Abstract
Müller cells are one of the primary glial cell types found in the retina and play a significant role in maintaining retinal function and health. Since Müller cells are the only cell type to span the entire width of the retina and have contact to almost every cell type in the retina they are uniquely positioned to perform a wide variety of functions necessary to maintaining retinal homeostasis. In the healthy retina, Müller cells recycle neurotransmitters, prevent glutamate toxicity, redistribute ions by spatial buffering, participate in the retinoid cycle, and regulate nutrient supplies by multiple mechanisms. Any disturbance to the retinal environment is going to influence proper Müller cell function and well being which in turn will affect the entire retina. This is evident in a disease like diabetic retinopathy where Müller cells contribute to neuronal dysfunction, the production of pro-angiogenic factors leading to neovascularization, the set up of a chronic inflammatory retinal environment, and eventual cell death. In this review, we highlight the importance of Müller cells in maintaining a healthy and functioning retina and discuss various pathological events of diabetic retinopathy in which Müller cells seem to play a crucial role. The beneficial and detrimental effects of cytokine and growth factor production by Müller cells on the microvasculature and retinal neuronal tissue will be outlined. Understanding Müller cell functions within the retina and restoring such function in diabetic retinopathy should become a cornerstone for developing effective therapies to treat diabetic retinopathy.
Keywords: Müller cells, retinal inflammation, cell death, diabetic retinopathy
Introduction
Müller cells are the principle glia of the retina. They are the only cells to span the entire width of the retina and have intimate contact with both the retinal blood vessels and retinal neurons. Because of this arrangement, Müller cells have a variety of important functions in the healthy retina. Functions of Müller cells can be divided into 3 major categories: (1) Uptake and recycling of neurotransmitters, retinoic acid compounds, and ions (such as potassium K+), (2) control of metabolism and supply of nutrients for the retina, and (3) regulation of blood flow and maintenance of the blood retinal barrier.
The extensive contact of Müller cells with retinal neurons allows Müller cells to actively participate in proper neurotransmission. They rapidly take up and clear glutamate and γ-aminobutryic acid (GABA) in the inner plexiform layer[1–4]. Studies have shown that Müller cells take up extracellular glutamate through the Glutamate Aspartate Transporter (GLAST) and indicate that glutamate removal and prevention of neurotoxicity in the retina is achieved primarily by this mechanism[5,6]. Once taken up, glutamate is converted to glutamine by glutamine synthetase and released back to neurons for re-synthesis of glutamate and GABA[7]. This process provides substrate for neurotransmitter synthesis and also prevents glutamate toxicity. Müller cells further maintain proper retinal function by participating in a process known as “potassium spatial buffering”, a process that redistributes and normalizes K+ in the surrounding microenvironment to avoid prolonged accumulation of K+[8]. It has been shown that Müller cells can take up K+ from the inner and outer plexiform layers where neuronal synapses occur and release the K+ into the vitreous humor in an effort to redistribute K+ ions[9]. This process is also involved in retinal fluid removal. Müller cells act as potassium shuttle by taking up potassium from the extracellular fluid through Kir2.1 potassium channels and depositing the potassium into the vasculature using Kir4.1 channels that are found on the Müller cell processes that encompass the blood vessels[10,11]. This leads to osmotic fluid removal through aquaporin-4[11–14].
In addition to regulating neurotransmitters and ion levels within the retina, Müller cells also participate in the retinoid cycle with cone photoreceptors by taking up all-trans retinol from the subretinal space[15–18]. During the visual cycle, photons of light lead to isomerization of 11-cis retinal to all-trans retinal in the rod and cone photoreceptors. Once isomerized, all-trans retinal is expelled from the opsin protein to be reduced by retinol dehydrogenases to all-trans retinol[19]. The all-trans retinol from the cones is then released into the extracellular space where it is taken up by Müller cells, isomerized back to 11-cis retinol by all-trans retinol isomerase, and released back to the extracellular space to be taken up by the cone photoreceptors where it can finally be oxidized from 11-cis retinol back to original 11-cis retinal to restart the visual cycle[15–17,20].
Müller cells seem a primary site of nutrient storage for the retina. It has been shown that ATP production in Müller cells drastically declines when glycolysis is inhibited. However, ATP levels remained equal in aerobic versus anaerobic conditions as long as glucose was provided, indicating that Müller cells live primarily from glycolysis rather than oxidative phosphorylation[21]. This is important as it spares oxygen for retinal neurons and other cell types that use oxidative phosphorylation for ATP production. Furthermore, Müller cells are the primary site of glycogen storage in the retina[21,22]. When nutrient supplies are low Müller cells can utilize this glycogen storage to provide metabolites for other cell types. Furthermore, the large amounts of lactate they produce via glycolysis and irreversible conversion of pyruvate to lactate due to a specific lactate dehydrogenase isoform can be transported to photoreceptors to be used as a potential alternative source of energy in case of need[21,23,24]. Interestingly, studies suggest that the metabolism of glucose and glycogen by Müller cells is regulated by light being absorbed by the photoreceptors[7]. This means that as photoreceptors absorb light, the Müller cells respond by metabolizing more glucose in order to provide more lactate for photoreceptors as needed, indicating that Müller cells and photoreceptors are tightly coupled in their respective functions by metabolism. In addition to providing lactate as a fuel source for photoreceptors, Müller cells can also regulate nutrient supplies to the retina via regulation of retinal blood flow. In a healthy retina, increased light stimulation results in increased retinal blood flow, which is required to supply the activated neurons with oxygen and other nutrients, a process termed neurovascular coupling. Müller cells play a crucial role in neurovascular coupling as they release metabolites controlling vasoconstriction and vasodilation of retinal blood vessels[25,26].
One of the most important functions of Müller cells is their regulation of retinal blood flow and contribution to the blood retinal barrier. The blood retinal barrier is essential for preventing leakage of blood and other potentially harmful stimuli such as pathogens from entering the retinal tissue. It has been shown that Müller cells induce blood-barrier properties in retinal endothelial cells[27,28]. Studies using conditional ablation of Müller cells showed severe blood retinal barrier breakdown[29]. The exact mechanism of how Müller cells maintain the blood retinal barrier is debated but includes the secretion of factors such as pigment epithelium-derived factor (PEDF) and thrombospondin-1 which are anti-angiogenic and increase the tightness of the endothelial barrier[30,31].
It is clear that Müller cells are an integral part of a healthy and well functioning retina. Any disturbance to these cells certainly affects cellular cross-talk within the retina and its proper function. However, despite their importance Müller cells are still an under-studied cell type in the context of diseases such as diabetic retinopathy. The following aims to provide an overview about the effects of diabetes on Müller cells and the role Müller cells play in pathological events in the diabetic retina.
Influence of diabetes on neurotransmitter and potassium regulation in Müller cells
Functional changes that have been determined in Müller cells begin early in the disease, with significant decreases in glutamate transport via GLAST beginning after just 4 weeks of diabetes in rats[32]. This is consistent with reports showing significantly increased glutamate accumulation in the retinas of diabetic rats[33,34]. Furthermore, these studies have shown that there is decreased glutamine synthetase activity and a subsequent decrease in the conversion of glutamate to glutamine necessary for neurotransmitter regeneration[33,34]. These results are in line with reports demonstrating glutamate increases to a potentially neurotoxic level in the vitreous of diabetic patients[35]. However, in neurological diseases such as stroke, therapies targeting glutamate increase have been ineffective indicating that increased glutamate levels might not play a pathophysiological role[36,37]. Whether increased glutamate levels actually cause neurotoxicity over time in diabetic retinopathy has yet to be determined.
It seems that Müller cells not only contribute to glutamate toxicity directly by decreased glutamate uptake, but Müller cells also contribute indirectly via decreased K+ uptake during the progression of diabetic retinopathy. There is decreased K+ conductance on the plasma membrane of Müller cells isolated from rat retinas after 4 months of experimental diabetes[38]. Redistribution of the Kir4.1 K+ channel has been identified as the mechanism of decreased K+ conductance[38]. This decrease in K+ conductance was also observed in Müller cells of patients with proliferative diabetic retinopathy[39]. Alteration of the Kir4.1 K+ channel localization in Müller cells in the diabetic retina has been attributed to the accumulation of advanced glycation endproducts (AGEs)[40]. Together, this can lead to an imbalance in K+ concentrations and altered K+ homeostasis leading to neuronal excitation and subsequent glutamate toxicity.
