Abstract
Aniline–peptide (FLDQV, FLDQVC, Dansyl-FLDQV, Dansyl-FLDQVC, and FLDQV-AMC) mixtures underwent oxidative chemical and electrochemical polymerization in excess of aniline. The products of the chemical polymerization were low molecular weight polymers containing more than 70% peptide. Electrochemically polymerized species polyaniline-FLDQV (PANI-FLDQV) consisted mainly of polyaniline units containing about 10% peptide. The solubility of the latter in 1,1,1,3,3,3-hexafluoro-2-propanol (HFP) was similar to the camphorsulfonic acid (CSA) doped emeraldine base (PANI-CSA) solubility, however the weight composition of the electrospun fibers produced from the two polymers was significantly different. 2D 1H–13C HSQC analyses were employed to analyze the binding between the aniline and peptide moieties. Binding of peptide to polyaniline is reflected by the appearance of extra cross-peaks which display line broadening between the free polyaniline and the free pentapeptide. Peptides may be chemically bonded to the polymer molecules, but they may also act as doping agents to the nitrogen atoms via hydrogen bonding.
Keywords: Polyaniline, Peptides, Polymerization, Electrospinning
1. Introduction
Synthetic polymers, such as polyaniline, polyethylene oxide, and polyethylene glycol, have often been used as matrixes for immobilization of proteins and peptides for catalytic, sensoring, synthetic, or therapeutic purposes [1–3]. While quite useful in many cases, these type of devices often have limited working range restricted by the solubility, structural stability, and pH sensitivity of the components. The search for more effective ways to achieve a synergistic combination of properties leads to the development of protein–polymer conjugates [4–7]. Synthetic strategies include utilization of common chemical reactions (Heck, Suzuki coupling, cycloaddition reactions) [8–10] with polymer-reactive peptides/proteins, or protein/peptide reactive polymers, under mild conditions, in order to render a product with the desired structure and properties. Modification of proteins and peptides occurs through the accessible amino acids and reactions may take place with the carboxyl and amino function as well as with the amino acid side chain [11,12].
Polyaniline, a conductive polymer subject to numerous studies because of easy synthesis, tunable properties, and plenty of potential practical applications, can also be obtained in chiral form in presence of chiral inducers [13–15]. Chiral PANI can be obtained by chemical, electrochemical, or enzymatic polymerization as well as by chiral doping-dedoping procedure [16–18]. Chiral acids, such as (+) or (−) 10-camphorsulphonic acid, have successfully induced chirality in the PANI polymer chains, however DNA, polysaccharide, and cellulose, have never been used for this purpose [19]. From another side, studies have shown that proteins, peptides, and even amino acids, present in the polymerization reaction mixture, can induce chirality and selective recognition sites. Polymerization of aniline monomers in presence of hemoglobin or bovine serum albumin, on DBSA template under mild conditions, produce enantio specificity of PANI probably due to the α-helical structure of the proteins [15]. PANI obtained from saturated solutions of L-phenylalanine, using ammonium persulfate as an oxidant, results in chiral PANI of flaky, spherical, and urchin-like morphologies. The latter research assumes also that L-phenylalanine is incorporated in the PANI chains as a dopant [20].
Electrospinning has been utilized to produce fiber mats with various sizes and properties. Polyaniline is notoriously difficult to electrospin since its low solubility in common solvents prevents the formation of solutions with appropriate concentration and viscosity [21]. De-doping with substances, such as camphorsulfonic acid, increases the solubility, however, electrospinning has been reported only in presence of supporting polymers [21–23].
We have previously reported an investigation on the attachment of dioxin selective pentapeptides on polyaniline matrix with or without a linker, and the stability of the chemosensors under different conditions [24]. Some of the peptides used in the study have been previously reported to selectively bind chlorinated toxins [25,26].
