Short abstract
The most common reasons for switching HIV-1 therapy in patients with virologic suppression are treatment regimen simplification and resolving tolerability issues. Single-pill regimens that include an integrase inhibitor are recommended options. A retrospective clinical audit was performed to determine the motivations for switching to dolutegravir (DTG)/abacavir (ABC)/lamivudine (3TC) at high HIV-caseload general practice clinics in Australia. The most common reasons for switching from a prior suppressive therapy to DTG/ABC/3TC were simplification of regimen, resolving toxicity/intolerance and patient preference (73%, 13% and 12%, respectively). Kaplan–Meier analysis showed that the probability of patients remaining on DTG/ABC/3TC therapy at 12 months was 95.1%. Switching to DTG/ABC/3TC from a range of other regimens was associated with a discontinuation rate of 3.2%, with 2.5% of patients discontinuing due to adverse events and no patients discontinuing due to virologic failure. Switching to DTG/ABC/3TC was a viable treatment strategy in this cohort of Australian patients.
Keywords: Dolutegravir, HIV, switch, real world
Introduction
The need to switch HIV-1 therapy because of virologic failure and drug resistance has decreased with improvements in antiretroviral therapy (ART). As a result of these improvements, there is now a rationale for switching therapy in some patients with virologic suppression. Reasons to consider switching therapy in such patients include adverse events (AEs) and simplification of regimen.1–3 Data on the outcomes of switching therapy are still evolving, therefore studies that provide insights into outcomes of patients post-switching therapy are important. The STRIIVING study demonstrated the non-inferiority of switching from a variety of antiretrovirals (ARVs) onto dolutegravir (DTG)/abacavir (ABC)/lamivudine (3TC) in comparison to staying on baseline (current) ART.4 The rate of AEs leading to discontinuation of DTG/ABC/3TC (4%) in this patient population was similar to that observed in DTG treatment-naive studies.4–9
The present paper describes a retrospective clinical audit at high HIV-caseload primary care practices in Australia to determine why virologically suppressed patients switched therapy to DTG/ABC/3TC fixed-dose combination and the clinical outcomes following this switch.
Methods
Patients identified across six primary care practices, who had received DTG/ABC/3TC alone for ≥1 day following a switch from suppressive ART (<50 HIV-1 RNA copies/ml), were included. These patients were required to have switched to DTG/ABC/3TC between 1 April 2015 and 31 March 2016, and to have been maintained on DTG/ABC/3TC alone after the switch. Patient files were reviewed by each general practice and individual cases submitted via a systematic electronic survey.
The primary outcome was the percentage of patients remaining on DTG/ABC/3TC therapy with HIV-1 RNA < 50 copies/ml. Time on treatment was calculated from the date of first DTG/ABC/3TC script and censored on 1 April 2016. Kaplan–Meier survival methods were used to determine probability of DTG/ABC/3TC continuation at 12 months of treatment. All other results are descriptive.
This project was approved by an Australian private not-for-profit ethics committee registered with the National Health and Medical Research Council and all patient data were de-identified.
Results
Data from 443 patients were included: 97% male and 45% ≥50 years. Of the 443 patients, two patients discontinued from DTG/ABC/3TC after 1 April 2016 and the data of one patient were received after the study closing date; the data from these three patients were included in the study. A summary of the most recent regimen prior to switching therapy is shown in Table 1.
Table 1.
Summary of ART history and most recent regimen prior to switch (N = 443).
| ART history | n (%) | |
|---|---|---|
| Historical ARV resistance | Any | 29 (6.5) |
| None | 414 (93.5) | |
| Time on ART | <5 years | 124 (28.0) |
| 5–10 years | 119 (26.9) | |
| >10 years | 200 (45.1) | |
| Number of previous ART regimens | ≥5 | 88 (19.9) |
| 3–4 | 125 (28.2) | |
|
|
1–2 |
230 (52.0) |
|
Most recent ART regimen |
|
n (%) |
| Time on prior therapy | <2 years | 242 (54.6) |
| 2–5 years | 81 (18.3) | |
| >5 years | 120 (27.1) | |
| Single-pill regimen | – | 50 (11.2) |
| Prior two NRTIa,b | ABC/3TC | 300 (67.7) |
| TDF/FTC | 119 (26.9) | |
| AZT/3TC | 5 (1.1) | |
| NRTI sparing | 5 (1.1) | |
| Prior core agenta,c | INSTI | 258 (58.2) |
| NNRTI | 118 (26.6) | |
| PI | 91 (20.5) | |
| EI | 2 (0.5) | |
| MI | 1 (0.2) | |
| Prior DTG-basedregimena | DTG + ABC/3TC (alone) | 165 (37.2) |
| Non DTG-based regimen | 242 (54.6) |
ABC/3TC: abacavir/lamivudine; ART: antiretroviral therapy; ARV: antiretroviral; AZT/3TC: zidovudine/lamivudine; DTG: dolutegravir; EI: entry inhibitor; INSTI: integrase strand transfer inhibitor; MI: maturation inhibitor; NNRTI: non-nucleoside reverse transcriptase inhibitor; NRTI: nucleoside reverse transcriptase inhibitor; PI: protease inhibitor; TDF/FTC: tenofovir/emtricitabine.
