SUMMARY
Testosterone (T) replacement is being increasingly offered to older men with age-related decline in testosterone levels. The effects of long-term testosterone replacement and aromatase inhibition (AI) on glucose homeostasis and cardiometabolic markers were determine in older non-diabetic men with low testosterone levels. Men ≥65 years, mean age 71 ± 3 years with serum total T < 350 ng/dL were randomized in a double-blind, placebo-controlled, parallel-group, proof-of-concept trial evaluating the effects of 5 g transdermal testosterone gel (TT) (n = 10), 1 mg anastrozole (n = 10) or placebo (n = 9) daily for 12 months. Homeostatic Model Assessment of insulin resistance (HOMAIR) was the primary outcome. Secondary outcomes included OGIS in response to OGTT, fasting lipids, C-reactive protein (CRP), adipokines, and abdominal and mid-thigh fat by computed tomography. All outcomes were assessed at baseline and 12 months. After 12 months, absolute changes in HOMAIR in both treatment arms (TT group: −0.05 ± 0.21); (AI group: 0.15 ± 0.10) were similar to placebo (−0.11 ± 0.26), as were CRP and fasting lipid levels. Adiponectin levels significantly decreased in the TT group (−1.8 ± 0.9 mg/L, p = 0.02) and abdominal subcutaneous fat (−60.34 ± 3.19 cm2, p = 0.003) and leptin levels (−1.5 ± 1.2 ng/mL, p = 0.04) were significantly lower with AI. Mid-thigh subcutaneous fat was reduced in both treatment arms (TT group: −4.88 ± 1.24 cm2, p = 0.008); (AI group: −6.05 ± 0.87 cm2, p = 0.0002). In summary, in this proof-of-concept trial, changes in HOMAIR AI were similar in all three groups while the effects of intervention on subcutaneous fat distribution and adipokines were variable. Larger efficacy and safety trials are needed before AI pharmacotherapy can be considered as a treatment option for low T levels in older men.
Keywords: aromatase inhibition, inflammation, insulin sensitivity, lipids, testosterone
INTRODUCTION
Serum testosterone levels decline as men age (Harman et al., 2001) and this decline has been associated with loss of muscle mass, reduced muscle strength, low bone mass, and sexual dysfunction (Katznelson et al., 1996). Hence, there has been great interest in testosterone replacement in older men with age-related decline in serum testosterone levels (Basaria, 2013). Indeed, testosterone prescriptions written for middle-age and older men with low testosterone level have risen exponentially in recent years (Basaria, 2013; Jasuja et al., 2015).
Although testosterone replacement in older men has resulted in improvement in body composition, muscle strength, and in some studies, physical function (Spitzer et al., 2013), recent reports have raised concerns regarding cardiovascular safety of exogenous testosterone therapy, especially in older men (Basaria et al., 2010, 2013; Vigen et al., 2013; Finkle et al., 2014; Layton et al., 2015). These findings are particularly important considering the pharmacokinetic shortcomings of available modalities of testosterone replacement. Indeed, there is significant variation in circulating serum testosterone concentrations in men on transdermal testosterone formulations while intramuscular injections, particularly with short-acting esters, are associated with peaks and troughs (Snyder & Lawrence, 1980; Swerdloff et al., 2015). Hence, stimulation of endogenous testosterone production in older men with aromatase inhibitors (AI) is an attractive option. As estradiol is a potent inhibitor of the pituitary/ gonadal axis, inhibition of its synthesis by AI results in an increase in circulating gonadotropins (Dias et al., 2015), which, in turn, stimulate testicular Leydig cells to increase endogenous testosterone production. We recently reported that 12-month intervention with AI in older men with low testosterone raised serum testosterone levels into the normal range and resulted in improvement in lean body mass and muscle strength (Dias et al., 2015). However, the long-term effects of AI on glucose homeostasis, insulin sensitivity and cardiovascular risk markers are unknown in this candidate population.
Population studies have found that low serum testosterone levels are associated with prevalent and incident type-2 diabetes (Stellato et al., 2000; Selvin et al., 2007). Similarly, a high percentage of men with prostate cancer undergoing androgen deprivation therapy develop diabetes and metabolic syndrome (Braga-Basaria et al., 2006) and acute testosterone withdrawal (that also lowers estrogen levels) in healthy young men reduces insulin sensitivity (Yialamas et al., 2007). Indeed, a few mechanistic studies have shown that testosterone replacement improves insulin sensitivity because of improved muscle glucose uptake and reductions in abdominal fat mass (Marin et al., 1992; Boyanov et al., 2003; Malkin et al., 2007; Yialamas et al., 2007). However, the relative contribution of estradiol to glucose homeostasis in male hypogonadism remains unclear. Furthermore, it also remains unclear whether the metabolic benefits seen with testosterone replacement are because of the direct action of testosterone or via its aromatization to estradiol, or some combination of both. Both laboratory and human studies indicate that testosterone and estradiol may have independent effects on glucose homeostasis and metabolism (Pitteloud et al., 2005; Faulds et al., 2012). Indeed, administration of estrogen to ob/ob mice improves glucose tolerance and insulin sensitivity (Gao et al., 2006). Similarly, administration of exogenous estrogen to men with congenital aromatase deficiency (who have normal testosterone levels) also improves insulin sensitivity (Rochira et al., 2007). These data suggest a distinct role of estradiol in glucose homeostasis in men. In contrast, two recent mechanistic studies in young men showed that withdrawal of estradiol had no impact on insulin sensitivity (Rubinow et al., 2012; Juang et al., 2014); however, the role of estradiol in older men with age-related low testosterone remains unclear. As treatment with AI is being considered as a viable option for increasing endogenous testosterone levels in hypogonadal elderly men, one must ensure that long-term inhibition of estradiol production does not have detrimental metabolic consequences.
Raising endogenous testosterone levels via administration of AI vs. exogenous testosterone replacement offers a unique opportunity to dissociate effects of testosterone from estradiol on metabolism. Previous studies using AI in men were short term, did not evaluate glucose homeostasis comprehensively, included a mixed population of diabetic and non-diabetic men, and did not include comparison groups given transdermal testosterone and placebo. Hence, we performed this proof-of-concept mechanistic trial of 12 months duration to determine the effects of long-term treatment with AI on glucose homeostasis and cardiovascular risk markers in nondiabetic elderly men.
