Abstract
Introduction
The aims of this study were to estimate all-cause and cause-specific mortality and years of life lost, investigated by disability-adjusted life-years (DALYs), due to colorectal cancer attributable to physical inactivity in Brazil and in the states; to analyze the temporal trend of these estimates over 25 years (1990–2015) compared with global estimates and according to the socioeconomic status of states of Brazil.
Methods
Databases from the Global Burden of Disease Study (GBD) for Brazil, Brazilian states and global information were used. It was estimated the total number and the age-standardized rates of deaths and DALYs for colorectal cancer attributable to physical inactivity in the years 1990 and 2015. We used the Socioeconomic Development Index (SDI).
Results
Physical inactivity was responsible for a substantial number of deaths (1990: 1,302; 2015: 119,351) and DALYs (1990: 31,121; 2015: 87,116) due to colorectal cancer in Brazil. From 1990 to 2015, the mortality and DALYs due to colorectal cancer attributable to physical inactivity increased in Brazil (0.6% and 0.6%, respectively) and decreased around the world (-0.8% and -1.1%, respectively). The Brazilian states with better socioeconomic indicators had higher rates of mortality and morbidity by colorectal cancer due to physical inactivity (p<0.01). Physical inactivity was responsible for deaths and DALYs due to colorectal cancer in Brazil.
Conclusions
Over 25 years, the Brazilian population showed more worrisome results than around the world. Actions to combat physical inactivity and greater cancer screening and treatment are urgent in the Brazilian states.
Introduction
Colon and rectum cancer (called colorectal cancer) is the third most lethal type of cancer in women and the fourth in men in Brazil [1]. In the United States, this type of cancer is the second most lethal [2]. This neoplasm has a multifactorial etiology that includes both genetic and modifiable lifestyle factors [3].
Among the lifestyle factors, physical inactivity stands out because it interacts with some genes that influence the onset of colorectal cancer, which potentiates the onset of the disease [3]. In addition, physical inactivity is a risk factor for obesity, which is another independent predictor of colorectal cancer [4,5]. Dose-response evidence has shown that regular physical activity can reduce the onset of colorectal cancer by 20–25% in both men and women.5 Mechanisms by which physical activity reduces the risk of colorectal cancer, were not entirely clear, albeit assumptions such as changes in the material in gastrointestinal transmit time, changes in immune function as well as changes in prostaglandin levels, insulin, insulin-like growth factors, bile acid secretion, serum cholesterol and pancreatic and gastrointestinal hormone profiles have been forwarded [4–6].
Although the benefits of physical activity for health are unequivocal, data from the Global Burden of Disease (GBD) study revealed that physical inactivity is the second greatest health risk factor to which the Brazilian population is exposed [7]. Complementing this information, the literature reinforces that physical inactivity is an independent risk factor for cancer morbidity and mortality and for non-communicable diseases more generally [8].
The aim of this study was to estimate the all-cause and cause-specific mortality and years of life lost, investigated by disability-adjusted life-years (DALYs), due to colorectal cancer attributable to physical inactivity in Brazil and in the states. A second aim was to analyze the temporal trend of these estimates over 25 years (1990–2015) compared with global estimates and according to the socioeconomic status of the states of Brazil.
Material and methods
Study overview
GBD 2015 includes an annual assessment covering 195 countries and territories from 1990 to 2015. It covers 310 diseases and injuries, 2,619 sequelae and 79 risk factors by age and sex. Detailed descriptions of the methodology and approach of GBD 2015 have been published elsewhere [9,10].
Colorectal cancer estimates
We mapped all neoplasms as defined by the 10th revision of the International Statistical Classification of Diseases (ICD-10) to one of the 29 GBD cancer groups. ICD-10 codes for mortality by colorectal cancer in GBD study were C18-C21.9, D01.0-D01.3, D12-D12.9, D37.3-D37.5 [3, 9, 11].
Input data for cancer mortality estimates came from Vital registry mortality and Cancer registry incidence data. Most cancer registries only report cancer incidence. However, if a cancer registry also reported cancer mortality, mortality data were also extracted from the source to be used in the mortality to incidence estimation. In the case when high quality mortality data were available but not reported by the registry, processed (post-redistribution) vital registration mortality data from the cause of death database were matched to the registry’s incidence data [3, 9, 11]. The accuracy of mortality data reported in cancer registries usually depends on the quality of the vital registration system. If the vital registration system is incomplete or of poor quality the mortality to incidence ratio can be biased to lower ratios. In this study, data were excluded if they were not representative of the coverage population (e.g., hospital based registries), if they did not cover all malignant neoplasms as defined in ICD9 (140–208) or ICD10 (C00-C96) (e.g., specialty cancer registry), if they did not include data for both sexes and all age groups, if the data were limited to years prior to 1980, or if the source did not provide details on the population covered. Preference was given to registries with national coverage over those with only local coverage, except those from countries where the GBD study provides sub-national estimates, as is the case in Brazil [3, 9, 11]. Studies of demography and statistics [12, 13] showed that there is a considerable improvement in the completeness of the death-count coverage in Brazil since 1980. In the Southeast and Southern Brazil there is complete coverage of the adult mortality registry. In the Northeast and North Brazil, there were still places with a low coverage, although there was a clear improvement in the quality of data. For all Brazilian states, the quality of the data from the vital and cancer registries is considered high and close to high-income countries [12, 13]. Cancer registry incidence data were transformed to mortality estimates using separately modeled mortality-to incidence ratios (MIR) [11]. Multiple logit random effect models were created. All models were run and the best model was selected. All models were tested at multiple stages before creating the final model output. The best model was selected based on the lowest mean out-of sample Root-Mean-Squared Error (RMSE) from those models remaining after checking the mean MIR. All of the modeling details have been published previously [3, 9, 11].
The raw data were processed to make them comparable and to account for ‘‘garbage codes”, which are codes assigned to causes that are not usable from a public health perspective [14]. These causes were redistributed to the most likely underlying cause of death based on a regression model [3]. Using a cause of death ensemble modeling (CODEm) approach with cause-specific covariates, we computed mortality estimates for each individual cause [15].