aMost recent regimen before DTG/ABC/3TC.
bIncludes patients on single-pill regimens containing the combination NRTI; 14 patients had alternative combination NRTIs or a single NRTI in their regimen.
cSome patients were on multiple core agents.
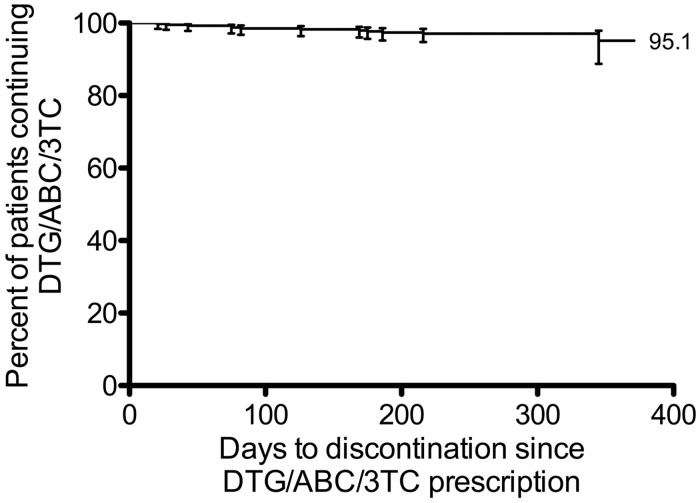
The probability of patients remaining on DTG/ABC/3TC therapy at 12 months was 95.1% (95% CI 88.8–97.9) by Kaplan–Meier estimate (Figure 1).
Figure 1.
Per cent of patients remaining on DTG/ABC/3TC therapy after switching, based on pre-specified censorship on 31 March 2016 (N = 443).
The most common reasons patients switched therapy to DTG/ABC/3TC were simplification, toxicity/intolerance and patient preference (73%, 13% and 12%, respectively). After excluding prior DTG-based regimens as the most recent before the switch, toxicity/intolerance as a reason for switching therapy increased to 21%. Few (2%) patients switched therapy for reasons attributed to cost, suboptimal adherence or drug–drug interactions. The most common toxicities/intolerances leading to the discontinuation of prior therapy and switching to DTG/ABC/3TC were nervous system disorders, renal and urinary disorders, and gastrointestinal disorders. Pre-existing toxicity/intolerance events resolved in 43 of 58 (74%) patients, including nine of 13 (69%) with nervous system disorders, eight of 12 (67%) with renal and urinary disorders and ten of ten (100%) with gastrointestinal disorders. Patients with toxicity/intolerance events that did not resolve after switch onto DTG/ABC/3TC had a median time on treatment that was lower (191 days [IQR 100–239.5]) than that of the overall population (266 days [IQR 176–325]).
Fourteen patients (3.2%) discontinued DTG/ABC/3TC; none of these were due to virologic failure (Table 2). Discontinuations were mainly related to AEs (2.5%); < 1% of patients discontinued due to a psychiatric event. The discontinuation rates for all subgroups were similar (0–5.6%), i.e.
Table 2.
Patients who discontinued DTG/ABC/3TC.
