Abstract
Background
For many environmental chemicals, concentrations in spot urine samples are considered valid surrogates of exposure and internal dose. To correct for urine dilution, spot urine concentrations are commonly adjusted for urinary creatinine. There are, however, several concerns about the use of urine creatinine. While urine osmolality is an attractive alternative; its characteristics and determinants in the general population remain unknown. Our objective was to describe the determinants of urine osmolality and to contrast the difference between osmolality and creatinine in urine.
Methods
From the National Health and Nutrition Examination Survey (NHANES) 2009–2012, 10,769 participants aged 16 years or older with measured urine osmolality and creatinine were used in the analysis. Very dilute and very concentrated urine was defined as urine creatinine lower than 0.3 g/l and higher than 3 g/l, respectively. Linear and logistic regression analyses were performed to investigate the associations of interest.
Results
Urine osmolality and creatinine were highly correlated (Pearson correlation coefficient = 0.75) and their respective median values were 648 mOsm/kg and 1.07 g/l. The prevalence of very dilute and very concentrated urine samples was 8.1% and 3.1%, respectively. Factors associated in the same direction with both urine osmolality and urine creatinine included age, sex, race, body mass index (BMI), hypertension, water intake, and blood osmolality. The magnitude of associations expressed as percent change was significantly stronger with creatinine than osmolality. Compared to urine creatinine, urine osmolality did not vary by diabetes status but was affected by daily total protein intake. Participants with chronic kidney disease (CKD) had significantly higher urine creatinine concentrations but lower urine osmolality. Both very dilute and concentrated urine were associated with a diverse array of sociodemographic, medical conditions, and dietary factors. For instance, females were approximately 3.3 times more likely to have urine over-dilution than male [the adjusted odds ratios (95% CI) = 3.27 (2.10–5.10)].
Conclusion
Although the determinants of urine osmolality were generally similar to those of urine creatinine, the relative influence of socio-demographic and medical conditions was less on urine osmolality than on urine creatinine. Protocols for spot urine sample collection could recommend avoiding excessive and insufficient water intake before urine sampling to improve urine adequacy. The feasibility of adopting urine osmolality adjustment and water intake recommendations before providing spot urine samples for environmental biomonitoring merits further investigation.
Index Words: Urine osmolality, urine creatinine, biomonitoring, urine adequacy, urine dilution
Background
For many environmental chemicals including metals, drugs, and pesticides, measurement in urine is a useful non-invasive method to assess external exposure and internal dose (Barr et al., 2005; Lin et al., 2005; Navas-Acien et al., 2005). Twenty-four hour urine samples are commonly regarded as the “gold standard” to quantify environmental exposures but are often cumbersome and expansive to collect, thereby, preventing its practical application in large population studies. Spot urine samples, with or without correction for urine dilution, are therefore commonly utilized in environmental monitoring. The adjustors commonly used to standardize the concentrations of chemicals in urine include urine creatinine, specific gravity, and osmolality. In the 1950–70s, urine specific gravity and osmolality were widely used in occupational medicine (Elkins, 1969; Elkins and Pagnotto, 1965). Following the validation of the urine albumin-creatinine ratio in clinical medicine in the early 1980s (Barratt et al., 1970; McCrory et al., 1959), urine creatinine adjustment gradually replaced urine specific gravity and osmolality in environmental science (Barr et al., 2005). However, there are growing concerns about the non-random variability introduced by correction with urine creatinine and the possibility that this correction biases exposure-response relationship estimates (Sieniawska et al., 2012; Suwazono et al., 2005).
Criticism of urine creatinine standardization stems from two fairly well-established observations. First, although relatively constant, since creatinine is metabolized from creatine in muscle, differences in muscle mass and diet cause variation in daily urine creatinine excretion that is depend on age, gender, race, and body mass index (Barr et al., 2005). Second, biosynthesis of creatinine is related to methylation processes including detoxification of many toxicants. For instance, the formation of creatinine may interfere with arsenic metabolism by competing for methyl groups yielded from one-carbon metabolism (Hall and Gamble, 2012; Nermell et al., 2008). Adjusting for urine creatinine may thus introduce measurement errors that are differential by disease status (e.g. kidney disease) or dependent on exposure levels or metabolism. Recent environmental studies have therefore explored correcting for urine dilution using urine specific gravity or osmolality (Shelley et al., 2014; Suwazono et al., 2005; Yassine et al., 2012). However, the determinants of these two markers of urine concentration remain largely unknown in population-based surveys. Also, the performance of these two historical methods to correct for urine dilution in environmental biomonitoring has not been systematically evaluated.
In 2009, the National Health Nutrition Examination Survey (NHANES), a representative sample of the civilian U.S. population, began measuring urine osmolality routinely in addition to urine creatinine. Our study objective was to characterize determinants of urine osmolality in NHANES 2009–2012 participants and to compare these determinants with those of urine creatinine. We also examined characteristics of participants who provided very dilute and concentrated urine samples as defined by the WHO guidelines for occupational biomonitoring(World Health Organization (WHO), 1996).
Methods
Study population
NHANES is a continuous nationally-representative survey conducted by the U.S. National Center for Health Statistics (Hyattsville, MD) using a stratified multistage sampling design. A total of 20,293 individuals participated in the NHANES 2009–2012 in-home interview and the medical evaluation at the mobile examination center. The participation rate was 73.6%. For urine osmolality, NHANES 2009–2012 selected study participants aged 6 years and older. For this study, we restricted the analysis to participants 16 years and older (N=13,064). Among 11,979 participants who had urine osmolality measured, we excluded participants who had missing urine creatinine (n=8), missing serum creatinine or blood osmolality level (n=746), those who were pregnant (n=109), and those with missing covariates of interest (e.g., body mass index or diabetes status, n=347), leaving a final sample of 10,769 participants for this analysis. For analyses of the association of urine osmolality or creatinine with dietary factors and medications, we further restricted our analysis to the 5,810 participants with information available on detailed dietary intake and daily medication use. The NHANES 2009–2011 cycles were approved by the institutional review board of the National Center for Health Statistics. Oral and written informed consent was obtained from all participants.
Measurement of urine osmolality and creatinine
In NHANES 2009–2012, osmolality was measured in spot or timed urine samples directly at the mobile examination center within 4 hours of collection, which conforms with current practice, to ensure the stability of measurements (Curria, 2011; The National Health and Nutrition Examination Survey (NHANES), 2010). In this study, 14.3% of urine specimens were derived from multiple urine collections. Urine osmolality was measured by Osmette II, Model 5005 Automatic Osmometer utilizing the freezing point depression method. Osmolality is expressed in units of milliOsmoles (mOsm) per kilogram of water. The interassay coefficients of variation of quality control–pooled samples analyzed throughout 2009–2010 ranged between 0.8% and 2.7%. For urine creatinine analysis, spot or timed urine samples were stored at 2–8 °C until analysis within 36 hours of receipt in the laboratory. Urine creatinine was measured by an enzymatic (creatinase) method by Roche/Hitachi Modular P Chemistry Analyzer in 2009–2012. The interassay coefficients of variation for urine creatinine throughout 2009–2010 ranged between 1.4% and 4.4%.
