Abstract
High salt diet elevates NaCl concentrations in the cerebrospinal fluid to increase sympathetic nerve activity (SNA) in salt-sensitive hypertension. The organum vasculosum of the lamina terminalis (OVLT) resides along the rostral wall of the third ventricle, lacks a complete blood-brain-barrier, and plays a pivotal role in body fluid homeostasis. Therefore, the present study used a multi-faceted approach to examine whether OVLT neurons of Sprague Dawley rats are intrinsically sensitive to changes in extracellular NaCl concentrations and mediate the sympathoexcitatory responses to central NaCl loading. Using in vitro whole-cell recordings, step-wise increases in extracellular NaCl concentrations (2.5–10mM) produced concentration-dependent excitation of OVLT neurons. Additionally, these excitatory responses were intrinsic to OVLT neurons as hypertonic NaCl evoked inward currents despite pharmacologic synaptic blockade. In vivo single-unit recordings demonstrate the majority of OVLT neurons (72%, 13/19) display concentration-dependent increases in neuronal discharge to intracarotid (50µL/15s) or intracerebroventricular infusion (5µL/10min) of hypertonic NaCl. Microinjection of hypertonic NaCl (30nL/60s) into the OVLT, but not adjacent areas, increased lumbar SNA, adrenal SNA, and ABP in a concentration-dependent manner. Renal SNA decreased, and splanchnic SNA remained unaffected. Finally, local inhibition of OVLT neurons with the GABAA receptor agonist muscimol (24nL/10s) significantly attenuated the sympathoexcitatory and pressor responses to intracerebroventricular infusion of 0.5M or 1.0M NaCl. Collectively, these findings indicate that OVLT neurons detect changes in extracellular NaCl concentrations to selectively alter SNA and raise ABP.
Keywords: sodium, blood pressure, sympathetic, hypothalamus, osmoreceptor
INTRODUCTION
Time-controlled studies in both humans and rodents suggest a high salt diet elevates cerebrospinal fluid (CSF) NaCl concentrations to subsequently increase sympathetic nerve activity (SNA) and arterial blood pressure (ABP).1, 2 For example, a high salt diet elevates plasma or CSF NaCl concentrations by 3–6mM in salt-sensitive subjects.3–6 Similarly, elevated CSF Na+ concentrations have been reported in Dahl Salt-Sensitive, Spontaneously Hypertensive, and Grollman Renal Wrap rats.7–9 Prior studies suggest the putative NaCl-sensing neurons reside within the anteroventral third ventricular region (AV3V).10–13 Lesion of AV3V prevents/attenuates the sympathoexcitatory and pressor response to central hypernatremia12–14 and neurogenic forms of salt-sensitive hypertension.15–17 AV3V is comprised of several hypothalamic nuclei bordering the rostral third ventricle including the periventricular preoptic, medial preoptic, ventral median preoptic, organum vasculosum of the lamina terminalis (OVLT), and fibers of passage from the subfornical organ (SFO).18 The OVLT and SFO have incomplete blood- and CSF- brain barriers, and are uniquely exposed to circulating neurohumoral factors.
Some evidence suggests that OVLT may contain neurons that detect elevations in NaCl to increase SNA and ABP in response to central hypernatremia. For instance, hypernatremia increase the number of Fos-positive neurons within OVLT.19, 20 Second, electrical stimulation of OVLT neurons increases SNA.21, 22 However, contemporary knowledge regarding OVLT neuronal responses to hyperosmotic stimuli are premised upon electrophysiological responses to hypertonic mannitol, but not NaCl.23 The distinction between hypertonic NaCl versus mannitol is important as central infusion of hypertonic NaCl elicits sympathoexcitation, but hypertonic mannitol does not.24–26
This study employed a multi-faceted approach to establish whether OVLT neurons are excited by physiologic elevations NaCl concentrations and mediate downstream NaCl-dependent sympathoexcitatory increases in ABP. Since these experiments represent the first step to identify a role for OVLT in NaCl-dependent responses, experiments were conducted in Sprague-Dawley rats. In vitro studies reveal that OVLT neurons display intrinsic, concentration-dependent excitation during 2–5% increases in NaCl concentrations. Additional in vivo neurophysiological experiments demonstrate central hypernatremia excites OVLT neurons to increase SNA and ABP.
METHODS
All of the experimental procedures conform to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the Pennsylvania State College of Medicine and University of Pittsburgh School of Medicine. Male Sprague-Dawley rats (250–400g, Charles River Laboratories) housed in a temperature-controlled room (22±1°C) with a 12-hour dark:light cycle, fed standard chow (Harlan Teklad Global Diet 2018), and given access to deionized water. A detailed methods section is available online (please see http://hyper.ahajournals.org).
In Vitro Electrophysiological Recording of OVLT Neurons
Whole-cell patch clamp recordings of spontaneously active OVLT neurons were obtained from coronal hypothalamic sections in adult Sprague Dawley rats. Slices were continuously perfused by oxygenated and heated (31°C) Krebs Buffer (composition in mM): 126 NaCl, 25 NaHCO3, 2.5 KCl, 1.2 MgCl2, 2.4 CaCl2, 1.2 NaH2PO4, and 11 d-glucose (pH 7.4 and 295 mOsm/L). Hypertonic solutions (+2.5, +5, or +10mM NaCl) were prepared by the addition of NaCl to the extracellular control solution (+0mM). OVLT neurons were classified as NaCl-sensitive by evidence of a >25% increase in peak action potential (AP) firing rate in response to a +5mM NaCl stimulus (3 min duration). This firing rate was calculated as the number of spikes averaged over a 60s interval. Only one OVLT slice was obtained per rat, and 1–2 OVLT neurons were recorded per slice. Different sets of slices were used for each experimental set.
Experiment 1: OVLT Neuron Excitation by Step-wise Hypertonic NaCl
In current clamp, mean membrane potential and firing rates were measured throughout step-wise increases in bath NaCl concentration during baseline (+0mM NaCl; 3–5min), +2.5mM NaCl (3min), +5mM NaCl (3min), and washout (+0mM hypertonic NaCl stimuli. These variables (final 30s for each stimulus) were compared across baseline, +2.5mM NaCl, and +5mM NaCl stimuli.
Experiment 2: OVLT Neuron Excitation by Sustained Hypertonic NaCl
In current clamp, mean firing rates were measured throughout a sustained increase in bath NaCl concentration during baseline (+0mM NaCl; 3–5min) and +5mM NaCl (10min) hypertonic NaCl stimuli. Mean firing rates (60s) were compared across baseline, the 5–6min midpoint of +5mM NaCl, and the final 9–10min of +5mM NaCl.
Experiment 3: Intrinsic NaCl Sensitivity of OVLT Neurons
These experiments were performed in the presence of 30µM bicuculline (BIC), 1mM kynurenic acid (KYN) and 1µM tetrodotoxin (TTX). In voltage clamp, mean current responses were measured throughout a sustained increase in bath NaCl concentration during baseline (+0mM NaCl; 3min) and in response to either +5mM or +10mM NaCl (10min). Mean current responses (60s) were compared across stimuli and time points.
