Abstract
Increased ambient temperature is inhibitory to plant immunity including auto-immunity. SNC1-dependent auto-immunity is, for example, fully suppressed at 28°C. We found that the Arabidopsis sumoylation mutant siz1 displays SNC1-dependent auto-immunity at 22°C but also at 28°C, which was EDS1 dependent at both temperatures. This siz1 auto-immune phenotype provided enhanced resistance to Pseudomonas at both temperatures. Moreover, the rosette size of siz1 recovered only weakly at 28°C, while this temperature fully rescues the growth defects of other SNC1-dependent auto-immune mutants. This thermo-insensitivity of siz1 correlated with a compromised thermosensory growth response, which was independent of the immune regulators PAD4 or SNC1. Our data reveal that this high temperature induced growth response strongly depends on COP1, while SIZ1 controls the amplitude of this growth response. This latter notion is supported by transcriptomics data, i.e. SIZ1 controls the amplitude and timing of high temperature transcriptional changes including a subset of the PIF4/BZR1 gene targets. Combined our data signify that SIZ1 suppresses an SNC1-dependent resistance response at both normal and high temperatures. At the same time, SIZ1 amplifies the dark and high temperature growth response, likely via COP1 and upstream of gene regulation by PIF4 and BRZ1.
Author summary
Ambient temperature is a major actor in plant immunity and in growth regulation. Foremost, high temperature (>27°C) is known to block plant defence responses. High temperature also alters the plant morphology by inducing elongation growth, which facilitates plant ‘cooling’. This process is called thermomorphogenesis. Importantly, the SUMO E3 ligase SIZ1 suppresses plant immunity at normal conditions (22°C), but its role in immunity at high temperature was unknown. SIZ1 was recently shown to sumoylate and activate the ubiquitin E3 ligase COP1, a key player in thermomorphogenesis affecting the accumulation and/or stability of key transcription factors for this process (PIF4 and HY5). At high temperature PIF4 suppresses SNC1-dependent growth defects and auto-immunity for the snc1-1 mutant. We report that part of the SNC1-dependent auto-immune response is retained and activated in the siz1 mutant at high temperature resulting in enhanced resistance to Pseudomonas. In addition, we find that SIZ1 controls the thermomorphogenesis response and it affects expression of a substantial subset of PIF4 and BZR1 gene targets in response to high temperature. Our data imply that SIZ1 acts upstream of the PIF4/BZR1 hub. Combined the data highlight that SIZ1 has a dual role in the trade-off between SNC1-dependent immunity and growth at elevated temperature, where the latter aspect potentially runs via COP1.
Introduction
Ambient temperature is a major factor that affects plant growth and development, but also plant immunity [1,2]. In particular, the temperature range of 16-32ºC modulates the output of many plant immune receptors. For example, the tobacco N (Necrosis) gene fails to trigger resistance against Tobacco mosaic virus (TMV) at 30°C, while conferring resistance at 23°C [3]. This is accompanied by the loss of the hypersensitive response (HR) above 27°C. This HR includes a localized cell death that appears to be associated with recognition of pathogen effectors resulting in effector-triggered immunity (ETI) [4–7]. Multiple examples of high temperature suppression of ETI have been described for the TNL-type of immune receptors (Toll Interleukin-1 receptor [TIR], NB-LRR-type) [2], including the tobacco immune receptor N against Tobacco mosaic virus (TMV) [7,8], but also resistance mediated by the Arabidopsis immune receptor RPS4, which recognizes the avirulence protein AvrRPS4 from Pseudomonas, is suppressed at high temperature [9]. Finally, SNC1 (Suppressor of npr1-1, constitutive 1) dependent auto-immunity in the gain-of-function mutant snc1-1 is suppressed at high temperature [10]. Auto-immunity in the snc1-1 mutant was caused by hyperaccumulation of a mutant variant of SNC1 resulting in a dwarf stature of the mutant plant with curly leaves at 22°C [11]; At 28°C this auto-immune phenotype of snc1-1 is fully suppressed yielding plants with wild type rosettes without any macroscopic lesions or microscopic cell death. Importantly, HR activation by SNC1 required nuclear localization of SNC1, which appeared to be compromised when plants were kept at 28°C [6,7,12].
In non-infected plants, SNC1 levels are tightly controlled at both the transcript and protein level to prevent spurious immune signalling [13]. The expression of SNC1 is, for example, indirectly negatively regulated by the plasma membrane-localized protein BON1 (Bonzai 1) [14], but also the protein levels of SNC1 are regulated e.g. by the immune adaptor SRFR1 (Suppressor of RPS4-RLD 1) [15,16], several protein folding chaperones [17], and the F-box protein CPR1 (Constitutive expressor of Pathogenesis-related (PR) proteins 1) [11,18]. Mutations in the corresponding genes (e.g. snc1-1, bon1, srfr1-4 and cpr1-2) cause SNC1-dependent auto-immunity (hereafter SNC1auto-I). SNC1auto-I relies on EDS1 and PAD4 (Enhanced disease susceptibility 1, Phytoalexin-deficient 4) [19]. Upon recognition of biotrophic pathogens, EDS1 translocates from the cytoplasm, where it is sequestered by the related protein PAD4, to the nucleus [20–23]. Nuclear localization of EDS1 is necessary for transcriptional reprogramming to trigger SA biosynthesis and other plant defence responses.
Strikingly, high temperature suppression of auto-immunity depends for the snc1-1 mutant on the central growth regulator PIF4 (Phytochrome Interacting Factor 4), a transcription factor (TF) that is essential for thermomorphogenesis at 28°C [24]. This implies that plant growth is prioritized over SNC1-dependent auto-immunity at 28°C via transcriptional regulation. High ambient temperature increases PIF4 activity by controlling both its transcript levels and protein levels in a diurnal dark/light cycle [25]. This process is directly affected by relocalization of the ubiquitin E3 ligase COP1 (Constitutive Photomorphogenesis 1) to the nucleus in dark conditions. In the nucleus COP1 targets key regulators of both PIF4 protein activity and PIF4 gene expression for degradation [26]. Recent data highlight that COP1 is not only essential for the dark-induced growth response, but also at high ambient temperature in a normal diurnal dark/light cycle [27].
Here we studied auto-immunity in a mutant of the Arabidopsis SUMO E3 ligase SIZ1. Auto-immunity of siz1 highly resembles SNC1auto-I [28,29], i.e. the mutant shows enhanced resistance to Pseudomonas infection due to high levels of SA, its rosette adopts a very similar morphology (including lesions and spontaneous cell death) as the SNC1auto-I mutants, and this auto-immune phenotype depends on PAD4. Auto-immunity in the siz1 mutant is likely caused by the absence of sumoylation on one or more of its substrates, as the sumo1/2KD knock-down mutant also displays auto-immunity [29]. SIZ1 is the major SUMO E3 ligase in Arabidopsis [30], affecting SUMO conjugation of many substrates including pivotal regulators of growth [31–33]. For example, COP1 is a direct substrate of SIZ1 and its sumoylation enhances the intrinsic ubiquitin E3 ligase activity of COP1 [34,35].
As PIF4 controls the high temperature-mediated recovery of snc1-1 auto-immunity and SIZ1 controls the activity of a key regulator of PIF4, namely COP1, we assessed here (i) whether the siz1 auto-immune phenotype requires a functional SNC1 gene copy at normal and high temperature. Moreover, we tested (ii) if loss of SIZ1 function suppresses the COP1/ PIF4 mediated growth response at high temperature and in dark conditions. We found that siz1 auto-immunity is sustained at 28°C resulting in enhanced resistance to bacteria, which depended on both SNC1 and EDS1. The dwarf stature of siz1 also hardly recovered at 28°C. Moreover, we found that siz1 shows a compromised thermosensory growth response, which was independent of SNC1 and PAD4. This positive regulatory role of SIZ1 in growth regulation was suppressed by the TF HY5 (Elongated hypocotyl 5) at 22°C, while it depended on COP1 function at 28°C (and in dark conditions). HY5 is a direct substrate for COP1 targeted protein degradation. Finally, we found that high temperature induced transcriptome changes are both attenuated and delayed in the siz1 and sumo1/2KD mutants and that a substantial subset of the affected genes are known genomic targets for PIF4 binding and regulation.
Results
Hallmarks of auto-immunity are not fully suppressed at high temperature in siz1
A hallmark of SNC1auto-I is a dwarf stature and curled leaves. These morphological defects disappear when SNC1auto-I mutants like cpr1-2, bon1, snc1-1, and srfr1-4 are grown at 28°C, adopting a wild type stature (Fig 1A and 1B). Here we tested if also for siz1 these morphological defects are rescued when it grows at high temperature. In contrast to the four aforementioned SNC1auto-I mutants, we observed that siz1 remains significantly smaller than the wild type control at 28°C (Fig 1A and Fig 1B, compare group ‘cd’ with group b). At 22°C, the rosette weight of siz1 was indistinguishable from these four SNC1auto-I mutants (Fig 1B, group ‘d’). Previous work by others had shown that the auto-immune phenotype of these SNC1auto-I mutants depends on (i) a functional gene copy of PAD4 and EDS1, and (ii) accumulation of the defence hormone SA [10,16,36,37]. Likewise, Lee and co-workers demonstrated that the siz1 phenotype (partially) depends on PAD4 and SA accumulation [28], but the role of EDS1 remained unknown. Since EDS1 is the major nuclear actor of the PAD4/EDS1 hub [22,38] and SIZ1 is considered to primarily act in the nucleus [39], we examined if siz1 auto-immunity depends on EDS1. The siz1 growth defect partially recovered when it was crossed with the eds1-2 mutation in the Col-0 background, but this recovery did not significantly differ from the recovery seen for the double mutants siz1 pad4 and siz1 NahG (a transgene encoding salicylate hydroxylase that effectively prevents SA accumulation by converting it to catechol) at 22°C (Fig 1C and 1E; all post hoc group ‘c’). We also crossed siz1 with a mutant for SID2 (Salicylic acid induction deficient 2), which encodes the key enzyme for SA synthesis in plant immunity [40]. As seen by others for other auto-immune mutants [41], introduction of the sid2 mutation did not rescue the siz1 growth defect seen at 22°C (Fig 1C and 1E, group d). Importantly, at 28°C none of the siz1 double mutants showed any additional growth recovery compared to siz1 alone (Fig 1D and 1E, group c). This suggests that the small growth recovery of siz1 seen at 28°C (Fig 1E, from only ‘d’ at 22°C to ‘cd’ at 28°C) is potentially linked to suppression of its auto-immune phenotype, which in turn would depend on EDS1/PAD4 and SA accumulation.
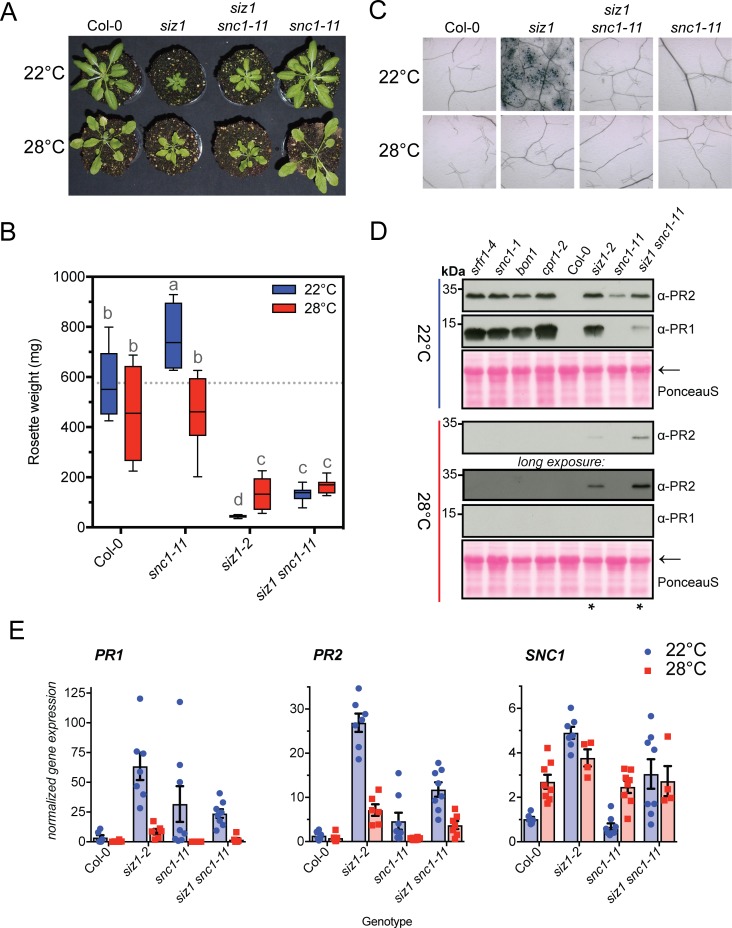
Fig 1. While growth retardation of siz1 is hardly rescued at 28°C, other hallmarks of auto-immunity fully recover at this temperature.