MATERIALS AND METHODS
Study subjects, recruitment, and eligibility
Community dwelling men aged 65 years and older with fasting morning (7–10 am) total T levels <350 ng/dL were enrolled. The trial was conducted at the National Institute on Aging (NIA) Intramural Research Program, where the first participant was screened in August 2004 and the last subject completed the trial in March 2012. The protocol was approved by MedStar Harbor Hospital Institutional Review Board (ClinicalTrials.gov Identifier: NCT00104572). The study was a double-blind, randomized, placebo-controlled, proof-of-concept trial of 12 months duration. Subjects were randomized to three groups: transdermal testosterone gel 5 g/day and placebo tablet (TT group, n = 10); Anastrozole 1 mg/day and placebo gel (AI group, n = 10), and placebo tablet and placebo gel daily (placebo, n = 9). The flow of participants, randomization scheme, and frequency of adverse events have been published (Dias et al., 2015). The participants were required to have normal levels of gonadotropins, prolactin, parathyroid hormone, and prostate-specific antigen levels ≤4.0 ng/dL. Men with hematocrit <36%, Mini-Mental Status Exam score <24, erythrocytosis, history of diabetes, uncontrolled high blood pressure, severe benign prostatic hypertrophy, or recent acute coronary syndrome were excluded. Men were also excluded if they were already prescribed AI, selective estrogen receptor modulators or anabolic agents. Subjects were requested to refrain from drinking more than 30 g of alcohol daily or smoking tobacco or cannabis products for the study duration. All participants provided written informed consent as previously described (13).
Outcome measures
The trial is registered in on clinicaltrials.gov (NCT 0010457), change in bone mineral density was the primary outcome of the trial (Dias et al., 2015). Secondary outcomes, that were determined a priori, included glucose tolerance and lipid metabolism, which we report in the current paper. In the current manuscript, HOMAIR is the primary outcome while secondary outcomes included OGIS, fasting lipids, adipokines (leptin and adiponectin), CRP, and total (visceral and subcutaneous) abdominal and mid-thigh subcutaneous fat. The measurement methods for these endpoints are detailed below.
Evaluation of glucose homeostasis
All participants underwent standardized oral glucose tolerance test (OGTT) at baseline and after 12 months. After 10 h of overnight fast, an intravenous catheter was inserted in the ante-cubital vein of the participants. Serum and plasma samples were obtained by centrifugation, whereby blood was placed in BD ‘Vacutainer’ gel tubes (Franklin Lakes, NJ) and centrifuged for 15 min at 2500 rpm (1416 g) (refrigerated centrifuge Sorvall Legend RT, ON, Canada). Samples were stored at −80 °C until assayed. An oral 75-g glucose tolerance test was performed and blood samples were obtained at 0 (before ingestion of glucose), and 5, 10, 15, 20, 40, 60, 80, 100, and 120 min after ingestion. Total body insulin sensitivity was calculated using the oral glucose insulin sensitivity [OGIS: 0 min, 90 min (mean of 80 and 100 min), and 120 min of OGTT] (Rubinow et al., 2012). The homeostasis model assessment (HOMAIR) was used to determine insulin sensitivity and was calculated as: fasting insulin (µU/mL) × fasting glucose (mmol/L)/22.5 (Pacini & Mari, 2003). HOMAIR is primarily a measure of liver insulin sensitivity because it utilizes fasting levels only (the lower the HOMA value, the greater the insulin sensitivity). The area under the curve (AUC) was calculated using the trapezoidal rule and was used as a proxy of the amount of glucose in the circulation or hormones secreted.
Laboratory methods
Measurement of fasting serum insulin, glucose, steroid hormones, and adipocytokines
All samples were stored at −80 °C until analysis. Assays were performed in batch after the trial completion and aliquots were thawed only once. Quantitation of plasma glucose levels was performed using a glucose and lactate analyzer (YSI 2300 stata plus, YSI incorporated, OH). Plasma insulin levels were measured by ELISA (Mercordia, Uppsala, Sweden). Both testosterone and estradiol were measured using liquid chromatography tandem mass spectroscopy (Dias et al., 2015). Sex hormone-binding globulin (SHBG) was measured using electrochemiluminescence as previously described (Selvin et al., 2007). Leptin was measured by ELISA with an inter-assay coefficient of variation (CV) of 2.6–6.2% and intra-assay CV of 2.6–4.6% (Millipore, Billerica, MA). Total adiponectin was measured by RIA with an inter-assay CV of 6.9–9.3% and an intra-assay CV of 1.8–6.2% (Millipore, Billerica, MA). Serum high-sensitivity CRP (hsCRP) was measured by ELISA with an inter-assay CV of 11.6–13.8% and intra-assay CV of 5.5–6.0%, lower limit of detection 1.9 ng/mL, sensitivity 0.124 ng/mL (Alpco, Salem, NH).
Quantitation of abdominal and mid-thigh fat
Abdominal fat
Computed tomography (CT) scans were acquired using a Somatom Sensation 10 CT, multislice, helical CT Scanner (Siemens, Malvern, PA). A cross-sectional 10-mm CT image of the abdomen at lumbar spine level L4–L5 was obtained from each participant. The total cross-sectional area of the abdomen was characterized as subcutaneous fat and visceral fat areas (cm2). geanie software version 2.1 (BonAlyse Oy, Jyvaskyla, Finland) was used to quantify the areas.
Mid-thigh fat
All scans were performed at the mid-thigh level, halfway between the femoral head and patella, with a 500 × 500 mm field-of-view, 512 × 512 image matrix, and 10 mm image thickness. The mid-thigh scans provided subcutaneous fat measurements (cm2) and were analyzed using Tibest version 1.4, a semi-automatic image quantification platform developed in-house (Makrogiannis et al., 2012).
Fasting serum lipid profile
Fasting levels of total cholesterol, LDL-cholesterol, HDL-cholesterol, and triglycerides were measured at baseline and at 12 months. Concentrations of lipids were determined enzymatically by using (ABA-200 ATC Biochromatic Analyzer; Abbott Laboratories) at Baltimore MedStar Harbor Hospital.
Statistical analysis
Assessment of normality
The data were assessed by plotting histograms and by performing the Shapiro–Wilk test and Kolmogorov–Smirnov test. All outcomes were approximately normally distributed.
Statistical power
The sample size was fixed since this is a secondary outcome of the study published (Dias et al., 2015). Therefore, we computed the minimal detectable difference based on two groups with n = 10 for each group, t-test/anova; the comparison between the treatment groups and placebo groups had 80% power with type one error (α) of 0·05 to detect minimal mean difference in change in HOMAIR from baseline to 12 months of 1.32 standard deviations.