In Vivo Electrophysiology Experiments
Rats were anesthetized with inaction (120 mg/kg, IV) and prepared for simultaneous recordings of ABP (brachial artery and vein) and SNA (lumbar, renal, splanchnic, and adrenal) as described previously.12, 27, 28 A brain cannula was implanted into the lateral ventricle for intracerebroventricular (ICV) Infusions of artificial CSF 0.15M, 0.5M or 1.0M NaCl (5µL/10 min). The OVLT was targeted through a dorsal or ventral approach (please see http://hyper.ahajournals.org). Surgical preparation required ~2hrs and was followed by 5–10hrs of experimental procedures. Different sets of rats were used per in vivo experimental set. Responses to each NaCl stimulus were measured in every rat within an experimental set.
Experiment 4: OVLT Single Unit Responses to Central Hypernatremia
To facilitate the identification of NaCl-responsive OVLT neurons, the tip of a non-occlusive intracarotid catheter was placed into the internal carotid artery at 1.5mm rostral to the carotid bifurcation by insertion through the ascending pharyngeal artery. Through a ventral surgical approach, extracellular neurons recordings of OVLT neurons were performed during intracarotid (0.15 or 0.5M NaCl, 50µL/15s) or ICV (0.15, 0.5, or 1.0M NaCl, 5µL/10 min) infusion of iso- or hypertonic NaCl. Intracarotid injection of 0.5M NaCl should increase carotid NaCl concentrations <6–7% (assuming carotid blood flow is 5.5mL/min), whereas ICV infusion of 0.5M or 1.0M NaCl increase CSF [Na+] by ~3mM (2%) and ~7mM (5%).13
Experiment 5: OVLT Activation by Direct Injection of Hypertonic NaCl
Hypertonic NaCl (0.5, 1.0. or 1.5M) or aCSF (30nL/60s) was microinjected in a randomized sequence into the OVLT or sites 500µm adjacent. Variables were recorded for an additional 45 min.
Experiment 6: OVLT Inhibition by Muscimol During ICV Infusion of Hypertonic NaCl
The GABAA agonist muscimol (5mM/24nL) or aCSF (24nL/10s) was injected into the OVLT or sites 500µm adjacent. At 10 min later, ICV infusion of 0.15M, 0.5M, or 1.0M NaCl (5µL/10 min) was performed in a randomized manner, and variables were recorded for an additional 60 min.
Statistics
Analyses and graphs were prepared with SigmaPlot 11 or Systat 10.2. All data are presented as mean±SEM. Step-wise paradigm AP firing rate and membrane potential data were analyzed by comparing firing rates at baseline and in response to hypertonic NaCl stimuli by two-way repeated measures ANOVA and post hoc Holm-Sidak tests to evaluate difference between groups. Sustained paradigm AP firing rates were analyzed with Friedman repeated measures ANOVA on ranks and post hoc Tukey tests. Inward current data were analyzed by one-way repeated measures ANOVA for +5mM and +10mM hypertonic NaCl stimuli and post hoc Bonferroni comparisons. Differences between inward current responses to +5mM and +10mM NaCl stimuli were compared via inward current averages from the final 1min of NaCl stimulation followed by Student’s t test. Passive and active membrane properties between NaCl-sensitive and non-sensitive OVLT neurons were compared with either Mann-Whitney U tests or Student’s t tests depending on whether or not the data satisfied normality. Data from in vivo experiments were averaged into 1 min bins. Peak changes in all variables were compared to a 5 min baseline segment and analyzed by one or two-way ANOVA. When significant F values were obtained, layered Bonferroni paired or independent t-tests were performed to identify differences. Data for intracarotid infusions were averaged in 1 s bins, and peak changes (2s) were compared to a 30s baseline segment using a t-test. P<0.05 was statistically significant for all comparisons.
RESULTS
Experiment 1: OVLT Neuron Excitation by Step-wise Hypertonic NaCl
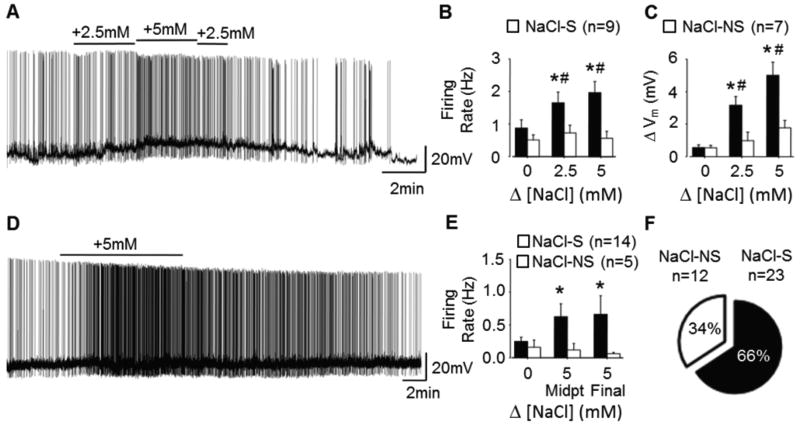
An initial goal was to establish whether OVLT neurons detect physiological changes in NaCl concentrations. Using whole-cell recordings, we identified 9/16 (56%) OVLT neurons as NaCl-sensitive (NaCl-S) defined by >25% increase in AP firing rate in response to +5mM NaCl. The remainder were NaCl-non-sensitive (NaCl-NS). Next, AP firing rates and membrane potentials were measured at baseline (+0mM) and in response to small, step-wise hypertonic NaCl stimuli (+2.5mM and +5mM; 3min each). Hypertonic NaCl induced concentration-dependent increases in NaCl-S, but not NaCl-NS, neuron AP firing rate (Figures 1A–B) and membrane depolarization (Figures 1A, C).
Figure 1.
OVLT neurons demonstrate concentration-dependent and sustained excitation in response to hypertonic NaCl. A, Current clamp trace of a NaCl-sensitive OVLT neuron held at approximately −55mV showing concentration-dependent excitation induced by hypertonic NaCl. B, Peak 30s AP firing rates in NaCl-S (9 neurons/7 rats) and NaCl-NS (7 neurons/5 rats) OVLT neurons during to hypertonic NaCl. C, Changes in membrane potential during peak firing rate intervals. D, Current clamp trace of NaCl-sensitive OVLT neuron held at approximately −55mV showing sustained excitation to a 10min hypertonic NaCl stimulus. E, AP firing rates during baseline, the 5–6min midpoint, and final 9–10min intervals in response to hypertonic NaCl in NaCl-S (14 neurons/12 rats) and NaCl-NS (5 neurons/4 rats) OVLT neurons. F, Proportion of NaCl-S versus NaCl-NS OVLT neurons. *p<0.05 compared to baseline #p<0.05 compared to NaCl-NS response to same stimulus.
