Abstract
Esophageal atresia (EA) is a relatively uncommon congenital anomaly, often observed in conjunction with tracheoesophageal fistula (TEF). Surgical repair in neonates typically takes place with little information about the pre-existing EA/TEF structure because there are currently no acceptable tools for evaluating EA/TEF anatomy prior to repair; chest x-ray radiograph does not identify malformation sub-type or gap length, while x-ray computed tomography (CT) demonstrate an unacceptably high exposure to ionizing radiation. There is a need for safe imaging methods to evaluate pre-operative EA/TEF anatomy, which would add value in surgical planning; this need may be met with high-resolution structural MRI.
We report three cases of Type-C EA/TEF in neonates. Patients were imaged prior to surgical repair using high-resolution ultrashort echo time (UTE) magnetic resonance imaging (MRI) to visualize tracheoesophageal anatomy and allow for informed surgical planning and risk management. One of the three patients was imaged post-repair to evaluate surgical efficacy and evolution of the tracheoesophageal anatomy.
Keywords: esophageal atresia, tracheoesophageal fistula, neonatal MRI, surgical planning
1. INTRODUCTION
Esophageal atresia (EA) is a relatively uncommon congenital anomaly that affects approximately 1 in 2500 to 4000 live births [1]. EA is most often observed in conjunction with a tracheal esophageal fistula (TEF), typically between the trachea and distal esophageal pouch (Type-C); other classification subtypes include Type-A (EA without TEF), Type-B (EA with proximal TEF), Type-D (EA with proximal and distal TEF), and Type-E (TEF without EA) [2–3]. EA/TEF is considered a surgical urgency, involving ligation of the fistula and anastomosis of the esophagus. Repair is required to avoid aspiration, reflux, pneumonitis, gaseous-distension of the stomach, and ultimately to establish a normal feeding mechanism [4].
Currently, there are no acceptable tools for evaluating EA/TEF anatomy prior to repair. In most patients, the EA/TEF diagnosis is confirmed only with a plain chest x-ray showing a coiled feeding tube within the upper esophageal pouch. Importantly, this approach to “confirmation” cannot determine the anatomic subtype of EA/TEF, the number or location of TEFs, the size of the gap between proximal and distal esophagus, or the presence of tracheomalacia. A few studies have evaluated computerized tomography (CT) scanning in pre-surgical evaluation of EA/TEF and found that CT could frequently identify the origin of the fistula and the size of the EA gap [5–6]. Although these studies suggested a benefit from preoperative CT scans improving surgical planning in 40% of cases, they also demonstrated a significant and unacceptably high exposure to ionizing radiation. Thus, there is a need for safe, noninvasive, non-ionizing imaging methods for visualizing and assessing pre-surgical EA/TEF anatomy, which would add value in surgical planning; this need may be met with high-resolution structural MRI. Such imaging may help identify infants at risk for complications and offer clinical teams an opportunity to reduce these risks. Additionally, post-operative imaging may provide an opportunity to evaluate surgical efficacy and developing function during post-surgical healing within the repaired anatomy.
2. DESCRIPTION OF CASE REPORTS
2.1. Case 1
A 38 3/7 week gestation male neonate (birth weight = 2520 g) presented to Cincinnati Children’s Hospital on day of life (DOL) 0 with cleft palate, increased secretions, and an inability to pass nasal gastric tube. Family history was significant with a sibling also diagnosed with EA/TEF and Pierre Robin sequence. A chest radiograph on DOL 0 demonstrated coiling of the feeding tube in the upper esophagus, suggesting EA with possible TEF; the chest radiograph also suggested a long proximal esophagus and a short esophageal gap length (Figure 1A).
Figure 1.
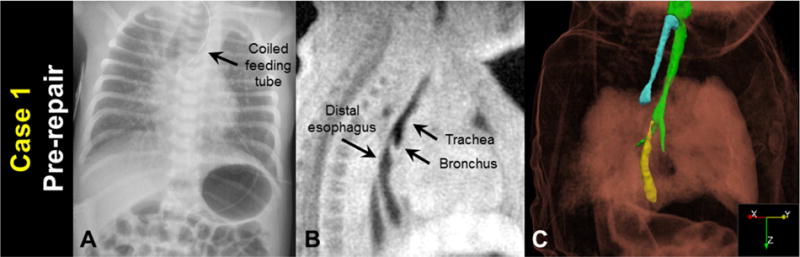
Case 1 before surgical repair: A) a clinical chest radiograph, demonstrating a coiled feeding tube; B) a sagittal MRI slice (3D isotropic resolution of 0.7 mm); and C) oblique view of a MRI-based 3D surface rendering of EA/TEF anatomy (airways in green, proximal esophagus in cyan, distal esophagus in yellow), shown within a volume rendering of the body MR image.