In diabetes and diabetic macular edema, Müller cells have been shown to downregulate the Kir4.1 channels, but not Kir2.1, leading to continued potassium uptake with no release into the microvasculature[38,41,42]. This leads to subsequent swelling of Müller cells contributing to Müller cell dysfunction and decreased fluid removal contributing to diabetic macular edema. Diabetic macular edema leads to thickening of the macula due to fluid accumulation and can be observed by optical coherence tomography (OCT). The thickening of the macula due to fluid accumulation typically leads to disruption of the retinal structure and changes in visual acuity.
Release of growth factors and pro-/anti-inflammatory cytokines from Müller cells in response to hyperglycemia – the bad and the potentially good
As already stated above, Müller cell have contact with every cell in the retina. Müller cell ablation leads to photoreceptor degeneration, vascular leak, and intraretinal neovascularization demonstrating that Müller cells are necessary for both neuronal and vascular function and viability[29,43]. Changes to their environment by hyperglycemia alters functional interaction with pericytes[44]. Deletion of the dystrophin-Dp71 protein within Müller cells caused extensive vascular leakage and edema in the mouse retina. It was suggested that breakdown of the blood retinal barrier was initiated by improper localization of proteins in the endfeet of Müller cells that are necessary for establishing barrier function[45]. Other studies have shown that Müller cells participate in regulation of vascular tone in a process of neurovascular coupling[25,26]. They are also seemingly involved in lactate exchange with neurons, glia, and vascular cells[46]. Given the intricate contact Müller cells have with other retinal cell types it is easy to see that any disturbance to Müller cells will certainly affect proper function and viability of neurons as well as cell of the microvasculature.
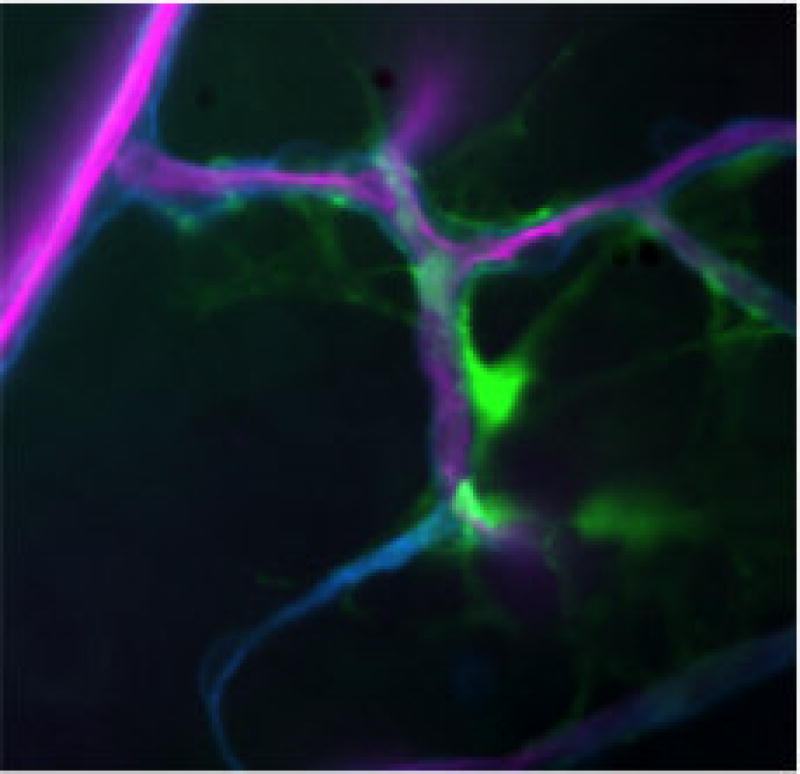
In diabetes, it has been well established that Müller cells become activated[47–50]. One of the most prominent signs that Müller cells are activated in diabetic retinopathy is the increased expression of glial fibrillary acidic protein (GFAP), a common marker of reactive gliosis[33,48,51]. In healthy conditions, Müller cells generally do not express GFAP[47,52]. Interestingly, while Müller cells upregulate GFAP expression in the diabetic retina astrocytes seemingly downregulate GFAP expression[53]. Figure 1 demonstrates the high level of GFAP expression in Müller cells in the diabetic retina. It also highlights the extensive contact that Müller cells have with the retinal microvasculature making it easy to comprehend the influence activated Müller cells have on proper function of the microvasculature.
Figure 1.
picture (4×) taken of a retinal flatmount obtained from a STZ (streptozotocin) diabetic mouse that expressed a GFP (green fluorescence protein) tagged GFAP specifically in Müller cells[49]. Duration of diabetes: 10 weeks.
Purple: microvasculature
Green: Müller cells
Despite GFAP several other markers might be more useful to determine early glial activation such as phospho-ERK (extracellular signal-regulated kinase)[54]. Although elevated GFAP expression happens early and persists throughout the disease, no study to date has been able to connect increased levels of GFAP to any functional outcome.
However, hyperglycemia-induced gliosis goes hand in hand with stimulation of growth factor, cytokine, and chemokine release by Müller cells at least in vitro. Hyperglycemia promotes release of (1) growth factors, such as vascular endothelial growth factor (VEGF) and pigment epithelium-derived factor (PEDF), and (2) cytokines and chemokines including interleukin-1β (IL-β), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and chemokine ligand-2 (CCL2)[52,55–61] [62–64]. In vitro studies have provided ample evidence that Müller cells are a potential source for growth factors and cytokines when stimulated with elevated glucose levels. Considering that most of the growth factors, cytokines, and chemokines released by Müller cells have been identified in the vitreous of diabetic patients it is fair to assume that Müller cells contribute to the overall synthesis of these factors in vivo[65–68].
Growth factors – the bad
How much Müller cell derived growth factors really contribute to the pathology of diabetic retinopathy in vivo is still not fully understood. The first studies to understand the contribution and effect of Müller cell derived VEGF to the development and progression of diabetic retinopathy were done by the group of Y.Z. Le. This group disrupted VEGF in Müller cells with an inducible Cre/lox system and examined diabetes-induced retinal inflammation and vascular leakage in these conditional VEGF knockout (KO) mice. The diabetic conditional VEGF KO mice exhibited an overall decrease in parameters associated with the pathology of diabetic retinopathy such as leukostasis, expression of inflammatory biomarkers, depletion of tight junction proteins, numbers of acellular capillaries, and vascular leakage compared to diabetic control mice[59,69,70]. Additional studies focusing on altering known regulators of VEGF production such as HIF-1 (hypoxia inducible factor 1)[71] and the Wnt signaling pathway[72] specifically in Müller cells have supported the notion that Müller cell derived VEGF is actually a major component in the process of retinal angiogenesis and pathology in diabetic retinopathy. Besides VEGF, Müller cell derived PEDF has also been suggested to have its part in diabetes-induced retinal angiogenesis[30]. Taken together, it seems that Müller cell derived growth factors contribute heavily to pathological vascular events in diabetic retinopathy.
Growth factors – the potentially good
Although Müller cell derived VEGF contributes to detrimental effects on the microvasculature in the diabetic retina, the intent of such growth factor production by Müller cells in the first place might have been to protect itself and the retinal neurons from a diabetic insult. This idea is supported by a study using mice that carry a disrupted VEGFR2 specifically in Müller cells. Loss of VEGFR2 caused a gradual reduction in Müller glial density, decreased of scotopic and photopic electroretinography amplitudes, and accelerated loss of photoreceptors, ganglion cells, and inner nuclear layer neurons in the diabetic retina[73]. More studies are needed to fully explore and understand the beneficial effects of Müller cell derived growth factors on Müller cells itself and retinal neurons in the context of disease. This is especially important since long-term anti-VEGF treatment might hamper functional integrity of Müller cells and neurons causing unexpected additional problems in treating diabetic retinopathy.
Cytokines – the bad
Besides growth factors, Müller cells release a variety of cytokines and chemokines under hyperglycemic conditions. For example, Müller cells are a major source of retinal interleukin-1beta (IL-1β) production[63,74–77]. Caspase-1, originally named interleukin-1β converting enzyme (ICE), produces the active cytokines IL-1β and IL-18 by cleavage of their inactive proform[78–81]. In Müller cells, hyperglycemia strongly induces the activation of the caspase-1/IL-1β signaling pathway as we have previously shown[63,77]. Increased caspase-1 activation and elevated IL-1β levels have also been identified in the retinas of diabetic mice and retinal tissue and vitreous fluid of diabetic patients[63,75,82–84]. We have identified that targeting this pathway by knocking down caspase-1 or the IL-1 receptor (IL-1R1) or by pharmacological intervention protects against the development of diabetic retinopathy in diabetic rats and mice[76,85]. Prolonged IL-1β production by Müller cells has been shown to affect endothelial cell viability in a paracrine fashion[75]. Endothelial cells are extremely susceptible to IL-1β and rapidly progress to cell death in response to this pro-inflammatory cytokine[75]. Endothelial cell death is detectable in the retinal microvasculature of diabetic animals and isolated retinal blood vessels of diabetic donors and has been associated with the formation of acellular capillaries, a hallmark of retinal pathology in diabetic retinopathy[86]. Besides IL-1β, Müller cells produce other well-known pro-inflammatory cytokines such as tumor necrosis factor alpha (TNFα) and interleukin-6 (IL-6)[76,77,85,87–90]. Anti-TNFα therapy has been proposed as a strategy to treat diabetic retinopathy in diabetic animals[91–94]. Detrimental effects of IL-6 have been associated with vascular dysfunction and promotion of angiogenesis[95–97] which is why IL-6 recently has become a new therapeutical target of interest to prevent diabetes-induced vascular damage. The production and release of pro-inflammatory cytokines by Müller cells strongly contributes to the chronic inflammatory environment detected in the diabetic retina that over time promotes drop-out of a retinal cells.
