Abstract
Construction of vascularized tissues is one of the major challenges of tissue engineering. The goal of this study was to engineer 3D microvascular tissues by incorporating the HUVEC-CS cells with a collagen/alginate-chitosan (AC) microcapsule scaffold. In the presence of AC microcapsules, a 3D vascular-like network was clearly observable. The results indicated the importance of AC microcapsules in engineering microvascular tissues -- providing support and guiding alignment of HUVEC-CS cells. This approach provides an alternative and promising method for constructing vascularized tissues.
Keywords: Microcapsule, Alginate, Chitosan, Tissue Engineering, Vascularization
There is an increasing need for organ/tissue for transplantation.1,2 Tissue engineering, using cells and a scaffold mimicking extracellular matrix (ECM), offers a promising approach for constructing clinically available organs/tissues.3 It is well known that ECM provides both structural integrity and biophysical and chemical signaling cues to direct cell behavior and function.4 Currently, there is a great interest in engineering ECM scaffolds, e.g., nano-scale scaffold 4 and perfusion decellualrization.5 The major challenge with tissue engineering is to construct vascularized organs/tissues.6 Different approaches have been developed for overcoming this obstacle, such as construction of 3D vascularized cardiac tissue with cell sheet engineering and vascular bed bioreactors.7 Interestingly, a nature-inspired microfluidic network (mimicking plant leaf venation) has been investigated for fabricating a perfusable tissue construct.8 Recently, a lung alveolus-like structure has been developed by using the collagen-matrigel/microcapsule scaffold.9 The alginate-ploy-L-lysine-alginate (APA) microcapsules were used and proven not only to have a space occupying effect but also served as a seeding cell growth scaffold. In this study, a collagen/alginate-chitosan (AC) microcapsule scaffold was developed to test its potential for constructing microvascular tissue for the first time. An AC microcapsule was chosen because of its positive surface charge, which is due to the protonated amino groups 10, since a positive charged surface is preferred for cell adhesion.11,12 Moreover, chitosan-based hydrogel have been developed for creating endothelial cell agregate-induced microvascular networks, respectively. 13
To fabricate alginate-chitosan (AC) microcapsules, the calcium alginate microcapsules (~ 100 micrometers) generated by electrostatic spray were washed using 0.5 M mannitol (in saline) for 6 minutes and suspended in chitosan (0.4% w/v in saline) solution (pH 6.5–6.6) for 6 minutes to obtain the alginate-chitosan (AC) microcapsules. 10, 14 For the vascular endotheial growth factor (VEGF; BD; Franklin Lakes, NJ) loaded AC microcapsules, VEGF (0.1 µg/mL) was added to the alginate solution. Form there, VEGF loaded C microcapsules were prepared in the same way as those without VEGF.
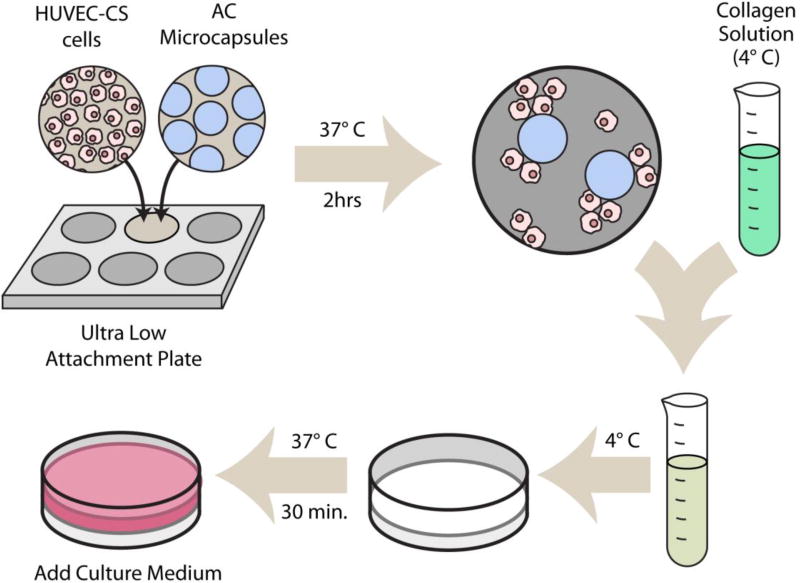
The prepared AC microcapsules were tested with different concentrations of collagen to determine the effect of collagen concentration on the formation of microvascularized 3D tissue structure. The scaffold fabrication process is shown in Scheme 1. The human umbilical vein endothelial cells (HUVEC-CS; ATCC, Manassas, VT) (0.7 M cells/mL) were incubated with AC microcapsules withut VEGF for 2 hours at 37 °C, using the ultralow attachment plates to enhance the adhesion of the HUVEC-CS cells to the microcapsules. The cells and microcapsules were then mixed with collagen of three different final concentrations: 0.5, 1 and 2 mg/mL at 4 °C. Thereafter, 0.5 mL of the different mixtures was transferred into individual wells of 6-well ultralow attachment plates (Corning). After 45 min. gelation to form the scaffold, 1 mL of pre-warmed medium was added into each well. At day 3, the cell viability was evaluated by using the cytotoxicity kit for mammalian cells (Invitrogen; Carlsbad, CA), and the samples were then examined using a Zeiss LSM 510 confocal microscope.