Analysis
Twenty-nine subjects formed the analytic sample and absolute change from baseline to 12 months was computed for all outcomes. Data are presented as means ± SEM (standard error of the mean). Baseline characteristics were compared across the three study groups using F-test from analysis of variance (anova). We used anova to estimate the effect of the treatment assignment on 12-month change scores for all endpoints; F-tests were used to evaluate the difference in changes between the groups, no post hoc tests were performed for most of the analysis. However, two groups t-test was performed if the F-test was significant. As a secondary analysis, paired t-tests were used to evaluate for within-group 12-month changes. Analyses were unadjusted for multiple comparisons based on the objective of this trial as a proof-of-concept mechanistic study, and a clear hierarchy of primary and secondary endpoints. p-values (p) <0.05 were considered statistically significant.
RESULTS
Baseline characteristics
Baseline characteristics among the three groups were similar. The participants were overweight and non-diabetic as reflected by their fasting and 2-h glucose levels after OGTT (Table 1). Serum levels of gonadal steroids, glycemic parameters, fasting lipids, and adipocytokines were also similar between the three groups (Table 1).
Table 1.
Baseline characteristic of the participants
| Parameters | Placebo (n = 9) | TT group (n = 10) | AI group (n = 10) | p-value |
|---|---|---|---|---|
| Age (years) | 72 ± 3 | 70 ± 3 | 70 ± 3 | 0.31 |
| BMI (kg/m2) | 27.6 ± 3.5 | 29.9 ± 1.2 | 27.7 ± 1.4 | 0.31 |
| Waist circumference (cm) | 97.8 ± 9.8 | 102.6 ± 3.6 | 99.8 ± 3.9 | 0.65 |
| Sex steroids | ||||
| Total testosterone (nmol/L) | 10.5 ± 3.3 | 10.4 ± 2.9 | 10.6 ± 2.9 | 0.25 |
| Estradiol (pmol/L) | 5.9 ± 1.5 | 7.3 ± 2.5 | 6.2 ± 1.8 | 0.28 |
| SHBG (nmol/L) | 58.6 ± 21.3 | 43.7 ± 23.6 | 39.9 ± 20.7 | 0.17 |
| LH (mLU/mL) | 12.2 ± 10.1 | 10.3 ± 7.8 | 7.1 ± 2.5 | 0.35 |
| FSH (mLU/mL) | 8.2 ± 10.8 | 5.6 ± 2.7 | 6.8 ± 5.9 | 0.73 |
| Metabolic parameters | ||||
| Fasting glucose (mmol/L) | 5.32 ± 8.3 | 5.35 ± 13.4 | 5.31 ± 8.4 | 0.99 |
| 2-h glucose (mmol/L) | 8.00 ± 1.9 | 7.21 ± 1.3 | 7.37 ± 1.7 | 0.55 |
| Fasting insulin (µU/mL) | 10.0 ± 9.6 | 9.3 ± 5.0 | 7.1 ± 2.6 | 0.58 |
| 2-h insulin (µU/mL) | 70.2 ± 51.9 | 53.4 ± 15.4 | 68.0 ± 50.5 | 0.64 |
| HOMAIR | 2.5 ± 1.2 | 2.2 ± 0.4 | 1.7 ± 0.6 | 0.61 |
| OGIS (mL/min/m2) | 378.41 ± 59.2 | 398.0 ± 56.8 | 375.7 ± 71.8 | 0.88 |
| Glucose total AUC0–120 (mg/dL × min) | 18,521.1 ± 3906.9 | 17,510.0 ± 2532.7 | 17,851.7 ± 3161.5 | 0.96 |
| Insulin total AUC0–120 (µU/mL × min) | 7082.9 ± 4866.5 | 6386.6 ± 2792.6 | 7600.6 ± 5264.3 | 0.55 |
| Lipid profile | ||||
| Cholesterol (mmol/L) | 4.2 ± 0.7 | 4.9 ± 0.7 | 4.5 ± 0.6 | 0.12 |
| Triglyceride (mmol/L) | 2.8 ± 0.7 | 2.9 ± 0.7 | 3.0 ± 0.7 | 0.95 |
| HDL (mmol/L) | 1.3 ± 0.3 | 1.1 ± 0.3 | 1.2 ± 0.3 | 0.39 |
| LDL (mmol/L) | 2.4 ± 0.5 | 3.5 ± 0.6 | 2.9 ± 0.7 | 0.03 |
| Adipokines and Inflammatory markers | ||||
| Leptin (ng/mL) | 7.4 ± 4.0 | 7.7 ± 5.6 | 7.0 ± 7.0 | 0.98 |
| Adiponectin (mg/L) | 12.51 ± 8.5 | 11.5 ± 5.6 | 8.81 ± 7.4 | 0.73 |
| C-reactive protein (mg/L) | 1.15 ± 1.0 | 2.38 ± 3.2 | 3.64 ± 2.1 | 0.13 |
| Abdominal and thigh fat | ||||
| Abdominal subcutaneous fat area(cm2) | 258.7 ± 61.8 | 282.3 ± 154.6 | 238.8 ± 97.3 | 0.70 |
| Abdominal visceral fat area (cm2) | 148.3 ± 80.4 | 152.5 ± 45.6 | 134.9 ± 49.2 | 0.79 |
| Mid-thigh subcutaneous fat area (cm2) | 61.8 ± 25.7 | 63.3 ± 26.7 | 56.2 ± 19.8 | 0.82 |
Data are expressed as mean ± SD, placebo n = 8, TT group (n = 7), AI group (n = 8) for thigh fat. Statistical analysis were done by anova F-test.
