Supplemental Digital Content is available in the text.
Keywords: 0/1-hour algorithm, chronic kidney disease, diagnosis of acute myocardial infarction, high-sensitivity cardiac troponin, renal dysfunction
Abstract
Background:
The European Society of Cardiology recommends a 0/1-hour algorithm for rapid rule-out and rule-in of non–ST-segment elevation myocardial infarction using high-sensitivity cardiac troponin (hs-cTn) concentrations irrespective of renal function. Because patients with renal dysfunction (RD) frequently present with increased hs-cTn concentrations even in the absence of non–ST-segment elevation myocardial infarction, concern has been raised regarding the performance of the 0/1-hour algorithm in RD.
Methods:
In a prospective multicenter diagnostic study enrolling unselected patients presenting with suspected non–ST-segment elevation myocardial infarction to the emergency department, we assessed the diagnostic performance of the European Society of Cardiology 0/1-hour algorithm using hs-cTnT and hs-cTnI in patients with RD, defined as an estimated glomerular filtration rate <60 mL/min/1.73 m2, and compared it to patients with normal renal function. The final diagnosis was centrally adjudicated by 2 independent cardiologists using all available information, including cardiac imaging. Safety was quantified as sensitivity in the rule-out zone, accuracy as the specificity in the rule-in zone, and efficacy as the proportion of the overall cohort assigned to either rule-out or rule-in based on the 0- and 1-hour sample.
Results:
Among 3254 patients, RD was present in 487 patients (15%). The prevalence of non–ST-segment elevation myocardial infarction was substantially higher in patients with RD compared with patients with normal renal function (31% versus 13%, P<0.001). Using hs-cTnT, patients with RD had comparable sensitivity of rule-out (100.0% [95% confidence interval {CI}, 97.6–100.0] versus 99.2% [95% CI, 97.6–99.8]; P=0.559), lower specificity of rule-in (88.7% [95% CI, 84.8–91.9] versus 96.5% [95% CI, 95.7–97.2]; P<0.001), and lower overall efficacy (51% versus 81%, P<0.001), mainly driven by a much lower percentage of patients eligible for rule-out (18% versus 68%, P<0.001) compared with patients with normal renal function. Using hs-cTnI, patients with RD had comparable sensitivity of rule-out (98.6% [95% CI, 95.0–99.8] versus 98.5% [95% CI, 96.5–99.5]; P=1.0), lower specificity of rule-in (84.4% [95% CI, 79.9–88.3] versus 91.7% [95% CI, 90.5–92.9]; P<0.001), and lower overall efficacy (54% versus 76%, P<0.001; proportion ruled out, 18% versus 58%, P<0.001) compared with patients with normal renal function.
Conclusions:
In patients with RD, the safety of the European Society of Cardiology 0/1-hour algorithm is high, but specificity of rule-in and overall efficacy are decreased. Modifications of the rule-in and rule-out thresholds did not improve the safety or overall efficacy of the 0/1-hour algorithm.
Clinical Trial Registration:
URL: https://www.clinicaltrials.gov. Unique identifier: NCT00470587.
Editorial, see p 452
Clinical Perspective.
What Is New?
The 0/1-hour algorithms using high-sensitivity cardiac troponin for rapid triage of patients with suspected myocardial infarction are increasingly used in clinical practice worldwide.
Although their high safety and efficacy could be shown in the general, mixed setting of emergency departments, their utility in patients with renal dysfunction, presenting with elevated high-sensitivity cardiac troponin levels often in the absence of acute myocardial ischemia, has been questioned.
For the first time, we demonstrated the excellent safety of the 0/1-hour algorithms using high-sensitivity cardiac troponin T and high-sensitivity cardiac troponin I also in patients with renal dysfunction, whereas overall efficacy and rule-in specificity were reduced compared with patients with normal renal function.
What Are the Clinical Implications?
The investigated 0/1-hour algorithms for rapid triage of patients with suspected myocardial infarction provide high safety irrespective of renal function and do not seem to require adjustment for renal function.
However, the proportion of patients eligible for rule-out is reduced in patients with renal dysfunction compared with patients with normal renal function (≈ factor 3) because of the substantially higher prevalence of myocardial infarction in patients with renal dysfunction (≈ factor 3).
Acute myocardial infarction (AMI) is a major cause of death and disability worldwide. Its rapid and accurate diagnosis is critical for the initiation of effective evidence-based medical management and treatment.1–3 In addition, its rapid and reliable rule-out has the potential to reduce the time spent in the emergency department (ED), accelerate the identification and treatment of the actual cause of chest pain, reduce patients’ anxiety, and avoid substantial costs for the healthcare system.4,5
For several reasons, patients with renal dysfunction merit particular attention.6,7 First, the incidence of AMI is increased in this vulnerable subgroup.8–10 Second, atypical clinical presentation of AMI may be more frequent.11,12 Third, left ventricular hypertrophy is common and often results in ECG changes that may mimic or obscure AMI. Fourth, patients with renal dysfunction are more prone to adverse events related to cardiovascular medication (eg, anticoagulation) as well as cardiovascular procedures, including coronary angiography and coronary intervention.1,2 Fifth, levels of cardiac troponin (cTn) are frequently chronically elevated even in the absence of AMI.6,10,13 Recently, sensitive and high-sensitivity cardiac troponin assays (hs-cTn) were demonstrated to be accurate tools in diagnosing AMI in patients with renal dysfunction, particularly when adjusted slightly higher cutoff levels are used for clinical decision making.10
The latest guidelines of the European Society of Cardiology (ESC) for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation recommend the use of a 0/1-hour algorithm to rapidly rule out and rule in non–ST-segment elevation myocardial infarction (NSTEMI) based on hs-cTn concentrations at presentation and their absolute 1-hour changes.1 Assay-specific cutoff values are recommended for uniform application irrespective of renal function. High safety and efficacy of the 0/1-hour algorithm were demonstrated in unselected patients, of which the vast majority had normal renal function. It is unknown whether these results also apply to patients with renal dysfunction (RD).1,14–19 Because patients with RD frequently present with increased hs-cTn concentrations even in the absence of NSTEMI, concern has been raised regarding the performance of the 0/1-hour algorithm in RD.10
We therefore aimed to assess the diagnostic performance of the ESC 0/1-hour algorithm in patients with RD in a large prospective multicenter diagnostic study.
Methods
Study Design and Population
APACE (Advantageous Predictors of Acute Coronary Syndrome Evaluation) is an ongoing prospective international multicenter diagnostic study with 12 centers in 5 European countries aiming to advance the early diagnosis of AMI (ClinicalTrials.gov. Unique identifier: NCT00470587).10,15–17,20,21 Adult patients presenting to the ED with symptoms suggestive of AMI (eg, acute chest discomfort and angina pectoris) with an onset or peak within the last 12 hours were recruited. Enrollment was independent of renal function, whereas patients with terminal kidney failure on chronic dialysis were excluded. For this analysis, patients with ST-segment elevation myocardial infarction, patients with missing creatinine measurement, patients in whom the final diagnosis remained unclear even after central adjudication and ≥1 elevated hs-cTnT concentration possibly indicating AMI, as well as patients with no available hs-cTnT (for dataset A) or hs-cTnI (for dataset B) concentrations determined on presentation to the ED and after 1 hour were also excluded. Dataset B represents a subset of dataset A. The most common reasons for misvsing samples after 1 hour were early transfer to the catheter laboratory or coronary care unit and diagnostic procedures around the 1-hour window that precluded blood draw at 1 hour.
The study was carried out according to the principles of the Declaration of Helsinki and approved by the local ethics committees. Written informed consent was obtained from all patients. The authors designed the study, gathered and analyzed the data, vouched for the data and analysis, wrote the paper, and decided to publish. The STARD Checklist (Standards for Reporting of Diagnostic Accuracy Studies) can be found in Table I in the online-only Data Supplement.22 The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Routine Clinical Assessment
Patients underwent clinical assessment that included medical history, physical examination, and standard blood tests including serial measurements of local hs-cTn, 12-lead ECG, chest radiography, continuous ECG rhythm monitoring, and pulse oximetry. Management of patients was left to the discretion of the attending physician.
Adjudicated Final Diagnosis
Adjudication of the final diagnosis was performed by 2 independent cardiologists at the core laboratory (University Hospital Basel) applying the universal definition of AMI using 2 datasets: (1) all available medical records obtained during clinical care, including history, physical examination, results of laboratory testing including serial clinical hs-cTn levels (according to onsite used hs-cTn assay obtained from clinical blood samples), radiological testing, ECG, echocardiography, cardiac exercise test, lesion severity, and morphology in coronary angiography pertaining to the patient from the time of ED presentation to 90-day follow-up; and (2) study-specific assessments, including detailed chest pain characteristics using 34 predefined criteria, serial hs-cTnT blood concentrations obtained from study samples, and clinical follow-up by telephone or mail. In situations of disagreement about the diagnosis, cases were reviewed and adjudicated in conjunction with a third cardiologist.
AMI was defined and hs-cTn interpreted as recommended in the current guidelines.1–3,14 In brief, myocardial infarction was diagnosed when there was evidence of myocardial necrosis in association with a clinical setting consistent with myocardial ischemia. Myocardial necrosis was diagnosed by ≥1 cTn value >99th percentile together with a significant rising or falling. The criteria used to define a rise or fall in conventional cTn and hs-cTnT are described in detail in the Methods section in the online-only Data Supplement. All other patients were classified in the categories of unstable angina, noncardiac chest pain, cardiac but noncoronary disease (eg, tachyarrhythmias, perimyocarditis), and symptoms of unknown origin with normal levels of hs-cTnT.
Assessment of Renal Function
Renal function was quantified by estimating glomerular filtration rate (eGFR) with the use of the chronic kidney disease epidemiology collaboration formula based on plasma creatinine level obtained at presentation to the ED, age, sex, and ethnicity.23 For this analysis, RD was defined as an eGFR of <60 mL/min/1.73 m.2 Creatinine measurements were performed on a Roche Modular P1 analyzer with the enzymatic creatinine-peroxidase-antiperoxidase PAP method for quantification (Roche Diagnostics). Serum creatinine can be converted from micromoles per liter to milligrams per deciliter by dividing by 88.4. Preexisting kidney dysfunction was documented based on previous hospital records and detailed patient history at the time of ED presentation.