(A) Picture of the rosettes of siz1 and SNC1-dependent auto-immune mutants (crp1-2, bon1, snc1-1, srfr1-4) grown for 5 weeks in SD conditions at 22°C or 28°C. The mutants are in the Col-0 background. (B) Box-plot (middle bar = median, box limit = upper and lower quartile, extremes = Min and Max values) depicting the rosette weight of the genotypes shown in (A). Plants were 5-week-old plants. Significant differences were detected using a two-way ANOVA with Tukey’s multiple comparisons test: Genotype p-value<0.0001 (29% of the variation), Temperature p-value<0.001 (37%); GxT interaction p-value<0.0001 (22%); letters indicate significantly different post hoc groups (n = 8–10). The experiment was repeated 3 times with similar result. (C, D) Similar to (A), plants were grown in parallel for 5-weeks at 22°C or 28°C. The top row depicts wild type Col-0 and the single mutants, while the bottom row depicts siz1 and siz1 crossed with the mutants of the top row. (E) Box-plot with the rosette weight of the plants of panels (C, D). The statistical test was similar to (B) with similar result (n = 8). siz pad4-1, siz1 eds1-2, and siz1 NahG show a small recovery at 22°C (C) without any additional effect at 28°C (D). The experiment was repeated three times with similar result. Left side, single mutants; right side, siz1 and the corresponding double mutants. (F) Spontaneous cell death is absent in siz1 at 28°C, but also 22°C when EDS1/PAD4 are mutated or SA accumulation is compromised (NahG). Fully elongated leaves of 5-week-old plants were stained with Trypan blue and examined under the microscope. (G) Accumulation of PR1/PR2 in siz1 double mutants grown at 22°C or 28°C. srfr1 and bon1 are shown as control for PR accumulation. Total protein was extracted from 5-week-old plants. PR proteins were detected with polyclonal antibodies. Blots were stained with Ponceau S to confirm equal protein loading (← = Rubisco). The blots shown are a single exposure on one film of gels run/blotted in parallel with the samples taken in parallel as well. The apparent Mw of marker proteins is shown on the left.
Hence, we assessed if other hallmarks of the SNC1auto-I phenotype are also partially rescued when siz1 is grown at 28°C. We looked at spontaneous cell death, expression of defence-related genes (PR1, PR2, and SNC1), and accumulation of the encoded PR proteins. Both spontaneous cell death and PR1 expression are known (i) to strongly depend on EDS1/PAD4 and SA accumulation, and (ii) to be suppressed at 28°C in the aforementioned SNC1auto-I mutants. Spontaneous cell death was fully suppressed when siz1 was grown at 28°C (Fig 1F). At 22°C spontaneous cell death was lost in the double mutants siz1 pad4, siz1 eds1 and siz1 NahG (Fig 1F), indicating that EDS1/PAD4 and SA accumulation are required for the spontaneous cell death in siz1. At 22°C expression of PR1 and PR2 was also strongly up-regulated in siz1 compared to the control (Col-0) and expression of both genes required EDS1, PAD4 and SA accumulation (Figs 2E, S1A and S1B). At 28°C, PR1 expression was completely suppressed in siz1, but PR2 expression partially remained (S1B Fig). This situation was reflected in their protein levels, i.e. PR1 levels were high in siz1 at 22°C while undetectable at 28°C (Fig 1G). In contrast, PR2 levels were elevated in siz1 both at 22°C and 28°C albeit to a lower level at 28°C. In the case of the four SNC1auto-I mutants, PR1 and PR2 did not accumulate when these mutants were grown at 28°C (Figs 1G and 2D). Thus, the siz1 auto-immune response is (partially) temperature sensitive, but it does not simply mimic the ‘classic’ behaviour of SNC1auto-I mutants.
Fig 2. The siz1 auto-immune phenotype is partially rescued by loss of SNC1.
(A) Picture of the rosettes of siz1 and the siz1 snc1-11 double at 22°C/28°C. At 22°C growth retardation of siz1 is partially recovered in the snc1-11 background, while at 28°C siz1 snc1-11 does not show any additional recovery to siz1. Plants were 5-weeks-old (SD). (B) Box plot showing the rosette weight of the plants in (A). Significant differences were determined using a two-ay ANOVA followed by a Tukey post-hoc test: Genotype p-value<0.0001 (75% of the variation), Temperature p-value = 0.0033 (2.1%); GxT interaction p-value<0.0001 (8.8%). The letters indicate statistically different post hoc groups (n = 8) (C) Spontaneous cell death in siz1 requires SNC1 function. Fully elongated leaves of 5-week-old plants were stained with Trypan blue. (D) Accumulation of PR1 and PR2 is partially suppressed in siz1 snc1-11. As control for thermosensitive accumulation of PR proteins, srf1-4, snc1-1, bon1 and cpr1-2 are shown. Total protein was extracted from 5-week-old plants. The blots were prepared in parallel and ECL detection was done on one film, except for the ‘long exposure’ to reveal PR2 accumulation. Blots were stained with Ponceau S to confirm equal protein loading. Asterisks mark enhanced PR2 accumulation in siz1-2 and siz1 snc1-11 at 28°C. (E) Normalized gene expression of PR1, PR2 and SNC1 (mean ± SE) in 5-week-old plants (fold change; Col-0 at 22°C = 1). PR1 and PR2 expression are still elevated in siz1 snc1-11 at 22°C. At 28°C PR1 expression is gone in siz1 snc1-11, while PR2 expression remains up regulated (7-fold up).
Spontaneous cell death but not accumulation of PR2 depends in siz1 on SNC1
As elevated expression of SNC1 triggers auto-immunity at 22°C [42], we measured SNC1 expression in siz1. SNC1 expression proved to be induced by nearly 5-fold in siz1 at 22°C (S1C Fig), suggesting that an increase in SNC1 transcript levels could be causal for the siz1 dwarf stature and auto-immunity. To determine if the SNC1 gene is indeed required for the siz1 phenotype at 22°C/28°C, we crossed siz1 with a loss-of-function mutant of SNC1, snc1-11 (SALK_04705). This mutant has a T-DNA insertion in the first exon, which results in a severely truncated transcript [42]. When grown at 22°C, the siz1 snc1-11 double mutant displayed a small but significant growth recovery compared to siz1 (Fig 2A and 2B; group ‘c’ and ‘d’, respectively), which is more apparent when the plants are flowering (S2 Fig). However, in our conditions the snc1-11 mutant itself also displayed a small but significant increase in biomass compared to the wild type control (Col-0) at 22°C (Fig 2B). More importantly, both siz1 and the siz1 snc1-11 double mutant largely kept their dwarf stature when grown at 28°C. This is striking, as the growth defects of the SNC1auto-I mutants cpr1-2, bon1 and srfr1-4 recovered strongly (to wild type levels) when the snc1-11 mutation was introduced in these mutants by crossing [10,15,18]. The increase in SNC1 transcript levels can, therefore, not be the main or sole cause of the dwarf stature of siz1.
Nonetheless, spontaneous cell death was fully suppressed in siz1 snc1-11 at 22°C (Fig 2C), while PR2 and to a lesser extent PR1 still accumulated in siz1 snc1-11 at 22°C (Fig 2D). Also at 28°C PR2 still accumulated to some extent in siz1 snc1-11, similar to siz1 (Fig 2D). The PR1 and PR2 protein levels were again mirrored by their gene expression levels (Fig 2E), i.e. at 22°C the expression of PR1 was roughly 50% in siz1 snc1-11 in comparison to siz1, which in both cases was fully suppressed when these two mutants were grown at 28°C. On the other hand, PR2 expression remained detectable when both mutants were grown at 28°C. Also the (truncated) transcript of SNC1 still accumulated to higher levels in siz1 snc1-11 than in siz1. For snc1-11, 2–3 samples showed up-regulation of PR1 and PR2, while the remaining samples 5 samples showed hardly any up-regulation suggesting that the latter samples reflect the general trend.
Increased SNC1 protein levels are known to trigger auto-immunity [11]. SNC1 levels are negatively controlled by the HSP90/SGT1/SRFR1 chaperone-complex of which some components were reported to be SUMO substrates [43,44]. We therefore examined whether siz1 auto-immunity was attenuated when mutants for SGT1a, SGT1b, and RAR1 were introduced by crossing. Introduction of these mutants in siz1 (i.e. siz1 rar1, siz1 sgt1aKO and siz1 sgt1beta3) partially compromised cell death induction (S3A Fig), while it hardly enhanced rosette growth in these siz1 chaperone double mutants (S3B Fig). Hence, the chaperones contribute to the siz1 phenotype, but they are not essential for spontaneous cell death. Clearly, the siz1 auto-immune phenotype partially depends on SNC1, but not all of the elements of the auto-immune phenotype disappear when SNC1 is non-functional.
SIZ1 auto-immunity confers resistance to Pseudomonas at high temperature in an EDS1- and SNC1-dependent manner
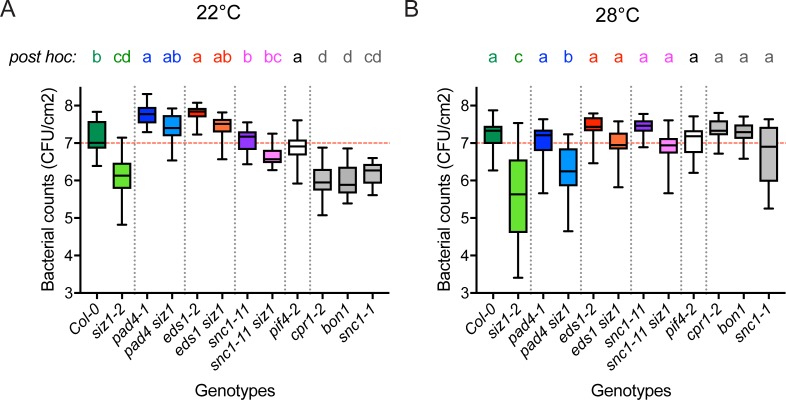
As the PR1 levels were down in siz1 at 28°C, we tested if enhanced resistance of siz1 to the pathogen Pseudomonas syringae pv. syringae strain DC3000 (PstDC3000) is compromised at high temperature. In order to inoculate similar looking plants, all plants were grown at 28°C and half of the plants was shifted to 22°C twenty-four hours prior to the inoculation. In this way extreme differences in rosette size, morphology, or tissue structure had no impact on the disease assay (compare the plants grown at 28°C in Fig 1A). The 24 hours pre-incubation at 22°C was sufficient to re-activate auto-immunity in the SNC1auto-I mutants tested (cpr1-2, bon1, snc1-1) resulting in reduced susceptibility to PstDC3000 (Fig 3A, post hoc groups ‘cd’ and ‘d’). As expected, the three tested SNC1auto-I mutants (cpr1-2, bon1, and snc1-1) were as susceptible as the wild type control (Col-0) at 28°C (Fig 3B). However, siz1 displayed enhanced resistance to PstDC3000 both at 22°C and 28°C (Fig 3A and 3B). This resistance was compromised in siz1 at 22°C when PAD4, EDS1 or SNC1 were mutated (Fig 3A). At high temperature, only siz1 pad4 retained enhanced resistance to PstDC3000, while siz1 eds1-2 and siz1 snc1-11 were both as susceptible as the wild type control (Fig 3B). This means that enhanced resistance of siz1 to the pathogen PstDC3000 at 28°C was still dependent on EDS1 and SNC1.
Fig 3. siz1 displays enhanced resistance to Pseudomonas at 22°C and 28°C in a SNC1- and EDS1-dependent manner.
(A) Disease resistance to Pseudomonas syringae pv. tomato strain DC3000 (box plot; n = 20–24; Bacterial growth was determined 3 dpi after syringe infiltration of the leaves (1×105 CFU/ml). Significant differences were detected using two-way ANOVA (genotype p-value <0.0001, temperature p-value = not significant, GxT p-value <0.0001) followed by a Tukey post-hoc test. Letters indicate statistically different groups at 22°C. Plants were grown for 5 weeks at 28°C and 24 hrs prior to the inoculation shifted to 22°C. Three independent experiments were combined with each replicate showing the same trend. (B) Similar to (A) except that the plants remained at 28°C during the experiment. The letters indicate statistically different groups at 28°C. This experiment was done in parallel with (A).
In the case of snc1-1, high temperature suppression of immunity and restoration of growth were both reported to depend on PIF4 [24]. Therefore, we also tested if the pif4-2 mutant showed altered resistance to PstDC3000 at 22°C/28°C. The pif4-2 plants showed a clearly compromised thermomorphogenesis response at 28°C, i.e. (i) the hypocotyl length was reduced (Fig 4A and 4B), (ii) the rosette showed no hyponasty and (iii) the leaf blades and petioles failed to elongate in comparison to Col-0. However, the pif4-2 mutant was as susceptible to PstDC3000 as the wild type control (Col-0) at either temperature in our conditions (Fig 3).