Scheme 1.
A schematic illustration of the procedure for fabricating scaffold formation for generating vascularized tissues: (1) incubating HUVEC-CS cells with AC microcapsules; (2) mixing with collagen solution; (3) gelling the mixture to form the scaffold.
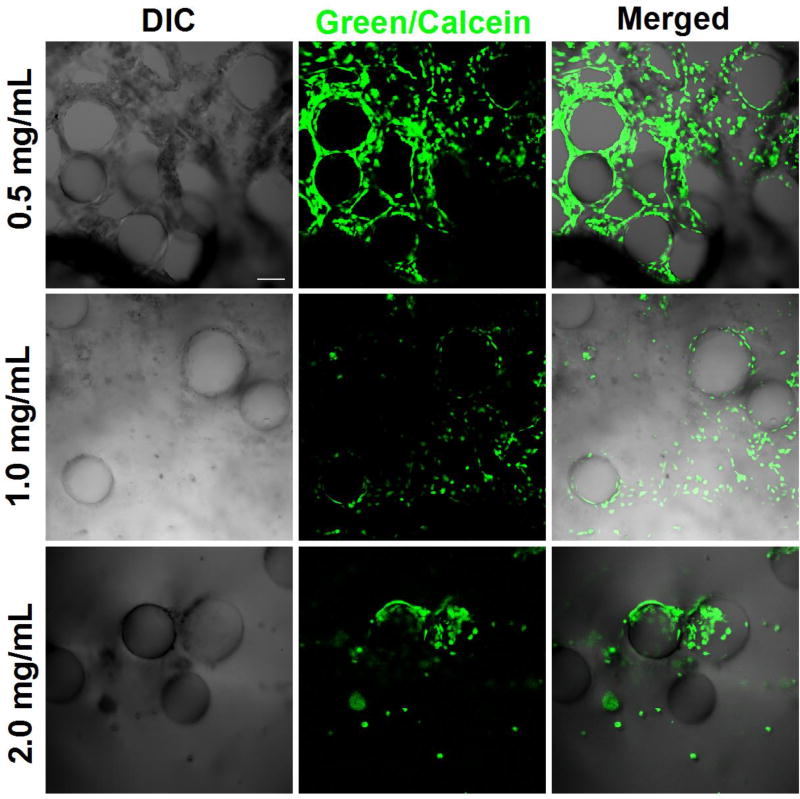
Live cells show up as green while dead cells were stained as red. As shown in Figure 1, cell viability of all the samples was high since the majority (> 97 %) showed bright green fluorescence. When the collagen concentration was 0.5 mg/mL, the HUVEC-CS cells self-assembled around the AC microcapsules and formed a three dimensional (3D) vascular-like network. However, this 3D microvascularized tissue structure was not observable when the collagen concentration was equal or greater than 1 mg/ml. Instead, the cells aggregated together. This might be due to the high collagen concentration which inhibits the cell migration that forms the vasculature network -- indicating the importance of the mechanical aspects of cell migration.15 The 0.5 mg/mL concentration was used for the rest of the study.
Fig. 1.
Confocal images of the HUVEC-CS cells within different collagen/AC microcapsule scaffolds. As the collagen concentrations were increased, the cells tended to aggregate together. Scale bar: 100 µm.