Cytokines – the potentially good
From a vascular perspective, IL-6 has been solely associated with detrimental effects[95–97]. However, we have previously shown that IL-6 prevents hyperglycemia-induced Müller cell dysfunction and loss clearly supporting a beneficial and protective nature of IL-6[77]. This observation is well in line with reports that in the retina IL-6 is an important cytokine responsible for maintaining proper neuronal function as well as stimulating neuroprotective effects[77,98–101]. Treatment with IL-6 has been shown to protect retinal ganglion cells from pressure-induced cell death[98]. Additionally, in an experimental model of retinal detachment, genetic ablation or neutralization of IL-6 led to a significant increase in photoreceptor cell death. However, treatment with exogenous IL-6 resulted in a significant increase in photoreceptor density in the outer nuclear layer[101]. These different effects of IL-6 can potentially be attributed to the two distinct signaling pathways IL-6 acts through. Classical IL-6 signaling - thought to be the anti-inflammatory and protective pathway - is mediated by the membrane-bound form of the IL-6 receptor (IL-6R) and the ubiquitously expressed glycoprotein 130 (gp130). Only cells such as Müller cells (but not endothelial cells) that express IL-6R are able to signal through classical IL-6 signaling. Conversely, IL-6 trans-signaling, which is mediated by binding of IL-6 to the soluble form of the IL-6 receptor (sIL-6R) and gp130, is thought to be the more pro-inflammatory and pro-angiogenic pathway[77,96,99,102–112]. In diabetic patients, correlations between increased levels of IL-6 and the development of complications in the eye have been made[113–118]. However, whether IL-6 levels are increased in diabetes as an attempt to protect from a pro-inflammatory environment or whether high levels of IL-6 synergistically exaggerate diabetes-induced inflammation has yet to be determined.
Müller cell loss in diabetic retinopathy
Whether Müller cells die in diabetic retinopathy has long been a matter of debate. It is easy to see that Müller cells are “sturdy” cells taking into account how well equipped these cells are to produce fair amounts of protective factors that shield them at least in the beginning from a chronic diabetic insult as discussed above. However, newer studies indicate that over time Müller cells actually do begin to die the longer diabetic retinopathy progresses. Frequency of Müller cell death in the diabetic retina rapidly accelerates when protective growth factors are blunted[73].
Better understanding of types of cell deaths has furthered studies to look for mechanisms other than apoptosis by which Müller cells can die in a diabetic environment. We have identified one particular mechanism of cell death that stands out and can explain histological features described for Müller cells in the diabetic retina. Pyroptosis is an inflammatory driven type of cell death that depends on caspase-1 activation[119–121]. Müller cells show increased caspase-1 activity and IL-1β production following exposure to hyperglycemic conditions and cells die as a consequence[122,123]. While it is known that initiation of pyroptosis is caspase-1 and IL-1β driven, the execution phase of pyroptosis is not yet completely understood. Execution of pyroptosis shares traits with both apoptosis and necrosis[124,125]. Since execution of pyropototic cell death lacks specific marker, identifying retinal cells dying by pyroptosis in vivo is a difficult task. Markers such as TUNEL staining used to detect apoptotic cell death may not adequately detect pyroptosis. Therefore, we have performed a study actually counting Müller cells in the healthy and diabetic retina and determined roughly 15% cell death at 7 months of diabetes[88]. Even more important, inhibition of the caspase-1/IL-1β pathway inhibited diabetes-induced Müller cell death in vivo as we had previously shown in vitro[76,77,88]. Several other studies are in line with our observation that Müller cells die in a hyperglycemic environment. The first study to describe dying Müller cells in diabetic retinopathy was done using EM analysis[126]. Dying Müller cells are described as being hypertrophic consistent with the notion that during pyroptosis, cells swell rather than shrink as observed in apoptotic cell death[48]. To collect more evidence for Müller cells death in the diabetic retina we looked at earlier markers of cell death and we have identified that GAPDH (glyceraldehyde-3-phosphate dehydrogenase) accumulates in the nucleus of Müller cells in the retinas of diabetic rats[50]. Nuclear accumulation of GAPDH has been closely associated with cell death induction[127–129]. Consistent with our finding that Müller cells die by pyroptotic cell death, hyperglycemia-induced nuclear accumulation of GAPDH depends on the activation of the caspase-1/IL-1β pathway[52,130]. The consequences of dying Müller cells are multi faceted. On the bad side – Müller cell death will promote loss of retinal blood barrier integrity, increased vascular permeability, and loss of neuroprotection affecting both neurons and vascular cells. Loss of Müller cells in diabetes has also been associated with aneurysm formation, a clinical characteristic of diabetic retinopathy[126]. However, one can also argue that on the good side – removal of activated and pro-inflammatory Müller cells might be a “shut off” mechanism to deal with an increasing inflammatory environment in the diabetic retina. A lot more studies are needed to determine the full pathway of Müller cells death and to identify whether all Müller cells are equally affected by hyperglycemia.
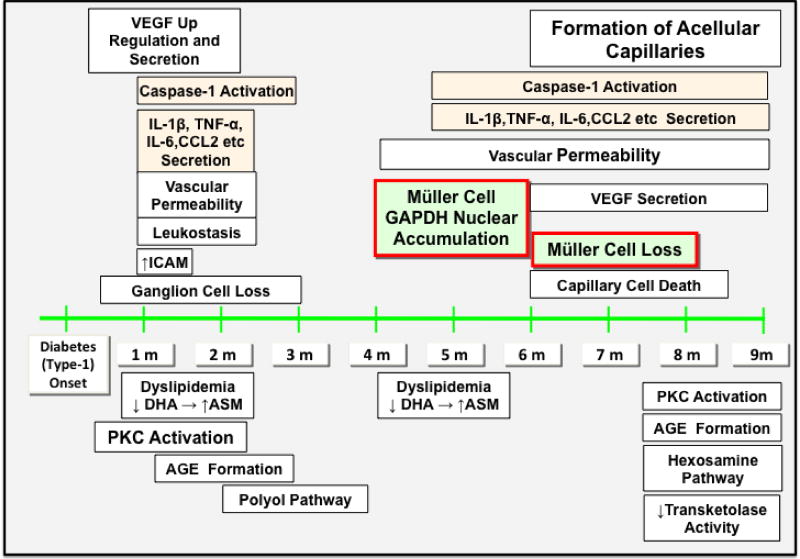
Figure 2.
Timeline of caspase-1 activation, cytokine secretion, Müller cell death initiation and execution in comparison to other prominent events associated with diabetic retinopathy in retinas of STZ diabetic mice and rats.
Conclusion
Müller cells are a major component of a healthy retinal environment. Once chronic hyperglycemia disturbs their environment, Müller cells become dysfunctional and start activating pathways to counter-regulate and “repair” the environment.
In order to do so, Müller cells release a large variety of growth factors and cytokines in a diabetic environment. Most of the research to date has focused on the detrimental effects the release of these growth factors and cytokines causes to the retina. When taking a closer look most of these effects are associated with vascular dysfunction and angiogenesis. On the other hand, it seems that production of these growth factors and cytokines by Müller cells are primarily intended to protect Müller cells and consequently retinal neurons from diabetic insult and might only secondarily turn into the damaging components observed in diabetic retinopathy. Very few studies have started to consider the protective nature of Müller cell derived growth factors and cytokines in regards to the integrity of glia cells and neurons. A lot more studies are needed to understand the nature of Müller cells derived growth factors and cytokines. For a successful development of a new therapy targeting these factors both detrimental as well as beneficial effects need to be considered.
Understanding Müller cell functions within the retina and restoring such function in diabetic retinopathy should become a cornerstone for developing effective therapies to treat diabetic retinopathy. Some approaches have been tested to increase Müller cell function by stimulating the beta-adrenergic pathway[131,132]. Whether these studies materialize into effective therapy strategies has to be seen in the future.