Investigational hs-cTn Measurements
Blood samples for determination of hs-cTnT and hs-cTnI were collected into tubes containing potassium EDTA (as an anticoagulant) or serum gel (as a clot activator) at presentation to the ED and serially thereafter. Serial sampling was discontinued when a patient was discharged or transferred to the catheter laboratory for treatment. After centrifugation, samples were either analyzed directly or frozen at −80°C until they were assayed in a blinded fashion in a dedicated core laboratory.
According to the manufacturer, the hs-cTnT assay (Elecsys 2010 high-sensitivity troponin T, Roche Diagnostics) has a 99th percentile concentration of 14 ng/L with a corresponding coefficient of variation of 10% at 13 ng/L.24 Limit of blank and limit of detection have been determined to be 3 ng/L and 5 ng/L. None of the hs-cTnT measurements in this analysis were affected by the 2010 to 2012 calibration shift.25–28
The hs-cTnI assay (ARCHITECT High Sensitive STAT Troponin I, Abbott Laboratories) has a 99th percentile concentration of 26.2 ng/L with a corresponding coefficient of variation of <5% and a limit of detection of 1.9 ng/L.29–31
Distributions of the latest study blood samples according to time since ED presentation and time since chest pain onset are listed in Tables II and III in the online-only Data Supplement.
ESC hs-cTn 0/1-Hour Algorithm
Recent studies have highlighted fundamental differences in mortality risk, pathophysiology, and benefit from early coronary angiography and intense dual-antiplatelet therapy between patients with NSTEMI and patients with true unstable angina (not including patients with small NSTEMIs missed by conventional cTn assays).1,32 Accordingly, the immediate task in the ED is to detect NSTEMI. Thus, the ESC 0/1-hour algorithm was designed to detect NSTEMI. The diagnosis of unstable angina is based on clinical assessment, ECG, and rule-out of NSTEMI in the ED, as well as cardiac imaging performed either in-hospital or on an outpatient basis.1,32
The ESC hs-cTn 0/1-hour algorithm, which should always be used in conjunction with all clinical information available, including the ECG, triages patients presenting with suspected NSTEMI toward rule-out, observe, and rule-in based on assay-specific levels of hs-cTn obtained at presentation and after 1 hour (Figure I in the online-only Data Supplement).1 The assay-specific cutoff levels were derived in diagnostic studies enrolling unselected patients with mostly normal renal function.1,14–19
Main Outcome Measures
The coprimary outcome measures were safety of rule-out, accuracy of rule-in, and overall efficacy of the ESC 0/1-hour algorithm in patients with RD. Safety was quantified as sensitivity for NSTEMI in the rule-out group, accuracy as specificity for NSTEMI in the rule-in group, and overall efficacy as the proportion of patients triaged to either rule-out or rule-in based on the 0- and 1-hour sample. Because prevalence of NSTEMI differs between patients with RD and patients with normal renal function,10 the negative predictive value (NPV) for NSTEMI in the rule-out group and the positive predictive value (PPV) in the rule-in group, which both depend on prevalence, were considered as secondary outcome measures. Additional secondary outcome measures included the proportion of patients assigned directly to rule-out or rule-in based on the single hs-cTn concentration measured at presentation.
Subgroup analyses assessing the diagnostic performance of the 0/1-hour algorithm were performed in early presenters (≤2 hours after chest pain onset), in patients with preexisting and new onset of RD, in women and men, and in the dataset after exclusion of patients who were part of the initial derivation cohort of the 1-hour algorithms.
To extend and corroborate the concept of the ESC 0/1-hour algorithm in patients with RD, diagnostic performance was further assessed using stepwise modified cutoff criteria optimized for patients with RD using hs-cTn concentrations at presentation or absolute changes within the first hour.
Follow-Up and Clinical End Points
Patients were contacted 3, 12, and 24 months after discharge by telephone calls or in written form. Information regarding death during follow-up was furthermore obtained from the patient’s hospital notes, the family physician’s records, and the national registry on mortality. The coprimary prognostic end points were overall survival after 30 days and 2 years. The secondary prognostic end point was major adverse cardiac events (MACEs), defined as the composite of all-cause mortality, AMI (including index event), cardiogenic shock, ventricular tachyarrhythmias, or higher degree atrioventricular block at 30 days.
Statistical Analysis
All data are expressed as medians (1st quartile, 3rd quartile) for continuous variables and for categorical variables as numbers and percentages. Continuous variables were compared with the Mann-Whitney U test, and categorical variables using the chi-square test or Fisher exact test as appropriate. Receiver operating characteristics curves were constructed to assess the discriminative performance throughout hs-cTn concentrations at presentation and their absolute changes in ≤1 hour to diagnose NSTEMI. The comparison of independent areas under the receiver operating characteristics curve was performed as recommended by Hanley and McNeil.33
We used the cross tables derived by the application of the official ESC assay-specific cutoff criteria for rule-out or rule-in to calculate diagnostic performance parameters and their 95% confidence intervals (CI).34 To compare sensitivity, specificity, NPV, PPV, and efficacy, we used a chi-square or Fisher exact test for unpaired samples and the McNemar test or the method described by Moskowitz and Pepe35 for paired samples, as appropriate. Correlations between renal function and concentrations/changes of hs-cTn were determined with the use of the Spearman rank correlation based on log-transformed hs-cTn values.
Overall survival and MACE-free survival during follow-up according to the classification provided by the respective 0/1-hour algorithm were plotted in Kaplan-Meier curves, and a log-rank test was used to assess differences in survival among groups.
Unless stated otherwise, results are reported based on dataset A. All hypothesis testing was 2-tailed, and P values of <0.05 were considered to indicate statistical significance without adjustments for multiple testing. All statistical analyses were performed with the use of IBM SPSS Statistics for Windows, version 23.0 (SPSS Inc), R statistical software version 3.4.1 (www.R-project.org, R Foundation for Statistical Computing), and MedCalc Statistical Software, version 17.8 (MedCalc Software bvba).
Results
Patient Characteristics
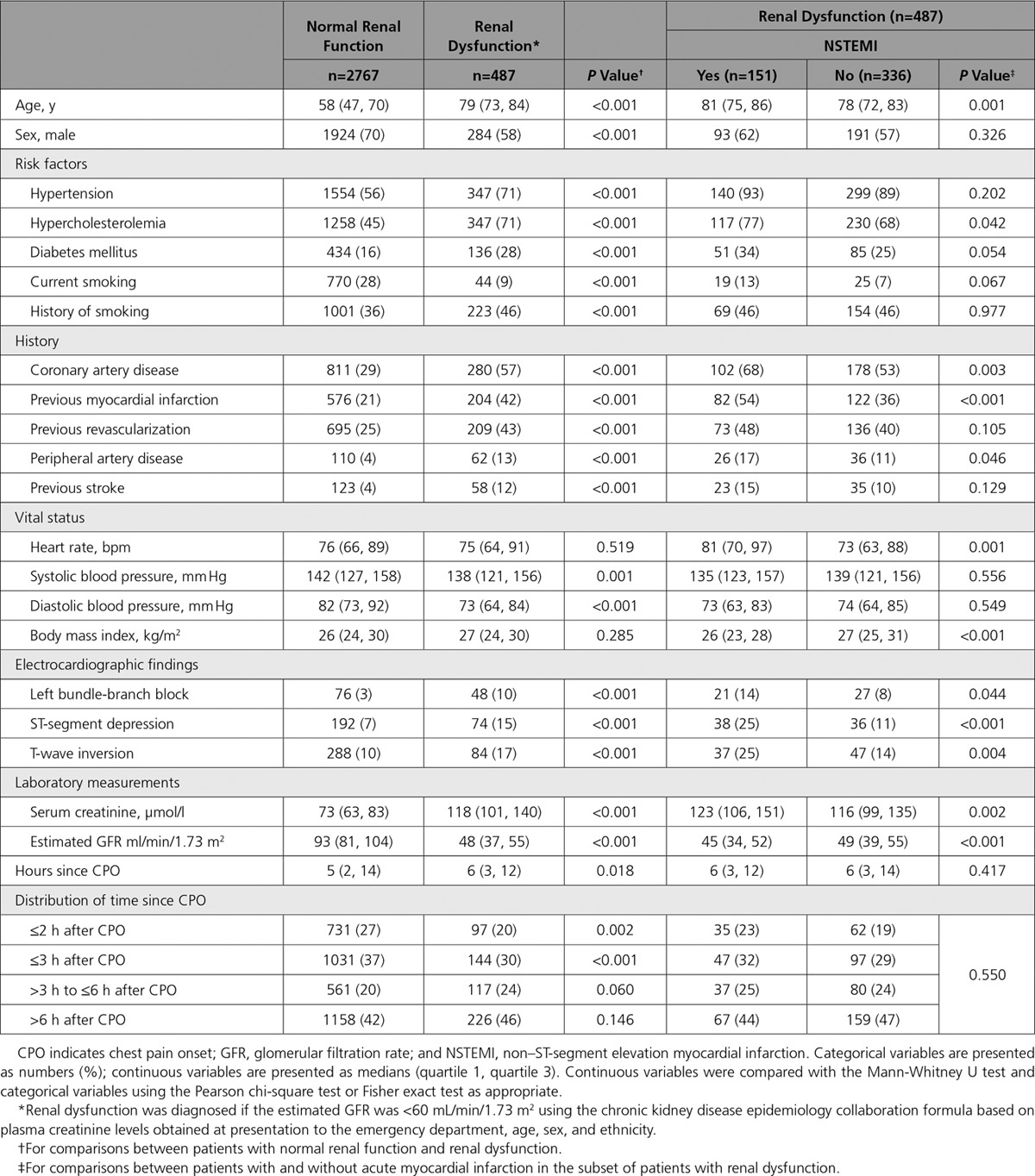
From 4323 consecutively recruited patients, serial hs-cTnT measurements at presentation and after 1 hour were available in 3254 patients (dataset A, 100%) and serial hs-cTnI measurements in 2949 patients (dataset B) (Figure II in the online-only Data Supplement). Baseline characteristics are depicted in the Table 1 and Table IV in the online-only Data Supplement. Dataset B represented a subset of dataset A (overlap, 91%) (Table V in the online-only Data Supplement). Prevalence of RD was 15% (487/3254 in dataset A, 445/2949 in dataset B) with a median eGFR of 48 (37, 55) ml/min/1.73 m2 as compared with 93 (81, 104) ml/min/1.73 m2 in patients with normal renal function. Patients with RD differed from patients with normal renal function in multiple baseline characteristics, including higher prevalence of cardiovascular risk factors, previous myocardial infarction, and ECG abnormalities.