HOMA-IR and other glucose homeostasis measures
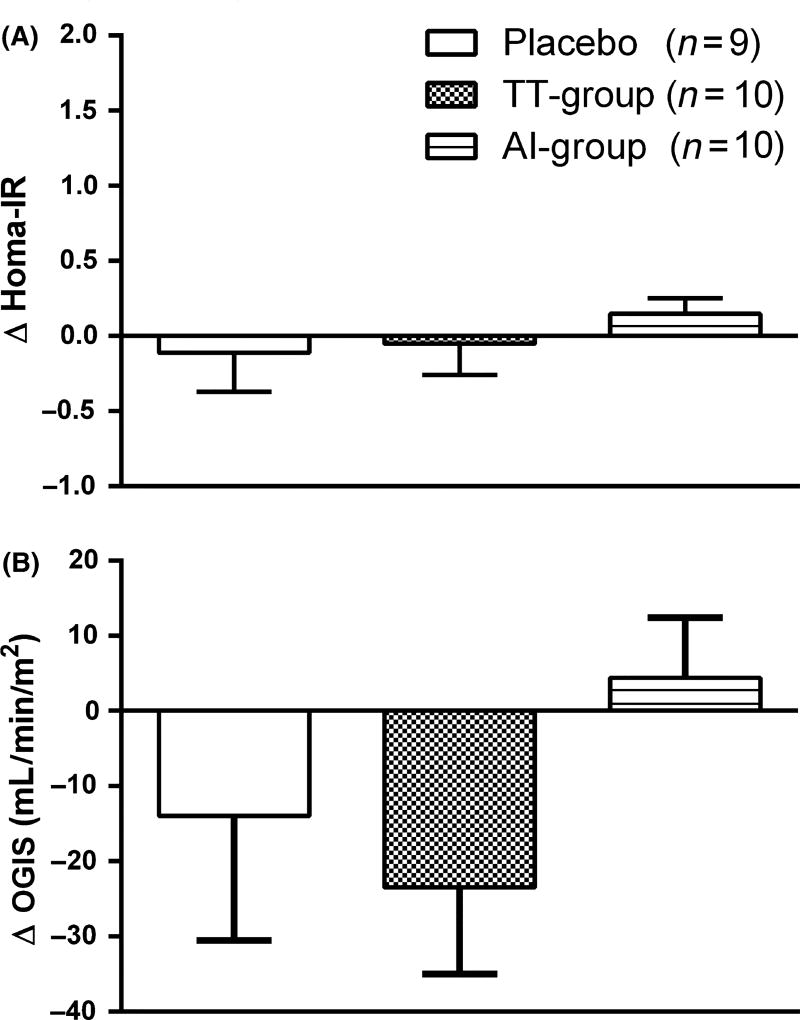
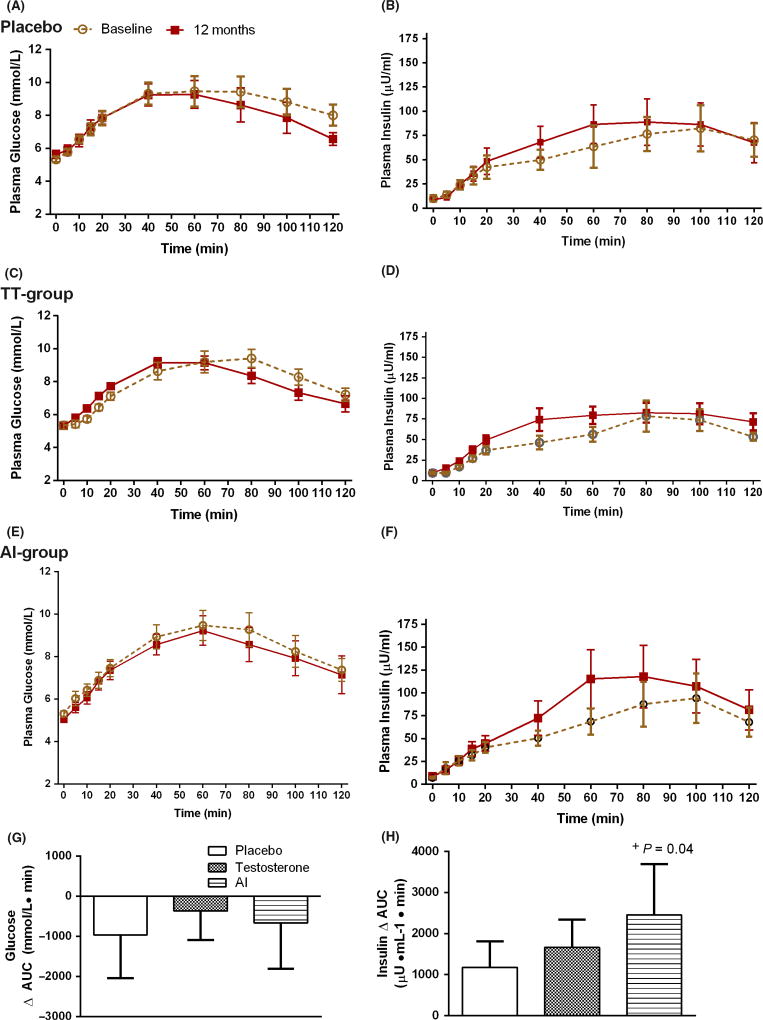
At the end of the 12-month intervention, HOMAIR levels were not significantly altered in any of the three groups (Fig. 1A). OGIS was also not statistically altered by any of the treatment regimens (Fig. 1B). The fasting glucose (Fig. 2A, C, E) and insulin levels remained unchanged (Fig. 2B, D, F). The total AUC0–120 min for plasma glucose for the three groups was similar (Fig. 2G). However, on analyzing the total insulin response to the glucose challenge, the total AUC0–120 min for plasma insulin was significantly increased in the AI group at 12 months, compared to baseline (p = 0.04) (Fig. 2H) and was not significantly different in the other groups (p > 0.05). Upon further analysis, this increase was evident in the late post-glucose period, which is the muscle glucose uptake phase of insulin action (AUC Insulin 40–120 min = 8707.08 ± 2399.11 post-treatment, compared to pre-treatment: 6390.12 ± 1579.27, p = 0.04). No increase was seen in the early post-glucose phase (AUC insulin 0–20 min).
Figure 1.
Changes in homeostasis model assessment (A) and oral glucose insulin sensitivity (B) after 12 months of treatment. Data are presented as mean±sem. Between group comparisons were performed with anova (F-test) and within-group comparisons were performed with paired t-test. F-test (A) p = 0.62, (B) p = 0.31.
Figure 2.
The profiles of fasting plasma glucose and insulin, and area under the curve for both glucose (A, C, E, G) and insulin (B, D, F, H) after 75 g oral glucose at baseline and 12 months. Data are presented as mean±sem. + p < 0.05 compared to baseline. Between group comparisons were performed with anova (F-test) and within-group comparisons were performed with paired t-test. F-test (G) p = 0.08, (H) p = 0.61.
Changes in gonadal hormones
In both the intervention groups, testosterone levels significantly increased from baseline into the target range, which was determined a priori to be 17.4–34.7 nmol/L. At 12 months, the fasting testosterone levels in the TT group were 16.4 ± 2.2 nmol/L and in the AI group were 17.7 ± 1.8 nmol/L (13). Serum estradiol levels significantly increased in the TT group by (100.6 ± 14.7 pmol/L) while a reduction was seen in the AI group of −29.7 ± 4.4 pmol/L; estradiol levels were significantly different between the two groups (p = 0.02). Serum SHBG levels did not change during intervention in any of the groups. As expected, gonadotropin levels were suppressed in the TT group, whereas they increased in the AI group. These data have been published (13).
