Abstract
The idea that interconnected neuronal ensembles code for specific behaviors has been around for decades; however, recent technical improvements allow studying these networks and their causal role in initiating and maintaining behavior. In particular, the role of ensembles in drug-seeking behaviors in the context of addiction is being actively investigated. Concurrent with breakthroughs in quantifying ensembles, research has identified a role for synaptic glutamate spillover during relapse. In particular, the transient relapse-associated changes in glutamatergic synapses on accumbens neurons, as well as in adjacent astroglia and extracellular matrix, are key elements of the synaptic plasticity encoded by drug use and the metaplasticity induced by drug-associated cues that precipitate drug seeking behaviors. Here, we briefly review the recent discoveries related to ensembles in the addiction field, and then endeavor to link these discoveries with drug-induced striatal plasticity and cue-induced metaplasticity towards deeper neurobiological understandings of drug-seeking.
Keywords: neuronal ensembles, cocaine self-administration, cued-reinstatement, nucleus accumbens, glutamate, synaptic plasticity, synaptic potentiation, spines
1. Introduction: ensembles in addiction
According to classic theory, neuronal networks adapt during brain plasticity, modifying firing probability within the network (Josselyn et al., 2017, Hebb, 1949). As a result, all of the neurons included in a specific network will respond to the same stimulus. One of the first proofs of this theory was found in brain slices from the developing rat neocortex where measures of calcium signaling revealed functional domains formed by neurons activated in synchrony (Yuste et al., 1995, Yuste et al., 1992). Concurrently, ensemble coding for location was measured in the hippocampus of rats in vivo (Wilson and McNaughton, 1993). A distributed coding model was first applied to the nucleus accumbens (NAc) by Pennartz and colleagues (Pennartz et al., 1994), who implemented the theory to explain how a cue is associated with drug delivery. In this case, the cue induces activation of an interconnected network between the cortex, amygdala and thalamus that converge to activate a neuronal ensemble in the NAc to induce long-term potentiation (LTP). More than 10 years later, Hope and colleagues established a causal link between an ensemble of neurons selectively activated by drugs, drug-associated cues and context and the expression of cocaine-induced behavioral sensitization (Koya et al., 2009). The researchers made use of the specific pattern of neuronal activation of the immediate early gene c-fos during expression of context-specific sensitization to cocaine (Mattson et al., 2008) and induced expression of a β–galactosidase reporter only in that c-fos defined ensemble, which represents a surprisingly low 2–3% of all NAc neurons. Subsequently, these investigators utilized the prodrug Daun02, which is converted to the Ca2+ reducing agent daunorubicin only in the neurons that express β–galactosidase. Critically, using this method of selectively lesioning the ensemble coding the context-drug association, context-specific locomotor sensitization was abolished (Koya et al., 2009). Moreover, animals expressing sensitization to cocaine formed silent synapses specifically in the neurons comprising the ensemble activated by cocaine (Koya et al., 2012). In a follow-up experiment, only animals receiving cocaine in a context dependent manner displayed sensitization to the context and had ensembles containing silent synapses, thus demonstrating that the ensemble selectively encodes the drug-context association (Whitaker et al., 2015).
This same approach has also been used in an increasing number of self-administration models to show the role of specific ensembles in operant responding for drugs. In addition to cocaine sensitization, context induced reinstatement of cocaine seeking is also driven by a selective NAc ensemble that consists largely of medium spiny neurons and parvalbumin positive interneurons (Cruz et al., 2014a). Furthermore, ensembles in the ventral medial prefrontal cortex (mPFC) and orbitofrontal cortex (OFC) were shown to encode the context-induced operant responding for heroin during relapse and after extended abstinence, respectively (Bossert et al., 2011, Fanous et al., 2012). Additionally, ensembles in the dorsal striatum were linked to voluntary abstinence from methamphetamine taking (Caprioli et al., 2017), ensembles in the central amygdala to craving alcohol and nicotine during abstinence (Funk et al., 2016, de Guglielmo et al., 2016), and ventral mPFC ensembles were found to suppress ethanol seeking and drive or inhibit seeking of natural rewards depending on environmental contingencies (Pfarr et al., 2015, Suto et al., 2016, Warren et al., 2016). Importantly, all these studies report a very small number of activated neurons in a given brain region (e.g., <5%), indicating that highly specific addictive behaviors are regulated by small ensembles of neurons throughout the brain.
An interesting question is whether neuronal networks activated by cocaine are specific for drug-related behavior or whether they overlap with other engrams, such as those inducing behaviors driven by natural rewards. By studying the pattern of phasic neuronal firing in MSNs in the NAc, it was found that the selective encoding of natural rewards (food, water or sucrose) involves largely distinct cells from those encoding cocaine, with only ~20% of neurons responding to both cocaine and sucrose or water reinforcements (Cameron and Carelli, 2012, Carelli et al., 2000). Remarkably, convergent findings using different biomarkers reveal that ~2–5% of cells encode a putative cocaine engram, including deltaFosB immune-labeling (Mattson et al., 2008), Daun02-inactivation of cFos expressing neurons (Koya et al., 2009), and the number of NAc MSNs exhibiting phasic activity NAc MSNs (Cameron and Carelli, 2012). Furthermore, the same relative proportion of NAc cells in the cocaine engram is reported between passive drug-context associations during sensitization and active cocaine self-administration and reinstatement (Koya, 2009; Cruz, 2012; Cameron, 2012).
2. Constitutive changes induced by drugs of abuse
Abused drugs share the property of modifying the extracellular levels of three behaviorally important monoamines: noradrenalin, serotonin and dopamine. This modulation is achieved by either blocking neurotransmitter plasmalemmal transporters (e.g. psychostimulants (Balster and Schuster, 1973, Crespi et al., 1997)) or via disinhibition of synaptic release (e.g. opioids (Khachaturian and Watson, 1982)). However, the release of dopamine in the NAc by all drugs of abuse (Di Chiara and Imperato, 1988) and the role of dopamine in reward prediction (Schultz, 1998) has placed dopamine as the most studied monoamine neuromodulator in the addiction field. Thus, dopamine release in the NAc and limbic cortical and allocortical regions is a necessary event for drug-mediated reward, and has therefore been proposed to be a necessary event in establishing learned associations between the rewarding effects of addictive drugs and the environment (Pascoli et al., 2015). Acute dopamine release during drug use reinforces learned associations between the environment and drug to the extent that the environmental associations become provocateurs for initiating drug seeking and relapse. While acute dopamine release by drug-associated cues and context can contribute to initiating drug seeking (Phillips et al., 2003, McFarland and Kalivas, 2001, McGlinchey et al., 2016, See et al., 2001), particularly in limbic cortical and allocortical regions, the constitutive synaptic plasticity and transient metaplasticity that creates the high level of motivation to obtain drugs relative to other natural rewards has been demonstrated most convincingly at glutamatergic synapses in the NAc (Kalivas, 2009).
Morphological (Anderson and Self, 2017) and functional (Luscher, 2013, Luscher and Malenka, 2011, Kourrich et al., 2015) changes induced by chronic exposure to drugs of abuse have been extensively studied (Mulholland et al., 2016), and in Table 1 we have assembled the major constitutive changes induced in the NAc by cocaine and heroin, two drugs actively being investigated at the engram level. Remarkably, different drug types induce distinct constitutive modifications, such as opposing changes in spine head diameter and AMPA currents. However, both drug classes share a constitutive down-regulation of the astroglial glutamate transporter, GLT-1, and a retraction of glial end feet from NAc synapses (Scofield et al., 2016). Given the overlap in the drug-seeking endophenotype produced by self-administration of opioids and psychostimulants, and the shared vulnerability to relapse in addicts, one interpretation is that the enduring synaptic changes in MSNs that are not shared between drug classes may be less important mediators of relapse than the shared changes in astroglia. In this respect, it would be particularly interesting to look specifically at astrocytes surrounding behaviorally relevant ensembles. We will describe in detail below how dysregulation of glutamate homeostasis in the core region of the NAc (NAcore) induces drug seeking and the findings suggesting that a transient potentiation of glutamatergic synapses might be the common denominator driving seeking and relapse behaviors.