The objectives of this research are the following: (1) to explore the chemical and electrochemical polymerization of aniline in presence of penta- and hexapeptides FLDQV, FLDQVC, as well as their Dansyl and AMC labeled derivatives. (2) To produce electrospun fibers from the PANI–peptide polymers and the compare the results with the PANI-CSA doped electrospun mats. (3) To perform preliminary testing of the polymers and the fibers for extraction of chlorinated toxins.
Our hypothesis is that the PANI–peptide polymers and fibers will be promising binding reagents which can be further utilized as solid state extraction tools.
2. Experimental
2.1. Materials and methods
Aniline (99.5%), ammonium persulfate (APS) (98%), phosphate buffer powder, 0.1 M, hydrochloric acid (37%), ammonium hydroxide (28–30%), 1,1,1,3,3,3-hexafluoro-2-propanol (HFP, HPLC grade), 1-methyl-2-pyrrolidinone (NMP, spectroscopic grade), and methanol (spectroscopic grade) were purchased from Sigma Aldrich. FLDQV, FLDQVC, FLDQV-AMC, Dansyl-FLDQV, FLDQVC-AMC, and Dansyl-FLDQVC, were obtained from Biomatik, Inc., Ontario, Canada.
The absorption spectra were recorded on Shimadzu 240IPC spectrophotometer. NMR spectra were recorded on a Brucker 700 MHz spectrometer at 298 K. SEM images were produced on Zeiss 1540EsB dual beam Scanning Electron Microscope.
2.2. Synthesis
2.2.1. Chemical synthesis
Emeraldine base and emeraldine salt were synthesized using the procedure of MacDiarmid et al. [13,14]. De-doping with camphorsulphonic acid was done before the electrospinning experiments [22,23].
The chemical polymerization of aniline in presence of non-labeled and labeled peptides, FLDQVC, FLDQV, Dansyl FLDQVC, and FLDQV AMC was carried out at room temperature. The mixture was stirred for 2–4 h. The aniline and the peptides were mixed at 0 °C and the mixtures were kept at room temperature for five days. The peptide was dissolved in methanol before mixing with aniline and 0.1 M APS.
The products of the polymerization reactions were characterized by solid state 1H and 13C NMR, UV/Vis Spectroscopy, Fluorescence Spectroscopy, and Scanning Electron Microscopy (SEM).
A typical polymerization of the peptide–aniline mixtures was done as follows [20]
400 μL (4.38 × 10−3 mol) of aniline was pipetted into 10 mL of 0.1 M ammonium persulfate (APS). The mixture was placed in an ice bath, stirred, and the peptide solution in 1.5–2 mL methanol was added. The peptide:aniline molar ratio was 1:25–1:30. After 5 days at room temperature, the dark brown precipitate was filtered, rinsed with distilled water and acetone, and dried in the air at room temperature. The reaction yield was 30–40%.
2.2.2. Electrochemical synthesis
The 100 mL electrochemical cell was equipped with a platinum foil counter electrode, and a KCl saturated calomel reference electrode (SCE), against which all potentials were measured. The working electrode was a rotating platinum disk (RDE, from Pine Instruments), 5 mm diameter, mounted in a Pine AFMSRCE rotator and speed controller. Potential ramps were generated with the aid of a Pine AFTP1 Wavenow potentiostat, and the resulting voltammograms were recorded using Aftermath software. The electrode was polished with 0.05 μm alumina (Buehler), sonicated for 30 s, rinsed with water, and then dried with a stream of nitrogen.
Adsorption of polyelectrolytes onto the RDE at 300 rpm was done with the aid of a robot (StratoSequence V, nanoStrata Inc.). The polymer deposition solutions contained 100 mg aniline (1.0 × 10−3 mol) in 100 mL 0.25 M HCl. FLDQV (peptide:aniline 1:125–1:15.6 molar ratio) was dissolved in minimum amount of methanol and added to the mixture. Between polyelectrolyte depositions (5 min each), there were three rinses with deionized water (1 min each). Rinse and polymer solution volumes were approximately 50 mL each. The thickness of the PANI was measured in a separate experiment using a 1 in. diameter single side polished silicon wafer and determined using a Gaertner Scientific L116S autogain ellipsometer with 632.8 nm radiation at 70 incident angle.