Acknowledgments
This work was supported by NIH Grants EY017206, EY007739, and EY024757 (SM). We thank Dr. Vijay Sarthy for supporting our research by providing us with the GFP-GFAP mouse model.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Matsui K, Hosoi N, Tachibana M. Active Role of Glutamate Uptake in the Synaptic Transmission from Retinal Nonspiking Neurons. J Neurosci. 1999 Aug 15;19(16):6755–66. doi: 10.1523/JNEUROSCI.19-16-06755.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Derouiche A, Rauen T. Coincidence of L-glutamate/L-aspartate transporter (GLAST) and glutamine synthetase (GS) immunoreactions in retinal glia: Evidence for coupling of GLAST and GS in transmitter clearance. J Neurosci Res. 1995 Sep 1;42(1):131–43. doi: 10.1002/jnr.490420115. [DOI] [PubMed] [Google Scholar]
- 3.Barbour B, Brew H, Attwell D. Electrogenic glutamate uptake in glial cells is activated by intracellular potassium. Nature. 1988 Sep 29;335(6189):433–5. doi: 10.1038/335433a0. [DOI] [PubMed] [Google Scholar]
- 4.Biedermann B, Bringmann A, Reichenbach A. High-affinity GABA uptake in retinal glial (Müller) cells of the guinea pig: Electrophysiological characterization, immunohistochemical localization, and modeling of efficiency. Glia. 2002 Sep 1;39(3):217–28. doi: 10.1002/glia.10097. [DOI] [PubMed] [Google Scholar]
- 5.Harada T, Harada C, Watanabe M, Inoue Y, Sakagawa T, Nakayama N, Sasaki S, Okuyama S, Watase K, Wada K, Tanaka K. Functions of the two glutamate transporters GLAST and GLT-1 in the retina. Proc Natl Acad Sci U S A. 1998 Apr 14;95(8):4663–6. doi: 10.1073/pnas.95.8.4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rauen T, Taylor WR, Kuhlbrodt K, Wiessner M. High-affinity glutamate transporters in the rat retina: a major role of the glial glutamate transporter GLAST-1 in transmitter clearance. Cell Tissue Res. 1998 Jan;291(1):19–31. doi: 10.1007/s004410050976. [DOI] [PubMed] [Google Scholar]
- 7.Poitry S, Poitry-Yamate C, Ueberfeld J, MacLeish PR, Tsacopoulos M. Mechanisms of Glutamate Metabolic Signaling in Retinal Glial (Müller) Cells. J Neurosci. 2000 Mar 1;20(5):1809–21. doi: 10.1523/JNEUROSCI.20-05-01809.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orkand RK, Nicholls JG, Kuffler SW. Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J Neurophysiol. 1966 Jul;29(4):788–806. doi: 10.1152/jn.1966.29.4.788. [DOI] [PubMed] [Google Scholar]
- 9.Karwoski CJ, Lu H-K, Newman EA. Spatial Buffering of Light-Evoked Potassium Increases by Retinal Müller (Glial) Cells. Science. 1989 May 5;244(4904):578–80. doi: 10.1126/science.2785716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bringmann A, Reichenbach A, Wiedemann P. Pathomechanisms of cystoid macular edema. Ophthalmic Res. 2004 Oct;36(5):241–9. doi: 10.1159/000081203. [DOI] [PubMed] [Google Scholar]
- 11.Nagelhus EA, Horio Y, Inanobe A, Fujita A, Haug FM, Nielsen S, Kurachi Y, Ottersen OP. Immunogold evidence suggests that coupling of K+ siphoning and water transport in rat retinal Müller cells is mediated by a coenrichment of Kir4.1 and AQP4 in specific membrane domains. Glia. 1999 Mar;26(1):47–54. doi: 10.1002/(sici)1098-1136(199903)26:1<47::aid-glia5>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 12.Iandiev I, Pannicke T, Reichel MB, Wiedemann P, Reichenbach A, Bringmann A. Expression of aquaporin-1 immunoreactivity by photoreceptor cells in the mouse retina. Neurosci Lett. 2005 Nov 11;388(2):96–9. doi: 10.1016/j.neulet.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 13.Nagelhus EA, Veruki ML, Torp R, Haug FM, Laake JH, Nielsen S, Agre P, Ottersen OP. Aquaporin-4 water channel protein in the rat retina and optic nerve: polarized expression in Müller cells and fibrous astrocytes. J Neurosci Off J Soc Neurosci. 1998 Apr 1;18(7):2506–19. doi: 10.1523/JNEUROSCI.18-07-02506.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patil RV, Saito I, Yang X, Wax MB. Expression of aquaporins in the rat ocular tissue. Exp Eye Res. 1997 Feb;64(2):203–9. doi: 10.1006/exer.1996.0196. [DOI] [PubMed] [Google Scholar]
- 15.Mata NL, Radu RA, Clemmons RC, Travis GH. Isomerization and oxidation of vitamin a in cone-dominant retinas: a novel pathway for visual-pigment regeneration in daylight. Neuron. 2002 Sep 26;36(1):69–80. doi: 10.1016/s0896-6273(02)00912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trevino SG, Villazana-Espinoza ET, Muniz A, Tsin ATC. Retinoid cycles in the cone-dominated chicken retina. J Exp Biol. 2005 Nov;208(Pt 21):4151–7. doi: 10.1242/jeb.01881. [DOI] [PubMed] [Google Scholar]
- 17.Kanan Y, Kasus-Jacobi A, Moiseyev G, Sawyer K, Ma J-X, Al-Ubaidi MR. Retinoid processing in cone and Müller cell lines. Exp Eye Res. 2008 Feb;86(2):344–54. doi: 10.1016/j.exer.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edwards RB, Adler AJ, Dev S, Claycomb RC. Synthesis of retinoic acid from retinol by cultured rabbit Müller cells. Exp Eye Res. 1992 Apr;54(4):481–90. doi: 10.1016/0014-4835(92)90126-d. [DOI] [PubMed] [Google Scholar]
- 19.Palczewski K, Jäger S, Buczyłko J, Crouch RK, Bredberg DL, Hofmann KP, Asson-Batres MA, Saari JC. Rod outer segment retinol dehydrogenase: substrate specificity and role in phototransduction. Biochemistry (Mosc) 1994 Nov 22;33(46):13741–50. doi: 10.1021/bi00250a027. [DOI] [PubMed] [Google Scholar]
- 20.Mata NL, Ruiz A, Radu RA, Bui TV, Travis GH. Chicken retinas contain a retinoid isomerase activity that catalyzes the direct conversion of all-trans-retinol to 11-cis-retinol. Biochemistry (Mosc) 2005 Sep 6;44(35):11715–21. doi: 10.1021/bi050942m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winkler BS, Arnold MJ, Brassell MA, Puro DG. Energy Metabolism in Human Retinal Müller Cells. Invest Ophthalmol Vis Sci. 2000 Sep;41(10):3183–90. [PMC free article] [PubMed] [Google Scholar]
- 22.Kuwabara T, Cogan DG. Retinal Glycogen. Trans Am Ophthalmol Soc. 1961;59:106–10. [PMC free article] [PubMed] [Google Scholar]
- 23.Poitry-Yamate CL, Tsacopoulos M. Glucose metabolism in freshly isolated Müller glial cells from a mammalian retina. J Comp Neurol. 1992 Jun 8;320(2):257–66. doi: 10.1002/cne.903200209. [DOI] [PubMed] [Google Scholar]
- 24.Poitry-Yamate CL, Poitry S, Tsacopoulos M. Lactate released by Müller glial cells is metabolized by photoreceptors from mammalian retina. J Neurosci Off J Soc Neurosci. 1995 Jul 15;(7 Pt 2):5179–91. doi: 10.1523/JNEUROSCI.15-07-05179.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Metea MR, Newman EA. Glial cells dilate and constrict blood vessels: a mechanism of neurovascular coupling. J Neurosci Off J Soc Neurosci. 2006 Mar 15;26(11):2862–70. doi: 10.1523/JNEUROSCI.4048-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newman EA. Glial cell regulation of neuronal activity and blood flow in the retina by release of gliotransmitters. Phil Trans R Soc B. 2015 Jul 5;370(1672):20140195. doi: 10.1098/rstb.2014.0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tout S, Chan-Ling T, Holländer H, Stone J. The role of müller cells in the formation of the blood-retinal barrier. Neuroscience. 1993 Jul;55(1):291–301. doi: 10.1016/0306-4522(93)90473-s. [DOI] [PubMed] [Google Scholar]