Cancer survival was calculated using a MIR-based scaling factor. We calculated 10-year prevalence of each cancer and each incidence cohort using these cancer survival estimates. The total prevalence was divided into four sequelae with variable disability weights: (1) diagnosis and treatment, (2) remission, (3) metastatic, and (4) terminal phase. We assumed a constant duration for sequelae (1), (3), and (4). Duration of sequela (2) was the remaining prevalence after subtracting the duration of the fixed sequelae. We computed years of life lost (YLLs) by multiplying deaths by the normative standard life expectancy at each age of death. For each sequela, Years Lost due to Disability (YLDs) were calculated by multiplying the prevalence of each sequela by its disability weight. Finally, DALYs were calculated by summing premature death (YLLs) and YLDs. More information about these estimates can be found elsewhere [9].
Physical inactivity estimate
Surveys of the general adult population, performed using random sampling procedures, were included that captured self-reported physical activity in all domains of life (leisure/recreation, work, household and transport). Due to the absence of a consistent relationship, on the individual level, between the amount of activity performed in each domain and total activity, it was not possible to use studies that included only recreational/leisure activities [16].
For the global estimates, data were primarily derived from two standardized questionnaires, The Global Physical Activity Questionnaire and the International Physical Activity Questionnaire, although any other survey instrument was included that asked about the intensity, frequency and duration of physical activities performed across all activity domains [16].
In the case of Brazil [7], we consulted existing surveys such the Telephone-based Surveillance of Risk and Protective Factors for Chronic Diseases, Brazil World Health Survey, and the International Prevalence Study on Physical Activity. More details can be found at http://ghdx.healthdata.org/gbd-2015/data-input-sources.
To standardize all estimates in Brazil and around the world, we considered data from the population aged 25 years or more. The physical activity was accumulated for durations of at least ten consecutive minutes, across all domains of life. The frequency, duration and intensity of activity were used to calculate the total metabolic equivalent (MET) minutes per week. The estimates were made for the subjects classified as physically inactive (<600 METS-min/week) [16].
Analytic methods
The contribution of physical inactivity in the mortality and DALYs by colorectal cancer was estimated using a comparative risk assessment approach in which observed health outcomes are compared to those that would have been observed with a counterfactual set of exposure where no one is exposed [16]. For this, we used the Cause of Death Ensemble Modeling-CODEm (CODEm) that is used to estimate indicators by age, sex, country, year, and cause, and is an analytical tool that tests several possible statistical models of causes of death and creates a combined set of models that offers the best predictive performance. The software DisMod-MR 2.1, a meta-regression tool, is used for simultaneous estimates of incidence, prevalence, remission, disability, and also mortality, attributable to risk factors, such as physical inactivity [16]. Modeling details can be found in the literature [9, 11, 16].
In this study, absolute numbers of deaths, the mortality rates and DALYs (per 100,000 inhabitants—crude and age-standardized) were used as the metric. The sum of DALYs across the population, or the burden of disease, can be thought of as a measurement of the gap between current health status and an ideal health situation [17]. The standard population used in this study in the corresponding age groups was estimated population from the World Population Prospect 2015 Revision by the United Nations Population Division [18]. For subnational locations, as is the case in Brazil, interpolation and extrapolation based on rate of change are used together with age specific population from censuses. Raking was applied to ensure consistency between subnational and national populations [9].
In the GBD study, 95% uncertainty intervals (95%U.I.) were calculated, to provide information on the variability of estimates resulting from errors due to the sampling process, and also non-sample errors due to adjustments of data sources and modeling [9].
The GBD 2015 created the Socioeconomic Development Index (SDI) [3, 9, 11] for all evaluated locations, by calculating per capita income, formal education at 15 years of age and fertility rate. In the present study, this index was used to compare the metrics used among the states of Brazil. For this, the Spearman correlation coefficient was applied. In all analyzes between colorectal cancer and physical inactivity, the population considered was aged ≥ 25 years. For the supplementary tables that present only the estimates for colorectal cancer we consider the entire population (i.e. ≥ 15 years old).
Results
Around the world, 487,860 deaths by colorectal cancer were estimated in 1990 and 832,048 in 2015. In Brazil, 6,894 deaths from colorectal cancer were estimated in 1990 and 21,419 in 2015. From 1990 to 2015, mortality from colorectal cancer increased more in Brazil than in the rest of the world (S1 Dataset and S1 Table).
Around the world, 10,777,181 DALYs for colorectal cancer were estimated in 1990 and 17,026,563 in 2015. In Brazil, 171,831 DALYs were estimated in 1990 and 467,421 in 2015. From 1990 to 2015, DALYs from colorectal cancer increased more in Brazil than in the rest of the world (S2 Table).
Regarding the mortality by physical inactivity due to all causes, 1,031,823 deaths were estimated in 1990 and 1,605,494 in 2015 around the world. In Brazil, 33,227 deaths were estimated in 1990 and 59,197 in 2015. From 1990 to 2015, mortality attributed to physical inactivity from all causes increased 33.6% (95%U.I.: 30.3–38.0) around the world and 19.6% (95%U.I.: 15.0–25.6) in Brazil (S3 Table).
Around the world, 22,318,505 DALYs from physical inactivity due to all causes were estimated in 1990 and 34,603,468 in 2015. In Brazil, 863,721 DALYs were estimated in 1990 and 1,401,966 in 2015. From 1990 to 2015, DALYs from physical inactivity due to all causes increased around the world, but did not increase in Brazil (S4 Table).
In relation to colorectal cancer mortality due to physical inactivity, 69,456 deaths were estimated in 1990 and 119,351 in 2015 around the world. These values represented, in 1990, an age-standardized rate of mortality of 2.1 (95%U.I.: 1.5–2.8) and of 1.9 (95%U.I: 1.3–2.4) in 2015. In Brazil, 1,302 deaths were estimated in 1990 and 4,143 in 2015 that represented, in 1990, an age-standardized rate of mortality of 2.0 (95%U.I: 1.4–2.5) and of 2.4 (95%U.I: 1.8–3.0) in 2015. From 1990 to 2015, the mortality by colorectal cancer due to physical inactivity increased in Brazil (0.6%; 95%U.I: 0.1–1.4) and decreased around the world (-0.8%; 95%U.I.: -1.5 - -0.2). The Brazilian state with the greatest increase (1990–2015) in colorectal cancer mortality due to physical inactivity was São Paulo (Table 1).
