Abstract
A low-cost electrochemical sensor with Nafion/Bi modification using adsorptive stripping voltammetry for Co and Ni determination in airborne particulate matter and welding fume samples is described. Carbon stencil-printed electrodes (CSPEs) manufactured on low-cost PET films were utilized. Dimethylglyoxime (DMG) was used as a Co(II) and Ni(II) chelator with selective chemical precipitation for trace electrochemical analysis. Electrochemical studies of the Nafion/Bi-modified CSPE indicated a diffusion-controlled redox reaction for Co and Ni measurements. The Nafion coating decreased the background current and enhanced the measured peak current. Repeatability tests based on changes in percent relative standard deviation (RSD) of peak current showed the electrode could be used at least 15 times before the RSD exceeded 15% (the reported value of acceptable repeatability from Association of Official Analytical Chemists (AOAC)) due to deterioration of electrode surface. Limits of detection were 1 μg L−1 and 5 μg L−1 for Co and Ni, respectively, which were comparable to electrochemical sensors requiring more complicated modification procedures. The sensor produced a working range of 1–250 and 5–175 μg L−1 for Co and Ni, respectively. Interference studies showed no other metal species interfered with Co and Ni measurements using the optimized conditions. Finally, the developed sensors were applied for Co and Ni determination in aerosol samples generated from Co rods and a certified welding-fume reference material, respectively. Validation with ICP-MS showed no statistically different results with 95% confidence between sensor and the ICP methods.
Highlights: Carbon stencil-printed electrode, Bi modified electrode, Co and Ni detection, Aerosol analysis, Adsorptive cathodic square-wave stripping voltammetry
1. Introduction
Co and Ni exposure are detrimental to human health depending on the magnitude and duration of exposure [1–7]. Occupational exposure to Co has been linked to a variety of respiratory tract and skin disorders such as skin lesions from allergy, inflammation of nasopharynx, and bronchial asthma [4]. Mortality from Co exposure can also occur when individuals reach to the final stage at which cor pulmonale and cardiorespiratory failure take place [4]. Long-term exposure to Ni has been associated with incidence of nasal cancer [8]. High occupational exposure of Co and Ni occurs primarily in industrial settings [9]. The amount of Co found in industrial areas can exceed 10 ng m−3, which is substantially higher than in remote areas (1 × 10−4 ng m−3) [10]. Similarly, Ni can be released from a variety of industrial processes such as welding (e.g., from stainless steel), leading to high occupational exposures [3, 11]. Therefore, measurement of Co and Ni in aerosols is important for understanding Co and Ni exposure.
Conventional measurements of Co and Ni measurements are performed using spectrophotometry coupled with flow injection analysis [12], atomic absorption spectrometry [13], x-ray fluorescence spectrometry [14], and inductively coupled plasma spectroscopy [15]. These traditional methods require expensive and/or complicated equipment and long, laboratory-based analysis. Several fast, low-cost sensors have been proposed for metal detection [16–21]. Recently, we have achieved colorimetric detection for Ni in particulate matter (PM) with microfluidic paper-based analytical devices (μPADs) [18, 22, 23]. Here, we describe a low-cost electrochemical sensor (less than $0.1) for Co and Ni with improved sensitivity and selectivity [16, 17, 24, 25]. Several other reports utilized Hg thin film electrodes [26, 27] or cation exchanger-modified electrodes [28] for detecting Co and Ni, but these electrodes require relatively complicated preparation procedures. Bi was also introduced to avoid the use of Hg while providing analogous analytical capability of forming metal amalgams to generate well-defined peaks and reproducible stripping signals [29–31]. For trace Co(II) and Ni(II) analysis, dimethylglyoxime (DMG) has been used as a chelator to selectively complex Co(II) and Ni(II) before detecting these complexes with adsorptive stripping voltammetry that could adsorptively accumulate sub-ppb level of complexes on the working electrode [32–34].
Here, carbon stencil-printed electrodes (CSPEs) were modified with bismuth, fabricated on polyethylene transparency (PET) sheets, and used to detect Co and Ni in particulate matter and welding fume. In the proposed method, DMG was employed as a chelating agent for complexing with Co and Ni and the complexes were detected by adsorptive cathodic stripping voltammetry. The ability of Bi-modified CSPE (BiCSPEs) to analyze Co(II)DMG and Ni(II)DMG was compared with that of unmodified CSPEs. Electrochemical characterization indicated a diffusion-controlled redox reaction for Co and Ni complexes. Nafion coating of the electrode surface enhanced peak current and lowered background current, improving the detection limit. Sensor precision was within the Association of Official Analytical Chemists (AOAC) relative standard deviation (RSD) limit of 15% [35]. Common metals that might interfere with Co and Ni measurements were analyzed and none of them showed significant interference. Finally, Nafion/BiCSPEs were applied for Co and Ni detection in aerosols and welding fume samples. Samples were validated with inductively coupled plasma mass spectrometry (ICP-MS) and the techniques provided statistically similar results. This work demonstrates the development of a low-cost, portable, and disposable sensor for Co and Ni with detection limits at ppb levels.
