Abstract
The current review re-conceptualizes seek and test strategies, particularly given the changing importance of HIV testing as care continuum entry for persons irrespective of their HIV status. Care continuum entry advances previous seek and test strategies for client engagement with two next-generation functions: 1) use of testing to engage (or re-engage) HIV negative clients in pre-exposure prophylaxis (PrEP) care; and 2) testing individuals who may already be known positives for care continuum re-entry. We review existing seek and test strategies for most impacted community members with a goal of optimizing care continuum entry as we move towards HIV transmission elimination. These strategies are context, sub-group, community and epidemic specific. This review is timely given the initiation of routine PrEP care, which shifts and broadens our conceptualization of care continuum entry triggered by the HIV testing event. In addition, as the epidemic becomes more concentrated, focusing on re-engagement of HIV infected persons becomes increasingly important given that transmission events involve both those acutely and newly infected as well as the large numbers who may not be virally suppressed. We start with examination of routine testing in healthcare settings, emphasizing its potential role in re-engagement for persons out of care. Subsequently, we describe risk-based testing to identify key populations. We then review network-based approaches and their impact on the epidemic. We close with future directions of individual and combination care continuum entry strategies most relevant for elimination of HIV transmission in the United States.
Keywords: HIV, key populations, seek and test strategies, HIV care continuum, implementation science, social network
Introduction
HIV remains a significant public health concern in the United States and internationally. Elimination of new sexual and injection transmission events requires optimization of effective intervention strategies, including finding marginalized populations most likely to acquire and transmit HIV and providing access to care and treatment. HIV currently has no cure; yet we do have effective antiretrovirals (ARV) that when used appropriately dramatically limit acquisition and transmission and also have the benefit of creating a manageable chronic disease [1, 2]. Transmission risk is thus directly related to engagement in care continuums. In turn, HIV prevention efforts require that persons living with HIV (PLWH) are not only identified via testing, but also have access to quality healthcare and ARV, and achieve and maintain viral suppression.
HIV prevention has rapidly evolved over the past 7 years with development of high-impact interventions such as treatment as prevention (TasP), or treating PLWH with ARV in order to decrease transmission events to uninfected partners [2]; HIV pre-exposure prophylaxis (PrEP) [1]; and test and treat strategies for rapid initiation of ARV to quickly lower viral load in acute and new HIV infections [3, 4]. Early identification and treatment clearly results in decreased mortality and ongoing transmission [2, 5]. While HIV incidence has declined in the general U.S. population, it has remained stable and even increased in certain vulnerable populations [6]; furthermore, a significant proportion of PLWH are unaware of their status, and even more are aware but not in care or virally suppressed [7]. The National HIV/AIDS Strategy (NHAS) goals of increasing linkage to care within one month of HIV diagnosis to 85%, increasing retention in care to 90%, and attaining 80% viral suppression still remain to be realized [8].
Social determinants of health are recognized for the crucial role they play in HIV prevention – stable housing, education, access to water and food, and criminal justice involvement all impact viral suppression and ongoing ARV-based prevention. Racial/ethnic inequity exists, exhibited by the burden of the epidemic, and in particular subpar healthcare access in certain US populations [9–12]. Furthermore, economic and social hardships influence HIV risk and highlight the role of structural factors in HIV prevention [13]. All of these contribute to the stigma that continues to persist 35 years into the epidemic that limits testing and engagement in care, as well as the extent to which HIV has become a priority on a national level [8, 14–16]. Despite these barriers, a mandate to halt HIV transmission remains.
“Seek, test, treat, and retain” describes a model of care introduced by the National Institutes on Drug Abuse (NIDA) in 2010, referring to approaches to reach at-risk groups for HIV testing that have not been diagnosed or recently tested for HIV, counsel and engage them in HIV testing, link those who test positive to medical care, treat with ARV, and retain in care [17–19]. While the model in its entirety is important for prevention and transmission, in this review we focus on seek and test aspects to engage and re-engage at-risk persons as care continuum entry strategies. Initially developed for drug misuse, this model has been expanded to apply to other vulnerable populations [20]; in this paper we re-conceptualize seek and test not only as the traditional approach to identification of newly infected persons, but importantly as a purposeful strategy to engage existing HIV infected persons who may not be retained as well as persons at risk for HIV acquisition.
Eliminating HIV transmission in well-resourced countries such as the United States will require that marginalized populations be more readily engaged. Accessible and widespread HIV testing is one component of HIV transmission elimination strategies and serves as the entry into the care continuum [21]. Interventions targeted towards this entry point are discussed here. Advancing HIV testing technology has assisted HIV prevention strategies in many cases and we acknowledge these technologies’ contributions; however in-depth discussion of these approaches is beyond our scope and has been reviewed elsewhere [22, 23]. Similarly, sampling methods in marginalized populations has also been described and will only be discussed here when relevant to seek and test strategies [24]. Many of these seek and test strategies have been applied to the HIV epidemic worldwide yet we choose to focus on concentrated epidemics and in particular the United States where many of the approaches were first described and developed.
Towards integrated care continuums
Engagement in HIV care is now recognized as a continuum, with points resembling a cascade including HIV diagnosis, linkage to care, initiation of ARV, retention in care, and viral suppression [7, 25–27]. Through simulations, Gardner et al. determined that significant improvement in engagement along the entire continuum of care is necessary to curb the HIV epidemic, finding that achievement of 90% engagement in care, treatment of 90% of engaged individuals, and 90% viral suppression would result in improvement in viral suppression and subsequent transmission [27]. The HIV care continuum provides a motivating visual for how care delivery needs to improve to achieve these goals and is widely used by public health departments in describing the state of the HIV epidemic on worldwide, national, and local levels [7].
PrEP offers an opportunity for primary prevention of HIV and subsequently there has been a paradigm shift in that both HIV positive and HIV at-risk individuals can be incorporated into the care continuum [28]. Several care continuums for HIV negative persons exist [29–31]. For example, Horn et al. present a comprehensive, integrated care continuum to illustrate both primary HIV prevention for negative individuals through HIV testing, identification of risk behaviors and needs assessment, counseling for risk reduction, linkage to care, retention and adherence, and re-testing at-risk individuals [28]. For both HIV positive and negative but at-risk individuals, the HIV testing event is a crucial entry into the healthcare system and serves as an important point in time where individuals can be evaluated for appropriate engagement interventions, which has the potential to impact downstream care continuum engagement. Care continuum entry interventions encompass a range of healthcare-based testing (HbT), community-based testing (CbT), and network interventions to engage and re-engage persons in care (Figure 1). These interventions overlap in their efforts to reach key populations. We will review HIV testing interventions as strategies to engage and re-engage in care continuums.
Figure 1.
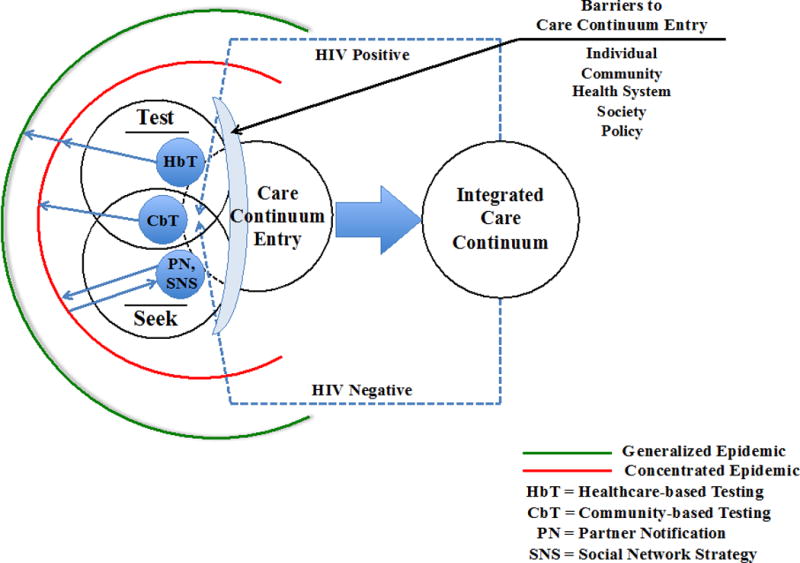
Conceptual model of care continuum entry strategies in the context of epidemic phase. The model depicts the intersection between seek and test strategies and care continuum entry. Seek and test strategies include healthcare-based testing (HbT), community-based testing (CbT), and network approaches of partner notification (PN) and social network strategy (SNS). Strategies are related to epidemic phase, as depicted by the blue arrows [138]. HIV testing is an entry point into the integrated care continuum for both HIV positive and at-risk individuals, who, if not retained, will require re-engagement via care continuum entry interventions [28]. The lens depicts barriers to care continuum entry, which occur on multiple levels: individual, community, health system, society, and policy [26].