Experiment 2: OVLT Neuron Excitation by Sustained Hypertonic NaCl
The next experiment assessed whether OVLT neuronal excitatory responses to hypertonic NaCl adapted with a more sustained stimulus exposure. Hypertonic NaCl (+5mM) induced sustained increases in AP firing rate of NaCl-S, but not NaCl-NS, OVLT neurons from baseline versus the 5–6min midpoint and the final 9–10min of stimulation (Figure 1D–E). Notably, there was no difference in AP firing rate between 5–6min versus 9–10min. One OVLT neuron hyperpolarized and reduced AP firing rate in response to hypertonic NaCl – that neuron was excluded from this analysis.
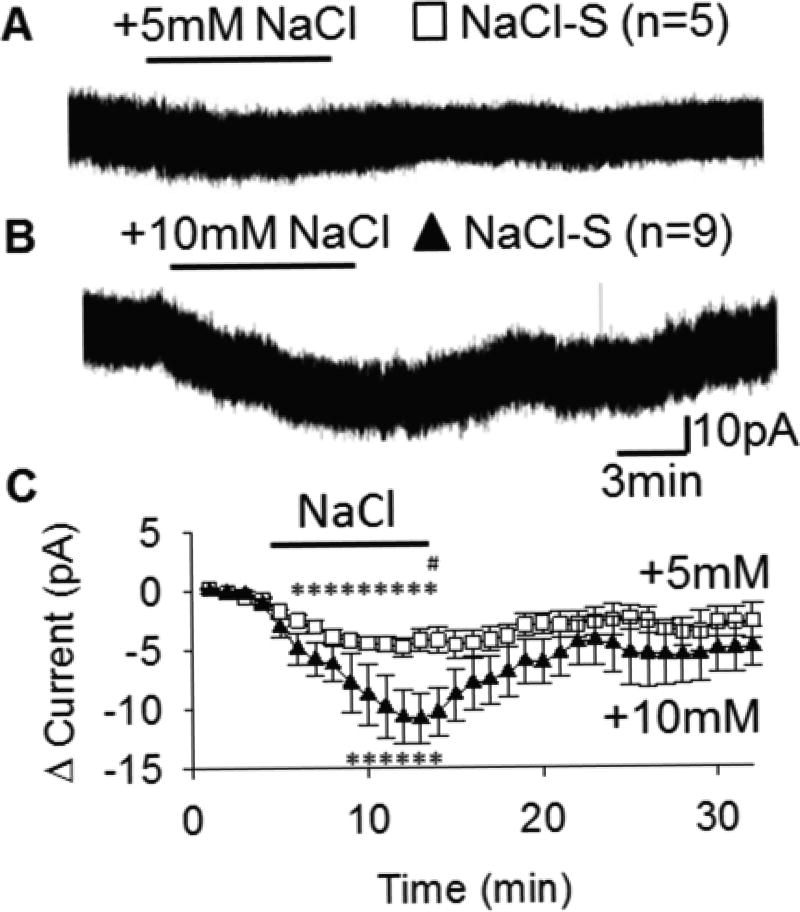
Experiment 3: Intrinsic NaCl Sensitivity of OVLT Neurons
We then interrogated whether these excitatory responses to hypertonic NaCl are intrinsic to OVLT neurons or mediated by synaptic neurotransmission onto OVLT. NaCl-S neurons were first identified in current clamp in the presence of an ionotropic glutamate receptor antagonist KYN (1mM), and a GABAA receptor antagonist BIC (30µM). Subsequently in voltage clamp (Vh = −50mV) with 1µM TTX to block action-potential dependent synaptic inputs, these NaCl-S neurons displayed concentration-dependent inward currents in response to either +5mM or +10mM NaCl (Figure 2A–B). Both +5mM and +10mM NaCl elicited significant inward currents compared to an isotonic NaCl baseline (Figure 2C). This inward current persisted for the remainder of the hypertonic NaCl stimulation. The average inward current during the final 1min of NaCl stimulation was significantly greater in response to +10mM versus +5mM NaCl (Figure 2C). Likewise, the magnitude of the integrated inward current (area above the curve) was significantly greater during +10mM NaCl (−75.25±16.98 pA*min) versus +5mM NaCl (−36.69±5.09 pA*min, P=0.023).
Figure 2.
NaCl-S OVLT neurons are intrinsically excited by hypertonic NaCl. NaCl-sensitive OVLT neurons voltage clamped at −50mV display intrinsic inward currents in response to +5mM (A) or +10mM (B) hypertonic NaCl in presence of 1µM TTX, 1mM KYN, 30µM BIC. C, 1-min changes in current from baseline among NaCl-S OVLT neurons in response to +5mM (5 neurons/5 rats) and +10mM (9 neurons/9 rats) hypertonic NaCl. *p<0.05 compared to baseline. #p<0.05 between +5mM and +10mM NaCl.
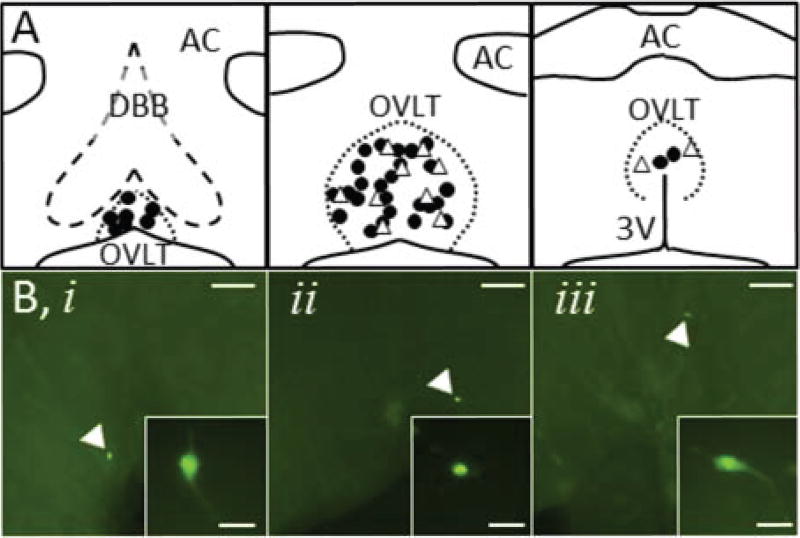
Post-hoc immunofluorescent identification of neurobiotin-filled OVLT neurons revealed that NaCl-S and NaCl-NS cells were distributed throughout all rostrocaudal levels of the OVLT (Figure 3). In addition, there were no significant differences between NaCl-S and NaCl-NS populations based on active and passive membrane properties derived from current pulse-stimulated APs and current-voltage relationships (Table S1, Figure S1).
Figure 3.