Adipocytokines
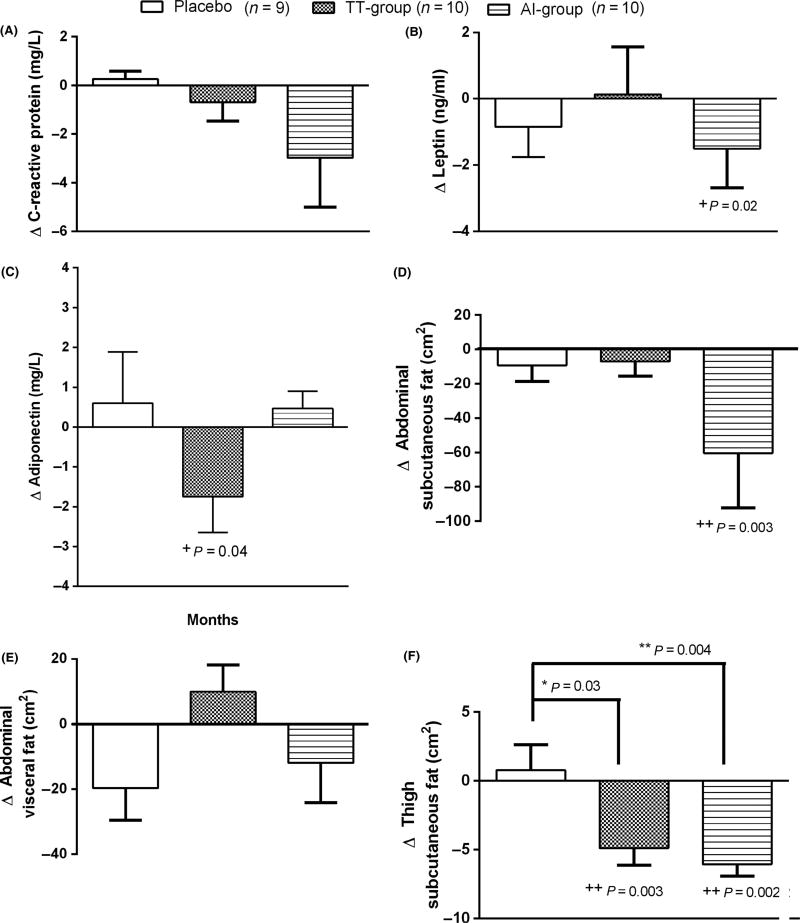
At 12 months, circulating CRP levels was not significantly altered in any of the groups (Fig. 3A). Circulating leptin levels, however, were significantly decreased in the AI group only compared to baseline (p = 0.04) (Fig. 3B). In contrast, circulating adiponectin levels significantly decreased only in the TT group (p = 0.02) (Fig. 3C).
Figure 3.
Serum changes in CRP (A), leptin (B), and adiponectin (C), and abdominal fat (D, E) and thigh fat quantity (F) after 12 months of treatment. Thigh fat was assessed in placebo (n = 8), TT group (n = 7), AI group (n = 8). Data are presented as mean±sem. *+p < 0.05, **++p < 0.01 compared to placebo (*) and to baseline values (+). Between group comparisons were performed with anova (F-test) and within-group comparisons were performed with paired t-test. F-test (A) p = 0.22, (B) p = 0.62, (C) p = 0.13, (D) p = 0.12, (E) p = 0.12, (F) p = 0.005, post hoc Dunnett Placebo vs. TT group p = 0.03; Placebo vs. AI group p = 0.004, TT group vs. AI group p = 0.83.
Abdominal and mid-thigh fat
However, abdominal subcutaneous fat was significantly decreased in the AI group (p = 0.003) (Fig. 3D) after 12 months while no changes in abdominal visceral fat was seen in any of the groups (Fig. 3E). Mid-thigh subcutaneous fat significantly decreased in both TT group (p = 0.03) and AI group (p = 0.004) compared to placebo (Fig. 3F).
Fasting lipid profile and BMI
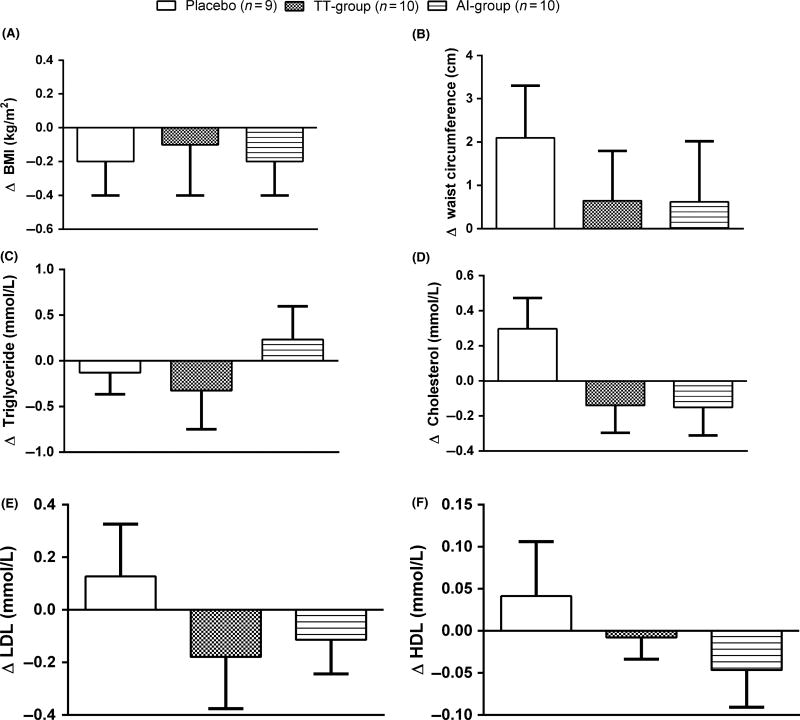
At 12 months, BMI and waist circumference were not significantly different in any of the groups (p > 0.05: Fig. 4A, B) from baseline measurements. Furthermore, no significant changes were seen in fasting lipids after 12 months (Fig. 4C–F).
Figure 4.
Changes in body mass index (A), waist circumference (B) and fasting lipids (C–F) after 12 months of treatment. Data are presented as mean±sem. Between group comparisons were performed with anova (F-test) and within-group comparisons were performed with paired t-test. F-test (A) p = 0.94, (B) p = 0.65, (C) p = 0.53, (D) p = 0.11, (E) p = 0.46, (F) p = 0.42.
