Abstract
Background:
Comfort may be an appropriate goal in advanced dementia. Longitudinal studies on physician decision-making and discomfort assessed by direct observation are rare, and intravenous rehydration therapy is controversial.
Methods:
To assess treatment decisions and discomfort in patients with advanced dementia and pneumonia and to compare by intravenous rehydration therapy, we used data from the observational multicenter Italian End of Life Observatory–Prospective Study On DEmentia patients Care. We analyzed 109 episodes of pneumonia, which involved decisions in 77 nursing home patients with Functional Assessment Staging Tool stage 7. We assessed decisions, decision-making, and treatments every fortnight. Trained observers assessed discomfort with the Discomfort Scale–Dementia Alzheimer Type (DS-DAT).
Results:
Most decisions referred to treatment with antibiotics (90%; 98 of 109) and intravenous rehydration therapy (53%; 58 of 109), but hospitalization was rare (1%). Selecting decisions with antibiotics, with rehydration therapy, the prognosis was more frequently <15 days (34% vs 5% without rehydration therapy; P = .001), and a goal to reduce symptoms/suffering was more common (96% vs 74%; P = .005) while there was no difference in striving for life prolongation (a minority). With rehydration therapy, the decision was more often discussed with family rather than communicated only. Mean DS-DAT scores over time proximate to the first decision ranged between 9.2 and 10.5.
Conclusions:
Italian nursing home patients with advanced dementia and pneumonia frequently received invasive rehydration therapy in addition to antibiotics, however, mostly with a palliative intent. Discomfort was high overall and symptom relief may be improved. Relations between invasive rehydration therapy and discomfort need further study.
Keywords: palliative care, comfort, dementia, pneumonia, fluid therapy, long-term care
Introduction
To improve comfort is an appropriate treatment goal for patients with advanced dementia.1 However, patients with dementia frequently develop pneumonia and often die from it,2,3 while pneumonia has been associated with high levels of discomfort.4,5 Observational studies in nursing home patients with dementia and pneumonia have shown that hydration status and antibiotics are associated with (dis)comfort and survival4–8 and hydration might affect survival more than antibiotics.9
However, it is unclear if rehydration therapy affects discomfort or survival. In the dying phase, the use of rehydration therapy in patients with dementia, especially intravenous rehydration, is controversial.1,10 For example, in an international Delphi study with experts from 23 countries, the experts achieved full consensus on almost all aspects of palliative care in dementia but not on rehydration being inappropriate in the dying phase.1 Lack of consensus may be indicated also by considerable variability in treatment practice in dementia at the end of life cross-nationally.11–13 Although parenteral rehydration and tube feeding are rarely provided to nursing home patients with dementia in the Netherlands,8,14 in Italy, many nursing home patients with advanced dementia die while receiving parenteral rehydration and tube feeding,11 and specific guidelines are lacking.
Our goal was to describe practice of treatment of pneumonia in Italian nursing home residents with advanced dementia in terms of decisions and decision-making including treatment goals and discomfort. We compared decisions and decision-making with and without intravenous rehydration therapy, and we described directly observed discomfort at several intervals close to treatment.
Methods
We used data from the prospective observational multicenter Italian End of Life Observatory–Prospective Study On DEmentia patients Care (EoLO PSODEC; June 2007 to May 2009) described elsewhere.15,16 The goal of the EoLO PSODEC cohort study was to describe, over time, end-of-life critical decisions taken for patients with advanced-stage dementia, treatments, and discomfort. Enrolled patients had a Functional Assessment Staging Tool (FAST) score of 7 (substages a to f)17 and an expected survival ≥2 weeks at baseline (upon enrollment) according to their primary doctor’s clinical judgment. Data were collected every 14 days until death or up to 6 months. EoLO PSODEC focused on critical decisions, that is, decisions perceived as critical by the physician because of possible impact on survival or quality of life, including rehydration therapy and antibiotics (a critical decision is the decision to start, withdraw, or withhold a treatment that the physician and/or the health-care team perceive as critical to a patient’s survival and/or quality of life16). Trained nursing staff coordinating the project’s data collection interviewed the physician on each critical decision. The study protocol was approved on 13 February 2007, by the ethics committee of the University of Reggio Emilia-Modena (protocol number 145/CE). The relatives provided informed consent for use of data for the study.