2. Experimental
2.1 Materials and Methods
Zinc(II) nitrate, chromium(III) chloride, cobalt(II) chloride, aluminum sulfate, bismuth(III) oxide, sodium dodecyl sulfate (SDS), sodium acetate trihydrate, and trimethylsilylated Nafion® were purchased from Sigma–Aldrich (St. Louis, MO). Potassium dichromate, iron(II) sulfate, iron(III) nitrate, manganese(II) chloride tetrahydrate, sodium nitrate, potassium nitrate, calcium nitrate tetrahydrate, hydrochloric acid, and ammonium chloride were purchased from Fisher Scientific (Waltham, MA). Copper(II) nitrate, ammonium hydroxide, sodium bicarbonate, and nitric acid were purchased from Mallinckrodt (St. Louis, MO). Nickel(II) sulfate hexahydrate was purchased from Acros (Morris, NJ). Dimethylglyoxime was purchased from Fluka (St. Louis, MO). Glacial acetic acid was purchased from EMD Millipore (Billerica, MA). Certified welding fume reference materials (SSWF-1 and MSWF-1) were obtained from Health & Safety Laboratory (Buxton, Derbyshire, UK). Milli-Q water from Millipore (R ≥ 18.2 MΩ cm) was used for all experiments. All chemicals were used as received without further purification. Carbon Ink purchased from Ercon (Wareham, MA), graphite powder (diameter <20 μm, Sigma–Aldrich, St. Louis, MO), and transparency film PP2200 (3M, St. Paul, MN) were used for electrode fabrication. A 30 W Epilog Zing Laser Cutter and Engraver (Golden, CO) was used to create electrode patterns on a transparency sheet using Corel Draw X4 program for stencil printing. A CHI1242B potentiostat (CH Instruments) was used for all electrochemical measurements. Electrodes were imaged using a JSM-6500F scanning electron microscope (JEOL USA Inc., Peabody, MA).
2.2 Fabrication of CSPEs
CSPEs were prepared as previously described [36–38]. Home-made electrode inks were created by adding 0.43 g graphite to 1.00 g of the commercial carbon ink followed by hand mixing until homogeneous. All of the working, counter, and reference electrodes were stencil printed on a PET sheet through a laser-cut stencil. The circle-shape working electrode had 3 mm diameter. After printing, the electrodes were dried at 65 °C for 1 h. A laser-cut, ring-shaped piece of adhesive tape was used for confining the solution droplet to the electrodes (Figure S1a). A photograph of a representative CSPE is shown in Figure S1b.
2.3 Electrode Modification
Electrode modification of Nafion/Bi CSPE was accomplished by dropcasting 1 μL of 0.5% Nafion dissolved in 50% v/v isopropanol/water onto the CSPE working electrode and allowing it to dry. 50 μL of 10 mg mL−1 Bi2O3 in 0.1 M acetate buffer pH 4.5 was electroplated on the CSPE surface using an optimum deposition potential of −1.4 V vs. carbon pseudo-reference electrode and deposition time of 20 min. After Bi modification, the CSPE was rinsed with 0.01 M ammonium buffer pH 9.0 prior to use.
2.4 Electrochemical Measurements
Cyclic voltammetry (CV) of 50 μg L−1 Co(II) and Ni(II) in 0.01 M ammonium buffer pH 9.0 (used as supporting electrolyte) containing 2 × 10−4 M DMG was performed using Nafion-modified BiCSPE (Nafion/BiCSPE). The potential was swept from −0.85 to −1.30 V versus a carbon pseudo-reference electrode with scan rates of 40–90 mV s−1. Square-wave cathodic stripping voltammetry (SWCSV) was carried out by pipetting 50.0 μL of standard Co(II) and Ni(II) in 0.01 M ammonium buffer pH 9.0 containing 2 × 10−4 M DMG onto the electrode. An optimum deposition potential was −0.85 V and the deposition time was varied from 15 s to 240 s as indicated in experimental details below. SWCSV was performed after a 10-s equilibration time from −0.9 to −1.5 V, and with an optimized step potential of 2 mV, amplitude of 25 mV, and frequency of 60 Hz.
2.4 Interference study
An interference study was performed using Cr(III), Cr(VI), Fe(II), Fe(III), Mn(II), Zn(II), Cu(II), Na(I), K(I), Ca(II), and Al(III) and the target metals, Co(II) and Ni(II). The mass ratios between the interfering metals and the target analytes were varied to determine tolerance ratios for potential interfering species. The tolerance ratio is defined as the mass ratio that creates a change in peak current of ±5% [39].
2.5 Sample collection and sample preparation
Cobalt aerosol was generated from a cobalt rod (ESPI Metals, Ashland, OR) using an arc-discharge generator with ultra-pure nitrogen as the flow. Aerosol was collected on 37-mm MCE filters (SKC Limited, Dorset, UK). The mass of the Co aerosol samples is shown in Table S1. A 5-mm diameter punch was removed from the 37-mm diameter filter for CSPE analysis. Before quantifying Co(II), punches were digested using a modification to a previously published procedure[22]. The digestion was performed by adding 8 μL of 5% w/v SDS in Milli-Q water to aid in filter wetting and 2 μL of concentrated nitric acid onto the 5-mm diameter punch. The punch was then placed in a microwave on high power for 15 s and repeated twice (i.e., a total of three heated digestions for 45 s total). A 15 μL aliquot of 5% SDS was added to the punch between each heating step. Each punch was then neutralized with 2 M Na2CO3 after the last digestion step. Verification that the punch was neutralized was performed with pH paper. A 50 μL of 0.01 M ammonium buffer pH 9.0 containing 2 × 10−4 M DMG was used to elute metals from the digested filter and the digestion container. 50 μL of the eluent was analyzed for Co(II) using the optimal settings described above from three punches of each sample filter to create replicate measurements.
Welding fume reference materials (SSWF-1 and MSWF-1) (the preparation was described in HSL report AS/2012/12 [40]) were digested using aqua regia (3:1 of hydrochloric acid: nitric acid). The sample masses and volumes of aqua regia solution, water, and 2 M sodium bicarbonate (for neutralization) used are shown in Table S2.
3. Results and Discussion
3.1 Co and Ni determinations using unmodified and Bi modified CSPEs
The analytical behavior of BiCSPEs for measuring Ni(II) and Co(II) DMG complexes was compared to that of the unmodified CSPEs. Ammonium buffer at pH 9.0 was used in this work because it was previously reported to provide a wide potential window for Bi thin-film electrodes generated by electroplating [31]. Figure 1a shows a cathodic peak current (23.0 ± 1.1 μA) at −1.16 ± 0.05 V (vs C pseudo-reference) by reducing Co(II)DMG with a BiCSPE; alternatively, no measurable peak is produced under these conditions with an unmodified CSPE. For detecting Ni(II)DMG with a BiCSPE (Figure 1b), the cathodic peak current (12.7 ± 0.8 μA) occurs at −1.07 ± 0.04 V and the peak is not present when using unmodified CSPE. These results demonstrate that BiCSPE can detect Co(II) and Ni(II) when these metals are complexed with DMG.