The patient was imaged on DOL 1 on a 1.5 tesla neonatal-sized MRI scanner [7–9] on room air without sedation using an ultrashort echo time (UTE) sequence [10–12] under an IRB-approved research protocol and with informed parental consent. Images had a 3D isotropic resolution of 0.7 mm (Figure 1B). Tracheoesophageal anatomy was semi-automatically segmented from UTE MR images (ITK-SNAP 3.6.0, 3D structure segmentation, University of Pennsylvania and University of Utah [13]), and 3D surface renderings of tracheoesophageal anatomy were visualized with volume-rendered MR images (ParaView 5.4.0, data analysis and visualization) (Figure 1C). MRI results indicated the patient had a Type-C EA/TEF with an esophageal gap length of approximately 1 cm, consistent with the chest radiograph. MRI also indicated a long, narrow fistula connecting the distal trachea to a caudally displaced distal esophagus, potentially increasing the effective esophageal gap length and increasing the surgical difficulty in connecting the distal and proximal esophagus. MRI also demonstrated a flattening of the trachea where it crosses the innominate artery, suggestive of a degree of tracheomalacia.
Due to the expected repair difficulty presented by the long, narrow fistula observed on MRI imaging, the surgical plan was changed from thoracoscopic to open approach, and the EA/TEF was repaired on DOL 2 by ligation of the TEF and primary anastomosis under mild tension. Bronchoscopy at the time of repair confirmed a TEF originating immediately proximal to the carina. The patient remained hospitalized until 3 months of age due to complications associated with his Pierre Robin sequence and his gastric tube. At the time of discharge he had feeding problems related to micrognathia, but no esophageal leak or esophageal stricture.
2.2. Case 2
A 38 3/7 week gestation female neonate (birth weight = 2450 g) presented at 0 DOL with respiratory distress, imperforate anus, and inability to pass a nasal gastric catheter past 10 cm. Radiograph demonstrated the catheter in the upper esophagus suggesting EA/TEF (Figure 2A).
Figure 2.
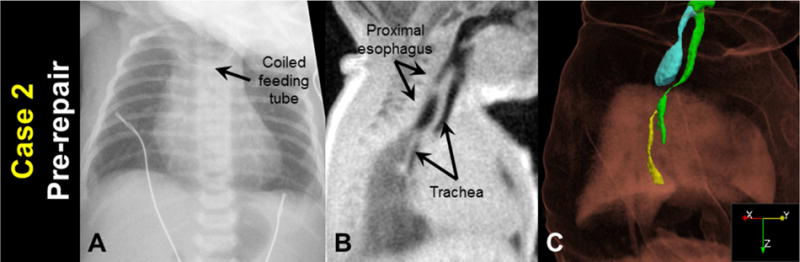
Case 2 before surgical repair: A) a clinical chest radiograph, demonstrating a coiled feeding tube; B) a sagittal MRI slice (3D isotropic resolution of 0.7 mm); and C) oblique view of a MRI-based 3D surface rendering of EA/TEF anatomy (airways in green, proximal esophagus in cyan, distal esophagus in yellow), shown within a volume rendering of the body MR image.
MRI was performed on DOL 1 with parameters and image processing identical to Case 1 (Figure 2B and 2C). MRI results indicated the patient had a Type-C EA/TEF with an esophageal gap length of approximately 2 cm. Furthermore, MRI results indicated that the trachea curved posteriorly below the large proximal esophageal pouch, and a significant narrowing of this curved tracheal section (approximately 1 mm2 cross-sectional area) was indicative of severe static tracheomalacia. Bronchoscopy at the time of repair confirmed curved tracheal anatomy and significant tracheomalacia.
Open repair on DOL 2 demonstrated an esophageal gap of approximately 2.5 cm. The TEF was ligated but primary esophageal anastomosis was not possible due to tension and the long esophageal gap. EA was repaired on DOL 47. The post-surgical clinical course was complicated by esophageal anastomotic leak, an esophageal stricture requiring multiple dilations, mechanical ventilation for the first 5 months of life, and two operations to repair tracheal defects that were noted on MRI and bronchoscopy.
2.3. Case 3
A 37 0/7 week gestation female neonate (birth weight = 3260 g) presented at 5 DOL with poor feeding and 600 g weight loss since birth. Chest radiograph on DOL 5 demonstrated coiling of the feeding tube in the upper esophagus, suggesting EA/TEF (Figure 3A). Bronchoscopy prior to repair indicated a Type-C EA/TEF. MRI was performed on DOL 6 with parameters and image processing identical to Case 1 (Figure 3B and 3C). MRI results also indicated the patient had a Type-C EA/TEF with a short esophageal gap length of approximately 1 cm.