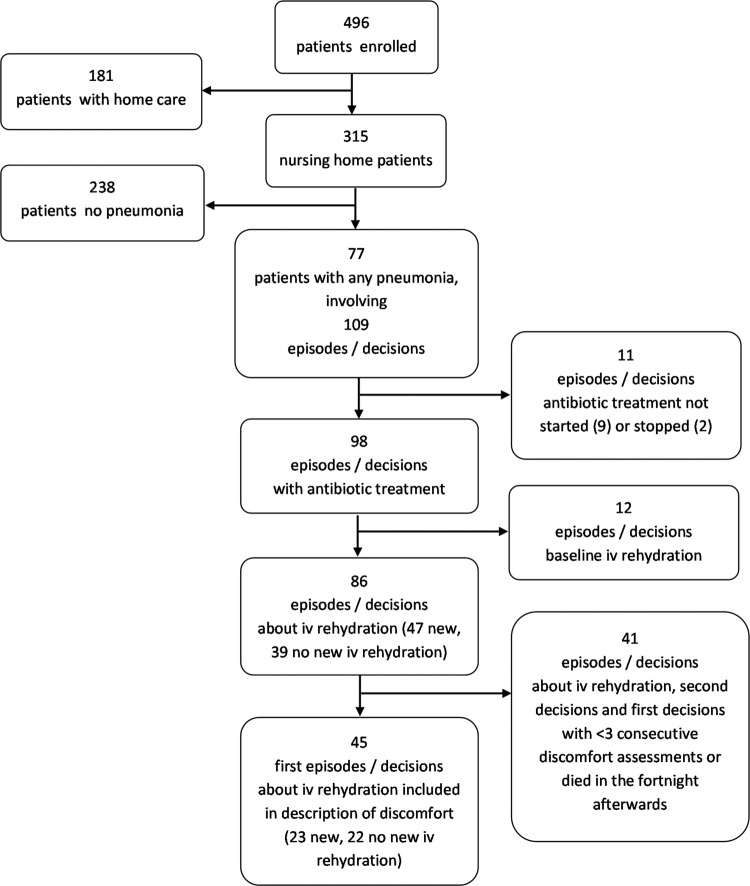
The study enrolled 496 patients from 34 nursing homes in the Lombardy region (315 patients) and home care in 2 provinces of Emilia-Romagna region (181 patients). We selected critical decisions around pneumonia in nursing home patients as the long-term care context differs from community settings (Figure 1). The physicians in nursing homes were on the staff, and intravenous fluid therapy was always available in the nursing homes.
Figure 1.
Selection of patients and decisions for description of treatment and decision-making, and discomfort.
We report demographics, dementia type, and FAST stage from the baseline assessment. At the time of a decision (the main unit of analyses) that was critical to the patient’s survival or quality of life, the physicians reported prognosis (whether less than 15 days), any treatments started or stopped from a prestructured list (including those not specific to treatment of the pneumonia such as blood transfusion). The list also included an open-ended option for any other treatments. The physician also reported treatment goals (aims), communication with family, and their perception of decision-making authority.
Discomfort was assessed every fortnight (not specifically timed around decisions) with the validated Discomfort Scale–Dementia Alzheimer Type (DS-DAT)18,19 by trained nurses. The theoretical range of the DS-DAT is 0 to 27, and scores during acute illness are generally normally distributed even though the highest score is rarely obtained as it implies discomfort and lack of comfort expressed extremely on all 9 items.
We compared decisions and decision-making between patients treated with and without intravenous rehydration therapy using χ2 or Fisher exact tests (treatment and aims) and t tests or nonparametric Mann-Whitney U test (number of aims) as appropriate. Antibiotics were controlled for (by stratifying and selection of those treated with antibiotics for analyses, related to the distribution; Figure 1) because antibiotics have been associated with more symptoms in the period around treatment by nursing report7 but also with lower observed discomfort following treatment.5 A power analysis based on data on discomfort levels observed 3 days after the treatment decision obtained in a Dutch study showed that to compare discomfort between 2 groups, 42 decisions in each group were needed to detect a clinically relevant difference of 3 DS-DAT points with 80% power and 90% 2-sided confidence intervals, and in case of 95% confidence intervals, 53 to compare between groups and 29 for paired analyses of change over time within treatment group. Because power was insufficient to compare different groups or the same group over time, we only describe the course of discomfort. For this, to avoid analyses of overlapping periods around multiple decisions per patient, we selected the first decisions in patients who did not receive rehydration therapy at baseline (Figure 1).