Care continuum entry interventions
Healthcare-based testing
With the development of the first HIV test in 1985, screening within healthcare settings was applied to blood donations [32]. This program was extremely successful in decreasing HIV infections transmitted via blood transfusion, virtually eliminating hemophiliacs as a group at risk of HIV infection and transmission in the US, and continues today in the form of donor screening and blood testing [33, 34]. More widespread HbT was not recommended until further knowledge about methods of transmission and risk factors was elucidated. In 1987 the Centers for Disease Control and Prevention (CDC) recommended that all individuals considered to be at high risk based on behavior or seeking treatment for STIs be tested for HIV, and over the years that followed broadened this recommendation in a step-wise approach to also include acute-care hospital settings in high prevalence areas (>1%), and then pregnant women for the prevention of perinatal transmission [32, 35, 36]. During this process, the CDC expanded its recommendation to make HIV testing a routine part of medical care, similar to other screening tests [32]. With advancement in the treatment of HIV, in 2006 the CDC endorsed conducting universal opt-out HIV screening during routine medical care in all healthcare settings for persons aged 13–64 years and all pregnant women on the basis that there is a clear benefit in morbidity, mortality, and transmission that is obtained by identifying early infections and initiating ARV [32]. Repeat screening was recommended for at-risk persons (people who inject drugs (PWID), persons who exchange sex for money or other goods, sex partners of HIV-infected individuals, men who have sex with men (MSM), and heterosexuals with multiple sexual partners) [32]. The USPSTF followed in 2012, aligning with CDC and NHAS goals [8, 32, 37, 38].
Universal opt-out screening has been very effective in nearly eliminating perinatal transmission risk but uptake has been inconsistent across other settings due to societal, legal, organizational and individual-level barriers [21, 39]. Mandated separate written consent and pre-test counseling requirements made it difficult to perform screening in busy clinical environments, therefore many laws have been successfully amended in recent years to promote acceptance through less burdensome procedures [40–42]. Continuing stigma associated with HIV contributes to suboptimal uptake of routine HbT [16, 43]. Providers’ concern for follow up on abnormal tests further inhibits the willingness to offer screening, particularly in settings such as the emergency department (ED) where they are more likely to encounter patients who do not otherwise access the healthcare system [39, 44–46]. Establishing institutional policies that support providers in these areas have been successful in overcoming these barriers, including personnel trained in notification and linkage procedures [47–50].
Best practices for implementation of universal opt-out screening processes in healthcare settings are still being explored, but the ability to leverage existing infrastructure and staff for HIV testing is propelled by the national move towards electronic health records (EHR) [51]. Bioinformatics tools using predictive analytics to incorporate EHR-based algorithms into workflow have enhanced testing rates and improved acceptability of universal screening programs [52, 53]. Algorithms that use sociodemographic characteristics and risk behaviors can be implemented to determine who needs more frequent testing and can help identify at-risk individuals who may qualify for further HIV prevention interventions, including PrEP [54–56].
PLWH who are aware of their HIV infection but not retained in care are now understood to contribute to a significant number of new transmission events, and efforts for re-engagement can begin with the testing event [57]. Universal screening programs implemented in places such as the ED have found that up to 50% of positive HIV tests occur in those already aware of their diagnosis, many of whom are out of care [47]. This group has been difficult to link back to care and novel strategies are being identified to address this problem such as the CDC’s Data to Care program funded through local health departments, as well as using surveillance data to identify and reach these individuals [58–60]. Shifting guidance around the CD4 level appropriate for treatment initiation can send mixed messages to clients not in care who are then re-tested; however, this offers an opportunity for discussions around treatment initiation or re-initiation.
Outreach
HIV testing in non-healthcare, or community-based, settings has become essential for identifying individuals within key populations who do not have frequent contact with the healthcare system [61, 62]. These groups who do not utilize mainstream services, yet are still at risk, are often considered “hidden” populations and are typically at increased risk of health inequity based on identity or socioeconomic status: sexual minorities including gay, bisexual and other MSM; racial minorities, particularly African Americans; gender minorities such as transgender individuals; recently incarcerated individuals; PWID; sex workers; and the unstably housed [63–65]. Frequently the most at-risk groups are dual or even triple minorities (e.g. gender, racial and/or sexual minority); at current rates, 1:2 black MSM will be diagnosed with HIV in their lifetime, the highest of any group [66, 67]. Localization of the epidemic within these vulnerable groups has given rise to tailored CbT strategies, which in addition to case-finding can deliver behavioral interventions to key populations [68–70].
Venue-based interventions have been particularly effective for MSM and PWID, who often display homophily in their choice of sexual and drug-sharing partners, allowing for seek and test efforts to be focused at certain sites such as specific bars and street corners where groups or individuals with risk behaviors are known to congregate [71]. Fujimoto et al. has used social and sexual network dynamics to delineate the relationship between HIV-susceptible individuals and venues; findings indicate centralized venues that have clustering of individuals by sexual behavior and HIV status, with overlap in individuals frequenting these sites [72]. This provides important opportunities for focused interventions and also avoiding duplication of efforts [72]. Furthermore, online venues such as mobile geo-social networking apps (Jack’d, Grindr, Scruff) have become popular for MSM seeking sexual partners [73]. These apps may provide a conduit for locating venues for care continuum entry interventions and distributing information on HIV prevention methods [74].
Additional CbT and outreach efforts have focused on a number of sites seeking susceptible populations such as the unstably housed, youth, the incarcerated, and PWID. Mobile testing has been employed to reach individuals in a wide range of high-risk settings, providing flexibility in testing sites and populations targeted [61]. HIV testing in jails and prisons is high yield for new diagnoses and frequently offers an opportunity for re-engagement [75, 76]. While universal screening in jails and prisons would be ideal, it is not always feasible; thus studies evaluating the relationship between arrest charge and HIV risk are promising to efficiently target testing [77]. Care continuum entry interventions targeting PWID, specifically HIV testing in needle exchange programs and opioid treatment programs, have been crucial to early identification of HIV [78].
Home-based testing has been explored through several different approaches. The first includes door-to-door CbT focusing on micro-epidemics within high prevalence neighborhoods [79]. Home-based self-testing has proven to be acceptable to groups of vulnerable individuals and can provide additional privacy, but has additional challenges to face in engagement in the care continuum after the testing event [80]. In addition, online interventions can tap into widespread groups of people who may be at risk for HIV but not otherwise be identified through other methods, particularly youth who frequently have a strong online presence [81, 82]. The expansion of home-based self-testing and online interventions may remove some barriers such as privacy and healthcare access, but must also be affordable and coupled with a plan for timely care continuum entry and engagement.
These targeted outreach strategies depend on fostering public health partnerships for surveillance and the flexibility to expand services to areas and populations as surveillance information evolves. Tools such as AIDSVu can help direct efforts to specific geographic areas, providing key information on where to deploy mobile units and which communities need allocation of resources. Using the geographic surveillance data to expand HIV testing services, including offering routine testing in non-traditional healthcare settings such as pharmacies and retail clinics in high-prevalence areas is a promising method of expanding access [83]. In addition, real-time surveillance allows for rapid response to emerging epidemics, such as that within PWID in southern Indiana, which prompted statewide mobilization of resources to curb ongoing transmission through a multi-pronged approach [84]. More granular information on community viral load may also further inform seek and test strategies, as well as innovative approaches such as network viral load [85, 86].