| Prior regimena | Reason for switch to DTG/ABC/3TC | Days on DTG/ABC/3TC | Reason for DTG/ABC/3TC discontinuationb | Event | Subsequent regimen | Returned to prior regimen | AE ceased | Regained/ maintained viral suppression |
|---|---|---|---|---|---|---|---|---|
| DTG + ABC/3TC | Simplification | 175 | Toxicity/intolerance | Headachec | DTG + ABC/3TC | Yes | Yes | Yes |
| ATV/r + ABC/3TC | Simplification | 75 | Blip | Single event of 63 copies/ml | ATV/r + ABC/3TC | Yes | Yes | |
| DTG + ABC/3TC | Simplification | 381 | Toxicity/intolerance | Creatinine | EVG/c/TDF/FTC | No | No | Yes |
| ATV/r + TDF/FTC | Simplification | 82 | Toxicity/intolerance | Abdominal discomfort | ATV/r + TDF/FTC | Yes | Yes | Yes |
| RAL + ABC/3TC | Simplification | 126 | Toxicity/intolerance | Insomnia | RAL + ABC/3TC | Yes | Yes | Yes |
| NVP + TDF/FTCd | Toxicity/intolerance | 75 | Toxicity/intolerance | Anxietye | NVP + TDF/FTC | Yes | Yes | Yes |
| RAL + ABC/3TCd | Toxicity/intolerance | 169 | Toxicity/intolerance | Dry mouth | RAL + ABC/3TC | Yes | Yes | Yes |
| RAL + TDF/FTC | Simplification | 27 | Toxicity/intolerance | Rash | RAL + TDF/FTC | Yes | Yes | Yes |
| DTG + ABC/3TC | Simplification | 43 | Drug–drug interactions | Cigarettes and risk of CVD | ATV/r + TDF/FTC | No | Yes | |
| DTG + ABC/3TCf | Patient preference | 304 | Toxicity/intolerance | Malaiseg | NVP + ABC/3TC | No | Yes | Yes |
| NVP + ABC/3TC | Simplification | 199 | Patient preference | NVP + ABC/3TC | Yes | Yes | ||
| DTG + ABC/3TC | Simplification | 345 | Toxicity/intolerance | Anxiety | DRV/r + ABC/3TC | No | Yes | Yes |
| RAL + DRV/r + ABC/3TC | Simplification | 186 | Toxicity/intolerance | Rhabdomyolysish | RPV/TDF/FTC | No | Yes | Yes |
| RAL + NVP + 3TC | Simplification | 21 | Toxicity/intolerance | Nausea | RAL + NVP + 3TC | Yes | Yes | Yes |
3TC: lamivudine; ABC: abacavir; AE: adverse event; ATV/r: atazanavir/ritonavir; c: cobicistat; CVD: cardiovascular disease; DRV/r: darunavir/ritonavir; DTG: dolutegravir; EVG: elvitegravir; NVP: nevirapine; RAL: raltegravir; RPV: rilpivirine; TDF/FTC: tenofovir/emtricitabine.
aSwitched from prior regimens for simplification, with three exceptions.
bMedian time on DTG/ABC/3TC treatment was 147.5 (IQR 75–195.75) days in these patients.
cThis subject was entered two days after study closure.
dDiscontinued prior regimen due to a pre-existing toxicity/intolerance.
eHistory of anxiety.
fDiscontinued prior regimen due to patient preference.
gAches, shortness of breath, tight chest, coughing, fatigue.
hSerious AE: onset bilateral arms, required hospitalisation.
history of ARV resistance (any, 3.4%; none, 3.1%)
number of previous regimens (≥5, 1.1%; 3–4, 5.6%; 1–2, 2.6%)
time on most recent regimen (<2 years, 3.3%; 2–5 years, 3.7%; 5 years, 2.5%)
most recent agent class in prior regimen (integrase strand transfer inhibitor, 3.9%; two nucleoside reverse transcriptase inhibitors [2NRTIs; TDF/FTC-ABC/3TC], 2.5–3.3%; non-NRTI, 2.5%; protease inhibitor, 3.2%)
rationale for regimen switch (simplification, 3.1%; toxicity/intolerance, 3.5%; patient preference, 2.0%)
DTG not used in prior regimen (3.7%) or a combination of DTG + ABC/3TC alone not used in prior regimen (3.0%)
Nine of 14 patients who discontinued DTG/ABC/3TC switched therapy back to their prior regimen (Table 2).
Discussion
This retrospective clinical audit demonstrated that the relative probability of staying on DTG/ABC/3TC therapy at 12 months after switching from another regimen was 95.1%.
The most common reason for switching from a prior suppressive therapy to DTG/ABC/3TC was simplification. For those who switched therapy due to toxicity/intolerance, most pre-existing events, including over two-thirds of nervous system disorders, resolved. Of patients who ceased DTG/ABC/3TC therapy, all remained virologically suppressed on their subsequent regimen, which was often the regimen they had switched from.