Results
There were 109 decisions (cases of pneumonia) in 77 nursing home patients residing in 28 nursing homes. In 58% of the cases (61 of 106, 3 missing values), the decision was taken by a female physician, and the mean age was 43.8 (SD 7.8; 3 missing values).
Most decisions (90%; 98 of 109) referred to starting treatment with antibiotics (Figure 1). In 9 cases, antibiotics were not started, and in 2 cases, they were stopped. In 53% (58 of 109) of cases, there was a decision to start intravenous rehydration therapy (Table 1, left column). In 11% of cases (12 of 109), patients had intravenous rehydration therapy at baseline. Hypodermoclysis, tube feeding, and hospitalization were rare (up to a few percentage only). However, (terminal) sedation and other potentially comfortable enhancing medical interventions were rare too, in spite of reducing symptoms/suffering being a treatment goal for the large majority of patients (89%) whether accompanied by a goal to prolong life (34% of total decisions; Table 2, left column). In 70% of cases, there was a single goal, and in the other cases, 2 to 4 goals. The mean number of goals per decision was 1.4, but in more than half of the cases, there was a single goal (median 1; Table 2).
Table 1.
Patient Characteristics and Treatments in 109 Decisions in 77 Patients With Advanced Dementia and Pneumonia.
| Total (n = 109) | Antibiotics—New IV Rehydration (n = 47) | Antibiotics—No New IV Rehydration (n = 39) | P | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Female, % | 72 | 74 | 79 | 0.58 |
| Age, mean (SD) | 84.9 (8.4) | 85.8 (8.4) | 83.6 (7.6) | 0.21 |
| Type of dementia, % | ||||
| Alzheimer | 39 | 32 | 54 | 0.07 |
| Vascular | 38 | 49 | 31 | |
| Mixed Alzheimer–vascular | 17 | 17 | 8 | |
| Any other type or combination | 6 | 2 | 7 | |
| FAST 7c or higher (vs a or b), % | 87 | 85 | 87 | 0.78 |
| Treatment, % | ||||
| IV rehydration therapy at baseline | 11 | – | – | |
| Treatment new/changes, % | ||||
| IV rehydration therapy | ||||
| Started | 53 | All | No | – |
| Stopped | 2 | 0 | 0 | NA |
| Hypodermoclysis | ||||
| Started | 4 | 6 | 0 | 0.25 |
| Stopped | 0 | 0 | 0 | NA |
| Tube feeding (nasogastric, PEG) | ||||
| Started | 1 | 0 | 0 | NA |
| Stopped | 2 | 2 | 0 | 1.0 |
| Hospitalization | 1 | 2 | 0 | 1.0 |
| Restraintsa | ||||
| Started | 0 | 0 | 0 | NA |
| Stopped | 0 | 0 | 0 | NA |
| Blood transfusion | ||||
| Started | 1 | 2 | 0 | 1.0 |
| Stopped | 0 | 0 | 0 | NA |
| Sedation | ||||
| Started | 2 | 0 | 0 | NA |
| Stopped | 3 | 2 | 5 | 0.59 |
| Terminal sedation | ||||
| Started | 1 | 2 | 0 | 1.0 |
| Stopped | 0 | 0 | 0 | NA |
| Other interventionsb | ||||
| Started (eg, oxygen, medication) | 9 | 9 | 8 | 1.0 |
| Stopped (eg, nutritional supplements, medication) | 8 | 13 | 5 | .28 |
| Not any intervention started or stopped | 1 (1 case) | 0 | 0 | NA |
Abbreviations: FAST, Functional Assessment Staging Tool; IV, intravenous; NA, not applicable; PEG, percutaneous endoscopic gastrostomy.
aRestraint use was unchanged around the decisions, but that restraints were probably already in use shortly before the decision, as baseline use was high overall, in the full sample of nursing home patients with FAST 7c or higher (93% any restraint; 49% abdominal restraints).15
bOther interventions from open-ended item. Oxygen therapy started in 5 cases. In the other cases, medication was added: heparin, cortisone, and anti-Parkinson medication. Decisions to stop included nutritional supplements, all oral medication, all medication except fentanyl, and morphine.
Table 2.