Table 1.
Constitutive changes in glutamate transmission induced by chronic exposure to cocaine and heroin/morphine
| Morphological changes | Functional changes | |||
|---|---|---|---|---|
| Spine Head Diameter | A:N Ratio | Plasticity | Glutamate Transporter | |
| Cocaine | ↑head diameter1, longevity of the changes controversial, see2 | ↑3 | ↓ LTD4 ↓ LTP5 | ↓ GLT-1 function6 |
| Heroin/Morphine | ↓ head diameter7 | ↓8 | ↓ LTD/LTP9 | ↓ GLT-1 function10 |
References:
3. Glutamate spillover and transient synaptic plasticity (t-SP), common to all drugs of abuse
Glutamate release is increased in the NAc of cocaine-sensitized animals in response to a cocaine challenge (Pierce et al., 1996) and following presentation of a cue paired with non-contingent cocaine exposure (Hotsenpiller et al., 2001). A large body of work has established that elevated synaptic glutamate spillover from prelimbic cortical afferents is measured in the accumbens during drug-seeking for cocaine, heroin, alcohol or nicotine, but not sucrose seeking (McFarland et al., 2003, LaLumiere and Kalivas, 2008, Gass et al., 2011, Gipson et al., 2013b). Under normal conditions, synaptic glutamate spillover is minimized by the patterned expression of the glial glutamate transporter GLT-1 on astroglial end feet adjacent to the synaptic cleft. Thus, GLT-1 strongly controls basal extracellular glutamate by negatively regulating synaptic glutamate spillover. However, after cocaine, heroin, alcohol and nicotine self-administration, GLT-1 expression and function are decreased (Knackstedt et al., 2009, Knackstedt et al., 2010, Gipson et al., 2013b, Shen et al., 2014b, Sari et al., 2011, Ducret et al., 2015).
The strong association between relapse in animal models and extracellular glutamate levels in the NAc across drug classes (cocaine, heroin, nicotine and alcohol) initiated many studies to understand the cellular mechanisms regulating extracellular glutamate levels and how these might be regulated by using addictive drugs. In addition to GLT-1, cocaine, but not other addictive drugs, reduces the cystineglutamate exchanger (Baker et al., 2003). Cystine-glutamate exchange involves a one-to-one stoichiometric exchange of intracellular glutamate for extracellular cystine and is rate-limiting in the synthesis of glutathione (GSH) (Uys et al., 2011). Also, a variety of addictive drugs alter signaling through presynaptic metabotropic glutamate 2/3 autoreceptors (mGluR2/3) with a net result of increasing synaptic glutamate release probability in the NAc. Taken together, addictive drug use produces enduring changes in key proteins or signaling cascades that result in a net increase in synaptic glutamate spillover, with the most widely shared adaptation being down-regulated GLT-1 (Kalivas, 2009) (Figure 1). Accordingly, compounds that negatively regulate glutamate spillover reduce drug seeking in animal models of relapse. Thus, stimulating mGluR2/3, to increase inhibitory presynaptic autoreceptor tone decreases cocaine, heroin and ethanol seeking (Peters and Kalivas, 2006, Baptista et al., 2004, Zhao et al., 2006, Bossert et al., 2006). Stimulation of mGluR2/3 by glial glutamate release in the NAcore also reduces cued-induced cocaine reinstatement (Scofield et al., 2015). The antibiotic ceftriaxone restores the levels of GLT-1 and xCT (catalytic subunit of the cystine-glutamate exchanger) in NAc, and normalizes the density of glial end feet adjacent to synapses, thereby inhibiting cue- and cocaine primed reinstated drug seeking (Knackstedt et al., 2010, Trantham-Davidson et al., 2012, Scofield et al., 2016), nicotine seeking (Alajaji et al., 2013) and alcohol-seeking (Weiland et al., 2015). Likewise, the acetylated amino acid N-acetylcysteine increases both xCT subunit and GLT-1 expression and prevents cocaine, nicotine, alcohol and heroin seeking (Baker et al., 2003, Zhou and Kalivas, 2008, Moussawi et al., 2009, Madayag et al., 2007, Murray et al., 2012, Ramirez-Nino et al., 2013). Using a selective protein knockdown strategy, the inhibition of cocaine reinstatement was shown to be mediated through the effect of N-acetylcysteine on GLT-1, not xCT (Reissner et al., 2015).
Figure 1.
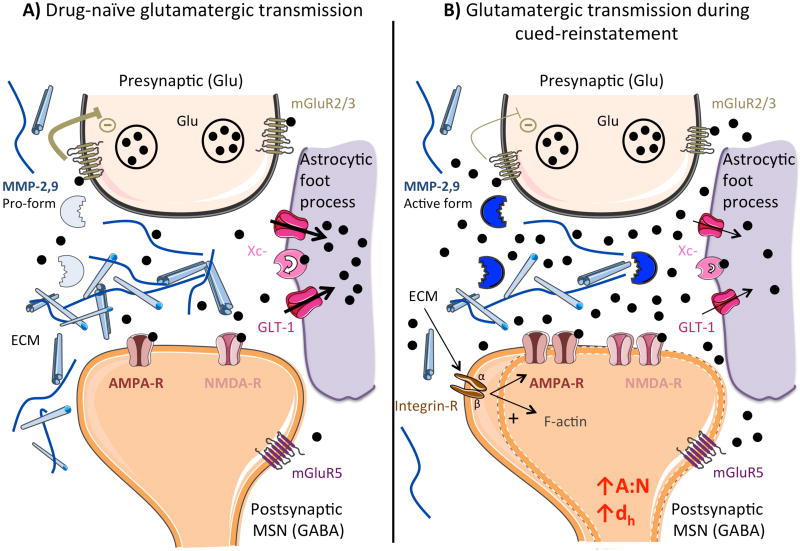
Model of the tetrapartite synapse and how it is altered after withdrawal from addictive drugs. A) Drug-naïve synaptic glutamate release probability is regulated by mGluR2/3 inhibitory autoreceptors, while the cystine-glutamate exchanger (Xc-) and glutamate transporter (GLT-1) expressed largely on astroglial cells, regulate the elimination of glutamate and determine how much glutamate spills out of the synapse. The extracellular matrix maintains synaptic structure and mediates synaptic plasticity by activating MMPs and signaling to the postsynapse via integrins. B) After drug self-administration and extinction training, presentation of the cues previously associated with the drug during acquisition of drug use induces a strong release of glutamate originating from prelimbic cortical afferents. Decreased function of mGluR2/3 and GLT-1, and withdrawal of astroglial end feet impair glutamate homeostasis and allow spillover of glutamate from the synaptic cleft. Extrasynaptic glutamate stimulates mGluR5 on nNOS interneurons (not shown), which activates matrix metalloproteases via nitrosylation. Catalytic signal transduction in the extracellular matrix by MMPs stimulates the expansion of postsynaptic spines and the insertion of AMPA receptors. AMPA-R: α-Amino-3-hydroxy-5-Methyl-4-isoxazole Propionic Acid Receptor
ECM: Extracellular Matrix GLT-1: Glutamate Transporter 1
Glu: Glutamate
MMP: Matrix Metalloprotease
MSN: Medium Spiny Neuron
NMDA-R: N-Methyl-D-Aspartate Receptor
Xc-: Cysteine/glutamate exchanger
At the post-synapse, another glutamate metabotropic receptor, mGluR5, also plays a role in drug-seeking, since its blockade though systemic or within the NAcore administration of antagonists prevents cued- and primed-reinstatement (Wang et al., 2013, Olive, 2009, Kenny and Markou, 2004). Since mGluR5 has predominantly perisynaptic localization, the efficacy of mGluR5 antagonists to inhibit drug seeking is hypothesized to arise from preventing the actions of synaptic glutamate spillover produced during a drug seeking event, and at least for cocaine reinstatement there is a critical involvement of mGluR5 expressed on accumbens interneurons that selectively express neuronal nitric oxide synthase (nNOS) (Smith et al., 2017).