Fig 4. SIZ1 enhances the temperature- and dark-induced hypocotyl elongation independent of PAD4 and SNC1.
(A) High temperature- and/or dark-induced hypocotyl elongation is compromised in siz1 and sumo1/2KD. Wild type (Col-0) seedlings and pif4-2 are shown as positive and negative control for thermosensory hypocotyl elongation, respectively. The pictures were taken 5 d post germination. (B) Bar graph (mean ± standard deviation) depicting the hypocotyl length 5 days post germination in 4 conditions: 22C L, 28C L = germination of the seedlings in SD growth conditions at 22°C or 28°C, respectively; 22C D, 28C D = germination of the seedlings in the dark at 22°C or 28°C, respectively. Hypocotyl elongation is significantly reduced in siz1 and sumo1/2KD in dark and/or high temperature conditions (compared to Col-0) independent of PAD4 or SNC1. Significant differences were determined using one-way ANOVA (for each condition separately) followed by a Tukey post-hoc test. The brackets indicate the result of the post hoc test for the connected genotypes; otherwise the asterisks denote the difference to the control line (Col-0, pad4 or scn1-11; ****, p≤0.0001; ***, p≤0.001, *, p≤0.05, ns, p>0.05; n = 40–43 samples). The experiment was repeated twice with similar results. (C) Bar graph (mean ± standard deviation) depicting the hypocotyl length of Col-0, siz1, hy5-215, siz1 hy5-215, cop1-4, and siz1 cop1-4 in response to the same four conditions. SIZ1 is important for hypocotyl elongation of hy5-215 in a normal dark/light cycles at 22°C (22C L). In dark conditions, loss of SIZ1 enhances the phenotype of the cop1-4 mutant (22C D, 28C D). Significant differences were determined using one-way ANOVA followed by Tukey post hoc test. The brackets indicate the result of the post hoc test for the connected genotypes. (D) Bar graph (mean± standard deviation) depicting the hypocotyl length of the SUMO mutants (siz1, sumo1/2KD) in response to GA3 (10 μM) or the GA3 biosynthesis inhibitor PAC (0.5 μM). Gibberellin biosynthesis is needed for hypocotyl growth at 28°C (+PAC), as PAC fully inhibits hypocotyl elongation. Significant differences were determined using two-way ANOVA followed by Tukey post-hoc test; significantly different groups are indicated by the letters (n = 41–44). Seeds were germinated on plate and hypocotyl lengths were measured 5 days post germination at 28°C in SD. siz1 and sumo1/2KD showed less germination on PAC (n = 8 and n = 32, respectively). All seeds were fresh and harvested simultaneously. The experiment was repeated twice with similar results (E) Similar to (D) except that seeds were germinated on 0.1 μM Brassinolide (BL) or the BR biosynthesis inhibitor PPZ (2.0 μM) with n = 41–52 samples.
Both SIZ1 and SUMO1/2 control dark and high temperature induced hypocotyl elongation
As snc1-1 auto-immunity is inhibited by PIF4 at high temperature [24], the enhanced immunity of siz1 to PstDC3000 at 28°C might also be due to reduced PIF4 function. In line with this notion, we found that siz1 and the sumo1/2KD mutant both showed reduced hypocotyl elongation at 28°C in normal diurnal dark/light cycles (Fig 4A, light; 4B, compare 22C L with 28C L), implying that SIZ1 and the two archetype SUMO proteins, SUMO1 and SUMO2 (hereafter SUMO1/2), act as positive regulators of thermomorphogenesis similar to PIF4 (pif4-2 was included as control for the loss of thermosensitive hypocotyl elongation; Fig 4A and 4B). SIZ1 and SUMO1/2 were both also needed for skotomorphogenesis (dark-induced hypocotyl elongation) at 22°C and 28°C (Fig 4A, dark; 4B, compare 22C L with 22C D). The compromised dark and high temperature growth responses were both independent of PAD4 and SNC1, as they still occurred to same extent in siz1 pad4 and siz1 snc1-11 (Fig 4B). This means that not the auto-immune phenotype of siz1 is responsible for the compromised thermo/skotomorphogenesis, but rather that SIZ1 itself acts as positive regulator of these growth responses. In support of this notion, we confirmed that the SNC1auto-I mutants cpr1, bon1, and srfr1-4 display a normal thermomorphogenesis response (S4 Fig), indicating that PIF4 function is unaffected in them. Moreover, the sumo1/2KD consistently showed a stronger reduction in hypocotyl elongation than siz1 nearing pif4-2 at the 28°C in a normal dark/light cycle (Fig 4B, 28°C L).
The mutants siz1 and sumo1/2KD also displayed a strong reduction in hypocotyl elongation when they were kept in the dark at 22°C and 28°C (Fig 4B; panels 22C D, 28C D). As SIZ1 stimulates COP1 activity and the nuclear function of COP1 is activated in the dark [34,35], we examined whether loss of SIZ1 function could enhance the thermo/skotomorphogenesis phenotype of a strong but not lethal COP1 mutant, cop1-4 [45]. Hypocotyl elongation was indeed more reduced in siz1 cop1-4 than in cop1-4 alone in dark conditions at 22°C and 28°C (Fig 4C; panels 22C D, 28C D). Thus, COP1 is critical for the thermosensory growth response–as recently reported [27], while SIZ1 appears to primarily enhance this response (as further detailed below).
In light conditions, the TF HY5 is known to inhibit hypocotyl elongation by inhibiting PIF4 expression [25]. COP1 targets HY5 for proteasomal degradation when COP1 is active in the nucleus. We found that SIZ1 function is needed for the full hypocotyl elongation of the HY5 loss-of-function mutant hy5-215 in a diurnal light/dark cycle at 22°C (Fig 4C, panel 22C L). This means that in a diurnal light/dark cycle at 22°C the stimulatory role of SIZ1 on hypocotyl growth is masked by the inhibitory role of HY5. We also compared the rosette size and morphology of siz1 cop1-4 and siz1 hy5-215 with the single mutants at both temperatures (S5 Fig). At 22°C siz1 cop1-4 and siz1 hy5-215 both adopted a siz1 rosette size/morphology. At 28°C growth was recovered for siz1 hy5-215, but to a lesser extent than for siz1. In contrast, siz1 cop1-4 failed to respond to the high temperature and this mutant still closely resembled cop1-4 mutant (having a compact rosette with hardly any petioles and no hyponasty; S5A Fig). This is consistent with a model in which COP1 primarily conveys the thermosensory growth response and that SIZ1 amplifies the output of this response.
Hormone biosynthesis is required for the SIZ1-dependent temperature induced growth
As biosynthesis of the hormones gibberellic acid (GA3) and the brassinosteroids is needed for the temperature induced hypocotyl elongation [46], we checked if the positive regulatory role of SIZ1 and SUMO1/2 in thermomorphogenesis requires these two hormones. First, we inhibited GA3 or BR biosynthesis by adding paclobutrazol (PAC) or propiconazole (PPZ), respectively. Irrespective of the genetic background, we found that biosynthesis of both hormones was essential for the temperature-induced hypocotyl elongation in the lines tested including the residual elongation in pif4-2 (Fig 4D and 4E). GA3 is known to reduce the abundance of the DELLAs by triggering their degradation [47]. In turn the DELLAs restrain cell growth by reducing protein abundance of the PIFs (including PIF4) and the TF BZR1 (Brassinazole resistance 1) [48,49]. A combined treatment of 28°C+GA3 resulted in increased hypocotyl elongation for each of the four tested lines compared to the 28°C control (-) (Fig 4D). However, hypocotyl elongation was still impaired for siz1, sumo1/2KD and pif4-2 in the combined treatment 28°C+GA3 (Fig 4D). This implies that the positive role of SIZ1 on temperature-induced hypocotyl growth is independent of DELLA accumulation. The combined treatment of 28°C plus the brassinosteroid Brassinolide (28°C+BL) triggered a small but significant increase in hypocotyl elongation in the control (Col-0) plants compared to the mock treatment (Fig 4E,—vs. BL). However, the SUMO mutants (siz1 and sumo1/2KD) showed no additional response to the combined treatment 28°C+BL (Fig 4E). Strikingly, the pif4-2 mutant did respond to the BL treatment (from post hoc group D to C), suggesting that in siz1 and sumo1/2KD brassinosteroid signalling is apparently already at its maximum physiological level.
SIZ1 controls amplitude and timing of the transcriptional response to high temperature in part via PIF4/BZR1
To elucidate how SIZ1 and SUMO1/2 conjugation affect high temperature-induced gene expression, we grew siz1 pad4 and the sumo1/2KD pad4 mutants for two weeks at 22°C and then shifted them to 28°C (4 hrs after light onset) to trigger a temperature induced transcriptional response. To avoid that constitutive (auto-)immune signalling impedes the thermosensory transcriptional response at t = 0, we performed the experiment in the pad4 background, which largely blocked siz1 auto-immunity at 22°C (i.e. the enhanced accumulation of PR1 and PR2, spontaneous cell death and the increased resistance to PstDC3000 are suppressed in siz1 pad4; Figs 1F, 1G and 3A), but it only partially restored the dwarf stature. Importantly, increased resistance to PstDC3000 was not lost in siz1 pad4 at 28°C, similar to siz1 (Fig 3B). The plants were sampled at the shift to 28°C (day 0) and 24hrs (day 1) and 96 hrs (day 4) after the shift. Catala et al. had previously shown that siz1 shows a strong up-regulation of defence-related genes (like PR genes and immune receptors), while genes involved in BR biosynthesis/signalling are down-regulated [50]. We first determined which genes are differentially expressed at 22°C in siz1 pad4 in comparison to the control (pad4). We found that a small set of genes encoding for TNL immune receptors, Receptor-like kinases (RLKs), and Receptor-like proteins (RLPs) remained up-regulated in siz1 pad4 in comparison to the control (pad4) at 22°C (S1A Table). SNC1 or immune receptors of the CNL type (Coiled-coil NB-LRR-type) were not amongst the up-regulated genes in the microarray data. Real time PCR revealed that SNC1 was roughly two-fold induced in siz1 pad4 (close to the cut-off value for differential gene expression), while SNC1 showed no up regulation in siz1 eds1 or siz1 NahG (S1C Fig). As SNC1 was 5-fold induced in siz1 at 22°C (S1C Fig), we conclude that this requires feedback regulation via EDS1 and SNC1.
There was no broad up-regulation of TF families linked to plant immunity (WKRY, TGA or MYC family) in siz1 pad4 at 22°C. Likewise, PR genes like PR2, PR3, or PR4 were no longer strongly up-regulated in siz1 pad4 at 22°C. The genes involved in BR biosynthesis and signalling were also no longer collectively down-regulated except for two genes, which encode for two rate-limiting enzymes of the Brassinosteroid (BR) biosynthesis pathway (DWF4 or DWARF 4; and BR6OX2 or BRASSINOSTEROID-6-OXIDASE 2) [51–53]. This suggests that the BR levels might be reduced in siz1 pad4. In agreement with this, we found that the TFs BEE1, BEE3, and TCP1 are down-regulated in siz1 pad4 (S1B Table). BEE1 and -3 are two closely related bHLH TFs that act as early response TFs required for the full BR response [54]. TCP1 encodes a TF that directly positively regulates the expression of DWF4 [55]. Combined, these data argue that the siz1 pad4 phenotype may be (partially) due to BR-deficiency.
