochrane systematic review and network meta-analysis. BMJ. 2016;353:i1777. doi: 10.1136/bmj.i1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63*.Kostenuik PJ, Smith SY, Samadfam R, Jolette J, Zhou L, Ominsky MS. Effects of denosumab, alendronate, or denosumab following alendronate on bone turnover, calcium homeostasis, bone mass and bone strength in ovariectomized cynomolgus monkeys. J Bone Miner Res. 2015;30(4):657–69. doi: 10.1002/jbmr.2401. Informative comparison of DMab and alendronate effects on bone. [DOI] [PubMed] [Google Scholar]
- 64.Xue Y, Cohen JM, Wright NA, Merola JF. Skin Signs of Rheumatoid Arthritis and its Therapy-Induced Cutaneous Side Effects. American journal of clinical dermatology. 2016;17(2):147–62. doi: 10.1007/s40257-015-0167-z. [DOI] [PubMed] [Google Scholar]
- 65.Baum R, Gravallese EM. Bone as a Target Organ in Rheumatic Disease: Impact on Osteoclasts and Osteoblasts. Clin Rev Allergy Immunol. 2015 doi: 10.1007/s12016-015-8515-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rossini M, Viapiana O, Adami S, Fracassi E, Idolazzi L, Dartizio C, et al. In patients with rheumatoid arthritis, Dickkopf-1 serum levels are correlated with parathyroid hormone, bone erosions and bone mineral density. Clin Exp Rheumatol. 2015;33(1):77–83. [PubMed] [Google Scholar]
- 67.Schett G, Gravallese E. Bone erosion in rheumatoid arthritis: mechanisms, diagnosis and treatment. Nat Rev Rheumatol. 2012;8(11):656–64. doi: 10.1038/nrrheum.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Edrees AF, Misra SN, Abdou NI. Anti-tumor necrosis factor (TNF) therapy in rheumatoid arthritis: correlation of TNF-alpha serum level with clinical response and benefit from changing dose or frequency of infliximab infusions. Clin Exp Rheumatol. 2005;23(4):469–74. [PubMed] [Google Scholar]
- 69.Manicourt DH, Triki R, Fukuda K, Devogelaer JP, Nagant de Deuxchaisnes C, Thonar EJ. Levels of circulating tumor necrosis factor alpha and interleukin-6 in patients with rheumatoid arthritis. Relationship to serum levels of hyaluronan and antigenic keratan sulfate. Arthritis and rheumatism. 1993;36(4):490–9. doi: 10.1002/art.1780360409. [DOI] [PubMed] [Google Scholar]
- 70.Sakito S, Ueki Y, Eguchi K, Kawabe Y, Nagataki S. Serum cytokines in patients with rheumatoid arthritis. Correlation of interferon gamma and tumor necrosis factor alpha with the characteristics of peripheral blood mononuclear cells. Rheumatol Int. 1995;15(1):31–7. doi: 10.1007/BF00286766. [DOI] [PubMed] [Google Scholar]
- 71.Yen JH, Chen JR, Tsai WJ, Liu HW. Correlation of tumor necrosis factor alpha levels with disease activity of rheumatoid arthritis. Zhonghua Min Guo Wei Sheng Wu Ji Mian Yi Xue Za Zhi. 1992;25(4):232–43. [PubMed] [Google Scholar]
- 72.Yeo L, Lom H, Juarez M, Snow M, Buckley CD, Filer A, et al. Expression of FcRL4 defines a pro-inflammatory, RANKL-producing B cell subset in rheumatoid arthritis. Ann Rheum Dis. 2015;74(5):928–35. doi: 10.1136/annrheumdis-2013-204116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meednu N, Zhang H, Owen T, Sun W, Wang V, Cistrone C, et al. Production of RANKL by Memory B Cells: A Link Between B Cells and Bone Erosion in Rheumatoid Arthritis. Arthritis & rheumatology. 2016;68(4):805–16. doi: 10.1002/art.39489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang JC, Sakata T, Pfleger LL, Bencsik M, Halloran BP, Bikle DD, et al. PTH differentially regulates expression of RANKL and OPG. J Bone Miner Res. 2004;19(2):235–44. doi: 10.1359/JBMR.0301226. [DOI] [PubMed] [Google Scholar]