- 28.Abukawa H, Tomi M, Kiyokawa J, Hori S, Kondo T, Terasaki T, Hosoya K. Modulation of retinal capillary endothelial cells by Müller glial cell-derived factors. Mol Vis. 2009 Feb 27;15:451–7. [PMC free article] [PubMed] [Google Scholar]
- 29.Shen W, Fruttiger M, Zhu L, Chung SH, Barnett NL, Kirk JK, Lee S, Coorey NJ, Killingsworth M, Sherman LS, Gillies MC. Conditional Müller Cell Ablation Causes Independent Neuronal and Vascular Pathologies in a Novel Transgenic Model. J Neurosci. 2012 Nov 7;32(45):15715–27. doi: 10.1523/JNEUROSCI.2841-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eichler W, Yafai Y, Keller T, Wiedemann P, Reichenbach A. PEDF derived from glial Müller cells: a possible regulator of retinal angiogenesis. Exp Cell Res. 2004 Sep 10;299(1):68–78. doi: 10.1016/j.yexcr.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 31.Bringmann A, Pannicke T, Grosche J, Francke M, Wiedemann P, Skatchkov SN, Osborne NN, Reichenbach A. Müller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006 Jul;25(4):397–424. doi: 10.1016/j.preteyeres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 32.Li Q, Puro DG. Diabetes-Induced Dysfunction of the Glutamate Transporter in Retinal Müller Cells. Invest Ophthalmol Vis Sci. 2002 Sep 1;43(9):3109–16. [PubMed] [Google Scholar]
- 33.Lieth E, Barber AJ, Xu B, Dice C, Ratz MJ, Tanase D, Strother JM. Glial reactivity and impaired glutamate metabolism in short-term experimental diabetic retinopathy. Penn State Retina Research Group. Diabetes. 1998 May 1;47(5):815–20. doi: 10.2337/diabetes.47.5.815. [DOI] [PubMed] [Google Scholar]
- 34.Lieth E, LaNoue KF, Antonetti DA, Ratz M. Diabetes reduces glutamate oxidation and glutamine synthesis in the retina. The Penn State Retina Research Group. Exp Eye Res. 2000 Jun;70(6):723–30. doi: 10.1006/exer.2000.0840. [DOI] [PubMed] [Google Scholar]
- 35.Ambati J, Chalam KV, Chawla DK, D’Angio CT, Guillet EG, Rose SJ, Vanderlinde RE, Ambati BK. Elevated gamma-aminobutyric acid, glutamate, and vascular endothelial growth factor levels in the vitreous of patients with proliferative diabetic retinopathy. Arch Ophthalmol Chic Ill 1960. 1997 Sep;115(9):1161–6. doi: 10.1001/archopht.1997.01100160331011. [DOI] [PubMed] [Google Scholar]
- 36.O’Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006 Mar;59(3):467–77. doi: 10.1002/ana.20741. [DOI] [PubMed] [Google Scholar]
- 37.Sloan SA, Barres BA. The detrimental role of glial acidification during ischemia. Neuron. 2014 Jan 22;81(2):221–3. doi: 10.1016/j.neuron.2013.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pannicke T, Iandiev I, Wurm A, Uckermann O, vom Hagen F, Reichenbach A, Wiedemann P, Hammes H-P, Bringmann A. Diabetes alters osmotic swelling characteristics and membrane conductance of glial cells in rat retina. Diabetes. 2006 Mar;55(3):633–9. doi: 10.2337/diabetes.55.03.06.db05-1349. [DOI] [PubMed] [Google Scholar]
- 39.Bringmann A, Pannicke T, Uhlmann S, Kohen L, Wiedemann P, Reichenbach A. Membrane conductance of Müller glial cells in proliferative diabetic retinopathy. Can J Ophthalmol J Can Ophtalmol. 2002 Jun;37(4):221–7. doi: 10.1016/s0008-4182(02)80113-2. [DOI] [PubMed] [Google Scholar]
- 40.Curtis TM, Hamilton R, Yong P-H, McVicar CM, Berner A, Pringle R, Uchida K, Nagai R, Brockbank S, Stitt AW. Müller glial dysfunction during diabetic retinopathy in rats is linked to accumulation of advanced glycation end-products and advanced lipoxidation end-products. Diabetologia. 2011 Mar;54(3):690–8. doi: 10.1007/s00125-010-1971-x. [DOI] [PubMed] [Google Scholar]
- 41.Reichenbach A, Wurm A, Pannicke T, Iandiev I, Wiedemann P, Bringmann A. Müller cells as players in retinal degeneration and edema. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Klin Exp Ophthalmol. 2007 May;245(5):627–36. doi: 10.1007/s00417-006-0516-y. [DOI] [PubMed] [Google Scholar]
- 42.Pannicke T, Iandiev I, Uckermann O, Biedermann B, Kutzera F, Wiedemann P, Wolburg H, Reichenbach A, Bringmann A. A potassium channel-linked mechanism of glial cell swelling in the postischemic retina. Mol Cell Neurosci. 2004 Aug;26(4):493–502. doi: 10.1016/j.mcn.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 43.Shen W, Lee S-R, Araujo J, Chung SH, Zhu L, Gillies MC. Effect of glucocorticoids on neuronal and vascular pathology in a transgenic model of selective Müller cell ablation. Glia. 2014 Jul;62(7):1110–24. doi: 10.1002/glia.22666. [DOI] [PubMed] [Google Scholar]
- 44.Muto T, Tien T, Kim D, Sarthy VP, Roy S. High glucose alters Cx43 expression and gap junction intercellular communication in retinal Müller cells: promotes Müller cell and pericyte apoptosis. Invest Ophthalmol Vis Sci. 2014 Jun 17;55(7):4327–37. doi: 10.1167/iovs.14-14606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vacca O, Charles-Messance H, El Mathari B, Sene A, Barbe P, Fouquet S, Aragón J, Darche M, Giocanti-Aurégan A, Paques M, Sahel J-A, Tadayoni R, Montañez C, Dalkara D, Rendon A. AAV-mediated gene therapy in Dystrophin-Dp71 deficient mouse leads to blood-retinal barrier restoration and oedema reabsorption. Hum Mol Genet. 2016 Jul 15;25(14):3070–9. doi: 10.1093/hmg/ddw159. [DOI] [PubMed] [Google Scholar]
- 46.Gerhart DZ, Leino RL, Drewes LR. Distribution of monocarboxylate transporters MCT1 and MCT2 in rat retina. Neuroscience. 1999;92(1):367–75. doi: 10.1016/s0306-4522(98)00699-x. [DOI] [PubMed] [Google Scholar]
- 47.Puro DG. Diabetes-induced dysfunction of retinal Müller cells. Trans Am Ophthalmol Soc. 2002;100:339–52. [PMC free article] [PubMed] [Google Scholar]
- 48.Mizutani M, Gerhardinger C, Lorenzi M. Müller cell changes in human diabetic retinopathy. Diabetes. 1998 Mar 1;47(3):445–9. doi: 10.2337/diabetes.47.3.445. [DOI] [PubMed] [Google Scholar]
- 49.Gerhardinger C, Costa MB, Coulombe MC, Toth I, Hoehn T, Grosu P. Expression of acute-phase response proteins in retinal Müller cells in diabetes. Invest Ophthalmol Vis Sci. 2005 Jan;46(1):349–57. doi: 10.1167/iovs.04-0860. [DOI] [PubMed] [Google Scholar]
- 50.Kusner LL, Sarthy VP, Mohr S. Nuclear Translocation of Glyceraldehyde-3-Phosphate Dehydrogenase: A Role in High Glucose-Induced Apoptosis in Retinal Müller Cells. Invest Ophthalmol Vis Sci. 2004 May 1;45(5):1553–61. [PubMed] [Google Scholar]
- 51.Rungger–Brändle E, Dosso AA, Leuenberger PM. Glial Reactivity, an Early Feature of Diabetic Retinopathy. Invest Ophthalmol Vis Sci. 2000 Jun 1;41(7):1971–80. [PubMed] [Google Scholar]
- 52.Yego ECK, Vincent JA, Sarthy V, Busik JV, Mohr S. Differential regulation of high glucose-induced glyceraldehyde-3-phosphate dehydrogenase nuclear accumulation in Müller cells by IL-1beta and IL-6. Invest Ophthalmol Vis Sci. 2009 Apr;50(4):1920–8. doi: 10.1167/iovs.08-2082. [DOI] [PubMed] [Google Scholar]
- 53.Barber AJ, Antonetti DA, Gardner TW. Altered Expression of Retinal Occludin and Glial Fibrillary Acidic Protein in Experimental Diabetes. Invest Ophthalmol Vis Sci. 2000 Oct 1;41(11):3561–8. [PubMed] [Google Scholar]