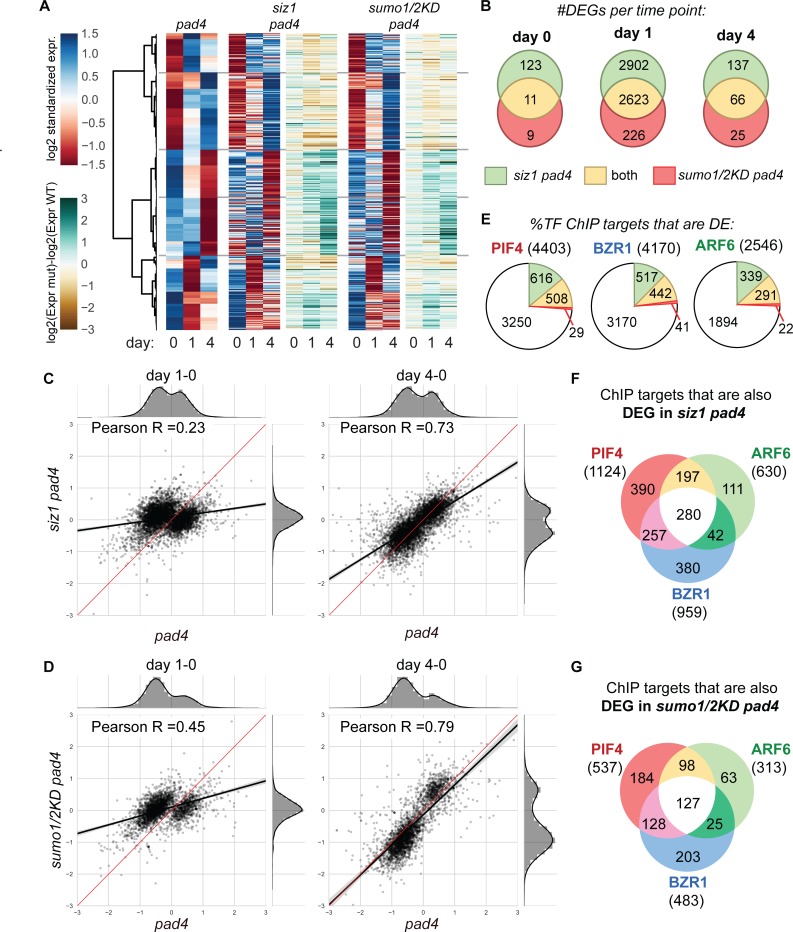
We then selected the set of thermosensitive genes by identifying the genes that are differentially expressed (DEGs, q ≤ 0.01) in pad4 in response to the shift to 28°C (comparing day 1 to day 0, day 4 to day 1, and day 4 to day 0). The DEGs were clustered based on their expression profile and their expression dynamics was revealed by plotting their standardized expression values in a clustered heat map (Fig 5A, red-to-blue). To detect differences in the gene expression profiles of siz1 pad4 and sumo1/2KD pad4 we plotted the same gene expression heat maps for the two mutants while retaining the gene clustering (Fig 5A). We also plotted the difference in gene expression (Δ) between the mutants (siz1 pad4 and sumo1/2KD pad4) and pad4 (brown-to-cyan heat maps). Fig 5A reveals that overall the gene expression profiles of the thermosensitive genes do not differ strongly between the two SUMO conjugation mutants and the control pad4 (blue-to-red heat maps). In other words, most of the thermosensitive genes also respond to the shift to 28°C in siz1 pad4 or sumo1/2KD as they do in pad4.
Fig 5. SIZ1 promotes the timing and amplitude of the expression of thermosensitive genes, including genomic targets of the transcription factors PIF4/BZR1.
(A) Heat maps representing thermosensitive genes, i.e. differentially expressed genes in pad4 in response to the shift to 28°C and their expression profiles in the siz1 pad4 and sumo1/2KD pad4 mutants (blue-to-red diagrams). Gene clustering was based on Pearson correlation of the expression profiles in pad4 (shown on the left). To reveal gene expression dynamics over time in the mutants versus pad4, the expression values were standardized (zero mean, unit variance per profile). Of note, the expression profiles of the thermosensitive genes were largely similar for the three genotypes. In addition, the relative difference-in-expression (log2 fold change, non-standardized) is shown for the thermosensitive genes in ‘siz1 pad 4 versus pad4’ and ‘sumo1/2KD versus pad4’ (brown-to-cyan depicting less expressed-to-more expressed in the mutant). (B) Venn diagrams showing the total number of differentially expressed genes (#DEGs, q≤0.01) in siz1 pad4 (green), sumo1/2KD pad4 (red) and their overlap (yellow) in comparison to pad4 at the three time points. The largest change in global gene induction/repression occurs at day 1 in both two mutants. (C) Scatter plot depicting the change-in-expression at day 1 or 4 with respect to the expression at day 0 (day 1–0; day 4–0) for all the DEGs in siz1 pad4 (y-axis) in comparison to their change in expression in pad4 (x-axis). The DEGs are the same as panel (B). For each DEG is shown the log2-fold change-in-expression. The density plots at the right and top depict the global change in expression for these DEGs in the mutant and the wild type, respectively. The black lines depict a Pearson linear regression analysis for all the DEGs with the 95% confidence interval indicated by the grey zone. The red line depicts an equal change in expression for all genes in siz1 pad4 versus pad4. (D) Similar to (C), but the scatter plot depicts the change in expression for the DEGs in sumo1/2KD pad4 in comparison to pad4. (E) Pie diagrams depicting the total number of direct genomic targets of PIF4, BZR1 and ARF6, retrieved from published chromatin immuno-precipitation (ChIP) datasets (Adapted from [56]), and the number these targets that are differentially expressed (DE) in siz1 pad4 (green+yellow) or sumo1/2KD pad4 (red+yellow). These two sets are a combination of the DEGs from panel B. The overlap between the genomic targets of these three TFs and the DEGs in siz1 pad4 and sumo1/2KD pad4 was significant (p-values for siz1 pad4: PIF4, 3.1e-24; BZR1, 2.1e-8; ARF6, 9.2e-11; p-values for sumo1/2KD pad4: PIF4, 1.2e-6; BZR1, 6.9e-4; ARF6, 1.5e-4; hypergeometric test). (F) Venn diagram depicting the overlap between the genomics targets of PIF4, BZR1 and ARF6 that are differentially expressed in siz1 pad4 over the course of the experiment. Most DEGs are a target of PIF4 and/or BZR1. (G) Venn diagram depicting the overlap between the genomics targets of PIF4, BZR1 and ARF6 that are differentially expressed in sumo1/2 KD pad4 over the course of the experiment.
However, most of the thermosensitive genes appear to show an attenuated response in siz1 pad4 and sumo1/2KD pad4 at day 1 and/or 4. For example the up-regulated genes (changing from red at day 0 to blue at day 4 in the heat maps) show less expression in siz1 pad4 and sumo1/2KD pad4 than pad4 at day 4 (brown colour in the ‘ΔExpr (mut-WT)’ heat maps). Likewise, the down-regulated genes (shift from blue at day 0 to red at day 4) show increased expression in siz1 pad4 and sumo1/2KD pad4 at day 4 (cyan colour in the ‘ΔExpr (mut-WT)’ heat maps). To confirm this notion, we selected for each time point the DEGs in siz1 pad4 and sumo1/2KD pad4 in comparison to pad4. A large set of these DEGs was shared between the two mutants (siz1 pad4 and sumo1/2KD pad4), as can be seen in the VENN diagrams (Fig 5B). Strikingly, the largest number of DEGs was obtained for both mutants at day 1 rather than at day 4. To visualize the dynamic response of these DEGs in response to high temperature, we plotted in a scatter plot the fold change in expression of these DEGs for siz1 pad4 and sumo1/2KD pad4 (both y-axis) versus pad 4 (x-axis) (by separately combining the DEGs for the different time points for the two mutants). The left panel in Fig 5C and 5D depicts the change in expression from day 0 to day 1, while the right panel depicts the change from day 0 to day 4. This revealed that primarily in the control (pad4) at day 1 the expression of the DEGs changed due to the increase in temperature, while in siz1 pad4 and sumo1/2KD pad4 these genes largely failed to respond at this time point (Fig 5C and 5D, panel day 1–0). This is best seen in the global expression profiles (top and right side of the scatter plot) revealing a double hump in pad4, while the expression profile displays a single Gaussian curve around zero for both SUMO mutants. In contrast, at day 4 we find a positive correlation for the change in expression of all DEGs (Pearson R = 0.73; linear regression) with a slope = 0.61 for siz1 pad4 versus pad4. This means that at day 4 the DEGs responded in siz1 pad4 to the high temperature, but their response was overall attenuated. A similar situation is seen for sumo1/2KD pad4 at day 4 (Pearson R = 0.79; slope = 0.87). Thus, SIZ1 and SUMO1/2 both appear to control in a similar manner both the timing and the amplitude of the temperature-induced transcriptional response.
We then examined if the direct genomic targets of the TFs PIF4/BZR1/ARF6 are differentially expressed in siz1 pad4 and sumo1/2KD pad4. The direct genomic targets of these tree TFs, which form a trimeric transcriptional hub, were obtained from published chromatin-immunoprecipitation (ChIP) datasets of these TFs [56,57]. As shown in Fig 5E, nearly 25% of the genomic targets of these three TFs was differentially expressed in siz1 pad4 during the course of the temperature shift experiment. This overlap was very significant with p-values of 3.07e-21 (PIF4), 2.11e-8 (BZR1), 9.24e-11 (ARF6) using a hypergeometric test (based on 26859 annotated probes; TAIR9). The overlap was still significant but less strong for sumo1/2KD pad4 (with an overlap of ±12%, Fig 5E) and p-values of 1.17e-6 (PIF4), 6.92e-4 (BZR1), 1.46e-4 (ARF6). Thus, there is a significant enrichment for the genomic targets of these three TFs amongst the DEGs in both our mutants in response to shift to temperature 28°C (Fig 5E). The change in expression of these genomic targets of these three TFs in the mutants versus the control (pad4) mirrored largely the global pattern seen for all the DEGs combined (S6 and S7 Figs). Thus, the response of the misexpressed genomic targets of PIF4, BZR1, and ARF6 in the siz1 pad4 and sumo1/2KD pad4 mutants follows the same trend as the global response (i.e. their expression is largely delayed till day 4 and the response remains attenuated at day 4). This corroborates our hypothesis that the PIF4-dependent high-temperature growth response is compromised in siz1 and sumo1/2KD. We also looked at the genomic targets of the ‘cold’ regulator HY5 that binds to and competes (at low temperature) for the same genomic targets as PIF4 [58,59]. The HY5 genomic targets largely failed to respond in siz1 pad4 at day 1, while at day 4 their response was largely attenuated in siz1 pad4 compared to pad4 (S8C and S8D Fig). This effect on the expression of the HY5 genomic targets was less clear for sumo1/2KD pad4 (S9C and S9D Fig). While examining the list of DEGs we noted that many SAUR (Small auxin up RNA) genes were present among the top of the gene lists. PIF4 is known to regulate auxin biosynthesis via the SAUR family [60]. The differentially expressed SAUR genes showed a strong deregulation in siz1 pad4 and sumo1/2KD pad4 at both time points, with very distinct global expression profiles in the mutants versus the control (pad4) (S8E, S8F, S9E and S9F Figs). Combined, our data revealed that the siz1 pad4 and the sumo1/2KD pad4 mutants display a delayed and attenuated transcriptional response to high temperature (in comparison to pad4), which runs in part over the PIF4/BZR1 transcriptional hub.
Discussion
Here, we describe an interconnected dual role for SIZ1 and SUMO1/2 conjugation in the switch between plant immunity and high temperature induced growth (as summarized in the model of Fig 6). Our data unveil that both SIZ1 and SUMO1/2 conjugation are positive regulators of thermo- and skotomorphogenesis upstream of the PIF4/BZR1 growth regulation hub. In this hub, BZR1 is activated by the hormone BL, while PIF4 is activated by dark conditions and high ambient temperature. In line, these two TFs share a large number of genomic targets that are synergistically regulated by them [56]. We find that loss of SIZ1 and SUMO1/2 both delays and attenuates this transcriptional response to high temperature affecting many targets of PIF4 and BZR1. This suggests that SIZ1 activity acts as a positive regulator of PIF4 function in thermomorphogenesis and that PIF4 function is apparently compromised/inhibited in siz1 at high temperature (Fig 6, siz1-2). Importantly, the PIF4 protein abundance is positively regulated by COP1 E3 ligase activity [58], while COP1 activity is stimulated by SIZ1-dependent sumoylation (Fig 6, wild type route c.) [34,35]. Our data unveil that COP1 is essential to convey this high temperature signal, as recently reported by others [27], while SIZ1 enhances the high temperature and dark signal. This role of SIZ1 in thermo/skotomorphogenesis is distinct from its reported role on cell elongation due to constitutive defence signalling [61], as hypocotyl elongation was still compromised at high temperature when PAD4 or SNC1 were mutated. Likewise, we noted that the rosette of siz1 pad4, siz1 eds1, and siz1 NahG remained compact at 28°C (without strong petiole elongation or hyponasty as seen for Col-0).
Fig 6. Model depicting the role of SIZ1 in (i) SNC1-dependent auto-immunity and (ii) thermosensory growth via COP1 and PIF4.
(A) Wild type situation; SIZ1 inhibits at the transcription and/or protein level SNC1-dependent auto-immunity (route a.). This involves PAD4, EDS1, SA accumulation, and transcriptional feedback regulation. SNC1 auto-immunity is suppressed at 28°C by PIF4 function, at least for the mutant snc1-1 (route b.) [24]. SIZ1 sumoylation of COP1 stimulates the intrinsic ubiquitin (Ub) E3 ligase activity of COP1 resulting in degradation of COP1 substrates, including HY5 and SIZ1 [34,35] (route c.). In this way, SIZ1 amplifies and tunes COP1 activity at high temperature and/or dark conditions (based on Fig 4). (B) In the siz1-2 mutant, auto-immunity is not inhibited at low and only partially at high temperature. This results in enhanced resistance to bacteria in siz1-2 at low and high temperature, while requiring EDS1 and SNC1 function (Fig 3). COP1 activity is required to convey thermosensing resulting in less hypocotyl elongation growth in cop1-4 mutant (Fig 4C). This (residual) COP1 activity is reduced when SIZ1 is mutated (based on Fig 4C; [34,35]). As inhibition of snc1-1 auto-immunity at high temperature requires PIF4 function, it appears that route b. is compromised or absent in the siz1-2 mutant. The blue/red/black arrows depict signalling routes at 22°C, 28°C, or that are independent of these temperatures, respectively. The thickness of the arrows marks the amount of protein activity.