A, Location of NaCl-S (filled circles) and NaCl-NS (open triangles) neurons after post hoc immunofluorescent processing of neurobiotin. B, Neurobiotin-labelled NaCl-S neurons (green) were found throughout the OVLT: (i) rostrolateral, (ii) lateral margin, (iii) dorsal cap. Scale bars represent 100µm in 4× images and 20µm in 40× inset images. Panels A and B are plotted left to right in a rostral to caudal orientation at ~250µm intervals
Experiment 4. OVLT Single Unit Responses to Central Hypernatremia
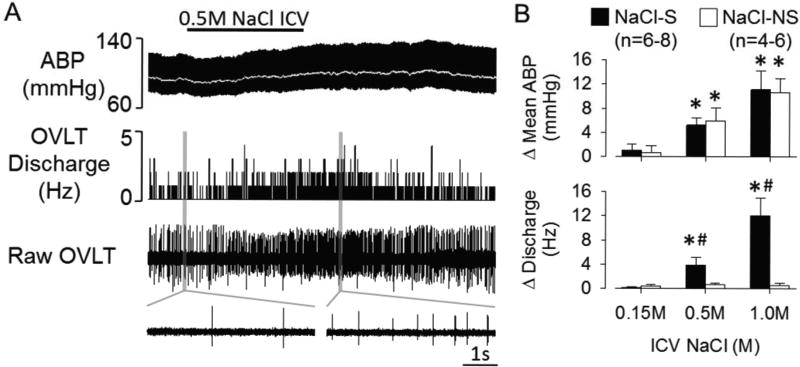
To determine the extent by which central hypernatremia alters the activity of OVLT neurons in vivo, we used a ventral approach to perform single-unit recordings of OVLT neurons during intracarotid or ICV infusion of iso- or hypertonic NaCl. NaCl-S or NaCl-NS OVLT neurons were initially distinguished by the discharge response to intracarotid injection of 0.5M NaCl (50µL/15s). Baseline mean ABP and heart rate were 92±4 and 387±12 bpm, respectively (n=12). The majority of OVLT neurons were NaCl-S (72%, 13/18) and increased discharge frequency to intracarotid injection of 0.5M NaCl (1.2±0.3 to 9.9±1.9 Hz, P<0.01). Intracarotid injection of 0.5M NaCl also produced a small increase in mean ABP (9±2 mmHg, P<0.05). Intracarotid injection of 0.15M NaCl did not alter cell discharge (1.1±0.2 to 1.3±0.3 Hz, P>0.5) or mean ABP (1±2 mmHg, P>0.6). These same NaCl-S neurons displayed concentration-dependent increases in cell discharge during ICV infusion of 0.5M or 1.0M NaCl (Figures 4A, B). The increased firing rate occurred within 2 min after the onset and remained elevated despite an increased mean ABP. In fact, both intracarotid and ICV infusion of hypertonic NaCl increased cell discharge before any significant changes in mean ABP. On the other hand, a subset of OVLT neurons (5/18, 28%) were NaCl-NS as intracarotid injection of 0.5M NaCl did not alter cell discharge (1.9±0.5 to 2.1±0.6 Hz, P>0.6). Similarly, ICV infusion of 0.5M or 1.0M NaCl did not alter cell discharge in NaCl-NS neurons (Figure 4). Juxtacellular labeling revealed NaCl-S neurons had processes that typically coursed caudally along the 3rd ventricle. NaCl-S or NaCl-NS OVLT neurons were anatomically distributed throughout the OVLT (Figure S2).
Figure 4.
A, ABP, mean ABP (grey line), rate meter histogram and raw OVLT neuronal discharge during ICV infusion of 0.5M NaCl (5µL/10 min). Insets below represent 5 s traces of baseline and peak OVLT neuronal discharge. B, Mean±SEM of peak change in mean ABP OVLT neuronal discharge for NaCl-S and NaCl-NS OVLT neurons. *P<0.05 vs 0.15M, #P<0.05 vs NaCl-NS.
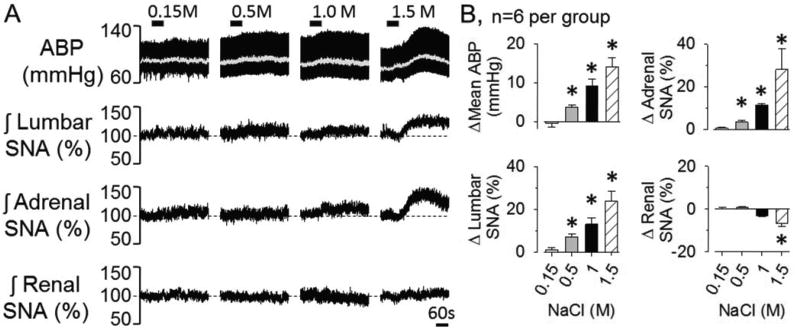
Experiment 5: OVLT Activation by Direct Injection of Hypertonic NaCl
The next experiment tested the extent by which local changes in extracellular NaCl concentrations within the OVLT elevated SNA and ABP. Again, half of the animals per group were performed using a dorsal (n=3) versus ventral (n=3) approach to target OVLT. Since there were no differences in baseline mean ABP, heart rate, or the responses to local injection of NaCl between ventral versus dorsal approaches, the data were combined. Baseline mean ABP and heart rate were 90±4 mmHg and 403±11 bpm, respectively. OVLT injection of NaCl produced a concentration-dependent increase in lumbar SNA, adrenal SNA and mean ABP (Figure 5). Typically, these variables began to change as the injection was performed. Renal and/or splanchnic SNA did not change from baseline values. Importantly, injection of 1.0M NaCl adjacent to the OVLT (rostral, lateral, and dorsal; n=4 per site) did not produce significant changes in any SNA or mean ABP. Injection sites are illustrated in the online supplement (Figure S3).
Figure 5.
(A) ABP, mean ABP (grey line) and integrated lumbar, adrenal, and renal SNA in response to OVLT microinjection of 0.15M, 0.5M, 1.0M, and 1.5M NaCl (30nL/60s). B, Peak changes, *P<0.05 vs 0.15M.