Table 1. Number and age-standardized rate (per 100,000 inhabitants) of deaths from colorectal cancer due to physical inactivity globally, in Brazil, and in the Brazilian states.
| Deaths due to colorectal cancer attributable to physical inactivity | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1990 | 2015 | 1990 | 2015 | Change (1990–2015) | |||||||||||
| Deaths | 95% U.I. | Deaths | 95% U.I. | Rate* | 95% U.I. | Rate* | 95% U.I. | %* | 95% U.I. | ||||||
| Global | 69,456 | 48,533 | 90,67 | 119,351 | 83,913 | 155,791 | 2.15 | 1.51 | 2.80 | 1.90 | 1.34 | 2.47 | -0.84 | -1.54 | -0.24 |
| Brazil | 1,302 | 966 | 1,639 | 4,143 | 3,099 | 5,189 | 2.00 | 1.49 | 2.50 | 2.41 | 1.81 | 3.02 | 0.66 | 0.15 | 1.41 |
| Acre | 02 | 01 | 02 | 06 | 05 | 08 | 1.18 | 0.88 | 1.50 | 1.58 | 1.17 | 2.03 | 0.65 | -0.08 | 1.43 |
| Alagoas | 12 | 09 | 15 | 33 | 24 | 43 | 1.19 | 0.88 | 1.50 | 1.48 | 1.11 | 1.93 | 0.22 | -0.75 | 1.25 |
| Amapá | 01 | 01 | 01 | 04 | 03 | 06 | 0.88 | 0.65 | 1.13 | 1.36 | 0.95 | 1.81 | 0.59 | -0.36 | 1.59 |
| Amazonas | 09 | 07 | 11 | 36 | 25 | 47 | 1.55 | 1.15 | 1.98 | 1.95 | 1.37 | 2.58 | 0.52 | -0.39 | 1.61 |
| Bahia | 78 | 58 | 99 | 238 | 174 | 308 | 1.56 | 1.18 | 1.99 | 2.02 | 1.48 | 2.61 | 0.25 | -0.67 | 1.22 |
| Ceará | 34 | 24 | 43 | 129 | 93 | 167 | 1.15 | 0.83 | 1.46 | 1.86 | 1.35 | 2.40 | 0.30 | -0.49 | 1.26 |
| Distrito Federal | 10 | 07 | 12 | 50 | 36 | 65 | 2.09 | 1.55 | 2.66 | 2.36 | 1.69 | 3.09 | 0.77 | -0.12 | 1.95 |
| Espírito Santo | 19 | 13 | 24 | 68 | 50 | 89 | 1.75 | 1.27 | 2.22 | 2.06 | 1.51 | 2.68 | 0.68 | -0.21 | 1.97 |
| Goiás | 27 | 20 | 34 | 107 | 77 | 138 | 1.88 | 1.40 | 2.35 | 2.20 | 1.59 | 2.83 | 0.42 | -0.61 | 1.62 |
| Maranhão | 27 | 19 | 35 | 65 | 45 | 89 | 1.38 | 0.98 | 1.80 | 1.52 | 1.07 | 2.06 | 0.88 | -0.05 | 2.13 |
| Mato Grosso | 09 | 07 | 12 | 43 | 31 | 55 | 1.60 | 1.20 | 2.04 | 2.03 | 1.48 | 2.64 | 0.69 | -0.37 | 1.96 |
| Mato Grosso do Sul | 11 | 08 | 15 | 45 | 32 | 60 | 1.71 | 1.26 | 2.21 | 2.25 | 1.62 | 2.96 | 0.12 | -0.97 | 1.42 |
| Minas Gerais | 126 | 93 | 159 | 430 | 315 | 553 | 1.79 | 1.32 | 2.26 | 2.18 | 1.59 | 2.80 | -0.07 | -1.06 | 0.93 |
| Paraná | 76 | 55 | 97 | 271 | 197 | 352 | 2.19 | 1.60 | 2.77 | 2.71 | 1.97 | 3.50 | 0.51 | -0.49 | 1.75 |
| Paraíba | 19 | 14 | 24 | 54 | 38 | 71 | 1.15 | 0.84 | 1.47 | 1.63 | 1.16 | 2.14 | 0.17 | -0.60 | 1.05 |
| Pará | 21 | 15 | 27 | 73 | 51 | 97 | 1.32 | 0.96 | 1.68 | 1.65 | 1.17 | 2.16 | 0.44 | -0.55 | 1.71 |
| Pernambuco | 44 | 33 | 57 | 120 | 86 | 158 | 1.28 | 0.95 | 1.64 | 1.67 | 1.21 | 2.19 | 0.85 | 0.03 | 2.01 |
| Piaui | 12 | 08 | 15 | 35 | 25 | 46 | 1.14 | 0.83 | 1.46 | 1.50 | 1.08 | 1.94 | 0.21 | -0.71 | 1.30 |
| Rio de Janeiro | 186 | 138 | 236 | 508 | 369 | 654 | 2.61 | 1.94 | 3.33 | 2.94 | 2.15 | 3.79 | 0.49 | -0.31 | 1.52 |
| Rio Grande do Norte | 15 | 11 | 19 | 46 | 33 | 61 | 1.26 | 0.95 | 1.60 | 1.69 | 1.20 | 2.22 | 0.20 | -0.87 | 1.50 |
| Rio Grande do Sul | 141 | 103 | 178 | 369 | 264 | 490 | 2.93 | 2.15 | 3.71 | 2.98 | 2.13 | 3.95 | 0.61 | -0.41 | 1.88 |
| Rondônia | 04 | 03 | 05 | 15 | 11 | 20 | 1.38 | 1.02 | 1.74 | 1.54 | 1.13 | 1.99 | 0.73 | -0.03 | 1.59 |
| Roraima | 00 | 00 | 01 | 03 | 02 | 04 | 1.05 | 0.79 | 1.31 | 1.34 | 0.99 | 1.75 | 0.82 | 0.02 | 1.81 |
| Santa Catarina | 39 | 28 | 49 | 136 | 96 | 175 | 2.17 | 1.61 | 2.78 | 2.36 | 1.69 | 3.03 | 0.61 | -0.39 | 1.90 |
| Sergipe | 08 | 06 | 11 | 25 | 18 | 33 | 1.27 | 0.93 | 1.64 | 1.67 | 1.19 | 2.19 | 0.57 | -0.42 | 1.78 |
| São Paulo | 370 | 272 | 470 | 1,218 | 891 | 1,574 | 2.51 | 1.86 | 3.19 | 2.96 | 2.17 | 3.82 | 0.90 | 0.01 | 2.07 |
| Tocantins | 03 | 02 | 04 | 15 | 11 | 20 | 1.06 | 0.73 | 1.41 | 1.59 | 1.13 | 2.11 | 0.76 | -0.28 | 2.03 |
*Age-standardized rate; U.I.: uncertainty interval
In relation to colorectal cancer DALYs due to physical inactivity, 1,391,037 DALYs were estimated in 1990 and 2,209,209 in 2015 around the world. These values represented, in 1990, an age-standardized rate of mortality of 38.8 (95%U.I.: 26.7–51.3) and of 33.2 (95%U.I: 23.0–43.6) in 2015. In Brazil, 31,121 DALYs were estimated in 1990 and 87,116 in 2015 that represented, in 1990, an age-standardized rate of mortality of 38.3 (95%U.I: 28.3–48.2) and of 45.7 (95%U.I: 34.0–57.3) in 2015. From 1990 to 2015, the DALYs by colorectal cancer due to physical inactivity increased in Brazil (0.6%; 95%U.I: 0.1–1.4) and decreased around the world (-1.1%; 95%U.I.: -2.0 - -0.3). The Brazilian state with the greatest increase (1990–2015) in colorectal cancer DALYs due to physical inactivity was São Paulo (Table 2).