Interestingly, part of the siz1 auto-immune phenotype is sustained at high temperature resulting in enhanced resistance to bacteria (Fig 3). This enhanced resistance still required SNC1 and EDS1 function at 28°C (Fig 3, Fig 6 wild type route a.). The latter is relevant, as both SNC1 and EDS1 immune signalling depend on their nuclear localization, while SNC1 nuclear localization is impaired at high temperature [7,12,22,62]. High temperature suppression of snc1-1 auto-immunity and concomitantly rescue of its growth phenotype requires PIF4 function [24] (Fig 6 wild type route b.). SNC1-dependent auto-immunity, including enhanced resistance to the bacterial pathogen Pseudomonas, is normally fully suppressed in the mutants bon1, crp1-2 and snc1-1 at 28°C, resulting in normal rosette growth (e.g. Figs 1–3) [7,63]. However, siz1 fails to resume normal growth at 28°C and this is independent of PAD4/EDS1, SNC1 or SA accumulation. This implies that the ‘high temperature’ signal is not properly conveyed in siz1. At the same time, SIZ1 suppresses expression of a small subset of immune receptors at 22°C, even when PAD4 is mutated. It remains an open question if elevated expression of one of these immune receptors (S1A Table) is causal for the auto-immune phenotype of siz1, rather than the misexpression of SNC1.
Biochemically, SUMO conjugation was already implied as a regulator of photomorphogenesis [34,35]. Our data suggest that the role of SIZ1 in thermomorphogenesis is mechanistically independent of light sensing, as hypocotyl elongation in siz1 was also reduced in the dark. Previous works had indicated that sumoylation of phyB allows PIF5 to bind its target promoters resulting in root growth stimulation. These authors demonstrated that sumoylation of the Pfr state (red light activated state) of phyB suppresses the interaction between phyB and PIF5, the closest homologue of PIF4 [32,64,65]. Our GA3 treatment experiment also suggests that SIZ1 controls thermomorphogenesis response independent of DELLA accumulation (Fig 4D). The DELLAs control the stability of the PIFs, while they themselves are also controlled by sumoylation [31,49]. Other (putative) sumoylation substrates implicated in PIF4 function are ELF3 (Early flowering 3) [43,66], HFR1 [67] and LAF1 [68], HY5 and HY5-like (HYL) [69]. The role of sumoylation has not yet been determined for ELF3. Both HFR1 and LAF1 are also targets for COP1-mediated degradation. The link between their degradation and sumoylation remains to be studied. Nevertheless, it is evident that (i) SUMO conjugation acts at multiple levels as a regulator of growth and that (ii) certain COP1 substrates are also targets for sumoylation.
Finally, we found that several actors in BR biosynthesis and signalling are still down-regulated (DWF4, BEE1, BEE3, and TCP1) in siz1 pad4. CESTA, a close homologue of BEE1 and BEE3, is another SUMO substrate that directly binds to BEE1 to control BR biosynthesis [70]. Catala and co-workers had previously reported that from the nearly 1600 differentially expressed genes in siz1 (>two-fold change), eleven down-regulated genes were known to be critical for BR biosynthesis and signalling [50]. In addition, they found in their genome-wide expression analysis that both PIF4 and PIF5 were underexpressed in siz1 [50]. These data warrant further research on the role of sumoylation on BL signalling and biosynthesis.
To conclude, SIZ1 and SUMO1/2 both act as important positive regulators of growth, while SIZ1 also acts as negative regulator of an SNC1-dependent immune response at high temperature. SIZ1 thus plays an interdependent dual role in growth and immunity at elevated ambient temperature.
Materials and methods
Plant materials and growth conditions
The genetic resources for this research were wild type Arabidopsis (Arabidopsis thaliana) ecotype Col-0, siz1-2 [71], cop1-4 [45], cpr1-2 [18], bon1-1 [10], hy5-215 [72], snc1-1 [19], srfr1-4 [15], pad4-1 [73], eds1-2 (backcrossed in Col-0) [74], sid2-1 [40], 35Spro::NahG [75], snc1-11 (SALK_047058) [10], sgt1a-3 [16], sgt1b(eta3) [76], rar1-21 [77], pif4-2 [78], and sumo1/2KD [aka sum1-1 amiR-SUMO2 line B][29,79]. The double mutants pad4 siz1, NahG siz1 [28], cop1-4 siz1-2 and hy5-215 siz1-2 [35] are described elsewhere. Arabidopsis plants were grown under white light with 120 μmol m-2 sec-1 under short-day (SD) light conditions (11 hr light, 13 hr dark) at 22°C or 28°C on a compost/perlite soil mixture. After crossing, the plants were genotyped according to the primer combinations and primer sequences presented in the S2 and S3 Tables, respectively.
Fresh rosette weight measurements
The fresh rosette weight of plants (minimum 8) grown individually in single pots was measured. The rosette was sampled from 5-week-old plants grown in parallel at 22°C or 28°C. Statistical analyses were made using two-way ANOVA (genotype, temperature, interaction GxT) followed by Tukey post hoc test in Prism7. Significantly different groups are indicated by letters.
Protein analysis
For immunoblot analysis, seedlings or leaf material was homogenized in liquid nitrogen, thawed on ice in extraction buffer (10% glycerol, 50 mM K2HPO4/KH2PO4 pH 7.5, 150 mM NaCl, 1 mM EDTA, 2% w/v polyvinylpolypyrrolidone K25, 1× protease inhibitors (Roche), 1% v/v Nonidet P-40, 0.1% SDS and 5 mM DTT), and centrifuged for 10 min at 13,000g. The supernatant was mixed 1:1 with 2× SB (125 mM Tris-HCl pH 6.8, 4% SDS, 20% v/v glycerol, and 100 mM DTT), and the samples were boiled for 10 min. Proteins were separated on 15% SDS-PAGE and blotted onto Polyvinylidene fluoride (Immobilon-P, MIllipore) membranes. Secondary immunoglobulins conjugated to horseradish peroxidase were visualized using ECL Plus (GE Healthcare). Primary antibodies against PR1 (αPR1) were described previously [80] and αPR2 was obtained from Agrisera (#AS12 2366, ~35kDA). Incubation of both primary and secondary antibodies were done in Tris-buffered saline with 0.05% Tween-20 (TBST) followed by three rinses of 10 minutes in TBS. Equal protein loading was confirmed for the samples by Ponceau S staining of the membranes and when needed the loaded total protein amounts were standardized using BCA protein analysis on the total protein extracts prior to protein loading of the gels. The primary antibodies αPR1, αPR2 and the secondary antibody Goat-anti-Rabbit HRP (Fisher) were used at 1:5000, 1:2000 and 1:5000 dilutions, respectively.
Quantitative gene expression analysis
For the gene expression analysis, total RNA was extracted from 100–200 mg of leaf material of 5-week-old plants grown at 22/28°C using TRIzol LS reagent (Fisher). The RNA was treated with DNase (ThermoFisher) according to the supplier’s protocol and RNA concentrations were determined by measuring the Abs(260) on a Nanodrop. cDNA was synthesised from 1 μg total RNA using RevertAid H reverse transcriptase in the presence of the RNAse inhibitor Ribolock (both ThermoFisher) following the supplier’s protocol. All biological samples were measured in technical replicate with 3–4 biological replicates per experiment. The PCR amplification was followed using Hot FIREPol EvaGreen qPCR (Solis Biodyne) in a QuantoStudio3 (ThermoFisher). Gene expression was normalized using two genes: Actin2 (At3g18780) and beta-Tub4 (At5g44340). The primers used are given in the S2 Table. The Ct values were corrected for primer efficiencies. All expression data were analysed using the pipeline in qBASE+ (Biogazelle).
Hypocotyl elongation measurements
Cold-stratified (3 days at 4°C) sterilized seeds (~50 per line) were placed on vertical plates with 1/2 MS medium supplemented with 1% w/v sucrose and 1% w/v Daishin agar (Duchefa). Seeds were irradiated with white light for 6 hrs to promote germination and then incubated in the specified light/temperature conditions for 5 days. The used seeds were fresh and from the same seed harvest. Seedlings were scanned and the hypocotyl lengths were measured using ImageJ (http://rsb.info.nih.gove/ij). Sensitivity to the Gibberellin biosynthesis inhibitor Paclobutrazol (Pac, Duchefa) and the hormone Gibberellic acid (GA3, Duchefa) was analysed by growing the seedlings on 0.5 μM PAC or 10 μM GA3, respectively. Likewise, sensitivity to the Brassinosteroid biosynthesis inhibitor Propiconazole (PPZ, Sigma-Aldrich) or the hormone 24-epiBrassinolide (BL, #b1439, Sigma-aldrich) was analysed by adding 2 μM PPZ or 0.1 μM BL to the plates, respectively.
Pseudomonas disease assay
Pseudomonas syringae pv. tomato DC3000 (PstDC3000) [81] (carrying the empty vector pVSP61) was freshly grown overnight at 28°C with 200 rpm in 10 mL Kings B broth [82] supplemented with rifampicin (50 μg/mL) and kanamycin (40 μg/mL) to reach an OD600 of ~0.9–1.2. Directly prior to infiltration, the bacterial suspensions were spun down, washed with 10 mM MgSO4, and resuspended at OD600 = 0.0002 (1×105 CFU/mL) in 10 mM MgSO4 for syringe leaf infiltrations. For the Pst disease assays the plants were germinated and grown at 28°C constant temperature (with 11L/13D) for 5 weeks in soil. Twenty-four hours prior to inoculation (9:00 am), one batch of plants was moved to 22°C (SD) and both plant sets were placed in propagators to increase humidity (>90%). The two plant batches were simultaneously infiltrated at 22°C and 28°C using the same bacterial suspension. Upon infiltration the plants were left to dry for 1.5–2 hrs after which they were again covered with lids for 72 hours to increase humidity (>90% relative humidity). Humidity and temperature was followed using a data logger inside the propagators for the duration of the experiment. Leaf discs were taken 1 hour after dipping (t = 0) at both temperatures and 72 hrs post infiltration (t = 3). At least 6 plants were infiltrated per condition. In total 8 samples were taken for each condition combining 2–3 leaf discs with a diameter of 5 mm. Leaf discs were taken from different leaves and only ‘mature’ fully elongated rosette leaves were sampled. The first-formed round shaped leaves were excluded from tissue sampling. The sampled intact leaves were surface-sterilized prior to taking leaf discs (10 sec dip in 70% ethanol followed by two washes with sterile water). The disease assays were performed with at least two independent replicates with similar results.
Cell death analyses using trypan blue staining
The rosette leaves were stained with a 1:1 mixture (v/v) of ethanol and lactic acid–phenol–trypan blue solution (2.5 mg mL−1 trypan blue, 25% v/v lactic acid, 25% phenol, 25% glycerol, and water) and boiled for 5 min. For destaining, the trypan blue solution was replaced with a chloral hydrate solution (2.5 g mL−1 in water), as described [83].
Microarray gene expression analysis
The siz1 pad4, sumo1/2KD pad4, and pad4 plants were grown on soil in SD conditions at 22°C for 2 weeks and then transferred to 28°C at noon (t = 0). Leaf samples were taken in triplicate for total RNA extraction at t = 0, 24 hrs (1d), and 96 hrs (4d). Total RNA was purified using the RNAeasy mini kit (QIAGEN). The RNA quality was examined by monitoring Abs(260/280) and the Abs(260/230) ratios. Total RNA (100 ng) was amplified using the GeneChip WT PLUS kit (Affymetrix) generating biotinylated sense-strand DNA targets. The labelled samples were hybridized to Arabidopsis Gene 1.1 ST arrays (Affymetrix). Washing, staining and scanning was performed using the GeneTitan Hybridization, wash, and stain kit for WT Array Plates, and the GeneTitan Instrument (both Affymetrix).
All arrays were subjected to a set of quality control checks, such as visual inspection of the scans, checking for spatial effects through pseudo-color plots, and inspection of pre- and post-normalized data with box plots, ratio-intensity plots and principal component analysis. Normalized expression values were calculated using the robust multi-array average (RMA) algorithm [84]. The experimental groups were contrasted to test for differential gene expression. Empirical Bayes test statistics were used for hypothesis testing [85] using the Limma package in R 3.2.1 (http://cran.r-project.org/), and all p-values were corrected for false discoveries according to Storey and Tibshirani [86]. Downstream statistical analyses (e.g. hypergeometric tests on enrichment) were performed in Python using the Scipy.stats module (https://scipy.org/scipylib/). The microarray data were deposited in Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/) under the accession number GSE97641 and Github (DEGs and scripts used to prepare Fig 5; https://github.com/LikeFokkens/Siz1_immunity-vs-growth_temperature).