Adequacy of urine specimens
The World Health Organization (WHO) has established guidelines to determine the adequacy of spot urine samples in occupational biomonitoring (World Health Organization (WHO), 1996). Following the WHO guidelines, we defined urine specimens as adequate for environmental biomonitoring if the urine creatinine concentrations were between 0.3 and 3 g/l. Very dilute and very concentrated urine was defined as urine creatinine level less than 0.3 g/l, and higher than 3 g/l, respectively (Barr et al., 2005; World Health Organization (WHO), 1996).
Dietary intake and medication databases
The NHANES 2009–2010 cycle included two 24-hour dietary recalls for about 82% of total study participants. Participants were asked to list the types and amounts of all food and beverages consumed during the previous 24-hours with the goal of obtaining data representative of usual dietary intake for the US population (Dwyer et al., 2003). Data from the first dietary recall completed at the mobile examination center following standardized protocols by NHANES trained dietary interviewers were used to estimate total intake of energy, nutrients, and non-nutrient food components from food and beverages that were consumed during the 24-hour period prior to the exam (midnight to midnight) for all participants. Total plain water intake (gm/day) was defined as the 24-hour amount of water consumed (including plain tap water, water from a drinking fountain, water from a water cooler, bottled water, and spring water). Total protein intake (gm/day) was defined as the daily aggregates of protein from all food and beverages as calculated using USDA’s FOOD and Nutrient Database for Dietary Studies 5 (FNDDS 5.0).
Information on prescription medications was collected during the household interview by trained interviewers using a Computer-Assisted Personal Interviewing (CAPI) system. In NHANES 2009–2010 cycle, 92% of all reported drugs were automatically matched to the data collection drug database (National Health and Nutrition Examination Survey, 2012). Diuretic usage was defined as participants taking thiazide-type, loop-active agents, and potassium-sparing agents given in monotherapy or in combination.
Other variables
ociodemographic variables collected during the interview included age, race/ethnicity, gender, education, cigarette smoking, and alcohol consumption. Smoking was categorized as current and non-current (including former or never smoking) by self-report. Alcohol consumption was categorized as never (< 12 drinks in any 1 year in life), former (≥ 12 drinks in any 1 year in life and not drinking now), and current (≥12 drinks in any 1 year in life and drinking now) drinking.
Body mass index was calculated as measured weight in kilograms divided by measured height in meters squared. Diabetes mellitus was defined by self-reported diagnosis by a physician, diabetes medication use, or glucose levels higher or equal than 126 mg/dL (fasting 8 hours or more) or than 200 mg/dL (fasting less than 8 hours). Hypertension was defined as a self-reported physician diagnosis, use of antihypertensive medication, mean systolic blood pressure > 140 mmHg or mean diastolic blood pressure > 90 mmHg. Urine albumin was determined using fluorescein immunoassay by Sequoia-Turner digital fluorometer (model 450). Albuminuria was defined as urine albumin-creatinine ratio greater than 30 gm/g creatinine. Serum creatinine was measured by the Jaffé rate method (kinetic alkaline picrate) at the Collaborative Laboratory Services at Ottumwa, Iowa, using a Beckman Coulter UniCel® DxC800 Synchron in 2009–2012. Kidney function was assessed by calculating the estimated glomerular filtration rate (eGFR) using the equation provided by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula. Chronic kidney disease (CKD) was defined as eGFR less than 60 ml/min/1.73m2(Levey et al., 2009).
Statistical analysis
Statistical analyses were performed using the sample survey commands in STATA version 12.0 statistical software (StataCorp LP, College Station, Texas) to account for the complex sampling design and to incorporate appropriate weights, primary sampling units, and strata in NHANES 2009–2012 and obtain unbiased point estimates and robust linearized standard errors. In the current study, sample weights for NHANES 2009–2012 analyses were computed by combining the sample weights for each individual survey cycle (2009–2010 and 2011–2012) following the NCHS analytical guidelines (National Health and Nutrition Examination Survey (NHANES), 2013). The 2-sided statistical significance level was set at α=0.05.
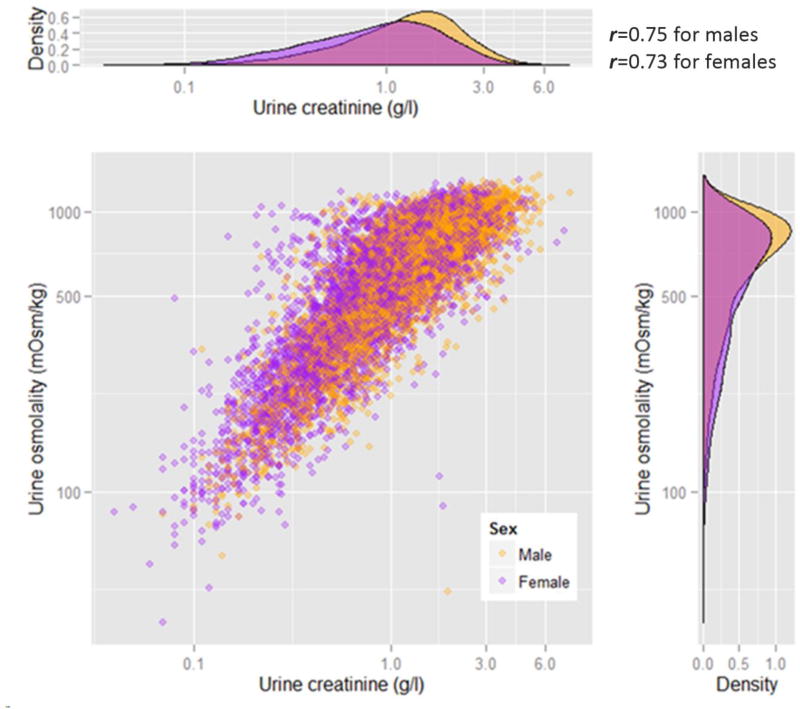
The levels of urine osmolality and creatinine were summarized in a scatter plot with density distributions (figure 1). Following the approach used by Barr et al (Barr et al., 2005), we used linear regression models to examine the influence of a set of proposed parameters on the levels of urine osmolality and creatinine. The proposed adjustment variables included age (per 10-year change), gender, race, body mass index, hypertension, diabetes, chronic kidney disease, diuretics usage, daily total protein intake (in tertiles), total water intake (in tertiles), and blood osmolality (in tertiles). Because both dependent variables (urine creatinine and urine osmolality) are right-skewed, we log-transformed them to improve normality and to estimate their percent change attributed to the proposed independent variables. We used multiple logistic regression models to evaluate the determinants of very dilute urine or very concentrated urine in separate analyses. Regression analyses were conducted with and without adjustment using the same variables mentioned above.
Figure 1.
The distribution of urine osmolality and urine creatinine by gender in NHANES 2009–2012.