CONCLUSIONS
Recent reports have raised concerns regarding cardiovascular adverse effects of testosterone therapy in older men (He et al., 2013; Vigen et al., 2013; Xu et al., 2013; Finkle et al., 2014; Layton et al., 2015). As testosterone is aromatized to estradiol, it has been suggested that estradiol might be contributing, at least in part, to these adverse effects. Hence, there is a growing interest in the exploration of alternative treatment modalities such as AI that increase endogenous testosterone production via stimulation of gonadotropins. However, estradiol has independent effects on male metabolism (Ding et al., 2006; Gao et al., 2006; Rochira et al., 2007), and the effects of long-term suppression of estradiol on metabolism in men remain unclear. In this first double-blind, randomized, proof-of-concept trial using TT and AI in non-diabetic older men with low testosterone, the change in HOMAIR after 12 months of treatment with AI did not significantly differ from other groups. The findings of this study are made all the more convincing by the strength of its design, including blinding, placebo and TT groups, concealed randomization, and parallel-group design. Randomization effectively generated three groups that were similar in their baseline glycemic parameters. Screening and on-treatment testosterone levels were measured using liquid chromatography–mass spectrometry (LC-MS), the current gold standard method for sex hormone measurements. At baseline, mean total and free testosterone levels were well below the lower limits of established norms in community-based samples (Bhasin et al., 2011) and both interventions effectively raised testosterone levels into the target range. Lastly, this is the first study using AI that has performed in-depth evaluation of glucose homeostasis, inflammatory markers, and evaluation of abdominal and thigh adipose tissue.
After 12 months of study, fasting glucose and insulin levels were not altered by any of the treatments. This suggests that insulin sensitivity at the level of the liver was not influenced by either of the treatment arms. Furthermore, the total AUC for glucose during OGTT also did not differ between the groups. However, and interestingly, an increase in insulin secretion was observed during the muscle glucose uptake phase (40–120 min) of the OGTT in the AI group, pointing to reduced insulin action and illustrating the importance of studying the whole body response to a metabolic challenge. These findings suggest that estradiol is a modulator of muscle insulin sensitivity and are consistent with some animal studies showing that estrogen receptor-α is important for insulin action (Ribas et al., 2010). These findings should be confirmed in future trials.
At 12 months, men in the AI group experienced significant reduction in subcutaneous fat in both abdominal and thigh region was reflected by the reduction in serum leptin levels. Men in the TT group showed a reduction in thigh fat only, suggesting differential sensitivity of regional fat depots to gonadal steroids (Bhasin et al., 2007; Frederiksen et al., 2012; Gianatti et al., 2014). Even though significant reduction in subcutaneous abdominal and thigh fat was seen in the AI group, muscle insulin sensitivity, as described above, was reduced in this group. This underscores a direct estrogen effect in muscle, independent of changes in fat mass. Our findings are complementary to two recent meta-analyses showing that both endogenous testosterone and exogenous testosterone supplementation is negatively associated with fat mass (Corona et al., 2016a,b). However, our findings are in contrast with another study using AI in elderly men in which reduction in abdominal subcutaneous fat was not seen (Lapauw et al., 2009); however, in that study AI was administered for only 28 days, and therefore unlikely to be of sufficient duration to appreciate any change in fat distribution.
Adiponectin is an adipokine which displays antidiabetic effects (Lanfranco et al., 2004). We observed a significant reduction in circulating adiponectin levels in the TT group, a finding consistent with other studies (Lanfranco et al., 2004; Ding et al., 2006), however, it was not accompanied by reduced insulin sensitivity. In contrast, no significant changes were seen in adiponectin in the AI group, a finding that is also in agreement with previous short-term studies (Lapauw et al., 2009). These observations suggest that suppression of adiponectin by testosterone administration might be mediated via estradiol. Serum concentration of CRP, a marker of inflammation, did not worsen in either intervention groups, findings consistent with previous short-term studies of administration of testosterone (Dougherty et al., 2005; Kapoor et al., 2007) and aromatase inhibitors (Dougherty et al., 2005).
The fasting lipid profile did not worsen in the TT group or AI group. Previous studies of testosterone administration have reported a slight reduction in HDL-cholesterol and either no change or reduction in total cholesterol, LDL-cholesterol, and triglycerides (Basaria, 2014), however, studies of AI in men have shown conflicting results. One short-term trial using AI in young eugonadal men showed an increase in triglyceride levels (Lapauw et al., 2009) while another short-term study in older men demonstrated no such effect (Dougherty et al., 2005).
This study has some limitations. First, this study has a relatively small sample size. However, this was a proof-of-concept trial that was designed to answer mechanistic questions and the sample size of this trial is larger than some other mechanistic studies (Pitteloud et al., 2005; Yialamas et al., 2007). Indeed, the findings of this trial will provide impetus for a larger trial with AI in older men. Second, we did not perform whole body magnetic resonance imaging which would have provided volumetric information regarding fat depots. However, the majority of previous trials of replacement with gonadal steroids have utilized CT scans to determine changes in fat depots. Third, we did not perform fat or muscle biopsies, which would have provided deeper mechanistic insights into signaling of gonadal steroids. We recommend that future mechanistic studies include these outcomes.
In this proof-of-concept trial, change in HOMAIR after 12 months of AI did not differ significantly from T replacement or placebo. The effects of the intervention on subcutaneous fat and adipokines were variable. Larger efficacy and safety trials are needed before AI pharmacotherapy can be considered as a treatment option to raise serum testosterone levels in older men with low testosterone.
Acknowledgments
This research was supported by the Intramural Research program at NIH, National Institute of Aging. Authors are most grateful to Dr. Sokratis Makrogiannis for his help with the software for quantitation of CT thigh. In addition, authors are most grateful to all the participants who took part in this study.
FUNDING
This research was supported by the Intramural Research Program of the NIH, National Institute on Aging.
Footnotes
CONFLICT OF INTEREST
Dr. Basaria has previously received investigator-initiated research grants from Abbvie Pharmaceuticals (previously Solvay Pharmaceuticals) and has previously consulted for Eli Lilly, Inc and Takeda Pharmaceuticals.
AUTHORS’ CONTRIBUTIONS
JPD collected the data, conducted blood sample analysis and statistical analysis, and wrote the manuscript; MDS reviewed statistical analysis and wrote manuscript; DM assigned nurse and clinical coordinator of the study; GC conducted quantification on abdominal fat computed tomography scans. OC conducted blood sample analysis; LF reviewed study findings and wrote manuscript; CWC contributed to clinical aspect of the trial; JME designed the study, wrote and reviewed the manuscript; SB designed the study, wrote and reviewed the manuscript.