Cyclic voltammograms (CVs) at the RDE were performed by sweeping the potential at a 20 mV s−1 scan rate in the range of 500–250 mV vs SCE, rotation rate 1000 rpm. Purging the solution for 10 min with Ar was necessary to remove dissolved oxygen; a blanket of Ar was maintained during the experiments [27].
2.3. Electrospinning
EB was mixed in a mortar with CSA in 1:2 ratio according to previously reported procedure. The dedoped PANI-CSA was mixed with HFP, sonicated for 30 min, stirred overnight, and filtered through 20 μm syringe filter to obtain 5% solution. A 20% solution of gelatin in HFP was prepared similarly. Equal volumes of the two solutions were mixed so that the final concentrations of PANI-CSA and gelatin in the mixture were 2.5% and 10% correspondingly. 5 mL of this mixture was electrospun at 14 kV, using 18 gauge needle, delivery rate of 0.8 mL/min, and 12 cm distance from the cathode. Same conditions were used to produce electrospun fibers from the electrochemically polymerized PANI-FLDQV/gelatin mixtures.
3. Results and discussion
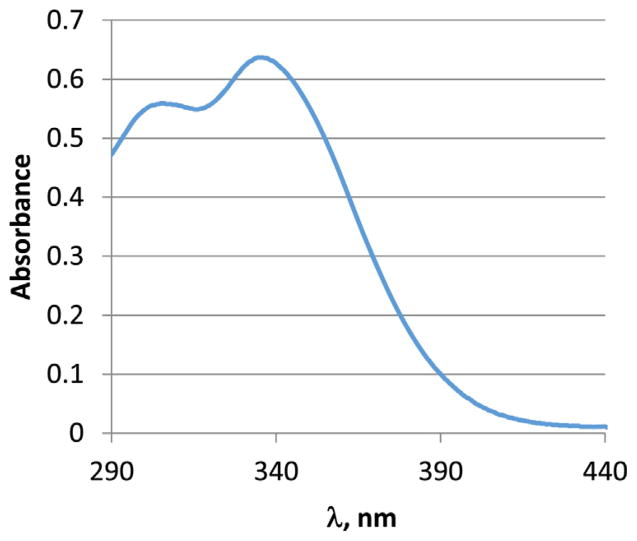
The absorption and emission spectra of the peptides and polymerization products were collected in N-methyl pyrollidinone (NMP) which has an optical cut-off at 285 nm. The spectra are shown in Figs. 1 and 2. Dansyl-FLDQVC peptide exhibits a broad absorption band at 346 nm. Two absorption bands were observed in the spectrum of the polymerized product. The long-wavelength absorption maximum undergoes slight hypsochomic shift (λmax 338 nm) and a new absorption band appears at 306 nm. The former is due to the presence of the dansyl chromophore, while the latter can be assigned to theπ–π* transitions of the polyaniline backbone. It is possible that polyaniline has another absorption transition which overlaps with the dansyl absorption band. The bands can be resolved using different band resolving approaches, however this goes beyond the purposes of this study. The emission spectra (λex 340 nm) show broad structure-less bands with maximum at 514 nm both for the peptide and peptide–polymer compound. The absorption and emission properties of the labeled peptides as well as the PANI–peptide polymers are very sensitive to the presence of chlorinated toxins probably due to a complex formation. Preliminary results have shown significant fluorescence quenching and molar absorptivity decrease together with a hypsochromic shift of the absorption maximum. Further studies are in progress and the results will be published elsewhere.
Fig. 1.
Absorption spectrum of polyaniline- Dansyl FLDQVC, 1.6 × 10−3 mg/mL, in NMP.
Fig. 2.
