Abstract
Background
Obesity is a well-known risk factor of breast cancer in post-menopausal women that also correlates with a diminished therapeutic response. The influence of adipocytes and their secretome, i.e. adipokines, on the efficacy of hormone therapy has yet to be elucidated.
Methods
We investigated, ex vivo, whether mature adipocytes, differentiated from adipose stem cells of normal-weight (MA20) or obese (MA30) women, and their secretions, were able to counteract the effects of tamoxifen (Tx) which is known to decrease neoplastic cell proliferation.
Results
In a tridimensional model and in a model of co-culture, the anti-proliferative effect of Tx on MCF-7 cancer cells was counteracted by MA30. These two models highlighted two different specific gene expression profiles for genes encoding cytokines or involved in angiogenesis based on the adipocyte microenvironment and the treatment. Thus it notably showed altered expression of genes such as TNFα that correlated with IL-6. In addition, leptin, IL-6 and TNFα, at concentrations reflecting plasma concentrations in obese patients, decreased the anti-proliferative efficacy of 4-hydroxytamoxifen (a major active metabolite of Tx).
Conclusions
These findings bring insights on adipocytes and mammary cancer cell interactions in Tx therapy, particularly in overweight/obese people. Indeed, patient’ adipokine status would give valuable information for developing individual strategies and avoid resistance to treatment.
Introduction
Obesity is a risk factor for breast cancer in postmenopausal women [1,2] and an increased BMI is associated with higher risk of metastasis, recurrence and poor final outcome [3–7]. Adipose tissue secretes adipokines, such as leptin, interleukin 6 (IL-6) and Tumor Necrosis Factor α (TNFα), whose plasma concentrations are increased in obese subjects [8–10]. In the breast, adipose tissue and more particularly adipocytes and their secretome, may play a major role in cancer development by surrounding the mammary gland. After menopause, circulating estrogens, which derive from adipose tissue (AT), are associated with an increase of both risk and progression of estrogen receptor positive (ER+) breast cancer [5] because estrogens are well known to be involved in breast cancer progression. Indeed, 75% of postmenopausal breast cancer patients develop ER+ breast cancer and women presenting ER+ breast cancer have poorer final outcome if they are obese compared to women with healthy weight [11]. According to the World Cancer Research Fund, an increase in fat mass of 5 kg/m2 among postmenopausal women increases the relative risk (RR) of developing breast cancer (RR = 1.13; 95% confidence interval (CI) = 1.08–1.18) and a weight gain of 10 to 20 kg induces a relative risk of mortality of 1.93 (95% CI = 1.43–2.73).
The higher risk of recurrence and mortality in obese patients could be related to a lesser efficacy of anti-cancer treatments probably due to plasma adipokine variations linked to overweight. Indeed, obese breast cancer women are less sensitive to chemotherapy [12] and present higher mortality rates [13–15]. MDA-MB-231 mammary cancer cells treated with adipose stem cell supernatants present resistance to doxorubicin [16]. By addition, leptin counteracts with the cytotoxic activity of the 5-fluorouracil in colorectal cancer cells [17]. Moreover, we demonstrated that leptin can reduce Tamoxifen (Tx) and chemotherapy efficacy (5-fluorouracil, taxol and vinblastin) in an assessment led on MCF-7 proliferation especially when leptin was used at concentrations reflecting circulating levels found in obese people [18]. Tx, a standard hormone therapy, increases serum leptin levels in postmenopausal breast cancer patients [19,20]. So, leptin may interfere with the efficacy of breast cancer treatments, especially anti-estrogens like Tx that targets ER.
The objective of this research was to evaluate the relationship between obesity and the efficacy of breast cancer treatment with tamoxifen. We evaluated ex vivo the impact of adipocyte secretome using human adipocytes from obese and healthy weight women on the efficacy of Tx hormone therapy and focused on specific biomarkers associated with a poor prognosis (leptin, IL-6 and TNFα). This would allow to better understand the risk associated with obesity which could participate to promote therapeutic escape.
Materials and methods
Cell culture and reagents
The human breast cancer cell line ERα+ MCF-7 and the human breast cells 184B5 from a healthy tissue removed during breast reduction (American Type Culture Collection (ATCC), Molsheim, France), were cultured as previously described [18] according to ATCC recommendations.
Human adipose stem cells (hASCs) were kindly provided by the Cell and Tissue Bank (Hôpital Edouard-Herriot, Lyon, France). hASCs were obtained from patients undergoing surgery for cosmetic purposes without associated pathology according to Helsinki declaration from anonymous healthy donors. Surgical residue was harvested according to French regulation including declaration to research ministry (DC n°2008162) and procurement of written informed consent from the patient. hASCs were extracted from subcutaneous AT from women undergoing optimized liposuction who presented a body mass index (BMI) corresponding to either a normal weight (BMI = 22.4, hASC20), or overweight (BMI = 27.7, hASC27) or obese (BMI = 30.3, hASC30) situations. hASCs were extracted [21] using a 3 mm cannula according to ethical and safety guidelines as approved by the local IRB and as described by Björntorp and differentiated into mature adipocytes (MA) [21].
All the cells used were under mycoplasma-free conditions (MycoAlert Plus, mycoplasma detection kit, Lonza, Bale, Switzerland) and cultured in a 5% CO2-humidified incubator at 37°C.
Influence of mature adipocyte secretions on tamoxifen efficacy in a monolayer system
MA obtained after differenciation of hASC [22] from normal (MA20) or obese (MA30) women were cultured (5x103cells/cm2, n = 3) for 5 days and conditioned media (CM) collected (CM20 or CM30 respectively). MCF-7 and 184B5 cells were plated in 96-well plates (5x103 cells) and the medium was replaced after 24h by CM20 or CM30 treated or not with Tx (12.5 μM, 72h). Cell proliferation was measured using the resazurin test (Exw = 530nm and Emw = 590nm, Fluoroskan Ascent FL®, Thermo Fisher Scientific, Wilmington, USA).
Influence of mature adipocyte secretions on tamoxifen efficacy in a co-culture system
Mammary cancer cells were co-cultured with adipocytes (Transwell culture system, porosity 0.4 μm, 5x103 cells/cm2; Merck Millipore, Molsheim, France). MCF-7 cells were seeded on the bottom of the system and MA20, MA27 or MA30 on the top chamber. After 24h of co-culture, cells were treated or not with Tx (12.5 μM) and cell proliferation was measured after 72h as described above (n = 3). Mammary and adipose cells cultured alone served as controls.
Mammary breast cells 184B5 (n = 3) were also co-cultured with MA from overweight women (MA27) to assess the role of adipocytes on normal cells.