References
- Basaria S. Testosterone therapy in older men with late-onset hypogonadism: a counter-rationale. Endocr Pract. 2013;19:853–863. doi: 10.4158/EP13318.RA. [DOI] [PubMed] [Google Scholar]
- Basaria S. Male hypogonadism. Lancet. 2014;383:1250–1263. doi: 10.1016/S0140-6736(13)61126-5. [DOI] [PubMed] [Google Scholar]
- Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, Jette AM, Eder R, Tennstedt S, Ulloor J, Zhang A, Choong K, Lakshman KM, Mazer NA, Miciek R, Krasnoff J, Elmi A, Knapp PE, Brooks B, Appleman E, Aggarwal S, Bhasin G, Hede-Brierley L, Bhatia A, Collins L, LeBrasseur N, Fiore LD, Bhasin S. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109–122. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basaria S, Davda MN, Travison TG, Ulloor J, Singh R, Bhasin S. Risk factors associated with cardiovascular events during testosterone administration in older men with mobility limitation. J Gerontol A Biol Sci Med Sci. 2013;68:153–160. doi: 10.1093/gerona/gls138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhasin S, Parker RA, Sattler F, Haubrich R, Alston B, Umbleja T, Shikuma CM. Effects of testosterone supplementation on whole body and regional fat mass and distribution in human immunodeficiency virus-infected men with abdominal obesity. J Clin Endocrinol Metab. 2007;92:1049–1057. doi: 10.1210/jc.2006-2060. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Pencina M, Jasuja GK, Travison TG, Coviello A, Orwoll E, Wang PY, Nielson C, Wu F, Tajar A, Labrie F, Vesper H, Zhang A, Ulloor J, Singh R, D’Agostino R, Vasan RS. Reference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community-based sample of healthy nonobese young men in the Framingham Heart Study and applied to three geographically distinct cohorts. J Clin Endocrinol Metab. 2011;96:2430–2439. doi: 10.1210/jc.2010-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyanov MA, Boneva Z, Christov VG. Testosterone supplementation in men with type 2 diabetes, visceral obesity and partial androgen deficiency. Aging Male. 2003;6:1–7. [PubMed] [Google Scholar]
- Braga-Basaria M, Muller DC, Carducci MA, Dobs AS, Basaria S. Lipoprotein profile in men with prostate cancer undergoing androgen deprivation therapy. Int J Impot Res. 2006;18:494–498. doi: 10.1038/sj.ijir.3901471. [DOI] [PubMed] [Google Scholar]
- Corona G, Giagulli VA, Maseroli E, Vignozzi L, Aversa A, Zitzmann M, Saad F, Mannucci E, Maggi M. Testosterone supplementation and body composition: results from a meta-analysis study. Eur J Endocrinol. 2016a;174:R99–R116. doi: 10.1530/EJE-15-0262. [DOI] [PubMed] [Google Scholar]
- Corona G, Giagulli VA, Maseroli E, Vignozzi L, Aversa A, Zitzmann M, Saad F, Mannucci E, Maggi M. Testosterone supplementation and body composition: results from a meta-analysis of observational studies. J Endocrinol Invest. 2016b;39:967–981. doi: 10.1007/s40618-016-0480-2. [DOI] [PubMed] [Google Scholar]
- Dias JP, Melvin D, Simonsick EM, Carlson O, Shardell MD, Ferrucci L, Chia CW, Basaria S, Egan JM. Effects of aromatase inhibitor vs. testosterone in older men with low testosterone: randomized-controlled trial. Andrology. 2015;4:33–40. doi: 10.1111/andr.12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2006;295:1288–1299. doi: 10.1001/jama.295.11.1288. [DOI] [PubMed] [Google Scholar]
- Dougherty RH, Rohrer JL, Hayden D, Rubin SD, Leder BZ. Effect of aromatase inhibition on lipids and inflammatory markers of cardiovascular disease in elderly men with low testosterone levels. Clin Endocrinol (Oxf) 2005;62:228–235. doi: 10.1111/j.1365-2265.2005.02205.x. [DOI] [PubMed] [Google Scholar]
- Faulds MH, Zhao C, Dahlman-Wright K, Gustafsson JA. The diversity of sex steroid action: regulation of metabolism by estrogen signaling. J Endocrinol. 2012;212:3–12. doi: 10.1530/JOE-11-0044. [DOI] [PubMed] [Google Scholar]
- Finkle WD, Greenland S, Ridgeway GK, Adams JL, Frasco MA, Cook MB, Fraumeni JF, Jr, Hoover RN. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS ONE. 2014;9:e85805. doi: 10.1371/journal.pone.0085805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen L, Hojlund K, Hougaard DM, Mosbech TH, Larsen R, Flyvbjerg A, Frystyk J, Brixen K, Andersen M. Testosterone therapy decreases subcutaneous fat and adiponectin in aging men. Eur J Endocrinol. 2012;166:469–476. doi: 10.1530/EJE-11-0565. [DOI] [PubMed] [Google Scholar]
- Gao H, Bryzgalova G, Hedman E, Khan A, Efendic S, Gustafsson JA, Dahlman-Wright K. Long-term administration of estradiol decreases expression of hepatic lipogenic genes and improves insulin sensitivity in ob/ob mice. a possible mechanism is through direct regulation of signal transducer and activator of transcription 3. Mol Endocrinol. 2006;20:1287–1299. doi: 10.1210/me.2006-0012. [DOI] [PubMed] [Google Scholar]
- Gianatti EJ, Dupuis P, Hoermann R, Strauss BJ, Wentworth JM, Zajac JD, Grossmann M. Effect of testosterone treatment on glucose metabolism in men with type 2 diabetes: a randomized controlled trial. Diabetes Care. 2014;37:2098–2107. doi: 10.2337/dc13-2845. [DOI] [PubMed] [Google Scholar]
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- He J, Bhasin S, Binder EF, Yarasheski KE, Castaneda-Sceppa C, Schroeder ET, Roubenoff R, Chou CP, Azen SP, Sattler FR. Cardiometabolic risks during anabolic hormone supplementation in older men. Obesity (Silver Spring) 2013;21:968–975. doi: 10.1002/oby.20081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasuja GK, Bhasin S, Reisman JI, Berlowitz DR, Rose AJ. Ascertainment of testosterone prescribing practices in the VA. Med Care. 2015;53:746–752. doi: 10.1097/MLR.0000000000000398. [DOI] [PubMed] [Google Scholar]
- Juang PS, Peng S, Allehmazedeh K, Shah A, Coviello AD, Herbst KL. Testosterone with dutasteride, but not anastrazole, improves insulin sensitivity in young obese men: a randomized controlled trial. J Sex Med. 2014;11:563–573. doi: 10.1111/jsm.12368. [DOI] [PubMed] [Google Scholar]