Non-targeted outreach programs include general educational efforts as well as public service and media campaigns. In the US, HIV prevention education provided in schools has potential to make an impact, but has been limited by political policies and quality of sexual education [87]. Public service and media campaigns focused on reaching the general public include CDC’s Act Against AIDS Campaign and Chicago’s PrEP4Love. These campaigns have the benefit of reaching a wide audience and can be effective if done in a culturally sensitive manner with a clear, sustained message [88, 89]. They also act to increase awareness among the general population of the ongoing public health implications of HIV. Although media campaigns appear to be less successful in developed countries as compared to developing countries, there is still a role for their use in key populations and specific geographic areas in need of health communication around HIV [89].
Network-based Approaches
The use of contact tracing for venereal disease control has been widespread since 1936, when the Public Health Service first recommended that sex contacts of those infected with syphilis be found, notified, and interviewed for their own protection [90]. Since that time, contact tracing has become the standard of care and primary method of control efforts employed by local Public Health Departments for syphilis and HIV across the United States [91, 92]. Contact tracing has been utilized effectively to eradicate other infectious diseases such as smallpox and is a key strategic element in ongoing polio eradication efforts. Typically, the process of contact tracing in the context of HIV involves Disease Interventionist Specialists querying newly infected clients about their sex or drug contacts and then locating those contacts in the field to inform them that they have been exposed. Models suggest that this approach could be effective in reducing transmission and it may be cost-effective compared to other Public Health Department control efforts [93–96]. For these reasons the approach has been adopted in several other international settings [97, 98].
In the standard-of-care network tracing strategy that has not changed much since 1937, staff interview infected people (called index cases) to elicit names of their sexual or needle-sharing partners (risk partners); notify risk partners of potential risk of infection; and provide prevention services, including HIV testing and linkage/referrals to medical care for partners who have been notified [90]. Partner notification (PN) is an efficacious method of diagnosing HIV-positive individuals, especially when facilitated by a health provider or trained professional [92, 99–104]; in a systematic review of nine studies, 8% of all sex partners listed by index clients were successfully diagnosed with HIV through PN [102]. Another systematic review demonstrated that one new HIV diagnosis was made for every 8–10 partners interviewed through PN [101]. PN reduces HIV burden through diagnosis and subsequent decreases in risky behavior [105–111], HIV/STI transmission [2, 109, 112–114], and improved health outcomes through earlier linkage to treatment [2, 113, 115–118]. The CDC therefore recommends that PN and comprehensive partner services be offered to all recently HIV-diagnosed individuals [92].
Other strategies include network services, referred to as the Social Network Strategy (SNS) by the CDC, where staff conduct similar activities to partner services but elicit names of HIV infected people’s social network rather than limiting it to recent risk partners [119]. Variations on how long the chains are continued from an index client such as 2-steps from an infected client or even 3-step partners services (or network services) where partner engagement occurs 3-steps from an infected client until terminal chains are 3-steps away [120]. Strategies beyond three steps include network mobilization approaches such as snowball or respondent driven sampling and are considered network interventions [121]. We do not include these larger network engagement strategies as they are less targeted and are analytically difficult in observational studies due to contamination and cross-over across clusters as more individuals are engaged.
Policies criminalizing transmission of HIV make contact tracing and partner services for HIV testing and prevention difficult to implement effectively; these laws remain in place in many states [42]. This stigmatizes HIV and prevents effective infection control measures. IDSA and HIVMA have released a statement against policies that criminalize HIV [122]. While it is promising that ongoing de-stigmatization efforts may create safe spaces to discuss sex partners; social network strategies are increasingly utilized given less stigma and may provide benefit in yielding clients infected and at risk for HIV infection. As we enter final HIV elimination efforts, the social network strategy may need to revert back to sexual network care continuum entry approaches.
Molecular care continuum entry approaches
Molecular HIV surveillance is increasingly recognized as a promising approach to both improve care continuum entry and target limited public health resources. Sexually transmitted infections diffuse through contact networks, and the number of onward transmissions varies widely [123, 124]. With limited public health resources, it is important to target care continuum entry efforts towards those most likely to transmit and their sexual partners. Simulations also demonstrate that targeting HIV-seropositive individuals already highly connected in molecular clusters disproportionately decreases onward transmissions [125]. In a recent first effort at intervening in phylogenetically derived networks, members of a rapidly expanding drug resistant HIV cluster in Canada were targeted with enhanced public health follow up to ensure linkage to care - and transmission of drug resistant HIV from this cluster was reduced [126]. In the United States in 2017, 27 jurisdictions participate in molecular HIV surveillance (MHS), an integrated component of CDC’s National HIV Surveillance System to which commercial laboratories report HIV pol nucleotide sequences from clinical drug resistance screening [127]. The HIV-1 pol region has limited length variation (insertions or deletions); this permits robust pairwise alignment, which in turn allows molecular cluster determination from aligned sequences, as well as drug resistance surveillance.
Secure HIV TRACE was launched in March 2017 by Joel Wertheim and others as a feasible approach to guide local health department HIV care continuum entry efforts. HIV-TRACE creates molecular clusters of HIV sequences by calculating all pairwise genetic distances between aligned sequences [128, 129]. Genetic distance is a proxy for epidemiological relatedness, because it increases as a function of time since transmission (recipient’s virus diverging from source’s virus, with each changing). The molecular clock underlying this sequence divergence between source-recipient pairs of HIVs, however, is highly imprecise due to immune/drug selection pressure, viral latency, and other factors. Furthermore, the virus can evolve at different rates in the donor and recipient, so the genetic distance between source and recipient strains is not simply translatable to a standardized time since they diverged. Clusters include sequences that diverge less than a pre-specified threshold (usually 0.5-2%). This threshold is chosen because it is an average estimate of within patient evolution [130], segregates well between the two distributions of distances seen in large sets of sequences [127], and agrees with the genetic distance seen between named HIV risk partners [131]. Clustered sequences can help target intervention based on concern for their size, associated epidemiological or clinical features, or growth.
Next generation care continuum entry strategies
Next generation care continuum entry strategies should include combination approaches that leverage strengths of each testing method to maximize case finding and engagement. Routine healthcare-based testing should trigger social and sexual network testing, particularly in individuals from vulnerable groups such as MSM or PWID. Linking care continuum entry interventions may be difficult to implement in practice, but has the potential to widen the scope of those receiving highly effective social and sexual network testing that identify vulnerable persons, as well as additional wraparound services available in clinical settings.
A second approach to combination care continuum entry includes combining multiple network approaches to best identify individuals who are transmitting as well those who are most at risk for HIV infection. A combined network approach includes social, sexual (both online and offline) and molecular. Such an approach ensures that individuals at greatest risk of acquisition and those who are least likely to be engaged in care or with acute/recent infection (both with high viremia) are getting engaged in HIV care continuum. Inclusion of the online and offline social and sexual networks allow public health practitioners to move beyond a network of positive individuals that is generated from molecular network, but also to include important ties of HIV negative individuals connected to clusters or other HIV infected persons. An example of such an approach (Figure 2), was developed from a population-based cohort of young Black MSM in Chicago from 2013–2016 [132, 133].
Figure 2.
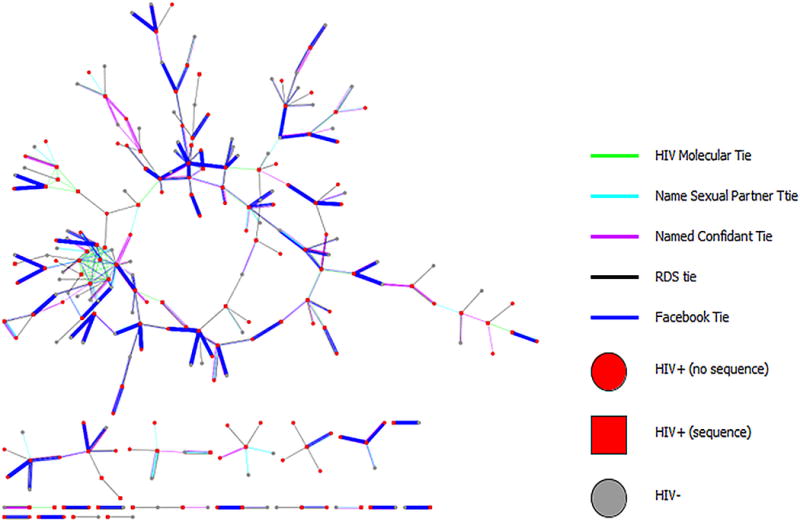
Combined social, sexual, digital and molecular networks among a population-based cohort of young Black MSM (n=618), Chicago IL 2013-2016. Visualization created by Ethan Morgan, PhD.