Table 1.
Baseline Characteristics of Patients in Dataset A
Adjudicated Final Diagnosis
NSTEMI was the adjudicated final diagnosis in 515 of 3254 (16%) patients. In patients with RD, prevalence of NSTEMI was 31% compared with 13% in patients with normal renal function (P<0.001). The prevalence of NSTEMI was significantly higher in those patients with RD who had preexisting kidney disease (37% versus 24%, P=0.002). Among all NSTEMIs, type 2 NSTEMI was more frequent in patients with RD compared with patients with normal renal function (22% versus 10%, P<0.001) (Table VI in the online-only Data Supplement), resulting in an overall type 2 NSTEMI prevalence of 6.8% (33/487) in patients with RD compared with 1.3% (35/2767) in patients with normal renal function. Also, cardiac causes other than coronary artery disease were more common in patients with RD and noncardiac causes less common compared with patients with normal renal function. Disagreement between the 2 independent cardiologists adjudicating the final diagnosis was more common in patients with RD compared with patients with normal renal function (13.1% versus 9.1%, P=0.006).
Hs-cTn Concentrations at Presentation and 1-Hour Changes According to Renal Function and Final Diagnosis
In patients with RD and patients with normal renal function, hs-cTn concentrations at presentation as well as absolute 1-hour changes were significantly higher in NSTEMI compared with other final diagnoses (P<0.001 for all comparisons, data not shown).
In patients with final diagnoses other than NSTEMI, hs-cTnT and hs-cTnI concentrations at presentation as well as absolute 1-hour changes showed a strong, inverse correlation with eGFR, which was not observed in NSTEMI (Figure III in the online-only Data Supplement). The diagnostic accuracy of hs-cTnT and hs-cTnI concentrations at presentation for NSTEMI, as quantified by the areas under the receiver operating characteristics curve, was high among patients with RD (for hs-cTnT, 0.87 [95% CI, 0.84–0.90]; for hs-cTnI, 0.86 [95% CI, 0.83–0.90]) but even significantly higher in patients with normal renal function (for hs-cTnT, 0.94 [95% CI, 0.93–0.95]; for hs-cTnI, 0.93 [95% CI, 0.92–0.95]) (Figure IV in the online-only Data Supplement). Smaller differences were observed for the diagnostic accuracy of the absolute 1-hour change in hs-cTn.
Performance of the ESC 0/1-Hour Algorithm Using hs-cTnT in RD
Safety of rule-out by the ESC 0/1-hour algorithm, quantified as the sensitivity for NSTEMI in the rule-out group, was high in patients with RD and similar to patients with normal renal function using hs-cTnT (100% [95% CI, 97.6–100] versus 99.2% [95% CI, 97.6–99.8], respectively; P=0.559) (Table 2 and Figure 1). NPV was 100% in patients with RD compared with 99.8% (95% CI, 99.5–100) in patients with normal renal function (P=1.0).
Table 2.
Performance of the European Society of Cardiology 0/1-Hour Algorithm in Patients With Renal Dysfunction and Normal Renal Function
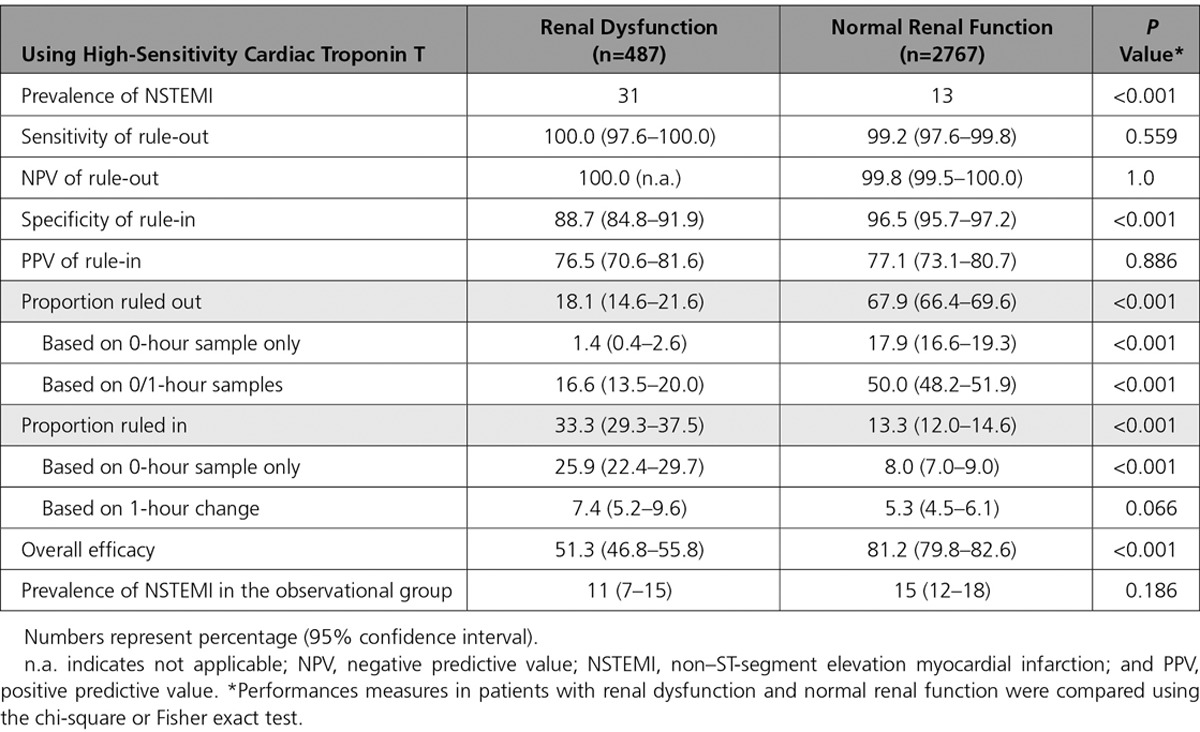
Figure 1.
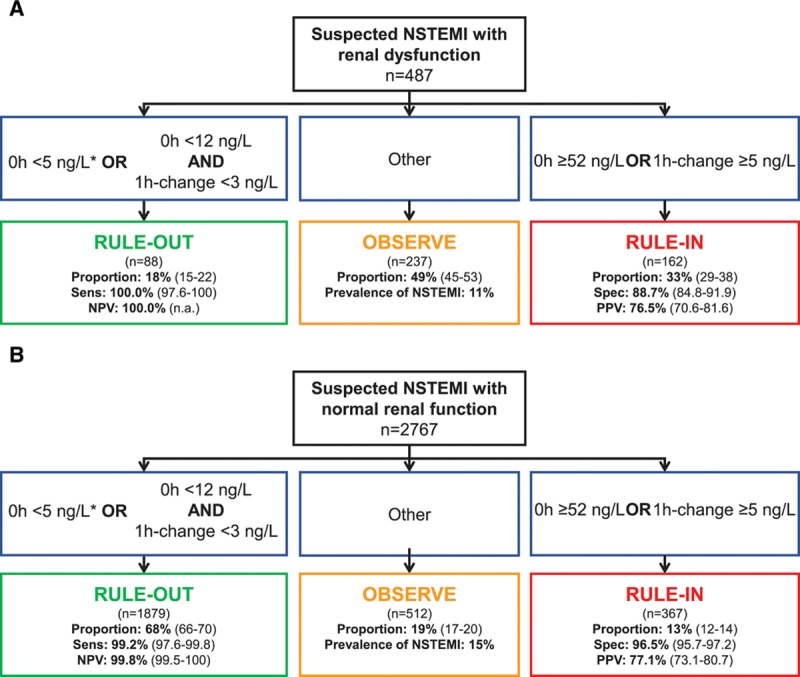
Performance of the European Society of Cardiology 0/1-hour algorithm using high-sensitivity cardiac troponin T in patients with renal dysfunction and normal renal function. Flow charts depicting the diagnostic performance of the European Society of Cardiology 0/1-hour algorithm for rule-out and rule-in of non–ST-segment elevation myocardial infarction in (A) patients with renal dysfunction (defined as an estimated glomerular filtration rate <60 mL/min/1.73 m2), and (B) patients with normal renal function using high-sensitivity cardiac troponin T (hs-cTnT, Elecsys analyzer). 1h-change indicates absolute (unsigned) change of high-sensitivity cardiac troponin within 1 hour; n.a., not applicable; NPV, negative predictive value; NSTEMI, non–ST-segment elevation myocardial infarction; PPV, positive predictive value; Sens, Sensitivity; and Spec, specificity. *If chest pain onset >3 hours before presentation to the emergency department.
Accuracy of rule-in, quantified as the specificity for NSTEMI in the rule-in group, was lower in patients with RD compared with patients with normal renal function (88.7% [95% CI, 84.8–91.9] versus 96.5% [95% CI, 95.7–97.2], P<0.001). Because of the higher prevalence of NSTEMI in patients with RD, accuracy of rule-in as quantified by PPV remained comparable in patients with RD and patients with normal renal function (PPV, 76.5% [95% CI, 70.6–81.6] versus 77.1% [95% CI, 73.1–80.7], P=0.886). Unstable angina (n=2 and 1), myocarditis (n=0 and 14), Tako-Tsubo cardiomyopathy (n=1 and 4), and acute heart failure (n=11 and 6) accounted for 37% and 30% of non-NSTEMI diagnoses in the rule-in groups of patients with RD and normal renal function, respectively (P=0.445 for comparison).