Prognosis and Decision-Making.a
| Prognosis and Decision-Making According to Physician Interview, % | Total (n = 109) | Antibiotics—New IV Rehydration (n = 47) | Antibiotics—No New IV Rehydration (n = 39) | P |
|---|---|---|---|---|
| Prognosis, % | ||||
| Less than 15 days according to the physician (3 missing values) | 21 | 34 | 5 | .001 |
| Aim(s) (more possible; 2 missing values due to missing interview)b | ||||
| To reduce symptoms/suffering | 89 | 96 | 74 | 0.005 |
| To prolong life | 34 | 34 | 42 | 0.50 |
| To avoid/stop futile treatment | 7 | 9 | 3 | 0.37 |
| To avoid prolonging life | 6 | 6 | 0 | 0.25 |
| To make the process of dying smoother | 3 | 2 | 0 | 1.0 |
| Number of aims, median (range) | 1 (1-3) | 1 (1-3) | 1 (1-2) | NAc |
| Decision discussed with family or legal representative according to physician (4 missing values) | 49 | 54 | 27 | 0.01 |
| Decision communicated to family or legal representative before or after the decision, according to physician (3 missing values) | 89 | 89 | 84 | .45 |
| Person who took the final decision (4 missing values)d | ||||
| Physician in full autonomy | 67 | 62 | 67 | 0.65 |
| Physician guided by family or legal representative | 7 | 11 | 3 | |
| The health care team | 23 | 23 | 23 | |
| Nurse only | 2 | 2 | 3 | |
| Others (physician on duty or missing specification) | 2 | 2 | 3 |
Abbreviations: NA, not applicable.
an = 109, decisions in 77 patients with advanced dementia and pneumonia.
bThe option “other aim” was not chosen (0%).
cDistributions were the same; nonparametric P value could not be computed.
dThe following response options were not chosen (0%): physician in consultation with specialist, physician guided by patient (advance directive, previous discussions), and the family or legal representative only.
The decision was often communicated to family but less often discussed with them (89% and 49%, respectively, Table 2). The final decision was taken by the physician alone in two-thirds (67%) of cases, and in other cases, it was mostly the health-care team (23%). The physician was never guided by (previous) patient wishes and rarely (7%) by family wishes. The physician found the treatment decisions corresponded with what the physician felt as the right thing to do in all but 2 cases (no reason provided; 6 missing values, not in Table 2).
Because the large majority of cases was treated with antibiotics, we restricted the comparison of treatment decisions between patients who did and did not receive intravenous rehydration therapy to those treated with antibiotics (right columns in Table 1), also excluding patients who received intravenous rehydration therapy at baseline. Demographics and dementia type did not differ, but patients who received intravenous rehydration therapy more frequently had a poor prognosis at the time of the decision as perceived by the physician (34% vs 5%; P = .001). Treatment other than antibiotics and intravenous rehydration was rare and comparisons therefore often not meaningful, but goals of care differed in that the most frequent goal, to reduce symptoms/suffering, applied to almost all cases treated with intravenous rehydration therapy (96%), whereas this was about three-quarters (74%) in untreated cases (P = .005). Most decisions did not aim at life prolongation, and there was no difference between cases treated with and without intravenous rehydration. Further, the patterns regarding the physicians taking the decisions and communicating it to families did not differ, but a decision to start intravenous rehydration therapy was more frequently discussed with family (54% vs 27%, P = .01).
The mean DS-DAT score in the period before the first decision, for all cases of pneumonia, was 9.6 (SD 6.6); it was 10.5 (SD 7.1) in the period of the decision and 9.2 (SD 6.6) in the period after the decision (Figure 2, solid line). Figure 2 visualizes that the means did not vary much over time and also that the DS-DAT score patterns did not differ much by having received intravenous rehydration therapy.
Figure 2.
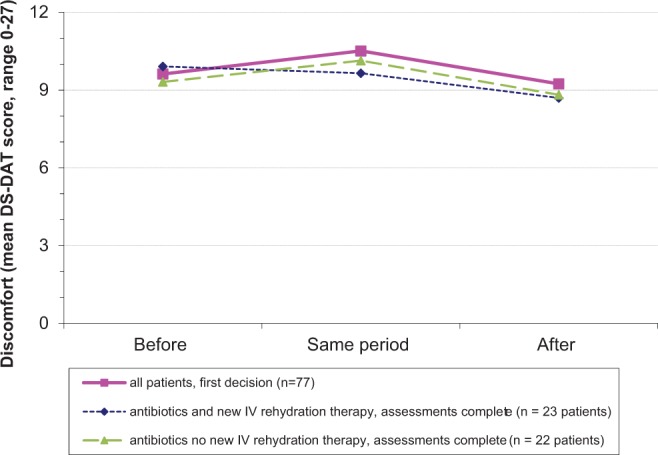
Discomfort around the time of the first pneumonia decision and by the decision to provide or withhold intravenous rehydration therapy. The same period covers the period in which the decision was made. The mean Discomfort Scale–Dementia Alzheimer Type (DS-DAT) score during the period of the decision for all 109 decisions was 10.8 (SD 7.0; 13 missing values), and for the 77 first decisions, it was 10.5 (SD 7.1; 10 missing values). To avoid presenting overlapping periods, the figure shows patterns of the first 77 decisions only. Further, 45 decisions referred to decisions to treat with antibiotics and with 3 consecutive discomfort assessments and not followed by death in the fortnight afterward and with no intravenous rehydration therapy at baseline. Statistical test results are not provided because of insufficient power. IV, intravenous.