- 54.Geller SF, Lewis GP, Fisher SK. FGFR1, signaling, and AP-1 expression after retinal detachment: reactive Müller and RPE cells. Invest Ophthalmol Vis Sci. 2001 May;42(6):1363–9. [PubMed] [Google Scholar]
- 55.Mu H, Zhang X-M, Liu J-J, Dong L, Feng Z-L. Effect of high glucose concentration on VEGF and PEDF expression in cultured retinal Müller cells. Mol Biol Rep. 2009 Nov 1;36(8):2147–51. doi: 10.1007/s11033-008-9428-8. [DOI] [PubMed] [Google Scholar]
- 56.Wang L-L, Chen H, Huang K, Zheng L. Elevated histone acetylations in Müller cells contribute to inflammation: a novel inhibitory effect of minocycline. Glia. 2012 Dec;60(12):1896–905. doi: 10.1002/glia.22405. [DOI] [PubMed] [Google Scholar]
- 57.Liu X, Ye F, Xiong H, Hu D-N, Limb GA, Xie T, Peng L, Zhang P, Wei Y, Zhang W, Wang J, Wu H, Lee P, Song E, Zhang DY. IL-1β induces IL-6 production in retinal Müller cells predominantly through the activation of p38 MAPK/NF-κB signaling pathway. Exp Cell Res. 2015 Feb 1;331(1):223–31. doi: 10.1016/j.yexcr.2014.08.040. [DOI] [PubMed] [Google Scholar]
- 58.Du Y, Sarthy VP, Kern TS. Interaction between NO and COX pathways in retinal cells exposed to elevated glucose and retina of diabetic rats. Am J Physiol Regul Integr Comp Physiol. 2004 Oct;287(4):R735–741. doi: 10.1152/ajpregu.00080.2003. [DOI] [PubMed] [Google Scholar]
- 59.Wang J, Xu X, Elliott MH, Zhu M, Le Y-Z. Müller Cell-Derived VEGF Is Essential for Diabetes-Induced Retinal Inflammation and Vascular Leakage. Diabetes. 2010;59(9):2297–305. doi: 10.2337/db09-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu Y, Chen H, Su SB. Neuroinflammatory responses in diabetic retinopathy. J Neuroinflammation. 2015;12:141. doi: 10.1186/s12974-015-0368-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou T, Che D, Lan Y, Fang Z, Xie J, Gong H, Li C, Feng J, Hong H, Qi W, Ma C, Yang Z, Cai W, Zhong J, Ma J, Yang X, Gao G. Mesenchymal marker expression is elevated in Müller cells exposed to high glucose and in animal models of diabetic retinopathy. Oncotarget. 2016 Dec 15; doi: 10.18632/oncotarget.13945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vincent JA, Mohr S. Inhibition of caspase-1/interleukin-1beta signaling prevents degeneration of retinal capillaries in diabetes and galactosemia. Diabetes. 2007 Jan;56(1):224–30. doi: 10.2337/db06-0427. [DOI] [PubMed] [Google Scholar]
- 63.Mohr S, Xi X, Tang J, Kern TS. Caspase Activation in Retinas of Diabetic and Galactosemic Mice and Diabetic Patients [Internet] [cited 2012 Jun 18]; doi: 10.2337/diabetes.51.4.1172. Available from: http://diabetes.diabetesjournals.org.proxy1.cl.msu.edu. [DOI] [PubMed]
- 64.Lei X, Zhang J, Shen J, Hu L-M, Wu Y, Mou L, Xu G, Li W, Xu G-T. EPO attenuates inflammatory cytokines by Muller cells in diabetic retinopathy. Front Biosci Elite Ed. 2011;3:201–11. doi: 10.2741/e234. [DOI] [PubMed] [Google Scholar]
- 65.Abu el Asrar AM, Maimone D, Morse PH, Gregory S, Reder AT. Cytokines in the vitreous of patients with proliferative diabetic retinopathy. Am J Ophthalmol. 1992 Dec 15;114(6):731–6. doi: 10.1016/s0002-9394(14)74052-8. [DOI] [PubMed] [Google Scholar]
- 66.Brooks HL, Jr, Caballero S, Jr, Newell CK, Steinmetz RL, Watson D, Segal MS, Harrison JK, Scott EW, Grant MB. Vitreous levels of vascular endothelial growth factor and stromal-derived factor 1 in patients with diabetic retinopathy and cystoid macular edema before and after intraocular injection of triamcinolone. Arch Ophthalmol. 2004 Dec;122(12):1801–7. doi: 10.1001/archopht.122.12.1801. [DOI] [PubMed] [Google Scholar]
- 67.Demircan N, Safran BG, Soylu M, Ozcan AA, Sizmaz S. Determination of vitreous interleukin-1 (IL-1) and tumour necrosis factor (TNF) levels in proliferative diabetic retinopathy. Eye. 2005 Nov 11;20(12):1366–9. doi: 10.1038/sj.eye.6702138. [DOI] [PubMed] [Google Scholar]
- 68.Hernández C, Segura RM, Fonollosa A, Carrasco E, Francisco G, Simó R. Interleukin-8, monocyte chemoattractant protein-1 and IL-10 in the vitreous fluid of patients with proliferative diabetic retinopathy. Diabet Med. 2005;22(6):719–722. doi: 10.1111/j.1464-5491.2005.01538.x. [DOI] [PubMed] [Google Scholar]
- 69.Bai Y, Ma J, Guo J, Wang J, Zhu M, Chen Y, Le Y-Z. Müller cell-derived VEGF is a significant contributor to retinal neovascularization. J Pathol. 2009 Dec;219(4):446–54. doi: 10.1002/path.2611. [DOI] [PubMed] [Google Scholar]
- 70.Wang J-J, Zhu M, Le Y-Z. Functions of Müller cell-derived vascular endothelial growth factor in diabetic retinopathy. World J Diabetes. 2015 Jun 10;6(5):726–33. doi: 10.4239/wjd.v6.i5.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lin M, Chen Y, Jin J, Hu Y, Zhou KK, Zhu M, Le Y-Z, Ge J, Johnson RS, Ma J-X. Ischaemia-induced retinal neovascularisation and diabetic retinopathy in mice with conditional knockout of hypoxia-inducible factor-1 in retinal Müller cells. Diabetologia. 2011 Mar 1;54:1554–66. doi: 10.1007/s00125-011-2081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou KK, Benyajati S, Le Y, Cheng R, Zhang W, Ma J. Interruption of Wnt signaling in Müller cells ameliorates ischemia-induced retinal neovascularization. PloS One. 2014;9(10):e108454. doi: 10.1371/journal.pone.0108454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fu S, Dong S, Zhu M, Sherry DM, Wang C, You Z, Haigh JJ, Le Y-Z. Müller Glia Are a Major Cellular Source of Survival Signals for Retinal Neurons in Diabetes. Diabetes. 2015 Oct;64(10):3554–63. doi: 10.2337/db15-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lei X, Zhang J, Shen J, Hu L-M, Wu Y, Mou L, Xu G, Li W, Xu G-T. EPO attenuates inflammatory cytokines by Muller cells in diabetic retinopathy. Front Biosci Elite Ed. 2011;3:201–11. doi: 10.2741/e234. [DOI] [PubMed] [Google Scholar]
- 75.Busik JV, Mohr S, Grant MB. Hyperglycemia-Induced Reactive Oxygen Species Toxicity to Endothelial Cells Is Dependent on Paracrine Mediators. Diabetes. 2008 Jul;57(7):1952–65. doi: 10.2337/db07-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vincent JA, Mohr S. Inhibition of caspase-1/interleukin-1beta signaling prevents degeneration of retinal capillaries in diabetes and galactosemia. Diabetes. 2007 Jan;56(1):224–30. doi: 10.2337/db06-0427. [DOI] [PubMed] [Google Scholar]
- 77.Yego ECK, Vincent JA, Sarthy V, Busik JV, Mohr S. Differential Regulation of High Glucose–Induced Glyceraldehyde-3-Phosphate Dehydrogenase Nuclear Accumulation in Müller Cells by IL-1β and IL-6. Invest Ophthalmol Vis Sci. 2009 Apr 1;50(4):1920–8. doi: 10.1167/iovs.08-2082. [DOI] [PubMed] [Google Scholar]
- 78.Cohen GM. Caspases: the executioners of apoptosis. Biochem J. 1997 Aug 15;326(Pt 1):1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Creagh EM, Conroy H, Martin SJ. Caspase-activation pathways in apoptosis and immunity. Immunol Rev. 2003 Jun;193:10–21. doi: 10.1034/j.1600-065x.2003.00048.x. [DOI] [PubMed] [Google Scholar]
- 80.Thornberry NA, Molineaux SM. Interleukin-1 beta converting enzyme: a novel cysteine protease required for IL-1 beta production and implicated in programmed cell death. Protein Sci Publ Protein Soc. 1995 Jan;4(1):3–12. doi: 10.1002/pro.5560040102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dinarello CA. Interleukin-1 beta, interleukin-18, and the interleukin-1 beta converting enzyme. Ann N Y Acad Sci. 1998 Sep 29;856:1–11. doi: 10.1111/j.1749-6632.1998.tb08307.x. [DOI] [PubMed] [Google Scholar]