Efficacy of rule-out, quantified as the proportion of patients assigned toward rule-out based on the 0- and 1-hour samples, was substantially lower in patients with RD compared with patients with normal renal function (18.1% [95% CI, 14.6–21.6] versus 67.9% [95% CI, 66.4–69.6], P<0.001). Direct rule-out, based on a single hs-cTn concentration measured at presentation in patients presenting >3 hours after chest pain onset, was feasible in 1.4% (95% CI, 0.4–2.6) of patients with RD compared with 17.9% (95% CI, 16.6–19.3) of patients with normal renal function (P<0.001). Efficacy of rule-in was substantially higher in patients with RD compared with patients with normal renal function (33.3% [95% CI, 29.3–37.5] versus 13.3% [95% CI, 12.0–14.6], P<0.001). Overall efficacy, quantified as the proportion of patients assigned to either rule-out or rule-in in ≤1 hour, was substantially lower in patients with RD compared with patients with normal renal function (51.3% [95% CI, 46.8–55.8] versus 81.2% [95% CI, 79.8–82.6], P<0.001). Prevalence of NSTEMI in the observe group was comparable in patients with RD compared with patients with normal renal function (11% versus 15%, P=0.186). No NSTEMI patient with RD was incorrectly ruled out by the ESC 0/1-hour algorithm, whereas 3 NSTEMI patients (0.1%) with normal renal function were missed (Table VII in the online-only Data Supplement). The diagnostic performance of the ESC hs-cTnT 0/1-hour algorithm according to different stages of renal dysfunction is depicted in Figure 2.
Figure 2.
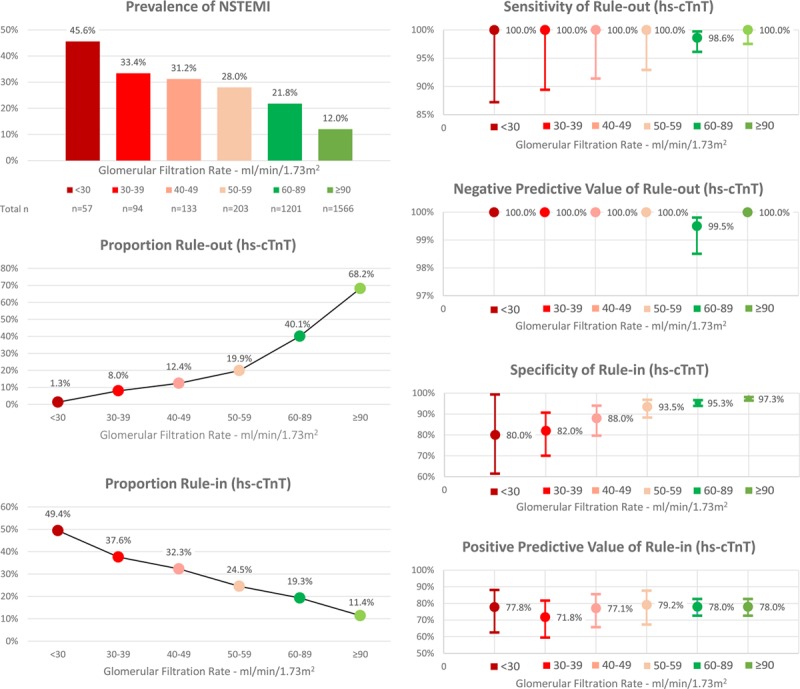
Performance of the European Society of Cardiology 0/1-hour algorithm using high-sensitivity cardiac troponin T in different stages of renal function. hs-cTnT indicates high-sensitivity cardiac troponin T; and NSTEMI, non–ST-segment elevation myocardial infarction.
Performance of the ESC 0/1-Hour Algorithm Using hs-cTnI in RD
Safety of rule-out by the ESC 0/1-hour algorithm was high in patients with RD and similar to patients with normal renal function using hs-cTnI (98.6% [95% CI, 95.0–99.8] versus 98.5% [95% CI, 96.5–99.5], respectively; P=1.0) (Table 3 and Figure 3). NPV (and the prevalence of non-NSTEMI) was lower in patients with RD (NPV, 97.4% [95% CI, 90.5–99.4]) compared with patients with normal renal function (NPV, 99.7% [95% CI, 99.2–99.9], P=0.046).
Table 3.
Performance of the European Society of Cardiology 0/1-Hour Algorithm in Patients With Renal Dysfunction and Normal Renal Function
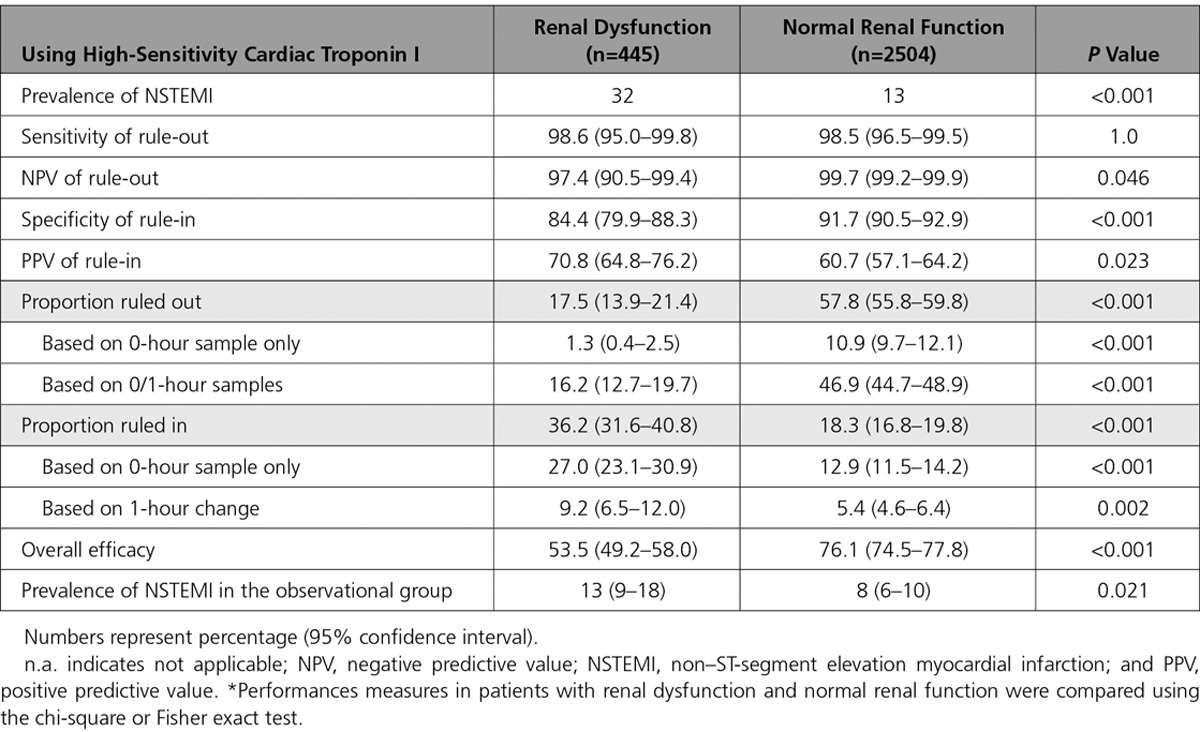
Figure 3.
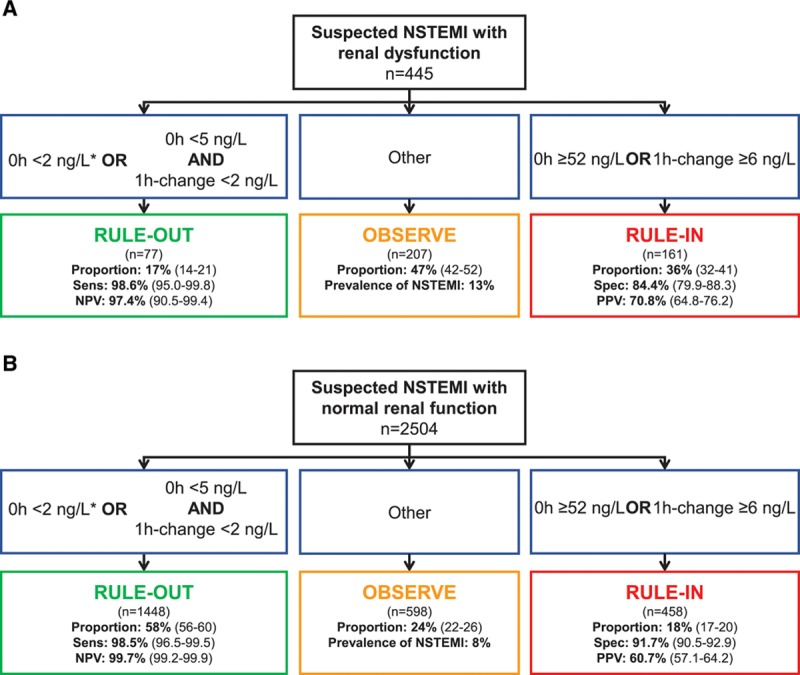
Performance of the European Society of Cardiology 0/1-hour algorithm using high-sensitivity cardiac troponin I in patients with renal dysfunction and normal renal function. Flow charts depicting the diagnostic performance of the European Society of Cardiology 0/1-hour algorithm for rule-out and rule-in of non–ST-segment elevation myocardial infarction in patients with (A) renal dysfunction and (B) normal renal function using high-sensitivity cardiac troponin I (hs-cTnI, Architect analyzer). 1-h change indicates absolute (unsigned) change of high-sensitivity cardiac troponin within 1 hour; NPV, negative predictive value; NSTEMI, non–ST-segment elevation myocardial infarction; PPV, positive predictive value; Sens, sensitivity; and Spec, specificity. *If chest pain onset >3 hours before presentation to the emergency department.