Supporting information
(A) List of genes encoding TNLs, Receptor-like kinases (RLKs), and Receptor-like proteins (RLPs) genes whose expression is induced in siz1 pad4 compared to pad4 at 22°C, ranked by fold change. Up-regulation of these genes is SIZ1-dependent while independent of PAD4. Statistical significant differences are indicated with q values.
(B) Similar to (A). Brassinosteroid biosynthesis is possibly reduced in siz1 pad4. Expression of the genes DWF4, BR6OX2, BEE1, BEE3, and TCP1 is down-regulated in siz1 pad4 relative to pad4 at 22°C. DWF4 and BR6OX2 catalyse two rate-limiting reactions of brassinosteroid biosynthesis. BEE1, BEE3 and TCP1 are TFs involved in brassinosteroid signalling. Statistical significances differences are indicated with q values.
(DOC)
(DOC)
(DOC)
Normalized gene expression of the defence marker genes PR1 (A), PR2 (B) and SNC1 (C) using qRT-PCR (mean ± SE, Col-0 at 22°C = 1). RNA was isolated from 5-week-old plants. 3–4 biological replicates were measured in technical replicate. The experiment was repeated twice and the data combined. Experiment is part of the same set shown in Fig 2E.
(JPG)
The picture was taken using 6-week-old flowering plants.
(JPG)
(A) Loss-of-function mutants of RAR1, SGT1a and SGT1b were introduced in siz1-2 by crossing. The double mutants show less cell death than the siz1 single mutant. siz1 snc1-11 is included as neg. control (see Fig 2C). Leaves of 5-week-old plants were stained with Trypan blue. To quantify cell death the number of lesions was counted per leaf size area for each genotype. At least 10 images were counted per genotype. Statistical analyses were made using an unpaired two-sided student t-test (grey lines) with ns for p>0.05; * for p≤0.05; ** for p≤0.01 and *** for p≤0.001.
(B) Introduction of loss-of-function mutants of RAR1, SGT1a and SGT1b in siz1 hardly rescues the growth retardation of siz1. Rosette weight was taken from 5-week-old plants (n = 8).
(JPG)
Whereas hypocotyl growth is compromised in siz1 and sumo1/2KD, the mutants cpr1, bon1, and srfr1-4 show normal hypocotyl elongation at elevated temperature (both in a diurnal cycle and in dark conditions; 28C L and 28C D, respectively). Only srfr1-4 shows less hypocotyl elongation in dark conditions at 22°C (28C D). Seeds were germinated on plates at 22°C/28°C in SD (L) or dark (D) conditions. Hypocotyl length was measured 5 days post germination. Significant differences were determined using ANOVA followed by Tukey post-hoc test (****, p≤0.0001; ***, p≤0.001; ns, p>0.05; n = 40–43). All significant differences indicated are in comparison to Col-0 (control). The result shown was part of the experiment in Fig 4A and 4B. Experiment was repeated two times with similar results. Error bars indicate standard deviation.
(JPG)
(A) Picture of the rosettes of siz1 hy5-215, siz1 cop1-4 and the single mutants. Plants were grown for 5 weeks at 22°C or 28°C (SD). The double mutants adopted a similar morphology as siz1-2 at 22°C. while the growth retardation of siz1 hy5-215 partially recovered at 28°C albeit slightly less than siz1 alone. The rosette of siz1 cop1-4 remained as compact as cop1-4 alone without petiole elongation at 28°C, indicative of a compromised thermomorphogenesis response.
(B) Box-plot (middle bar = median, box limit = upper and lower quartile, extremes = Min and Max values) showing the rosette weight of the genotypes depicted in (A). Weight was taken from 5-week-old plants. Significant differences were detected using a two-way ANOVA with Tukey’s multiple comparisons test; the letters indicate significantly different groups (n = 8–10). The experiment was repeated twice times with similar result.
(JPG)
(A, B) Scatter plot showing the log2 fold change in expression of all DEGs (black spots) at the three time points in siz1 pad4 versus pad4 (identical to Fig 5C and 5D) for [day 1–0] and [day 4–0], respectively. The black line depicts a Pearson linear regression result on the DEGs with the 95% confidence interval indicated by the grey zone.
(C, D) Similar to (A, B) except that only the DEGs are shown that are also genomic targets for binding of PIF4 (red spots), BZR1 (blue spots) or ARF6 (green spots), top-to-bottom. The red, blue and green lines depict the Pearson linear regression analysis on these DEGs that are also genomic targets of these different TFs with the 95% confidence interval indicated by the red, blue or green zone.
(JPG)
(A, B) Scatter plot showing the log2 fold change in expression of all DEGs (black spots) at the three time points in sumo1/2KD pad4 versus pad4 (identical to Fig 5C and 5D) for [day 1–0] and [day 4–0], respectively. The black lines depict a linear Pearson regression analysis on the DEGs with the 95% confidence interval indicated by the grey zone.
(C, D) Similar to (A, B) except that only the DEGs are shown that are also genomic targets for binding of PIF4 (red spots), BZR1 (blue spots) or ARF6 (green spots), top-to-bottom. The red, blue and green lines depict a Pearson linear regression analysis on these DEGs that are also genomic targets of PIF4, BZR1 or ARF6, respectively, with the 95% confidence interval indicated by the red, blue or green zone.
(JPG)
(A, B) Scatter plot showing the log2 fold change in expression of all DEGs (black spots) at the three time points in siz1 pad4 versus pad4 (identical to Fig 5C and 5D) for [day 1–0] and [day 4–0], respectively. The black lines depict a Pearson linear regression analysis of the differentially expressed genes with the 95% confidence interval indicated by the grey zone.
(C, D) Similar to (A, B) except that only the DEGs are shown that are also a genomic target for binding of HY5 (purple spots). The purple line depicts a Pearson linear regression analysis on these DEGs that are also genomic targets of HY5, with the 95% confidence interval indicated by the purple zone.
(E, F) Similar to (A, B) except that only the DEGs are shown that encode for SAUR genes (yellow spots). These SAUR genes are clearly up-regulated in pad4 at day 1 and 4, but they fail to respond at day 1 and their response is irregular at 4 day in siz pad4. The yellow line depicts a Pearson linear regression analysis on these DEGs that encode SAURs with the 95% confidence interval indicated by the yellow zone.
(JPG)
(A, B) Scatter plot showing the log2 fold change in expression of all DEGs (black spots) at the three time points in sumo1/2KD pad4 versus pad4 (identical to Fig 5C and 5D) for [day 1–0] and [day 4–0], respectively. The black lines depict a Pearson linear regression analysis on the differentially expressed genes with the 95% confidence interval indicated by the grey zone.
(C, D) Similar to (A, B) except that only the DEGs are shown that are also a genomic target for binding of HY5 (purple spots). The purple line depicts a Pearson linear regression analysis on the DEGs that are also genomic targets of HY5, with the 95% confidence interval indicated by the purple zone.
(E, F) Similar to (A, B) except that only the DEGs are shown that encode for SAUR genes (yellow spots). The yellow line depicts a Pearson linear regression analysis on these DEGs that encode SAURs with the 95% confidence interval indicated by the yellow zone.
(JPG)
Acknowledgments
We would like to thank J. Ham for plant pheno- and genotyping. We are grateful to L. Tikovsky and H. Lemereis for taking care of our plants. We kindly acknowledge M. van Zanten (Utrecht University, Netherlands), J. Parker (MPI Plant Breeding, Cologne), W. Gassmann (University of Missouri, Columbia, MI), Jian Hua (Cornell University, Ithaca, NY), T. Wroblewski (UC Davids, CA) for providing materials. We would like to thank T. Helderman, M. van Hulten and M. Kwaaitaal for assisting with the disease assays.
Data Availability
All relevant data are within the paper and its Supporting Information files, except for the the microarray data, which were deposited in Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/) under the accession number GSE97641 and Github (https://github.com/LikeFokkens/Siz1_immunity-vs-growth_temperature).
Funding Statement
This work was supported by Vernieuwingsimpuls VIDI grant (864.10.004) from the Netherlands Scientific Organisation (NWO) awarded to HAvdB and VH and the Dutch Topsector program Horticulture and Starting Materials (HAvdB, SC, MAM). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Wigge PA (2013) Ambient temperature signalling in plants. Curr Opin Plant Biol 16: 661–6. doi: 10.1016/j.pbi.2013.08.004 [DOI] [PubMed] [Google Scholar]
- 2.Hua J (2013) Modulation of plant immunity by light, circadian rhythm, and temperature. Curr Opin Plant Biol 16: 406–13. doi: 10.1016/j.pbi.2013.06.017 [DOI] [PubMed] [Google Scholar]
- 3.Whitham S, McCormick S, Baker B (1996) The N gene of tobacco confers resistance to tobacco mosaic virus in transgenic tomato. Proc Natl Acad Sci USA 93: 8776–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng C, Gao XQ, Feng BM, Sheen J, Shan LB, et al. (2013) Plant immune response to pathogens differs with changing temperatures. Nat Commun 4: 2530 doi: 10.1038/ncomms3530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menna A, Nguyen D, Guttman DS, Desveaux D (2015) Elevated temperature differentially influences effector-triggered immunity outputs in Arabidopsis. Front Plant Sci 6: 995 doi: 10.3389/fpls.2015.00995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Bao ZL, Zhu Y, Hua J (2009) Analysis of temperature modulation of plant defense against biotrophic microbes. Mol Plant Microbe Interact 22: 498–506. doi: 10.1094/MPMI-22-5-0498 [DOI] [PubMed] [Google Scholar]
- 7.Zhu Y, Qian W, Hua J (2010) Temperature modulates plant defense responses through NB-LRR proteins. PLoS Pathog 6: e1000844 doi: 10.1371/journal.ppat.1000844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erickson FL, Holzberg S, Calderon-Urrea A, Handley V, Axtell M, et al. (1999) The helicase domain of the TMV replicase proteins induces the N-mediated defence response in tobacco. 18: 67–75. [DOI] [PubMed] [Google Scholar]
- 9.Heidrich K, Tsuda K, Blanvillain-Baufume S, Wirthmueller L, Bautor J, et al. (2013) Arabidopsis TNL-WRKY domain receptor RRS1 contributes to temperature-conditioned RPS4 auto-immunity. Front Plant Sci 4: doi: 10.3389/fpls.2013.00403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang S, Hua J (2004) A haplotype-specific resistance gene regulated by BONZAI1 mediates temperature-dependent growth control in Arabidopsis. Plant Cell 16: 1060–71. doi: 10.1105/tpc.020479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng YT, Li YZ, Huang SA, Huang Y, Dong XN, et al. (2011) Stability of plant immune-receptor resistance proteins is controlled by SKP1-CULLIN1-F-box (SCF)-mediated protein degradation. Proc Natl Acad Sci USA 108: 14694–9. doi: 10.1073/pnas.1105685108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mang HG, Qian WQ, Zhu Y, Qian J, Kang HG, et al. (2012) Abscisic aAcid deficiency antagonizes high-temperature inhibition of disease resistance through enhancing nuclear accumulation of resistance proteins SNC1 and RPS4 in Arabidopsis. Plant Cell 24: 1271–84. doi: 10.1105/tpc.112.096198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gou M, Hua J (2012) Complex regulation of an R gene SNC1 revealed by auto-immune mutants. Plant Signal Behav 7: 213–6. doi: 10.4161/psb.18884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li YQ, Yang SH, Yang HJ, Hua J (2007) The TIR-NB-LRR gene SNC1 is regulated at the transcript level by multiple factors. Mol Plant Microbe Interact 20: 1449–56. doi: 10.1094/MPMI-20-11-1449 [DOI] [PubMed] [Google Scholar]
- 15.Kim SH, Gao F, Bhattacharjee S, Adiasor JA, Nam JC, et al. (2010) The Arabidopsis resistance-like gene SNC1 Is activated by mutations in SRFR1 and contributes to resistance to the bacterial Effector AvrRps4. PLoS Pathog 6: e1001172 doi: 10.1371/journal.ppat.1001172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li YZ, Li SX, Bi DL, Cheng YT, Li X, et al. (2010) SRFR1 negatively regulates plant NB-LRR resistance protein accumulation to prevent autoimmunity. PloS Pathog 6: e1001111 doi: 10.1371/journal.ppat.1001111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang S, Monaghan J, Zhong XH, Lin L, Sun TJ, et al. (2014) HSP90s are required for NLR immune receptor accumulation in Arabidopsis. Plant J 79: 427–39. doi: 10.1111/tpj.12573 [DOI] [PubMed] [Google Scholar]