Discussion
Pneumonia in Italian nursing home patients with advanced dementia was frequently but not always (90%) treated with antibiotics, and over half of the patients (53%) received intravenous rehydration therapy. However, other treatments, including not only sedation but also hospitalization, were notably rare. Focusing on intravenous rehydration therapy in antibiotic-treated patients, strikingly, rehydration therapy was more common when the patient was near death according to the physician (prognosis <15 days, 34% vs 5%). Moreover, for almost all (96%) patients treated with intravenous rehydration therapy, to reduce symptoms or suffering was a treatment goal (vs 74% of patients treated with antibiotics only).
With DS-DAT scores between 9.2 and 10.5, the levels of directly observed discomfort were high at all intervals proximate to the decision. A mean level of discomfort of around 10 points has been observed as a peak level with pneumonia in an older study in the Netherlands4 and with fever in an older US study—only when comfort care was not provided.20 In the more recent Dutch PneuMonitor study, the highest mean DS-DAT score was 8.1 and occurred 1 day after diagnosis after which it decreased to a mean of 4.5 at day 10.21
Regarding potential effects of intravenous rehydration therapy, it is possible that 2 processes affect comfort in opposite directions: invasive procedures that induce discomfort and antibiotics being more effective when combined with rehydration possibly resolving symptoms sooner, at least, in the context of probably inadequate treatment to relieve symptoms.6 The finding of high discomfort overall and the figure not showing a substantially lower discomfort over time without invasive rehydration therapy is in contrast to the earlier Dutch work,8 where directly observed discomfort decreased after a decision to withhold artificial nutrition and hydration (ANH). However, the Dutch study lacked a predecision discomfort assessment, lacked a comparison with treated patients, and the patients had various acute conditions, with only few having a respiratory tract infection.5,8 Later analyses found increased discomfort in dying patients who received rehydration therapy.5 In the current study, fewer patients died, so we could not explore discomfort close to death, which might have been high also due to increased secretions that are not cleared anymore.
Antibiotics were withheld in 10% of cases, which was a lower percentage than found in patients with severe dementia in previous studies in the United States (24%) and the Netherlands (33%), but hospitalization was equally rare as in the Netherlands.13 This may be related to the fact that in the Lombardy region, similar as in the Netherlands, physicians are on the staff. In the US study, 28% received invasive rehydration, which was, however, strongly associated with hospitalization. Other studies have also shown that the Italian setting is dissimilar regarding treatment of intake problems, for example, Italian physicians were the least likely to forgo ANH (not specific to dementia) compared with 5 other European countries.22 In a survey, Valentini et al23 found that most Italian physicians (74%) agreed with the provision of artificial hydration in patients with advanced dementia, and support was substantially greater than for artificial nutrition (42%), which has been studied far more often than artificial hydration in dementia. However, hypodermoclysis, which is short-lived and more generally accepted in case of infections in dementia,1,24 was uncommon in our study.
