Abstract
Patients with psychotic disorders regularly use natural medicines, although it is unclear whether these are effective and safe. The aim of this study was to provide an overview of evidence for improved outcomes by natural medicines. A systematic literature search was performed through Medline, PsycINFO, CINAHL, and Cochrane until May 2015. In 110 randomized controlled trials, evidence was found for glycine, sarcosine, N-acetylcysteine, some Chinese and ayurvedic herbs, ginkgo biloba, estradiol, and vitamin B6 to improve psychotic symptoms when added to antipsychotics. Ginkgo biloba and vitamin B6 seemed to reduce tardive dyskinesia and akathisia. Results on other compounds were negative or inconclusive. All natural agents, except reserpine, were well tolerated. Most study samples were small, study periods were generally short, and most results need replication. However, there is some evidence for beneficial effects of certain natural medicines.
Key Words: Psychosis, natural products, complementary medicine
Despite much progress in treatment options in the last century, the pharmacological treatment of psychotic disorders is often unsatisfactory, as expressed in persistent positive, negative, cognitive and affective symptoms, and problems in social functioning (Kane and Correll, 2010). Psychotic symptoms are often only partially resolved (Rummel-Kluge et al., 2010), especially cognitive and negative symptoms (Buckley and Stahl, 2007). Apart from clozapine, second-generation antipsychotics are generally as effective as first-generation antipsychotics for positive symptoms, but the promise of greater efficacy for negative symptoms has not been fulfilled (Leucht et al., 2012). Many patients continue experiencing persistent symptoms and relapses during treatment with antipsychotics, particularly when they fail to adhere to prescribed medications (Van Os and Kapur, 2009). Psychiatric medication adherence is a problem because many patients do not want them or consider them unnecessary (Cooper et al., 2007), or experience undesired adverse effects (Pai and Vella, 2012). For antipsychotics, these adverse effects include weight gain, sexual dysfunction, glycemic and lipid dysfunction, extrapyramidal symptoms (EPS), and sedation (Stahl, 2008).
Many patients with psychotic disorders use nonconventional medicines or treatments in the hope of decreasing undesired adverse effects or a more successful recovery (Hazra et al., 2010; Stevinson, 2001). Nonconventional medicine includes therapeutic lifestyle changes and complementary and alternative medicine (CAM) (Hoenders, 2013). Complementary medicine comprises diagnostics, treatments, and prevention strategies based on theories accepted in biomedicine and substantiated by some scientific evidence (two or more randomized controlled trials [RCTs]), but for various (cultural or practical) reasons are no part of biomedicine (Hoenders et al., 2011). Alternative medicine comprises diagnostics, treatments, and prevention strategies using other than the basic concepts of biomedicine. So far, there is little proof for the efficacy of the latter treatments and/or considerable controversy about their scientific validation (Lake, 2007). Natural medicine is part of complementary medicine, using agents produced by living organisms (plant, tree, seed, vegetable, fruit, animal, and human) instead of nonnatural (i.e., chemical) agents only being obtained from laboratory experiments (Porter, 1998). Some patients prefer natural medicines, assuming that natural is better and will cause fewer adverse effects. This is obviously not (always) true, as the natural environment contains agents that can be toxic to humans. The molecular structure and dosage of a substance rather than its source determine its effect on human health (Topliss et al., 2002). Besides, herbal medicines can cause undesired effects including interactions with prescription medication (Ernst, 2003a, 2003b).
Hazra et al. (2010) reported a lifetime and 1-year prevalence rate of CAM use in Canadian psychotic outpatients of 88% and 68%, respectively. A major difficulty these patients encounter is the heterogeneity in treatment options with CAM, ranging from possibly interesting agents to useless, or even dangerous, ones (Ernst, 2003b). For instance, the concomitant use of antipsychotics and Chinese herbs was found to induce significantly improved clinical outcomes compared with antipsychotics only (Rathbone et al., 2007). However, a small but significant number of patients concomitantly treated with Chinese herbs have a greater risk of developing worse outcomes (Zhang et al., 2011b).