- 18.Gou MY, Shi ZY, Zhu Y, Bao ZL, Wang GY, et al. (2012) The F-box protein CPR1/CPR30 negatively regulates R protein SNC1 accumulation. Plant J 69: 411–20. doi: 10.1111/j.1365-313X.2011.04799.x [DOI] [PubMed] [Google Scholar]
- 19.Zhang YL, Goritschnig S, Dong XN, Li X (2003) A gain-of-function mutation in a plant disease resistance gene leads to constitutive activation of downstream signal transduction pathways in suppressor of npr1-1, constitutive 1. Plant Cell 15: 2636–46. doi: 10.1105/tpc.015842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia AV, Blanvillain-Baufume S, Huibers RP, Wiermer M, Li G, et al. (2010) Balanced nuclear and cytoplasmic activities of EDS1 are required for a complete plant innate immune response. PLoS Pathog 6: e1000970 doi: 10.1371/journal.ppat.1000970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiermer M, Feys BJ, Parker JE (2005) Plant immunity: the EDS1 regulatory node. Curr Opin Plant Biol 8: 383–9. doi: 10.1016/j.pbi.2005.05.010 [DOI] [PubMed] [Google Scholar]
- 22.Heidrich K, Wirthmueller L, Tasset C, Pouzet C, Deslandes L, et al. (2011) Arabidopsis EDS1 connects pathogen effector recognition to cell compartment-specific immune responses. Science 334: 1401–4. doi: 10.1126/science.1211641 [DOI] [PubMed] [Google Scholar]
- 23.Wagner S, Stuttmann J, Rietz S, Guerois R, Brunstein E, et al. (2013) Structural basis for signaling by exclusive EDS1 heteromeric complexes with SAG101 or PAD4 in plant innate immunity. Cell Host Microbe 14: 619–30. doi: 10.1016/j.chom.2013.11.006 [DOI] [PubMed] [Google Scholar]
- 24.Gangappa SN, Berriri S, Kumar SV (2017) PIF4 coordinates thermosensory growth and immunity in Arabidopsis. Curr Biol 27: 243–9. doi: 10.1016/j.cub.2016.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quint M, Delker C, Franklin KA, Wigge PA, Halliday KJ, et al. (2016) Molecular and genetic control of plant thermomorphogenesis. Nat Plants 2: 15190 doi: 10.1038/nplants.2015.190 [DOI] [PubMed] [Google Scholar]
- 26.Hoecker U (2017) The activities of the E3 ubiquitin ligase COP1/SPA, a key repressor in light signaling. Curr Opin Plant Biol 37: 63–9. doi: 10.1016/j.pbi.2017.03.015 [DOI] [PubMed] [Google Scholar]
- 27.Park YJ, Lee HJ, Ha JH, Kim JY, Park CM (2017) COP1 conveys warm temperature information to hypocotyl thermomorphogenesis. 215: 269–80. doi: 10.1111/nph.14581 [DOI] [PubMed] [Google Scholar]
- 28.Lee J, Nam J, Park HC, Na G, Miura K, et al. (2007) Salicylic acid-mediated innate immunity in Arabidopsis is regulated by SIZ1 SUMO E3 ligase. Plant J 49: 79–90. doi: 10.1111/j.1365-313X.2006.02947.x [DOI] [PubMed] [Google Scholar]
- 29.Van den Burg HA, Kini RK, Schuurink RC, Takken FLW (2010) Arabidopsis Small Ubiquitin-like Modifier paralogs have distinct functions in development and defense. Plant Cell 22: 1998–2016. doi: 10.1105/tpc.109.070961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheong MS, Park HC, Hong MJ, Lee J, Choi W, et al. (2009) Specific domain structures control abscisic acid-, salicylic acid-, and stress-mediated SIZ1 phenotypes. Plant Physiol 151: 1930–42. doi: 10.1104/pp.109.143719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conti L, Nelis S, Zhang CJ, Woodcock A, Swarup R, et al. (2014) Small Ubiquitin-like Modifier protein SUMO enables plants to control growth independently of the phytohormone Gibberellin. Dev Cell 28: 102–10. doi: 10.1016/j.devcel.2013.12.004 [DOI] [PubMed] [Google Scholar]
- 32.Sadanandom A, Adam E, Orosa B, Viczian A, Klose C, et al. (2015) SUMOylation of phytochrome-B negatively regulates light-induced signaling in Arabidopsis thaliana. Proc Natl Acad Sci USA 112: 11108–13. doi: 10.1073/pnas.1415260112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crozet P, Margalha L, Butowt R, Fernandes N, Elias CA, et al. (2016) SUMOylation represses SnRK1 signaling in Arabidopsis. Plant J 85: 120–33. doi: 10.1111/tpj.13096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim JY, Jang IC, Seo HS (2016) COP1 controls abiotic stress responses by modulating AtSIZ1 function through its E3 Ubiquitin ligase activity. Front Plant Sci 7: 1182 doi: 10.3389/fpls.2016.01182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin XL, Niu D, Hu ZL, Kim DH, Jin YH, et al. (2016) An Arabidopsis SUMO E3 ligase, SIZ1, negatively regulates photomorphogenesis by promoting COP1 activity. PLoS Genet 12: e1006016 doi: 10.1371/journal.pgen.1006016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jirage D, Zhou N, Cooper B, Clarke JD, Dong XN, et al. (2001) Constitutive salicylic acid-dependent signaling in cpr1 and cpr6 mutants requires PAD4. Plant J 26: 395–407. [DOI] [PubMed] [Google Scholar]
- 37.Bhattacharjee S, Halane MK, Kim SH, Gassmann W (2011) Pathogen effectors target Arabidopsis EDS1 and alter its interactions with immune regulators. 334: 1405–8. doi: 10.1126/science.1211592 [DOI] [PubMed] [Google Scholar]
- 38.Stuttmann J, Peine N, Garcia AV, Wagner C, Choudhury SR, et al. (2016) Arabidopsis thaliana DM2h (R8) within the Landsberg RPP1-like Resistance Locus Underlies Three Different Cases of EDS1-Conditioned Autoimmunity. 12: e1005990 doi: 10.1371/journal.pgen.1005990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ishida T, Yoshimura M, Miura K, Sugimoto K (2012) MMS21/HPY2 and SIZ1, two Arabidopsis SUMO E3 ligases, have distinct functions in development. PLoS One 7: e46897 doi: 10.1371/journal.pone.0046897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wildermuth MC, Dewdney J, Wu G, Ausubel FM (2001) Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 414: 562–5. doi: 10.1038/35107108 [DOI] [PubMed] [Google Scholar]
- 41.Brodersen P, Malinovsky FG, Hematy K, Newman MA, Mundy J (2005) The role of salicylic acid in the induction of cell death in Arabidopsis acd11. Plant Physiol 138: 1037–45. doi: 10.1104/pp.105.059303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stokes TL, Kunkel BN, Richards EJ (2002) Epigenetic variation in Arabidopsis disease resistance. Genes Dev 16: 171–82. doi: 10.1101/gad.952102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller MJ, Barrett-Wilt GA, Hua Z, Vierstra RD (2010) Proteomic analyses identify a diverse array of nuclear processes affected by small ubiquitin-like modifier conjugation in Arabidopsis. Proc Natl Acad Sci USA 107: 16512–7. doi: 10.1073/pnas.1004181107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller MJ, Scalf M, Rytz TC, Hubler SL, Smith LM, et al. (2013) Quantitative proteomics reveals factors regulating RNA biology as dynamic targets of stress-induced SUMOylation in Arabidopsis. Mol Cell Proteomics 12: 449–63. doi: 10.1074/mcp.M112.025056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McNellis TW, von Arnim AG, Araki T, Komeda Y, Misera S, et al. (1994) Genetic and molecular analysis of an allelic series of cop1 mutants suggests functional roles for the multiple protein domains. Plant Cell 6: 487–500. doi: 10.1105/tpc.6.4.487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stavang JA, Gallego-Bartolome J, Gomez MD, Yoshida S, Asami T, et al. (2009) Hormonal regulation of temperature-induced growth in Arabidopsis. Plant J 60: 589–601. doi: 10.1111/j.1365-313X.2009.03983.x [DOI] [PubMed] [Google Scholar]
- 47.Fu X, Richards DE, Fleck B, Xie D, Burton N, et al. (2004) The Arabidopsis mutant sleepy1gar2-1 protein promotes plant growth by increasing the affinity of the SCFSLY1 E3 ubiquitin ligase for DELLA protein substrates. Plant Cell 16: 1406–18. doi: 10.1105/tpc.021386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li QF, Wang C, Jiang L, Li S, Sun SS, et al. (2012) An interaction between BZR1 and DELLAs mediates direct signaling crosstalk between brassinosteroids and gibberellins in Arabidopsis. Sci Signal 5: ra72 doi: 10.1126/scisignal.2002908 [DOI] [PubMed] [Google Scholar]
- 49.Li K, Yu R, Fan LM, Wei N, Chen H, et al. (2016) DELLA-mediated PIF degradation contributes to coordination of light and gibberellin signalling in Arabidopsis. Nat Commun 7: 11868 doi: 10.1038/ncomms11868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Catala R, Ouyang J, Abreu IA, Hu Y, Seo H, et al. (2007) The Arabidopsis E3 SUMO ligase SIZ1 regulates plant growth and drought responses. Plant Cell 19: 2952–66. doi: 10.1105/tpc.106.049981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Choe S, Fujioka S, Noguchi T, Takatsuto S, Yoshida S, et al. (2001) Overexpression of DWARF4 in the brassinosteroid biosynthetic pathway results in increased vegetative growth and seed yield in Arabidopsis. Plant J 26: 573–82. [DOI] [PubMed] [Google Scholar]
- 52.Choe SW, Dilkes BP, Fujioka S, Takatsuto S, Sakurai A, et al. (1998) The DWF4 gene of Arabidopsis encodes a cytochrome P450 that mediates multiple 22 alpha-hydroxylation steps in brassinosteroid biosynthesis. Plant Cell 10: 231–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chung Y, Choe S (2013) The regulation of Brassinosteroid biosynthesis in Arabidopsis. Crit Rev Plant Sci 32: 396–410. [Google Scholar]
- 54.Friedrichsen DM, Nemhauser J, Muramitsu T, Maloof JN, Alonso J, et al. (2002) Three redundant brassinosteroid early response genes encode putative bHLH transcription factors required for normal growth. Genetics 162: 1445–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guo ZX, Fujioka S, Blancaflor EB, Miao S, Gou XP, et al. (2010) TCP1 modulates Brassinosteroid biosynthesis by regulating the expression of the key biosynthetic Gene DWARF4 in Arabidopsis thaliana. Plant Cell 22: 1161–73. doi: 10.1105/tpc.109.069203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oh E, Zhu JY, Bai MY, Arenhart RA, Sun Y, et al. (2014) Cell elongation is regulated through a central circuit of interacting transcription factors in the Arabidopsis hypocotyl. Elife 3: e03031 doi: 10.7554/eLife.03031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oh E, Zhu JY, Wang ZY (2012) Interaction between BZR1 and PIF4 integrates brassinosteroid and environmental responses. Nat Cell Biol 14: 802–U64. doi: 10.1038/ncb2545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gangappa SN, Kumar SV (2017) DET1 and HY5 control PIF4-mediated thermosensory elongation growth through distinct mechanisms. Cell Rep 18: 344–51. doi: 10.1016/j.celrep.2016.12.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Toledo-Ortiz G, Johansson H, Lee KP, Bou-Torrent J, Stewart K, et al. (2014) The HY5-PIF regulatory module coordinates light and temperature control of photosynthetic gene transcription. PLoS Genet 10: doi: 10.1371/journal.pgen.1004416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Franklin KA, Lee SH, Patel D, Kumar SV, Spartz AK, et al. (2011) Phytochrome-interacting factor 4 (PIF4) regulates auxin biosynthesis at high temperature. Proc Natl Acad Sci USA 108: 20231–5. doi: 10.1073/pnas.1110682108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miura K, Lee J, Miura T, Hasegawa PM (2010) SIZ1 controls cell growth and plant development in Arabidopsis through salicylic acid. Plant Cell Physiol 51: 103–13. doi: 10.1093/pcp/pcp171 [DOI] [PubMed] [Google Scholar]
- 62.Zhu Z, Xu F, Zhang Y, Cheng YT, Wiermer M, et al. (2010) Arabidopsis resistance protein SNC1 activates immune responses through association with a transcriptional corepressor. Proc Natl Acad Sci U S A 107: 13960–5. doi: 10.1073/pnas.1002828107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang S, Yang H, Grisafi P, Sanchatjate S, Fink GR, et al. (2006) The BON/CPN gene family represses cell death and promotes cell growth in Arabidopsis. Plant J 45: 166–79. doi: 10.1111/j.1365-313X.2005.02585.x [DOI] [PubMed] [Google Scholar]
- 64.Huq E, Quail PH (2002) PIF4, a phytochrome-interacting bHLH factor, functions as a negative regulator of phytochrome B signaling in Arabidopsis. EMBO J 21: 2441–50. doi: 10.1093/emboj/21.10.2441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Koini MA, Alvey L, Allen T, Tilley CA, Harberd NP, et al. (2009) High temperature-medated adaptations in plant architecture require the bHLH transcription factor PIF4. Curr Biol 19: 408–13. doi: 10.1016/j.cub.2009.01.046 [DOI] [PubMed] [Google Scholar]
- 66.Nieto C, Lopez-Salmeron V, Daviere JM, Prat S (2015) ELF3-PIF4 interaction regulates plant growth independently of the Evening Complex. Curr Biol 25: 187–93. doi: 10.1016/j.cub.2014.10.070 [DOI] [PubMed] [Google Scholar]