Table 2. Number and age-standardized rate (per 100,000 inhabitants) of deaths from colorectal cancer due to physical inactivity globally, in Brazil, and in the Brazilian states.
| DALYs due to colorectal cancer attributable to physical inactivity | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1990 | 2015 | 1990 | 2015 | Change (1990–2015) | |||||||||||
| DALYs | 95% U.I. | DALYs | 95% U.I. | Rate* | 95% U.I. | Rate* | 95% U.I. | %* | 95% U.I. | ||||||
| Global | 1,391,037 | 952,242 | 1,850,921 | 2,209,209 | 1,528,502 | 2,915,924 | 38.86 | 26.74 | 51.37 | 33.20 | 23.06 | 43.69 | -1.10 | -2.05 | -0.36 |
| Brazil | 31,121 | 22,933 | 39,367 | 87,116 | 64,619 | 109,15 | 38.34 | 28.36 | 48.27 | 45.77 | 34.03 | 57.32 | 0.68 | 0.11 | 1.48 |
| Acre | 40 | 29 | 51 | 145 | 107 | 190 | 22.84 | 16.81 | 28.80 | 30.48 | 22.66 | 39.74 | 0.49 | -0.39 | 1.48 |
| Alagoas | 288 | 212 | 367 | 763 | 551 | 991 | 23.59 | 17.44 | 29.87 | 29.63 | 21.52 | 38.32 | -0.13 | -1.34 | 1.13 |
| Amapá | 18 | 13 | 23 | 106 | 74 | 146 | 16.61 | 12.16 | 21.30 | 25.93 | 18.08 | 35.00 | 0.48 | -0.57 | 1.59 |
| Amazonas | 234 | 173 | 301 | 881 | 604 | 1,169 | 30.15 | 22.40 | 38.62 | 38.07 | 26.40 | 50.38 | 0.21 | -0.87 | 1.42 |
| Bahia | 1,744 | 1,306 | 2,21 | 5,07 | 3,643 | 6,585 | 29.44 | 22.07 | 37.26 | 38.75 | 27.91 | 50.23 | 0.17 | -1.01 | 1.29 |
| Ceará | 759 | 544 | 969 | 2,760 | 2,004 | 3,572 | 21.80 | 15.76 | 27.73 | 36.58 | 26.53 | 47.22 | 0.44 | -0.44 | 1.44 |
| Distrito Federal | 269 | 192 | 346 | 1,011 | 718 | 1,328 | 39.27 | 28.61 | 50.09 | 41.80 | 29.70 | 54.82 | 0.56 | -0.41 | 1.71 |
| Espírito Santo | 456 | 323 | 586 | 1,447 | 1,038 | 1,918 | 33.41 | 23.85 | 42.49 | 39.08 | 28.17 | 51.53 | 0.47 | -0.57 | 1.87 |
| Goiás | 713 | 527 | 903 | 2,458 | 1,739 | 3,165 | 36.94 | 27.44 | 46.57 | 42.80 | 30.57 | 54.93 | 0.13 | -1.05 | 1.46 |
| Maranhão | 657 | 463 | 867 | 1,483 | 1,012 | 2,059 | 28.05 | 19.85 | 36.99 | 30.73 | 21.10 | 42.64 | 0.66 | -0.43 | 1.96 |
| Mato Grosso | 250 | 184 | 321 | 1,008 | 725 | 1,320 | 30.78 | 22.92 | 39.03 | 38.93 | 28.13 | 50.90 | 0.68 | -0.67 | 2.22 |
| Mato Grosso do Sul | 288 | 207 | 373 | 986 | 693 | 1,3 | 32.82 | 23.69 | 42.45 | 42.81 | 30.30 | 56.21 | -0.16 | -1.39 | 1.18 |
| Minas Gerais | 2,994 | 2,176 | 3,825 | 9,046 | 6,542 | 11,817 | 34.06 | 25.06 | 43.26 | 42.44 | 30.76 | 55.29 | -0.36 | -1.41 | 0.62 |
| Paraná | 1,874 | 1,342 | 2,382 | 5,758 | 4,116 | 7,559 | 41.72 | 30.10 | 52.93 | 51.75 | 37.14 | 67.56 | 0.38 | -0.75 | 1.63 |
| Paraíba | 426 | 309 | 543 | 1,159 | 816 | 1,553 | 22.49 | 16.34 | 28.53 | 32.55 | 22.93 | 43.37 | 0.13 | -0.76 | 1.09 |
| Pará | 531 | 382 | 684 | 1,746 | 1,205 | 2,334 | 25.42 | 18.44 | 32.67 | 32.22 | 22.38 | 42.86 | 0.29 | -0.85 | 1.61 |
| Pernambuco | 1,059 | 776 | 1,371 | 2,655 | 1,886 | 3,528 | 25.76 | 18.87 | 33.25 | 32.89 | 23.44 | 43.54 | 0.72 | -0.27 | 1.96 |
| Piaui | 277 | 199 | 358 | 792 | 558 | 1,041 | 22.15 | 16.01 | 28.34 | 29.72 | 21.06 | 38.89 | 0.20 | -0.96 | 1.48 |
| Rio de Janeiro | 4,498 | 3,330 | 5,718 | 10,379 | 7,526 | 13,368 | 50.85 | 37.54 | 64.36 | 56.55 | 41.05 | 72.92 | 0.81 | -0.12 | 2.03 |
| Rio Grande do Norte | 312 | 228 | 397 | 973 | 689 | 1,286 | 23.25 | 17.23 | 29.53 | 32.38 | 22.98 | 42.60 | 0.10 | -1.05 | 1.47 |
| Rio Grande do Sul | 3,220 | 2,328 | 4,085 | 7,299 | 5,141 | 9,769 | 54.51 | 39.71 | 68.91 | 55.93 | 39.34 | 74.61 | 0.42 | -0.67 | 1.70 |