Glutamate spillover into the NAcore during drug or cue-induced reinstatement is paralleled by transient synaptic potentiation (t-SP) of medium spiny neurons. This pairing has been shown with many addictive drugs, including cocaine (Gipson et al., 2013a), heroin (Shen et al., 2011) and nicotine (Gipson et al., 2013b). t-SP is a transitory event that is measured by two main biomarkers of synaptic potentiation, i.e. (i) increases in spine head diameter and (ii) the AMPA/NMDA (A/N) ratio, an index of the strength of AMPA receptor-mediated transmission, at the excitatory synapses in the NAcore during the first 15 min minutes of cue-induced reinstatement (Gipson et al., 2014). The increase in either A/N ratio or spine head diameter positively correlates with the intensity of cue-reinstated active lever pressing and, akin to synaptic glutamate spillover, is not seen during cue-induced sucrose seeking (Gipson et al., 2013a). Matrix metalloproteases (MMPs), a family of enzymes acting in the extracellular matrix, are essential to the induction of transient synaptic plasticity (Smith et al., 2014, Smith et al., 2015). In particular, activity of the gelatinase family of MMPs, MMP-2 and -9, is transiently increased during reinstatement induced by cues previously paired with cocaine, nicotine and heroin, and inhibiting either MMP-2 or MMP-9 reduces the transient increases in both spine head diameter and A/N ratio, as well as cue-induced cocaine or heroin reinstatement (Smith et al., 2014). Moreover, intra-ventricular microinjection of a nonspecific MMP antagonist reduces cue-induced reinstatement of heroin seeking (Van den Oever et al., 2010). MMPs also appear to also be important in the transition to escalated ethanol self-administration (Smith et al., 2011), and MMP-9’s role in spine remodeling was shown during electrically-induced long-term potentiation in the hippocampus (Wang et al., 2008, Huntley, 2012). MMP activity signals to cells by catalytically creating ligands that bind to membrane receptors. The membrane receptor targets of MMP catalytic products that are required for t-SP are unknown, but one probable target is the adhesion molecule integrin. The β3 subunit of integrin is upregulated in the NAcore after cocaine self-administration and extinction (Wiggins et al., 2011), and modulating integrin receptor activation with RGD motif microinjections, a peptide ligand mimicking ECM binding, prevents ECM binding to integrin receptors and inhibits cocaine primed-reinstatement (Wiggins et al., 2011). Integrin stimulation induces activation of several kinases, among which, integrin-linked kinase (ILK) and the focal adhesion kinase (FAK) (Niu and Chen, 2011) promote filamentous actin, a cytoskeletal protein critical for spine remodeling (Ghatak et al., 2013). Finally, we recently found that antisense knock-down of the β3 subunit inhibits t-SP and reinstated cocaine seeking (Constanza Garcia-Keller and Peter Kalivas, unpublished observations).
Regardless of the signaling involved, MMP-2,9 activation is necessary to allow spine restructuring and t-SP in the NAcore initiated during cue-induced reinstatement (Smith et al., 2014). These results led to the idea of a critical role for the tetrapartite synapse in the NAcore in addiction-related mechanisms, including the canonical pre- and post-synaptic elements, astroglial end feet adjacent to the synaptic cleft and the ECM surrounding the synapse, which is catalytically regulated by MMPs (Smith et al., 2015, Dityatev and Rusakov, 2011, Mulholland et al., 2016) (Figure 1).
In conclusion, there is strong evidence showing that presenting cues previously paired with drugs during self-administration induces release of glutamate from prelimbic cortex afferents in the NAcore. Due to drug-induced impairments of glutamate homeostasis (a combination of one or more: decrease of mGluR2/3 function, decrease in cysteine/glutamate exchanger, and/or GLT-1 expression, and withdrawal of astroglial end feet from the synapse), glutamate release is not as tightly restricted to the synaptic cleft, and spills more readily into the extracellular space. Given the capacity of mGluR5 antagonists in the NAcore to prevent reinstated drug seeking (Olive, 2009) and the location of these receptors largely outside of the synaptic cleft (Mitrano and Smith, 2007), mGluR5 is a likely target of glutamate spillover. Recently it was shown that cue-induced cocaine seeking is critically dependent on mGluR5 located on a small population of NAc interneurons expressing nNOS, and that mGluR5 stimulation of nitric oxide production activates MMP-2,9 via nitrosylation (Smith et al., 2017). As described above, activated MMP-2,9 signals transient potentiation in NAcore MSNs. Below we discuss how the induction of t-SP might expand the ensemble of neurons in the NAc that code cue-induced drug seeking, and thereby cause drug-associated cues to be more potent behavioral motivators than natural rewards.
4. Could the t-SP be embedded in a neuronal network specific to drug seeking?
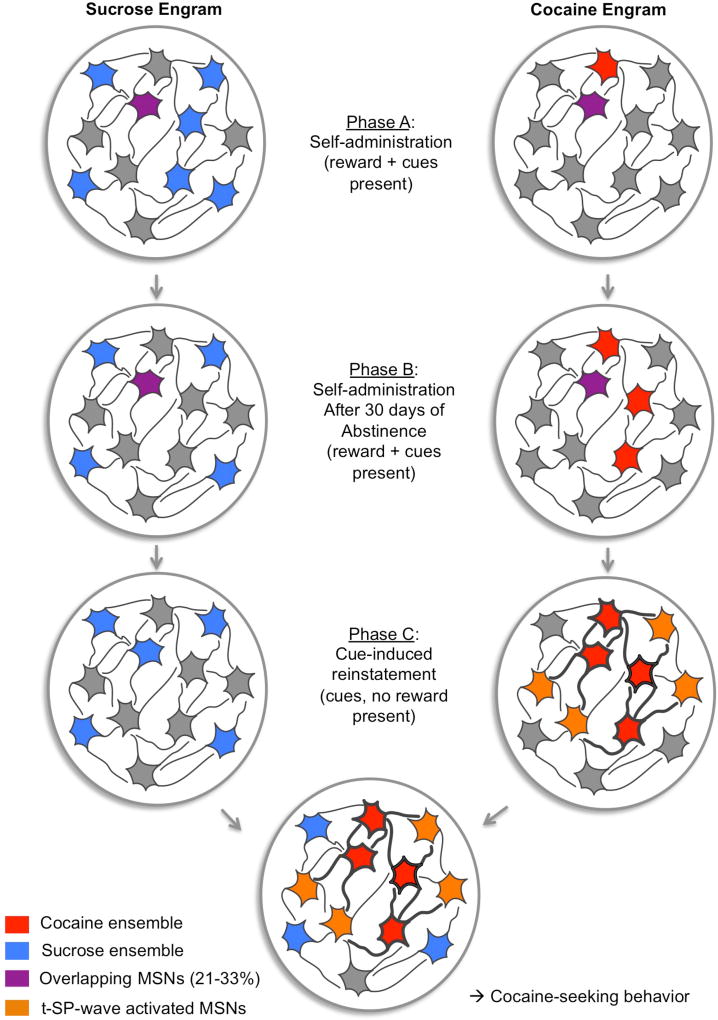
The results described above on how t-SP drives drug-seeking do not distinguish subpopulations of MSNs in the NAcore, and in particular do not specifically identify an engram activated by reinstated drug-seeking. Indeed, the A/N ratio and spine density measurements to date have been made indiscriminately from all the MSNs in the NAcore (Gipson et al., 2013a). Interestingly, according to the available studies on engrams and responses to drugs (Koya et al., 2009, Carelli et al., 2000), the percentage of neurons included in the engram responding to cocaine is estimated to be only 2–5% of NAc cells. It is surprising that a robust and reproducible potentiation of both A/N and spine diameter can be measured in a large number of neurons throughout the NAc, both inside and outside of the cocaine engram. The fact that t-SP occurs in neurons that are not specific to the engram could argue for a transient enlargement of the engram in the NAcore involved in linking the drug-associated cue with the operant drug seeking response (Figure 2). We hypothesize that the network (or engram) activated by drug-associated cues harbors synapses with poor glutamate homeostasis, resulting in the spillover of synaptically released glutamate. As a result, the original engram is enlarged during drug seeking through glutamate spillover inducing transient synaptic potentiation in near adjacent MSNs (represented as orange neurons in Figure 2). This transient “potentiation wave” serves to either prevent further activation of the network by stimuli signaling alternate behaviors, or more simply, by recruiting a larger ensemble of NAcore neurons for the drug cue initiated response (craving in humans), causing cues associated with biological stimuli that are not potentiated to become outcompeted.