Figure 1.
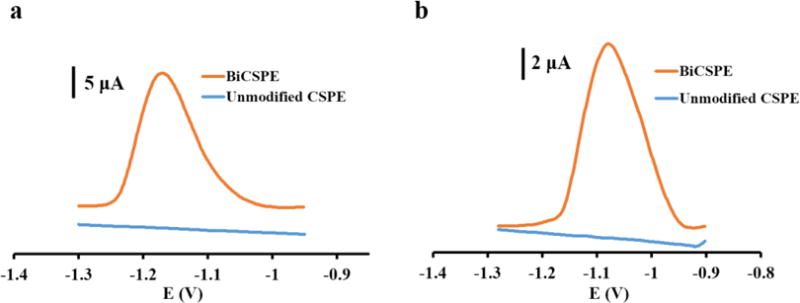
(a) Square wave voltammograms of 100 μg L−1 Co(II)DMG complex using unmodified CSPE and Bi modified CSPE with 120 s deposition time. (b) Square wave voltammograms of 100 μg L−1 Ni(II)DMG complex using unmodified CSPE and Bi modified CSPE with 120 s deposition time.
3.2 Electrochemical Characterization
The mass transfer process of Co(II) and Ni(II) to BiCSPEs was studied as shown in Figure 2. In a diffusion-controlled electrochemical redox reaction, the peak current (ip) shows a linear relationship with the square root of the scan rate as described by the Randles-Sevcik equation [41]:
where n is the number of electrons transferred in the redox reaction, A is the effective electrode area in cm2, D is the diffusion coefficient in cm2 s−1, C is theconcentration in mol cm−3 and υ is the scan rate of the cyclic voltammogram in V s−1. Figure 2 shows cyclic voltammograms at various scan rates of Co(II)DMG complex (Figure 2a) and Ni(II)DMG complex (Figure 2c). The peak currents (ip) at various square roots of scan rate of Co(II)DMG and Ni(II)DMG complexes detection are shown in Figures 2b and 2d, respectively. The peak current increases linearly with the square root of the scan rate for both complexes, suggesting that the mass transfer process is diffusion-controlled. Moreover, the adsorption of Co(II)DMG and Ni(II)DMG existed on BiCSPE that was observed from the linear relationship between peak currents and scan rates as shown in Figure S2a and S2b. However, the correlation coefficients (R2) of the linear fit from diffusion controlled process (R2 = 0.994 for Co and 0.965 for Ni) were better than those from the adsorption process (R2 = 0.982 for Co and 0.943 for Ni). Therefore, the mass transfer process for Co and Ni was predominantly controlled by the diffusion process. Additionally, cyclic voltammograms of both Co(II)DMG (Figure 2a) and Ni(II)DMG (Figure 2c) show one peak during cathodic scan and no peak during anodic scan, indicating the reduction of the complexes is irreversible. The peak potential appears to shift with increasing scan rate caused by the decrease of electron transfer rate constant [42]. In addition, a CV was recorded in 0.1 M ammonium buffer pH 9.0 containing 2 × 10−4 M DMG (Figure S3) demonstrated that the appearance of the slopped background in the cyclic voltammograms of Co(II)DMG and Ni(II)DMG was due to the onset of oxygen reduction at −1.25 V [43].
Figure 2.
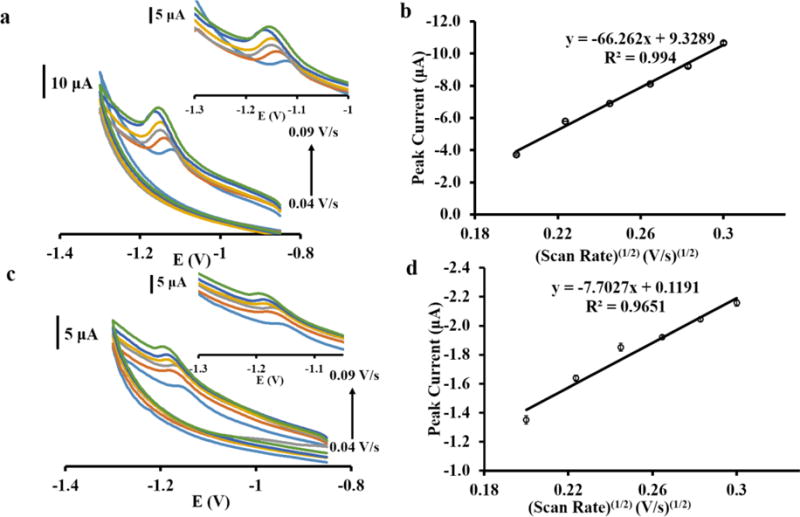
(a) Cyclic voltammograms of 50 μg L−1 Co(II)DMG complex using Bi modified CSPE with different scan rates (40–90 mV s−1). The expansion of reduction peaks of Co(II)DMG is shown in inset. (b) Relationship between peak current and square root of scan rate from (a) (n=3). (c) Cyclic voltammograms of 50 μg L−1 Ni(II)DMG complex using Bi modified CSPE with different scan rates (40–90 mV s−1). The expansion of reduction peaks of Ni(II)DMG is shown in inset. (d) Relationship between peak current and square root of scan rate from (c) (n=3).
3.3 Effect of Nafion coating and Bi electroplating time
Nafion was utilized to enhance detection current. Nafion, as a cation exchange polymer, is insoluble in water, electrochemically inert, and non-electroactive making it suitable for electrode modification [44]. The sulfonate group in Nafion allows selective preconcentration of cations resulting in improved detection performance [45]. Figure 3 shows coating Nafion onto CSPE before Bi-electroplating increased the peak current of Co(II)DMG to 12.2 μA from 4.8 μA without Nafion coating.