Cell density also influenced microvascularized tissue formation greatly. HUVEC-CS cells of different density, 0.2, 0.5 and 1 M cells/mL, were incubated with AC microcapsules (made out of 50 µl alginate solution) for 2 hours at 37 °C using ultralow attachment plates. The scaffolds were then prepared in the same way as described above.
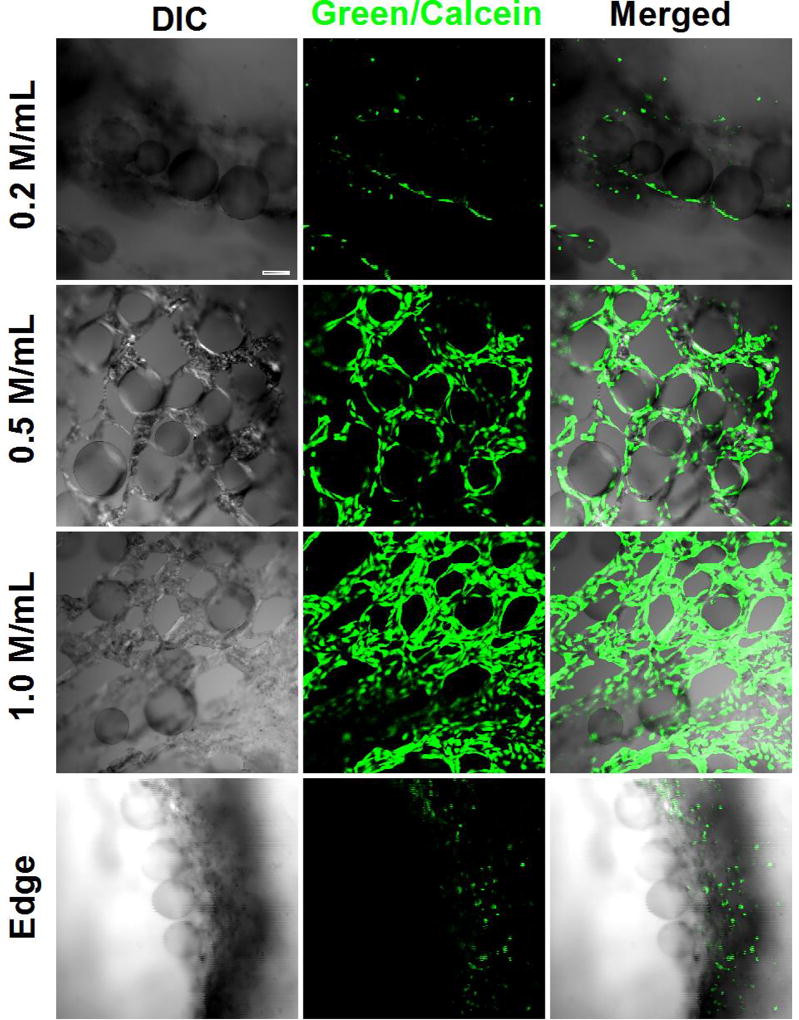
A cell density of ~ 0.5 M cells/mL was found to be optimal for the given amount of AC microcapsules (without VEGF) used. Cells aligned well within the engineered ECM with a small percentage of cells present on the edge. However, when the cell density was low, 0.2 M cells/mL, no clear 3D microvascular structure was observable (top panel of Figure 2). On the contrary, at a high cell density of 1.0 M cells/mL, most of the cells accumulated at the edge of the engineered matrix rather than forming the vascular structure (bottom panel of Figure 2).
Fig. 2.
Confocal images show the influences of HUVECS-CS density on 3D microvascular tissue formation. When the cell density is lower than 0.5 M/cells (top panel), no continuous vascular structure could be observed. On the contrary, HVCES-CS cells tend to migrate to the edge of the dish without contributing to the network formation (bottom panel). Scale bar: 100 µm.
AC microcapsule and VEGF’s influences on microvascularized 3D tissue formation was then studied. HUVEC-CS cells, 0.7 M cells/ mL, were incubated with the same amount of AC microcapsules with or without VEGF for 2 hours using ultralow attachment plates. Thereafter, 0.2 mL of different mixtures was transferred into individual wells of a 24-well ultralow attachment plates. After 45 min. gelation, 0.4 mL pre-warmed medium was added into each well. The samples were examined using a confocal microscope at different times, after culturing in the 24-well plates, to observe the vascularized tissue formation and development.