We found that the providing of intravenous rehydration therapy was associated with a comfort goal and a poor prognosis. This is surprising; some assume that rehydration therapy is not for comfort. For example, a recent Dutch study reported on the practice of withholding of ANH in older people, and the authors suggested it was often withheld because the physicians wanted to avoid “burdensome treatment solely to prolong life.”25 Valentini et al suggest that, in Italy, rehydration is regarded as basic care. Solarino et al,26 in another survey involving 22 219 Italian physicians, found divergence among the physicians as 37% of respondents considered ANH as a basic need (“only a life-sustaining measure”; 61% regarded ANH a medical treatment, and 2% was unsure). Miccinesi et al,27 in a 6-country study about palliative sedation in the general population, found that when provided, it was mostly with ANH in Italy and Belgium, but mostly without in Denmark, Sweden, the Netherlands, and Switzerland. As also found in the study comparing Italy with other countries,22 the physicians often made the decision without discussing it with nursing staff or other physicians. Physicians who are on the staff may be more paternalistic than physicians with more distant practices as suggested by US-Dutch cross-national work,28 but we found that in Italy, there was also little shared decision-making with nurses and with families, unlike in the Netherlands.29 This, however, may not be uncommon or unique to Italy, as a recent US study found that US families were often unaware of nursing home patients with advanced dementia experiencing a respiratory tract infection and were not involved in decision-making.30 Internationally, there is no consensus about providing artificial hydration in dementia in the dying phase, and our study increases understanding of how hydration may be perceived as a comfort measure.
Treatment to relieve symptoms was rarely provided in the current Italian study, for example, only 4.5% of the patients enrolled in the study with FAST 7c or higher (which applied to 87% of the patients with pneumonia) received paracetamol (acetaminophen) at baseline and 4.9% received opioids.15 No patients received new restraints at the time of the decisions, but 49% already had abdominal restraints at baseline and 16% already had rehydration therapy at baseline.
Limitations and Strengths
The analyses were limited to a single region in Italy where physicians are on the staff, whereas other regions use other models such as with fewer and smaller nursing homes but more home care or with general practitioners visiting nursing homes. We focused on critical decisions which were decisions perceived as important with regard to survival and quality of life. The incidence of decisions around pneumonia in our study was 0.84 per year, which was rather high compared to incidence of nursing home-acquired pneumonia more generally (0.1-0.9 episodes per year),31 which suggest that our approach is unlikely to have resulted in missing of many cases of pneumonia. We did not collect data on fluid intake. We also did not ask physicians to report the type of medication to relieve symptoms, and we asked to report any other treatment with an open-ended item, which might have resulted in underreporting of medication to relieve symptoms. However, medication to relieve symptoms was equally rare in the baseline assessment where we inventoried all medication provided, which suggests that under treatment to relieve symptoms is a genuine concern.
The Italian prospective EoLO PSODEC study provided unique data on discomfort, with, to our best knowledge, the only directly observed assessments of discomfort ahead of a decision or treatment, and more such studies are recommended.32 The data do not allow for answering ethical or other questions about benefit from rehydration therapy. From observational data, we cannot conclude whether invasive rehydration therapy affects discomfort. Discomfort was not observed at the time of the decision, and the exact date of the decision was not recorded. Variable time between the decision and the discomfort assessments probably explains that we did not observe a high peak of discomfort in the period in which the decision was made. Nevertheless, previous work found that mean discomfort returned to stable levels within 10 days,4,21 and in line with this, similar discomfort levels before and after the period in which the patient experienced the pneumonia suggest that elevated discomfort as a result of the pneumonia or its treatment, if any, does not persist. It is possible that patients who received rehydration therapy had more symptoms or suffered more before the pneumonia, but the course of discomfort suggests there were no major differences. Although the EoLO PSODEC study followed as many as 496 cases, the power for comparing treatment groups was insufficient, and we therefore refrained from testing difference between treatment groups. This illustrates the difficulty of efficiently assessing discomfort ahead of incident pneumonia, and this may explain why this, to our best knowledge, has not been done before.
Conclusion
Our findings are important for a debate on the utility of invasive rehydration therapy in patients with advanced dementia because, compared to feeding tubes, controversies around artificial rehydration therapy that exist across the world is a relatively unexplored area. Regarding Italian nursing home patients with dementia and pneumonia, there is room to decrease high discomfort levels by providing treatment that probably improves comfort, in line with palliative treatment goals. New work should not limit to a focus on inappropriate life-prolonging treatment but focus on comfort enhancing pharmacological as well as nonpharmacological treatment. It should examine treatment goals in more detail, such as possible relief of feelings of thirst, or increase the effectiveness of antibiotics, and future intervention studies should consider how to address beliefs that may be rooted deeply in some cultures. Our study in which discomfort was observed directly several times around the decision, and taking into account antibiotics and proximity to death, needs to be replicated with larger sample sizes, more frequent independent observations, and in settings with different treatment practices and populations varying in antibiotic use, prognosis, and dementia severity. Patient, family, and professional caregivers’ perspectives on benefits of rehydration therapy may be examined more in-depth with qualitative research. In the absence of clear evidence on its possible life-prolonging effects and unclear effects on discomfort in patients with advanced dementia and pneumonia, local policies regarding rehydration therapy may be developed which take into account goals of care and sensitivities and perceptions about how patients may benefit from fluid therapy.