Accuracy of rule-in as quantified by specificity was lower in patients with RD compared with patients with normal renal function (specificity, 84.4% [95% CI, 79.9–88.3] versus 91.7% [95% CI, 90.5–92.9], P<0.001). However, because of the higher prevalence of NSTEMI in patients with RD, accuracy as quantified by PPV of rule-in was higher in patients with RD compared with patients with normal renal function (PPV, 70.8% [95% CI, 64.8–76.2] versus 60.7% [95% CI, 57.1–64.2], P=0.023). Unstable angina (n=8 and 30), myocarditis (n=0 and 15), Tako-Tsubo cardiomyopathy (n=1 and 4), and acute heart failure (n=10 and 15) accounted for 40% and 36% of non-NSTEMI diagnoses in the rule-in groups of patients with RD and patients with normal renal function, respectively (P=0.614 for comparison).
Efficacy of rule-out was substantially lower in patients with RD compared with patients with normal renal function (17.5% [95% CI, 13.9–21.4] versus 57.8% [95% CI, 55.8–59.8], P<0.001). Direct rule-out was feasible in 1.3% (95% CI, 0.4–2.5) of patients with RD compared with 10.9% (95% CI, 9.7–12.1) of patients with normal renal function (P<0.001). Efficacy of rule-in was higher in patients with RD compared with patients with normal renal function (36.2% [95% CI, 31.6–40.8] versus 18.3% [95% CI, 16.8–19.8], P<0.001). Overall efficacy was substantially lower in patients with RD compared with patients with normal renal function (53.5% [95% CI, 49.2–58.0] versus 76.1% [95% CI, 74.5–77.8], P<0.001). Prevalence of NSTEMI in the observational group was lower in patients with RD compared with patients with normal renal function (13% versus 18%, P=0.021). Two patients with NSTEMI (0.4%) with RD were incorrectly ruled out by the ESC 0/1-hour algorithm, whereas 5 patients with NSTEMI (0.2%) with normal renal function were missed (Table VIII in the online-only Data Supplement). Diagnostic performance of the ESC hs-cTnI 0/1-hour algorithm according to different stages of RD is depicted in Figure 4.
Figure 4.
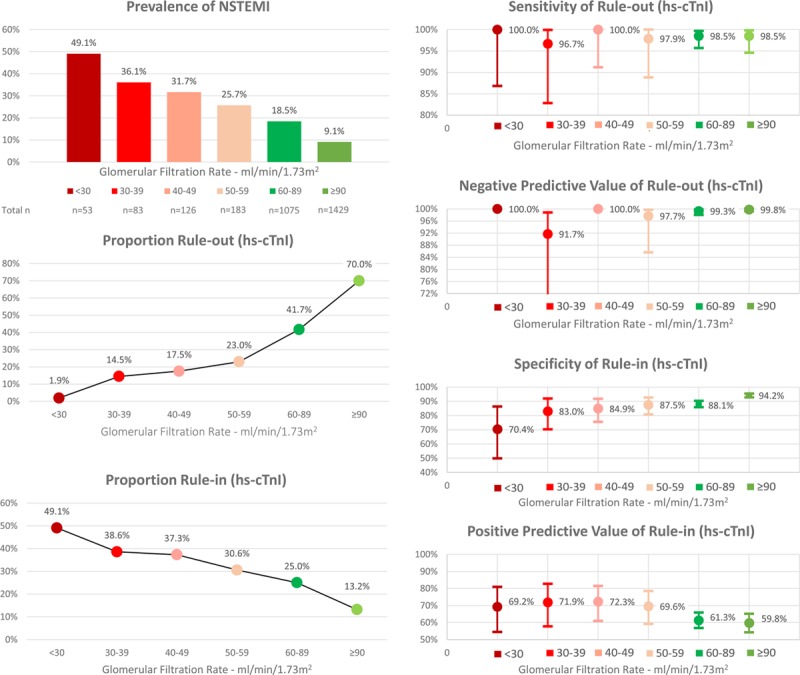
Performance of the European Society of Cardiology 0/1-hour algorithm using high-sensitivity cardiac troponin I in different stages of renal function. hs-cTnI indicates high-sensitivity cardiac troponin I; and NSTEMI, non–ST-segment elevation myocardial infarction.
Performance of the ESC 0/1-Hour Algorithm in Different Subgroups
Robust and highly comparable findings were observed in subgroup and sensitivity analyses performed in patients presenting within the first 2 hours after chest pain onset, in patients with preexisting and new-onset of renal dysfunction, and in women and men as well as in the study dataset after exclusion of patients who were part of the original derivation cohorts of the 2 investigated 0/1-hour algorithms. Details on the diagnostic performance of the ESC 0/1-hour algorithms in the various subgroups are listed in Tables IX–XII in the online-only Data Supplement.
Modifications of the 0/1-Hour Algorithm to Optimize Rule-Out Efficacy and Rule-In Specificity in Patients With RD
Stepwise increase of the official ESC assay-specific cutoff criteria for rule-out of NSTEMI resulted in increasing rule-out efficacy, however at the cost of rule-out safety. Stepwise increase of the official ESC assay-specific cutoff criteria for rule-in of NSTEMI resulted in increasing specificity of rule-in, however at the cost of rule-in efficacy (Tables XIII and XIV in the online-only Data Supplement). Among the numerous possible cutoff criteria combinations, 1 specific cutoff value combination for rule-out, preserving the same sensitivity as the official ESC cutoff value combination, as well as 1 specific cutoff value combination for rule-in, was chosen for each hs-cTn assay to compare its performance with the official ESC 0/1-hour algorithm (Table XV and Figures V and VI in the online-only Data Supplement). Cutoff concentrations optimized for RD increased rule-out efficacy and rule-in specificity by 4.5% (P<0.001) and 3.9% (P<0.001), respectively, for hs-cTnT and by 4.7% (P<0.001) and 3.7%, (P=0.001) respectively, for hs-cTnI. However, because improved rule-in specificity was obtained at the cost of rule-in efficacy, overall efficacy could not be optimized with the modified 0/1-hour algorithm (for hs-cTnT, −1.0%, P=0.568; for hs-cTnI, +1.1%, P=0.500).
Prognostic Performance of the ESC 0/1-Hour Algorithm
Median follow-up time was 749 days (418, 847). Estimated overall-survival was 99.2% at 30 days and 94.3% at 2 years. Particularly in patients with RD, the ESC 0/1-hour algorithm using hs-cTnT and hs-cTnI allowed a powerful discrimination between high versus moderate and low probability of short-term (30 days) and midterm (2 years) overall survival and short-term (30 days) MACE-free survival in the respective rule-out, observe, and rule-in groups (all log-rank P values <0.001) (Figure 5 and Figure VII in the online-only Data Supplement).
Figure 5.
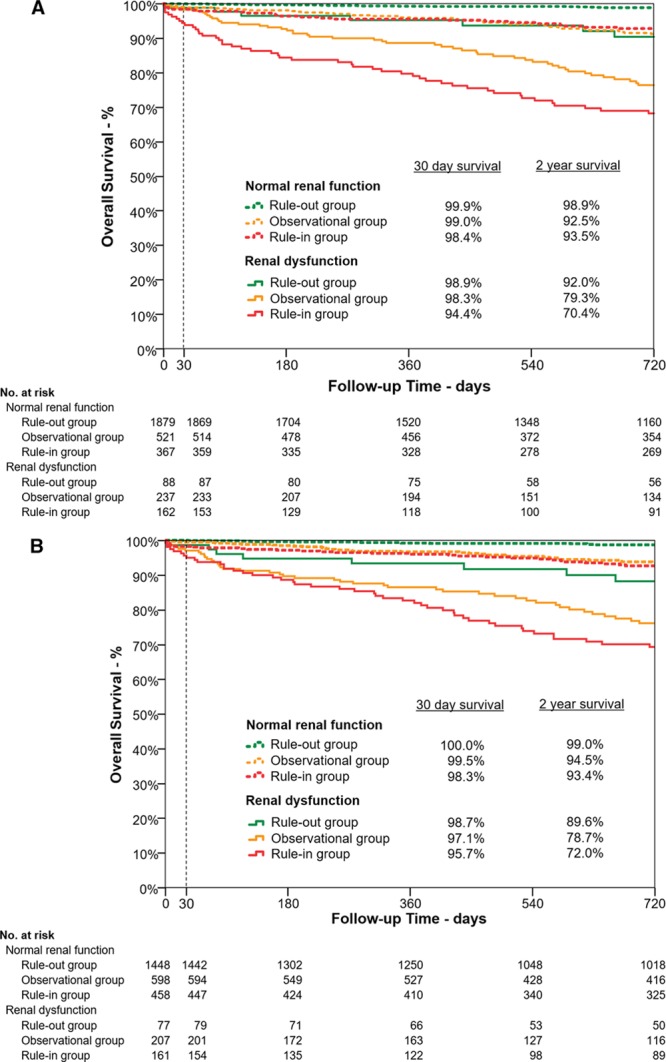
Short- and midterm survival according to risk stratification group by the European Society of Cardiology 0/1-hour algorithm using high-sensitivity cardiac troponin T and I in patients with normal renal function and renal dysfunction. Kaplan-Meier curves depicting overall survival within 30 and 720 days for patients with normal renal function (dashed lines) and renal dysfunction (solid lines) stratified by the European Society of Cardiology 0/1-hour algorithm to the rule-out (green lines), observational (orange lines), and rule-in (red lines) groups. A, Using high-sensitivity cardiac troponin T. B, Using high-sensitivity cardiac troponin I.
Discussion
This prospective, multicenter diagnostic study enrolling unselected patients presenting with acute chest discomfort to the ED used central adjudication to assess the performance of the ESC 0/1-hour algorithm in patients with RD. We report 8 major findings.