Figure 3.
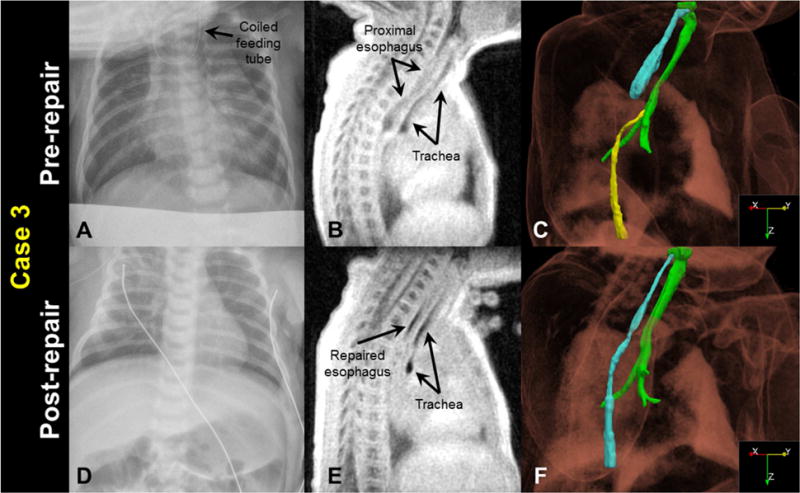
Case 3 before and after surgical repair: A&D) a clinical chest radiograph, demonstrating a coiled feeding tube prior to repair; B&E) a sagittal MRI slice (3D isotropic resolution of 0.7 mm); and C&F) oblique view of a MRI-based 3D surface rendering of EA/TEF anatomy (airways in green, pre-repair proximal esophagus in cyan, pre-repair distal esophagus in yellow, and post-repair esophagus in cyan), shown within a volume rendering of the body MR image. Note the post-repair collapse of the trachea, indicating some degree of tracheomalacia.
Open repair of the EA/TEF was performed on DOL 9. MRI at 12 days after repair (DOL 21) (Figure 3E and 3F) demonstrated an intact esophageal repair with a non-dilated proximal esophagus, and revealed a compression of the upper intrathoracic trachea by the innominate vein anteriorly and the proximal esophagus posteriorly, compatible with a degree of tracheomalacia. There were no detectable esophageal leaks or strictures prior to discharge, but the infant did develop an esophageal stricture at 2 months of life that required dilation. Despite the tracheomalacia observed on MRI, respiratory support was not required, and the infant was weaned to room air 5 days after surgery.
3. DISCUSSION
To our knowledge, this is the first visualization and assessment of pre- and post-repair EA/TEF anatomy via MRI. This non-invasive, non-ionizing imaging technique does not require sedation/anesthesia and provides valuable structural information for both pre-operative planning and post-operative evaluation of surgical efficacy and subsequent evolution of the tracheoesophageal anatomy.
In Case 1, chest radiograph suggested a short esophageal gap length, but MRI indicated the effective gap length would be longer due to the caudally displaced distal esophagus and long, narrow fistula. Primary repair was completed in this patient, but the esophageal anastomosis was under mild tension. In Case 2, MRI demonstrated a long esophageal gap and also significant tracheomalacia. Subsequent bronchoscopy and direct surgical visualization confirmed these findings, and the patient experienced multiple esophageal and tracheal complications. In Case 3, MRI clearly demonstrated a short esophageal gap and as a result, the clinical team and parents prepared for a primary esophageal repair. Post-repair imaging in Case 3 suggested an intact repair and, importantly, revealed a mild to moderate degree of tracheomalacia that will require long-term monitoring.
This technique is novel in its use of UTE MRI to assess EA/TEF structures. MRI is ideal for this application due to its tomographic imaging results, without requiring ionizing radiation. Further, UTE MRI is robust to motion due to its radial k-space acquisition scheme (9), and retrospective UTE MRI image reconstruction allows for discarding of image data acquired during periods of patient non-compliance (10), which obviates the need for sedation/anesthesia in a free-breathing neonate. The primary limitation of this MRI application is similar to that of x-ray CT — that the esophagus with a closed lumen can be challenging to segment from surrounding tissue.
This approach has three potential benefits. First, the pre-operative structural information provided by 3D MRI renderings allow for surgical planning with detail not provided by chest radiograph, and not possible via CT without significant ionizing radiation and sedation/anesthesia. Second, this approach allows for improved parental counseling with more thorough information on the infant’s condition and potential surgical recovery. Finally, the ability to image patients both before and after repair provides the potential to evaluate surgical efficacy and to identify infants at risk for other complications, such as tracheomalacia and esophageal stricture.