Evaluation of cell interactions in 3D model
Development of a tridimensional adipose skin equivalent model
Primary cultures of keratinocytes and fibroblasts were established in accordance to ethical and safety guidelines (French regulation n° DC-2008-162) from patients undergoing surgery (child donors, age<10 years), to prepare an adipose skin equivalent model as previously described [22,23].
hASCs (105 cells/skin) from thin and obese women were seeded on the top of a collagen-glycosaminoglycan-chitosan scaffold to constitute a dermal substrate. Fibroblasts (105 cells/skin) were seeded on it to obtain a fatty equivalent dermis after 3 weeks. MCF7 cells or keratinocytes (skin equivalent control) (106 cells) were seeded on the surface and treated or not with Tx (2.5 μM) to obtain 3D adipose skin equivalents (n = 3) [23].
Histological analysis
3D adipose skin equivalents were fixed in O.C.T compound (Tissue Teck, Sakura, Netherlands) and frozen (−20°C) [23–25]. Tissue sections were stained with Hematoxylin Phloxin Safran (HPS, Sigma-Aldrich, Saint-Louis, United States) to visualize nucleus, cytoplasm and extracellular matrix formation or with Oil Red O and counterstained with Hematoxylin (Sigma-Aldrich).
Evaluation of protein expressions by immunohistochemistry
For immunohistochemical and immunofluorescence staining, Ki67 expression was investigated using affinity-purified polyclonal biotinylated antibodies (Merck Millipore, 1 μg/mL) or monoclonal-mouse primary antibodies to Ki67 respectively (clone MIB1, EnVision, Dakocytomation, Glostrup, Denmark, 1/50). Quantification of proliferative capacity was done by Ki67 positive-cell numeration (n = 3, 3 different lecturers). Nuclear counterstaining using Hoescht was carried out routinely.
Evaluation of gene expressions by qRT-PCR
Mammary cells were collected at the surface of the skin after a thermolysin treatment and RNA was extracted with Trizol (Invitrogen, Thermo Fisher Scientific, Waltham, United States). After the evaluation of the quantity and purity (NanoDrop 2000, Thermo Fisher Scientific), DNase treatment (DNase I Amplification grade, Invitrogen) and cDNAs retrotranscription (HighCap cDNA RT Kit RNAse inhib, Invitrogen) were made according to the manufacturer’s recommendations.
Quantitative Real-Time PCR (qPCR) assays were performed on plates designed by Applied Biosystems (TaqMan® Array 96 well Fast Plate, Customformat 48, Part N° 4413257, Lot N°1307140–0001) using SDS7900HT automaton (Applied Biosystems, Thermo Fisher Scientific) with TaqMAN® (Applied Biosystems). The analysis was conducted on 44 genes and 3 references genes with TaqMAN® Array Fast Plates (18S; UBC; ACTB; LEPR; LEP; ADIPOQ; ADIPOR1; ADIPOR2; AKT1; BAX; BCL2; BRCA1; CCND1; CYP19A1; ESR1; ESR2; IL6; MAPK1; MMP2; MMP9; MYC; PGR; PPARA; PPARG; STAT3; TNF; TP53; GSR; PTGS2; HMOX1; GPX1; GPX4; VEGFA; KDR; THBS1; HIF1A; CDH1; PCNA; ERBB2; AURKA; BIRC5; CCNB1; MYBL2; GRB7; BAG1; MMP11; NME1; CA9). Actin B (ACTB) and Ubiquitin C (UBC) were used for normalization. The comparative cycle threshold (CT) method (2-ΔΔCT) was used to calculate the relative gene expression of a given sample, normalized within the sample to two reference genes, and relative to the expression of the same gene in another sample: 2-ΔΔCT method with ΔΔCT = [ΔCT (sample1) - ΔCT (sample2)] and ΔCT = [CT(target gene)–geometric mean CT (reference genes)].
Influence of leptin, IL-6 and TNFα on 4-OH-Tx efficacy in a monolayer system
MCF-7 cells (5x103 cells, 96-well plates, n = 6) were treated or not with leptin (10 ng.mL-1, 100 ng.mL-1), Il-6 (2.3 pg.mL-1, 83 pg.mL-1) and TNFα (0.7 pg.mL-1, 3.5 pg.mL-1) in the presence or not of 4-OH-Tx (12.5 μM). Cell proliferation was measured as described above. The choosen concentrations reflected plasma concentrations in thin or obese women [10,26,27].
Statistics
Results were expressed as mean +/- SEM. Statistical analysis was performed using the paired, bilateral Student’s t-test with StatView® Software (SAS Institute Inc.) excepted concerning the Ki67 positive-cells (Mann-Withney test).
Results
Crosstalk between breast cancer cells and adipocytes or their secretome decreased Tx efficacy on MCF-7 and 184B5 cells, especially in case of obesity
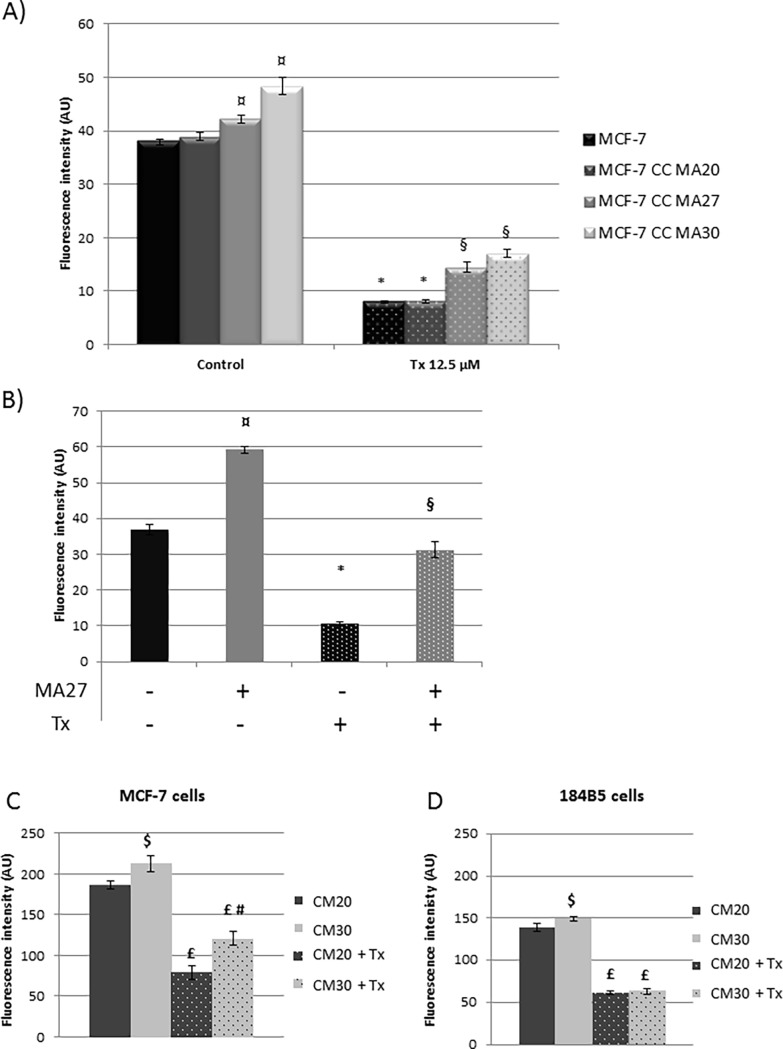
MA27 or MA30 induced a significantly increase of MCF-7 cell proliferation (+15% and 28% respectively, p<0.05) (Fig 1A) and a less efficacy of Tx treatment (-68% or -65% respectively, p<0.05). In 184B5 mammary normal cells, MA increased the proliferation (+61%, p<0.05) (Fig 1B). Tx treatment decreased cell proliferation (-71%, p<0.05), this effect was counteracted with MA (-84%, p<0.05).