What can be seen from this network visualization is that there is limited overlap between social and molecular networks and that various network typologies fill in ties and clusters of individuals ideal for care continuum-entry. While Facebook has the most numbers of ties in this network; other online (i.e. Jack’d) and offline (i.e. gay family) networks could fill in important ties that would further risk stratify individuals most in need of care continuum entry.
Targeting interventions for an evolving epidemic
Comprehensive national policies and procedures that integrate healthcare-based HIV testing into routine care and establish performance metrics for health systems around HIV screening will help continue to decrease numbers of PLWH who are unaware of their infection currently, but will have less of an impact as the epidemic becomes increasingly concentrated. Routine HbT has not supplanted the need for ongoing targeted and non-targeted outreach. Sub-populations affected by the HIV epidemic that do not regularly access healthcare may be better served by CbT and outreach, the social network strategy, or partner services, which identify some of the most susceptible individuals. Studies have reported testing strategies’ ability to detect new HIV diagnoses in populations that may be harder to reach due to infrequent or unequal access to healthcare and suggest that these populations may benefit from employing a combination of strategies [134–137]. To be successful in eliminating HIV, systems will need to pivot towards using a combination of evidence-based approaches to reach vulnerable individuals both within and outside of the healthcare system, and closely integrate these approaches for biomedical prevention and care. More research is needed to understand how testing strategy can best be matched to epidemic phase.
Here we focus on strategies to identify and test key populations for HIV in an effort to engage these populations in continuums of care, keeping in mind the dynamic nature of the entire continuum as a cyclical process [28]. Together, those who are HIV infected but undiagnosed and those who are diagnosed but not retained in care account for a significant proportion of new transmission events [57]. Improving care at each point in the continuum, particularly increasing the use of ARVs for prevention, the proportion of PLWH who are virally suppressed, and engagement in biomedical prevention, is the key to HIV elimination. As we develop and hone these strategies for care continuum entry, in turn decreasing the number of PLWH who are unaware of their infection, we must do the same for each step in the continuum. Care continuum entry is only effective in stemming the HIV epidemic if it ultimately leads to decreasing transmission of HIV.
As we move to HIV elimination in the United States, cost-efficient strategies that combine, modify or enhance existing care continuum entry strategies are critical to engage the most vulnerable populations, in which the epidemic is concentrating. The number of new HIV diagnoses in the US is still high and while decreasing overall, it is rising in some groups such as young Black MSM. Real time surveillance by the public health department is promising and incorporating phylogenetic information about transmission patterns may lead to the ability to quickly adapt services and create an early warning system to mobilize resources to areas of the community with high viral loads and active transmission networks. Developing models utilizing this real-time information about where new clusters of diagnoses are occurring and then mobilizing the system to allocate available resources such as mobile testing, partner services, condom distribution, and education may get us closer to identifying the newest cases, and most importantly, those connected to them in order for early intervention. Providing these resource-intensive services will be limited by the stage of the epidemic and only feasible if numbers of new transmissions continue to decline.
Acknowledgments
We would like to thank Joel Wertheim and Manon Ragonnet-Cronin for their input on molecular networks and Ethan Morgan for creating the multiplex network visualization. We would like to thank Jared Kerman for his contribution to creation of the conceptual model. We would also like to thank the National Institutes of Health (R01 DA039934), the Third Coast Center for AIDS Research (P30 AI 117943), and the Center for Prevention Implementation Methodology at Northwestern University Feinberg School of Medicine (P30 DA027828) for their support.
References
- 1.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dieffenbach CW, Fauci AS. Universal voluntary testing and treatment for prevention of HIV transmission. JAMA. 2009;301(22):2380–2382. doi: 10.1001/jama.2009.828. [DOI] [PubMed] [Google Scholar]
- 4.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373(9657):48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 5.Insight Start Study Group. Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373(9):795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. CDC Fact Sheet: Trends in US HIV diagnoses, 2005–2014. Centers for Disease Control and Prevention; 2016. [Google Scholar]
- 7.Centers for Disease Control and Prevention. Understanding the HIV care continuum. Centers for Disease Control and Prevention; 2014. [Google Scholar]
- 8.White House Office of National AIDS Policy. National HIV/AIDS strategy for the United States: updated to 2020. Washington, D.C.: Office of National AIDS Policy; 2015. [Google Scholar]
- 9.Adimora AA, Schoenbach VJ, Floris-Moore MA. Ending the epidemic of heterosexual HIV transmission among African Americans. Am J Prev Med. 2009;37(5):468–471. doi: 10.1016/j.amepre.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall HI, Frazier EL, Rhodes P, Holtgrave DR, Furlow-Parmley C, Tang T, et al. Differences in human immunodeficiency virus care and treatment among subpopulations in the United States. JAMA Intern Med. 2013;173(14):1337–1344. doi: 10.1001/jamainternmed.2013.6841. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Characteristics associated with HIV infection among heterosexuals in urban areas with high AIDS prevalence—24 cities, United States, 2006–2007. MMWR Recomm Rep. 2011;60(31):1045–1049. [PubMed] [Google Scholar]
- 12.Greenberg AE, Purcell DW, Gordon CM, Barasky RJ, del Rio C. Addressing the challenges of the HIV continuum of care in high-prevalence cities in the United States. J Acquir Immune Defic Syndr. 2015;69(Suppl 1):S1–7. doi: 10.1097/QAI.0000000000000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson LE, Wilton L, Moineddin R, Zhang N, Siddiqi A, Sa T, et al. Economic, legal, and social hardships associated with HIV risk among black men who have sex with men in six US cities. J Urban Health. 2016;93(1):170–188. doi: 10.1007/s11524-015-0020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaiser Family Foundation. HIV/AIDS at 30: a public opinion perspective. Kaiser Family Foundation; 2011. [Google Scholar]
- 15.Padamsee TJ. The politics of prevention: lessons from the neglected history of US HIV/AIDS policy. J Health Polit Policy Law. 2017;42(1):73–122. doi: 10.1215/03616878-3702782. [DOI] [PubMed] [Google Scholar]
- 16.Mahajan AP, Sayles JN, Patel VA, Remien RH, Ortiz D, Szekeres G, et al. Stigma in the HIV/AIDS epidemic: a review of the literature and recommendations for the way forward. AIDS. 2008;22(Suppl 2):S67–S79. doi: 10.1097/01.aids.0000327438.13291.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gwadz M, Cleland CM, Hagan H, Jenness S, Kutnick A, Leonard NR, et al. Strategies to uncover undiagnosed HIV infection among heterosexuals at high risk and link them to HIV care with high retention: a “seek, test, treat, and retain” study. BMC Public Health. 2015;15:481. doi: 10.1186/s12889-015-1816-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Institute on Drug Abuse. Seek, test, treat and retain. National Institute on Drug Abuse; 2012. [Google Scholar]