- 82.Liu Y, Biarnés Costa M, Gerhardinger C. IL-1β Is Upregulated in the Diabetic Retina and Retinal Vessels: Cell-Specific Effect of High Glucose and IL-1β Autostimulation. PLoS ONE. 2012 May 16;7(5):e36949. doi: 10.1371/journal.pone.0036949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mohr S. Potential new strategies to prevent the development of diabetic retinopathy. Expert Opin Investig Drugs. 2004 Mar;13(3):189–98. doi: 10.1517/13543784.13.3.189. [DOI] [PubMed] [Google Scholar]
- 84.Demircan N, Safran BG, Soylu M, Ozcan AA, Sizmaz S. Determination of vitreous interleukin-1 (IL-1) and tumour necrosis factor (TNF) levels in proliferative diabetic retinopathy. Eye Lond Engl. 2006 Dec;20(12):1366–9. doi: 10.1038/sj.eye.6702138. [DOI] [PubMed] [Google Scholar]
- 85.Krady JK, Basu A, Allen CM, Xu Y, LaNoue KF, Gardner TW, Levison SW. Minocycline reduces proinflammatory cytokine expression, microglial activation, and caspase-3 activation in a rodent model of diabetic retinopathy. Diabetes. 2005 May;54(5):1559–65. doi: 10.2337/diabetes.54.5.1559. [DOI] [PubMed] [Google Scholar]
- 86.Mizutani M, Kern TS, Lorenzi M. Accelerated death of retinal microvascular cells in human and experimental diabetic retinopathy. J Clin Invest. 1996 Jun 15;97(12):2883–90. doi: 10.1172/JCI118746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mohr S, Xi X, Tang J, Kern TS. Caspase Activation in Retinas of Diabetic and Galactosemic Mice and Diabetic Patients. Diabetes. 2002 Apr 1;51(4):1172–9. doi: 10.2337/diabetes.51.4.1172. [DOI] [PubMed] [Google Scholar]
- 88.Feenstra DJ, Yego ECK, Mohr S. Modes of Retinal Cell Death in Diabetic Retinopathy. J Clin Exp Ophthalmol. 2013;4:298. doi: 10.4172/2155-9570.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Trueblood KE, Mohr S, Dubyak GR. Purinergic regulation of high-glucose-induced caspase-1 activation in the rat retinal Müller cell line rMC-1. Am J Physiol - Cell Physiol. 2011 Nov 1;301(5):C1213–23. doi: 10.1152/ajpcell.00265.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yoshida S, Sotozono C, Ikeda T, Kinoshita S. Interleukin-6 (IL-6) production by cytokine-stimulated human Müller cells. Curr Eye Res. 2001 May;22(5):341–7. doi: 10.1076/ceyr.22.5.341.5498. [DOI] [PubMed] [Google Scholar]
- 91.Yoshida S, Yoshida A, Ishibashi T. Induction of IL-8, MCP-1, and bFGF by TNF-α in retinal glial cells: implications for retinal neovascularization during post-ischemic inflammation. Graefes Arch Clin Exp Ophthalmol. 2004 May 1;242(5):409–13. doi: 10.1007/s00417-004-0874-2. [DOI] [PubMed] [Google Scholar]
- 92.Sfikakis PP, Markomichelakis N, Theodossiadis GP, Grigoropoulos V, Katsilambros N, Theodossiadis PG. Regression of Sight-Threatening Macular Edema in Type 2 Diabetes Following Treatment With the Anti–Tumor Necrosis Factor Monoclonal Antibody Infliximab. Diabetes Care. 2005 Feb 1;28(2):445–7. doi: 10.2337/diacare.28.2.445. [DOI] [PubMed] [Google Scholar]
- 93.Joussen AM, Doehmen S, Le ML, Koizumi K, Radetzky S, Krohne TU, Poulaki V, Semkova I, Kociok N. TNF-α mediated apoptosis plays an important role in the development of early diabetic retinopathy and long-term histopathological alterations. Mol Vis. 2009 Jul 25;15:1418–28. [PMC free article] [PubMed] [Google Scholar]
- 94.Li J, Wang JJ, Yu Q, Wang M, Zhang SX. Endoplasmic reticulum stress is implicated in retinal inflammation and diabetic retinopathy. FEBS Lett. 2009 May 6;583(9):1521–7. doi: 10.1016/j.febslet.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Barnes TC, Anderson ME, Moots RJ. The Many Faces of Interleukin-6: The Role of IL-6 in Inflammation, Vasculopathy, and Fibrosis in Systemic Sclerosis. Int J Rheumatol. 2011;2011:1–6. doi: 10.1155/2011/721608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Neurath MF, Finotto S. IL-6 signaling in autoimmunity, chronic inflammation and inflammation-associated cancer. Cytokine Growth Factor Rev. 2011 Apr;22(2):83–9. doi: 10.1016/j.cytogfr.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 97.Rojas M, Zhang W, Lee DL, Romero MJ, Nguyen DT, Al-Shabrawey M, Tsai N-T, Liou GI, Brands MW, Caldwell RW, Caldwell RB. Role of IL-6 in Angiotensin II-Induced Retinal Vascular Inflammation. Invest Ophthalmol Vis Sci. 2009 Oct 15;51(3):1709–18. doi: 10.1167/iovs.09-3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sappington RM, Chan M, Calkins DJ. Interleukin-6 Protects Retinal Ganglion Cells from Pressure-Induced Death. Invest Ophthalmol Vis Sci. 2006 Jul 1;47(7):2932–42. doi: 10.1167/iovs.05-1407. [DOI] [PubMed] [Google Scholar]
- 99.Zhao X-F, Wan J, Powell C, Ramachandran R, Myers MG, Goldman D. Leptin and IL-6 family cytokines synergize to stimulate Müller glia reprogramming and retina regeneration. Cell Rep. 2014 Oct 9;9(1):272–84. doi: 10.1016/j.celrep.2014.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sims SM, Holmgren L, Cathcart HM, Sappington RM. Spatial regulation of interleukin-6 signaling in response to neurodegenerative stressors in the retina. Am J Neurodegener Dis. 2012;1(2):168–79. [PMC free article] [PubMed] [Google Scholar]
- 101.Chong DY, Boehlke CS, Zheng Q-D, Zhang L, Han Y, Zacks DN. Interleukin-6 as a Photoreceptor Neuroprotectant in an Experimental Model of Retinal Detachment. Invest Ophthalmol Vis Sci. 2008 Jul 1;49(7):3193–200. doi: 10.1167/iovs.07-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011 May;1813(5):878–88. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 103.Barnes TC, Anderson ME, Moots RJ. The Many Faces of Interleukin-6: The Role of IL-6 in Inflammation, Vasculopathy, and Fibrosis in Systemic Sclerosis. Int J Rheumatol. 2011;2011:1–6. doi: 10.1155/2011/721608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Naugler WE, Karin M. The wolf in sheep’s clothing: the role of interleukin-6 in immunity, inflammation and cancer. Trends Mol Med. 2008 Mar;14(3):109–19. doi: 10.1016/j.molmed.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 105.Tilg H, Trehu E, Atkins MB, Dinarello CA, Mier JW. Interleukin-6 (IL-6) as an anti-inflammatory cytokine: induction of circulating IL-1 receptor antagonist and soluble tumor necrosis factor receptor p55. Blood. 1994 Jan 1;83(1):113–8. [PubMed] [Google Scholar]
- 106.Ropelle ER, Flores MB, Cintra DE, Rocha GZ, Pauli JR, Morari J, de Souza CT, Moraes JC, Prada PO, Guadagnini D, Marin RM, Oliveira AG, Augusto TM, Carvalho HF, Velloso LA, Saad MJA, Carvalheira JBC. IL-6 and IL-10 Anti-Inflammatory Activity Links Exercise to Hypothalamic Insulin and Leptin Sensitivity through IKKβ and ER Stress Inhibition. In: Vidal-Puig AJ, editor. PLoS Biol. 8. Vol. 8. 2010. Aug 24, p. e1000465. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 107.Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei XF, Achong MK. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest. 1998 Jan 15;101(2):311–20. doi: 10.1172/JCI1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yasukawa H, Ohishi M, Mori H, Murakami M, Chinen T, Aki D, Hanada T, Takeda K, Akira S, Hoshijima M, Hirano T, Chien KR, Yoshimura A. IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat Immunol. 2003;4(6):551–6. doi: 10.1038/ni938. [DOI] [PubMed] [Google Scholar]