Results
Characteristics of urine osmolality
Urine osmolality and creatinine were strongly correlated with each other (Pearson correlation coefficient = 0.75). The median (interquartile range [IQR]) were 648 (393, 838) mOsm/kg for urine osmolality and 1.07 (0.6, 1.66) g/l for urine creatinine (Figure 1). The median (IQR) urine osmolality concentrations were summarized by age groups across sociodemographic factors, medical conditions, dietary intake and diuretics usage (Table 1–3). An inverse association of decreasing urine osmolality with increasing age was observed (ptrend < 0.001). Regardless of age, male participants generally had higher urine osmolality than females (Figure 1). Non-Hispanic blacks and Mexican Americans tended to have significantly higher urine osmolality compared to Non-Hispanic whites across all age categories(Table 1). Urine osmolality was also higher in adult obese participants (BMI ≥ 30 kg/m2) compared to those with normal BMI (p < 0.001) (Table 2).
Table 1.
Median (interquartile range) urine osmolality concentrations (mOsm/kg) in NHANES 2009–2012 participants >16 years old by sociodemographic characteristics. The first number for each cell corresponds to the sample size in that category.
| Age categories (years) | All | 16–20 | 21–29 | 30–39 | 40–49 | 50–59 | 60–69 | ≥70 |
|---|---|---|---|---|---|---|---|---|
| Overall | 10769 | 1053 | 1671 | 1652 | 1720 | 1570 | 1578 | 1525 |
| 648 (393–838) | 811(469–946) | 745(444–925) | 709 (392–887) | 678 (391–842) | 614 (370–775) | 591 (375–740) | 532(386–678) | |
| Sex | ||||||||
| Male | 5486 | 581 | 854 | 853 | 837 | 798 | 806 | 757 |
| 697 (458–874) | 860 (551–973) | 772 (503–935) | 753 (495–906) | 729 (472–866) | 647 (438–822) | 652 (441–780) | 589 (443–729) | |
| Female | 5283 | 472 | 817 | 799 | 883 | 772 | 772 | 768 |
| 588 (340–791) | 730 (399–912) | 724 (372–912) | 644 (326–848) | 600 (345–816) | 559 (310–736) | 528 (311–702) | 491 (351–620) | |
| p-value | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| Race | ||||||||
| Non-Hispanic white | 4548 | 287 | 612 | 741 | 720 | 631 | 576 | 981 |
| 618 (371–809) | 742 (424–923) | 716 (412–890) | 650 (326–855) | 652 (373–830) | 589 (349–768) | 590 (362–734) | 529 (386–678) | |
| Non-Hispanic black | 2295 | 290 | 374 | 279 | 339 | 376 | 402 | 235 |
| 727 (503–906) | 882 (625–1006) | 848 (632–1008) | 782 (556–949) | 728 (496–882) | 670 (458–816) | 606 (430–770) | 574 (426–687) | |
| Mexican American | 1649 | 242 | 275 | 256 | 285 | 236 | 250 | 105 |
| 756 (492–913) | 887 (594–990) | 777 (505–936) | 815 (578–926) | 741 (431–873) | 684 (487–824) | 648 (418–778) | 546 (366–716) | |
| Others | 2277 | 234 | 410 | 376 | 376 | 327 | 350 | 204 |
| 662 (392–853) | 827 (481–945) | 751 (392–926) | 735 (462–874) | 688 (431–845) | 564 (352–759) | 592 (359–745) | 500 (334–638) | |
| p-value | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.14 | 0.02 |
| Smoking | ||||||||
| Non-current | 7668 | — | 1232 | 1236 | 1281 | 1210 | 1307 | 1402 |
| 642 (398–824) | — | 740 (430–930) | 705 (388–890) | 688 (419–848) | 630 (396–782) | 602 (386–753) | 534 (386–678) | |
| Current | 2042 | — | 437 | 416 | 437 | 359 | 270 | 123 |
| 625 (366–832) | — | 753 (485–909) | 713 (405–871) | 588 (363–807) | 522 (310–734) | 523 (294–696) | 488 (381–676) | |
| p-value | 0.14 | 0.46 | 0.92 | 0.04 | <0.01 | 0.02 | 0.69 | |
| Alcohol | ||||||||
| Never | 2062 | — | 231 | 291 | 326 | 357 | 411 | 446 |
| 602 (360–786) | — | 670 (389–914) | 575 (351–820) | 678 (397–838) | 589 (310–763) | 602 (358–756) | 535 (374–669) | |
| Former | 2478 | — | 532 | 507 | 463 | 415 | 349 | 212 |
| 653 (390–831) | — | 722 (412–890) | 688 (347–878) | 675 (391–840) | 614 (362–789) | 607 (438–762) | 580 (454–703) | |
| Current | 5176 | — | 908 | 854 | 931 | 798 | 818 | 867 |
| 652 (405–841) | — | 776 (492–942) | 753 (468–901) | 678 (390–846) | 616 (394–773) | 569 (339–716) | 509 (376–668) | |
| p-value | <0.01 | <0.01 | <0.01 | 0.67 | 0.48 | 0.01 | <0.01 |
P-trend across age categories for each sociodemographic factors were all significant (p<0.001).
Table 3.
Median (interquartile range) urine osmolality concentrations (mOsm/kg) in NHANES 2009–2012 participants >16 years old by dietary factors and hydration status (Total protein intake, total water intake, and diuretics usage were only available in the NHANES 2009–2010 at the time of analysis). The first number for each cell corresponds to the sample size in that category.