- 19.Burns DN, DeGruttola V, Pilcher CD, Kretzschmar M, Gordon CM, Flanagan EH, et al. Toward an endgame: finding and engaging people unaware of their HIV-1 infection in treatment and prevention. AIDS Res Hum Retroviruses. 2014;30(3):217–224. doi: 10.1089/aid.2013.0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Volkow ND, Baler RD, Normand JL. The unrealized potential of addiction science in curbing the HIV epidemic. Curr HIV Res. 2011;9(6):393–395. doi: 10.2174/157016211798038605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The Lancet HIV. The role of testing in HIV prevention. Lancet HIV. 2017;4(5):e189. doi: 10.1016/S2352-3018(17)30076-0. [DOI] [PubMed] [Google Scholar]
- 22.Daskalakis D. HIV diagnostic testing: evolving technology and testing strategies. Top Antivir Med. 2011;19(1):18–22. [PMC free article] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention and Association of Public Health Laboratories. Laboratory testing for diagnosis of HIV infection: updated recommendations. Centers for Disease Control and Prevention; 2014. [Google Scholar]
- 24.Magnani R, Sabin K, Saidel T, Heckathorn D. Review of sampling hard-to-reach and hidden populations for HIV surveillance. AIDS. 2005;19(Suppl 2):S67–72. doi: 10.1097/01.aids.0000172879.20628.e1. [DOI] [PubMed] [Google Scholar]
- 25.Greenberg AE, Hader SL, Masur H, Young AT, Skillicorn J, Dieffenbach CW. Fighting HIV/AIDS in Washington, D.C. Health Aff (Millwood) 2009;28(6):1677–1687. doi: 10.1377/hlthaff.28.6.1677. [DOI] [PubMed] [Google Scholar]
- 26.Mugavero MJ, Amico KR, Horn T, Thompson MA. The state of engagement in HIV care in the United States: from cascade to continuum to control. Clin Infect Dis. 2013;57(8):1164–1171. doi: 10.1093/cid/cit420. [DOI] [PubMed] [Google Scholar]
- 27.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52(6):793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horn T, Sherwood J, Remien RH, Nash D, Auerbach JD, Treatment Action G, et al. Towards an integrated primary and secondary HIV prevention continuum for the United States: a cyclical process model. J Int AIDS Soc. 2016;19(1):21263. doi: 10.7448/IAS.19.1.21263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNairy ML, El-Sadr WM. A paradigm shift: focus on the HIV prevention continuum. Clin Infect Dis. 2014;59(Suppl_1):S12–15. doi: 10.1093/cid/ciu251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelley CF, Kahle E, Siegler A, Sanchez T, Del Rio C, Sullivan PS, et al. Applying a PrEP continuum of care for men who have sex with men in Atlanta, Georgia. Clin Infect Dis. 2015;61(10):1590–1597. doi: 10.1093/cid/civ664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith DK, Beltrami J. A proposed framework to monitor daily oral antiretroviral pre-exposure prophylaxis in the U.S. Am J Prev Med. 2013;44(1 Suppl 2):S141–146. doi: 10.1016/j.amepre.2012.09.040. [DOI] [PubMed] [Google Scholar]
- 32.Branson BM, Handsfield HH, Lampe MA, Janssen RS, Taylor AW, Lyss SB, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1–17. quiz CE11-14. [PubMed] [Google Scholar]
- 33.U.S. Food and Drug Administration. Keeping blood transfusions safe: FDA’s multi-layered protections for donated blood. U.S. Food and Drug Administration; 2015. [Google Scholar]
- 34.Kroner BL, Rosenberg PS, Aledort LM, Alvord WG, Goedert JJ. HIV-1 infection incidence among persons with hemophilia in the United States and western Europe, 1978–1990. Multicenter Hemophilia Cohort Study. J Acquir Immune Defic Syndr. 1994;7(3):279–286. [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention. U.S. Public Health Service recommendations for human immunodeficiency virus counseling and voluntary testing for pregnant women. MMWR Recomm Rep. 1995;44(RR-7) [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention. Public health service guidelines for counseling and antibody testing to prevent HIV infection and AIDS. MMWR Recomm Rep. 1987;36:509–515. [PubMed] [Google Scholar]
- 37.U.S. Preventive Services Task Force. Final recommendation statement: human immunodeficiency virus (HIV) infection: screening. U.S. Preventive Services Task Force; 2016. [Google Scholar]
- 38.Chou R, Selph S, Dana T, Bougatsos C, Zakher B, Blazina I, et al. AHRQ Publication No 12-05173-EF-1. Rockville, MD: Agency for Healthcare Research and Quality; 2012. Screening for HIV: systematic review to update the U.S. Preventive Services Task Force recommendation. Evidence Synthesis No. 95. [PubMed] [Google Scholar]
- 39.Haukoos JS, Hopkins E. Understanding HIV screening in the emergency department: is perception reality? Acad Emerg Med. 2013;20(3):309–312. doi: 10.1111/acem.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frieden TR, Das-Douglas M, Kellerman SE, Henning KJ. Applying public health principles to the HIV epidemic. N Engl J Med. 2005;353(22):2397–2402. doi: 10.1056/NEJMsb053133. [DOI] [PubMed] [Google Scholar]
- 41.Centers for Disease Control and Prevention. State HIV testing laws: consent and counseling requirements. Centers for Disease Control and Prevention; 2015. [Google Scholar]
- 42.Centers for Disease Control and Prevention. State HIV laws. Centers for Disease Control and Prevention; 2017. [Google Scholar]
- 43.Stringer KL, Turan B, McCormick L, Durojaiye M, Nyblade L, Kempf M-C, et al. HIV-related stigma among healthcare providers in the Deep South. AIDS Behav. 2016;20(1):115–125. doi: 10.1007/s10461-015-1256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gardner EM, Haukoos JS. At the crossroads of the HIV care continuum: emergency departments and the HIV epidemic. Ann Emerg Med. 2015;66(1):79–81. doi: 10.1016/j.annemergmed.2015.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schnall R, Clark S, Olender S, Sperling JD. Providers’ perceptions of the factors influencing the implementation of the New York State mandatory HIV testing law in two urban academic emergency departments. Acad Emerg Med. 2013;20(3):279–286. doi: 10.1111/acem.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arbelaez C, Wright EA, Losina E, Millen JC, Kimmel S, Dooley M, et al. Emergency provider attitudes and barriers to universal HIV testing in the emergency department. J Emerg Med. 2012;42(1):7–14. doi: 10.1016/j.jemermed.2009.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bares S, Eavou R, Bertozzi-Villa C, Taylor M, Hyland H, McFadden R, et al. Expanded HIV testing and linkage to care: conventional vs. point-of-care testing and assignment of patient notification and linkage to care to an HIV care program. Public Health Rep. 2016;131(Suppl 1):107–120. doi: 10.1177/00333549161310S113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kelen GD, Hsieh YH, Rothman RE, Patel EU, Laeyendecker OB, Marzinke MA, et al. Improvements in the continuum of HIV care in an inner-city emergency department. AIDS. 2016;30(1):113–120. doi: 10.1097/QAD.0000000000000896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schrantz SJ, Babcock CA, Theodosis C, Brown S, Mercer S, Pillow MT, et al. A targeted, conventional assay, emergency department HIV testing program integrated with existing clinical procedures. Ann Emerg Med. 2011;58(1 Suppl 1):S85–88 e81. doi: 10.1016/j.annemergmed.2011.03.031. [DOI] [PubMed] [Google Scholar]
- 50.Burke RC, Sepkowitz KA, Bernstein KT, Karpati AM, Myers JE, Tsoi BW, et al. Why don’t physicians test for HIV? A review of the US literature. AIDS. 2007;21(12):1617–1624. doi: 10.1097/QAD.0b013e32823f91ff. [DOI] [PubMed] [Google Scholar]