Fig 1. Effect of mature adipocytes, their secretions and Tamoxifen (Tx) on cell proliferation.
(A) MCF-7 cells were co-cultured with MA obtained from women of normal weight (MA20), overweight (MA27) or obese (MA30) women and treated with Tx. (B) 184B5 cells were co-cultured with MA27 with or without Tx. For A) and B), the proliferation was quantified after 72h using a resazurine test (Fluoroskan Ascent® FL). (C) MCF-7 and (D) 184B5 cells were also cultured with conditioned media from the culture of MA20 (CM20) and AM30 (CM30) with or without Tx treatment. Results were expressed as Mean ± SEM, ¤ MA vs Control, * Tx vs Control, § MA+Tx vs Tx; $ CM30 vs CM20, £ CM+Tx vs CM, # CM30+Tx vs CM20+Tx (n = 3, Student's t test).
Concerning the impact of the secretome, CM30 increased MCF-7 proliferation (+14%, p<0.05) and diminished Tx efficacy compared to CM20 (-43% and -58% respectively, p<0.05) (Fig 1C). Concerning 184B5 cells, CM30 slightly increased their proliferation (+15%, p<0.05) but not affected the antiproliferative effect of Tx (Fig 1D).
Adipocytes and their secretome decreased Tx efficacy on MCF-7 cells in a tridimensional model and induced variations of gene expressions
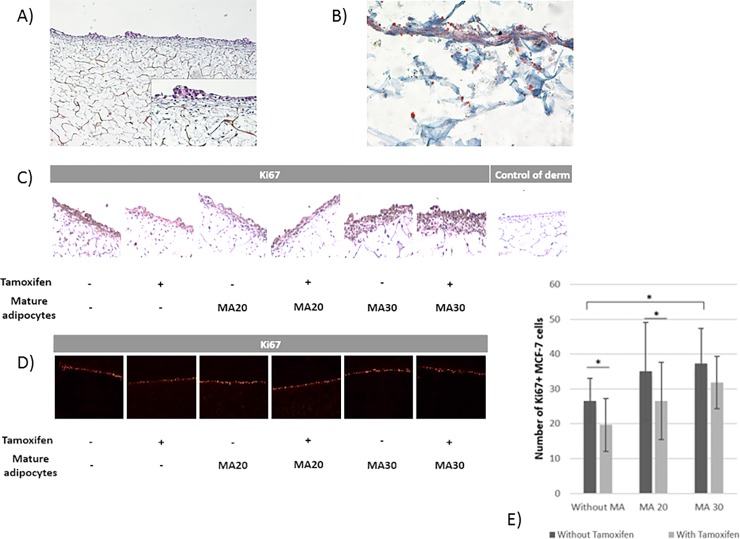
The validation of the 3D model was checked by histological analysis as previously described [23]. This analysis was made in order to show the presence of fibroblasts colonizing the porous scaffold surrounded by their extracellular matrix via HPS staining (Fig 2A). Oil Red O staining showed the presence of MA differentiated from hASC (Fig 2B).
Fig 2. Tridimensional adipose mammary equivalent model.
(A) Hematoxylin Phloxin Safran staining showed a pluristratified and differentiated epithelium on a connective underlying tissue made of fibroblasts surrounded by their ECM coloured in yellow in the porous scaffold. (B) Mature adipocytes were visible after Oil Red O staining coloring their vesicles in pink. (C) Ki67 immunohistochemical staining of the tridimensional adipose equivalent model using affinity-purified polyclonal biotinylated antibodies raised against Ki67 (Magnification: ×400). Positive staining appeared in brown. (D) Ki67 immunofluorescent staining of the tridimensional adipose equivalent model. Positive staining appeared in red. (E) Comparison of cellular proliferation in tridimensional adipose equivalent model with or without mature adipocytes and tamoxifen treatment.
Without MA, Tx decreased Ki67+ MCF7 cell number (Fig 2C). MA30 increased Ki67+ MCF7 number but no significant decrease was observed after Tx treatment (Fig 2C, 2D and 2E).