- 75.Moller Dohn U, Boonen A, Hetland ML, Hansen MS, Knudsen LS, Hansen A, et al. Erosive progression is minimal, but erosion healing rare, in patients with rheumatoid arthritis treated with adalimumab. A 1 year investigator-initiated follow-up study using high-resolution computed tomography as the primary outcome measure. Ann Rheum Dis. 2009;68(10):1585–90. doi: 10.1136/ard.2008.097048. [DOI] [PubMed] [Google Scholar]
- 76.Kanzaki H, Shinohara F, Kanako I, Yamaguchi Y, Fukaya S, Miyamoto Y, et al. Molecular regulatory mechanisms of osteoclastogenesis through cytoprotective enzymes. Redox Biol. 2016;8:186–91. doi: 10.1016/j.redox.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Humphrey MB, Daws MR, Spusta SC, Niemi EC, Torchia JA, Lanier LL, et al. TREM2, a DAP12-associated receptor, regulates osteoclast differentiation and function. J Bone Miner Res. 2006;21(2):237–45. doi: 10.1359/JBMR.051016. [DOI] [PubMed] [Google Scholar]
- 78.Nimmerjahn F, Ravetch JV. Fc-receptors as regulators of immunity. Adv Immunol. 2007;96:179–204. doi: 10.1016/S0065-2776(07)96005-8. [DOI] [PubMed] [Google Scholar]
- 79**.Luo J, Yang Z, Ma Y, Yue Z, Lin H, Qu G, et al. LGR4 is a receptor for RANKL and negatively regulates osteoclast differentiation and bone resorption. Nat Med. 2016;22(5):539–46. doi: 10.1038/nm.4076. In this important contribution, a second receptor, LGR4 for RANKL is described and therapeutic potential discussed. [DOI] [PubMed] [Google Scholar]
- 80.Zaidi M, Iqbal J. Closing the loop on the bone-resorbing osteoclast. Nat Med. 2016;22(5):460–1. doi: 10.1038/nm.4104. [DOI] [PubMed] [Google Scholar]
- 81.Bengtsson AK, Ryan EJ. Immune function of the decoy receptor osteoprotegerin. Crit Rev Immunol. 2002;22(3):201–15. [PubMed] [Google Scholar]
- 82.Kelesidis T, Currier JS, Yang OO, Brown TT. Role of RANKL-RANK/osteoprotegerin pathway in cardiovascular and bone disease associated with HIV infection. AIDS Rev. 2014;16(3):123–33. [PMC free article] [PubMed] [Google Scholar]
- 83.Kohli SS, Kohli VS. Role of RANKL-RANK/osteoprotegerin molecular complex in bone remodeling and its immunopathologic implications. Indian J Endocrinol Metab. 2011;15(3):175–81. doi: 10.4103/2230-8210.83401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Walsh MC, Choi Y. Biology of the RANKL-RANK-OPG System in Immunity, Bone, and Beyond. Front Immunol. 2014;5:511. doi: 10.3389/fimmu.2014.00511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85*.Nicolin Vanessa, DI D, Valentini Roberto. Osteoimmunology represents a link between skeletal and immune system. Italian Journal of Anatomy and Embryology. 2016;121(1):37–42. [PubMed] [Google Scholar]