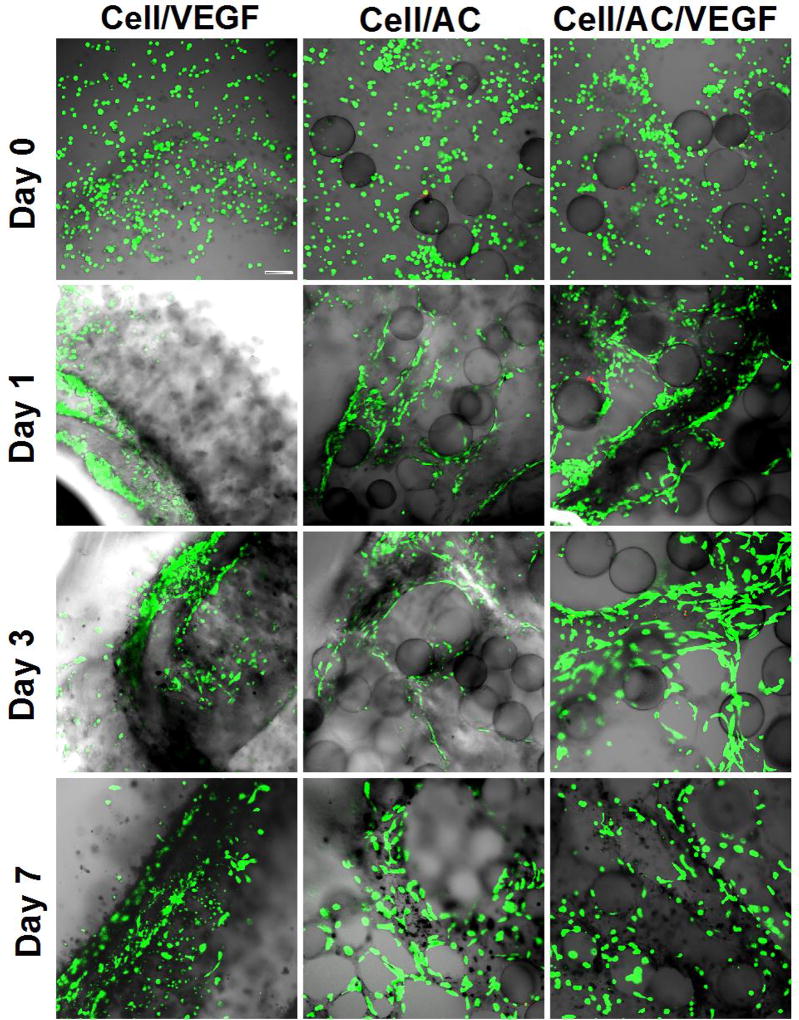
As shown in Figure 3, the cells were homogeneously distributed within the engineered ECM. Without AC microcapsules, only a single loop of the vessel-like structure was formed in each well. In the presence of AC microcapsules, a 3D vascular-like microvascular network was clearly observable at day 1, 3, and 7 (Figure 3). VEGF was used to enhance network formation, although no significant visual difference could be observed between the two groups with AC microcapsules. These results indicate that AC microcapsules, both with or without VEGF, are crucial in guiding the self-assembly or alignment of HUVEC cells to form complex 3D microvascularized tissue.
Fig. 3.
Confocal images of different samples at different time points. Microvascular structure formed within the samples with AC (alginate-chitosan) microcapsules. Staining of the cells: green for live cells (calcein) and red for dead cells (EthD-1). Cell: HUVEC-CS and AC: AC microcapsule. Scale bar: 100 µm.