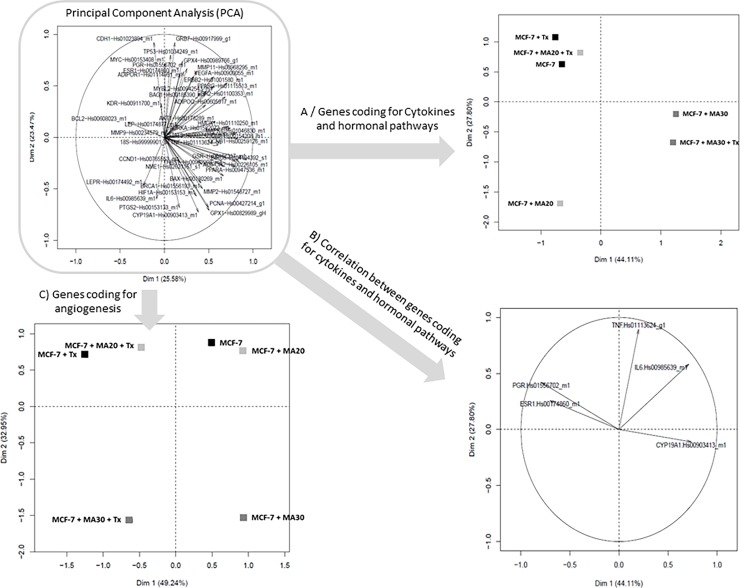
A principal component analysis (PCA) was performed on all the studied genes (Fig 3). In view of the complexity of these results, we decided to carry out this analysis according to the biological functions of the genes eg “adipokines, cytokines and hormonal pathway” (LEP, LEPR, ADIPOQ, ADIPOR1, ADIPOR2, ESR1, ESR2, PGR, IL6, TNF, CYP19A1), “cell cycle and proliferation” (MYC, AKT1, BAX, BCL2, BRCA1, CCND1, MAPK1, PPARA, PPARG, TP53, STAT3, PCNA, ERBB2, AURKA, BIRC5, CCNB1, MYBL2, GRB7, BAG1), “angiogenesis” (CDH1, MMP9, MMP2, VEGFA, KDR, THBS1 HIF1A), “oxidative stress” (GSR, PTGS2, HMOX1, GPX1, GPX4), “treatment response” (MMP11, NME1, CA9, HIF1A, CDH1, PCNA, ERBB2, AURKA, BIRC5, CCND1, MYBL2, GRB7, BAG1).
Fig 3. Principal component analysis (PCA) to explore the relationships among genes on 3D adipose skin equivalent model.
The expression of genes in MCF-7 mammary cell line co-cultured or not with mature adipocytes from women with a normal weight (MA20) or obese women (MA30) with or without tamoxifen treatment were analyzed by PCA. A first global PCA was carried out (A) followed by a second one taking into account the biological function of the different studied genes (B, C, D, E).
The restricted PCA analysis concerning “Cytokines and hormonal pathways” (Fig 3A) permitted to segregate cells exposed to an obese environment. In addition, closed correlations have been identified notably between PGR and ESR1 and between IL-6 and TNFα (Fig 3B). When the PCA focused on “angiogenesis”, clusters were obtained according both to an obese environment and to the Tx treatment exposition (Fig 3C).
When the effect of obesity was investigated using expression of specific genes, we observed that Leptin, PGR and VEGF expressions were significantly decreased in MCF-7 co-cultured with MA30 versus MCF-7 co-cultured with MA20 (Leptin: 0.09-fold, p<0.001; PGR: 0.27-fold, p = 0.05; VEGF: 0.48-fold, p<0.05, vs MA20) contrary to TNFα gene expression which was increased by MA30 (2.71-fold, p = 0.01) (Table 1, column A).
Table 1. qRT-PCR assays on 3D adipose equivalent model.
| Column A | Column B | Column C | Column D | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Obesity effect | Tamoxifen effect | Adipocyte effect with Tx treatment | Obesity effect with Tx | |||||||||
| MCF7_MA30 / MCF7_MA20 | MCF-7_Tx / MCF-7 | MCF-7_MA20-Tx/MCF-7_Tx | MCF-7_MA30-Tx/MCF-7_Tx | MCF7_MA30_Tx / MCF7_MA20_Tx | ||||||||
| R (quantity) |
p value |
R (quantity) | p value | R (quantity) | p value | R (quantity) | p value | R (quantity) | p value | |||
| Adipokines, cytokines, hormonal pathway | LEP | 0,09 | 0,00001 | 1,00 | 0,99 | 1,13 | 0,68 | 0,49 | 0,04 | 0,44 | 0,02 | |
| TNF | 2,71 | 0,01 | 1,07 | 0,83 | 1,02 | 0,96 | 0,65 | 0,20 | 0,64 | 0,18 | ||
| PGR | 0,27 | 0,05 | 1,26 | 0,70 | 0,77 | 0,68 | 0,23 | 0,03 | 0,30 | 0,07 | ||
| ESR1 | 0,59 | 0,16 | 0,68 | 0,30 | 0,57 | 0,14 | 0,48 | 0,06 | 0,84 | 0,64 | ||
| IL6 | 1,34 | 0,50 | 1,25 | 0,61 | 0,99 | 0,98 | 1,02 | 0,97 | 1,03 | 0,95 | ||
| CYP19A1 | 1,34 | 0,93 | 0,57 | 0,25 | 0,79 | 0,62 | 1,62 | 0,33 | 2,05 | 0,16 | ||
| ADIPOR1 | 1,05 | 0,87 | 0,95 | 0,84 | 0,72 | 0,25 | 0,65 | 0,14 | 0,91 | 0,73 | ||
| ADIPOR2 | 0,77 | 0,87 | 0,84 | 0,55 | 0,89 | 0,70 | 0,83 | 0,53 | 0,93 | 0,81 | ||
| PPARA | 0,84 | 0,61 | 0,72 | 0,34 | 1,13 | 0,71 | 0,92 | 0,80 | 0,81 | 0,54 | ||
| PPARG | 0,74 | 0,32 | 0,79 | 0,43 | 0,93 | 0,79 | 0,87 | 0,63 | 0,94 | 0,79 | ||
| cell cycle and proliferation, oncogene | BRCA1 | 0,77 | 0,49 | 0,40 | 0,03 | 1,35 | 0,43 | 0,94 | 0,86 | 1,35 | 0,82 | |
| CCNB1 | 1,12 | 0,73 | 0,63 | 0,17 | 0,50 | 0,05 | 0,74 | 0,36 | 1,50 | 0,23 | ||
| MYBL2 | 1,08 | 0,76 | 0,77 | 0,33 | 0,63 | 0,10 | 0,81 | 0,43 | 1,29 | 0,34 | ||
| ERBB2 | 0,56 | 0,09 | 1,39 | 0,30 | 0,58 | 0,10 | 0,66 | 0,19 | 1,14 | 0,68 | ||