- 86.Wong BR, Josien R, Lee SY, Sauter B, Li HL, Steinman RM, et al. TRANCE (tumor necrosis factor [TNF]-related activation-induced cytokine), a new TNF family member predominantly expressed in T cells, is a dendritic cell-specific survival factor. J Exp Med. 1997;186(12):2075–80. doi: 10.1084/jem.186.12.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kikuta J, Wada Y, Kowada T, Wang Z, Sun-Wada GH, Nishiyama I, et al. Dynamic visualization of RANKL and Th17-mediated osteoclast function. J Clin Invest. 2013;123(2):866–73. doi: 10.1172/JCI65054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Komatsu N, Okamoto K, Sawa S, Nakashima T, Oh-hora M, Kodama T, et al. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nature medicine. 2014;20(1):62–8. doi: 10.1038/nm.3432. [DOI] [PubMed] [Google Scholar]
- 89.Onal M, Xiong J, Chen X, Thostenson JD, Almeida M, Manolagas SC, et al. Receptor activator of nuclear factor kappaB ligand (RANKL) protein expression by B lymphocytes contributes to ovariectomy-induced bone loss. J Biol Chem. 2012;287(35):29851–60. doi: 10.1074/jbc.M112.377945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Green EA, Choi Y, Flavell RA. Pancreatic lymph node-derived CD4(+)CD25(+) Treg cells: highly potent regulators of diabetes that require TRANCE-RANK signals. Immunity. 2002;16(2):183–91. doi: 10.1016/s1074-7613(02)00279-0. [DOI] [PubMed] [Google Scholar]
- 91.Totsuka T, Kanai T, Nemoto Y, Tomita T, Okamoto R, Tsuchiya K, et al. RANK-RANKL signaling pathway is critically involved in the function of CD4+CD25+ regulatory T cells in chronic colitis. J Immunol. 2009;182(10):6079–87. doi: 10.4049/jimmunol.0711823. [DOI] [PubMed] [Google Scholar]
- 92.Loser K, Mehling A, Loeser S, Apelt J, Kuhn A, Grabbe S, et al. Epidermal RANKL controls regulatory T-cell numbers via activation of dendritic cells. Nat Med. 2006;12(12):1372–9. doi: 10.1038/nm1518. [DOI] [PubMed] [Google Scholar]
- 93.Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402(6759):304–9. doi: 10.1038/46303. [DOI] [PubMed] [Google Scholar]
- 94.Pettit AR, Ji H, von Stechow D, Muller R, Goldring SR, Choi Y, et al. TRANCE/RANKL knockout mice are protected from bone erosion in a serum transfer model of arthritis. Am J Pathol. 2001;159(5):1689–99. doi: 10.1016/S0002-9440(10)63016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Boyce BF, Xing L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res Ther. 2007;9(Suppl 1):S1. doi: 10.1186/ar2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang XF, Zhang YK, Yu ZS, Zhou JL. The role of the serum RANKL/OPG ratio in the healing of intertrochanteric fractures in elderly patients. Mol Med Rep. 2013;7(4):1169–72. doi: 10.3892/mmr.2013.1335. [DOI] [PubMed] [Google Scholar]
- 97.Wasilewska A, Rybi-Szuminska A, Zoch-Zwierz W. Serum RANKL, osteoprotegerin (OPG), and RANKL/OPG ratio in nephrotic children. Pediatr Nephrol. 2010;25(10):2067–75. doi: 10.1007/s00467-010-1583-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T, et al. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999;13(18):2412–24. doi: 10.1101/gad.13.18.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bachmann MF, Wong BR, Josien R, Steinman RM, Oxenius A, Choi Y. TRANCE, a tumor necrosis factor family member critical for CD40 ligand-independent T helper cell activation. J Exp Med. 1999;189(7):1025–31. doi: 10.1084/jem.189.7.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ashcroft AJ, Cruickshank SM, Croucher PI, Perry MJ, Rollinson S, Lippitt JM, et al. Colonic dendritic cells, intestinal inflammation, and T cell-mediated bone destruction are modulated by recombinant osteoprotegerin. Immunity. 2003;19(6):849–61. doi: 10.1016/s1074-7613(03)00326-1. [DOI] [PubMed] [Google Scholar]