- 109.Leibinger M, Müller A, Gobrecht P, Diekmann H, Andreadaki A, Fischer D. Interleukin-6 contributes to CNS axon regeneration upon inflammatory stimulation. Cell Death Dis. 2013;4:e609. doi: 10.1038/cddis.2013.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Atreya R, Mudter J, Finotto S, Müllberg J, Jostock T, Wirtz S, Schütz M, Bartsch B, Holtmann M, Becker C, Strand D, Czaja J, Schlaak JF, Lehr HA, Autschbach F, Schürmann G, Nishimoto N, Yoshizaki K, Ito H, Kishimoto T, Galle PR, Rose-John S, Neurath MF. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nat Med. 2000 May;6(5):583–8. doi: 10.1038/75068. [DOI] [PubMed] [Google Scholar]
- 111.Campbell IL, Erta M, Lim SL, Frausto R, May U, Rose-John S, Scheller J, Hidalgo J. Trans-signaling is a dominant mechanism for the pathogenic actions of interleukin-6 in the brain. J Neurosci Off J Soc Neurosci. 2014 Feb 12;34(7):2503–13. doi: 10.1523/JNEUROSCI.2830-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chalaris A, Schmidt-Arras D, Yamamoto K, Rose-John S. Interleukin-6 trans-signaling and colonic cancer associated with inflammatory bowel disease. Dig Dis Basel Switz. 2012;30(5):492–9. doi: 10.1159/000341698. [DOI] [PubMed] [Google Scholar]
- 113.Koskela UE, Kuusisto SM, Nissinen AE, Savolainen MJ, Liinamaa MJ. High vitreous concentration of IL-6 and IL-8, but not of adhesion molecules in relation to plasma concentrations in proliferative diabetic retinopathy. Ophthalmic Res. 2013;49(2):108–14. doi: 10.1159/000342977. [DOI] [PubMed] [Google Scholar]
- 114.Funk M, Schmidinger G, Maar N, Bolz M, Benesch T, Zlabinger GJ, Schmidt-Erfurth UM. ANGIOGENIC AND INFLAMMATORY MARKERS IN THE INTRAOCULAR FLUID OF EYES WITH DIABETIC MACULAR EDEMA AND INFLUENCE OF THERAPY WITH BEVACIZUMAB. Retina. 2010 Oct;30(9):1412–9. doi: 10.1097/IAE.0b013e3181e095c0. [DOI] [PubMed] [Google Scholar]
- 115.Wegner M, Araszkiewicz A, Piorunska-Stolzmann M, Wierusz-Wysocka B, Zozulinska-Ziolkiewicz D. Association Between IL-6 Concentration and Diabetes-Related Variables in DM1 Patients with and without Microvascular Complications. Inflammation. :1–6. doi: 10.1007/s10753-013-9598-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Funatsu H, Yamashita H, Noma H, Mimura T, Yamashita T, Hori S. Increased levels of vascular endothelial growth factor and interleukin-6 in the aqueous humor of diabetics with macular edema. Am J Ophthalmol. 2002 Jan;133(1):70–7. doi: 10.1016/s0002-9394(01)01269-7. [DOI] [PubMed] [Google Scholar]
- 117.Funatsu H, Yamashita H, Shimizu E, Kojima R, Hori S. Relationship between vascular endothelial growth factor and interleukin-6 in diabetic retinopathy. Retina Phila Pa. 2001;21(5):469–77. doi: 10.1097/00006982-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 118.Funatsu H, Yamashita H, Ikeda T, Mimura T, Eguchi S, Hori S. Vitreous levels of interleukin-6 and vascular endothelial growth factor are related to diabetic macular edema. Ophthalmology. 2003 Sep;110(9):1690–6. doi: 10.1016/S0161-6420(03)00568-2. [DOI] [PubMed] [Google Scholar]
- 119.Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El-Deiry WS, Fulda S, Gottlieb E, Green DR, Hengartner MO, Kepp O, Knight RA, Kumar S, Lipton SA, Lu X, Madeo F, Malorni W, Mehlen P, Nuñez G, Peter ME, Piacentini M, Rubinsztein DC, Shi Y, Simon H-U, Vandenabeele P, White E, Yuan J, Zhivotovsky B, Melino G, Kroemer G. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012 Jan;19(1):107–20. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009 Feb;7(2):99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Denes A, Lopez-Castejon G, Brough D. Caspase-1: is IL-1 just the tip of the ICEberg? Cell Death Dis. 2012 Jul;3(7):e338. doi: 10.1038/cddis.2012.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Trueblood KE, Mohr S, Dubyak GR. Purinergic regulation of high-glucose-induced caspase-1 activation in the rat retinal Müller cell line rMC-1. Am J Physiol Cell Physiol. 2011 Nov;301(5):C1213–1223. doi: 10.1152/ajpcell.00265.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Küser-Abalı G, Ozcan F, Ugurlu A, Uysal A, Fuss SH, Bugra-Bilge K. SIK2 Is Involved in the Negative Modulation of Insulin-Dependent Müller Cell Survival and Implicated in Hyperglycemia-Induced Cell Death. Invest Ophthalmol Vis Sci. 2013 May 1;54(5):3526–37. doi: 10.1167/iovs.12-10729. [DOI] [PubMed] [Google Scholar]
- 124.Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS, Golstein P, Green DR, Hengartner M, Knight RA, Kumar S, Lipton SA, Malorni W, Nuñez G, Peter ME, Tschopp J, Yuan J, Piacentini M, Zhivotovsky B, Melino G Nomenclature Committee on Cell Death 2009. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009 Jan;16(1):3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Labbé K, Saleh M. Cell death in the host response to infection. Cell Death Differ. 2008 Jun 20;15(9):1339–49. doi: 10.1038/cdd.2008.91. [DOI] [PubMed] [Google Scholar]
- 126.Hori S, Mukai DN. Ultrastructural lesions of retinal pericapillary Müller cells in streptozotocin-induced diabetic rats. Albrecht Von Graefes Arch Für Klin Exp Ophthalmol. 1980 Mar 1;213(1):1–9. doi: 10.1007/BF02391205. [DOI] [PubMed] [Google Scholar]
- 127.Hara MR, Agrawal N, Kim SF, Cascio MB, Fujimuro M, Ozeki Y, Takahashi M, Cheah JH, Tankou SK, Hester LD, Ferris CD, Hayward SD, Snyder SH, Sawa A. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nat Cell Biol. 2005;7(7):665–74. doi: 10.1038/ncb1268. [DOI] [PubMed] [Google Scholar]
- 128.Leisner TM, Moran C, Holly SP, Parise LV. CIB1 prevents nuclear GAPDH accumulation and non-apoptotic tumor cell death via AKT and ERK signaling. Oncogene. 2012 Sep 10; doi: 10.1038/onc.2012.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nakajima H, Amano W, Kubo T, Fukuhara A, Ihara H, Azuma Y-T, Tajima H, Inui T, Sawa A, Takeuchi T. GAPDH aggregate formation participates in oxidative stress-induced cell death. [cited 2013 Aug 8];J Biol Chem [Internet] 2009 Oct 16; doi: 10.1074/jbc.M109.027698. Available from: http://www.jbc.org/content/early/2009/10/16/jbc.M109.027698. [DOI] [PMC free article] [PubMed]
- 130.Yego ECK, Mohr S. Siah-1 Protein Is Necessary for High Glucose-Induced Glyceraldehyde-3-Phosphate Dehydrogenase Nuclear Accumulation and Cell Death in Müller Cells. J Biol Chem. 2010 Jan 29;285(5):3181–90. doi: 10.1074/jbc.M109.083907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Walker RJ, Steinle JJ. Role of β-Adrenergic Receptors in Inflammatory Marker Expression in Müller Cells. Invest Ophthalmol Vis Sci. 2007 Nov 1;48(11):5276–81. doi: 10.1167/iovs.07-0129. [DOI] [PubMed] [Google Scholar]
- 132.Walker RJ, Anderson NM, Jiang Y, Bahouth S, Steinle JJ. Role of β-Adrenergic Receptor Regulation of TNF-α and Insulin Signaling in Retinal Müller Cells. Invest Ophthalmol Vis Sci. 2011 Dec 1;52(13):9527–33. doi: 10.1167/iovs.11-8631. [DOI] [PMC free article] [PubMed] [Google Scholar]