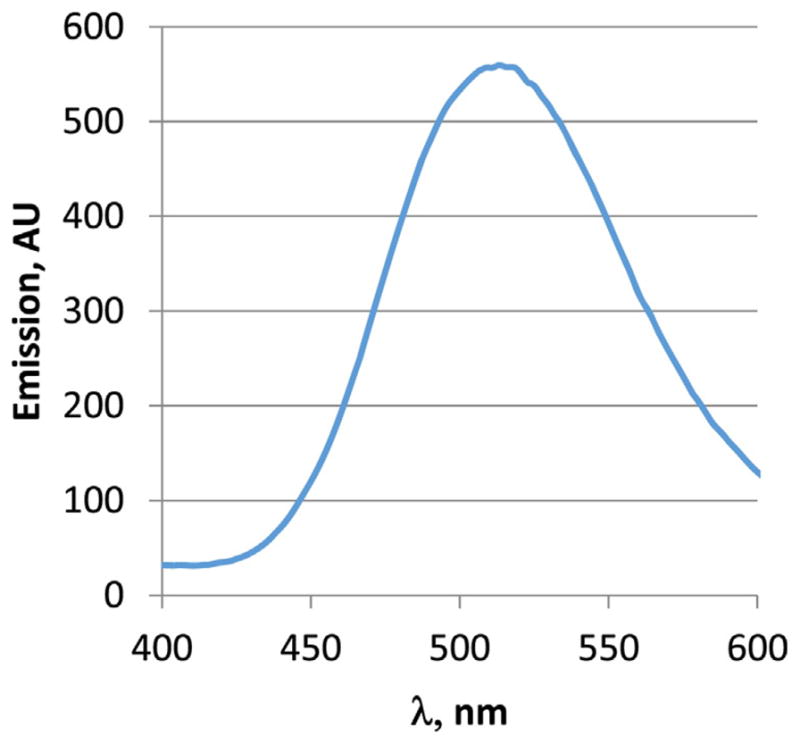
Emission spectrum of Dansyl-FLDQVC, 1.6 × 10−3 mg/mL, in NMP.
Solid state 1H NMR spectra indicated presence of significant amount of peptide in the chemically polymerized product. The latter was quantified using absorption and emission spectra. Standard curves were constructed from serial dilutions from 0.05 mg/mL to 0.35 mg/mL for Dansyl FLDQVC and 0.02–0.14 mg/mL for FLDQV-AMC. The amount of peptide in the polymer varied between 70 and 80% for the different peptides used in the experiment.
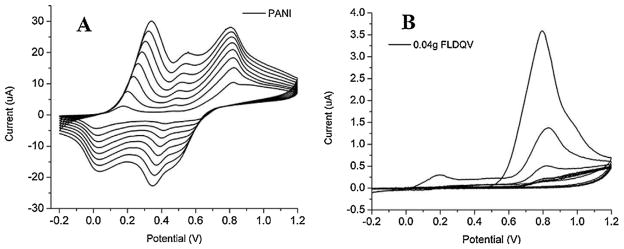
Fig. 3 shows the electrochemical growth of aniline and aniline-FLDQV in 0.1 M HCl. First anodic peak corresponds to the oxidation of monomer and reduction peak appears which shows that the formation of polymer on electrode surface. The subsequent swipes show three anodic oxidation peaks corresponding to the three different oxidation states of polyaniline (leucoemeraldine, emeraldine and pernigraniline). The current values of each oxidation and reduction peaks are greater than that of a previous cycle which indicates that the polymer thickness is increasing on the electrode surface. The peak potentials slightly shift as the film becomes thicker due to a decrease in electrical conductivity and increase in conjugation length. The effect of an increasing concentration of FLDQV on the voltamograms is presented in Fig. 3. No significant qualitative difference is observed until the 40 mg FLDQV sample other then expected decrease in the current values.
Fig. 3.
Cyclic voltammograms from the electrochemical polymerization of aniline (A) and aniline-FLDQV (B).
Analyzing in comparison the two extremes Fig. 3, it can be observed that, the shape of cyclovoltammograms from 40 mg FLDQV/PANI differ very much comparative to shape of pure PANI confirming the different redox processes in the two electrochemically obtained polymers due to composition and structural differences. That means that the FLDQV was entrapped in PANI backbone. It should be also noted that the incorporation of FLDQV do not infringe in the oxidation of the aniline monomer as can be seen in Fig. 3B.