| Age categories (years) | All | 16–20 | 21–29 | 30–39 | 40–49 | 50–59 | 60–69 | ≥70 |
|---|---|---|---|---|---|---|---|---|
| Overall | 10769 | 1053 | 1671 | 1652 | 1720 | 1570 | 1578 | 1525 |
| 648 (393–838) | 811(469–946) | 745(444–925) | 709 (392–887) | 678 (391–842) | 614 (370–775) | 591 (375–740) | 532(386–678) | |
| Total protein intake | ||||||||
| Tertile 1 | 1997 | 193 | 274 | 252 | 295 | 258 | 309 | 416 |
| < 61.7 mg/day | 569 (335–782) | 729 (434–927) | 684 (319–895) | 599 (369–837) | 596 (306–804) | 522 (310–730) | 525(289–702) | 504 (375–631) |
| Tertile 2 | 1824 | 169 | 263 | 242 | 314 | 264 | 295 | 277 |
| 61.7–92.0 mg/day | 631 (375–823) | 796 (348–917) | 727 (418–928) | 629 (306–875) | 720 (455–838) | 581 (334–754) | 554 (395–760) | 545 (374–691) |
| Tertile 3 | 1788 | 193 | 306 | 333 | 335 | 275 | 200 | 146 |
| >92.0 mg/day | 701 (454–883) | 870 (560–980) | 762 (469–936) | 751 (473–897) | 697 (459–883) | 666 (441–838) | 631 (448–781) | 558 (401–690) |
| p-trend | <0.01 | 0.05 | 0.03 | <0.01 | <0.01 | <0.01 | <0.01 | 0.02 |
| Total water intake | ||||||||
| Tertile 1 | 1974 | 198 | 278 | 268 | 326 | 281 | 282 | 341 |
| <340.7 mg/day | 660 (412–853) | 804 (399–953) | 782 (517–960) | 750 (461–912) | 726 (492–859) | 618 (353–789) | 579 (378–746) | 545 (387–671) |
| Tertile 2 | 1884 | 200 | 273 | 240 | 282 | 265 | 304 | 320 |
| 340.7–1184.0 mg/day | 654 (411–844) | 820 (522–957) | 783 (466–942) | 702 (341–903) | 707 (465–873) | 624 (399–775) | 534 (380–752) | 524 (376–669) |
| Tertile 3 | 1751 | 157 | 292 | 319 | 336 | 251 | 218 | 178 |
| >1184.0 mg/day | 576 (336–799) | 744 (441–920) | 548 (294–853) | 605 (343–823) | 580 (351–797) | 559 (326–746) | 603 (351–719) | 522 (380–664) |
| p-trend | <0.01 | 0.17 | <0.01 | <0.01 | <0.01 | 0.21 | 0.64 | 0.32 |
| Blood osmolality | ||||||||
| Tertile 1 | 4007 | 510 | 867 | 795 | 747 | 430 | 369 | 289 |
| <276 mmol/Kg | 576 (311–807) | 709 (394–906) | 672 (360–872) | 606 (310–836) | 562 (293–801) | 529 (281–722) | 474 (269–655) | 426 (315–549) |
| Tertile 2 | 2886 | 325 | 475 | 488 | 512 | 461 | 371 | 254 |
| 276–279 mmol/Kg | 669 (409–847) | 842 (517–959) | 771 (508–954) | 752 (481–904) | 677 (422–839) | 574 (364–749) | 588 (354–732) | 512 (366–641) |
| Tertile 3 | 3876 | 218 | 329 | 369 | 461 | 679 | 838 | 982 |
| >279 mmol/Kg | 685 (494–857) | 927 (672–1045) | 848 (624–995) | 794 (561–941) | 799 (619–921) | 660 (449–816) | 632 (441–777) | 572 (444–705) |
| p-trend | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| Diuretics | ||||||||
| Yes | 719 | 1 | 4 | 22 | 84 | 107 | 195 | 306 |
| 553 (395–721) | 557 (557–557) | 771 (492–771) | 725 (461–804) | 731 (486–796) | 647 (459–788) | 524 (358–681) | 491 (380–594) | |
| No | 5091 | 566 | 869 | 839 | 891 | 707 | 645 | 574 |
| 651 (381–845) | 797 (461–946) | 738 (400–927) | 685 (369–875) | 677 (402–842) | 590 (337–767) | 578 (370–757) | 556 (369–695) | |
| p-value | <0.01 | <0.01 | 0.61 | 0.49 | 0.76 | 0.06 | 0.15 | <0.01 |
P-trend across age categories for each dietary factor, hydration indicator, and medication variable are all significant (p<0.001).
Table 2.
Median (interquartile range) urine osmolality concentrations (mOsm/kg) in NHANES 2009–2012 participants >16 years old by medical conditions. The first number for each cell corresponds to the sample size in that category.
| Age categories (years) | All | 16–20 | 21–29 | 30–39 | 40–49 | 50–59 | 60–69 | ≥70 |
|---|---|---|---|---|---|---|---|---|
| Overall | 10769 | 1053 | 1671 | 1652 | 1720 | 1570 | 1578 | 1525 |
| 648 (393–838) | 811(469–946) | 745(444–925) | 709 (392–887) | 678 (391–842) | 614 (370–775) | 591 (375–740) | 532(386–678) | |
| Body mass index | ||||||||
| <25 kg/m2 | 3529 | 620 | 752 | 522 | 452 | 371 | 361 | 451 |
| 580 (321–813) | 808 (462–945) | 683 (336–892) | 629 (293–855) | 558 (286–794) | 442 (280–645) | 532 (317–666) | 506 (367–657) | |
| ≥25 & <30 kg/m2 | 3494 | 244 | 445 | 528 | 589 | 568 | 553 | 567 |
| 636 (392–831) | 842 (465–971) | 750 (472–916) | 702 (428–912) | 674 (378–834) | 614 (349–775) | 578 (350–762) | 524 (373–684) | |
| ≥30 kg/m2 | 3746 | 189 | 474 | 602 | 679 | 631 | 664 | 507 |
| 706 (492–859) | 800 (557–941) | 837 (613–963) | 758 (494–891) | 749 (517–873) | 704 (516–820) | 638 (418–762) | 546 (429–686) | |
| p-value | <0.01 | 0.57 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.05 |
| Hypertension | ||||||||
| Yes | 4318 | — | 236 | 434 | 610 | 802 | 1048 | 1188 |
| 607 (402–780) | — | 743 (530–876) | 750 (460–891) | 695 (422–831) | 632 (422–783) | 586 (392–730) | 511 (377–665) | |
| No | 5398 | — | 1435 | 1218 | 1110 | 768 | 530 | 337 |
| 659 (384–854) | — | 747 (426–933) | 692 (381–885) | 674 (386–845) | 580 (335–768) | 604 (354–760) | 562 (433–727) | |
| p-value | <0.01 | — | 0.98 | 0.11 | 0.28 | 0.18 | 0.91 | <0.01 |
| Diabetes mellitus | ||||||||
| Yes | 1397 | 6 | 28 | 72 | 167 | 280 | 426 | 418 |
| 609 (443–765) | 644 (152–1007) | 705 (576–923) | 754 (474–885) | 745 (465–865) | 645 (455–779) | 604 (403–734) | 545 (409–657) | |
| No | 4470 | 505 | 770 | 751 | 755 | 620 | 527 | 542 |
| 654 (418–836) | 761 (426–938) | 733 (458–914) | 727 (394–884) | 670 (419–831) | 631 (410–773) | 607 (406–754) | 531 (415–676) | |
| p-value | <0.01 | 0.81 | 0.33 | 0.54 | 0.03 | 0.14 | 0.88 | 0.83 |
| CKD | ||||||||
| Yes | 816 | — | 3 | 7 | 18 | 57 | 177 | 554 |
| 520 (391–656) | — | 361 (278–504) | 408 (382–943) | 501 (236–662) | 574 (345–656) | 532 (391–666) | 517 (403–626) | |
| No | 8900 | — | 1668 | 1645 | 1702 | 1513 | 1401 | 971 |
| 652 (390–837) | — | 745 (444–925) | 709 (392–887) | 678 (392–842) | 616 (371–775) | 600 (372–754) | 542 (374–692) | |
| p-value | <0.01 | <0.01 | 0.69 | 0.04 | 0.20 | 0.17 | 0.22 | |
| Albuminuria | ||||||||
| Yes | 1133 | 91 | 73 | 84 | 117 | 152 | 244 | 372 |
| 566 (371–762) | 691 (372–916) | 797 (490–893) | 704 (474–870) | 662 (447–808) | 571 (370–803) | 529 (358–681) | 479 (347–609) | |
| No | 9636 | 962 | 1598 | 1568 | 1603 | 1418 | 1334 | 1153 |
| 655 (396–842) | 819 (473–949) | 743 (443–926) | 709 (388–887) | 678 (390–843) | 614 (370–775) | 600 (375–754) | 545 (405–688) | |
| p-value | <0.01 | 0.34 | 0.72 | 0.61 | 0.72 | 0.83 | 0.16 | <0.01 |
P-trend across age categories for each medical condition are all significant (p<0.001) except among participants with CKD (p-trend=0.54).