Figure 3.
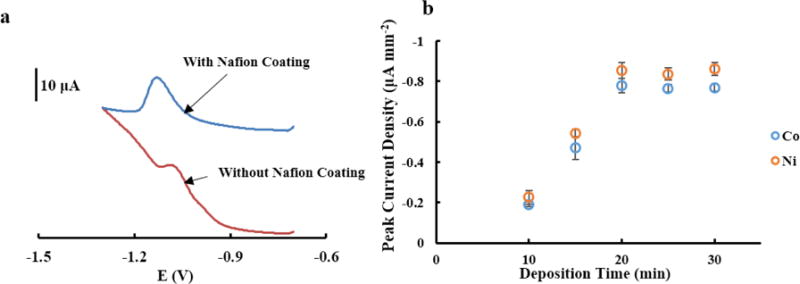
(a) Square-wave voltammograms of 100 μg L−1 Co(II)DMG complex using Bi modified CSPE with/without Nafion coating using 120 s deposition time. (b) Representative graph for 100 μg L−1 Co(II)DMG and 100 μg L−1 Ni(II)DMG at various electrodeposition times of Bi (n=3).
We also investigated the influence of electrochemical deposition time for electroplating Bi on CSPEs for Co(II) and Ni(II) detection (Figure 3b). As expected, when increasing the deposition time, the current density (defined as the ratio of peak current [μA] to area of the working electrode [28.3 mm2]) for both Co(II)DMG and Ni(II)DMG increases until reaching a plateau at 20 min. Therefore, 20 min was chosen as an optimum time for electroplating Bi on CSPEs.
3.4 Repeatability of Nafion/BiCSPE for Co(II) and Ni(II) detections
After optimizing the detection conditions of Nafion/BiCSPE, the electrode lifetime was tested by determining how many runs could be performed with a single low-cost electrode system. Repeated runs using the same electrode for standard Co and Ni measurements were performed as shown in Figure 4. Three separate Nafion/BiCSPE electrodes were used to determine repeatability for each metal (labelled in different colors in Figure 4). The peak currents were stable for 15 runs as shown in Figure 4a (Co(II)) and Figure 4b (Ni(II)). The %RSDs (7.3 ± 0.5 % for Co(II) and 9.1 ± 0.6 % for Ni(II)) of 15 runs for Co(II) are less than the reported value from AOAC (for the detection in μg L−1 level) (15%) on three separated Nafion/BiCSPEs [35]. The results indicate that Nafion/BiCSPEs can be used for up to 15 times. No attempt was made to extend the system beyond 15 runs given the low-cost of the electrodes. For Ni(II) detection (Figure 4b), the modified electrodes also provided acceptable repeatability with %RSDs of 15 runs <15%. However, %RSDs of Nafion/BiCSPEs at run 12 to 15 for Ni(II) detection increased slightly, which is different from Co(II) detection where %RSDs maintained stable for 15 runs. We hypothesized that the deposition time of each metal caused a change in the surface morphology leading to smaller peak currents. This assumption was verified by imaging the surface of the Nafion/BiCSPE with scanning electron microscopy (Figure 5). When comparing the surface after measuring Co(II) (Figure 5c) and Ni(II) (Figure 5d), Nafion (represented as the bright flat sheets) and Bi (represented as the small crystals) were more deteriorated relative to those on the surface of the unused CSPE (Figure 5b). Moreover, as hypothesized, the electrode morphology when detecting Ni(II) with 45 s deposition time (Figure 5d) changed more than that for the Co(II) measurement that required a 15 s deposition time (Figure 5c).
Figure 4.
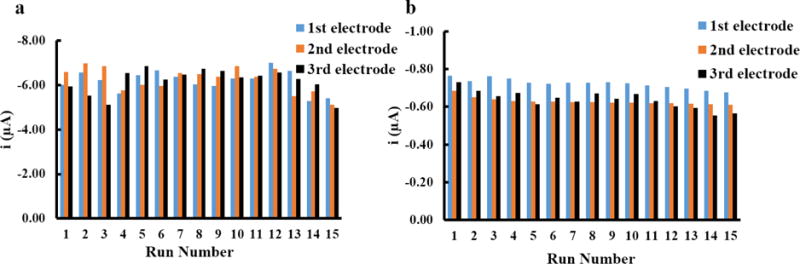
(a) Repeatability of three Nafion/BiCSPEs to measure current of 50 μg L−1 Co(II)DMG complex using 15 s deposition time. (b) Repeatability of three electrodes to measure current of 50 μg L−1 Ni(II)DMG complex using 45 s deposition time.
Figure 5.
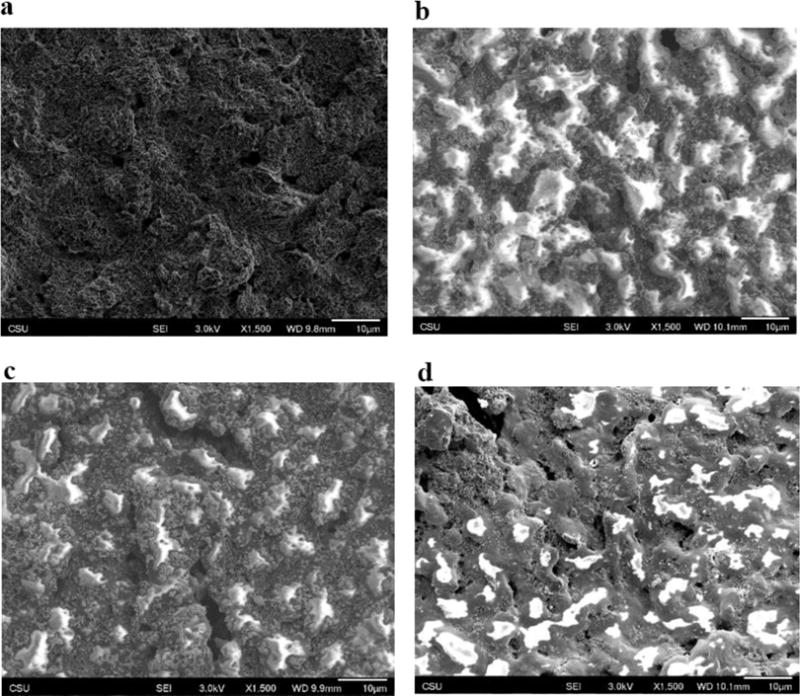
(a) SEM images of CSPE. (b) Nafion/BiCSPE. (c) Nafion/BiCSPE after 20 runs of 50 μg L−1 Co(II)DMG complex. (d) Nafion/BiCSPE after 20 runs of 50 μg L−1 Ni(II)DMG complex.