Figure 2.
Schematic of the wave of engram recruitment in the NAcore induced by a cocaine cue. We hypothesize that the cocaine/cue-associated ensemble, formed during cocaine self-administration, undergoes transient synaptic potentiation (t-SP) that spreads through the NAcore due to NO production and activation of MMPs (see figure 1). The local recruitment of a larger number of MSNs reduces the size of the ensemble activated by a sucrose cue, thereby promoting drug seeking over sucrose seeking.
Cocaine-selective cells in red, sucrose-selective cells in blue, cells showing overlapping activity for cocaine and sucrose in purple, non-responding cells in grey, cells activated during reinstatement in orange. Phases A and B based on Cameron & Carelli, 2012.
Interestingly, measurement of the electrophysiological properties of engram neurons showed a depotentiation of these cells (decrease in A/N ratio and sEPSC frequency) after chronic cocaine exposure (Koya et al., 2012, Whitaker et al., 2015). However, these measures were obtained in animals repeatedly exposed to cocaine 90 min after a cocaine challenge and cocaine-paired context exposure, which may have resulted in these studies missing t-SP. The time course of t-SP in MSNs of reinstated cocaine withdrawn rats is <45 min for spine head expansion and <120 min for increased AMPA/NMDA (Gipson et al., 2013a). Also, LTD is observed 24 hours after the last cocaine injection (Kourrich et al., 2007).
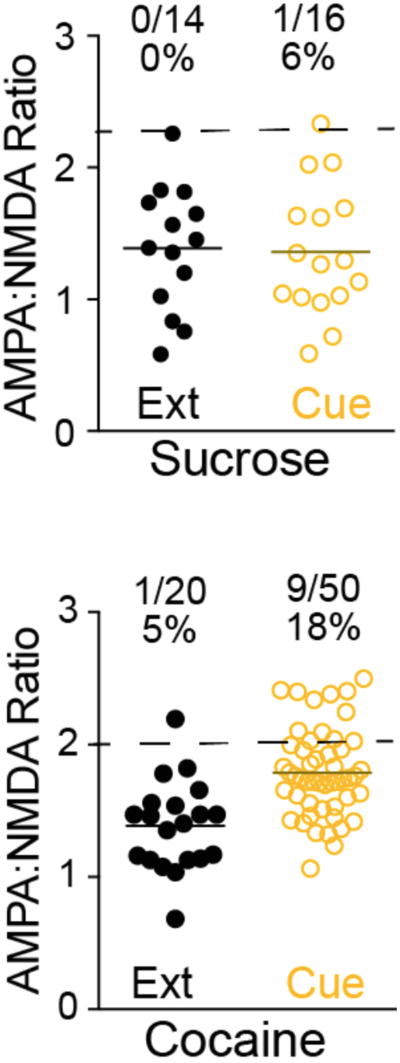
The concept of engram expansion receives behavioral support from a study showing that while rats prefer a food reinforcement over a cocaine reinforcement, in a cued reinstatement session where animals respond only to the reward associated cue, they respond quantitatively more to the cocaine cue (Tunstall and Kearns, 2016). In this study, animals presented with a choice between grain pellets or cocaine reward overwhelmingly chose food when the reward was present. After extinction training, animals underwent cued-reinstatement, in the absence of rewards where they were presented with a choice between cocaine or food lever leading to either drug- or food-associated cue delivery. In this case, animals pressed the cocaine-associated lever at a high rate, hence seeking cocaine more intensely than food (Tunstall and Kearns, 2016). The authors conclude that after the drug-free period, the cocaine cue becomes more salient than the food cue. Based on the fact that glutamate spillover specifically occurs in synapses following cocaine and not sucrose self-administration (Gipson et al., 2013a) due to downregulated GLT-1, the induction of t-SP is initiated only in the cocaine engram. Thus, we propose that glutamate spillover and the widespread induction of t-SP represents the recruitment of additional synapses on neurons near adjacent to the cocaine engram neurons, and the recruitment of these neurons creates a stronger engram that mediates the strengthened behavioral response to a cocaine cue. Electrophysiological studies support the general idea that cocaine cues recruit a larger engram than natural reward-associated cues. For example, selective encoding of natural rewards (food, water or sucrose) is different from the encoding for cocaine, with only 20% of measured cells responding to both type of reinforcers during the task, but after 30 days of cocaine abstinence, the percentage of cells overlapping increases to 33% as a result of the engram encoding cocaine reward having enlarged, and the food reward engram involving a smaller number of neurons (Cameron and Carelli, 2012, Carelli et al., 2000) (Figure 2). The relative enlargement of neuronal coding for the cocaine cue is consistent with the incubation of drug craving seen after cocaine withdrawal (Grimm et al., 2001). Another study used prolonged access to cocaine self-administration, and in vivo electrophysiological recordings in the NAc were performed during escalation of cocaine intake, after 30 days of forced abstinence, and during cocaine re-exposure (Guillem et al., 2014). The authors concluded that the incubation of cocaine seeking observed after the abstinence phase was significantly and selectively correlated to an increase in the proportion of neurons that fired phasically during cocaine seeking. Finally, a re-analysis of our earlier data (Gipson et al., 2013a) shows that if we arbitrarily define recruitment of neurons to an engram when the neurons have an A/N that is two standard deviations above the mean A/N value, reinstatement of lever pressing induced by a sucrose cue does not enlarge the engram size over 6%, while cue-induced cocaine seeking potentiates cells creating an engram constituting 18% of the MSNs recorded (Figure 3). Concurrent with this observation, relapse to a cocaine context increased the number of cFos positive neurons in the NAc by a similar magnitude compared to extinction baseline (Cruz et al., 2014a).
Figure 3.
AMPA:NMDA ratio measured in rats that underwent sucrose self-administration (sucrose) or cocaine self-administration (cocaine). In the extinction group (Ext), rats underwent an extinction session 24h before taking NAcore tissue slices and measuring A/N, in the cue group, animals were tested after 15 min of cue-induced reinstatement. Numbers on top of the data recapitulate the number of cells with ratios two standard deviations above the mean ratio over total number of cells measured, as well as the corresponding percentages. These data were originally published in (Gipson et al., 2013a).