| CCND1 | 0,71 | 0,18 | 0,90 | 0,68 | 1,06 | 0,80 | 1,21 | 0,43 | 1,14 | 0,59 | ||
| STAT3 | 0,84 | 0,51 | 0,98 | 0,95 | 0,55 | 0,04 | 0,85 | 0,54 | 1,54 | 0,11 | ||
| MAPK1 | 0,91 | 0,74 | 0,78 | 0,38 | 0,77 | 0,35 | 1,06 | 0,83 | 1,39 | 0,25 | ||
| TP53 | 1,04 | 0,89 | 0,92 | 0,78 | 0,88 | 0,66 | 0,87 | 0,65 | 0,99 | 0,98 | ||
| AKT1 | 1,04 | 0,87 | 0,97 | 0,89 | 0,83 | 0,41 | 1,02 | 0,93 | 1,23 | 0,36 | ||
| BAX | 1,04 | 0,89 | 0,81 | 0,47 | 1,05 | 0,86 | 1,31 | 0,36 | 1,24 | 0,45 | ||
| BIRC5 | 1,34 | 0,53 | 0,56 | 0,23 | 0,66 | 0,38 | 1,04 | 0,93 | 1,59 | 0,33 | ||
| Angiogenesis | VEGFA | 0,48 | 0,03 | 1,01 | 0,98 | 0,81 | 0,50 | 0,41 | 0,01 | 0,5 | 0,1 | |
| HIF1A | 0,67 | 0,32 | 0,42 | 0,05 | 1,79 | 0,16 | 1,13 | 0,75 | 0,63 | 0,27 | ||
| THBS1 | 1,04 | 0,89 | 0,80 | 0,67 | 1,21 | 0,72 | 0,98 | 0,97 | 0,81 | 0,69 | ||
| MMP2 | 1,31 | 0,31 | 0,85 | 0,53 | 0,95 | 0,83 | 1,31 | 0,31 | 1,38 | 0,23 | ||
| Oxidative stress | HMOX1 | 0,61 | 0,13 | 0,81 | 0,49 | 0,51 | 0,1 | 0,81 | 0,50 | 1,58 | 0,16 | |
| GSR | 0,80 | 0,41 | 0,83 | 0,48 | 0,90 | 0,69 | 0,95 | 0,85 | 1,06 | 0,83 | ||
| GPX1 | 0,78 | 0,60 | 0,80 | 0,64 | 1,08 | 0,87 | 1,71 | 0,26 | 1,58 | 0,33 | ||
| GPX4 | 1,30 | 0,39 | 1,02 | 0,95 | 0,80 | 0,46 | 0,75 | 0,35 | 0,94 | 0,84 | ||
| PTGS2 | 0,69 | 0,47 | 0,75 | 0,57 | 1,44 | 0,48 | 1,05 | 0,93 | 0,73 | 0,54 | ||
| Treatment response | CDH1 | 1,13 | 0,82 | 1,25 | 0,67 | 0,46 | 0,17 | 0,41 | 0,11 | 0,89 | 0,82 | |
| CA9 | 0,65 | 0,16 | 0,82 | 0,51 | 0,71 | 0,25 | 0,92 | 0,79 | 1,30 | 0,37 | ||
| NME1 | 0,54 | 0,14 | 0,92 | 0,83 | 1,15 | 0,73 | 0,80 | 0,58 | 0,70 | 0,38 | ||
| AURKA | 1,02 | 0,94 | 0,86 | 0,58 | 0,60 | 0,08 | 0,58 | 0,07 | 0,97 | 0,91 | ||
| GRB7 | 0,89 | 0,80 | 1,95 | 0,16 | 0,45 | 0,09 | 0,36 | 0,04 | 0,80 | 0,62 | ||
qRT-PCR analysis was conducted on MCF-7 cells co-cultured with MA from normal (MA20) or obese (MA30) women with or without tamoxifen treatment (TaqMAN® (Applied Biosystems)). ACTB and UBC were used for normalization. The comparative cycle threshold (CT) method (2-ddCT) was used to calculate the relative gene expression of a given sample, normalized within the sample to two endogenous reference genes. The results are significant when p value is inferior to 0.05 (indicated in the table).
When cells were treated with Tx, Leptin expression remained unchanged whereas a significant decrease of BRCA1 and HIF1A expression was observed (Table 1, column B).
When adipose microenvironment was evaluated on Tx efficacy (MCF-7 co-cultured with both adipocytes and tamoxifen versus MCF-7 cultured only with Tx) (Table 1, column C), Leptin expression was unchanged in comparison with MCF-7 cultured only with Tx. A decrease of HMOX1 was observed for MCF-7 co-cultured with MA20 (R = 0.51, p = 0.05, vs without MA) whereas a decrease of VEGFA, PGR and GRB7 was observed for MCF-7 with MA30 (R = 0.41, p = 0.01; R = 0.23, p<0.05; R = 0.36, p<0.05 respectively, vs without MA).
When we investigated the impact of both obesity and Tx treatment, Leptin and VEGF expressions were decreased in MCF-7 cells co-cultured with MA30 and Tx (R = 0.44, p<0.05; R = 0.51, p = 0.05, vs co-cultured with MA20 and Tx). These results confirmed the results obtained in the 3D system.
Other gene expressions like ESR1, IL-6, CYP19A1, AdipoR1, AdipoR2, PPARα and PPARγ were not altered by MA or Tx treatment.
Leptin, IL-6 and TNFα decreased 4-OH-Tx efficacy on neoplasic MCF-7 cells
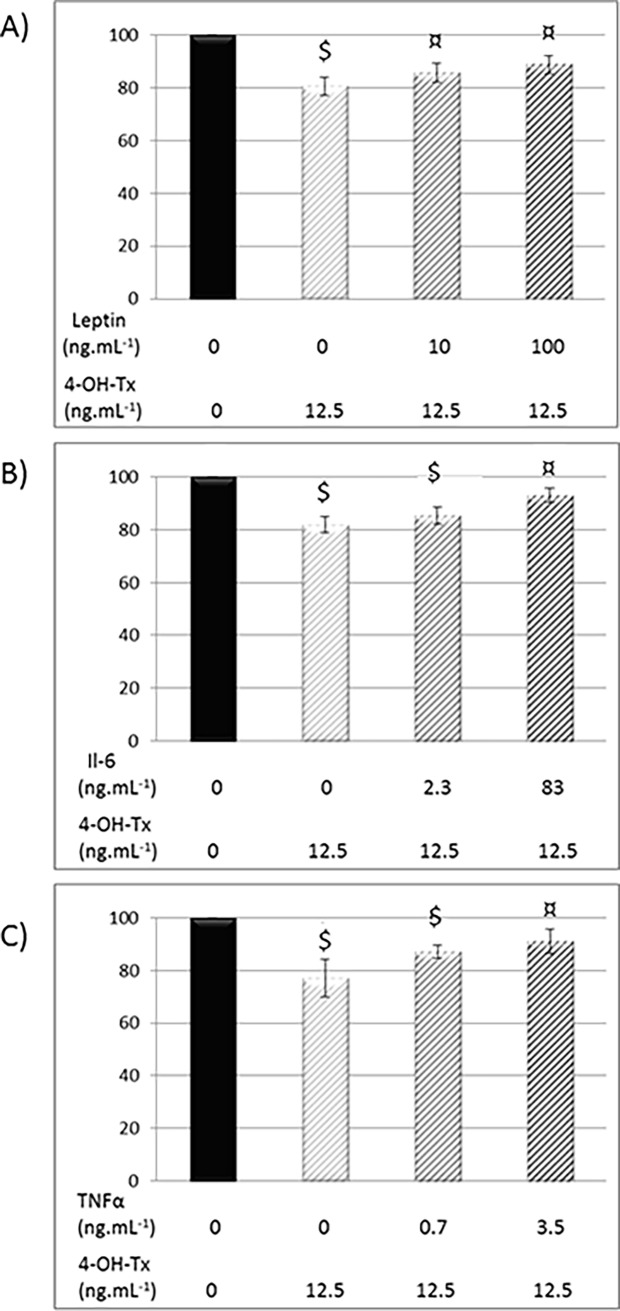
We previously showed that leptin (100 and 1,000 ng/ml) increased MCF7 proliferation and reduced Tx efficacy [18]. In the present study, we demonstated that the presence of leptin, IL-6 and TNFα, used at concentrations reflected plasmatic levels in obese people, also diminished the efficacy of 4-OH-Tx, an active metabolite of Tx (-11%, -7%, -9% respectively) (Fig 4).