3.5 Electrochemical measurement of Co(II) and Ni(II)
The linear working ranges for measuring Co(II) and Ni(II) at Nafion/BiCSPE are shown in Figure 6. The decrease of current density at high concentration of Ni(II)DMG (200 μg L−1) in Figure 6d was caused by electrode fouling bringing about incomplete reduction of Ni(II)DMG [46]. The Bi deposition time significantly influenced the linear ranges of Co(II) and Ni(II) as shown in Table 1. The widest linear range was observed when using the deposition time of 15 s and 45 s for Co(II) (20–250 μg L−1) and Ni(II) (50–175 μg L−1), respectively. Longer Bi deposition time allowed detection at lower concentration ranges for Co(II) and Ni(II) (1–50 μg L−1 for Co(II)) and 5–50 μg L−1 for Ni(II)) at 240 s deposition time). Likewise, a longer deposition time provided lower LODs than a shorter deposition time. LODs for Co(II) and Ni(II) using 240 s deposition time were 1 μg L−1 and 5 μg L−1, respectively, where LOD was defined as the concentration giving a peak height of three times the root-mean-square of the baseline noise. The LODs of Co(II) and Ni(II) detection at each deposition time are summarized in Table 1. The voltammograms and calibration curves for Co and Ni using 120 s and 240 s deposition time are shown in Figures S4 and S5, respectively.
Figure 6.
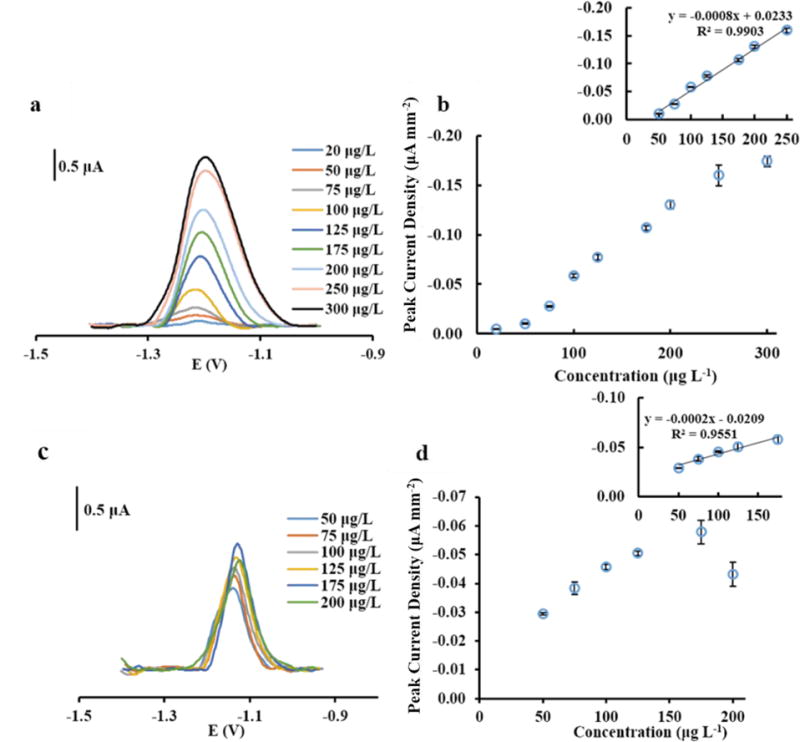
(a) Square-wave voltammograms of Co(II)DMG complex from 1–100 μg L−1 using 15 s deposition time. (b) Representative calibration graph for Co(II)DMG complex. Linear fit of calibration graph for Co(II)DMG complex (b inset) (n=3). (c) Square-wave voltammograms of Ni(II)DMG complex from 5–100 μg L−1 using 45 s deposition time. (d) Representative calibration graph for Ni(II)DMG complex. Linear fit of calibration graph for Co(II)DMG complex (d inset) (n=3).
Table 1.
Linearity range with various deposition times
| Deposition Time (s) | Co(II) (μg L−1) | Ni(II) (μg L−1) | ||
|---|---|---|---|---|
| Linearity range | LOD | Linearity range | LOD | |
| 15 | 20–250 | 20 | – | – |
| 45 | – | – | 50–175 | 50 |
| 120 | 20–100 | 20 | 20–75 | 20 |
| 240 | 1–50 | 1 | 5–50 | 5 |
3.6 Interference Study
As the goal of this work is to detect Ni and Co in sample matrices such as welding fume, the tolerance ratio for key interferences was determined. The tolerance ratio is defined as the mass ratio of an interfering species relative to the target metal that gives a change in peak current of ±5% [39]. The tolerance ratios between interfering species and Co(II) and Ni(II) are shown in Table 2. The results indicated that none of the tested interfering elements affected Co(II) and Ni(II) detection at a significant level except Cu(II). The tolerance ratio between Cu(II) and Ni(II) was low because DMG can also chelate with Cu(II) [47]. While there are known strategies to address Cu interferences, Cu(II) is present at very low levels in welding fume and related samples making removal of the interference unnecessary. As a result, the proposed method showed selectivity and sensitivity toward Co(II) and Ni(II) to enable analysis of aerosol samples and welding fume samples.