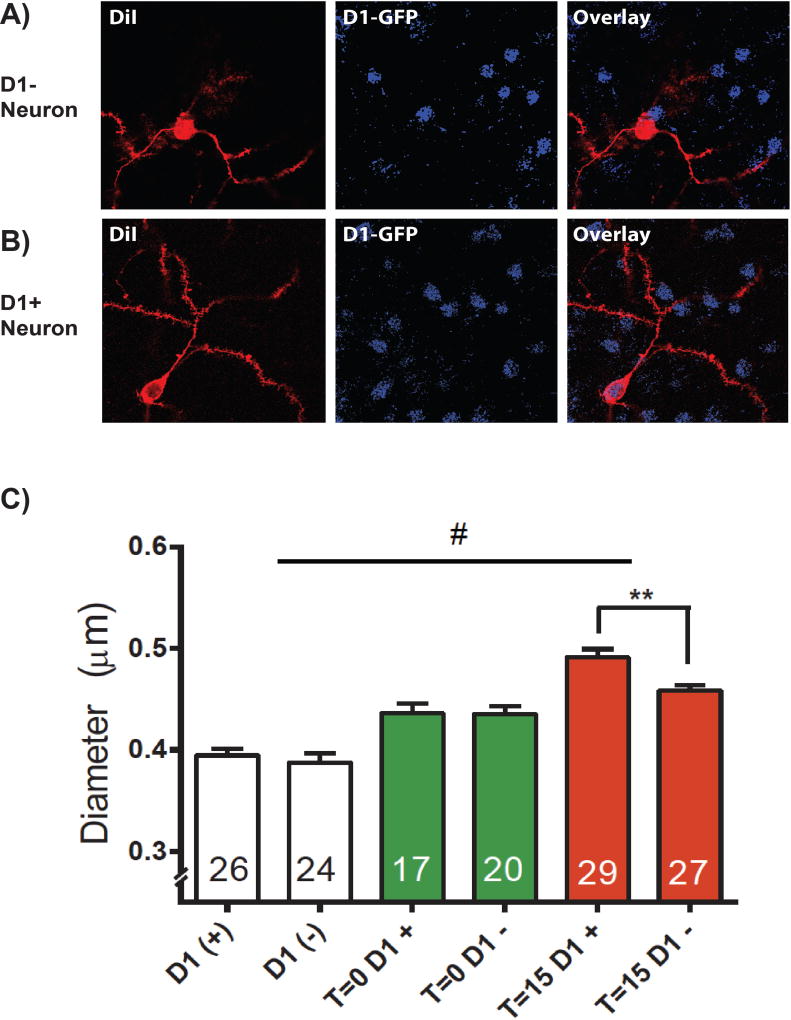
A final consideration is that MSNs in the NAcore are divided in two subtypes, depending on the expression of dopamine D1 or D2 receptors that have opposing roles in cocaine taking and reinstatement (Bock et al., 2013, Heinsbroek et al., 2017). This raises the question that ensembles triggered by drug associated cues might be limited to D1 or D2 MSNs. The data presented in Figure 4, shows that while both D1 and D2 MSN spine heads are enlarged in cocaine-extinguished animals compared to yoked saline controls, the transient increase in spine head diameter of NAcore MSNs produced during cue-induced drug seeking is significantly greater in D1 than in D2 MSN spines. These data were collected in D1-eGFP reporter mice that underwent standard intravenous saline or cocaine (0.8 mg/kg/infusion) self-administration, followed by extinction training and subsequent cue-induced reinstatement (specific protocol described in (Heinsbroek et al., 2017)). Cells were diolistically labeled, and spine head diameter quantified as described by (Shen et al., 2008).
Figure 4.
DiI labeling coupled with Immunohistochemistry enhanced labeling of GFP allowed visualization of A) putative D2 neurons (D1 negative, D1−) and B) D1 positive neurons in the NAc C) Spine head diameter on D1+ and D1− neurons. After extinction from cocaine self administration (T=0, green bars), a potentiation in dh is observed in both D1+ and D1− neurons compared to saline controls (white bars). During cue-induced reinstatement (T=15, red bars), dh is elevated specifically on D1+ dendritic spines. N shown in bars is the number of neurons quantified, and the data were analyzed using a 2-way ANOVA F(1,137) = 4.613, p < 0.05 (main effect); ** D1 vs D2 p<0.01; # Between groups p< 0.001)
5. Concluding remarks
Changes in NAc plasticity after drug exposure are critical to seeking behaviors. Particularly, the transient deregulation of glutamate homeostasis observed in NAcore tetrapartite synapses (constituted by pre- and post-synaptic elements, astroglial end feet and extracellular matrix encompassing the synapse) has been shown to be necessary to initiate drug seeking during cue-induced reinstatement. Although dissecting the different types of neurons in the NAc and their respective projections has moved the addiction field forward (Lenz and Lobo, 2013), a growing movement campaigns for a shift from focusing on the neuron as the brain unit to integrated neuronal networks (Cruz et al., 2013, Yuste, 2015). We hypothesize here that transient glutamate overflow occurs in the NAcore during reinstatement within the engram specific to drug seeking behavior, which was formed during drug self-administration. We speculate that this engram, constituted at first of a small number of neurons, is enlarged during reinstatement by synaptic glutamate spillover that produces a mGluR5-dependent NO activation of MMPs to induce t-SP in MSNs. This pathological process results in a prepotent behavioral response for drug-associated cues that inhibits the initiation of competing behavioral responses by stimuli associated with behaviors other than drug seeking. The development of novel tools that employ the expression of immediate early genes like c-fos (Cruz et al., 2014b, Reijmers et al., 2007) and recent advancements in narrowing the window of cell tagging around a specific behavior using the TRAP technology (Guenthner et al., 2013), allows for the study of specific cells activated during a behavior and will lead to a better understanding of how engrams steer behavior.
Acknowledgments
ACB was funded by a post-doctoral study grant from the French Fyssen Foundation, CDG by NIH R00 DA036569, CDF by NIH DA032543, PJK by NIH DA025983, and PWK was funded by NIH DA003906, DA12513 and DA015369. The authors would like to thank all the members of the Kalivas lab for helpful discussions and comments on this manuscript. Thank you also to the Servier Medical Art for providing free open source designed medical elements used in the illustrations.