First, patients with RD presenting with acute chest discomfort to the ED had NSTEMI >2 times as often and type 2 NSTEMI even >5 times as often as patients with normal renal function. This observation extends and corroborates previous studies indicating that RD is not only commonly associated with coronary artery disease but also hypertensive heart disease and other structural cardiac disorders prone to developing the triggers of type 2 myocardial infarction, such as tachyarrhythmias, hypertension, and anemia.8–10,36,37
Second, hs-cTn concentrations at presentation and their absolute 1-hour changes correlated strongly and inversely with eGFR in patients with diagnoses other than NSTEMI but not NSTEMI. Third, in patients with RD, the diagnostic performance of hs-cTn concentrations at presentation was high (areas under the receiver operating characteristics curve, 0.86–0.87) and further increased on using absolute 1-hour hs-cTn changes (areas under the receiver operating characteristics curve, 0.88–0.92).
Fourth, and likely of utmost importance, the safety of the official ESC 0/1-hour algorithm was high in patients with RD (sensitivity, 98.6–100) and comparable to patients with normal renal function (sensitivity, 98.5–99.2) irrespective of whether hs-cTnT or hs-cTnI was used. However, the efficacy of rule-out was substantially reduced in patients with RD and allowed the early rule-out in 18% of patients only.
Fifth, because of the higher proportions of patients with elevated levels of hs-cTn even in the absence of NSTEMI, specificity of rule-in was lower in patients with RD (84.4–88.7) compared with patients with normal renal function (91.7–96.5). However, the higher prevalence of NSTEMI in patients with RD also increased rule-in efficacy while maintaining high PPV of rule-in. The performance measures (mainly the PPV) of the ESC hs-cTnT 0/1-hour algorithm and the ESC hs-cTnI 0/1-hour algorithm showed subtle but consistent differences to the advantage of hs-cTnT. These differences are at least in part caused by the fact that serial measurements of hs-cTnT but not hs-cTnI were part of the extensive clinical information available for the adjudication of the final diagnosis in all patients. Accordingly, our methodology provided the most accurate and valid estimates for the ESC hs-cTnT 0/1-hour algorithm but possibly slightly underestimated the true performance of the ESC hs-cTnI 0/1-hour algorithm.
Sixth, overall efficacy allowing triage toward rule-out or rule-in based on the 0/1-hour samples was substantially reduced in patients with RD (51.3–53.5) compared with patients with normal renal function (76.1–81.2). This difference was driven by the substantial reduction in rule-out efficacy that could only partly be compensated for by the increase of rule-in efficacy. As a consequence, the percentage of patients remaining in the observe zone and usually requiring additional diagnostic tests including a 3-hour sample of hs-cTn and cardiac imaging is nearly twice as high in patients with RD compared with patients with normal renal function.
Seventh, using slightly higher cutoff concentrations of hs-cTn as an attempt to increase rule-out efficacy and rule-in specificity only partly helped to overcome the challenges posed by RD. The high pretest probability for NSTEMI in patients with RD challenges the derivation of an alternative 0/1-hour algorithm that would balance rule-out efficacy and rule-in specificity substantially better than the official ESC 0/1-hour algorithm without losing safety. It is a matter of debate how much increase of rule-out efficacy at the cost of rule-out safety or how much increase of rule-in specificity at the cost of rule-in efficacy would be acceptable. The use of alternative cutoff criteria combinations yielded rather small improvements even though they were tested in a derivation setting unblinded to the outcome NSTEMI. Accordingly, the observed small improvements in efficacy when using alternative cutoffs are associated with a potential systematic bias toward overestimating the real improvements, which might be even smaller in subsequent external validation in an independent study. Therefore, and because safety and simplicity are the most important characteristics of any diagnostic algorithm, the findings of this study recommend the use of the official ESC 0/1-hour algorithm in patients with RD until information technology-based decision tools integrating all available information (eg, age, sex, serial hs-cTn measurements, renal function) become available in clinical routine. The PPV for NSTEMI in patients assigned toward rule-in and thereby early coronary angiography would still be considered high enough by most experts, particularly given the difficulty of obtaining similar diagnostic certainty in patients with moderate elevations in cTn without coronary angiography.
Eighth, the ESC 0/1-hour algorithm allowed a powerful discrimination between high versus moderate and lower probability of short- and midterm overall survival as well as short-term MACE-free survival in the respective rule-out, observe, and rule-in groups also in patients with RD. The rather high rate of all-cause mortality during follow-up and MACE within 30 days of patients in the observe zone can be explained by the high incidence of chronic diseases in those patients, such as chronic heart failure, which are associated with high rates of both overall mortality and MACE within 30 days. These findings extend and corroborate previous studies addressing the multitude of major unmet clinical needs in the often elderly patients with RD.8–10,38
Many of these challenges are related to the high prevalence of common yet undiagnosed cardiac comorbidities including hypertensive heart disease and diabetic cardiomyopathy associated with chronic cardiomyocyte injury and therefore increases in hs-cTn plasma concentrations and an increased prevalence of ECG abnormalities in patients with RD. The exact underlying pathophysiological mechanisms are incompletely understood. The contribution of cardiomyocyte injury to elevated plasma concentrations of hs-cTn in RD seems to be far greater than that of impaired renal clearance, particularly because the molecular size of the intact molecule is too large to be filtrated by glomeruli.36,37,39–41 Although cTn molecules may be degraded into smaller fragments that are small enough to be filtered by the kidney,42 the renal elimination and half-life of these cTn fragments seem to be similar in patients with RD and patients with normal renal function.43 In addition, in patients with end-stage renal disease and only minimal remaining endogenous renal function, successful renal transplantation leads to a substantial reduction and often normalization of serum creatinine but no relevant change in plasma concentrations of cTnI.39 It has been hypothesized that the underlying mechanism of chronic cTn release is associated with a cardiorenal syndrome triggered by some inflammatory processes leading to chronic cardiomyocyte injury and cTn release in RD.44,45
Initial pilot studies evaluating the use of single cutoff concentrations suggested that in patients with RD, adjusted higher hs-cTn concentrations might provide a better balance between sensitivity and specificity compared with the 99th percentiles or the optimal single-cutoff concentration derived in patients with normal renal function.10 Meanwhile, the clinical use of hs-cTn has advanced, and current guidelines recommend the integrated use of baseline hs-cTn concentrations and their absolute changes during serial sampling, as incorporated in the ESC 0/1-hour algorithm.1 In contrast to a single cutoff strategy, the ESC 0/1-hour algorithm triages patients toward 1 of 3 strata: rule-out, observe, or rule-in. Assessing the possible use of adjusted higher hs-cTn concentrations within this state-of-the-art concept in patients with RD revealed pros and cons.
To the best of our knowledge, this is the first study investigating in detail the diagnostic performance of the ESC 0/1-hour algorithm in the vulnerable patient population with RD, extending the excellent performance characteristics observed in patients with overwhelmingly normal renal function.16–21 We cannot generalize our findings to patients with terminal kidney failure on chronic dialysis because they were excluded from this study. Additionally, our study was conducted in patients at the ED with symptoms suggestive of AMI. Further studies are required to quantify the utility of the ESC 0/1-hour algorithm in patients with either higher (eg, in a coronary care unit setting) or lower (eg, in a general practitioner setting) pretest probability for AMI.
Some limitations merit consideration when interpreting these findings. First, although we used the most stringent methodology to adjudicate the presence or absence of NSTEMI, including central adjudication by experienced cardiologists, imaging, and serial measurements of hs-cTn, we still may have misclassified a small number of patients.3,14 Second, to reflect the clinical information available to the ED physician when interpreting hs-cTn concentrations, we classified RD according to eGFR based on the serum creatinine concentrations obtained at ED presentation. Accordingly, this classification differs from the definition of chronic kidney disease, which would require RD to be present for 3 months.46 Third, the chronic kidney disease epidemiology collaboration formula was used to estimate GFR irrespective of age. However, the chronic kidney disease epidemiology collaboration formula was primarily validated in patients <70 years of age.
In conclusion, in patients with RD, the safety of the ESC 0/1-hour algorithm is high, but the specificity of rule-in and overall efficacy are decreased. Modifications of cutoffs can only partly overcome the challenges of RD.
Sources of Funding
This work was supported by research grants from the Swiss National Science Foundation, the Swiss Heart Foundation, the Kommission für Technologie und Innovation, the Stiftung für kardiovaskuläre Forschung Basel, Abbott, Beckman Coulter, Biomerieux, Brahms, Roche, Siemens, and Singulex.
Disclosures
The authors designed the study, gathered and analyzed the data, vouch for the data and analysis, wrote the paper, and decided to publish. Drs Twerenbold and Mueller had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. All authors have read and approved the manuscript. The sponsors had no role in designing or conducting the study and no role in gathering or analyzing the data or writing the manuscript. The manuscript and its contents have not been published previously and are not being considered for publications elsewhere in whole or in part in any language, including publicly accessible web sites or e-print servers. Dr Twerenbold received research support from the Swiss National Science Foundation (P300PB_167803), the University Hospital Basel, the University of Basel, and the Cardiovascular Research Foundation Basel, as well as speaker honoraria/consulting honoraria from Abbott, Brahms, Siemens, Singulex, and Roche. Dr Boeddinghaus received speaker honoraria from Siemens. Dr Rubini received speaker honoraria from Abbott and research grants from the Swiss Heart Foundation. Dr Reichlin received research grants from the Goldschmidt-Jacobson-Foundation, the Swiss National Science Foundation (PASMP3-136995), the Swiss Heart Foundation, the Professor Max Cloëtta Foundation, the Uniscientia Foundation Vaduz, the University of Basel, and the Department of Internal Medicine, University Hospital Basel, as well as speaker honoraria from Brahms and Roche. Dr Mueller received research support from the Swiss National Science Foundation, the Swiss Heart Foundation, the Kommission für Technologie und Innovation, the Stiftung für kardiovaskuläre Forschung Basel, Abbott, Alere, AstraZeneca, Beckman Coulter, Biomerieux, Brahms, Roche, Siemens, Singulex, Sphingotec, and the Department of Internal Medicine, University Hospital Basel, as well as speaker honoraria/consulting honoraria from Abbott, Alere, AstraZeneca, Biomerieux, Boehringer Ingelheim, Bristol-Myers Squibb, Brahms, Cardiorentis, Novartis, Roche, Siemens, and Singulex. The other authors report no conflicts.