- 51.Signer D, Peterson S, Hsieh YH, Haider S, Saheed M, Neira P, et al. Scaling up HIV testing in an academic emergency department: an integrated testing model with rapid fourth-generation and point-of-care testing. Public Health Rep. 2016;131(Suppl 1):82–89. doi: 10.1177/00333549161310S110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McGuire R, Moore E. Using a configurable EMR and decision support tools to promote process integration for routine HIV screening in the emergency department. J Am Med Inform Assoc. 2016;23(2):396–401. doi: 10.1093/jamia/ocv031. [DOI] [PubMed] [Google Scholar]
- 53.Avery AK, Del Toro M, Caron A. Increases in HIV screening in primary care clinics through an electronic reminder: an interrupted time series. BMJ Qual Saf. 2014;23(3):250–256. doi: 10.1136/bmjqs-2012-001775. [DOI] [PubMed] [Google Scholar]
- 54.Haukoos JS, Hopkins E, Bucossi MM, Lyons MS, Rothman RE, White DAE, et al. Validation of a quantitative HIV risk prediction tool using a national HIV testing cohort. Jaids-J Acq Imm Def. 2015;68(5):599–603. doi: 10.1097/QAI.0000000000000518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin J, Mauntel-Medici C, Heinert S, Baghikar S. Harnessing the power of the electronic medical record to facilitate an opt-out HIV screening program in an urban academic emergency department. J Public Health Manag Pract. 2017;23(3):264–268. doi: 10.1097/PHH.0000000000000448. [DOI] [PubMed] [Google Scholar]
- 56.Lancki N, Holman D, Eavou R, Pitrak D, Schneider J, Ridgway J. Use of an electronic medical record (EMR)-based algorithm to identify patients for prevention follow-up among patients tested for human immunodeficiency virus (HIV) in the emergency department. Open Forum Infect Dis. 2016;3(suppl_1):472. [Google Scholar]
- 57.Skarbinski J. Human immunodeficiency virus transmission at each step of the care continuum in the United States. JAMA Intern Med. 2015;175:588–596. doi: 10.1001/jamainternmed.2014.8180. [DOI] [PubMed] [Google Scholar]
- 58.Dombrowski JC, Carey JW, Pitts N, Craw J, Freeman A, Golden MR, et al. HIV provider and patient perspectives on the development of a health department “Data to Care” program: a qualitative study. BMC Public Health. 2016;16 doi: 10.1186/s12889-016-3152-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Centers for Disease Control and Prevention. Data to Care: Improving Health and Prevention. Centers for Disease Control and Prevention; 2015. [Google Scholar]
- 60.Buchacz K, Chen MJ, Parisi MK, Yoshida-Cervantes M, Antunez E, Delgado V, et al. Using HIV surveillance registry data to re-link persons to care: the RSVP Project in San Francisco. PLoS One. 2015;10(3):e0118923. doi: 10.1371/journal.pone.0118923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suthar AB, Ford N, Bachanas PJ, Wong VJ, Rajan JS, Saltzman AK, et al. Towards universal voluntary HIV testing and counselling: a systematic review and meta-analysis of community-based approaches. PLoS Med. 2013;10(8):e1001496. doi: 10.1371/journal.pmed.1001496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thornton AC, Delpech V, Kall MM, Nardone A. HIV testing in community settings in resource-rich countries: a systematic review of the evidence. HIV Med. 2012;13(7):416–426. doi: 10.1111/j.1468-1293.2012.00992.x. [DOI] [PubMed] [Google Scholar]
- 63.Institute of Medicine Committee on Lesbian G, Bisexual, and Transgender Health Issues and Research Gaps and Opportunities; Board on Health of Select Populations. The Health of Lesbian, Gay, Bisexual, and Transgender People: Building a Foundation for Better Understanding. Washington, D.C: National Academies Press; 2011. [PubMed] [Google Scholar]
- 64.Institute of Medicine. Unequal treatment: confronting racial and ethnic disparities in health care. Washington, D.C.: National Academies Press. Institute of Medicine; 2003. [PubMed] [Google Scholar]
- 65.Makadon H, Mayer K, Potter J, Goldhammer H. The Fenway Guide to Lesbian, Gay, Bisexual and Transgender Health. Philadelphia, PA: American College of Physicians; 2008. [Google Scholar]
- 66.Kates J, Ranji U, Beamesderfer A, Salganicoff A, Dawson L. Health and access to care and coverage for lesbian, gay, bisexual, and transgender individuals in the US. Kaiser Family Foundation; 2016. [Google Scholar]
- 67.Centers for Disease Control and Prevention. Lifetime risk of HIV diagnosis. Centers for Disease Control and Prevention; 2016. [Google Scholar]
- 68.Centers for Disease Control and Prevention. Advancing HIV prevention: new strategies for a changing epidemic – United States, 2003. MMWR Recomm Rep. 2003;52(15):329–332. [PubMed] [Google Scholar]
- 69.Centers for Disease Control and Prevention. Implementing HIV testing in nonclinical settings: a guide for HIV testing providers. Centers for Disease Control and Prevention; 2016. [Google Scholar]
- 70.Coleman SM, Rajabiun S, Cabral HJ, Bradford JB, Tobias CR. Sexual risk behavior and behavior change among persons newly diagnosed with HIV: the impact of targeted outreach interventions among hard-to-reach populations. AIDS Patient Care STDs. 2009;23(8):639–645. doi: 10.1089/apc.2008.0092. [DOI] [PubMed] [Google Scholar]
- 71.Fujimoto K, Wang P, Ross MW, Williams ML. Venue-mediated weak ties in multiplex HIV transmission risk networks among drug-using male sex workers and associates. Am J Public Health. 2015;105(6):1128–1135. doi: 10.2105/AJPH.2014.302474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fujimoto K, Williams ML, Ross MW. Venue-based affiliation networks and HIV risk-taking behavior among male sex workers. Sex Transm Dis. 2013;40(6):453–458. doi: 10.1097/OLQ.0b013e31829186e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beymer MR, Weiss RE, Bolan RK, Rudy ET, Bourque LB, Rodriguez JP, et al. Sex on demand: geosocial networking phone apps and risk of sexually transmitted infections among a cross-sectional sample of men who have sex with men in Los Angeles County. Sex Transm Infect. 2014;90(7):567–572. doi: 10.1136/sextrans-2013-051494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goedel WC, Duncan DT. Geosocial-networking app usage patterns of gay, bisexual, and other men who have sex with men: survey among users of Grindr, a mobile dating app. JMIR Public Health Surveill. 2015;1(1):e4. doi: 10.2196/publichealth.4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Harawa N, Adimora A. Incarceration African Americans and HIV: advancing a research agenda. J Natl Med Assoc. 2008;100(1):57–63. doi: 10.1016/s0027-9684(15)31175-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eastment MC, Toren KG, Strick L, Buskin SE, Golden MR, Dombrowski JC. Jail booking as an occasion for HIV care reengagement: a surveillance-based study. Am J Public Health. 2017;107(5):717–723. doi: 10.2105/AJPH.2017.303668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Harawa NT, Bingham TA, Butler QR, Dalton KS, Cunningham WE, Behel S, et al. Using arrest charge to screen for undiagnosed HIV infection among new arrestees: a study in Los Angeles County. J Correct Health Care. 2009;15(2):105–117. doi: 10.1177/1078345808330038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Metsch LR, Feaster DJ, Gooden L, Matheson T, Mandler RN, Haynes L, et al. Implementing rapid HIV testing with or without risk-reduction counseling in drug treatment centers: results of a randomized trial. Am J Public Health. 2012;102(6):1160–1167. doi: 10.2105/AJPH.2011.300460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nunn A, Yolken A, Cutler B, Trooskin S, Wilson P, Little S, et al. Geography should not be destiny: focusing HIV/AIDS implementation research and programs on microepidemics in US neighborhoods. Am J Public Health. 2014;104(5):775–780. doi: 10.2105/AJPH.2013.301864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lippman SA, Moran L, Sevelius J, Castillo LS, Ventura A, Treves-Kagan S, et al. Acceptability and feasibility of HIV self-testing among transgender women in San Francisco: a mixed methods pilot study. AIDS Behav. 