Table 2.
Tolerance ratio of interfering ions in the electrochemical determination of 100 μg L−1 of Co(II)DMG complex and Ni(II)DMG complex
| Interference | Tolerance Ratio for Co(II)DMG Complex | Tolerance Ratio for Ni(II)DMG Complex |
|---|---|---|
| Cr3+ | 500 | 500 |
| Cr6+ | 100 | 100 |
| Fe2+/Fe3+ | 500 | 50 |
| Mn2+ | >500 | 50 |
| Zn2+ | 100 | >500 |
| Cu2+ | 100 | 10 |
| Na+ | 100 | 500 |
| K+ | 100 | 100 |
| Ca2+ | 100 | 500 |
| Al3+ | >500 | >500 |
3.7 Co(II) and Ni(II) detections in environmental samples
Adsorptive cathodic stripping square-wave voltammetry was applied for detecting Co(II) and Ni(II) through complexing with DMG in Cobalt-generated aerosol and welding fume samples. The voltammograms for Co(II) determination are shown in Figure S6a. The amount of Co(II) measured by Nafion/BiCSPE and the validation method (ICP-MS) was summarized in Table 3. In the case of Ni(II) determination, the signal of Ni(II)DMG in welding fume reference materials (SSWF-1 and MSWF-1) is shown in Figure S6b. The amount of Ni(II) detected in SSWF-1 was 3.3 ± 0.2 %, close to the certified value (3.7%) as shown in Table 4. For MSWF-1, the amount of Ni(II) was under detection limit and corresponded to the reference data showing that the sample did not contain Ni(II). Quantitation of Co(II) and Ni(II) in different samples is summarized in Table 3 and 4, respectively. A paired Student’s t-test was used to compare measured Co(II) values between Nafion/BiCSPE and ICP-MS. For Co, the t value (−9.00) is less than the critical t value (3.182, P=0.05) for n-1=3 degrees freedom when n = 4 implying that the null hypothesis is not rejected. Therefore, the proposed method does not provide significantly different results with 95% confidence for Co detection.
Table 3.
Co(II) Determination in aerosol samples (n=3)
| Sample | Concentration of Co (μg) | |
|---|---|---|
| BiCSPE | ICP-MS | |
| 1 | 36 ± 1.4 | 41 ± 0.9 |
| 2 | 40 ± 1.2 | 43 ± 1.0 |
| 3 | 37 ± 1.1 | 42 ± 0.8 |
| 4 | 59 ± 1.9 | 64 ± 1.3 |
Table 4.
Ni(II) Determination in welding fume samples (n=3)
| Sample (Certified Reference Material) | %Ni (Experimental value) | %Ni (theoretical value) |
|---|---|---|
| SSWF-1 | 3.3±0.2 | 3.7 |
| MSWF-1 | below LOD | 0 |
4. Conclusion
A home-made Nafion/BiCSPE was fabricated for trace Co(II) and Ni(II) determination by chelating with DMG. The proposed sensors provided LODs of 1 μg L−1 and 5 μg L−1 for Co(II) and Ni(II), respectively. The key factor leading to improved performance was the electroplating of a thin film of Bi onto the electrode surface. Nafion coating also enhanced peak current and decreased background current. The resulting electrodes and chemistry allowed for repeated Co(II) and Ni(II) measurements up to 15 times based on changes in %RSD. Furthermore, the Nafion/BiCSPE was selective for Co(II) and Ni(II) against other possible metal interferences. Cu(II) was the only element that caused a significant change in signal but was not a problem with the target samples because of its low concentration. The resulting system was used to measure Co(II) and Ni(II) in aerosol samples and welding fume, respectively. Results from the electroanalytical system were statistically similar with the results from ICP-MS (for Co) and close to certified values (for Ni). The results show that the Nafion/BiCSPEs using adsorptive stripping voltammetry have great potential for selective and sensitive determination of Co(II) and Ni(II) in environmental applications.
Supplementary Material
Highlights.
Low-cost, simple, and portable electrochemical sensors were proposed.
Adsorptive cathodic stripping voltammetry of Co and Ni was carried out using Bi modified carbon stencil-printed electrodes.
Low detection limits (1 ppb for Co and 5 ppb for Ni) were achieved with the low-cost sensors.
High repeatable use of the electrodes (up to 15 times with no problem (%RSD < 15%)) was obtained when compared with the reported value of acceptable repeatability from Association of Official Analytical Chemists.
The sensors were applied for Co and Ni detection in aerosols.