References
- Alajaji M, Bowers MS, Knackstedt L, Damaj MI. Effects of the beta-lactam antibiotic ceftriaxone on nicotine withdrawal and nicotine-induced reinstatement of preference in mice. Psychopharmacology (Berl) 2013;228:419–26. doi: 10.1007/s00213-013-3047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EM, Self DW. It's only a matter of time: longevity of cocaine-induced changes in dendritic spine density in the nucleus accumbens. Current Opinion in Behavioral Sciences. 2017;13:117–123. doi: 10.1016/j.cobeha.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DA, Mcfarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–9. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Balster RL, Schuster CR. A comparison of d-amphetamine, l-amphetamine, and methamphetamine self-administration in rhesus monkeys. Pharmacol Biochem Behav. 1973;1:67–71. doi: 10.1016/0091-3057(73)90057-9. [DOI] [PubMed] [Google Scholar]
- Baptista MA, Martin-Fardon R, Weiss F. Preferential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on conditioned reinstatement versus primary reinforcement: comparison between cocaine and a potent conventional reinforcer. J Neurosci. 2004;24:4723–7. doi: 10.1523/JNEUROSCI.0176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock R, Shin JH, Kaplan AR, Dobi A, Markey E, Kramer PF, Gremel CM, Christensen CH, Adrover MF, Alvarez VA. Strengthening the accumbal indirect pathway promotes resilience to compulsive cocaine use. Nat Neurosci. 2013;16:632–8. doi: 10.1038/nn.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Gray SM, Lu L, Shaham Y. Activation of group II metabotropic glutamate receptors in the nucleus accumbens shell attenuates context-induced relapse to heroin seeking. Neuropsychopharmacology. 2006;31:2197–209. doi: 10.1038/sj.npp.1300977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Stern AL, Theberge FR, Cifani C, Koya E, Hope BT, Shaham Y. Ventral medial prefrontal cortex neuronal ensembles mediate context-induced relapse to heroin. Nat Neurosci. 2011;14:420–2. doi: 10.1038/nn.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron CM, Carelli RM. Cocaine abstinence alters nucleus accumbens firing dynamics during goal-directed behaviors for cocaine and sucrose. Eur J Neurosci. 2012;35:940–51. doi: 10.1111/j.1460-9568.2012.08024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprioli D, Venniro M, Zhang M, Bossert JM, Warren BL, Hope BT, Shaham Y. Role of Dorsomedial Striatum Neuronal Ensembles in Incubation of Methamphetamine Craving after Voluntary Abstinence. J Neurosci. 2017;37:1014–1027. doi: 10.1523/JNEUROSCI.3091-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli RM, Ijames SG, Crumling AJ. Evidence that separate neural circuits in the nucleus accumbens encode cocaine versus "natural" (water and food) reward. J Neurosci. 2000;20:4255–66. doi: 10.1523/JNEUROSCI.20-11-04255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespi D, Gobbi M, Mennini T. 5-HT3 serotonin hetero-receptors inhibit [3H]acethylcholine release in rat cortical synaptosomes. Pharmacol Res. 1997;35:351–4. doi: 10.1006/phrs.1997.0143. [DOI] [PubMed] [Google Scholar]
- Cruz FC, Babin KR, Leao RM, Goldart EM, Bossert JM, Shaham Y, Hope BT. Role of nucleus accumbens shell neuronal ensembles in context-induced reinstatement of cocaine-seeking. J Neurosci. 2014a;34:7437–46. doi: 10.1523/JNEUROSCI.0238-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz FC, Javier Rubio F, Hope BT. Using c-fos to study neuronal ensembles in corticostriatal circuitry of addiction. Brain Res. 2014b doi: 10.1016/j.brainres.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz FC, Koya E, Guez-Barber DH, Bossert JM, Lupica CR, Shaham Y, Hope BT. New technologies for examining the role of neuronal ensembles in drug addiction and fear. Nat Rev Neurosci. 2013;14:743–54. doi: 10.1038/nrn3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Guglielmo G, Crawford E, Kim S, Vendruscolo LF, Hope BT, Brennan M, Cole M, Koob GF, George O. Recruitment of a Neuronal Ensemble in the Central Nucleus of the Amygdala Is Required for Alcohol Dependence. J Neurosci. 2016;36:9446–53. doi: 10.1523/JNEUROSCI.1395-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–8. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dityatev A, Rusakov DA. Molecular signals of plasticity at the tetrapartite synapse. Curr Opin Neurobiol. 2011;21:353–9. doi: 10.1016/j.conb.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducret E, Puaud M, Lacoste J, Belin-Rauscent A, Fouyssac M, Dugast E, Murray JE, Everitt BJ, Houeto JL, Belin D. N-Acetylcysteine Facilitates Self-Imposed Abstinence After Escalation of Cocaine Intake. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanous S, Goldart EM, Theberge FR, Bossert JM, Shaham Y, Hope BT. Role of orbitofrontal cortex neuronal ensembles in the expression of incubation of heroin craving. J Neurosci. 2012;32:11600–9. doi: 10.1523/JNEUROSCI.1914-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Smith KD, Houston AC, Rebec GV. Differential effects of cocaine access and withdrawal on glutamate type 1 transporter expression in rat nucleus accumbens core and shell. Neuroscience. 2012;210:333–9. doi: 10.1016/j.neuroscience.2012.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk D, Coen K, Tamadon S, Hope BT, Shaham Y, Le AD. Role of Central Amygdala Neuronal Ensembles in Incubation of Nicotine Craving. J Neurosci. 2016;36:8612–23. doi: 10.1523/JNEUROSCI.1505-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Sinclair CM, Cleva RM, Widholm JJ, Olive MF. Alcohol-seeking behavior is associated with increased glutamate transmission in basolateral amygdala and nucleus accumbens as measured by glutamate-oxidase-coated biosensors. Addict Biol. 2011;16:215–28. doi: 10.1111/j.1369-1600.2010.00262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghatak S, Morgner J, Wickstrom SA. ILK: a pseudokinase with a unique function in the integrin-actin linkage. Biochem Soc Trans. 2013;41:995–1001. doi: 10.1042/BST20130062. [DOI] [PubMed] [Google Scholar]
- Gipson CD, Kupchik YM, Kalivas PW. Rapid, transient synaptic plasticity in addiction. Neuropharmacology. 2014;76(Pt B):276–86. doi: 10.1016/j.neuropharm.2013.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, Kupchik YM, Shen H, Reissner KJ, Thomas CA, Kalivas PW. Relapse induced by cues predicting cocaine depends on rapid, transient synaptic potentiation. Neuron. 2013a;77:867–72. doi: 10.1016/j.neuron.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, Reissner KJ, Kupchik YM, Smith AC, Stankeviciute N, Hensley-Simon ME, Kalivas PW. Reinstatement of nicotine seeking is mediated by glutamatergic plasticity. Proc Natl Acad Sci U S A. 2013b;110:9124–9. doi: 10.1073/pnas.1220591110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–2. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenthner CJ, Miyamichi K, Yang HH, Heller HC, Luo L. Permanent genetic access to transiently active neurons via TRAP: targeted recombination in active populations. Neuron. 2013;78:773–84. doi: 10.1016/j.neuron.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillem K, Ahmed SH, Peoples LL. Escalation of cocaine intake and incubation of cocaine seeking are correlated with dissociable neuronal processes in different accumbens subregions. Biol Psychiatry. 2014;76:31–9. doi: 10.1016/j.biopsych.2013.08.032. [DOI] [PubMed] [Google Scholar]
- Hebb DO. The organization of behavior : a neuropsychological theory. Mahwah, N.J.L: Erlbaum Associates; 1949. [Google Scholar]
- Heinsbroek JA, Neuhofer DN, Griffin WC, 3rd, Siegel GS, Bobadilla AC, Kupchik YM, Kalivas PW. Loss of Plasticity in the D2-Accumbens Pallidal Pathway Promotes Cocaine Seeking. J Neurosci. 2017;37:757–767. doi: 10.1523/JNEUROSCI.2659-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotsenpiller G, Giorgetti M, Wolf ME. Alterations in behaviour and glutamate transmission following presentation of stimuli previously associated with cocaine exposure. Eur J Neurosci. 2001;14:1843–55. doi: 10.1046/j.0953-816x.2001.01804.x. [DOI] [PubMed] [Google Scholar]