The rate of discontinuations due to AEs in this study was low and comparable to that of the phase 3b stable switch study STRIIVING (2.5% and 4.0%, respectively).4 Other randomised controlled trials assessing stable switch strategies to single-pill regimens containing rilpivirine or elvitegravir have reported similar discontinuation rates due to AEs.10–14 The rates of discontinuations due to psychiatric AEs observed in this Australian cohort were consistent with the phase 3 clinical trials of DTG in treatment-naïve patients5–9 as well as many real-world evidence studies.15–19 Some real-world evidence studies have reported higher rates of discontinuations due to psychiatric AEs,20–22 possibly because of differences in study populations, or perhaps due to reporting and/or channelling bias. This tenet is supported, in part, by an analysis of the OPERA cohort study, in which patients receiving DTG were found to be more likely to have a history of psychiatric disorder at baseline than patients receiving other core agents.19
This audit report was subject to the general limitations of a retrospective cohort analysis, including limits to data collection imposed by pre-specified end points in the study protocol. Consequently, data were not collected for a range of parameters including other regimens switched to during similar time frames, AEs that did not lead to discontinuation and the severity of AEs leading to discontinuation. In addition, there are limitations associated with use of DTG/ABC/3TC, including the need to test for HLA-B*5701 allele status before initiating therapy and, since DTG/ABC/3TC is a fixed dose tablet, it should not be prescribed for patients requiring dose adjustment.
In this real-world retrospective audit, switching to DTG/ABC/3TC from a range of other regimens showed a low rate of discontinuation. Few patients were reported to discontinue due to AEs and no patients discontinued due to virologic failure, supporting DTG/ABC/3TC as a viable treatment strategy in this Australian patient population.
Acknowledgement
The authors would like to acknowledge site staff for study and data input management, in particular Trina Vincent and Shikha Agrawal from Holdsworth House Medical Practice, David Ninham from Taylor Square Private Clinic, Vicki Ieroklis from East Sydney Doctors and David Youds from Gladstone Road Medical Centre. The authors thank Kim Magner of Lateral Connections, Melbourne, VIC, Australia for providing medical writing support, which was funded by ViiV Healthcare, Melbourne, VIC, Australia in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3), and Andrew Sakko, PhD, CMPP, of ViiV Healthcare, Melbourne, VIC, Australia for editorial support.
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: David Baker, Mark Bloch, Robert Finlayson and Norman Roth have served on advisory boards for ViiV Healthcare. Dannae Brown, Fraser Drummond and Pedro Eitz Ferrer are full-time employees of ViiV Healthcare. Rimgaile Urbaityte is a full-time employee of GlaxoSmithKline. The remaining authors have nothing to declare.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by ViiV Healthcare. Study sites received financial support from ViiV Healthcare to perform electronic medical record reviews and data entry. The authors received no financial support for authorship or publication of this article.
References
- 1.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services, http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf (accessed 21 April 2017).
- 2.European AIDS Clinical Society. Guidelines. Version 8.1. October 2016, http://www.eacsociety.org/files/guidelines_8.1-english.pdf (accessed 21 April 2017).
- 3.Günthard HF, Saag MS, Benson CA, et al. International Antiviral Society–USA Panel. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the International Antiviral Society–USA Panel. JAMA 2016; 316: 191–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trottier B, Lake J, Logue K, et al. Dolutegravir/abacavir/lamivudine versus current ART in virally suppressed patients (STRIIVING): a 48-week randomized, non-inferiority, open-label, Phase IIIb study. Antiviral Therapy. Epub ahead of print 12 April 2017. DOI: 10.3851/IMP3166. [DOI] [PubMed]
- 5.Raffi F, Rachlis A, Brinson C, et al. Dolutegravir efficacy at 48 weeks in key subgroups of treatment-naïve HIV-infected individuals in three randomized trials. AIDS 2015; 29: 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walmsley S, Baumgarten A, Berengueret J, et al. Dolutegravir plus abacavir/lamivudine for the treatment of HIV-1 infection in antiretroviral therapy-naive patients: week 96 and week 144 results from the SINGLE randomized clinical Trial. J Acquir Immune Defic Syndr 2015; 70: 515–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Molina J-M, Clotet B, van Lunzen J, et al. Once-daily dolutegravir versus darunavir plus ritonavir for treatment-naive adults with HIV-1 infection (FLAMINGO): 96 week results from a randomised, open-label, phase 3b study. Lancet HIV 2015; 2: e127–e136. [DOI] [PubMed] [Google Scholar]