Fig 4. Effects of Leptin, IL-6 and TNFα on MCF-7 cell proliferation after 4-OH-tamoxifen treatment.
After adhesion, MCF-7 were treated with leptin (0; 10; 100 ng/mL) (A), IL-6 (0, 2.3; 83 pg/mL) (B), TNFα (0; 0.7; 12.5 pg/mL) (C) and with 12.5 μM of 4-OH-Tx. After 72h, cell proliferation was quantified using a resazurine test (Fluoroskan Ascent® FL). Mean ± SEM, $ 4-OH-Tx +/- adipokine vs Control, ¤ 4-OH-Tx +/- adipokine vs 4-OH-Tx (n = 3, Student's t test).
Discussion
Obesity is a risk factor for breast cancer development in postmenopausal women and increases metastasis and recurrence associated with changes in serum adipokines [1,3,6]. A lesser therapeutic response is always described for obese patients and could be related to obesity or change in body weight. Indeed, an increase of relapses or mortality is described in obese women receiving chemotherapy [13,28]. An hyperleptinemia is also described in women treated by Tx [19]. In our paper, the relationship between obesity, adipokines and Tx therapy was investigated by evaluating interactions between cancer and adipose cells, and the specific role of adipocyte secretome. The strength of our study is to use ex-vivo human mature adipocytes from women of normal weight, overweight and obese women.
With the model of co-culture between MA and mammary cells, we brought the evidence of the role of MA in breast cancer growth. In our results, women MA obtained after hASCs differentiation were able, ex vivo, to significantly increase the proliferation of both mammary cancer cells (MCF-7) and normal mammary cells (184B5). Co-culture experiments between MA isolated from rat subcutaneous adipose tissue have also demonstrated their ability to stimulate the proliferation of breast cancer cell lines (MCF-7, T47D cells…) [29]. It was also shown that adipose tissue co-cultured with rat mammary tumor cells (CRL1743 cells) resulted in an increase in growth and migration of tumor cells [30].
To highlight the influence of adipocyte secretome on Tx efficacy, we used conditioned media obtained from the culture of MA20 or MA30 (CM20 and CM30) and showed that CM30 raised MCF-7 and 184B5 proliferation compared to CM20 treatment. Similarly, the effects of adipocyte differentiation on proliferation and migration of normal (NMuMG) and tumoral (LM3) murine breast epithelial cells were increased with 3T3-L1 conditioned media [31]. In studies using human samples, the incubation of normal (MCF10a) and malignant (MCF-10CA1) breast epithelial cells with breast adipocyte conditioned media (Adip-CM) increased cell motility [32].
Until now, only few studies focus on the relationship between human mammary and adipose cells in case of obesity. So, we investigated obesity effect using a co-culture model between MCF-7 and MA obtained from normal weight (MA20), overweight (MA27) and obese (MA30) women on Tx efficacy, to bring out the influence of the dialogue between these two cell types on treatment resistance. To the best of our knowledge, our study is the first to report an ex vivo impact of adipocytes obtained from obese women compared to women of normal weight.
We showed that human MA, differentiated from hASCs, were able to induce MCF-7 proliferation by increasing the number of Ki67 positive-cells. This effect was more pronounced with MA27 and MA30. A recent study using hASC, derived from the abdominal subcutaneous AT of obese subject (BMI>30), has shown an enhanced breast cancer cell proliferation (MCF-7 and MDA-MB-231 cells) and tumorogenesis in immunodeficient mice [33]. As expected, we observed that Tx treatment was efficient to reduce the MCF-7 cell proliferation, whereas its effect was significantly counteracted in the presence of MA27 or MA30. Our results could be correlated with the breast cancer cell (SUM159PT) radioresistance observed when MA obtained from 3T3 murine preadipocyte differentiation were added [34].
To highlight the impact of cell-cell interaction and obesity in a more physiological model, we developed an original 3D model [23], wich mimic breast tumor in contact with a connective tissue containing AT assessing the interactions between the different cell types in the presence or not of Tx treatment. Similarly to monolayer co-culture, the adipose microenvironment from obese women induced a higher proliferation of breast cancer cells. Tx treatment decreased MCF-7 proliferation and interestingly the presence of MA from obese women reduced its efficacy. A gene expression analysis permitted us to classify genes according to their biological functions such as “angiogenesis process”, “cytokines and hormonal pathways”. The PCA highlighted two groups from the panel of genes according to adipocyte microenvironment and Tx treatment. Considering “cytokines and hormone” gene expression, only a positive correlation between PGR and ESR1 may be identified independently of adipocyte microenvironment and Tx treatment. In addition, in this 3D model, the expression of TNFα was increased, in case of obesity, and a positive correlation was highlighted between genes coding for inflammatory proteins such as IL-6 and TNFα. Indeed, TNFα was demonstrated as being able to regulate the expression of other cytokines such as IL-6 [35]. It was consistent with our results because, i) an increase of TNFα expression was found in MCF-7 cells after co-culture with MA30, compared to MA20; ii) analysis of conditioned media of co-culture between MCF-7 and MA30 compared to those of MCF-7 showed that IL-6 secretion was increased.
To investigate further which adipokine can be involved in this effect, we focused on leptin, IL-6 and TNFα. We previously described that leptin decreases Tx efficacy [18] and we used in the present study the 4-OH-Tx, a Tx active metabolite. Leptin, IL-6 and TNFα decreased the anti-proliferative effect of 4-OH-Tx on MCF7 cells. This crosstalk between leptin, IL-6 and TNFα may be considered since for example, epithelial ovarian cancer which presenting an autocrine production of TNFα have greater release of IL-6 [36]. Moreover, the stimulatory effect of adipose stromal cells on migration and invasion of breast tumor cells is abrogated by a depletion of IL-6 [37].