In conclusion, structural MRI can be safely and effectively used to visualize and evaluate the pre- and post-surgical anatomy of neonatal EA/TEF. Ultimately, larger studies are needed to determine the validity and benefit of structural MRI in the evaluation of EA/TEF, but this case series suggests that this approach has strong potential.
4. CONCLUSION
In cases of suspected neonatal EA/TEF, structural MRI is a safe, non-ionizing, non-sedated imaging method that allows for improved pre-operative planning, parental counseling, and post-operative evaluation of repair efficacy.
HIGHLIGHTS.
Esophageal atresia/tracheal esophageal fistula is a rare congenital anomaly.
Structural MRI allows for improved surgical planning and parental counseling.
Detailed anatomical MRI may identify infants at risk for EA/TEF complications.
UTE MRI provides high-quality images without sedation or ionizing radiation.
Acknowledgments
GRANT SUPPORT
This work was supported by The Perinatal Institute at Cincinnati Children’s Hospital Medical Center and the NIH (P01 HL070831). Sources of financial support had no involvement in conducting of this research or preparation of this article.
ABBREVIATIONS
- CT
x-ray computed tomography
- DOL
day of life
- EA/TEF
esophageal atresia/tracheoesophageal fistula
- MRI
magnetic resonance imaging
- UTE
ultrashort echo time
References
- 1.Sfeir R, Michaud L, Salleron J, Gottrand F. Epidemiology of esophageal atresia. Dis Esophagus. 2013;26(4):354–355. doi: 10.1111/dote.12051. [DOI] [PubMed] [Google Scholar]
- 2.Spitz L. Oesophageal atresia. Orphanet J Rare Dis. 2007 May 11;2:24. doi: 10.1186/1750-1172-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gross RE. The surgery of infancy and childhood. WB Saunders; 1953. [Google Scholar]
- 4.Gupta A, Gupta N. Ineffective Ventilation in A Neonate with A Large Pre-Carinal Tracheoesophageal Fistula and Bilateral Pneumonitis-Microcuff Endotracheal Tube to Our Rescue! J Neonatal Surg. 2017;6(1):14. doi: 10.21699/jns.v6i1.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahalik SK, Sodhi KS, Narasimhan KL, Rao KL. Role of preoperative 3D CT reconstruction for evaluation of patients with esophageal atresia and tracheoesophageal fistula. Pediatr Surg Int. 2012;28(10):961–966. doi: 10.1007/s00383-012-3111-9. [DOI] [PubMed] [Google Scholar]
- 6.Ngerncham M, Lee EY, Zurakowski D, Tracy DA, Jennings R. Tracheobronchomalacia in pediatric patients with esophageal atresia: comparison of diagnostic laryngoscopy/bronchoscopy and dynamic airway multidetector computed tomography. J Pediatr Surg. 2015;50(3):402–407. doi: 10.1016/j.jpedsurg.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 7.Tkach JA, Hillman NH, Jobe AH, et al. An MRI system for imaging neonates in the NICU: initial feasibility study. Pediatr Radiol. 2012;42(11):1347–56. doi: 10.1007/s00247-012-2444-9. [DOI] [PubMed] [Google Scholar]
- 8.Tkach JA, Merhar SL, Kline-Fath BM, et al. MRI in the neonatal ICU: initial experience using a small-footprint 1.5-T system. AJR Am J Roentgenol. 2014;202(1):W95–W105. doi: 10.2214/AJR.13.10613. [DOI] [PubMed] [Google Scholar]
- 9.Merhar SL, Tkach JA, Woods JC, South AP, Wiland EL, Rattan MS, Dumoulin CL, Kline-Fath BM. Neonatal imaging using an on-site small footprint MR scanner. Pediatr Radiol. 2017;47(8):1001–1011. doi: 10.1007/s00247-017-3855-4. [DOI] [PubMed] [Google Scholar]
- 10.Hahn AD, Higano NS, Walkup LL, et al. Pulmonary MRI of neonates in the intensive care unit using 3D ultrashort echo time and a small footprint MRI system. J Magn Reson Imaging. 2017;45(2):463–471. doi: 10.1002/jmri.25394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higano NS, Hahn AD, Tkach JA, et al. Retrospective respiratory self-gating and removal of bulk motion in pulmonary UTE MRI of neonates and adults. Magn Reson Med. 2017;77(3):1284–1295. doi: 10.1002/mrm.26212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higano NS, Fleck RJ, Spielberg DR, et al. Quantification of neonatal lung parenchymal density via ultrashort echo time MRI with comparison to CT. J Magn Reson Imaging. 2017 Feb 3; doi: 10.1002/jmri.25643. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage. 2006;31(3):1116–28. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]