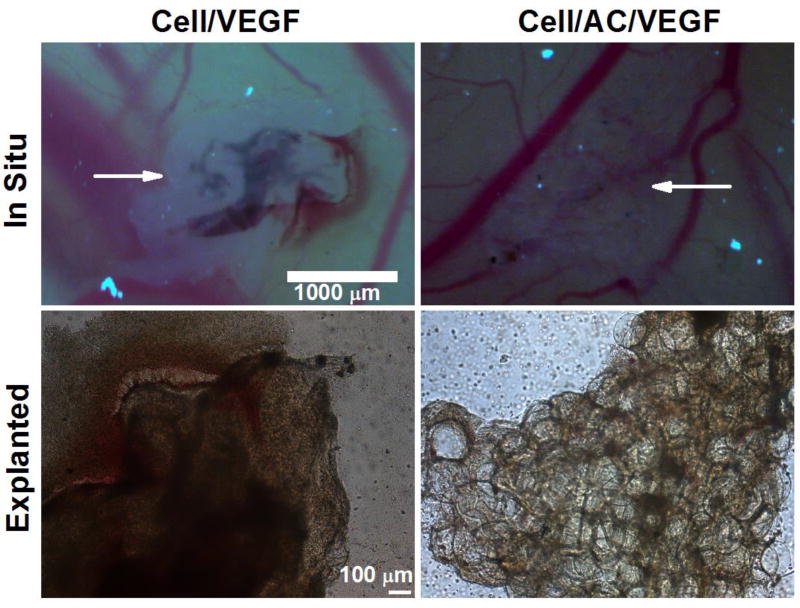
Lastly, chick chorioallantoic membrane (CAM) assay was performed to test the in vivo microvascularized 3D tissue structure formation. The protocol was designed according to literature 16 and 17. In brief, fertilized chicken eggs, obtained from the College of Veterinary Medicine at The Ohio State University, were incubated at 37 °C in an egg incubator (ThermoFisher) for 3 days. Then, a total of 2–3 mL of albumin was then removed from the egg using a 3 mL syringe. One day later, a round window was cut in the eggshell to confirm that the blood vessels in the chorioallantoic membrane were clearly visible, and then the window was sealed. On day 6, the window was re-opened and the samples were placed on the chorioallantoic membrane and covered with a ThermanoxTM coverslip after a gentle laceration was made to the membrane surface. After 48 hours, the samples were examined microscopically in situ and after being explanted from the chorioallantoic membrane.
The microscopic images of the HUVEC-CS/free VEGF (left column) and HUVEC-CS/VEGF loaded AC microcapsules (right column) samples are shown in Figure 4. Blood was observed in both samples, indicating the vasculature in the sample was anastomosed with blood vessels in the chorioallantoic membrane. In the HUVEC-CS/free VEGF sample, blood was visible only in the loop-like area where the HUVEC-CS cells assembled to form a single loop-like capillary (Figure 4). However, the sample with VEGF loaded AC microcapsules showed many spotty areas of blood were visible which indicated the formation of a complex 3D microvascular network in the engineered ECM.
Fig. 4.
Microscopic images of the CAM assay samples. Top panel: images of the samples in situ. Bottom panel: images of samples explanted from the membrane. Arrows indicate the location of the samples.
In summary, microvascular tissues were successfully developed by using the engineered ECM composed of collagen, vein endothelial cells, and alginate-chitosan (AC) microcapsules with/without VEGF in this study. Endothelial cells were incubated with AC microcapsules to enhance the cell adhesion before scaffold formation. AC microcapsules play an important role in vascular network formation by providing the support for cells and guiding the cell alignment/organization. Future studies will be performed on the cell and AC microcapsules interaction, optimization of the scaffold components, e.g. VEGF concentration, scaffold stability studies, as well as the cryopreservation of the scaffold.
Acknowledgments
This work was partially supported by grants from NSF (CBET-1154965) and NIH (R01EB012108). We thank Stacey Opperwall and Victoria Pink for assisting with the proofreading of this manuscript.