The investigated hs-cTn assays were donated by the manufacturers, which had no role in the design of the study, analysis of the data, preparation of the manuscript, or decision to submit the manuscript for publication.
Supplementary Material
Footnotes
The online-only Data Supplement, podcast, and transcript are available with this article at http://circ.ahajournals.org/lookup/suppl/doi:10.1161/CIRCULATIONAHA.117.028901/-/DC1.
Continuing medical education (CME) credit is available for this article. Go to http://cme.ahajournals.org to take the quiz.
Circulation is available at http://circ.ahajournals.org.
References
- 1.Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S, Baumgartner H, Gaemperli O, Achenbach S, Agewall S, Badimon L, Baigent C, Bueno H, Bugiardini R, Carerj S, Casselman F, Cuisset T, Erol Ç, Fitzsimons D, Halle M, Hamm C, Hildick-Smith D, Huber K, Iliodromitis E, James S, Lewis BS, Lip GY, Piepoli MF, Richter D, Rosemann T, Sechtem U, Steg PG, Vrints C, Luis Zamorano J Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:267–315. doi: 10.1093/eurheartj/ehv320. doi: 10.1093/eurheartj/ehv320. [DOI] [PubMed] [Google Scholar]
- 2.Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Jr, Ganiats TG, Holmes DR, Jr, Jaffe AS, Jneid H, Kelly RF, Kontos MC, Levine GN, Liebson PR, Mukherjee D, Peterson ED, Sabatine MS, Smalling RW, Zieman SJ Members AATF, Society for Cardiovascular A, Interventions and the Society of Thoracic S. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:2354–2394. doi: 10.1161/CIR.0000000000000133. doi: 10.1161/CIR.0000000000000133. [DOI] [PubMed] [Google Scholar]
- 3.Thygesen K, Alpert JS, White HD, Jaffe AS, Apple FS, Galvani M, Katus HA, Newby LK, Ravkilde J, Chaitman B, Clemmensen PM, Dellborg M, Hod H, Porela P, Underwood R, Bax JJ, Beller GA, Bonow R, Van der Wall EE, Bassand JP, Wijns W, Ferguson TB, Steg PG, Uretsky BF, Williams DO, Armstrong PW, Antman EM, Fox KA, Hamm CW, Ohman EM, Simoons ML, Poole-Wilson PA, Gurfinkel EP, Lopez-Sendon JL, Pais P, Mendis S, Zhu JR, Wallentin LC, Fernández-Avilés F, Fox KM, Parkhomenko AN, Priori SG, Tendera M, Voipio-Pulkki LM, Vahanian A, Camm AJ, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Morais J, Brener S, Harrington R, Morrow D, Lim M, Martinez-Rios MA, Steinhubl S, Levine GN, Gibler WB, Goff D, Tubaro M, Dudek D, Al-Attar N Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. Circulation. 2007;116:2634–2653. doi: 10.1161/CIRCULATIONAHA.107.187397. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 4.Taylor MJ, Scuffham PA, McCollam PL, Newby DE. Acute coronary syndromes in Europe: 1-year costs and outcomes. Curr Med Res Opin. 2007;23:495–503. doi: 10.1185/030079906X167462. doi: 10.1185/030079906X167462. [DOI] [PubMed] [Google Scholar]
- 5.Tiemann O. Variations in hospitalisation costs for acute myocardial infarction: a comparison across Europe. Health Econ. 2008;17(s)(uppl 1):S33–S45. doi: 10.1002/hec.1322. doi: 10.1002/hec.1322. [DOI] [PubMed] [Google Scholar]
- 6.deFilippi CR, Herzog CA. Interpreting cardiac biomarkers in the setting of chronic kidney disease. Clin Chem. 2017;63:59–65. doi: 10.1373/clinchem.2016.254748. doi: 10.1373/clinchem.2016.254748. [DOI] [PubMed] [Google Scholar]
- 7.Twerenbold R, Boeddinghaus J, Nestelberger T, Wildi K, Rubini Gimenez M, Badertscher P, Mueller C. Clinical use of high-sensitivity cardiac troponin in patients with suspected myocardial infarction. J Am Coll Cardiol. 2017;70:996–1012. doi: 10.1016/j.jacc.2017.07.718. doi: 10.1016/j.jacc.2017.07.718. [DOI] [PubMed] [Google Scholar]
- 8.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 9.Anavekar NS, McMurray JJ, Velazquez EJ, Solomon SD, Kober L, Rouleau JL, White HD, Nordlander R, Maggioni A, Dickstein K, Zelenkofske S, Leimberger JD, Califf RM, Pfeffer MA. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351:1285–1295. doi: 10.1056/NEJMoa041365. doi: 10.1056/NEJMoa041365. [DOI] [PubMed] [Google Scholar]
- 10.Twerenbold R, Wildi K, Jaeger C, Gimenez MR, Reiter M, Reichlin T, Walukiewicz A, Gugala M, Krivoshei L, Marti N, Moreno Weidmann Z, Hillinger P, Puelacher C, Rentsch K, Honegger U, Schumacher C, Zurbriggen F, Freese M, Stelzig C, Campodarve I, Bassetti S, Osswald S, Mueller C. Optimal cutoff levels of more sensitive cardiac troponin assays for the early diagnosis of myocardial infarction in patients with renal dysfunction. Circulation. 2015;131:2041–2050. doi: 10.1161/CIRCULATIONAHA.114.014245. doi: 10.1161/CIRCULATIONAHA.114.014245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aronow WS, Ahn C, Mercando AD, Epstein S. Prevalence of coronary artery disease, complex ventricular arrhythmias, and silent myocardial ischemia and incidence of new coronary events in older persons with chronic renal insufficiency and with normal renal function. Am J Cardiol. 2000;86:1142–1143. doi: 10.1016/s0002-9149(00)01176-0. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura S, Uzu T, Inenaga T, Kimura G. Prediction of coronary artery disease and cardiac events using electrocardiographic changes during hemodialysis. Am J Kidney Dis. 2000;36:592–599. doi: 10.1053/ajkd.2000.16198. doi: 10.1053/ajkd.2000.16198. [DOI] [PubMed] [Google Scholar]
- 13.deFilippi C, Seliger SL, Kelley W, Duh SH, Hise M, Christenson RH, Wolf M, Gaggin H, Januzzi J. Interpreting cardiac troponin results from high-sensitivity assays in chronic kidney disease without acute coronary syndrome. Clin Chem. 2012;58:1342–1351. doi: 10.1373/clinchem.2012.185322. doi: 10.1373/clinchem.2012.185322. [DOI] [PubMed] [Google Scholar]
- 14.Thygesen K, Mair J, Giannitsis E, Mueller C, Lindahl B, Blankenberg S, Huber K, Plebani M, Biasucci LM, Tubaro M, Collinson P, Venge P, Hasin Y, Galvani M, Koenig W, Hamm C, Alpert JS, Katus H, Jaffe AS Study Group on Biomarkers in Cardiology of ESC Working Group on Acute Cardiac Care. How to use high-sensitivity cardiac troponins in acute cardiac care. Eur Heart J. 2012;33:2252–2257. doi: 10.1093/eurheartj/ehs154. doi: 10.1093/eurheartj/ehs154. [DOI] [PubMed] [Google Scholar]
- 15.Reichlin T, Twerenbold R, Wildi K, Rubini Gimenez M, Bergsma N, Haaf P, Druey S, Puelacher C, Moehring B, Freese M, Stelzig C, Krivoshei L, Hillinger P, Jäger C, Herrmann T, Kreutzinger P, Radosavac M, Weidmann ZM, Pershyna K, Honegger U, Wagener M, Vuillomenet T, Campodarve I, Bingisser R, Miró Ò, Rentsch K, Bassetti S, Osswald S, Mueller C. Prospective validation of a 1-hour algorithm to rule-out and rule-in acute myocardial infarction using a high-sensitivity cardiac troponin T assay. CMAJ. 2015;187:E243–E252. doi: 10.1503/cmaj.141349. doi: 10.1503/cmaj.141349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reichlin T, Schindler C, Drexler B, Twerenbold R, Reiter M, Zellweger C, Moehring B, Ziller R, Hoeller R, Rubini Gimenez M, Haaf P, Potocki M, Wildi K, Balmelli C, Freese M, Stelzig C, Freidank H, Osswald S, Mueller C. One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Arch Intern Med. 2012;172:1211–1218. doi: 10.1001/archinternmed.2012.3698. doi: 10.1001/archinternmed.2012.3698. [DOI] [PubMed] [Google Scholar]
- 17.Rubini Gimenez M, Twerenbold R, Jaeger C, Schindler C, Puelacher C, Wildi K, Reichlin T, Haaf P, Merk S, Honegger U, Wagener M, Druey S, Schumacher C, Krivoshei L, Hillinger P, Herrmann T, Campodarve I, Rentsch K, Bassetti S, Osswald S, Mueller C. One-hour rule-in and rule-out of acute myocardial infarction using high-sensitivity cardiac troponin I. Am J Med. 2015;128:861.e4–870.e4. doi: 10.1016/j.amjmed.2015.01.046. doi: 10.1016/j.amjmed.2015.01.046. [DOI] [PubMed] [Google Scholar]
- 18.Mueller C, Giannitsis E, Christ M, Ordóñez-Llanos J, deFilippi C, McCord J, Body R, Panteghini M, Jernberg T, Plebani M, Verschuren F, French J, Christenson R, Weiser S, Bendig G, Dilba P, Lindahl B TRAPID-AMI Investigators. Multicenter evaluation of a 0-hour/1-hour algorithm in the diagnosis of myocardial infarction with high-sensitivity cardiac troponin T. Ann Emerg Med. 2016;68:76.e4–87.e4. doi: 10.1016/j.annemergmed.2015.11.013. doi: 10.1016/j.annemergmed.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 19.Pickering JW, Greenslade JH, Cullen L, Flaws D, Parsonage W, Aldous S, George P, Worster A, Kavsak PA, Than MP. Assessment of the European Society of cardiology 0-hour/1-hour algorithm to rule-out and rule-in acute myocardial infarction. Circulation. 2016;134:1532–1541. doi: 10.1161/CIRCULATIONAHA.116.022677. doi: 10.1161/CIRCULATIONAHA.116.022677. [DOI] [PubMed] [Google Scholar]