Concerning normal mammary cells 184B5 cells, the decrease of proliferation could be due to another Tx target since it was demonstrated that ligands which bind to antiestrogen-binding site (AEBS) could inhibit cell proliferation in a dose dependent manner [38]. Another hypothesis was that Tx could induce Protein Kinase C inhibition, which resulted in oxidative stress, and was followed by an inhibition of proliferation [39].
Conclusions
Our data showed that adipocyte secretome may reduce the efficacy of Tx therapy in case of overweight/obesity. Further studies are therefore needed to better understand the precise role played by adipose tumor microenvironment and by adipokines such as IL-6, leptin and TNFα in breast cancer progression. Indeed, the identification of specific biomarkers may allow a personalized management of overweight breast cancer patients.
Abbreviations
- BMI
Body Mass Index
- MA20
Mature adipocytes differentiated from stem cells of normal-weight (BMI = 20) women
- MA27
Mature adipocytes differentiated from stem cells of overweight (BMI = 27) women
- MA30
Mature adipocytes differentiated from stem cells of obese (BMI = 30) women
Data Availability
All relevant data are within the paper.
Funding Statement
This study received financial support from the Canceropôle Lyon Auvergne Rhône-Alpes (Oncostarter 2012), from the Institut National du Cancer (Mammadipo 2013, INCA n°6666) and from the league of cancer research (CD63). Lauriane Bougaret is supported by a fellowship from the Ministry of Research and Technology.
References
- 1.Lahmann PH, Hoffmann K, Allen N, van Gils CH, Khaw K-T, Tehard B, et al. Body size and breast cancer risk: findings from the European Prospective Investigation into Cancer And Nutrition (EPIC). Int J Cancer J Int Cancer. 2004;111: 762–771. doi: 10.1002/ijc.20315 [DOI] [PubMed] [Google Scholar]
- 2.Björntorp P. Adipose tissue distribution and function. Int J Obes. 1991;15 Suppl 2: 67–81. [PubMed] [Google Scholar]
- 3.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348: 1625–1638. doi: 10.1056/NEJMoa021423 [DOI] [PubMed] [Google Scholar]
- 4.Harvie M, Hooper L, Howell AH. Central obesity and breast cancer risk: a systematic review. Obes Rev Off J Int Assoc Study Obes. 2003;4: 157–173. [DOI] [PubMed] [Google Scholar]
- 5.Stephenson GD, Rose DP. Breast cancer and obesity: an update. Nutr Cancer. 2003;45: 1–16. doi: 10.1207/S15327914NC4501_1 [DOI] [PubMed] [Google Scholar]
- 6.Petrelli JM, Calle EE, Rodriguez C, Thun MJ. Body mass index, height, and postmenopausal breast cancer mortality in a prospective cohort of US women. Cancer Causes Control CCC. 2002;13: 325–332. [DOI] [PubMed] [Google Scholar]
- 7.Biglia N, Peano E, Sgandurra P, Moggio G, Pecchio S, Maggiorotto F, et al. Body mass index (BMI) and breast cancer: impact on tumor histopathologic features, cancer subtypes and recurrence rate in pre and postmenopausal women. Gynecol Endocrinol Off J Int Soc Gynecol Endocrinol. 2013;29: 263–267. doi: 10.3109/09513590.2012.736559 [DOI] [PubMed] [Google Scholar]
- 8.Lorincz AM, Sukumar S. Molecular links between obesity and breast cancer. Endocr Relat Cancer. 2006;13: 279–292. doi: 10.1677/erc.1.00729 [DOI] [PubMed] [Google Scholar]
- 9.Maury E, Brichard SM. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol. 2010;314: 1–16. doi: 10.1016/j.mce.2009.07.031 [DOI] [PubMed] [Google Scholar]
- 10.Bougoulia M, Triantos A, Koliakos G. Plasma interleukin-6 levels, glutathione peroxidase and isoprostane in obese women before and after weight loss. Association with cardiovascular risk factors. Horm Athens Greece. 2006;5: 192–199. [DOI] [PubMed] [Google Scholar]
- 11.Maehle BO, Tretli S. Pre-morbid body-mass-index in breast cancer: reversed effect on survival in hormone receptor negative patients. Breast Cancer Res Treat. 1996;41: 123–130. [DOI] [PubMed] [Google Scholar]
- 12.Ewertz M, Jensen M-B, Gunnarsdóttir KÁ, Højris I, Jakobsen EH, Nielsen D, et al. Effect of obesity on prognosis after early-stage breast cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2011;29: 25–31. doi: 10.1200/JCO.2010.29.7614 [DOI] [PubMed] [Google Scholar]
- 13.Litton JK, Gonzalez-Angulo AM, Warneke CL, Buzdar AU, Kau S-W, Bondy M, et al. Relationship between obesity and pathologic response to neoadjuvant chemotherapy among women with operable breast cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2008;26: 4072–4077. doi: 10.1200/JCO.2007.14.4527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kyselova Z, Mechref Y, Kang P, Goetz JA, Dobrolecki LE, Sledge GW, et al. Breast cancer diagnosis and prognosis through quantitative measurements of serum glycan profiles. Clin Chem. 2008;54: 1166–1175. doi: 10.1373/clinchem.2007.087148 [DOI] [PubMed] [Google Scholar]
- 15.Schönherr A, Aivazova-Fuchs V, Annecke K, Jückstock J, Hepp P, Andergassen U, et al. Toxicity Analysis in the ADEBAR Trial: Sequential Anthracycline-Taxane Therapy Compared with FEC120 for the Adjuvant Treatment of High-Risk Breast Cancer. Breast Care Basel Switz. 2012;7: 289–295. doi: 10.1159/000341384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen D-R, Lu D-Y, Lin H-Y, Yeh W-L. Mesenchymal stem cell-induced doxorubicin resistance in triple negative breast cancer. BioMed Res Int. 2014;2014: 532161 doi: 10.1155/2014/532161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mełeń-Mucha G, Lawnicka H. Leptin promotes the growth of Colon 38 cancer cells and interferes with the cytotoxic effect of fluorouracil in vitro. Endokrynol Pol. 2007;58: 2–6. [PubMed] [Google Scholar]