Medical conditions including diabetes, hypertension, and albuminuria were not associated with the level of urine osmolality. However, participants with chronic kidney disease (CKD) tended to have lower urine osmolality. Among dietary factors, higher protein intake was significantly associated with higher urine osmolality across all age groups. For daily water intake, urine osmolality was significantly lower only among participants 20 to 59 years of age with higher daily water intake (Table 3).
Effect size comparison: Urine osmolality vs. urine creatinine
In multiple linear regression analysis with urine osmolality as the dependent variable in the original scale among participants with complete data available, independent variables that were statistically significant included age, gender, race/ethnicity, body mass index, daily total protein, water intake, blood osmolality and CKD. Diabetes was not associated with urine osmolality. For urine creatinine concentration in the original scale, the independent variables that were statistically significant included age, gender, race/ethnicity, body mass index, water intake, diabetes and CKD. Daily total protein intake and diuretics were not statistically significant. For CKD, the association with urine osmolality was negative and with urine creatinine it was positive. The estimated coefficients for urine osmolality (in mOsm/kg) and for urine creatinine (in g/l) are summarized in table 4.
Table 4.
Statistical difference of percent change in the influence of common determinants on urine osmolality and urine creatinine.
| Leading influential factors | Percent change in urine osmolality (%) |
Percent change in urine creatinine (%) |
p-value* |
|---|---|---|---|
| Age (per 10 years) | −2.8 (−3.8~−1.8) | −4.9 (−6.6~−3.3) | <0.01 |
| Sex (Male vs. female) | −19.4 (−22.6~−16.1) | −36.8 (−40.8~−32.9) | <0.01 |
| Race | |||
| Non-Hispanic whites | 0 (Ref) | 0 (Ref) | |
| Non-Hispanic blacks | 10.6 (6.2–15.1) | 31.1 (26.3–36.0) | <0.01 |
| Mexican Americans | 7.1 (3.0–11.2) | −1.7 (−7.4–4.1) | <0.01 |
| Others | 2.0 (−0.03–7.2) | −3.4 (−9.8–3.1) | 0.01 |
| Body mass index | |||
| <25 kg/m2 | 0 (Ref) | 0 (Ref) | |
| ≥25 & <30 kg/m2 | 11.0 (6.0–16.0) | 13.5 (6.6–20.5) | 0.15 |
| ≥30 kg/m2 | 21.7 (17.2–26.3) | 26.3 (20.1–32.4) | 0.04 |
| Hypertension (Yes vs. No) | −2.0 (−7.2~3.2) | −2.0 (−8.1~3.9) | 0.96 |
| Diabetes (Yes vs. No) | −1.1 (−4.7~2.4) | −12.6 (−17.7~−7.5) | <0.01 |
| Chronic kidney disease (Yes vs. No) | −5.0 (−9.7~−0.4) | 14.1 (6.5–21.8) | <0.01 |
| Diuretic use (Yes vs. No) | −4.1 (−9.7~1.5) | −1.8 (−11.9~8.2) | 0.43 |
| Protein intake | |||
| 1st tertile | 0 (Ref) | 0 (Ref) | |
| 2nd tertile | 7.3 (1.6–13.0) | 1.1 (−4.8–7.0) | <0.01 |
| 3rd tertile | 12.3 (6.5–18.0) | −1.8 (−10.1–6.4) | <0.01 |
| Water intake | |||
| 1st tertile | 0 (Ref) | 0 (Ref) | |
| 2nd tertile | 3.8 (−1.7–9.3) | 4.2 (−3.1–11.5) | 0.78 |
| 3rd tertile | −12.8 (−18.4~−7.3) | −13.9 (−21.2~−6.6) | 0.49 |
| Blood osmolality | |||
| 1st tertile | 0 (Ref) | 0 (Ref) | |
| 2nd tertile | 18.7 (10.8–26.7) | 15.9 (5.0–26.7) | 0.18 |
| 3rd tertile | 33.3 (26.9–39.6) | 26.2 (18.0–34.4) | <0.01 |
This p-value compared the effect estimates for urine osmolality and urine creatinine. It was estimated based on adjusted Wald test.
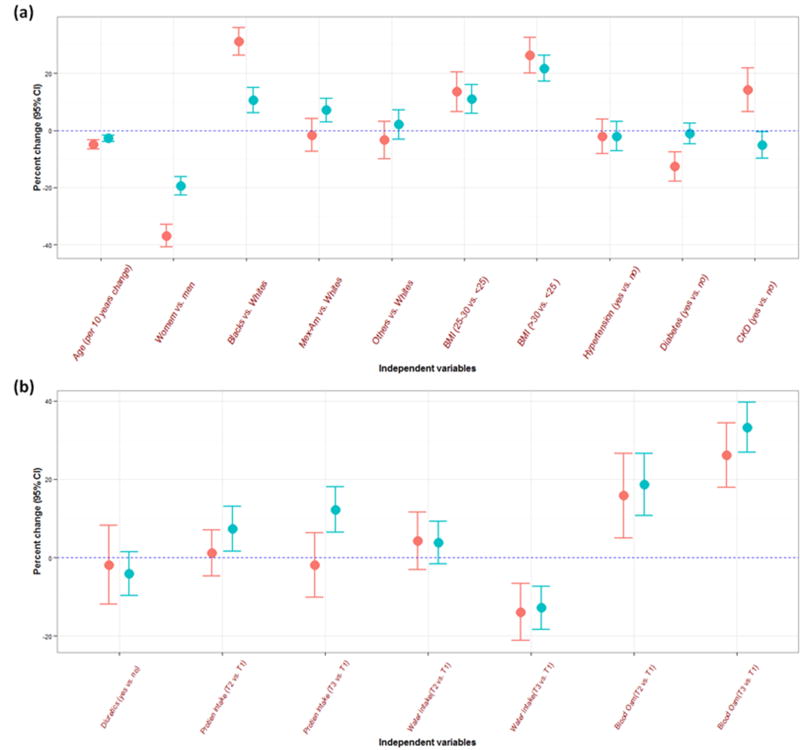
When using log-transformed dependent variables (urine osmolality and urine creatinine) in multiple linear regression modeling, we can evaluate percent change due to unit increase or categorical change in the independent variables (Figure2a and 2b). Compared to urine creatinine, the percent change in urine osmolality was less noticeable in participants that were female, non-Hispanic blacks, obese, diabetic, and with CKD (Table 4).
Figure 2.
Percent change (95% confidence interval) in urine dilution markers (urine creatinine in red and urine osmolality in blue) by participant characteristics. Panel A shows the results for sociodemographic characteristics and comorbidities (NHANES 2009–2012, N=5,867). Panel B shows the results for medication use and dietary intake (NHANES 2009–2010, N=3,023). BMI: body mass index, CKD: chronic kidney disease, T: tertile.