- 8.Orrell C, Hagins DP, Belonosovaet E, et al. Fixed-dose combination dolutegravir, abacavir, and lamivudine versus ritonavir-boosted atazanavir plus tenofovir disoproxil fumarate and emtricitabine in previously untreated women with HIV-1 infection (ARIA): week 48 results from a randomised, open-label, non-inferiority, phase 3b study. Lancet HIV. Epub ahead of print 17 July 2017. DOI: 10.1016/S2352-3018(17)30095-4 [DOI] [PubMed]
- 9.Raffi F, Jaeger H, Quiros-Roldan E, et al. Once-daily dolutegravir versus twice-daily raltegravir in antiretroviral-naive adults with HIV-1 infection (SPRING-2 study): 96 week results from a randomised, double-blind, non-inferiority trial. Lancet Infect Dis 2013; 13: 927–935. [DOI] [PubMed] [Google Scholar]
- 10.Palella FJ, Jr, Fisher M, Tebas P, et al. Simplification to rilpivirine/emtricitabine/tenofovir disoproxil fumarate from ritonavir-boosted protease inhibitor antiretroviral therapy in a randomized trial of HIV-1 RNA-suppressed participants. AIDS 2014; 28: 335–344. [DOI] [PubMed] [Google Scholar]
- 11.Pozniak A, Markowitz M, Mills A, et al. Switching to coformulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus continuation of non-nucleoside reverse transcriptase inhibitor with emtricitabine and tenofovir in virologically suppressed adults with HIV (STRATEGY-NNRTI): 48 week results of a randomised, open-label, phase 3b non-inferiority trial. Lancet Infect Dis 2014; 14: 590–599. [DOI] [PubMed] [Google Scholar]
- 12.Arribas JR, Pialoux G, Gathe J, et al. Simplification to coformulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus continuation of ritonavir-boosted protease inhibitor with emtricitabine and tenofovir in adults with virologically suppressed HIV (STRATEGY-PI): 48 week results of a randomised, open-label, phase 3b, non-inferiority trial. Lancet Infect Dis 2014; 14: 581–589. [DOI] [PubMed] [Google Scholar]
- 13.DeJesus E, Ramgopal M, Crofoot G, et al. Switching from efavirenz, emtricitabine, and tenofovir disoproxil fumarate to tenofovir alafenamide coformulated with rilpivirine and emtricitabine in virally suppressed adults with HIV-1 infection: a randomised, double-blind, multicentre, phase 3b, non-inferiority study. Lancet HIV 2017; 4: e205–e213. [DOI] [PubMed] [Google Scholar]
- 14.Rijnders B, Stephan C, Lazzarin A, et al. Switching from ritonavir or cobicistat boosted atazanavir plus emtricitabine/tenofovir disoproxil fumarate to a tenofovir alafenamide-based single tablet regimen: week 48 data in virologically suppressed adults. In: Fifteenth European AIDS conference, Barcelona, Spain, 21–24 October 2015. Abstract PS10/3.
- 15.Peñafiel J, de Lazzari E, Padilla M, et al. Tolerability of integrase inhibitors in a real-life setting. J Antimicrob Chemother 2017; 72: 1752–1759. [DOI] [PubMed]
- 16.Bonfanti P, Madeddu G, Gulminetti R, et al. Discontinuation of treatment and adverse events in an Italian cohort of patients on dolutegravir. AIDS 2017: 31: 455–457 [DOI] [PubMed]
- 17.Vivancos-Gallego MJ, Moreno A, Perez-Elias MJ, et al. Discontinuation of dolutegravir (DTG)-based regimens in clinical practice. In: International congress of drug therapy in HIV infection, Glasgow, UK, 23–26 October 2016. Abstract P116.
- 18.Postel N, Mueller M, Wyen C, et al. The DOL-ART Cohort: providing evidence from real world data – use of dolutegravir-based regimens in routine clinical care in Germany. In: International congress of drug therapy in HIV infection, Glasgow, UK, 23–26 October 2016. Abstract P133.
- 19.Fettiplace A, Stainsby C, Winston A, et al. Psychiatric symptoms in patients receiving dolutegravir. J Acquir Immune Defic Syndr 2017; 74: 423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffmann C, Welz T, Sabranski M, et al. Higher rates of neuropsychiatric adverse events leading to dolutegravir discontinuation in women and older patients. HIV Med 2017; 18: 56–63 [DOI] [PubMed]
- 21.Baldwin G, Borghetti A, Capetti A, et al. A comparison between tenofovir/emtricitabine/elvitegravir/cobicistat and dolutegravir-based three-drug regimens as switch strategies for virologically controlled, HIV-infected patients. In: International congress of drug therapy in HIV infection, Glasgow, UK, 23–26 October 2016. Abstract P106.
- 22.de Boer MG, van den Berk GE, van Holten N, et al. Intolerance of dolutegravir-containing combination antiretroviral therapy regimens in real-life clinical practice. AIDS 2016; 30: 2831–2834. [DOI] [PubMed] [Google Scholar]