2016;20(4):928–938. doi: 10.1007/s10461-015-1236-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rice E, Tulbert E, Cederbaum J, Barman Adhikari A, Milburn NG. Mobilizing homeless youth for HIV prevention: a social network analysis of the acceptability of a face-to-face and online social networking intervention. Health Educ Res. 2012;27(2):226–236. doi: 10.1093/her/cyr113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mustanski B, Garofalo R, Monahan C, Gratzer B, Andrews R. Feasibility, acceptability, and preliminary efficacy of an online HIV prevention program for diverse young men who have sex with men: the Keep It Up! intervention. AIDS Behav. 2013;17(9):2999–3012. doi: 10.1007/s10461-013-0507-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Weidle PJ, Lecher S, Botts LW, Jones L, Spach DH, Alvarez J, et al. HIV testing in community pharmacies and retail clinics: a model to expand access to screening for HIV infection. J Am Pharm Assoc. 2014;54(5):486–492. doi: 10.1331/JAPhA.2014.14045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Conrad C, Bradley HM, Broz D, Buddha S, Chapman EL, Galang RR, et al. Community outbreak of HIV infection linked to injection drug use of oxymorphone–Indiana, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(16):443–444. [PMC free article] [PubMed] [Google Scholar]
- 85.Das M, Chu PL, Santos GM, Scheer S, Vittinghoff E, McFarland W, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS One. 2010;5(6):e11068. doi: 10.1371/journal.pone.0011068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Livak B, Schneider JA. International Network for Social Network Analysis, Sunbelt XXXIV. St Pete Beach, Florida: 2014. The network viral load: a novel HIV risk assessment strategy. [Google Scholar]
- 87.Schalet AT, Santelli JS, Russell ST, Halpern CT, Miller SA, Pickering SS, et al. Invited commentary: broadening the evidence for adolescent sexual and reproductive health and education in the United States. J Youth Adolesc. 2014;43(10):1595–1610. doi: 10.1007/s10964-014-0178-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Romer D, Sznitman S, DiClemente R, Salazar LF, Vanable PA, Carey MP, et al. Mass media as an HIV-prevention strategy: using culturally sensitive messages to reduce HIV-associated sexual behavior of at-risk African American youth. Am J Public Health. 2009;99(12):2150–2159. doi: 10.2105/AJPH.2008.155036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.LaCroix JM, Snyder LB, Huedo-Medina TB, Johnson BT. Effectiveness of mass media interventions for HIV prevention, 1986–2013: a meta-analysis. J Acquir Immune Defic Syndr. 2014;66:S329–S340. doi: 10.1097/QAI.0000000000000230. [DOI] [PubMed] [Google Scholar]
- 90.United States Public Health Service. The eradication of syphilis. Washington D.C.: U.S. Dept. of Health, Education and Welfare; 1961. [Google Scholar]
- 91.Samoff E, Koumans EH, Katkowsky S, Shouse RL, Markowitz LE. Contact-tracing outcomes among male syphilis patients in Fulton County, Georgia, 2003. Sex Transm Dis. 2007;34(7):456–460. doi: 10.1097/01.olq.0000251203.34805.28. [DOI] [PubMed] [Google Scholar]
- 92.Centers for Disease Control and Prevention. Recommendations for partner services programs for HIV infection, syphilis, gonorrhea, and chlamydial infection. MMWR Recomm Rep. 2008;57(RR-9):1–83. quiz CE81-84. [PubMed] [Google Scholar]
- 93.Cohen DA, Wu SY, Farley TA. Comparing the cost-effectiveness of HIV prevention interventions. J Acquir Immune Defic Syndr. 2004;37(3):1404–1414. doi: 10.1097/01.qai.0000123271.76723.96. [DOI] [PubMed] [Google Scholar]
- 94.Holtgrave DR, Valdiserri RO, Gerber AR, Hinman AR. Human immunodeficiency virus counseling, testing, referral, and partner notification services. A cost-benefit analysis. Archives of internal medicine. 1993;153(10):1225–1230. [PubMed] [Google Scholar]
- 95.Hyman JM, Li J, Stanley EA. Modeling the impact of random screening and contact tracing in reducing the spread of HIV. Mathematical biosciences. 2003;181(1):17–54. doi: 10.1016/s0025-5564(02)00128-1. [DOI] [PubMed] [Google Scholar]
- 96.Landis SE, Schoenbach VJ, Weber DJ, Mittal M, Krishan B, Lewis K, et al. Results of a randomized trial of partner notification in cases of HIV infection in North-Carolina. New Engl J Med. 1992;326(2):101–106. doi: 10.1056/NEJM199201093260205. [DOI] [PubMed] [Google Scholar]
- 97.Hsieh YH, Wang YS, de Arazoza H, Lounes R. Modeling secondary level of HIV contact tracing: its impact on HIV intervention in Cuba. BMC infectious diseases. 2010;10:194. doi: 10.1186/1471-2334-10-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brown LB, Miller WC, Kamanga G, Nyirenda N, Mmodzi P, Pettifor A, et al. HIV partner notification is effective and feasible in sub-Saharan Africa: opportunities for HIV treatment and prevention. J Acquir Immune Defic Syndr. 2011;56(5):437–442. doi: 10.1097/qai.0b013e318202bf7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Golden MR, Dombrowski JC, Wood RW, Fleming M, Harrington RD. A controlled study of the effectiveness of public health HIV partner notification services. AIDS. 2009;23(1):133–135. doi: 10.1097/QAD.0b013e32831fb52f. [DOI] [PubMed] [Google Scholar]
- 100.Golden MR, Hogben M, Potterat JJ, Handsfield HH. HIV partner notification in the United States: a national survey of program coverage and outcomes. Sex Transm Dis. 2004;31(12):709–712. doi: 10.1097/01.olq.0000145847.65523.43. [DOI] [PubMed] [Google Scholar]
- 101.Brewer DD. Case-finding effectiveness of partner notification and cluster investigation for sexually transmitted diseases/HIV. Sex Transm Dis. 2005;32(2):78–83. doi: 10.1097/01.olq.0000153574.38764.0e. [DOI] [PubMed] [Google Scholar]
- 102.Hogben M, McNally T, McPheeters M, Hutchinson AB. The effectiveness of HIV partner counseling and referral services in increasing identification of HIV-positive individuals a systematic review. Am J Prev Med. 2007;33(2 Suppl):S89–100. doi: 10.1016/j.amepre.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 103.Task Force on Community Preventive Services. Recommendations to increase testing and identification of HIV-positive individuals through partner counseling and referral services. Am J Prev Med. 2007;33(2 Suppl):S88. doi: 10.1016/j.amepre.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 104.Macke BA, Maher JE. Partner notification in the United States: an evidence-based review. Am J Prev Med. 1999;17(3):230–242. doi: 10.1016/s0749-3797(99)00076-8. [DOI] [PubMed] [Google Scholar]
- 105.Gorbach PM, Weiss RE, Jeffries R, Javanbakht M, Drumright LN, Daar ES, et al. Behaviors of recently HIV-infected men who have sex with men in the year postdiagnosis: effects of drug use and partner types. J Acquir Immune Defic Syndr. 2011;56(2):176–182. doi: 10.1097/QAI.0b013e3181ff9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gorbach PM, Drumright LN, Daar ES, Little SJ. Transmission behaviors of recently HIV-infected men who have sex with men. J Acquir Immune Defic Syndr. 2006;42(1):80–85. doi: 10.1097/01.qai.0000196665.78497.f1. [DOI] [PubMed] [Google Scholar]
- 107.Khanna AS, Goodreau SM, Gorbach PM, Daar E, Little SJ. Modeling the impact of post-diagnosis behavior change on HIV prevalence in Southern California men who have sex with men (MSM) AIDS Behav. 2014;18(8):1523–1531. doi: 10.1007/s10461-013-0646-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fenton KA, Peterman TA. HIV partner notification: taking a new look. AIDS. 1997;11(13):1535–1546. doi: 10.1097/00002030-199713000-00001. [DOI] [PubMed] [Google Scholar]
- 109.Fox J, White PJ, Macdonald N, Weber J, McClure M, Fidler S, et al. Reductions in HIV transmission risk behaviour following diagnosis of primary HIV infection: a cohort of high-risk men who have sex with men. HIV Med. 2009;10(7):432–438. doi: 10.1111/j.1468-1293.2009.00708.x. [DOI] [PubMed] [Google Scholar]