Very dilute and very concentrated urine specimens
The estimated prevalence for very dilute and very concentrated urine was 8.1% and 3.1%, respectively (Table 5). Overall only 88.8% of urine samples in NHANES would be considered adequate specimens based on WHO guidelines for occupational monitoring. Very dilute urine tended to be more prevalent among the elderly, females, and subjects with BMI <25 kg/m2. These factors were associated in the opposite direction with very concentrated urine. Compared to race/ethnicity of participants providing adequate urine samples, very dilute urine was more common among non-Hispanic whites and less common among non-Hispanic blacks and Mexican Americans. Very concentrated urine was more common among non-Hispanic blacks and less common among non-Hispanic whites and Mexican-Americans.
Table 5.
Characteristics of NHANES 2009–2012 participants by adequacy of the collected spot urine sample following the WHO guidelines based on urine creatinine concentrations.
| Over-diluted urine specimen (Urine creatinine <0.3 g/l) N (%) =756 (8.1) |
Adequate urine specimen (urine creatinine ≥0.3 g/l &≤3 g/l) N (%) =9601 (88.8) |
Over-condensed urine specimen (Urine creatinine > 3 g/l) N (%) =412 (3.1) |
p-value | |
|---|---|---|---|---|
| Percentage (%) in Category | ||||
| Male (%) | 26.1 | 51.5 | 69.2 | <0.01 |
| Race (%) | <0.01 | |||
| Non-Hispanic white | 76.7 | 67.4 | 50.3 | |
| Non-Hispanic black | 3.3 | 10.7 | 34.8 | |
| Mexican American | 4.3 | 9.0 | 5.8 | |
| Others | 15.7 | 13.0 | 9.1 | |
| Hypertension (%) | 66.5 | 62.0 | 68.3 | 0.05 |
| Diabetes (%) | 14.3 | 18.8 | 12.6 | 0.08 |
| Chronic kidney disease (%) | 4.2 | 6.3 | 4.2 | 0.01 |
| Diuretics usage (%) | 9.4 (N=405) | 11.1 (N=5193) | 4.2 (N=212) | 0.01 |
| Median (Interquartile range) | ||||
|
| ||||
| Age (year) | 48 (35–59) | 45 (30–59) | 31 (23–46) | <0.01 |
| Body mass index (kg/m2) | 25.3 (22.3–28.9) | 27.5 (23.9–32.0) | 28.7 (24.6–33.1) | <0.01 |
| eGFR (ml/min/1.73m2) | 98.8 (86.5–111.4) | 97.3 (82.3–112.0) | 100.4 (86.6–117.3) | <0.01 |
| Blood glucose (mg/dL) | 96 (89–103) (N=278) | 98 (92–107) (N=4809) | 98 (90–104) (N=171) | 0.004 |
| Fasting time (min) | 721 (637–781) (N=278) | 701 (632–790) (N=4815) | 687 (620–784) (N=171) | 0.83 |
| Urine osmolality (mOsm/kg) | 176 (132–227) | 666 (453–840) | 990 (858–1076) | <0.01 |
| Blood osmolality (mmol/kg) | 276 (273–279) | 278 (275–281) | 278 (275–281) | <0.01 |
| Total protein intake (gm/day) | 73.4 (53.1–94.8) (N=384) | 77.6 (55.2–104.1) (N=5023) | 73.7 (49.0–104.5) (N=202) | 0.11 |
| Total water intake (gm/day) | 948 (296.3–1866.4) (N=384) | 681.4 (177.8–1481.3) (N=5023) | 414.8 (0–1184) (N=202) | <0.01 |
Median (interquartile range) and frequencies (percentages) are presented for continuous and categorical variables, respectively. P-values were derived from ANOVA and chi-square test for continuous and categorical variables, respectively.
Abbreviation: eGFR, estimated glomerular filtration rate
The sample sizes for diuretic use and nutrient and water intake were smaller as the information was only available for participants in NAHNES 2009–2010 at the time of analysis.
Subjects who provided either very dilute or very concentrated urine were less likely to have CKD (Table 5). Blood glucose and blood osmolality were significantly lower among participants with very dilute urine. Compared to adequate urine samples, urine osmolality was 176 mOsm/kg and 990 mOsm in very dilute and very concentrated samples, respectively. On average, total daily plain water intake in participants with very dilute urine was 267 gram greater than participants providing adequate urine specimens and 533.2 gram greater than those providing over-condensed urine samples.
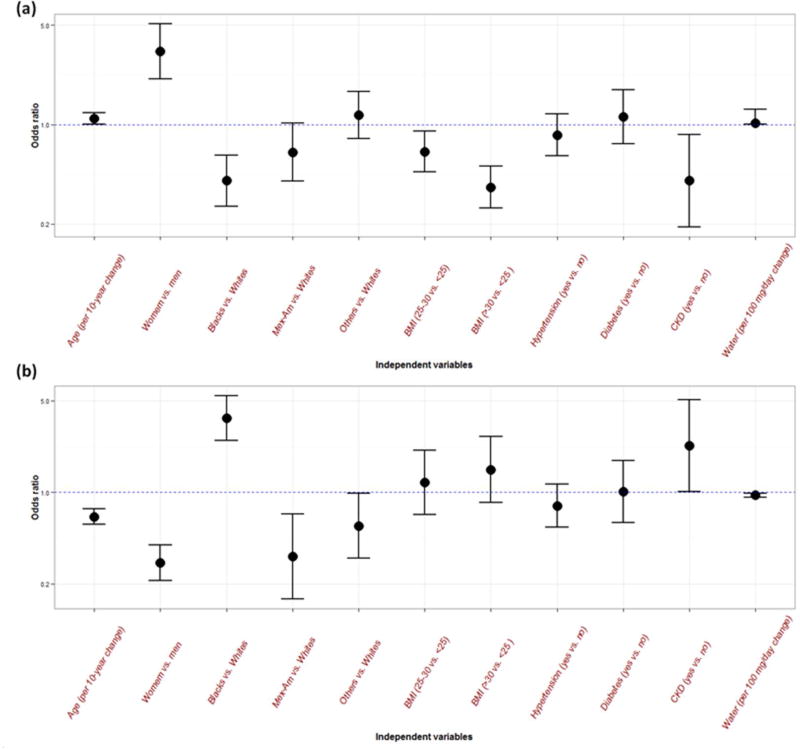
The adjusted odds ratios (95% CI) of urine over-dilution were 1.10 (1.00–1.21) for every 10-year increase in age, 3.27 (2.10–5.10) for female versus male, 0.40 (0.27–0.61) for non-Hispanic blacks versus non-Hispanic whites, 0.36 (0.26–0.51) for obese participants versus normal BMI, 0.40 (0.19–0.85) for CKD versus non-CKD, and 1.02 (1.00–1.05) for every 100 gram increase in daily total plain water intake (Figure 3a). The adjusted odds ratios (95% CI) of providing over-condensed urine specimens were 0.65 (0.570.75) every 10-year increase in age, 0.29 (0.21–0.40) for female versus male, 3.69 (2.50–5.45) for non-Hispanic blacks versus non-Hispanic whites, 1.49 (0.84–2.66) for obese participants versus normal BMI, 2.27 (1.01–5.10) for CKD versus non-CKD, and 0.95 (0.92–0.98) for every 100 gram increase in daily total plain water intake (Figure 3b).