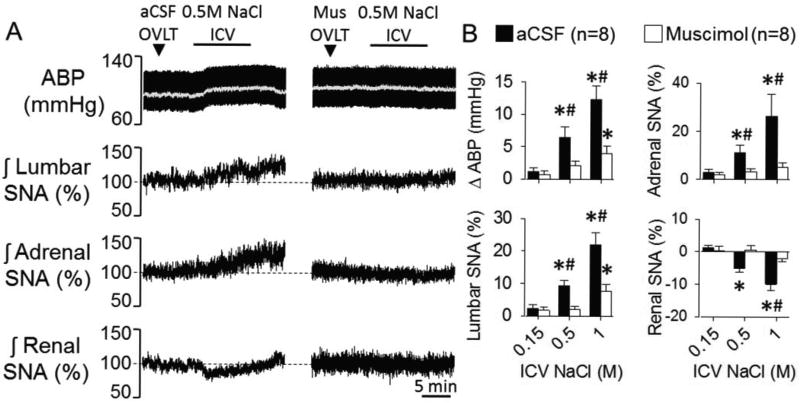
Experiment 6: OVLT Inhibition by Muscimol During ICV Infusion of Hypertonic NaCl
To evaluate whether OVLT neurons mediate the sympathoexcitatory response to changes in CSF NaCl concentrations, OVLT neurons were inhibited by injection of the GABAA agonist muscimol prior to ICV infusion of 0.5 or 1.0M NaCl. Half of the animals per group were performed using a dorsal (n=4) versus ventral (n=4) approach to target OVLT. Since there were no differences in baseline mean ABP, heart rate, or the responses to ICV infusion of NaCl between ventral versus dorsal approaches, the data were combined. Baseline mean ABP and heart rate were 95±4 mmHg and 399±13 bpm, respectively. Injection of aCSF into the OVLT did not alter SNA or mean ABP. As previously reported,13 ICV infusion of NaCl produced concentration-dependent increase in lumbar SNA, adrenal SNA, and ABP (Figure 6). Renal SNA decreased (Figure 6), and splanchnic SNA did not change (data not shown). Inhibition of OVLT neurons with local injection of the GABAA agonist did not alter any SNA or mean ABP. However, muscimol pretreatment significantly attenuated the sympathoexcitatory and pressor response to ICV infusion of 0.5M and 1.0M NaCl (Figure 6). In a separate set of animals, injection of muscimol rostral, lateral, or dorsal to OVLT did not alter the responses to ICV infusion of 0.5M or 1.0M NaCl (data not shown). Injection sites are illustrated in the online supplement (Figure S4).
Figure 6.
A, ABP, mean ABP (grey line) and integrated lumbar, adrenal, and renal SNA during ICV infusion of 0.5M NaCl (5µL/10 min) after microinjection of aCSF or the GABAA agonist muscimol (2.5mM/24nL). Splanchnic SNA did not change during any injection or infusion (data not shown). B, Mean±SEM of peak changes, *P<0.05 vs 0.15M, #P<0.05 vs muscimol.
DISCUSSION
This study provides the first evidence that physiologic elevations in NaCl concentrations excite OVLT neurons to increase SNA and ABP. We have made multiple novel observations that support this conclusion: (1) in vitro whole-cell recordings demonstrate OVLT neurons display concentration-dependent excitation to hypertonic NaCl (+2.5–10mM), (2) OVLT neurons are intrinsically NaCl-sensitive, as hypertonic NaCl stimulates an inward current in the presence of synaptic blockade, (3) intracarotid or ICV infusion of hypertonic NaCl produced concentration-dependent increases in OVLT neuronal discharge in vivo, (4) local OVLT microinjection of hypertonic NaCl produced concentration-dependent increases in lumbar SNA, adrenal SNA, and ABP, and (5) inhibition of OVLT neurons with local injection of the GABAA receptor agonist muscimol prevented the sympathoexcitatory response to ICV hypertonic NaCl.
CSF NaCl concentrations are elevated by +3–8mM in salt-sensitive hypertension.4, 7–9 Moreover, the elevation in CSF Na+ concentration may precede the development of salt-sensitive hypertension in Dahl Salt-Sensitive and Spontaneously Hypertensive rats fed a high salt diet.7, 8 Prior studies employing electrolytic lesions or Fos immunocytochemistry suggest the AV3V region, and more specifically OVLT, contain putative NaCl-responsive cells14, 29–31 ; yet evidence to indicate such neurons directly sensed physiological changes in NaCl concentrations was absent. The present study provides the first evidence on a cellular level that hypertonic NaCl (+2.5–10mM) causes concentration-dependent increases in OVLT neuron AP discharge frequency in vitro. These responses are likely intrinsic to OVLT neurons as hypertonic NaCl produced an inward current during pharmacologic blockade of synaptic neurotransmission. The response onsets to hypertonic NaCl were stimulus-locked, which suggests metabotropic receptor activation is less likely to initiate these responses. Still, this does not exclude possible contributions from atypical signaling molecules (e.g. ATP, lactate, hydrogen sulfide) originating from ependymal, astrocytic, or vascular elements within OVLT. These in vitro cellular responses were confirmed by in vivo single-unit recordings to demonstrate OVLT neurons were very sensitive to physiological changes in plasma or CSF NaCl concentrations (2–5%). Although the response magnitude to hypertonic NaCl was greater in vivo versus in vitro, these differences could be attributed to experimental preparation (ie, slice temperature, intact synaptic inputs). Nevertheless, both approaches provide clear evidence that OVLT neurons can sense and respond to discrete changes in extracellular NaCl concentrations.
Lesion of the AV3V region attenuates sympathoexcitatory responses to acute hypernatremia and also attenuates/prevents every neurogenic experimental model of salt-sensitive hypertension.10, 12, 13, 15–17 The current findings provide novel evidence to suggest OVLT neurons mediate these effects. First, local injection of hypertonic NaCl into the OVLT, but not adjacent to the OVLT, produced concentration-dependent increases in SNA and ABP. Second, inhibition of OVLT neurons by local injection of the GABAA agonist muscimol attenuated the sympathoexcitatory responses to ICV NaCl. Inhibition of neurons immediately adjacent to OVLT did not affect the sympathoexcitatory effect of central NaCl loading. Altogether, these site-specific effects indicate that the OVLT is a key neural substrate for NaCl-dependent regulation of ABP. The contribution of OVLT neurons to salt-sensitive hypertension is limited with the exception of one investigation in which OVLT lesion attenuated angiotensin II plus high salt hypertension.32
While local injection of muscimol into OVLT largely attenuated the sympathoexcitatory responses to ICV NaCl, the response was not completely eliminated. A second potential NaCl-sensing site is the SFO. Prior studies have reported the SFO contains NaCl-responsive neurons,31, 33, 34 and interruption of SFO neurotransmission attenuated pressor responses to ICV infusion of hypertonic NaCl.25 Although SFO lesions in rats only attenuate salt-sensitive hypertension in angiotensin II plus high salt model35–37, manipulation of SFO signaling in mice attenuates DOCA-salt hypertension.38 Hence, the SFO may also contribute to NaCl-sensing and salt-sensitive hypertension.
Central or peripheral infusion of hypertonic NaCl increases lumbar or muscle SNA in rodents and humans, respectively,12, 13, 39–41 but either has no effect or decreases renal SNA.26 Although a few studies report that intracarotid infusion of 0.75–1.50M NaCl (300µL) increases renal SNA, these renal sympathoexcitatory may be attributed to the extremely large increases in forebrain NaCl concentration (~15–100%).21, 42, 43 Indeed, our preliminary data indicate intracarotid infusion of 1.0M NaCl (50µL/15s) raises the firing rate of OVLT neurons to >40Hz (unpublished observation). Thus, these high magnitude intracarotid NaCl stimuli may activate OVLT and other sympathoregulatory centers. In the present study, ICV infusion of hypertonic NaCl decreased renal SNA. As reported and discussed previously,12, 13 this renal sympathoinhibitory response os mediated by a specific subset of RVLM neurons and attributed to a baroreflex-mediated inhibition or a centrally-mediated natriuretic response.