- Kapoor D, Clarke S, Stanworth R, Channer KS, Jones TH. The effect of testosterone replacement therapy on adipocytokines and C-reactive protein in hypogonadal men with type 2 diabetes. Eur J Endocrinol. 2007;156:595–602. doi: 10.1530/EJE-06-0737. [DOI] [PubMed] [Google Scholar]
- Katznelson L, Finkelstein JS, Schoenfeld DA, Rosenthal DI, Anderson EJ, Klibanski A. Increase in bone density and lean body mass during testosterone administration in men with acquired hypogonadism. J Clin Endocrinol Metab. 1996;81:4358–4365. doi: 10.1210/jcem.81.12.8954042. [DOI] [PubMed] [Google Scholar]
- Lanfranco F, Zitzmann M, Simoni M, Nieschlag E. Serum adiponectin levels in hypogonadal males: influence of testosterone replacement therapy. Clin Endocrinol (Oxf) 2004;60:500–507. doi: 10.1111/j.1365-2265.2004.02007.x. [DOI] [PubMed] [Google Scholar]
- Lapauw B, T’Sjoen G, Mahmoud A, Kaufman JM, Ruige JB. Short-term aromatase inhibition: effects on glucose metabolism and serum leptin levels in young and elderly men. Eur J Endocrinol. 2009;160:397–402. doi: 10.1530/EJE-08-0881. [DOI] [PubMed] [Google Scholar]
- Layton JB, Meier CR, Sharpless JL, Sturmer T, Jick SS, Brookhart MA. Comparative safety of testosterone dosage forms. JAMA Intern Med. 2015;175:1187–1196. doi: 10.1001/jamainternmed.2015.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makrogiannis S, Serai S, Fishbein KW, Schreiber C, Ferrucci L, Spencer RG. Automated quantification of muscle and fat in the thigh from water-, fat-, and nonsuppressed MR images. J Magn Reson Imaging. 2012;35:1152–1161. doi: 10.1002/jmri.22842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkin CJ, Jones TH, Channer KS. The effect of testosterone on insulin sensitivity in men with heart failure. Eur J Heart Fail. 2007;9:44–50. doi: 10.1016/j.ejheart.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Marin P, Holmang S, Jonsson L, Sjostrom L, Kvist H, Holm G, Lindstedt G, Bjorntorp P. The effects of testosterone treatment on body composition and metabolism in middle-aged obese men. Int J Obes Relat Metab Disord. 1992;16:991–997. [PubMed] [Google Scholar]
- Pacini G, Mari A. Methods for clinical assessment of insulin sensitivity and beta-cell function. Best Pract Res Clin Endocrinol Metab. 2003;17:305–322. doi: 10.1016/s1521-690x(03)00042-3. [DOI] [PubMed] [Google Scholar]
- Pitteloud N, Mootha VK, Dwyer AA, Hardin M, Lee H, Eriksson KF, Tripathy D, Yialamas M, Groop L, Elahi D, Hayes FJ. Relationship between testosterone levels, insulin sensitivity, and mitochondrial function in men. Diabetes Care. 2005;28:1636–1642. doi: 10.2337/diacare.28.7.1636. [DOI] [PubMed] [Google Scholar]
- Ribas V, Nguyen MT, Henstridge DC, Nguyen AK, Beaven SW, Watt MJ, Hevener AL. Impaired oxidative metabolism and inflammation are associated with insulin resistance in ERalpha-deficient mice. Am J Physiol Endocrinol Metab. 2010;298:E304–E319. doi: 10.1152/ajpendo.00504.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochira V, Madeo B, Zirilli L, Caffagni G, Maffei L, Carani C. Oestradiol replacement treatment and glucose homeostasis in two men with congenital aromatase deficiency: evidence for a role of oestradiol and sex steroids imbalance on insulin sensitivity in men. Diabet Med. 2007;24:1491–1495. doi: 10.1111/j.1464-5491.2007.02304.x. [DOI] [PubMed] [Google Scholar]
- Rubinow KB, Snyder CN, Amory JK, Hoofnagle AN, Page ST. Acute testosterone deprivation reduces insulin sensitivity. Clin Endocrinol. 2012;76:281–288. doi: 10.1111/j.1365-2265.2011.04189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvin E, Feinleib M, Zhang L, Rohrmann S, Rifai N, Nelson WG, Dobs A, Basaria S, Golden SH, Platz EA. Androgens and diabetes in men: results from the Third National Health and Nutrition Examination Survey (NHANES III) Diabetes Care. 2007;30:234–238. doi: 10.2337/dc06-1579. [DOI] [PubMed] [Google Scholar]
- Snyder PJ, Lawrence DA. Treatment of male hypogonadism with testosterone enanthate. J Clin Endocrinol Metab. 1980;51:1335–1339. doi: 10.1210/jcem-51-6-1335. [DOI] [PubMed] [Google Scholar]
- Spitzer M, Huang G, Basaria S, Travison TG, Bhasin S. Risks and benefits of testosterone therapy in older men. Nat Rev Endocrinol. 2013;9:414–424. doi: 10.1038/nrendo.2013.73. [DOI] [PubMed] [Google Scholar]
- Stellato RK, Feldman HA, Hamdy O, Horton ES, McKinlay JB. Testosterone, sex hormone-binding globulin, and the development of type 2 diabetes in middle-aged men. prospective results from the Massachusetts male aging study. Diabetes Care. 2000;23:490–494. doi: 10.2337/diacare.23.4.490. [DOI] [PubMed] [Google Scholar]
- Swerdloff RS, Pak Y, Wang C, Liu PY, Bhasin S, Gill TM, Matsumoto AM, Pahor M, Surampudi P, Snyder PJ. Serum testosterone (T) level variability in T gel-treated older hypogonadal men: treatment monitoring implications. J Clin Endocrinol Metab. 2015;100:3280–3287. doi: 10.1210/JC.2015-1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigen R, O’Donnell CI, Baron AE, Grunwald GK, Maddox TM, Bradley SM, Barqawi A, Woning G, Wierman ME, Plomondon ME, Rumsfeld JS, Ho PM. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829–1836. doi: 10.1001/jama.2013.280386. [DOI] [PubMed] [Google Scholar]
- Xu L, Freeman G, Cowling BJ, Schooling CM. Testosterone therapy and cardiovascular events among men: a systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med. 2013;11:108. doi: 10.1186/1741-7015-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yialamas MA, Dwyer AA, Hanley E, Lee H, Pitteloud N, Hayes FJ. Acute sex steroid withdrawal reduces insulin sensitivity in healthy men with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2007;92:4254–4259. doi: 10.1210/jc.2007-0454. [DOI] [PubMed] [Google Scholar]