Acknowledgments
This work was supported in part by grants from the U.S. National Institute for Occupational Safety and Health (OH010662) and the National Institute of Environmental Health Sciences (ES024719). JM thanks the Development and Promotion of Science and Technology Talents Project, Thailand. The authors gratefully acknowledge the technical assistance, generous support, and manuscript preparation from the Henry group, Colorado State University, and specifically Yuanyuan Yang.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Léonard A, Lauwerys R. Mutagenicity, carcinogenicity and teratogenicity of cobalt metal and cobalt compounds. Mutation Research/Reviews in Genetic Toxicology. 1990;239(1):17–27. doi: 10.1016/0165-1110(90)90029-b. [DOI] [PubMed] [Google Scholar]
- 2.Denkhaus E, Salnikow K. Nickel essentiality, toxicity, and carcinogenicity. Critical Reviews in Oncology/Hematology. 2002;42(1):35–56. doi: 10.1016/s1040-8428(01)00214-1. [DOI] [PubMed] [Google Scholar]
- 3.Schaumlöffel D. Nickel species: Analysis and toxic effects. Journal of Trace Elements in Medicine and Biology. 2012;26(1):1–6. doi: 10.1016/j.jtemb.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Lauwerys R, Lison D. Health risks associated with cobalt exposure — an overview. Science of The Total Environment. 1994;150(1–3):1–6. doi: 10.1016/0048-9697(94)90125-2. [DOI] [PubMed] [Google Scholar]
- 5.Goldoni M, Catalani S, De Palma G, Manini P, Acampa O, Corradi M, Bergonzi R, Apostoli P, Mutti A. Exhaled Breath Condensate as a Suitable Matrix to Assess Lung Dose and Effects in Workers Exposed to Cobalt and Tungsten. Environmental Health Perspectives. 2004;112(13):1293–1298. doi: 10.1289/ehp.7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MASTROMATTEO E. Nickel: A Review of Its Occupational Health Aspects. Journal of Occupational and Environmental Medicine. 1967;9(3):127–136. doi: 10.1097/00043764-196703000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Paustenbach DJ, Tvermoes BE, Unice KM, Finley BL, Kerger BD. A review of the health hazards posed by cobalt. Critical Reviews in Toxicology. 2013;43(4):316–362. doi: 10.3109/10408444.2013.779633. [DOI] [PubMed] [Google Scholar]
- 8.Costa M, Davidson TL, Chen H, Ke Q, Zhang P, Yan Y, Huang C, Kluz T. Nickel carcinogenesis: Epigenetics and hypoxia signaling. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2005;592(1–2):79–88. doi: 10.1016/j.mrfmmm.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Scansetti G, Maina G, Botta GC, Bambace P, Spinelli P. Exposure to cobalt and nickel in the hard-metal production industry. International Archives of Occupational and Environmental Health. 1998;71(1):60–63. doi: 10.1007/s004200050251. [DOI] [PubMed] [Google Scholar]
- 10.Lison D. Chapter 34 - Cobalt A2 - Nordberg, Gunnar F. In: Fowler BA, Nordberg M, editors. Handbook on the Toxicology of Metals. Fourth. Academic Press; San Diego: 2015. pp. 743–763. [Google Scholar]
- 11.Sjögren B, Hansen KS, Kjuus H, Persson PG. Exposure to stainless steel welding fumes and lung cancer: a meta-analysis. Occupational and Environmental Medicine. 1994;51(5):335–6. doi: 10.1136/oem.51.5.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martelli PB, Reis BF, Kronka EAM, F HB, Korn M, Zagatto EAG, Lima JFC, Araujo AN. Multicommutation in flow analysis. Part 2. Binary sampling for spectrophotometric determination of nickel, iron and chromium in steel alloys. Analytica Chimica Acta. 1995;308(1):397–405. [Google Scholar]
- 13.Profumo A, Spini G, Cucca L, Pesavento M. Determination of inorganic nickel compounds in the particulate matter of emissions and workplace air by selective sequential dissolutions. Talanta. 2003;61(4):465–472. doi: 10.1016/S0039-9140(03)00313-8. [DOI] [PubMed] [Google Scholar]
- 14.Dzubay TG, Stevens RK. Ambient air analysis with dichotomous sampler and x-ray fluorescence spectrometer. Environmental Science & Technology. 1975;9(7):663–668. [Google Scholar]
- 15.Kulkarni P, Chellam S, Flanagan JB, Jayanty RKM. Microwave digestion—ICP-MS for elemental analysis in ambient airborne fine particulate matter: Rare earth elements and validation using a filter borne fine particle certified reference material. Analytica Chimica Acta. 2007;599(2):170–176. doi: 10.1016/j.aca.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 16.Meredith NA, Quinn C, Cate DM, Reilly TH, Volckens J, Henry CS. Paper-based analytical devices for environmental analysis. Analyst. 2016;141(6):1874–1887. doi: 10.1039/c5an02572a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cate DM, Adkins JA, Mettakoonpitak J, Henry CS. Recent Developments in Paper-Based Microfluidic Devices. Analytical Chemistry. 2015;87(1):19–41. doi: 10.1021/ac503968p. [DOI] [PubMed] [Google Scholar]
- 18.Cate DM, Noblitt SD, Volckens J, Henry CS. Multiplexed paper analytical device for quantification of metals using distance-based detection. Lab on a Chip. 2015;15(13):2808–18. doi: 10.1039/c5lc00364d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinez AW, Phillips ST, Whitesides GM, Carrilho E. Diagnostics for the Developing World: Microfluidic Paper-Based Analytical Devices. Analytical Chemistry. 2010;82(1):3–10. doi: 10.1021/ac9013989. [DOI] [PubMed] [Google Scholar]
- 20.Mentele MM, Cunningham J, Koehler K, Volckens J, Henry CS. Microfluidic Paper-Based Analytical Device for Particulate Metals. Analytical Chemistry. 2012;84(10):4474–4480. doi: 10.1021/ac300309c. [DOI] [PubMed] [Google Scholar]