Figure 3.
Odds ratios adjusted for all other variables for urine over-dilution in the figure (a) and for urine over-condensation (b) with 95% confidence interval by demographics, comorbidities, and daily water intake.
Discussion
To our knowledge, this is the first large scale population-based study to characterize urine osmolality and explore predisposing factors that influence spot urine samples that are very dilute and very concentrated. The current analyses showed that urine osmolality was associated with age, gender, race, body mass index, chronic kidney disease status, daily total protein intake, plain water intake, and blood osmolality. Our study also found that older age, female gender, non-Hispanic white race/ethnicity and increased water intake were associated with higher likelihood of having a urine sample that was very dilute. In contrast, younger age, male gender, non-Hispanic blacks, CKD, and decreased water intake were associated with urine samples that were very concentrated. The influence of demographic factors and medical conditions on urine osmolality was less dramatic compared to the associations between those factors and urine creatinine. However, urine osmolality was more strongly associated with daily protein intake.
The success of using urine creatinine to standardize spot urine albumin concentration in clinical medicine makes it a widely adopted method in urine dilution correction in environmental biomonitoring. However, the biosynthesis and degradation of creatinine is tightly coupled with creatine metabolism, which mainly depends on the mass of muscular tissues, dietary creatine input, the synthesis capacity of the liver, and overall kidney function (Wyss and Kaddurah-Daouk, 2000). The kidney plays a dominant role in creatinine excretion. In addition to being filtered by glomerulus, creatinine is actively secreted into the proximal tubule adding another process through which its concentration in urine can be affected (Breyer and Qi, 2010; Ciarimboli et al., 2012). Urine creatinine concentrations are, therefore, very different depending on age, gender, race/ethnicity, body mass index, medical conditions including diabetes and kidney disease, and nutritional condition (Barr et al., 2005). This raises concern about the validity of using urine creatinine to standardize spot urine toxicant concentration, particularly at low exposure levels, most common among the general population. Some researchers suggest that urine specific gravity may perform better than urine creatinine based on limited evidence, mostly cross-sectional and descriptive in design and lacking a reference 24-hour urine specimen for measurement comparison (Ikeda et al., 2003; Nermell et al., 2008; Suwazono et al., 2005; Yassine et al., 2012). Specific gravity is the ratio of the density of urine compared to distilled water and is commonly measured in urine to approximate osmolality (Fogazzi, 2005). In general, urine osmolality is more accurate than urine specific gravity to quantify urine concentration and there is generally a good correlation between them (Cook et al., 2000). Urine osmolality is also less interfered by glucosuria and/or albuminuria (Fogazzi, 2005).
The performance and validity of urine osmolality adjustment in environmental biomonitoring has not been evaluated, although a few studies have supported that albumin-to-osmolality ratio or protein-to-osmolality ratio correlates closely with 24-hour measurements (Gyamlani et al., 2003; Kim et al., 2001; Morgenstern et al., 2003). In our study, we found that the variation in urine osmolality related to socio-demographic background and medical conditions was less dramatic compared to urine creatinine. However, urine osmolality varied significantly by daily total protein intake. In addition, urine osmolality and urine creatinine were associated in opposite directions in participants with and without CKD. The higher urine creatinine concentrations in CKD patients is likely reflective of higher plasma concentrations and can be explained by hyperfiltrated remnant nephrons and compensatory increases in proximal tubular secretion when blood creatinine concentration reaches a higher steady state (Breyer and Qi, 2010; Ciarimboli et al., 2012). Urine concentrating ability declines in the early stages of CKD; therefore, urine osmolality in participants with CKD is lower compared to that of participants with normal renal function (Combs and Berl, 2014). This finding has important implications on studying CKD in environmental science because the impact of CKD on urine creatinine and urine osmolality may introduce differential measurement error when we apply osmolality- or creatinine-based adjustment, especially in cross-sectional studies. It is not possible to conclude whether urine osmolality adjustment is more appropriate than urine creatinine according to our findings. More systematic research using 24-hour urine samples is necessary to assess the validity of spot urine osmolality adjustment in environmental biomonitoring.
Adequate urine sampling is the cornerstone to minimize information bias in both environmental biomonitoring and clinical studies. In occupational monitoring, urine samples with urine creatinine less than 0.3 g/l and higher than 3 g/l are classified as very dilute and very concentrated urine specimens, respectively (World Health Organization (WHO), 1996). Nevertheless, the definitions of very dilute and concentrated urine remain under debate and, currently, there are no cut-offs for extremes of urine concentration based on urine osmolality in environmental biomonitoring. For instance, for urine drug screens in the health care and criminal justice systems, urine samples with urine creatinine levels of less than 0.2g/l are considered very dilute (Moeller et al., 2008). To be consistent with WHO, urine creatinine of 0.3 g/l was used as a cut-off to define very dilute urine in this study. The prevalence of very dilute urine was 8.0% in NHANES III (Barr et al., 2005) and 8.1% in NHANES 2009–2012 samples. Our study showed that participants with female gender, non-Hispanic whites, BMI less than 25 kg/m2, and greater daily water intake were more likely to provide very dilute urine specimens. In contrast, factors including younger age, male gender, non-Hispanic blacks, CKD status, and less daily water intake were associated with very concentrated urine specimens. Whether different cut-offs should be applied to these specific subgroups remain controversial (e.g., age- or sex-dependent cut-offs). However, the only external factor that could be practically modified is water intake. In population-based research, participants are commonly asked to fast to analyze glucose and lipids. Generally, drinking water during the fasting period is not restricted, as it does not affect the blood test results. This is because the stability of blood concentration is tightly regulated through adjusting the amount of water in the urine. Therefore, this practice may introduce non-random variation into measurements of urine concentration. Prescripted water intake before urine sampling during fasting period may be considered to improve urine adequacy. More studies are needed to evaluate the performance and applicability of this approach and the amount of water that should be recommended.
Some limitations of our study should be considered. First, we had no information on some factors that are important for urine osmolality such as sodium concentrations in urine. Second, despite the biological plausibility and the efforts to control for confounding variables (e.g., body mass index), the study is cross-sectional and it only allows the investigation of associations, not causation, of host factors (e.g., CKD) with urine osmolality and creatinine. Third, although this study benefits from standardized measurements from a broad sample of the general population, the findings may not generalize to populations of different socioeconomic backgrounds, nutrition status, and medical status. For instance, as the study was conducted in a general non-institutionalized population, individuals with serious medical conditions were less likely to be recruited into the study. Fourth, urine creatinine or osmolality were measured in random urine samples (first sample in mobile examination center (MEC) at a single time point (spot sample) or timed (pooled up to three urine samples) urine collected in the MEC without information of water intake amount during the fasting period before biospecimen collection. Also, no 24-hour urine samples were available. Within-person variation in urine osmolality and creatinine measurements, and likewise variations in the measurements of urine determinants (e.g., water intake), could result in non-differential misclassification and might bias the observation towards null (Thomas et al., 1993). Further study is warranted to validate the current findings, especially the timing and frequency of urine collections, in larger datasets or cohort studies/clinical trials.