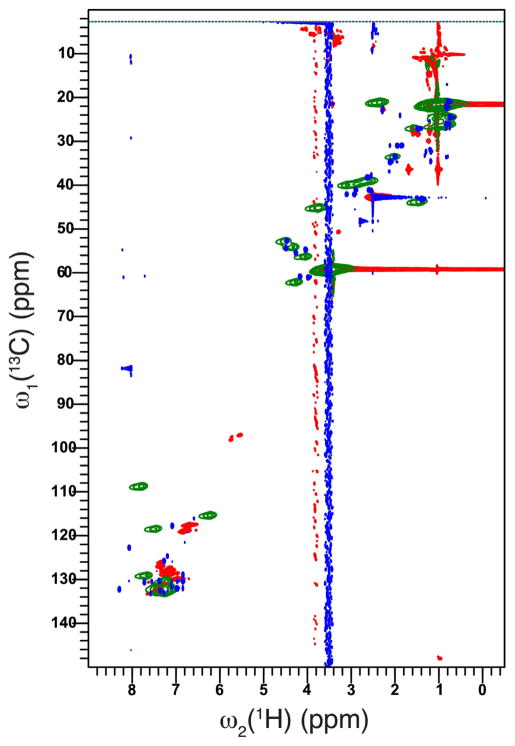
To assess the binding between polyaniline and FLDQV pentapeptide a 2D 1H–13C HSQC analyses has been employed. Fig. 4 shows the 2D 1H–13C heteronuclear single-quantum coherence (HSQC) of free polyaniline (green) with broad cross-peaks in the region of 6–8 ppm and 115–135 ppm on C dimension coming from polymerized aniline. By contrast, the FLDQV pentapeptide (blue cross-peaks) displays sharp cross-peaks in the region expected when compared with the simulated 1D spectra. Binding of peptide to polyaniline is reflected by the appearance of extra cross-peaks (red), which display line broadening between the free polyaniline and the free pentapeptide. The binding of FLDQV pentapeptide to polyaniline is independent of the method used as seen in Fig. 7, supplemental materials.
Fig. 4.
Overlay of 2D 1H–13C HSQC spectra of free polyaniline (green) with free pentapeptide (blue) and peptide-bound polyaniline (red). Spectra were recorded on a 700 MHz spectrometer at 298 K. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
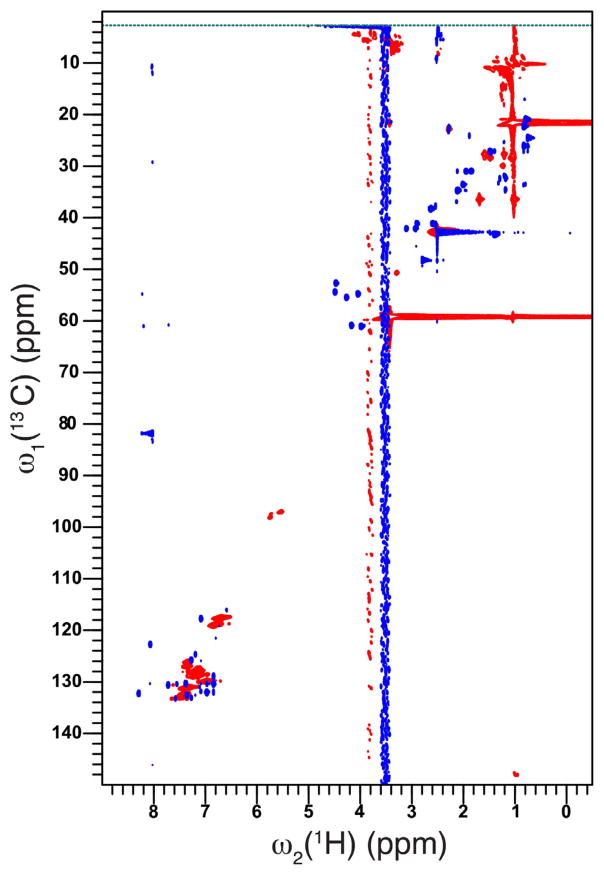
Simulated 1D NMR spectra of FLDQV pentapeptide (Fig. 9, supplemental) are in good agreement with the expected correlations shown in the 2D 1H–13C HSQC spectra (Fig. 4, blue spectrum) (Fig. 5).