References
- 1.Vogiatzi P. Some considerations on the current debate about typing resolution in solid organ transplantation. Transplant. Res. 2016;5:3. doi: 10.1186/s13737-016-0032-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rouchi A, Mahdavi-Mazdeh M. Regenerative Medicine in Organ and Tissue Transplantation: Shortly and Practically Achievable? Int. J. Organ Transplant. Med. 2015;6:93. [PMC free article] [PubMed] [Google Scholar]
- 3.Bajaj P, Schweller RM, Khademhosseini A, West JL, Bashir R. 3D biofabrication strategies for tissue engineering and regenerative medicine. Annu. Rev. Biomed. Eng. 2014;16:247. doi: 10.1146/annurev-bioeng-071813-105155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakayama KH, Hong G, Lee JC, Patel J, Edwards B, Zaitseva TS, Paukshto MV, Dai H, Cooke JP, Woo YJ, Huang NF. Aligned-Braided Nanofibrillar Scaffold with Endothelial Cells Enhances Arteriogenesis. ACS Nano. 2015;9:6900. doi: 10.1021/acsnano.5b00545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Q, Bao J, Zhou YJ, Wang YJ, Du ZG, Shi YJ, Li L, Bu H. Optimizing perfusion-decellularization methods of porcine livers for clinical-scale whole-organ bioengineering. Biomed. Res. Int. 2015;2015:785474. doi: 10.1155/2015/785474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Novosel EC, Kleinhans C, Kluger PJ. Vascularization is the key challenge in tissue engineering. Adv. Drug Deliv. Rev. 2011;63:300. doi: 10.1016/j.addr.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Sakaguchi K, Shimizu T, Okano T. Construction of three-dimensional vascularized cardiac tissue with cell sheet engineering. J. Control. Release. 2015;205:83. doi: 10.1016/j.jconrel.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 8.He J, Mao M, Liu Y, Shao J, Jin Z, Li D. Fabrication of nature-inspired microfluidic network for perfusable tissue constructs. Adv. Healthc. Mater. 2013;2:1108. doi: 10.1002/adhm.201200404. [DOI] [PubMed] [Google Scholar]
- 9.Zhang WJ, Lin QX, Zhang Y, Liu CT, Wang HB, Wang YM, Duan CM, Liu ZQ, Zhou J, Wang CY. The reconstruction of lung alveolus-like structure in collagen-matrigel/microcapsules scaffolds in vitro. J. Cell Mol. Med. 2011;15:1878. doi: 10.1111/j.1582-4934.2010.01189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang W, Zhao S, Rao W, Snyder J, Choi JK, Choi J, Wang J, Khan IA, Saleh NB, Mohler PJ, Yu J, Hund TJ, Tang C, He X. A Novel Core-Shell Microcapsule for Encapsulation and 3D Culture of Embryonic Stem Cells. J. Mater. Chem. B Mater. Biol. Med. 2013;2013:1002. doi: 10.1039/C2TB00058J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ko JH, Kim YH, Jeong SH, Lee S, Park SN, Shim IK, Kim SC. Collagen esterification enhances the function and survival of pancreatic beta cells in 2D and 3D culture systems. Biochem. Biophys. Res. Commun. 2015;463:1084. doi: 10.1016/j.bbrc.2015.06.062. [DOI] [PubMed] [Google Scholar]
- 12.Suga T, Osada S, Narita T, Oishi Y, Kodama H. Promotion of cell adhesion by low-molecular-weight hydrogel by Lys based amphiphile. Mater. Sci. Eng. C Mater. Biol. Appl. 2015;47:345. doi: 10.1016/j.msec.2014.11.032. [DOI] [PubMed] [Google Scholar]
- 13.Kim S, Kawai T, Wang D, Yang YP. Engineering a Dual-Layer Chitosan-Lactide Hydrogel to Create Endothelial Cell Aggregate-induced Microvascular Networks In Vitro and Increase Blood Perfusion In Vivo. ACS Appl. Mater. Interfaces. 2016 doi: 10.1021/acsami.6b04431. In Press. [DOI] [PubMed] [Google Scholar]
- 14.Zhang W, Zhao S, He X. Proliferation and Differentiation of Mesenchymal Stem Cells Encapsulated in Miniaturized 3D Liquid Core of Alginate-Chitosan-Alginate (ACA) Microcapsules. Arch. of Stem Cell Res. 2015;2:1004. [Google Scholar]
- 15.Kaufman LJ, Brangwynne CP, Kasza KE, Filippidi E, Gordon VD, Deisboeck TS, Weitz DA. Glioma expansion in collagen I matrices: analyzing collagen concentration-dependent growth and motility patterns. Biophys. J. 2005;89:635. doi: 10.1529/biophysj.105.061994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dohle DS, Pasa SD, Gustmann S, et al. Chick ex ovo culture and ex ovo CAM assay: how it really works. J. Vis. Exp. 2009;33:1620. doi: 10.3791/1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takigawa M, Enomoto M, Nishida Y, Pan HO, Kinoshita A, Suzuki F. Tumor angiogenesis and polyamines: alpha-difluoromethylornithine, an irreversible inhibitor of ornithine decarboxylase, inhibits B16 melanoma-induced angiogenesis in ovo and the proliferation of vascular endothelial cells in vitro. Cancer Res. 1990;50:4131. [PubMed] [Google Scholar]