- 110.Steward WT, Remien RH, Higgins JA, Dubrow R, Pinkerton SD, Sikkema KJ, et al. Behavior change following diagnosis with acute/early HIV infection-a move to serosorting with other HIV-infected individuals. The NIMH Multisite Acute HIV Infection Study: III. AIDS Behav. 2009;13(6):1054–1060. doi: 10.1007/s10461-009-9582-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vallabhaneni S, McConnell JJ, Loeb L, Hartogensis W, Hecht FM, Grant RM, et al. Changes in seroadaptive practices from before to after diagnosis of recent HIV infection among men who have sex with men. PLoS One. 2013;8(2):e55397. doi: 10.1371/journal.pone.0055397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Khanna A, Goodreau SM, Wohlfeiler D, Daar E, Little S, Gorbach PM. Individualized diagnosis interventions can add significant effectiveness in reducing human immunodeficiency virus incidence among men who have sex with men: insights from Southern California. Ann Epidemiol. 2015;25(1):1–6. doi: 10.1016/j.annepidem.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dieffenbach CW. Preventing HIV transmission through antiretroviral treatment-mediated virologic suppression: aspects of an emerging scientific agenda. Curr Opin HIV AIDS. 2012;7(2):106–110. doi: 10.1097/COH.0b013e32834f3f13. [DOI] [PubMed] [Google Scholar]
- 114.Donnell D, Baeten JM, Kiarie J, Thomas KK, Stevens W, Cohen CR, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet. 2010;375(9731):2092–2098. doi: 10.1016/S0140-6736(10)60705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Egger M, May M, Chene G, Phillips AN, Ledergerber B, Dabis F, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360(9327):119–129. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 116.Le T, Wright EJ, Smith DM, He W, Catano G, Okulicz JF, et al. Enhanced CD4+ T-cell recovery with earlier HIV-1 antiretroviral therapy. N Engl J Med. 2013;368(3):218–230. doi: 10.1056/NEJMoa1110187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Collins SE, Jean Juste MA, Koenig SP, Secours R, Ocheretina O, Bernard D, et al. CD4 deficit and tuberculosis risk persist with delayed antiretroviral therapy: 5-year data from CIPRA HT-001. Int J Tuberc Lung Dis. 2015;19(1):50–57. doi: 10.5588/ijtld.14.0217. [DOI] [PubMed] [Google Scholar]
- 118.Grinsztejn B, Hosseinipour MC, Ribaudo HJ, Swindells S, Eron J, Chen YQ, et al. Effects of early versus delayed initiation of antiretroviral treatment on clinical outcomes of HIV-1 infection: results from the phase 3 HPTN 052 randomised controlled trial. Lancet Infect Dis. 2014;14(4):281–290. doi: 10.1016/S1473-3099(13)70692-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Centers for Disease Control and Prevention. Effective behavioral interventions: social network strategy. Centers for Disease Control and Prevention; [Google Scholar]
- 120.Nikolopoulos GK, Pavlitina E, Muth SQ, Schneider J, Psichogiou M, Williams LD, et al. A network intervention that locates and intervenes with recently HIV-infected persons: the Transmission Reduction Intervention Project (TRIP) Sci Rep. 2016;6:38100. doi: 10.1038/srep38100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Valente TW. Network Interventions. Science. 2012;337(6090):49–53. doi: 10.1126/science.1217330. [DOI] [PubMed] [Google Scholar]
- 122.Infectious Diseases Society of America, HIV Medicine Association. Infectious Diseases Society of America (IDSA) and HIV Medicine Association (HIVMA) position on the criminalization of HIV, sexually transmitted infections and other communicable diseases. HIV Medicine Association; 2015. [Google Scholar]
- 123.Hamilton DT, Handcock MS, Morris M. Degree distributions in sexual networks: A framework for evaluating evidence. Sexually Transmitted Diseases. 2008;35(1):30–40. doi: 10.1097/olq.0b013e3181453a84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Anderson RM, May RM. Infectious Diseases of Humans: Dynamics and Control. Oxford University Press; 1992. [Google Scholar]
- 125.Little SJ, Kosakovsky Pond SL, Anderson CM, Young JA, Wertheim JO, Mehta SR, et al. Using HIV networks to inform real time prevention interventions. PLoS One. 2014;9(6):e98443. doi: 10.1371/journal.pone.0098443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Poon AF, Gustafson R, Daly P, Zerr L, Demlow SE, Wong J, et al. Near real-time monitoring of HIV transmission hotspots from routine HIV genotyping: an implementation case study. Lancet HIV. 2016;3(5):e231–238. doi: 10.1016/S2352-3018(16)00046-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Centers for Disease Control and Prevention. Detecting, investigating, and responding to HIV transmission clusters, version 1.0. Centers for Disease Control and Prevention; 2017. [Google Scholar]
- 128.Wertheim JO, Leigh Brown AJ, Hepler NL, Mehta SR, Richman DD, Smith DM, et al. The global transmission network of HIV-1. J Infect Dis. 2014;209(2):304–313. doi: 10.1093/infdis/jit524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Oster AM, Wertheim JO, Hernandez AL, Ocfemia MCB, Saduvala N, Hall HI. Using molecular HIV surveillance data to understand transmission between subpopulations in the United States. J Acquir Immune Defic Syndr. 2015;70(4):444–451. doi: 10.1097/QAI.0000000000000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hue S, Clewley JP, Cane PA, Pillay D. HIV-1 pol gene variation is sufficient for reconstruction of transmissions in the era of antiretroviral therapy. AIDS. 2004;18(5):719–728. doi: 10.1097/00002030-200403260-00002. [DOI] [PubMed] [Google Scholar]
- 131.Wertheim JO, Pond SLK, Forgione LA, Mehta SR, Murrell B, Shah S, et al. Social and genetic networks of HIV-1 transmission in New York City. Plos Pathog. 2017;13(1) doi: 10.1371/journal.ppat.1006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Schneider JA, C B, Jonas A, Behler R, Lancki N, Skaathun B, Michaels S, Khanna AS, Young LE, Morgan E, Duvoisin R, Friedman S, Schumm P, Laumann EO. Network dynamics of HIV risk and prevention in a population-based cohort of young Black men who have sex with men. Network Science. 2017 [Google Scholar]
- 133.Schneider JA, Kozloski M, Michaels S, Skaathun B, Voisin D, Lancki N, et al. Criminal justice involvement history is associated with better HIV care continuum metrics among a population-based sample of young black MSM. AIDS. 2017;31(1):159–165. doi: 10.1097/QAD.0000000000001269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gaiter JL, Johnson WD, Taylor E, Thadiparthi S, Duncan-Alexander T, Lemon C, et al. Sisters empowered, sisters aware: three strategies to recruit African American women for HIV testing. AIDS Educ Prev. 2013;25(3):190–202. doi: 10.1521/aeap.2013.25.3.190. [DOI] [PubMed] [Google Scholar]
- 135.Halkitis PN, Kupprat SA, McCree DH, Simons SM, Jabouin R, Hampton MC, et al. Evaluation of the relative effectiveness of three HIV testing strategies targeting African American men who have sex with men (MSM) in New York City. Ann Behav Med. 2011;42(3):361–369. doi: 10.1007/s12160-011-9299-4. [DOI] [PubMed] [Google Scholar]
- 136.Baytop C, Royal S, Hubbard McCree D, Simmons R, Tregerman R, Robinson C, et al. Comparison of strategies to increase HIV testing among African-American gay, bisexual, and other men who have sex with men in Washington, DC. AIDS Care. 2014;26(5):608–612. doi: 10.1080/09540121.2013.845280. [DOI] [PubMed] [Google Scholar]
- 137.Ellen JM, McCree DH, Muvva R, Chung SE, Miazad RM, Arrington-Sanders R, et al. Recruitment approaches to identifying newly diagnosed HIV infection among African American men who have sex with men. Int J STD AIDS. 2013;24(5):335–339. doi: 10.1177/0956462412472459. [DOI] [PubMed] [Google Scholar]
- 138.Blanchard JF. Populations, pathogens, and epidemic phases: closing the gap between theory and practice in the prevention of sexually transmitted diseases. Sex Transm Infect. 2002;78(Suppl 1):i183–i188. doi: 10.1136/sti.78.suppl_1.i183. [DOI] [PMC free article] [PubMed] [Google Scholar]