Conclusion
In this analysis of the US population, the characteristics of urine osmolality were generally similar to urine creatinine. The relative influence of socio-demographic and medical conditions on urine osmolality was generally less dramatic than on urine creatinine. However, urine osmolality differed significantly with total protein intake in contrast to urine creatinine. Given the known limitations of urine creatinine, the validity of urine osmolality adjustment in environmental biomonitoring is worth further investigation. As CKD status differentially affect levels of urine creatinine and osmolality, creatinine- or osmolality-based adjustment strategy should be cautiously used to determine internal dose in participants with CKD. Avoiding excessive and too limited water intake before urine sampling may improve the adequacy of spot urine samples. More research is needed to understand the usefulness of urine osmolality to adjust for urine dilution and to develop an appropriate urine collection protocol for environmental biomonitoring in general populations.
Footnotes
Potential conflicts of interest: All authors: no conflicts.
References
- Barr DB, et al. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect. 2005;113:192–200. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt TM, et al. Albumin excretion as a measure of glomerular dysfunction in children. Arch Dis Child. 1970;45:496–501. doi: 10.1136/adc.45.242.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breyer MD, Qi Z. Better nephrology for mice--and man. Kidney Int. 2010;77:487–9. doi: 10.1038/ki.2009.544. [DOI] [PubMed] [Google Scholar]
- Ciarimboli G, et al. Proximal tubular secretion of creatinine by organic cation transporter OCT2 in cancer patients. Clin Cancer Res. 2012;18:1101–8. doi: 10.1158/1078-0432.CCR-11-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs S, Berl T. Dysnatremias in patients with kidney disease. Am J Kidney Dis. 2014;63:294–303. doi: 10.1053/j.ajkd.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JD, et al. The characterization of human urine for specimen validity determination in workplace drug testing: a review. J Anal Toxicol. 2000;24:579–88. doi: 10.1093/jat/24.7.579. [DOI] [PubMed] [Google Scholar]
- Curria A. Refrigerated and room temperature storage stability of urine osmolality measurements. Advanced Instruments, Inc; Norwood, MA: 2011. [Google Scholar]
- Dwyer J, et al. Collection of food and dietary supplement intake data: What We Eat in America-NHANES. J Nutr. 2003;133:590S–600S. doi: 10.1093/jn/133.2.590S. [DOI] [PubMed] [Google Scholar]
- Elkins HB. Exposure tests in industrial toxicology. Pure Appl Chem. 1969;18:143–50. doi: 10.1351/pac196918010143. [DOI] [PubMed] [Google Scholar]
- Elkins HB, Pagnotto LD. Is the 24-hour urine sample a fallacy? Am Ind Hyg Assoc J. 1965;26:456–60. doi: 10.1080/00028896509342757. [DOI] [PubMed] [Google Scholar]
- Fogazzi G. Urinalysis and microscopy. In: Davison A, et al., editors. Oxford Textbook of Clinical Nephrology. Oxford University Press; New York: 2005. pp. 23–46. [Google Scholar]
- Gyamlani GG, et al. Urinary albumin to osmolality ratio predicts 24-hour urine albumin excretion in diabetes mellitus. Am J Kidney Dis. 2003;42:685–92. doi: 10.1016/s0272-6386(03)00830-8. [DOI] [PubMed] [Google Scholar]
- Hall MN, Gamble MV. Nutritional manipulation of one-carbon metabolism: effects on arsenic methylation and toxicity. J Toxicol. 2012;2012:595307. doi: 10.1155/2012/595307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M, et al. Bias induced by the use of creatinine-corrected values in evaluation of beta2-microgloblin levels. Toxicol Lett. 2003;145:197–207. doi: 10.1016/s0378-4274(03)00320-5. [DOI] [PubMed] [Google Scholar]
- Kim HS, et al. Quantification of proteinuria in children using the urinary protein-osmolality ratio. Pediatr Nephrol. 2001;16:73–6. doi: 10.1007/s004670000486. [DOI] [PubMed] [Google Scholar]
- Levey AS, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YS, et al. Air samples versus biomarkers for epidemiology. Occup Environ Med. 2005;62:750–60. doi: 10.1136/oem.2004.013102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory WW, et al. Estimation of severity of the nephrotic syndrome in childhood as a guide to therapy and prognosis. Pediatrics. 1959;23:861–73. [PubMed] [Google Scholar]
- Moeller KE, et al. Urine drug screening: practical guide for clinicians. Mayo Clin Proc. 2008;83:66–76. doi: 10.4065/83.1.66. [DOI] [PubMed] [Google Scholar]
- Morgenstern BZ, et al. Validity of protein-osmolality versus protein-creatinine ratios in the estimation of quantitative proteinuria from random samples of urine in children. Am J Kidney Dis. 2003;41:760–6. doi: 10.1016/s0272-6386(03)00023-4. [DOI] [PubMed] [Google Scholar]
- National Health and Nutrition Examination Survey. 2009 – 2010 Data Documentation, Codebook, and Frequencies: Prescription Medications (RXQ_RX_F) 2012;2014 [Google Scholar]
- National Health and Nutrition Examination Survey (NHANES) Analytic guidelines. Vol. 2014. Centers for Disease Control and Prevention; Atlanta, GA: 2013. [Google Scholar]
- Navas-Acien A, et al. Metals in urine and peripheral arterial disease. Environ Health Perspect. 2005;113:164–9. doi: 10.1289/ehp.7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nermell B, et al. Urinary arsenic concentration adjustment factors and malnutrition. Environ Res. 2008;106:212–8. doi: 10.1016/j.envres.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Shelley R, et al. Uranium associations with kidney outcomes vary by urine concentration adjustment method. J Expo Sci Environ Epidemiol. 2014;24:58–64. doi: 10.1038/jes.2013.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieniawska CE, et al. Twenty-four-hour urinary trace element excretion: reference intervals and interpretive issues. Ann Clin Biochem. 2012;49:341–51. doi: 10.1258/acb.2011.011179. [DOI] [PubMed] [Google Scholar]
- Suwazono Y, et al. Creatinine versus specific gravity-adjusted urinary cadmium concentrations. Biomarkers. 2005;10:117–26. doi: 10.1080/13547500500159001. [DOI] [PubMed] [Google Scholar]
- The National Health and Nutrition Examination Survey (NHANES) Laboratory Procedure Manual: Urine osmolality. The Centers for Disease Control (CDC); 2010. [Google Scholar]
- Thomas D, et al. Exposure measurement error: influence on exposure-disease. Relationships and methods of correction. Annu Rev Public Health. 1993;14:69–93. doi: 10.1146/annurev.pu.14.050193.000441. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) Biological monitoring of chemical exposure in the workplace. Vol. 1. Geneva: World Health Organization; 1996. pp. 20–41. [Google Scholar]
- Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80:1107–213. doi: 10.1152/physrev.2000.80.3.1107. [DOI] [PubMed] [Google Scholar]
- Yassine H, et al. Adjusting for Urinary Creatinine Overestimates Arsenic Concentrations in Diabetics. Cardiorenal Med. 2012;2:26–32. doi: 10.1159/000334225. [DOI] [PMC free article] [PubMed] [Google Scholar]