Acknowledgments
The authors thank the nursing homes involved in data collection.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by financial contributions from the Associazione Cremonese per la Cura del Dolore-ONLUS (ACCD), Italy. JTS was supported by a career award from the Netherlands Organisation for Scientific Research (NWO, the Hague; Innovational Research Incentives Scheme [grant number Vidi 91711339]).
References
- 1. van der Steen JT, Radbruch L, Hertogh CM, et al. White paper defining optimal palliative care in older people with dementia: a Delphi study and recommendations from the European Association for Palliative Care. Palliat Med. 2014;28(3):197–209. [DOI] [PubMed] [Google Scholar]
- 2. Mitchell SL, Teno JM, Kiely DK, et al. The clinical course of advanced dementia. N Engl J Med. 2009;361(16):1529–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van der Steen JT, Mehr DR, Kruse RL, et al. Predictors of mortality for lower respiratory infections in nursing home residents with dementia were validated transnationally. J Clin Epidemiol. 2006;59(9):970–979. [DOI] [PubMed] [Google Scholar]
- 4. van der Steen JT, Ooms ME, van der Wal G, Ribbe MW. Pneumonia: the demented patient’s best friend? Discomfort after starting or withholding antibiotic treatment. J Am Geriatr Soc. 2002;50(10):1681–1688. [DOI] [PubMed] [Google Scholar]
- 5. van der Steen JT, Pasman HR, Ribbe MW, van der Wal G, Onwuteaka-Philipsen BD. Discomfort in dementia patients dying from pneumonia and its relief by antibiotics. Scand J Infect Dis 2009;41(2):143–151. [DOI] [PubMed] [Google Scholar]
- 6. van der Steen JT, Lane P, Kowall NW, Knol DL, Volicer L. Antibiotics and mortality in patients with lower respiratory infection and advanced dementia. J Am Med Dir Assoc. 2012;13(2):156–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Givens JL, Jones RN, Shaffer ML, Kiely DK, Mitchell SL. Survival and comfort after treatment of pneumonia in advanced dementia. Arch Intern Med. 2010;170(13):1102–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pasman HR, Onwuteaka-Philipsen BD, Kriegsman DM, Ooms ME, Ribbe MW, van der Wal G. Discomfort in nursing home patients with severe dementia in whom artificial nutrition and hydration is forgone. Arch Intern Med. 2005;165(15):1729–1735. [DOI] [PubMed] [Google Scholar]
- 9. van der Steen JT, Sternberg S, Volicer L. Palliative care in dementia 1986-2016: progress and remaining challenges. J Am Med Dir Assoc. 2017;18(2):190–191. [DOI] [PubMed] [Google Scholar]
- 10. van der Steen JT, Hertogh CMPM, de Graas T, Nakanishi M, Toscani F, Arcand M. Translation and cross-cultural adaptation of a family booklet on comfort care in dementia: sensitive topics revised before implementation. J Med Ethics. 2013;39(2):104–109. [DOI] [PubMed] [Google Scholar]
- 11. Di Giulio P, Toscani F, Villani D, Brunelli C, Gentile S, Spadin P. Dying with advanced dementia in long-term care geriatric institutions: a retrospective study. J Palliat Med. 2008;11(7):1023–1028. [DOI] [PubMed] [Google Scholar]
- 12. van der Steen JT. Dying with dementia: what we know after more than a decade of research. J Alzheimers Dis. 2010;22(1):37–55. [DOI] [PubMed] [Google Scholar]
- 13. van der Steen JT, Kruse RL, Ooms ME, et al. Treatment of nursing home residents with dementia and lower respiratory tract infection in the United States and the Netherlands: an ocean apart. J Am Geriatr Soc. 2004;52(5):691–699. [DOI] [PubMed] [Google Scholar]
- 14. van der Steen JT, Ooms ME, Adèr HJ, Ribbe MW, van der Wal G. Withholding antibiotic treatment in pneumonia patients with dementia: a quantitative observational study. Arch Intern Med. 2002;162(15):1753–1760. [DOI] [PubMed] [Google Scholar]
- 15. Toscani F, Di Giulio P, Villani D, et al. Treatments and prescriptions in advanced dementia patients residing in long-term care institutions and at home. J Palliat Med. 2013;16(1):31–37. [DOI] [PubMed] [Google Scholar]