| Rondônia | 112 | 82 | 142 | 376 | 263 | 491 | 27.61 | 20.25 | 34.91 | 29.73 | 21.26 | 38.32 | 0.71 | -0.17 | 1.72 |
| Roraima | 13 | 10 | 17 | 69 | 50 | 89 | 20.14 | 15.11 | 25.28 | 24.92 | 18.33 | 32.28 | 0.82 | -0.10 | 2.05 |
| Santa Catarina | 920 | 671 | 1,178 | 2,849 | 1,989 | 3,715 | 40.21 | 29.62 | 50.94 | 43.48 | 30.67 | 56.68 | 0.18 | -0.89 | 1.47 |
| Sergipe | 188 | 135 | 244 | 578 | 403 | 773 | 24.82 | 18.01 | 32.08 | 32.99 | 23.26 | 43.91 | 0.54 | -0.52 | 1.77 |
| São Paulo | 8,900 | 6,460 | 11,434 | 24,980 | 18,051 | 32,107 | 47.64 | 34.95 | 60.55 | 55.16 | 40.07 | 70.88 | 1.11 | 0.20 | 2.42 |
| Tocantins | 82 | 55 | 111 | 337 | 238 | 448 | 20.37 | 13.94 | 27.41 | 30.54 | 21.63 | 40.42 | 0.74 | -0.46 | 2.14 |
*Age-standardized rate; U.I.: uncertainty interval
Mortality and DALYs due to colorectal cancer due to physical inactivity in Brazil increased with increasing age in 1990 and 2015. Table 3 shows the information according to age for absolute number and age-standardized rate of deaths and DALYs.
Table 3. Absolute number and rate non-standardized of death and DALYs from colorectal cancer due to all causes and attributed to physical inactivity in Brazil in 1990 and 2015 by age.
| Deaths due to colorectal cancer attributable to physical inactivity | DALYs due to colorectal cancer attributable to physical inactivity | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 15–49 years | 50–69 years | 70+ years | 15–49 years | 50–69 years | 70+ years | |||||||||||||
| Metric | 95% U.I. | Metric | 95% U.I. | Metric | 95% U.I. | Metric | 95% U.I. | Metric | 95% U.I. | 95% U.I. | ||||||||
| Males (1990) | ||||||||||||||||||
| Number | 84 | 60 | 108 | 263 | 195 | 335 | 243 | 182 | 306 | 3,965 | 2,868 | 5,104 | 7,222 | 5,371 | 9,213 | 3,326 | 2,484 | 4,188 |
| Rate* | 0.22 | 0.16 | 0.28 | 3.54 | 2.63 | 4.51 | 17.12 | 12.85 | 21.57 | 10.24 | 7.41 | 13.18 | 97.06 | 72.17 | 123.80 | 234.18 | 174.94 | 294.86 |
| Males (2015) | ||||||||||||||||||
| Number | 192 | 139 | 248 | 798 | 585 | 1,025 | 1,004 | 745 | 1,264 | 8,954 | 6,471 | 11,565 | 22,166 | 16,302 | 28,361 | 12,252 | 9,121 | 15,557 |
| Rate* | 0.34 | 0.25 | 0.44 | 4.65 | 3.42 | 5.98 | 23.63 | 17.54 | 29.75 | 15.88 | 11.47 | 20.51 | 129.28 | 95.08 | 165.41 | 288.34 | 214.64 | 366.13 |
| Female (1990) | ||||||||||||||||||
| Number | 99 | 72 | 125 | 282 | 206 | 357 | 329 | 246 | 414 | 4,668 | 3,418 | 5,926 | 7,786 | 5,679 | 9,841 | 4,151 | 3,086 | 5,231 |
| Rate* | 0.25 | 0.18 | 0.32 | 3.44 | 2.51 | 4.34 | 15.80 | 11.82 | 19.88 | 11.88 | 8.70 | 15.08 | 94.63 | 69.02 | 119.60 | 199.03 | 148.03 | 250.88 |
| Female (2015) | ||||||||||||||||||
| Number | 212 | 155 | 273 | 749 | 546 | 945 | 1,186 | 891 | 1,488 | 12,872 | 9,631 | 16,065 | 9,837 | 7,215 | 12,621 | 21,033 | 15,352 | 26,505 |
| Rate* | 0.37 | 0.27 | 0.48 | 3.87 | 2.82 | 4.88 | 19.50 | 14.63 | 24.46 | 17.34 | 12.72 | 22.24 | 108.67 | 79.32 | 136.94 | 211.57 | 158.31 | 264.07 |
* non-standardized rate (per 100,000 inhabitants)
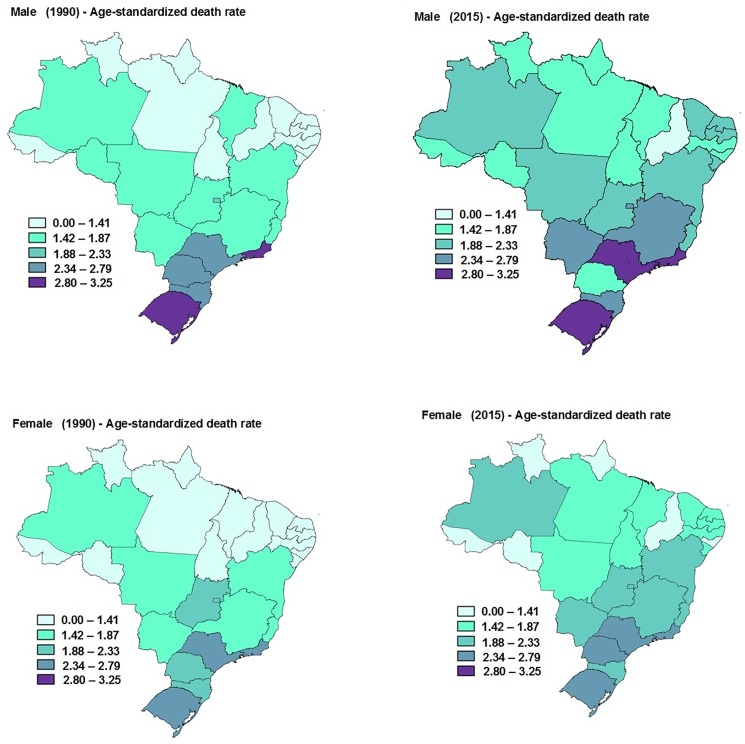
The age-standardized rate of mortality by colorectal cancer due to physical inactivity was similar between men and women in Brazil, both in 1990 (men: 2.0, 95%U.I.: 1.5–2.6; women: 1.9, 95%U.I.: 1.4–2.4) and 2015 (men: 2.7, 95%U.I: 2.0–3.4; women: 2.1, 95%U.I.: 1.6–2.7). From 1990 to 2015, colorectal cancer mortality rates due to physical inactivity did not change significantly among Brazilian states when analyses were stratified by sex (Fig 1).