Fig. 5.
Overlay of 2D 1H–13C HSQC spectra of free FLDQV pentapeptide (blue) with peptide-bound polyaniline (red). Spectra were recorded on a 700 MHz spectrometer at 298 K. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
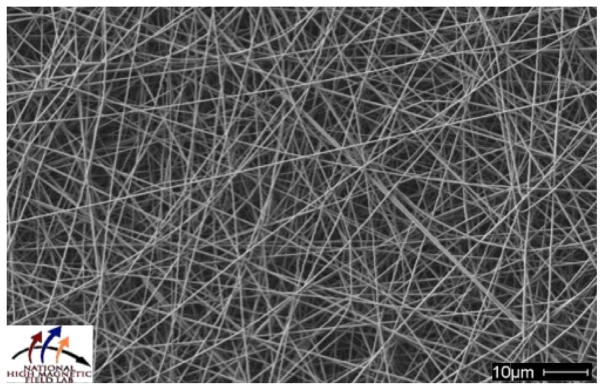
The CSA doped PANI and electrochemically synthesized PANI-FLDQV were electrospun with from mixtures with gelatin. In both cases we used saturated solutions of PANI mixed with 10% gelatin. SEM analysis of the fibers revealed formation of uniform, non-woven fiber texture with fiber sizes ranging from 50 to 100 nm. The fiber morphology of the resulting fiber mats is similar, however UV/Vis analysis showed very different composition. PANI-CSA/gelatin fibers contained 25–30% PANI-CSA by weight while the electrospun PANI-FLDQV/gelatin contained 2–3% PANI-FLDQV (Fig. 6).
Fig. 6.
SEM image of the electrospun PANI-FLDQV/gelatin fibers. The electrospinning solution contained 2.5% PANI-FLDQV and 10% gelatin. 5 mL of this mixture was electrospun at 14 kV, using 18-gauge needle, delivery rate of 0.8 mL/min, and 12 cm distance from the receiving plate.
4. Conclusions
Aniline–peptide mixtures were successfully polymerized by chemical and electrochemical methods resulting in products with different composition and properties. The presence of the peptide and the interactions with the polymer matrix were confirmed by means of UV/Vis and NMR spectroscopy. Electrochemically synthesized PANI-FLDQV polymer was electrospun with gelatin as a supporting polymer resulting in non-beaded, non-woven fiber mat containing 2–3% PANI-FLDQV. The polymers and the fibers reported in this paper are subjected to tests for extraction and determination of chlorinated toxins in water.
Supplementary Material
Acknowledgments
The authors want to thank Dr. Eric Palm and Mr. Bob Goddard from NHMFL, Tallahassee, FL. Funding for this research was provided by NSF DMR-1437417 grant as well as by NIH NCRR grant 2 G12 RR003020 and NIH NIMHD grant 8 G12 MD007582.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.apmt.2016.07.004.