If the OVLT is the putative NaCl-sensitive site to regulate SNA and ABP, then what is the underlying mechanism by which these neurons detect changes in extracellular NaCl concentrations? Since ICV and intracarotid administration of hypertonic NaCl stimulates SNA and raises ABP, but infusion of equiosmolar mannitol does not24–26, there are likely distinct cellular processes that differentiate between hyperosmolality versus hypertonic NaCl. For example, supraoptic nucleus neurons respond to hyperosmotic mannitol stimuli via a mixed cationic inward current, whereas hypertonic NaCl evokes an inward current without clear ionic reversal potential.44 There are several potential mechanisms that may underlie cellular NaCl-sensing. First, within the supraoptic nucleus, chronic NaCl-loading collapses the chloride gradient, converts synaptic GABAergic inputs from inhibitory to excitatory, and causes a partially vasopressin-dependent elevation in ABP.45 Second, the NaX channel has also been implicated in Na+-sensing along the lamina terminalis. Within the rat median preoptic nucleus, NaX mediates a sodium leak current that is regulated by the Na+/K+ ATPase α1 isoform to facilitate neuronal sodium-sensing.46 Within the mouse SFO, hypertonic NaCl was found to augment a NaX-mediated sodium leak current in ependycytes to stimulate lactate release and excite proximal GABAergic interneurons.47 Third, ICV administration of benzamil, a non-voltage gated sodium channel blocker, attenuates vasopressin secretion, sympathoexcitatory responses to CSF hypernatremia, and salt-sensitive hypertension.48–50 This data suggests that one of several benzamil-sensitive channels (i.e. sodium-calcium antiporters, sodium-proton pumps, acid sensing ion channels, or epithelial sodium channels) represents the putative NaCl sensor. Nevertheless, the above mechanisms have not yet been identified to function within OVLT neurons.
Limitations
In the current study, OVLT neurons were classified as NaCl-S based on a ≥25% increase in peak firing rate during +5mM NaCl. This classification method may underestimate the number of NaCl-S OVLT neurons. Inter-neuronal differences in the timing and magnitude of the response may reflect differences in expression or function of the unidentified NaCl-sensing protein(s). Second, multiple in vivo methods were employed to raise central NaCl concentrations (ICV, intracarotid, OVLT-microinjection). Although it is difficult to directly measure local NaCl concentrations in the OVLT, NaCl concentrations were estimated or measured in the CSF to change 3–5%.13 Finally, in vivo experiments were conducted in anesthetized animal preparations. Although anesthetics will alter the magnitude of the responses, the distinct advantage of the preparation was the novel insight gained from simultaneous recording of multiple sympathetic nerves and single-unit recordings. However, future experiments are needed to extend these observations into awake and chronic models of salt-sensitive hypertension.
Perspectives
Herein, we have provided the first electrophysiological evidence that a majority of OVLT neurons are intrinsically excited by 2–5% elevations in NaCl concentrations. These responses are functionally significant as hypernatremia-induced activation of OVLT neurons elevates lumbar SNA, adrenal SNA, and ABP. The implication of the current findings is that ingestion of a high salt diet elevates CSF NaCl concentrations to activate OVLT neurons, which elevate blood pressure. Surprisingly, very little data exists regarding the function of OVLT neurons in salt-sensitive hypertension. Consequently, follow-up studies are needed to evaluate alterations in OVLT neuron function in salt-sensitive models and the chronic contribution of OVLT to salt-sensitive hypertension. For now, the undiscovered mechanism(s) of NaCl-sensing by OVLT neurons remains an enticing prospect of future investigation.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
1. What is new?
OVLT neurons are intrinsically excited by physiologically-relevant elevations in extracellular NaCl concentrations.
In vivo, site-specific activation of OVLT neurons by hypertonic NaCl selectively increases SNA and ABP.
In vivo, central hypernatremia increased the discharge frequency of OVLT neurons and to subsequently to elevate lumbar SNA, adrenal SNA, and ABP.
2. What is relevant?
Rodents and humans affected by salt-sensitive hypertension demonstrate similar (3–8mM) elevations in blood and CSF NaCl concentrations in response to a high salt diet. This suggests pathologic activation of OVLT neurons could contribute to salt-sensitive hypertension.
3. Summary
OVLT neurons are excited by physiologic increases in NaCl concentrations to selectively regulate SNA and elevate ABP.
Acknowledgments
None.
SOURCES OF FUNDING
The research was supported by National Heart, Lung, and Blood Institute Grant HL-113270 (S.D.S.) American Heart Association Established Investigator Grant (S.D.S.) and Great Rivers Predoctoral Fellowship 14PRE19530001 (B.J.K).
Footnotes
CONFLICTS OF INTEREST/DISCLOSURES
None.