- 21.Lin Y, Gritsenko D, Feng S, Teh YC, Lu X, Xu J. Detection of heavy metal by paper-based microfluidics. Biosensors and Bioelectronics. 2016;83:256–266. doi: 10.1016/j.bios.2016.04.061. [DOI] [PubMed] [Google Scholar]
- 22.Cate DM, Nanthasurasak P, Riwkulkajorn P, L’Orange C, Henry CS, Volckens J. Rapid Detection of Transition Metals in Welding Fumes Using Paper-Based Analytical Devices. Annals of Occupational Hygiene. 2014 doi: 10.1093/annhyg/met078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rattanarat P, Dungchai W, Cate D, Volckens J, Chailapakul O, Henry CS. Multilayer Paper-Based Device for Colorimetric and Electrochemical Quantification of Metals. Analytical Chemistry. 2014;86(7):3555–3562. doi: 10.1021/ac5000224. [DOI] [PubMed] [Google Scholar]
- 24.Adkins J, Boehle K, Henry C. Electrochemical paper-based microfluidic devices. ELECTROPHORESIS. 2015;36(16):1811–1824. doi: 10.1002/elps.201500084. [DOI] [PubMed] [Google Scholar]
- 25.Mettakoonpitak J, Boehle K, Nantaphol S, Teengam P, Adkins JA, Srisa-Art M, Henry CS. Electrochemistry on Paper-based Analytical Devices: A Review. Electroanalysis. 2016;28(7):1420–1436. [Google Scholar]
- 26.Zen JM, Lee ML. Determination of traces of nickel(II) at a perfluorinated ionomer/dimethylglyoxime mercury film electrode. Analytical Chemistry. 1993;65(22):3238–3243. [Google Scholar]
- 27.Economou A, Fielden PR. Adsorptive stripping voltammetry on mercury film electrodes in the presence of surfactants. Analyst. 1993;118(11):1399–1404. [Google Scholar]
- 28.González P, Cortıńez VA, Fontán CA. Determination of nickel by anodic adsorptive stripping voltammetry with a cation exchanger-modified carbon paste electrode. Talanta. 2002;58(4):679–690. [PubMed] [Google Scholar]
- 29.Królicka A, Bobrowski A. Bismuth film electrode for adsorptive stripping voltammetry – electrochemical and microscopic study. Electrochemistry Communications. 2004;6(2):99–104. [Google Scholar]
- 30.Wang J, Lu J, Hocevar SB, Farias PAM, Ogorevc B. Bismuth-Coated Carbon Electrodes for Anodic Stripping Voltammetry. Analytical Chemistry. 2000;72(14):3218–3222. doi: 10.1021/ac000108x. [DOI] [PubMed] [Google Scholar]
- 31.Hutton EA, Ogorevc B, Hočevar SB, Weldon F, Smyth MR, Wang J. An introduction to bismuth film electrode for use in cathodic electrochemical detection. Electrochemistry Communications. 2001;3(12):707–711. [Google Scholar]
- 32.Morfobos M, Economou A, Voulgaropoulos A. Simultaneous determination of nickel(II) and cobalt(II) by square wave adsorptive stripping voltammetry on a rotating-disc bismuth-film electrode. Analytica Chimica Acta. 2004;519(1):57–64. [Google Scholar]
- 33.Tartarotti FO, de Oliveira MF, Balbo VR, Stradiotto NR. Determination of Nickel in Fuel Ethanol Using a Carbon Paste Modified Electrode Containing Dimethylglyoxime. Microchimica Acta. 2006;155(3):397–401. [Google Scholar]
- 34.Baldwin RP, Christensen JK, Kryger L. Voltammetric determination of traces of nickel(II) at a chemically modified electrode based on dimethylglyoxime-containing carbon paste. Analytical Chemistry. 1986;58(8):1790–1798. [Google Scholar]
- 35.A. International, Official methods of analysis of AOAC International, Official methods of analysis of AOAC International
- 36.Ruecha N, Rodthongkum N, Cate DM, Volckens J, Chailapakul O, Henry CS. Sensitive electrochemical sensor using a graphene–polyaniline nanocomposite for simultaneous detection of Zn(II), Cd(II), and Pb(II) Analytica Chimica Acta. 2015;874:40–48. doi: 10.1016/j.aca.2015.02.064. [DOI] [PubMed] [Google Scholar]
- 37.Berg KE, Adkins JA, Boyle SE, Henry CS. Manganese Detection Using Stencil-printed Carbon Ink Electrodes on Transparency Film. Electroanalysis. 2016;28(4):679–684. [Google Scholar]
- 38.Mettakoonpitak J, Mehaffy J, Volckens J, Henry CS. AgNP/Bi/Nafion-modified Disposable Electrodes for Sensitive Zn(II), Cd(II), and Pb(II) Detection in Aerosol Samples. Electroanalysis. 2017;29(3):880–889. [Google Scholar]
- 39.Chaiyo S, Chailapakul O, Sakai T, Teshima N, Siangproh W. Highly sensitive determination of trace copper in food by adsorptive stripping voltammetry in the presence of 1,10-phenanthroline. Talanta. 2013;108:1–6. doi: 10.1016/j.talanta.2013.02.031. [DOI] [PubMed] [Google Scholar]
- 40.Butler O, Musgrove D. Certification report reference material HSL SSWF-01 elements in stainless steel welding fume. 2013 Fabruary; [Google Scholar]
- 41.Bard AJ, Faulkner LR. Electrochemical Methods: Fundamentals and Applications. Wiley; 2000. [Google Scholar]
- 42.Nicholson RS. Theory and Application of Cyclic Voltammetry for Measurement of Electrode Reaction Kinetics. Analytical Chemistry. 1965;37(11):1351–1355. [Google Scholar]
- 43.Wang J, Lu J. Bismuth film electrodes for adsorptive stripping voltammetry of trace nickel. Electrochemistry Communications. 2000;2(6):390–393. [Google Scholar]
- 44.Torma F, Grün A, Bitter I, Tóth K. Calixarene/Nafion-Modified Bismuth-Film Electrodes for Adsorptive Stripping Voltammetric Determination of Lead. Electroanalysis. 2009;21(17–18):1961–1969. [Google Scholar]
- 45.Wang T, Zhao D, Guo X, Correa J, Riehl BL, Heineman WR. Carbon nanotube-loaded Nafion film electrochemical sensor for metal ions: europium. Analytical Chemistry. 2014;86(9):4354–61. doi: 10.1021/ac500163f. [DOI] [PubMed] [Google Scholar]
- 46.Davis DG, Boudreaux EA. Nickel(IV) dimethylglyoxime. Journal of Electroanalytical Chemistry (1959) 1964;8(6):434–441. [Google Scholar]
- 47.Bobrowski A. The nature of voltammetric waves of copper complexes with dimethylglyoxime in ammonia and borate buffer solutions. Electroanalysis. 1996;8(1):79–88. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