Fig 1. Age-standardized death rate (per 100,000 inhabitants) from colorectal cancer due to physical inactivity in males and females in the Brazilian states.
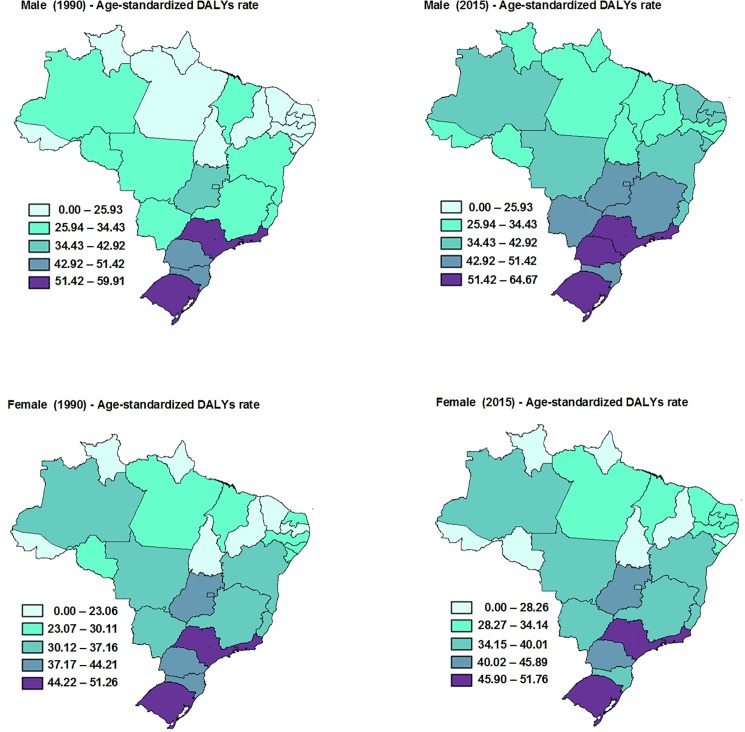
The age-standardized rate of DALYs by colorectal cancer due to physical inactivity was similar between men and women in Brazil, both in 1990 (men: 39.7, 95%U.I.: 29.6–50.3; women: 37.4, 95%U.I.: 27.4–47.1) and 2015 (men: 50.9, 95%U.I: 37.8–64.7; women: 41.8, 95%U.I.: 31.0–52.3). When the analyses were stratified by Brazilian state, men from São Paulo presented an increase age-standardized rate of DALYs from 1990 to 2015 (1.5%; 95%U.I.: 0.3–3.3), but for women there was no increase. In the other states of Brazil there were no significant changes in age-standardized rate of DALYs from 1990 to 2015 (Fig 2).
Fig 2. Age-standardized DALYs (per 100,000 inhabitants) from colorectal cancer due to physical inactivity in males and females in the Brazilian states.
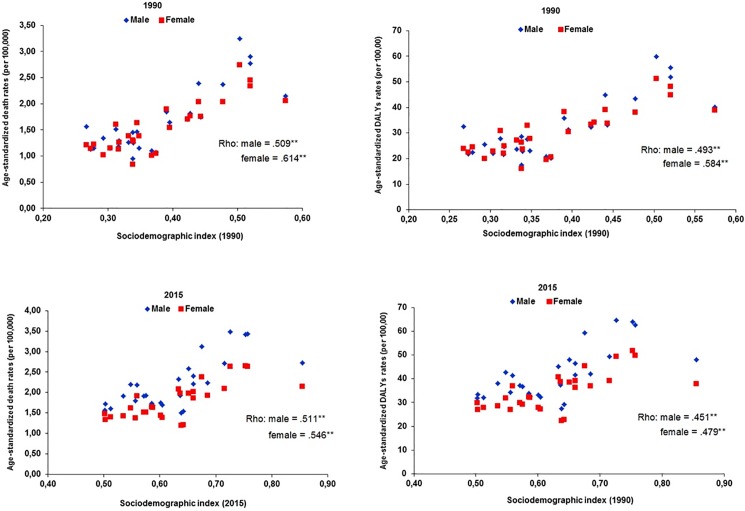
The states of Brazil with better socioeconomic indicators had higher mortality rates and DALYs by colorectal cancer due to physical inactivity (p<0.01). The magnitudes of association between mortality and DALYs rates with SDI were relatively higher for women than for men (Fig 3).
Fig 3. Relationship between age-standardized rate and DALYs from colorectal cancer due to physical inactivity and sociodemographic index of the Brazilian states in 1990 and 2015 according to sex.
Rho: Spearman's correlation coefficient; ** p <0.01.
Discussion
The main finding of this study was that in Brazil, the growth of colorectal cancer mortality rates, between 1990 and 2015, was higher than the overall global rates (68.47% vs 24.56%). Physical inactivity was responsible for 1,302 deaths in 1990 and 4,143 in 2015 due to colorectal cancer. For DALYs, the growth of colorectal cancer rates was higher in Brazil (67.19%) than overall global rates (22.92%), and physical inactivity was responsible for 31,121 cases in 1990 and 87,116 cases in 2015 due to colorectal cancer. In addition, there were more worrisome estimates for Brazil compared to around the world, because in Brazil there was an increase in mortality (0.66%) and DALYs (0.68%) rates by colorectal cancer due to physical inactivity over 25 years (1990–2015) and globally there was a decline (deaths: -0.84%; DALYs: -1.10%). This information reinforces physical inactivity is an independent risk factor for colorectal cancer and raises particular cause for concern in Brazil. One of the possible explanations for this increase observed in the present study may be the fact that in this period the risk of exposure to physical inactivity in adults in Brazil was 70% and around the world was 40% [7]. That is, in Brazil, the population has a higher risk of exposure to physical inactivity.