- 67.Tan CM, Li MY, Yang PY, Chang SH, Ho YP, et al. (2015) Arabidopsis HFR1 Is a potential nuclear substrate regulated by the Xanthomonas type III effector XopD(Xcc8004). PloS ONE 10: e0117067 doi: 10.1371/journal.pone.0117067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ballesteros ML, Bolle C, Lois LM, Moore JM, Vielle-Calzada JP, et al. (2001) LAF1, a MYB transcription activator for phytochrome A signaling. Genes Dev 15: 2613–25. doi: 10.1101/gad.915001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mazur MJ, Spears BJ, Djajasaputra A, van der Gragt M, Vlachakis G, et al. (2017) Arabidopsis TCP transcription factors interact with the SUMO conjugating machinery in nuclear foci 8: 2043 doi: 10.3389/fpls.2017.02043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Khan M, Rozhon W, Unterholzner SJ, Chen T, Eremina M, et al. (2014) Interplay between phosphorylation and SUMOylation events determines CESTA protein fate in brassinosteroid signalling. Nat Commun 5: 4687 doi: 10.1038/ncomms5687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miura K, Rus A, Sharkhuu A, Yokoi S, Karthikeyan AS, et al. (2005) The Arabidopsis SUMO E3 ligase SIZ1 controls phosphate deficiency responses. Proc Natl Acad Sci USA 102: 7760–5. doi: 10.1073/pnas.0500778102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oyama T, Shimura Y, Okada K (1997) The Arabidopsis HY5 gene encodes a bZIP protein that regulates stimulus-induced development of root and hypocotyl. Gene Dev 11: 2983–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Glazebrook J, Zook M, Mert F, Kagan I, Rogers EE, et al. (1997) Phytoalexin-deficient mutants of Arabidopsis reveal that PAD4 encodes a regulatory factor and that four PAD genes contribute to downy mildew resistance. Genetics 146: 381–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bartsch M, Gobbato E, Bednarek P, Debey S, Schultze JL, et al. (2006) Salicylic acid-independent ENHANCED DISEASE SUSCEPTIBILITY1 signaling in Arabidopsis immunity and cell death is regulated by the monooxygenase FMO1 and the nudix hydrolase NUDT7. Plant Cell 18: 1038–51. doi: 10.1105/tpc.105.039982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Delaney TP, Uknes S, Vernooij B, Friedrich L, Weymann K, et al. (1994) A central role of salicylic-acid in plant-disease resistance. Science 266: 1247–50. doi: 10.1126/science.266.5188.1247 [DOI] [PubMed] [Google Scholar]
- 76.Gray WM, Muskett PR, Chuang HW, Parker JE (2003) Arabidopsis SGT1b is required for SCF(TIR1)-mediated auxin response. Plant Cell 15: 1310–9. doi: 10.1105/tpc.010884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tornero P, Merritt P, Sadanandom A, Shirasu K, Innes RW, et al. (2002) RAR1 and NDR1 contribute quantitatively to disease resistance in Arabidopsis, and their relative contributions are dependent on the R gene assayed. Plant Cell 14: 1005–15. doi: 10.1105/tpc.001032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leivar P, Monte E, Al-Sady B, Carle C, Storer A, et al. (2008) The Arabidopsis phytochrome-interacting factor PIF7, together with PIF3 and PIF4, regulates responses to prolonged red light by modulating phyB levels. Plant Cell 20: 337–52. doi: 10.1105/tpc.107.052142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hammoudi V, Vlachakis G, de Jonge R, Breit TM, van den Burg HA (2017) Genetic characterization of T-DNA insertions in the genome of the Arabidopsis thaliana sumo1/2 knock-down line. Plant Signal Behav 12: e1293216 doi: 10.1080/15592324.2017.1293216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.March-Diaz R, Garcia-Dominguez M, Lozano-Juste J, Leon J, Florencio FJ, et al. (2008) Histone H2A.Z and homologues of components of the SWR1 complex are required to control immunity in Arabidopsis. Plant J 53: 475–87. doi: 10.1111/j.1365-313X.2007.03361.x [DOI] [PubMed] [Google Scholar]
- 81.Cuppels DA (1986) Generation and Characterization of Tn5 Insertion mutations in Pseudomonas-syringae pv tomato. Appl Environ Microb 51: 323–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.King EO, Ward MK, Raney DE (1954) Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med 44: 301–7. [PubMed] [Google Scholar]
- 83.van Wees S (2008) Phenotypic analysis of Arabidopsis mutants: trypan blue stain for fungi, oomycetes, and dead plant cells. CSH Protoc 2008: pdb prot4982. [DOI] [PubMed] [Google Scholar]
- 84.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, et al. (2003) Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4: 249–64. doi: 10.1093/biostatistics/4.2.249 [DOI] [PubMed] [Google Scholar]
- 85.Smyth GK (2004) Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 3: Article3. doi: 10.2202/1544-6115.1027 [DOI] [PubMed] [Google Scholar]
- 86.Storey JD, Tibshirani R (2003) Statistical significance for genomewide studies. Proc Natl Acad Sci USA 100: 9440–5. doi: 10.1073/pnas.1530509100 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) List of genes encoding TNLs, Receptor-like kinases (RLKs), and Receptor-like proteins (RLPs) genes whose expression is induced in siz1 pad4 compared to pad4 at 22°C, ranked by fold change. Up-regulation of these genes is SIZ1-dependent while independent of PAD4. Statistical significant differences are indicated with q values.
(B) Similar to (A). Brassinosteroid biosynthesis is possibly reduced in siz1 pad4. Expression of the genes DWF4, BR6OX2, BEE1, BEE3, and TCP1 is down-regulated in siz1 pad4 relative to pad4 at 22°C. DWF4 and BR6OX2 catalyse two rate-limiting reactions of brassinosteroid biosynthesis. BEE1, BEE3 and TCP1 are TFs involved in brassinosteroid signalling. Statistical significances differences are indicated with q values.
(DOC)
(DOC)
(DOC)
Normalized gene expression of the defence marker genes PR1 (A), PR2 (B) and SNC1 (C) using qRT-PCR (mean ± SE, Col-0 at 22°C = 1). RNA was isolated from 5-week-old plants. 3–4 biological replicates were measured in technical replicate. The experiment was repeated twice and the data combined. Experiment is part of the same set shown in Fig 2E.
(JPG)
The picture was taken using 6-week-old flowering plants.
(JPG)
(A) Loss-of-function mutants of RAR1, SGT1a and SGT1b were introduced in siz1-2 by crossing. The double mutants show less cell death than the siz1 single mutant. siz1 snc1-11 is included as neg. control (see Fig 2C). Leaves of 5-week-old plants were stained with Trypan blue. To quantify cell death the number of lesions was counted per leaf size area for each genotype. At least 10 images were counted per genotype. Statistical analyses were made using an unpaired two-sided student t-test (grey lines) with ns for p>0.05; * for p≤0.05; ** for p≤0.01 and *** for p≤0.001.
(B) Introduction of loss-of-function mutants of RAR1, SGT1a and SGT1b in siz1 hardly rescues the growth retardation of siz1. Rosette weight was taken from 5-week-old plants (n = 8).
(JPG)
Whereas hypocotyl growth is compromised in siz1 and sumo1/2KD, the mutants cpr1, bon1, and srfr1-4 show normal hypocotyl elongation at elevated temperature (both in a diurnal cycle and in dark conditions; 28C L and 28C D, respectively). Only srfr1-4 shows less hypocotyl elongation in dark conditions at 22°C (28C D). Seeds were germinated on plates at 22°C/28°C in SD (L) or dark (D) conditions. Hypocotyl length was measured 5 days post germination. Significant differences were determined using ANOVA followed by Tukey post-hoc test (****, p≤0.0001; ***, p≤0.001; ns, p>0.05; n = 40–43). All significant differences indicated are in comparison to Col-0 (control). The result shown was part of the experiment in Fig 4A and 4B. Experiment was repeated two times with similar results. Error bars indicate standard deviation.
(JPG)
(A) Picture of the rosettes of siz1 hy5-215, siz1 cop1-4 and the single mutants. Plants were grown for 5 weeks at 22°C or 28°C (SD). The double mutants adopted a similar morphology as siz1-2 at 22°C. while the growth retardation of siz1 hy5-215 partially recovered at 28°C albeit slightly less than siz1 alone. The rosette of siz1 cop1-4 remained as compact as cop1-4 alone without petiole elongation at 28°C, indicative of a compromised thermomorphogenesis response.
(B) Box-plot (middle bar = median, box limit = upper and lower quartile, extremes = Min and Max values) showing the rosette weight of the genotypes depicted in (A). Weight was taken from 5-week-old plants. Significant differences were detected using a two-way ANOVA with Tukey’s multiple comparisons test; the letters indicate significantly different groups (n = 8–10). The experiment was repeated twice times with similar result.
(JPG)
(A, B) Scatter plot showing the log2 fold change in expression of all DEGs (black spots) at the three time points in siz1 pad4 versus pad4 (identical to Fig 5C and 5D) for [day 1–0] and [day 4–0], respectively. The black line depicts a Pearson linear regression result on the DEGs with the 95% confidence interval indicated by the grey zone.
(C, D) Similar to (A, B) except that only the DEGs are shown that are also genomic targets for binding of PIF4 (red spots), BZR1 (blue spots) or ARF6 (green spots), top-to-bottom. The red, blue and green lines depict the Pearson linear regression analysis on these DEGs that are also genomic targets of these different TFs with the 95% confidence interval indicated by the red, blue or green zone.
(JPG)
(A, B) Scatter plot showing the log2 fold change in expression of all DEGs (black spots) at the three time points in sumo1/2KD pad4 versus pad4 (identical to Fig 5C and 5D) for [day 1–0] and [day 4–0], respectively. The black lines depict a linear Pearson regression analysis on the DEGs with the 95% confidence interval indicated by the grey zone.
(C, D) Similar to (A, B) except that only the DEGs are shown that are also genomic targets for binding of PIF4 (red spots), BZR1 (blue spots) or ARF6 (green spots), top-to-bottom. The red, blue and green lines depict a Pearson linear regression analysis on these DEGs that are also genomic targets of PIF4, BZR1 or ARF6, respectively, with the 95% confidence interval indicated by the red, blue or green zone.
(JPG)
(A, B) Scatter plot showing the log2 fold change in expression of all DEGs (black spots) at the three time points in siz1 pad4 versus pad4 (identical to Fig 5C and 5D) for [day 1–0] and [day 4–0], respectively. The black lines depict a Pearson linear regression analysis of the differentially expressed genes with the 95% confidence interval indicated by the grey zone.
(C, D) Similar to (A, B) except that only the DEGs are shown that are also a genomic target for binding of HY5 (purple spots). The purple line depicts a Pearson linear regression analysis on these DEGs that are also genomic targets of HY5, with the 95% confidence interval indicated by the purple zone.
(E, F) Similar to (A, B) except that only the DEGs are shown that encode for SAUR genes (yellow spots). These SAUR genes are clearly up-regulated in pad4 at day 1 and 4, but they fail to respond at day 1 and their response is irregular at 4 day in siz pad4. The yellow line depicts a Pearson linear regression analysis on these DEGs that encode SAURs with the 95% confidence interval indicated by the yellow zone.
(JPG)
(A, B) Scatter plot showing the log2 fold change in expression of all DEGs (black spots) at the three time points in sumo1/2KD pad4 versus pad4 (identical to Fig 5C and 5D) for [day 1–0] and [day 4–0], respectively. The black lines depict a Pearson linear regression analysis on the differentially expressed genes with the 95% confidence interval indicated by the grey zone.
(C, D) Similar to (A, B) except that only the DEGs are shown that are also a genomic target for binding of HY5 (purple spots). The purple line depicts a Pearson linear regression analysis on the DEGs that are also genomic targets of HY5, with the 95% confidence interval indicated by the purple zone.
(E, F) Similar to (A, B) except that only the DEGs are shown that encode for SAUR genes (yellow spots). The yellow line depicts a Pearson linear regression analysis on these DEGs that encode SAURs with the 95% confidence interval indicated by the yellow zone.
(JPG)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files, except for the the microarray data, which were deposited in Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/) under the accession number GSE97641 and Github (https://github.com/LikeFokkens/Siz1_immunity-vs-growth_temperature).