- 101.Bone HG, Bolognese MA, Yuen CK, Kendler DL, Wang H, Liu Y, et al. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women. J Clin Endocrinol Metab. 2008;93(6):2149–57. doi: 10.1210/jc.2007-2814. [DOI] [PubMed] [Google Scholar]
- 102.Baud’huin M, Lamoureux F, Duplomb L, Redini F, Heymann D. RANKL, RANK, osteoprotegerin: key partners of osteoimmunology and vascular diseases. Cellular and molecular life sciences : CMLS. 2007;64(18):2334–50. doi: 10.1007/s00018-007-7104-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kiechl S, Werner P, Knoflach M, Furtner M, Willeit J, Schett G. The osteoprotegerin/RANK/RANKL system: a bone key to vascular disease. Expert review of cardiovascular therapy. 2006;4(6):801–11. doi: 10.1586/14779072.4.6.801. [DOI] [PubMed] [Google Scholar]
- 104.Rossini M, Viapiana O, Adami S, Idolazzi L, Ghellere F, Tripi G, et al. Effects of denosumab on peripheral lymphocyte subpopulations. Endocrine. 2015 doi: 10.1007/s12020-015-0723-6. [DOI] [PubMed] [Google Scholar]
- 105.Panizo S, Cardus A, Encinas M, Parisi E, Valcheva P, Lopez-Ongil S, et al. RANKL increases vascular smooth muscle cell calcification through a RANK-BMP4-dependent pathway. Circulation research. 2009;104(9):1041–8. doi: 10.1161/CIRCRESAHA.108.189001. [DOI] [PubMed] [Google Scholar]
- 106.Helas S, Goettsch C, Schoppet M, Zeitz U, Hempel U, Morawietz H, et al. Inhibition of receptor activator of NF-kappaB ligand by denosumab attenuates vascular calcium deposition in mice. Am J Pathol. 2009;175(2):473–8. doi: 10.2353/ajpath.2009.080957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Iolascon G, Napolano R, Gioia M, Moretti A, Riccio I, Gimigliano F. The contribution of cortical and trabecular tissues to bone strength: insights from denosumab studies. Clin Cases Miner Bone Metab. 2013;10(1):47–51. doi: 10.11138/ccmbm/2013.10.1.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ostertag A, Cohen-Solal M, Audran M, Legrand E, Marty C, Chappard D, et al. Vertebral fractures are associated with increased cortical porosity in iliac crest bone biopsy of men with idiopathic osteoporosis. Bone. 2009;44(3):413–7. doi: 10.1016/j.bone.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 109.Kleyer A, Finzel S, Rech J, Manger B, Krieter M, Faustini F, et al. Bone loss before the clinical onset of rheumatoid arthritis in subjects with anticitrullinated protein antibodies. Ann Rheum Dis. 2014;73(5):854–60. doi: 10.1136/annrheumdis-2012-202958. [DOI] [PubMed] [Google Scholar]
- 110.Yue J, Griffith JF, Xiao F, Shi L, Wang D, Shen J, et al. Repair of bone erosion in rheumatoid arthritis by denosumab: A high-resolution peripheral quantitative computed tomography study. Arthritis Care Res (Hoboken) 2016 doi: 10.1002/acr.23133. [DOI] [PubMed] [Google Scholar]