Regular physical activity is known to have a protective effect against colorectal cancer [19, 20], even in individuals with different values of body mass index [21], for different domains of physical activity [19,20] and after controlling for many lifestyle factors [21]. However, there are few studies on the influence of socioeconomic level in relation to physical inactivity and colorectal cancer. Analyses of mortality and DALYs according to socioeconomic status of the city are provided in the GBD study and identify iniquities in health [9]. In this study we observed that in the Brazilian states with better socioeconomic conditions the mortality and DALYs rates from colorectal cancer due to physical inactivity were higher in comparison with the states of worst socioeconomic conditions. This result is contrary to that presented to the United States in a study that analyzed mortality from colorectal cancer due to all causes for a period of 30 years [22]. The authors reported that in the 1980s, colorectal cancer mortality was more prominent in the population with a high socioeconomic level. However, from the mid-1990s this was reversed, and higher mortality rates were observed in the population with lower socioeconomic status. According to the authors, this was due to advances in medical procedures and the early detection of colorectal cancer being more accessible to the population with a high socioeconomic level [22].
Evidence exists that reductions in colorectal cancer mortality can be achieved through detection and treatment of early-stage colorectal cancer and the identification and removal of adenomatous polyps, the precursor to these cancers [23]. In the WHO Noncommunicable Diseases Plan, countries were recommended to implement population-based colorectal cancer screening, including using a fecal occult blood test as appropriate) at age >50, linked with timely treatment [23]. However, this measure, which could lead to the detection and reduction of mortality, was not implemented in Brazil. It is necessary to broaden this discussion and accelerate measures for greater population prevention and early treatment of colorectal cancer.
Mortality and DALYs rates for colorectal cancer due to physical inactivity were similar between men and women from Brazil. When we analyzed the trend from 1990 to 2015 an increase in mortality and DALYs rates in both sexes was observed. There are no other studies that analyzed the mortality and DALYs trend by colorectal cancer due to physical inactivity, which does not allow us to make comparisons with other countries. We expected that men had higher mortality and higher DALYs than in women because the prevalence of sufficient physical activity during leisure time among Brazilian women increased more than in men over the last decade [24,25], and this may result in a decrease in morbidity and mortality by colorectal cancer. In addition to this, women more frequently participate in preventive tests compared to men [26,27]. In Brazil, for example, this situation resulted in the Men's Health Policy which aims to show men the importance of preventive health examinations and of the healthy lifestyle throughout life [27]. However, our hypothesis has not been confirmed and for Brazil more actions are necessary to stimulate the practice of physical activity and preventive tests for colorectal cancer for the whole population.
This present study has important limitations worth noting. The analysis of one type of cancer does not allow inferences to other types of cancer where physical inactivity is considered a risk factor, such as breast cancer [5]. Non-distinction of the tumor site in the colon (proximal, distal, or other) may be considered a limitation because it did not allow us to identify for which of these sites the mortality or DALYs were higher, as done in other studies [2]. Also, the present study only includes physical activity measured by questionnaires which are considered subjective measures of physical activity and associated with measurement bias [28]. The non-stratification of physical activity by domains is another limitation.
The strength of this study was to work with mortality and physical inactivity information from all over Brazil. From this information it was possible to estimate mortality rates and DALYs by colorectal cancer due to physical inactivity. Until now it was known that physical inactivity was a risk factor for colorectal cancer, but it had not been estimated how much it caused of morbidity (DALYs) and mortality, especially in a trend analysis of 25 years.
It can be concluded that physical inactivity was responsible for a substantial number of deaths and DALYs due to colorectal cancer in Brazil. Estimates of mortality and morbidity from colorectal cancer as a result of physical inactivity in Brazil increased between 1990 and 2015 compared to global estimates. The Brazilian states with better socioeconomic indicators had higher rates of mortality and morbidity by colorectal cancer due to physical inactivity.
Supporting information
(XLSX)
*Age-standardized rate; U.I.: uncertainty interval.
(PDF)
*Age-standardized rate; U.I.: uncertainty interval.
(PDF)
*Age-standardized rate; U.I.: uncertainty interval.
(PDF)
*Age-standardized rate; U.I.: uncertainty interval.