- Huang CC, Yeh CM, Wu MY, Chang AY, Chan JY, Chan SH, Hsu KS. Cocaine withdrawal impairs metabotropic glutamate receptor-dependent long-term depression in the nucleus accumbens. J Neurosci. 2011;31:4194–203. doi: 10.1523/JNEUROSCI.5239-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntley GW. Synaptic circuit remodelling by matrix metalloproteinases in health and disease. Nat Rev Neurosci. 2012;13:743–57. doi: 10.1038/nrn3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josselyn SA, Kohler S, Frankland PW. Heroes of the Engram. J Neurosci. 2017;37:4647–4657. doi: 10.1523/JNEUROSCI.0056-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–72. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kasanetz F, Deroche-Gamonet V, Berson N, Balado E, Lafourcade M, Manzoni O, Piazza PV. Transition to addiction is associated with a persistent impairment in synaptic plasticity. Science. 2010;328:1709–12. doi: 10.1126/science.1187801. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. The ups and downs of addiction: role of metabotropic glutamate receptors. Trends Pharmacol Sci. 2004;25:265–72. doi: 10.1016/j.tips.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Khachaturian H, Watson SJ. Some perspectives on monoamine-opioid peptide interaction in rat central nervous system. Brain Res Bull. 1982;9:441–62. doi: 10.1016/0361-9230(82)90154-x. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Larowe S, Mardikian P, Malcolm R, Upadhyaya H, Hedden S, Markou A, Kalivas PW. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol Psychiatry. 2009;65:841–5. doi: 10.1016/j.biopsych.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol Psychiatry. 2010;67:81–4. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourrich S, Calu DJ, Bonci A. Intrinsic plasticity: an emerging player in addiction. Nat Rev Neurosci. 2015;16:173–84. doi: 10.1038/nrn3877. [DOI] [PubMed] [Google Scholar]
- Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27:7921–8. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koya E, Cruz FC, Ator R, Golden SA, Hoffman AF, Lupica CR, Hope BT. Silent synapses in selectively activated nucleus accumbens neurons following cocaine sensitization. Nat Neurosci. 2012;15:1556–62. doi: 10.1038/nn.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koya E, Golden SA, Harvey BK, Guez-Barber DH, Berkow A, Simmons DE, Bossert JM, Nair SG, Uejima JL, Marin MT, Mitchell TB, Farquhar D, Ghosh SC, Mattson BJ, Hope BT. Targeted disruption of cocaine-activated nucleus accumbens neurons prevents context-specific sensitization. Nat Neurosci. 2009;12:1069–73. doi: 10.1038/nn.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J Neurosci. 2008;28:3170–7. doi: 10.1523/JNEUROSCI.5129-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz JD, Lobo MK. Optogenetic insights into striatal function and behavior. Behav Brain Res. 2013;255:44–54. doi: 10.1016/j.bbr.2013.04.018. [DOI] [PubMed] [Google Scholar]
- Luscher C. Drug-evoked synaptic plasticity causing addictive behavior. J Neurosci. 2013;33:17641–6. doi: 10.1523/JNEUROSCI.3406-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–63. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madayag A, Lobner D, Kau KS, Mantsch JR, Abdulhameed O, Hearing M, Grier MD, Baker DA. Repeated N-acetylcysteine administration alters plasticity-dependent effects of cocaine. J Neurosci. 2007;27:13968–76. doi: 10.1523/JNEUROSCI.2808-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson BJ, Koya E, Simmons DE, Mitchell TB, Berkow A, Crombag HS, Hope BT. Context-specific sensitization of cocaine-induced locomotor activity and associated neuronal ensembles in rat nucleus accumbens. Eur J Neurosci. 2008;27:202–12. doi: 10.1111/j.1460-9568.2007.05984.x. [DOI] [PubMed] [Google Scholar]
- Mcfarland K, Kalivas PW. The Circuitry Mediating Cocaine-Induced Reinstatement of Drug-Seeking Behavior. The Journal of Neuroscience. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcfarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–7. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcglinchey EM, James MH, Mahler SV, Pantazis C, Aston-Jones G. Prelimbic to Accumbens Core Pathway Is Recruited in a Dopamine-Dependent Manner to Drive Cued Reinstatement of Cocaine Seeking. J Neurosci. 2016;36:8700–11. doi: 10.1523/JNEUROSCI.1291-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrano DA, Smith Y. Comparative analysis of the subcellular and subsynaptic localization of mGluR1a and mGluR5 metabotropic glutamate receptors in the shell and core of the nucleus accumbens in rat and monkey. J Comp Neurol. 2007;500:788–806. doi: 10.1002/cne.21214. [DOI] [PubMed] [Google Scholar]
- Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, Kalivas PW. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci. 2009;12:182–9. doi: 10.1038/nn.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland PJ, Chandler LJ, Kalivas PW. Signals from the Fourth Dimension Regulate Drug Relapse. Trends Neurosci. 2016;39:472–85. doi: 10.1016/j.tins.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JE, Everitt BJ, Belin D. N-Acetylcysteine reduces early-and late-stage cocaine seeking without affecting cocaine taking in rats. Addict Biol. 2012;17:437–40. doi: 10.1111/j.1369-1600.2011.00330.x. [DOI] [PubMed] [Google Scholar]
- Niu G, Chen X. Why integrin as a primary target for imaging and therapy. Theranostics. 2011;1:30–47. doi: 10.7150/thno/v01p0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF. Metabotropic glutamate receptor ligands as potential therapeutics for addiction. Curr Drug Abuse Rev. 2009;2:83–98. doi: 10.2174/1874473710902010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoli V, Terrier J, Hiver A, Luscher C. Sufficiency of Mesolimbic Dopamine Neuron Stimulation for the Progression to Addiction. Neuron. 2015;88:1054–66. doi: 10.1016/j.neuron.2015.10.017. [DOI] [PubMed] [Google Scholar]
- Pennartz CM, Groenewegen HJ, Lopes da Silva FH. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Prog Neurobiol. 1994;42:719–61. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Peters J, Kalivas PW. The group II metabotropic glutamate receptor agonist, LY379268, inhibits both cocaine- and food-seeking behavior in rats. Psychopharmacology (Berl) 2006;186:143–9. doi: 10.1007/s00213-006-0372-9. [DOI] [PubMed] [Google Scholar]
- Pfarr S, Meinhardt MW, Klee ML, Hansson AC, Vengeliene V, Schonig K, Bartsch D, Hope BT, Spanagel R, Sommer WH. Losing Control: Excessive Alcohol Seeking after Selective Inactivation of Cue-Responsive Neurons in the Infralimbic Cortex. J Neurosci. 2015;35:10750–61. doi: 10.1523/JNEUROSCI.0684-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422:614–8. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16:1550–60. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Nino AM, D'souza MS, Markou A. N-acetylcysteine decreased nicotine self-administration and cue-induced reinstatement of nicotine seeking in rats: comparison with the effects of N-acetylcysteine on food responding and food seeking. Psychopharmacology (Berl) 2013;225:473–82. doi: 10.1007/s00213-012-2837-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijmers LG, Perkins BL, Matsuo N, Mayford M. Localization of a stable neural correlate of associative memory. Science. 2007;317:1230–3. doi: 10.1126/science.1143839. [DOI] [PubMed] [Google Scholar]