- 18.Dubois V, Delort L, Billard H, Vasson M-P, Caldefie-Chezet F. Breast cancer and obesity: in vitro interferences between adipokines and proangiogenic features and/or antitumor therapies? PloS One. 2013;8: e58541 doi: 10.1371/journal.pone.0058541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marttunen MB, Andersson S, Hietanen P, Karonen SL, Koistinen HA, Koivisto VA, et al. Antiestrogenic tamoxifen and toremifene increase serum leptin levels in postmenopausal breast cancer patients. Maturitas. 2000;35: 175–179. [DOI] [PubMed] [Google Scholar]
- 20.Ozet A, Arpaci F, Yilmaz MI, Ayta H, Ozturk B, Komurcu S, et al. Effects of tamoxifen on the serum leptin level in patients with breast cancer. Jpn J Clin Oncol. 2001;31: 424–427. [DOI] [PubMed] [Google Scholar]
- 21.Mojallal A, Auxenfans C, Lequeux C, Braye F, Damour O. Influence of negative pressure when harvesting adipose tissue on cell yield of the stromal-vascular fraction. Biomed Mater Eng. 2008;18: 193–197. [PubMed] [Google Scholar]
- 22.Lequeux C, Auxenfans C, Mojallal A, Sergent M, Damour O. Optimization of a culture medium for the differentiation of preadipocytes into adipocytes in a monolayer. Biomed Mater Eng. 2009;19: 283–291. doi: 10.3233/BME-2009-0593 [DOI] [PubMed] [Google Scholar]
- 23.Delort L, Lequeux C, Dubois V, Dubouloz A, Billard H, Mojallal A, et al. Reciprocal interactions between breast tumor and its adipose microenvironment based on a 3D adipose equivalent model. PloS One. 2013;8: e66284 doi: 10.1371/journal.pone.0066284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dubois V, Delort L, Mishellany F, Jarde T, Billard H, Lequeux C, et al. Zinc-alpha2-glycoprotein: a new biomarker of breast cancer? Anticancer Res. 2010;30: 2919–2925. [PubMed] [Google Scholar]
- 25.Jardé T, Caldefie-Chézet F, Damez M, Mishellany F, Perrone D, Penault-Llorca F, et al. Adiponectin and leptin expression in primary ductal breast cancer and in adjacent healthy epithelial and myoepithelial tissue. Histopathology. 2008;53: 484–487. doi: 10.1111/j.1365-2559.2008.03121.x [DOI] [PubMed] [Google Scholar]
- 26.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334: 292–295. doi: 10.1056/NEJM199602013340503 [DOI] [PubMed] [Google Scholar]
- 27.Dandona P, Weinstock R, Thusu K, Abdel-Rahman E, Aljada A, Wadden T. Tumor necrosis factor-alpha in sera of obese patients: fall with weight loss. J Clin Endocrinol Metab. 1998;83: 2907–2910. doi: 10.1210/jcem.83.8.5026 [DOI] [PubMed] [Google Scholar]
- 28.Thivat E, Thérondel S, Lapirot O, Abrial C, Gimbergues P, Gadéa E, et al. Weight change during chemotherapy changes the prognosis in non metastatic breast cancer for the worse. BMC Cancer. 2010;10: 648 doi: 10.1186/1471-2407-10-648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manabe Y, Toda S, Miyazaki K, Sugihara H. Mature adipocytes, but not preadipocytes, promote the growth of breast carcinoma cells in collagen gel matrix culture through cancer-stromal cell interactions. J Pathol. 2003;201: 221–228. doi: 10.1002/path.1430 [DOI] [PubMed] [Google Scholar]
- 30.Salameh TS, Le TT, Nichols MB, Bauer E, Cheng J, Camarillo IG. An ex vivo co-culture model system to evaluate stromal-epithelial interactions in breast cancer. Int J Cancer J Int Cancer. 2013;132: 288–296. doi: 10.1002/ijc.27672 [DOI] [PubMed] [Google Scholar]
- 31.Creydt VP, Sacca PA, Tesone AJ, Vidal L, Calvo JC. Adipocyte differentiation influences the proliferation and migration of normal and tumoral breast epithelial cells. Mol Med Rep. 2010;3: 433–439. doi: 10.3892/mmr_00000276 [DOI] [PubMed] [Google Scholar]
- 32.Carter JC, Church FC. Mature breast adipocytes promote breast cancer cell motility. Exp Mol Pathol. 2012;92: 312–317. doi: 10.1016/j.yexmp.2012.03.005 [DOI] [PubMed] [Google Scholar]
- 33.Strong AL, Strong TA, Rhodes LV, Semon JA, Zhang X, Shi Z, et al. Obesity associated alterations in the biology of adipose stem cells mediate enhanced tumorigenesis by estrogen dependent pathways. Breast Cancer Res BCR. 2013;15: R102 doi: 10.1186/bcr3569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bochet L, Meulle A, Imbert S, Salles B, Valet P, Muller C. Cancer-associated adipocytes promotes breast tumor radioresistance. Biochem Biophys Res Commun. 2011;411: 102–106. doi: 10.1016/j.bbrc.2011.06.101 [DOI] [PubMed] [Google Scholar]
- 35.Frühbeck G, Gómez-Ambrosi J, Muruzábal FJ, Burrell MA. The adipocyte: a model for integration of endocrine and metabolic signaling in energy metabolism regulation. Am J Physiol Endocrinol Metab. 2001;280: E827–847. doi: 10.1152/ajpendo.2001.280.6.E827 [DOI] [PubMed] [Google Scholar]
- 36.Kulbe H, Thompson R, Wilson JL, Robinson S, Hagemann T, Fatah R, et al. The inflammatory cytokine tumor necrosis factor-alpha generates an autocrine tumor-promoting network in epithelial ovarian cancer cells. Cancer Res. 2007;67: 585–592. doi: 10.1158/0008-5472.CAN-06-2941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walter M, Liang S, Ghosh S, Hornsby PJ, Li R. Interleukin 6 secreted from adipose stromal cells promotes migration and invasion of breast cancer cells. Oncogene. 2009;28: 2745–2755. doi: 10.1038/onc.2009.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fargin A, Bayard F, Faye JC, Traore M, Poirot M, Klaebe A, et al. Further evidence for a biological role of anti-estrogen-binding sites in mediating the growth inhibitory action of diphenylmethane derivatives. Chem Biol Interact. 1988;66: 101–109. [DOI] [PubMed] [Google Scholar]
- 39.Gundimeda U, Chen ZH, Gopalakrishna R. Tamoxifen modulates protein kinase C via oxidative stress in estrogen receptor-negative breast cancer cells. J Biol Chem. 1996;271: 13504–13514. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.