- 20.Reichlin T, Hochholzer W, Bassetti S, Steuer S, Stelzig C, Hartwiger S, Biedert S, Schaub N, Buerge C, Potocki M, Noveanu M, Breidthardt T, Twerenbold R, Winkler K, Bingisser R, Mueller C. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361:858–867. doi: 10.1056/NEJMoa0900428. doi: 10.1056/NEJMoa0900428. [DOI] [PubMed] [Google Scholar]
- 21.Twerenbold R, Jaeger C, Rubini Gimenez M, Wildi K, Reichlin T, Nestelberger T, Boeddinghaus J, Grimm K, Puelacher C, Moehring B, Pretre G, Schaerli N, Campodarve I, Rentsch K, Steuer S, Osswald S, Mueller C. Impact of high-sensitivity cardiac troponin on use of coronary angiography, cardiac stress testing, and time to discharge in suspected acute myocardial infarction. Eur Heart J. 2016;37:3324–3332. doi: 10.1093/eurheartj/ehw232. doi: 10.1093/eurheartj/ehw232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig L, Lijmer JG, Moher D, Rennie D, de Vet HC, Kressel HY, Rifai N, Golub RM, Altman DG, Hooft L, Korevaar DA, Cohen JF STARD Group. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. Clin Chem. 2015;61:1446–1452. doi: 10.1373/clinchem.2015.246280. doi: 10.1373/clinchem.2015.246280. [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giannitsis E, Kurz K, Hallermayer K, Jarausch J, Jaffe AS, Katus HA. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin Chem. 2010;56:254–261. doi: 10.1373/clinchem.2009.132654. doi: 10.1373/clinchem.2009.132654. [DOI] [PubMed] [Google Scholar]
- 25.Hallermayer K, Jarausch J, Menassanch-Volker S, Zaugg C, Ziegler A. Implications of adjustment of high-sensitivity cardiac troponin T assay. Clin Chem. 2013;59:572–574. doi: 10.1373/clinchem.2012.197103. doi: 10.1373/clinchem.2012.197103. [DOI] [PubMed] [Google Scholar]
- 26.Kuster N, Dupuy AM, Monnier K, Baptista G, Bargnoux AS, Badiou S, Jeandel C, Cristol JP. Implications of adjustment of high-sensitivity cardiac troponin T assay. Clin Chem. 2013;59:570–572. doi: 10.1373/clinchem.2012.197020. doi: 10.1373/clinchem.2012.197020. [DOI] [PubMed] [Google Scholar]
- 27.Kavsak PA, Hill SA, McQueen MJ, Devereaux PJ. Implications of adjustment of high-sensitivity cardiac troponin T assay. Clin Chem. 2013;59:574–576. doi: 10.1373/clinchem.2012.197434. doi: 10.1373/clinchem.2012.197434. [DOI] [PubMed] [Google Scholar]
- 28.Wildi K, Twerenbold R, Jaeger C, Rubini Giménez M, Reichlin T, Stoll M, Hillinger P, Puelacher C, Boeddinghaus J, Nestelberger T, Grimm K, Grob M, Rentsch K, Arnold C, Mueller C. Clinical impact of the 2010-2012 low-end shift of high-sensitivity cardiac troponin T. Eur Heart J Acute Cardiovasc Care. 2016;5:399–408. doi: 10.1177/2048872616642952. doi: 10.1177/2048872616642952. [DOI] [PubMed] [Google Scholar]
- 29.Apple FS, Ler R, Murakami MM. Determination of 19 cardiac troponin I and T assay 99th percentile values from a common presumably healthy population. Clin Chem. 2012;58:1574–1581. doi: 10.1373/clinchem.2012.192716. doi: 10.1373/clinchem.2012.192716. [DOI] [PubMed] [Google Scholar]
- 30.Koerbin G, Tate J, Potter JM, Cavanaugh J, Glasgow N, Hickman PE. Characterisation of a highly sensitive troponin I assay and its application to a cardio-healthy population. Clin Chem Lab Med. 2012;50:871–878. doi: 10.1515/cclm-2011-0540. doi: 10.1515/cclm-2011-0540. [DOI] [PubMed] [Google Scholar]
- 31.Krintus M, Kozinski M, Boudry P, Capell NE, Köller U, Lackner K, Lefèvre G, Lennartz L, Lotz J, Herranz AM, Nybo M, Plebani M, Sandberg MB, Schratzberger W, Shih J, Skadberg Ø, Chargui AT, Zaninotto M, Sypniewska G. European multicenter analytical evaluation of the Abbott ARCHITECT STAT high sensitive troponin I immunoassay. Clin Chem Lab Med. 2014;52:1657–1665. doi: 10.1515/cclm-2014-0107. doi: 10.1515/cclm-2014-0107. [DOI] [PubMed] [Google Scholar]
- 32.Braunwald E, Morrow DA. Unstable angina: is it time for a requiem? Circulation. 2013;127:2452–2457. doi: 10.1161/CIRCULATIONAHA.113.001258. doi: 10.1161/CIRCULATIONAHA.113.001258. [DOI] [PubMed] [Google Scholar]
- 33.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 34.Simel DL, Samsa GP, Matchar DB. Likelihood ratios with confidence: sample size estimation for diagnostic test studies. J Clin Epidemiol. 1991;44:763–770. doi: 10.1016/0895-4356(91)90128-v. [DOI] [PubMed] [Google Scholar]
- 35.Moskowitz CS, Pepe MS. Comparing the predictive values of diagnostic tests: sample size and analysis for paired study designs. Clin Trials. 2006;3:272–279. doi: 10.1191/1740774506cn147oa. doi: 10.1191/1740774506cn147oa. [DOI] [PubMed] [Google Scholar]
- 36.Irfan A, Twerenbold R, Reiter M, Reichlin T, Stelzig C, Freese M, Haaf P, Hochholzer W, Steuer S, Bassetti S, Zellweger C, Freidank H, Peter F, Campodarve I, Meune C, Mueller C. Determinants of high-sensitivity troponin T among patients with a noncardiac cause of chest pain. Am J Med. 2012;125:491.e1–498.e1. doi: 10.1016/j.amjmed.2011.10.031. doi: 10.1016/j.amjmed.2011.10.031. [DOI] [PubMed] [Google Scholar]
- 37.Mueller C, Laule-Kilian K, Scholer A, Nusbaumer C, Zeller T, Staub D, Perruchoud AP. B-type natriuretic peptide for acute dyspnea in patients with kidney disease: insights from a randomized comparison. Kidney Int. 2005;67:278–284. doi: 10.1111/j.1523-1755.2005.00079.x. doi: 10.1111/j.1523-1755.2005.00079.x. [DOI] [PubMed] [Google Scholar]
- 38.Reiter M, Twerenbold R, Reichlin T, Haaf P, Peter F, Meissner J, Hochholzer W, Stelzig C, Freese M, Heinisch C, Breidthardt T, Freidank H, Winkler K, Campodarve I, Gea J, Mueller C. Early diagnosis of acute myocardial infarction in the elderly using more sensitive cardiac troponin assays. Eur Heart J. 2011;32:1379–1389. doi: 10.1093/eurheartj/ehr033. doi: 10.1093/eurheartj/ehr033. [DOI] [PubMed] [Google Scholar]
- 39.Bozbas H, Korkmaz ME, Atar I, Eroglu S, Ozin B, Yildirir A, Muderrisoglu H, Colak T, Karakayali H, Haberal M. Serum levels of cardiac enzymes before and after renal transplantation. Clin Cardiol. 2004;27:559–562. doi: 10.1002/clc.4960271007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fridén V, Starnberg K, Muslimovic A, Ricksten SE, Bjurman C, Forsgard N, Wickman A, Hammarsten O. Clearance of cardiac troponin T with and without kidney function. Clin Biochem. 2017;50:468–474. doi: 10.1016/j.clinbiochem.2017.02.007. doi: 10.1016/j.clinbiochem.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 41.van der Linden N, Cornelis T, Kimenai DM, Klinkenberg LJJ, Hilderink JM, Lück S, Litjens EJR, Peeters FECM, Streng AS, Breidthardt T, van Loon LJC, Bekers O, Kooman JP, Westermark PO, Mueller C, Meex SJR. Origin of cardiac troponin T elevations in chronic kidney disease. Circulation. 2017;136:1073–1075. doi: 10.1161/CIRCULATIONAHA.117.029986. doi: 10.1161/CIRCULATIONAHA.117.029986. [DOI] [PubMed] [Google Scholar]
- 42.Diris JH, Hackeng CM, Kooman JP, Pinto YM, Hermens WT, van Dieijen-Visser MP. Impaired renal clearance explains elevated troponin T fragments in hemodialysis patients. Circulation. 2004;109:23–25. doi: 10.1161/01.CIR.0000109483.45211.8F. doi: 10.1161/01.CIR.0000109483.45211.8F. [DOI] [PubMed] [Google Scholar]
- 43.Ellis K, Dreisbach AW, Lertora JL. Plasma elimination of cardiac troponin I in end-stage renal disease. South Med J. 2001;94:993–996. [PubMed] [Google Scholar]
- 44.Wong CK, Szeto CC, Chan MH, Leung CB, Li PK, Lam CW. Elevation of pro-inflammatory cytokines, C-reactive protein and cardiac troponin T in chronic renal failure patients on dialysis. Immunol Invest. 2007;36:47–57. doi: 10.1080/08820130600745505. doi: 10.1080/08820130600745505. [DOI] [PubMed] [Google Scholar]
- 45.D’Marco L, Bellasi A, Raggi P. Cardiovascular biomarkers in chronic kidney disease: state of current research and clinical applicability. Dis Markers. 2015;2015:586569. doi: 10.1155/2015/586569. doi: 10.1155/2015/586569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G National Kidney Foundation. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]