References
- 1.Stocker SD, Monahan KD, Browning KN. Neurogenic and sympathoexcitatory actions of nacl in hypertension. Curr Hypertens Rep. 2013;15:538–546. doi: 10.1007/s11906-013-0385-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blaustein MP, Leenen FH, Chen L, Golovina VA, Hamlyn JM, Pallone TL, Van Huysse JW, Zhang J, Wier WG. How nacl raises blood pressure: A new paradigm for the pathogenesis of salt-dependent hypertension. Am J Physiol Heart Circ Physiol. 2012;302:H1031–1049. doi: 10.1152/ajpheart.00899.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmidlin O, Forman A, Sebastian A, Morris RC., Jr Sodium-selective salt sensitivity: Its occurrence in blacks. Hypertension. 2007;50:1085–1092. doi: 10.1161/HYPERTENSIONAHA.107.091694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawano Y, Yoshida K, Kawamura M, Yoshimi H, Ashida T, Abe H, Imanishi M, Kimura G, Kojima S, Kuramochi M, et al. Sodium and noradrenaline in cerebrospinal fluid and blood in salt-sensitive and non-salt-sensitive essential hypertension. Clin Exp Pharmacol Physiol. 1992;19:235–241. doi: 10.1111/j.1440-1681.1992.tb00444.x. [DOI] [PubMed] [Google Scholar]
- 5.Kawasaki T, Delea CS, Bartter FC, Smith H. The effect of high-sodium and low-sodium intakes on blood pressure and other related variables in human subjects with idiopathic hypertension. Am J Med. 1978;64:193–198. doi: 10.1016/0002-9343(78)90045-1. [DOI] [PubMed] [Google Scholar]
- 6.He FJ, Markandu ND, Sagnella GA, de Wardener HE, MacGregor GA. Plasma sodium: Ignored and underestimated. Hypertension. 2005;45:98–102. doi: 10.1161/01.HYP.0000149431.79450.a2. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura K, Cowley AW., Jr Sequential changes of cerebrospinal fluid sodium during the development of hypertension in dahl rats. Hypertension. 1989;13:243–249. doi: 10.1161/01.hyp.13.3.243. [DOI] [PubMed] [Google Scholar]
- 8.Huang BS, Van Vliet BN, Leenen FH. Increases in csf [na+] precede the increases in blood pressure in dahl s rats and shr on a high-salt diet. Am J Physiol Heart Circ Physiol. 2004;287:H1160–1166. doi: 10.1152/ajpheart.00126.2004. [DOI] [PubMed] [Google Scholar]
- 9.Haywood JR, Buggy J, Fink GD, DiBona GF, Johnson AK, Brody MJ. Alterations in cerebrospinal fluid sodium and osmolality in rats during one-kidney, one-wrap renal hypertension. Clin Exp Pharmacol Physiol. 1984;11:545–549. doi: 10.1111/j.1440-1681.1984.tb00865.x. [DOI] [PubMed] [Google Scholar]
- 10.Buggy J, Fink GD, Johnson AK, Brody MJ. Prevention of the development of renal hypertension by anteroventral third ventricular tissue lesions. Circ Res. 1977;40:I110–117. [PubMed] [Google Scholar]
- 11.McKinley MJ, Mathai ML, Pennington G, Rundgren M, Vivas L. Effect of individual or combined ablation of the nuclear groups of the lamina terminalis on water drinking in sheep. Am J Physiol. 1999;276:R673–683. doi: 10.1152/ajpregu.1999.276.3.R673. [DOI] [PubMed] [Google Scholar]
- 12.Simmonds SS, Lay J, Stocker SD. Dietary salt intake exaggerates sympathetic reflexes and increases blood pressure variability in normotensive rats. Hypertension. 2014;64:583–589. doi: 10.1161/HYPERTENSIONAHA.114.03250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stocker SD, Lang SM, Simmonds SS, Wenner MM, Farquhar WB. Cerebrospinal fluid hypernatremia elevates sympathetic nerve activity and blood pressure via the rostral ventrolateral medulla. Hypertension. 2015;66:1184–1190. doi: 10.1161/HYPERTENSIONAHA.115.05936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veerasingham SJ, Leenen FH. Excitotoxic lesions of the ventral anteroventral third ventricle and pressor responses to central sodium, ouabain and angiotensin ii. Brain Res. 1997;749:157–160. doi: 10.1016/s0006-8993(96)01381-9. [DOI] [PubMed] [Google Scholar]
- 15.Goto A, Ganguli M, Tobian L, Johnson MA, Iwai J. Effect of an anteroventral third ventricle lesion on nacl hypertension in dahl salt-sensitive rats. Am J Physiol. 1982;243:H614–618. doi: 10.1152/ajpheart.1982.243.4.H614. [DOI] [PubMed] [Google Scholar]
- 16.Berecek KH, Barron KW, Webb RL, Brody MJ. Vasopressin-central nervous system interactions in the development of doca hypertension. Hypertension. 1982;4:131–137. [PubMed] [Google Scholar]
- 17.Marson O, Saragoca MA, Ribeiro AB, Bossolan D, Tufik S, Ramos OL. Anteroventral third ventricle and renin-angiotensin system interaction in the two-kidney, one clip hypertensive rat. Hypertension. 1983;5:V90–93. doi: 10.1161/01.hyp.5.6_pt_3.v90. [DOI] [PubMed] [Google Scholar]
- 18.McKinley MJ, Pennington GL, Oldfield BJ. Anteroventral wall of the third ventricle and dorsal lamina terminalis: Headquarters for control of body fluid homeostasis? Clin Exp Pharmacol Physiol. 1996;23:271–281. doi: 10.1111/j.1440-1681.1996.tb02823.x. [DOI] [PubMed] [Google Scholar]
- 19.Oldfield BJ, Bicknell RJ, McAllen RM, Weisinger RS, McKinley MJ. Intravenous hypertonic saline induces fos immunoreactivity in neurons throughout the lamina terminalis. Brain Res. 1991;561:151–156. doi: 10.1016/0006-8993(91)90760-s. [DOI] [PubMed] [Google Scholar]
- 20.Larsen PJ, Mikkelsen JD. Functional identification of central afferent projections conveying information of acute "stress" to the hypothalamic paraventricular nucleus. J Neurosci. 1995;15:2609–2627. doi: 10.1523/JNEUROSCI.15-04-02609.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi P, Stocker SD, Toney GM. Organum vasculosum laminae terminalis contributes to increased sympathetic nerve activity induced by central hyperosmolality. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2279–2289. doi: 10.1152/ajpregu.00160.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stocker SD, Osborn JL, Carmichael SP. Forebrain osmotic regulation of the sympathetic nervous system. Clin Exp Pharmacol Physiol. 2008;35:695–700. doi: 10.1111/j.1440-1681.2007.04835.x. [DOI] [PubMed] [Google Scholar]
- 23.Ciura S, Bourque CW. Transient receptor potential vanilloid 1 is required for intrinsic osmoreception in organum vasculosum lamina terminalis neurons and for normal thirst responses to systemic hyperosmolality. J Neurosci. 2006;26:9069–9075. doi: 10.1523/JNEUROSCI.0877-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bunag RD, Miyajima E. Sympathetic hyperactivity elevates blood pressure during acute cerebroventricular infusions of hypertonic salt in rats. J Cardiovasc Pharmacol. 1984;6:844–851. doi: 10.1097/00005344-198409000-00017. [DOI] [PubMed] [Google Scholar]
- 25.Tiruneh MA, Huang BS, Leenen FH. Role of angiotensin ii type 1 receptors in the subfornical organ in the pressor responses to central sodium in rats. Brain Res. 2013;1527:79–86. doi: 10.1016/j.brainres.2013.06.028. [DOI] [PubMed] [Google Scholar]