- 16. Toscani F, van der Steen JT, Finetti S, et al. Critical decisions for older people with advanced dementia: a prospective study in long-term institutions and district home care. J Am Med Dir Assoc. 2015;16(6):535.e13–e20. [DOI] [PubMed] [Google Scholar]
- 17. Sclan SG, Reisberg B. Functional assessment staging (FAST) in Alzheimer’s disease: reliability, validity, and ordinality. Int Psychogeriatr. 1992;4(suppl 1):55–69. [DOI] [PubMed] [Google Scholar]
- 18. Hurley AC, Volicer BJ, Hanrahan PA, Houde S, Volicer L. Assessment of discomfort in advanced Alzheimer’s patients. Res Nurs Health. 1992;15(5):369–377. [DOI] [PubMed] [Google Scholar]
- 19. Dello Russo C, Di Giulio P, Brunelli C, et al. Validation of the Italian version of the Discomfort Scale–Dementia of Alzheimer Type. J Adv Nurs. 2008;64(3):298–304. [DOI] [PubMed] [Google Scholar]
- 20. Hurley AC, Volicer B, Mahoney MA, Volicer L. Palliative fever management in Alzheimer patients. Quality plus fiscal responsibility. ANS Adv Nurs Sci. 1993;16(1):21–32. [DOI] [PubMed] [Google Scholar]
- 21. van der Maaden T, van der Steen JT, de Vet HC, Hertogh CM, Koopmans RT. Prospective observations of discomfort, pain, and dyspnea in nursing home residents with dementia and pneumonia. J Am Med Dir Assoc. 2016;17(2):128–135. [DOI] [PubMed] [Google Scholar]
- 22. Buiting HM, van Delden JJ, Rietjens JA, et al. Forgoing artificial nutrition or hydration in patients nearing death in six European countries. J Pain Symptom Manage. 2007;34(3):305–314. [DOI] [PubMed] [Google Scholar]
- 23. Valentini E, Giantin V, Voci A, et al. Artificial nutrition and hydration in terminally ill patients with advanced dementia: opinions and correlates among Italian physicians and nurses. J Palliat Med. 2014;17(10):1143–1149. [DOI] [PubMed] [Google Scholar]
- 24. Lima Ribeiro SM, Morley JE. Dehydration is difficult to detect and prevent in nursing homes. J Am Med Dir Assoc. 2015;16(3):175–176. [DOI] [PubMed] [Google Scholar]
- 25. Martins Pereira S, Pasman HR, van der Heide A, van Delden JJ, Onwuteaka-Philipsen BD. Old age and forgoing treatment: a nationwide mortality follow-back study in the Netherlands. J Med Ethics. 2015;41(9):766–770. [DOI] [PubMed] [Google Scholar]
- 26. Solarino B, Bruno F, Frati G, et al. A national survey of Italian physicians’ attitudes towards end-of-life decisions following the death of Eluana Englaro. Intensive Care Med. 2011;37(3):542–549. [DOI] [PubMed] [Google Scholar]
- 27. Miccinesi G, Rietjens JA, Deliens L, et al. Continuous deep sedation: physicians’ experiences in six European countries. J Pain Symptom Manage. 2006;31(2):122–129. [DOI] [PubMed] [Google Scholar]
- 28. Helton MR, van der Steen JT, Daaleman TP, Gamble GR, Ribbe MW. A cross-cultural study of physician treatment decisions for demented nursing home patients who develop pneumonia. Ann Fam Med. 2006;4(3):221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. The AM, Pasman R, Onwuteaka-Philipsen B, Ribbe M, van der Wal G. Withholding the artificial administration of fluids and food from elderly patients with dementia: ethnographic study. BMJ. 2002;325(7376):1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Givens JL, Spinella S, Ankuda CK, et al. Healthcare proxy awareness of suspected infections in nursing home residents with advanced dementia. J Am Geriatr Soc. 2015;63(6):1084–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Muder RR. Pneumonia in residents of long-term care facilities: epidemiology, etiology, management, and prevention. Am J Med. 1998;105(4):319–330. [DOI] [PubMed] [Google Scholar]
- 32. Levy C. Expectation conversations about the very predictable events in advanced dementia. J Am Med Dir Assoc. 2015;16(9):724–727. [DOI] [PubMed] [Google Scholar]