(PDF)
Acknowledgments
The author (DASS and DCM) thanks the National Council for Scientific and Technological Development from Brazil for the research grant (PQ). Bill & Melinda Gates Foundation (GBD Global) and Ministry of Health from Brazil (GBD 2015 Brazil-states; Process No. 25000192049/2014-14). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author (DASS and DCM) thanks the National Council for Scientific and Technological Development from Brazil for the research grant (PQ). Bill & Melinda Gates Foundation (GBD Global) and Ministry of Health from Brazil (GBD 2015 Brazil-states; Process No. 25000192049/2014-14). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Guerra MR, Bustamante-Teixeira MT, Corrêa CSL, Abreu DMX, Curado MP, Mooney M, et al. Magnitude and variation of the burden of cancer mortality in Brazil and Federation Units, 1990 and 2015. Rev Bras Epidemiol. 2017;20(Suppl 01):102–115. doi: 10.1590/1980-5497201700050009 [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):104–17. doi: 10.3322/caac.21220 [DOI] [PubMed] [Google Scholar]
- 3.Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3(4):524–548. doi: 10.1001/jamaoncol.2016.5688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedenreich CM, Shaw E, Neilson HK, Brenner DR. Epidemiology and biology of physical activity and cancer recurrence. J Mol Med. In press. doi: 10.1007/s00109-017-1558-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kerr J, Anderson C, Lippman SM. Physical activity, sedentary behaviour, diet, and cancer: an update and emerging new evidence. Lancet Oncol. 2017;18(8):e457–e471. doi: 10.1016/S1470-2045(17)30411-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quadrilatero J, Hoffman-Goetz L. Physical activity and colon cancer: A systemic review of potential mechanisms. J Sports Med Phys Fitness 2003;43(2):121–8. [PubMed] [Google Scholar]
- 7.Malta DC, Felisbino-Mendes MS, Machado ÍE, Passos VMA, Abreu DMX, Ishitani LH, et al. Risk factors related to the global burden of disease in Brazil and its Federated Units, 2015. Rev Bras Epidemiol. 2017;20(Suppl 01):217–232. doi: 10.1590/1980-5497201700050018 [DOI] [PubMed] [Google Scholar]
- 8.Ekelund U, Steene-Johannessen J, Brown WJ, Fagerland MW, Owen N, Powell KE, et al. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet. 2016; 388(10051):1302–10. doi: 10.1016/S0140-6736(16)30370-1 [DOI] [PubMed] [Google Scholar]
- 9.Global Burden of Disease Mortality and Causes of Death Collaborators, Wang H, Naghavi M, Allen C, Barber RM, Bhutta ZA, et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1459–1544. doi: 10.1016/S0140-6736(16)31012-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Souza MFM, França EB, Cavalcante A. Burden of disease and health situation analysis: results of the Global Burden of Disease (GBD) Brazil network. Rev Bras Epidemiol. 2017;20(Suppl 01):1–3. doi: 10.1590/1980-5497201700050001 [DOI] [PubMed] [Google Scholar]
- 11.Global Burden of Disease Eastern Mediterranean Region Cancer Collaborators, Alsharif U, El Bcheraoui C, Khalil I, Charara R, Moradi-Lakeh M, et al. Burden of cancer in the Eastern Mediterranean Region, 2005–2015: findings from the Global Burden of Disease 2015 Study. Int J Public Health. In press. doi: 10.1007/s00038-017-0999-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishitani LH, Teixeira RA, Abreu DMX, Paixão LMMM, França EB. Quality of mortality statistics' information: garbage codes as causesof death in Belo Horizonte, 2011–2013. Rev Bras Epidemiol. 2017;20 (Suppl 01):34–45. doi: 10.1590/1980-5497201700050004 [DOI] [PubMed] [Google Scholar]
- 13.Queiroz BL, Freire FHMA, Gonzaga MR, Lima EEC. Completeness of death-count coverage and adult mortality (45q15) for Brazilian states from 1980 to 2010. Rev Bras Epidemiol. 2017;20(Suppl 01):21–33. doi: 10.1590/1980-5497201700050003 [DOI] [PubMed] [Google Scholar]
- 14.Naghavi M, Makela S, Foreman K, O'Brien J, Pourmalek F, Lozano R. Algorithms for enhancing public health utility of national causes-of-death data. Popul Health Metr. 2010;8:9 doi: 10.1186/1478-7954-8-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foreman KJ, Lozano R, Lopez AD, Murray CJ. Modeling causes of death: an integrated approach using CODEm. Popul Health Metr. 2012;10:1 doi: 10.1186/1478-7954-10-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Global Burden of Disease Risk Factors Collaborators, Forouzanfar MH, Afshin A, Alexander LT, Anderson HR, Bhutta ZA, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1659–1724. doi: 10.1016/S0140-6736(16)31679-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murray CJL, Salomon JA, Mathers CD, Lopez AD. Summary Measures in Population Health: Concepts, Ethics, Measurement, and Applications. Geneva: World Health Organization; 2002. [Google Scholar]
- 18.United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects: The 2015 Revision, Key Findings and Advance Tables. Working Paper No. ESA/P/WP; 2015.
- 19.Farris MS, Mosli MH, McFadden AA, Friedenreich CM, Brenner DR. The association between leisure time physical activity and pancreatic cancer risk in adults: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2015;24(10):1462–73. doi: 10.1158/1055-9965.EPI-15-0301 [DOI] [PubMed] [Google Scholar]
- 20.Kyu HH, Bachman VF, Alexander LT, Mumford JE, Afshin A, Estep K, et al. Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: systematic review and dose-response meta-analysis for the Global Burden of Disease Study 2013. BMJ. 2016;354:i3857 doi: 10.1136/bmj.i3857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore SC, Lee IM, Weiderpass E, Campbell PT, Sampson JN, Kitahara CM, et al. Association of Leisure-Time Physical Activity With Risk of 26 Types of Cancer in 1.44 Million Adults. JAMA Intern Med. 2016;176(6):816–25. doi: 10.1001/jamainternmed.2016.1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Breen N, Lewis DR, Gibson JT, Yu M, Harper S. Assessing disparities in colorectal cancer mortality by socioeconomic status using new tools: health disparities calculator and socioeconomic quintiles. Cancer Causes Control. 2017;28(2):117–125. doi: 10.1007/s10552-016-0842-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization. Global Action Plan for the Prevention and Control of NCDs 2013–2020. Geneva: World Health Organization; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Del Duca GF, Nahas MV, Silva DA, Hallal PC, Malta DC, Peres MA. Physical activity indicators in adults from a state capital in the South of Brazil: a comparison between telephone and face-to-face surveys. Cad Saude Publica 2013;9:2119–29. doi: 10.1590/0102-311X00130412 [DOI] [PubMed] [Google Scholar]
- 25.Malta DC, Andrade SSA, Santos MAS, Rodrigues G, Mielke G. Trends of physical activity indicators in adults: State Capitals of Brazil 2006–2013. Rev Bras Ativ Fis Saude 2015;20(2):141–51. doi: 10.12820/RBAFS.V.20N2P141 [Google Scholar]
- 26.Stopa SR, Malta DC, Monteiro CN, Szwarcwald CL, Goldbaum M, Cesar CLG. Use of and access to health services in Brazil, 2013 National Health Survey. Rev Saude Publica. 2017;51(suppl 1):3s doi: 10.1590/S1518-8787.2017051000074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwarz E, Gomes R, Couto MT, Moura EC, Carvalho SA, Silva SF. Men's Health Policy. Rev Saude Publica 2012;46(suppl 01):108–16. doi: 10.1590/S0034-89102012005000061 [DOI] [PubMed] [Google Scholar]
- 28.Strath SJ, Kaminsky LA, Ainsworth BE, et al. Guide to the assessment of physical activity: Clinical and research applications: a scientific statement from the American Heart Association. Circulation. 2013;128(20):2259–79. doi: 10.1161/01.cir.0000435708.67487.da [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
*Age-standardized rate; U.I.: uncertainty interval.
(PDF)
*Age-standardized rate; U.I.: uncertainty interval.
(PDF)
*Age-standardized rate; U.I.: uncertainty interval.
(PDF)
*Age-standardized rate; U.I.: uncertainty interval.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.