- 26.Frithiof R, Xing T, McKinley MJ, May CN, Ramchandra R. Intracarotid hypertonic sodium chloride differentially modulates sympathetic nerve activity to the heart and kidney. Am J Physiol Regul Integr Comp Physiol. 2014;306:R567–575. doi: 10.1152/ajpregu.00460.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ward KR, Bardgett JF, Wolfgang L, Stocker SD. Sympathetic response to insulin is mediated by melanocortin 3/4 receptors in the hypothalamic paraventricular nucleus. Hypertension. 2011;57:435–441. doi: 10.1161/HYPERTENSIONAHA.110.160671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steiner JL, Bardgett ME, Wolfgang L, Lang CH, Stocker SD. Glucocorticoids attenuate the central sympathoexcitatory actions of insulin. J Neurophysiol. 2014;112:2597–2604. doi: 10.1152/jn.00514.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buggy J, Jonhson AK. Preoptic-hypothalamic periventricular lesions: Thirst deficits and hypernatremia. Am J Physiol. 1977;233:R44–52. doi: 10.1152/ajpregu.1977.233.1.R44. [DOI] [PubMed] [Google Scholar]
- 30.Thrasher TN, Keil LC, Ramsay DJ. Lesions of the organum vasculosum of the lamina terminalis (ovlt) attenuate osmotically-induced drinking and vasopressin secretion in the dog. Endocrinology. 1982;110:1837–1839. doi: 10.1210/endo-110-5-1837. [DOI] [PubMed] [Google Scholar]
- 31.Hochstenbach SL, Ciriello J. Effect of lesions of forebrain circumventricular organs on c-fos expression in the central nervous system to plasma hypernatremia. Brain Res. 1996;713:17–28. doi: 10.1016/0006-8993(95)01425-x. [DOI] [PubMed] [Google Scholar]
- 32.Collister JP, Olson MK, Nahey DB, Vieira AA, Osborn JW. Ovlt lesion decreases basal arterial pressure and the chronic hypertensive response to angii in rats on a high-salt diet. Physiol Rep. 2013;1:e00128. doi: 10.1002/phy2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oldfield BJ, Badoer E, Hards DK, McKinley MJ. Fos production in retrogradely labelled neurons of the lamina terminalis following intravenous infusion of either hypertonic saline or angiotensin ii. Neuroscience. 1994;60:255–262. doi: 10.1016/0306-4522(94)90219-4. [DOI] [PubMed] [Google Scholar]
- 34.Gutman MB, Ciriello J, Mogenson GJ. Effects of plasma angiotensin ii and hypernatremia on subfornical organ neurons. Am J Physiol. 1988;254:R746–754. doi: 10.1152/ajpregu.1988.254.5.R746. [DOI] [PubMed] [Google Scholar]
- 35.Knuepfer MM, Johnson AK, Brody MJ. Effect of subfornical organ ablation on the development of renal hypertension. Clin Exp Hypertens A. 1984;6:1027–1034. doi: 10.3109/10641968409044054. [DOI] [PubMed] [Google Scholar]
- 36.Osborn JW, Jacob F, Hendel M, Collister JP, Clark L, Guzman PA. Effect of subfornical organ lesion on the development of mineralocorticoid-salt hypertension. Brain Res. 2006;1109:74–82. doi: 10.1016/j.brainres.2006.06.073. [DOI] [PubMed] [Google Scholar]
- 37.Osborn JW, Hendel MD, Collister JP, Ariza-Guzman PA, Fink GD. The role of the subfornical organ in angiotensin ii-salt hypertension in the rat. Exp Physiol. 2012;97:80–88. doi: 10.1113/expphysiol.2011.060491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hilzendeger AM, Cassell MD, Davis DR, Stauss HM, Mark AL, Grobe JL, Sigmund CD. Angiotensin type 1a receptors in the subfornical organ are required for deoxycorticosterone acetate-salt hypertension. Hypertension. 2013;61:716–722. doi: 10.1161/HYPERTENSIONAHA.111.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weiss ML, Claassen DE, Hirai T, Kenney MJ. Nonuniform sympathetic nerve responses to intravenous hypertonic saline infusion. J Auton Nerv Syst. 1996;57:109–115. doi: 10.1016/0165-1838(95)00108-5. [DOI] [PubMed] [Google Scholar]
- 40.Wenner MM, Rose WC, Delaney EP, Stillabower ME, Farquhar WB. Influence of plasma osmolality on baroreflex control of sympathetic activity. Am J Physiol Heart Circ Physiol. 2007;293:H2313–2319. doi: 10.1152/ajpheart.01383.2006. [DOI] [PubMed] [Google Scholar]
- 41.Greaney JL, Ray CA, Prettyman AV, Edwards DG, Farquhar WB. Influence of increased plasma osmolality on sympathetic outflow during apnea. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1091–1096. doi: 10.1152/ajpregu.00341.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen QH, Toney GM. At(1)-receptor blockade in the hypothalamic pvn reduces central hyperosmolality-induced renal sympathoexcitation. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1844–1853. doi: 10.1152/ajpregu.2001.281.6.R1844. [DOI] [PubMed] [Google Scholar]
- 43.Son SJ, Filosa JA, Potapenko ES, Biancardi VC, Zheng H, Patel KP, Tobin VA, Ludwig M, Stern JE. Dendritic peptide release mediates interpopulation crosstalk between neurosecretory and preautonomic networks. Neuron. 2013;78:1036–1049. doi: 10.1016/j.neuron.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Voisin DL, Chakfe Y, Bourque CW. Coincident detection of csf na+ and osmotic pressure in osmoregulatory neurons of the supraoptic nucleus. Neuron. 1999;24:453–460. doi: 10.1016/s0896-6273(00)80858-2. [DOI] [PubMed] [Google Scholar]
- 45.Choe KY, Han SY, Gaub P, Shell B, Voisin DL, Knapp BA, Barker PA, Brown CH, Cunningham JT, Bourque CW. High salt intake increases blood pressure via bdnf-mediated downregulation of kcc2 and impaired baroreflex inhibition of vasopressin neurons. Neuron. 2015;85:549–560. doi: 10.1016/j.neuron.2014.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berret E, Nehme B, Henry M, Toth K, Drolet G, Mouginot D. Regulation of central na+ detection requires the cooperative action of the nax channel and alpha1 isoform of na+/k+-atpase in the na+-sensor neuronal population. J Neurosci. 2013;33:3067–3078. doi: 10.1523/JNEUROSCI.4801-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shimizu H, Watanabe E, Hiyama TY, Nagakura A, Fujikawa A, Okado H, Yanagawa Y, Obata K, Noda M. Glial nax channels control lactate signaling to neurons for brain [na+] sensing. Neuron. 2007;54:59–72. doi: 10.1016/j.neuron.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 48.Gomez-Sanchez EP, Gomez-Sanchez CE. Effect of central infusion of benzamil on dahl s rat hypertension. Am J Physiol. 1995;269:H1044–1047. doi: 10.1152/ajpheart.1995.269.3.H1044. [DOI] [PubMed] [Google Scholar]
- 49.Nishimura M, Ohtsuka K, Nanbu A, Takahashi H, Yoshimura M. Benzamil blockade of brain na+ channels averts na(+)-induced hypertension in rats. Am J Physiol. 1998;274:R635–644. doi: 10.1152/ajpregu.1998.274.3.R635. [DOI] [PubMed] [Google Scholar]
- 50.Huang BS, Leenen FH. Brain amiloride-sensitive phe-met-arg-phe-nh(2)--gated na(+) channels and na(+)-induced sympathoexcitation and hypertension. Hypertension. 2002;39:557–561. doi: 10.1161/hy02t2.103004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.