- Reissner KJ, Gipson CD, Tran PK, Knackstedt LA, Scofield MD, Kalivas PW. Glutamate transporter GLT-1 mediates N-acetylcysteine inhibition of cocaine reinstatement. Addiction Biology. 2014:n/a–n/a. doi: 10.1111/adb.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissner KJ, Gipson CD, Tran PK, Knackstedt LA, Scofield MD, Kalivas PW. Glutamate transporter GLT-1 mediates N-acetylcysteine inhibition of cocaine reinstatement. Addict Biol. 2015;20:316–23. doi: 10.1111/adb.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Sakai M, Weedman JM, Rebec GV, Bell RL. Ceftriaxone, a beta-lactam antibiotic, reduces ethanol consumption in alcohol-preferring rats. Alcohol Alcohol. 2011;46:239–46. doi: 10.1093/alcalc/agr023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Scofield MD, Boger HA, Smith RJ, Li H, Haydon PG, Kalivas PW. Gq-DREADD Selectively Initiates Glial Glutamate Release and Inhibits Cue-induced Cocaine Seeking. Biol Psychiatry. 2015;78:441–51. doi: 10.1016/j.biopsych.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield MD, Li H, Siemsen BM, Healey KL, Tran PK, Woronoff N, Boger HA, Kalivas PW, Reissner KJ. Cocaine Self-Administration and Extinction Leads to Reduced Glial Fibrillary Acidic Protein Expression and Morphometric Features of Astrocytes in the Nucleus Accumbens Core. Biol Psychiatry. 2016;80:207–15. doi: 10.1016/j.biopsych.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE, Kruzich PJ, Grimm JW. Dopamine, but not glutamate, receptor blockade in the basolateral amygdala attenuates conditioned reward in a rat model of relapse to cocaine-seeking behavior. Psychopharmacology (Berl) 2001;154:301–10. doi: 10.1007/s002130000636. [DOI] [PubMed] [Google Scholar]
- Shen H, Kalivas PW. Reduced LTP and LTD in prefrontal cortex synapses in the nucleus accumbens after heroin self-administration. Int J Neuropsychopharmacol. 2013;16:1165–7. doi: 10.1017/S1461145712001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Moussawi K, Zhou W, Toda S, Kalivas PW. Heroin relapse requires long-term potentiation-like plasticity mediated by NMDA2b-containing receptors. Proc Natl Acad Sci U S A. 2011;108:19407–12. doi: 10.1073/pnas.1112052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Sesack SR, Toda S, Kalivas PW. Automated quantification of dendritic spine density and spine head diameter in medium spiny neurons of the nucleus accumbens. Brain Struct Funct. 2008;213:149–158. doi: 10.1007/s00429-008-0184-2. [DOI] [PubMed] [Google Scholar]
- Shen HW, Gipson CD, Huits M, Kalivas PW. Prelimbic cortex and ventral tegmental area modulate synaptic plasticity differentially in nucleus accumbens during cocaine-reinstated drug seeking. Neuropsychopharmacology. 2014a;39:1169–77. doi: 10.1038/npp.2013.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HW, Scofield MD, Boger H, Hensley M, Kalivas PW. Synaptic glutamate spillover due to impaired glutamate uptake mediates heroin relapse. J Neurosci. 2014b;34:5649–57. doi: 10.1523/JNEUROSCI.4564-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AC, Kupchik YM, Scofield MD, Gipson CD, Wiggins A, Thomas CA, Kalivas PW. Synaptic plasticity mediating cocaine relapse requires matrix metalloproteinases. Nat Neurosci. 2014;17:1655–7. doi: 10.1038/nn.3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AC, Scofield MD, Heinsbroek JA, Gipson CD, Neuhofer D, Roberts-Wolfe DJ, Spencer S, Garcia-Keller C, Stankeviciute NM, Smith RJ, Allen NP, Lorang MR, Griffin WC, 3rd, Boger HA, Kalivas PW. Accumbens nNOS Interneurons Regulate Cocaine Relapse. J Neurosci. 2017;37:742–756. doi: 10.1523/JNEUROSCI.2673-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AC, Scofield MD, Kalivas PW. The tetrapartite synapse: Extracellular matrix remodeling contributes to corticoaccumbens plasticity underlying drug addiction. Brain Res. 2015 doi: 10.1016/j.brainres.2015.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AW, Nealey KA, Wright JW, Walker BM. Plasticity associated with escalated operant ethanol self-administration during acute withdrawal in ethanol-dependent rats requires intact matrix metalloproteinase systems. Neurobiol Learn Mem. 2011;96:199–206. doi: 10.1016/j.nlm.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankeviciute NM, Scofield MD, Kalivas PW, Gipson CD. Rapid, transient potentiation of dendritic spines in context-induced relapse to cocaine seeking. Addict Biol. 2014;19:972–4. doi: 10.1111/adb.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto N, Laque A, De Ness GL, Wagner GE, Watry D, Kerr T, Koya E, Mayford MR, Hope BT, Weiss F. Distinct memory engrams in the infralimbic cortex of rats control opposing environmental actions on a learned behavior. Elife. 2016;5 doi: 10.7554/eLife.21920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trantham-Davidson H, Lalumiere RT, Reissner KJ, Kalivas PW, Knackstedt LA. Ceftriaxone normalizes nucleus accumbens synaptic transmission, glutamate transport, and export following cocaine self-administration and extinction training. J Neurosci. 2012;32:12406–10. doi: 10.1523/JNEUROSCI.1976-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunstall BJ, Kearns DN. Cocaine can generate a stronger conditioned reinforcer than food despite being a weaker primary reinforcer. Addict Biol. 2016;21:282–93. doi: 10.1111/adb.12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uys JD, Knackstedt L, Hurt P, Tew KD, Manevich Y, Hutchens S, Townsend DM, Kalivas PW. Cocaine-induced adaptations in cellular redox balance contributes to enduring behavioral plasticity. Neuropsychopharmacology. 2011;36:2551–60. doi: 10.1038/npp.2011.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Oever MC, Lubbers BR, Goriounova NA, Li KW, Van der Schors RC, Loos M, Riga D, Wiskerke J, Binnekade R, Stegeman M, Schoffelmeer AN, Mansvelder HD, Smit AB, De Vries TJ, Spijker S. Extracellular matrix plasticity and GABAergic inhibition of prefrontal cortex pyramidal cells facilitates relapse to heroin seeking. Neuropsychopharmacology. 2010;35:2120–33. doi: 10.1038/npp.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Moussawi K, Knackstedt L, Shen H, Kalivas PW. Role of mGluR5 neurotransmission in reinstated cocaine-seeking. Addict Biol. 2013;18:40–9. doi: 10.1111/j.1369-1600.2011.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XB, Bozdagi O, Nikitczuk JS, Zhai ZW, Zhou Q, Huntley GW. Extracellular proteolysis by matrix metalloproteinase-9 drives dendritic spine enlargement and long-term potentiation coordinately. Proc Natl Acad Sci U S A. 2008;105:19520–5. doi: 10.1073/pnas.0807248105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren BL, Mendoza MP, Cruz FC, Leao RM, Caprioli D, Rubio FJ, Whitaker LR, Mcpherson KB, Bossert JM, Shaham Y, Hope BT. Distinct Fos-Expressing Neuronal Ensembles in the Ventromedial Prefrontal Cortex Mediate Food Reward and Extinction Memories. J Neurosci. 2016;36:6691–703. doi: 10.1523/JNEUROSCI.0140-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland A, Garcia S, Knackstedt LA. Ceftriaxone and cefazolin attenuate the cue-primed reinstatement of alcohol-seeking. Front Pharmacol. 2015;6:44. doi: 10.3389/fphar.2015.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker LR, Carneiro de Oliveira PE, Mcpherson KB, Fallon RV, Planeta CS, Bonci A, Hope BT. Associative Learning Drives the Formation of Silent Synapses in Neuronal Ensembles of the Nucleus Accumbens. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins A, Smith RJ, Shen HW, Kalivas PW. Integrins modulate relapse to cocaine-seeking. J Neurosci. 2011;31:16177–84. doi: 10.1523/JNEUROSCI.3816-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, Mcnaughton BL. Dynamics of the hippocampal ensemble code for space. Science. 1993;261:1055–8. doi: 10.1126/science.8351520. [DOI] [PubMed] [Google Scholar]
- Yuste R. From the neuron doctrine to neural networks. Nat Rev Neurosci. 2015;16:487–97. doi: 10.1038/nrn3962. [DOI] [PubMed] [Google Scholar]
- Yuste R, Nelson DA, Rubin WW, Katz LC. Neuronal domains in developing neocortex: mechanisms of coactivation. Neuron. 1995;14:7–17. doi: 10.1016/0896-6273(95)90236-8. [DOI] [PubMed] [Google Scholar]
- Yuste R, Peinado A, Katz LC. Neuronal domains in developing neocortex. Science. 1992;257:665–9. doi: 10.1126/science.1496379. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Dayas CV, Aujla H, Baptista MA, Martin-Fardon R, Weiss F. Activation of group II metabotropic glutamate receptors attenuates both stress and cue-induced ethanol-seeking and modulates c-fos expression in the hippocampus and amygdala. J Neurosci. 2006;26:9967–74. doi: 10.1523/JNEUROSCI.2384-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Kalivas PW. N-acetylcysteine reduces extinction responding and induces enduring reductions in cue- and heroin-induced drug-seeking. Biol Psychiatry. 2008;63:338–40. doi: 10.1016/j.biopsych.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]