References
- 1.Date T, Tanaka K, Nagamura T, Serizawa T. Chem Mater. 2008;20:4536. [Google Scholar]
- 2.Kyprianou D, Guerreiro AR, Chianella I, Piletska EV, Fowler SA, Karim K, Whitcombe MJ, Turner APF, Piletsky SA. Biosens Bioelectron. 2009;24:1365. doi: 10.1016/j.bios.2008.07.070. [DOI] [PubMed] [Google Scholar]
- 3.Yao C, Qi L, Hu W, Wang F, Yang G. Anal Chim Acta. 2011;692:131. doi: 10.1016/j.aca.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Gauthier MA, Klok H-A. Chem Commun. 2008:2591. doi: 10.1039/b719689j. [DOI] [PubMed] [Google Scholar]
- 5.Hooker JM, Esser-Kahn AP, Francis MB. J Am Chem Soc. 2006;128:15558. doi: 10.1021/ja064088d. [DOI] [PubMed] [Google Scholar]
- 6.Jung B, Theato P. Adv Polym Sci. 2013;253:37. [Google Scholar]
- 7.Matsumura Y, Maeda H. Cancer Res. 1986;46:6387. [PubMed] [Google Scholar]
- 8.Kodama K, Fukuzawa S, Nakayama H, Sakamoto K, Kigawa T, Yabuki T, Matsuda N, Shirouzu M, Takio K, Yokoyama S, Tachibana K. Chem Biol Chem. 2007;8:232. doi: 10.1002/cbic.200600432. [DOI] [PubMed] [Google Scholar]
- 9.Ojida A, Tsutsumi H, Kasagi N, Hamachi I. Tetrahedron Lett. 2005;46:3301. [Google Scholar]
- 10.Dantas de Araujo A, Palomo JM, Cramer J, Seitz O, Alexandrov K, Waldmann H. Chem Eur J. 2006;12:6095. doi: 10.1002/chem.200600148. [DOI] [PubMed] [Google Scholar]
- 11.Tam A, Soellner MB, Raines RT. J Am Chem Soc. 2007;129:11421. doi: 10.1021/ja073204p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilmore JM, Sheck RA, Ester-Kahn AP, Joshi NS, Francis MB. Angew Chem Int Ed. 2006;45:5307. doi: 10.1002/anie.200600368. [DOI] [PubMed] [Google Scholar]
- 13.MacDiarmid AG, Epstein AJ. Faraday Discuss Chem Soc. 1989;88:317. [Google Scholar]
- 14.Asturias GE, MacDiarmid AG, McCall RP, Epstein AJ. Synth Met. 1989;29:E157. [Google Scholar]
- 15.Guo H, Chen J, Xu Y. ACS Macro Lett. 2014;3:295. doi: 10.1021/mz500008f. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, Song W. Polymer. 2007;48:5473. [Google Scholar]
- 17.Majidi MR, Kane-Maguire LAP, Wallace GG. Polymer. 1994;35:3113. [Google Scholar]
- 18.Li WG, Wang HL. J Am Chem Soc. 2004;126:2278. doi: 10.1021/ja039672q. [DOI] [PubMed] [Google Scholar]
- 19.Vasilieva IS, Morozova OV, Shumakovich GP, Shleev SV, Sakharov I, Yaropolov AI. Synth Met. 2007;157:684. [Google Scholar]
- 20.Li Y, Wang B, Feng W. Synth Met. 2009;159:1597. [Google Scholar]
- 21.Li M, Guo Y, Wei Y, MacDiarmid AG, Lelkes PI. Biomaterials. 2006;27:2705. doi: 10.1016/j.biomaterials.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 22.Xia Y, Wiesinger JM, Macdiarmid AG. Chem Mater. 1995;7:443. [Google Scholar]
- 23.Dominis AJ, Spinks GM, Kane-Maguire LAP, Wallace GG. Synth Met. 2002;129:165. [Google Scholar]
- 24.Archibong E, Wang L, Ivanov I, Lita A, Redda K, Mateeva N. Synth Met. 2012;162:1255. doi: 10.1016/j.synthmet.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamura C, Inuyama Y, Goto H, Obataya I, Kaneko N, Nakamura N, Santo N, Miyake J. Anal Chem. 2005;77:7750. doi: 10.1021/ac051151t. [DOI] [PubMed] [Google Scholar]
- 26.Inuyama Y, Nakamura C, Oka T, Yoneda Y, Obataya I, Santo N, Miyake J. Biosens Bioelectron. 2007;22:2093. doi: 10.1016/j.bios.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 27.Guimard N, Schmidt C, Gomez N. Conducting polymers in biomedical engineering. Polymer. 2007;32(8/9):876. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.