- 111.Dore RK. The RANKL pathway and denosumab. Rheum Dis Clin North Am. 2011;37(3):433–52. vi–vii. doi: 10.1016/j.rdc.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 112.Takeuchi T, Tanaka Y, Ishiguro N, Yamanaka H, Yoneda T, Ohira T, et al. Effect of denosumab on Japanese patients with rheumatoid arthritis: a dose-response study of AMG 162 (Denosumab) in patients with RheumatoId arthritis on methotrexate to Validate inhibitory effect on bone Erosion (DRIVE)-a 12-month, multicentre, randomised, double-blind, placebo-controlled, phase II clinical trial. Ann Rheum Dis. 2015 doi: 10.1136/annrheumdis-2015-208052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113**.Curtis JR, Xie F, Yun H, Saag KG, Chen L, Delzell E. Risk of hospitalized infection among rheumatoid arthritis patients concurrently treated with a biologic agent and denosumab. Arthritis Rheumatol. 2015;67(6):1456–64. doi: 10.1002/art.39075. This retrospective study from an administative database demonstrated that the combination of an anti-TNF agent and DMab was not associated with a higher risk of infection in hospitalized patients. [DOI] [PubMed] [Google Scholar]
- 114.Boers M, Verhoeven AC, van der Linden S. Combination therapy in early rheumatoid arthritis: the COBRA study. Ned Tijdschr Geneeskd. 1997;141(50):2428–32. [PubMed] [Google Scholar]
- 115.Bathon JM, Martin RW, Fleischmann RM, Tesser JR, Schiff MH, Keystone EC, et al. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med. 2000;343(22):1586–93. doi: 10.1056/NEJM200011303432201. [DOI] [PubMed] [Google Scholar]
- 116.Anandarajah A, Thiele R, Giampoli E, Monu J, Seo GS, Feng C, et al. Patients with rheumatoid arthritis in clinical remission manifest persistent joint inflammation on histology and imaging studies. J Rheumatol. 2014;41(11):2153–60. doi: 10.3899/jrheum.140411. [DOI] [PubMed] [Google Scholar]
- 117.Brown AK, Quinn MA, Karim Z, Conaghan PG, Peterfy CG, Hensor E, et al. Presence of significant synovitis in rheumatoid arthritis patients with disease-modifying antirheumatic drug-induced clinical remission: evidence from an imaging study may explain structural progression. Arthritis Rheum. 2006;54(12):3761–73. doi: 10.1002/art.22190. [DOI] [PubMed] [Google Scholar]
- 118.Schett G, Emery P, Tanaka Y, Burmester G, Pisetsky DS, Naredo E, et al. Tapering biologic and conventional DMARD therapy in rheumatoid arthritis: current evidence and future directions. Ann Rheum Dis. 2016;75(8):1428–37. doi: 10.1136/annrheumdis-2016-209201. [DOI] [PubMed] [Google Scholar]
- 119.Humphrey MB, Lanier LL, Nakamura MC. Role of ITAM-containing adapter proteins and their receptors in the immune system and bone. Immunological reviews. 2005;208:50–65. doi: 10.1111/j.0105-2896.2005.00325.x. [DOI] [PubMed] [Google Scholar]
- 120.Negishi-Koga T, Takayanagi H. Ca2+-NFATc1 signaling is an essential axis of osteoclast differentiation. Immunological reviews. 2009;231(1):241–56. doi: 10.1111/j.1600-065X.2009.00821.x. [DOI] [PubMed] [Google Scholar]
- 121.Nakashima T, Takayanagi H. Osteoimmunology: crosstalk between the immune and bone systems. J Clin Immunol. 2009;29(5):555–67. doi: 10.1007/s10875-009-9316-6. [DOI] [PubMed] [Google Scholar]
- 122**.Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3(6):889–901. doi: 10.1016/s1534-5807(02)00369-6. This paper was the 1st paper which demonstrated that RANKL regulates osteoclastogenesis through the NFATc1 axis. [DOI] [PubMed] [Google Scholar]
- 123.Chiu YH, Mensah KA, Schwarz EM, et al. Regulation of human osteoclast development by dendritic cell-specific transmembrane protein (DC-STAMP) J Bone Miner Res. 2012 Jan;27(1):79–92. doi: 10.1002/jbmr.531. [DOI] [PMC free article] [PubMed] [Google Scholar]