In recent years, patients' preferences and views have received more attention in making treatment choices (e.g., shared decision making [Elwyn et al., 2000] and “patient-centered care” [Gill, 2013]). The introduction of patient's choice in deciding which antipsychotic to choose has been proposed (Morrison et al., 2012). However, it is difficult for both patients and physicians to make informed decisions in the absence of reliable information on the emerging evidence for CAM or natural medicine. Considering its high usage in psychotic patients, there is an urgent need for readily available scientific information.
This article reviews the literature on the efficacy and safety of natural medicines for psychotic disorders.
REVIEW
Materials and Methods
Literature Search and Study Selection
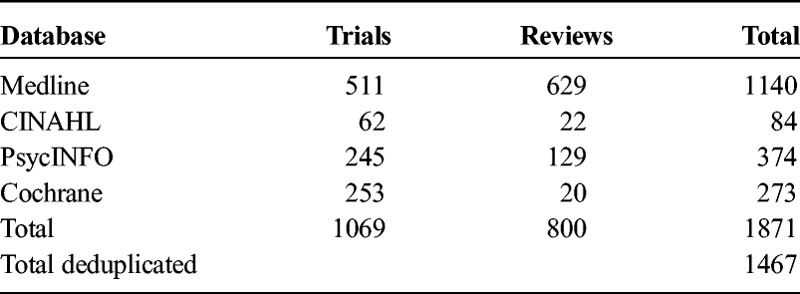
Studies were identified by a literature search in Medline, PsycINFO, CINAHL, and Cochrane, until May 2015, in accordance with the Medline RCT filter. The search terms (MeSH Thesaurus and free search terms) used were schizophrenia, psychosis, psychoses, psychotic (disorder), schizophreniform AND (R)CT, review AND complementary medicines, herbs, vitamins, supplements (search terms, in alphabetical order: alpha lipoic acid [ALA], artemisinin, ascorbic acid, Ayurveda, brahmyadiyoga, branched-chain amino acids [BCAA], Chinese herbs, d-cycloserine, d-serine, daotan decoction, dehydroepiandrosterone [DHEA], docosahexaenoic acid [DHA], eicosapentaenoic acid [EPA], estradiol, fatty acid, fish oil, folic acid, ginkgo biloba, glycine, jiawei lingguizhugan tang, jieyu anshen decoction, l-stepholidine, l-theanine, manganese, methylfolate, N-acetylcysteine [NAC], N-methylglycine, niacine, omega-3, orengedokuto, rauwolfia serpentina, saikokaryukotsuboreito, sarcosine, sarsasapogenin, selenium, shakuyakukanzoto, shuizhi dahuang mixture, suo quan, tongdatang serial recipe [TDT], traditional Chinese medicine [TCM], vitamin B complex, vitamin B3, vitamin C, vitamin D, vitamin E, and zinc). After systematic deduplication, 1465 hits (abstracts) were retrieved (Table 1).
TABLE 1.
Sources of Literature Retrieval and Included Number of Studies
Next, abstracts about the following topics were included: a) effects of natural medicines on psychotic symptoms in schizophrenia spectrum nonaffective disorders and b) effects of natural medicines on the adverse effects of antipsychotics. Those excluded were a) nonrandomized (controlled) trials; b) mechanism studies exploring the effects of natural medicines; c) animal studies; d) affective disorders/other disorders/no disorder/(relapse) prevention; e) conference abstracts; f) book chapters; g) clinical trial registrations; h) comments, addenda, corrigenda, and letters; i) non-English languages (e.g., Chinese, Japanese, Hebrew, German, and Spanish); and (j) duplicate hits that had not been removed systematically. Second, two authors (H.J.R.H. and A.A.B.V.) independently indicated whether papers—based on the abstracts—should (possibly) be included. Consultation followed about dubious cases and in case of discordance. Thereupon, 427 studies remained, of which the full papers on RCTs were retrieved and studied. Of these, another 160 were excluded. A flowchart of the study selection is presented in Figure 1. We found 147 reviews and checked whether RCTs in their reference lists matching our inclusion criteria were included. Eight RCTs with a Jadad score of 3 or higher (see paragraph on risk of bias assessment and Table 2) found through cross-references were added. Eighteen RCTs were excluded because of a Jadad score less than 3. The reviews (not shown in Table 3) will be contrasted to our findings in the Discussion section.
FIGURE 1.
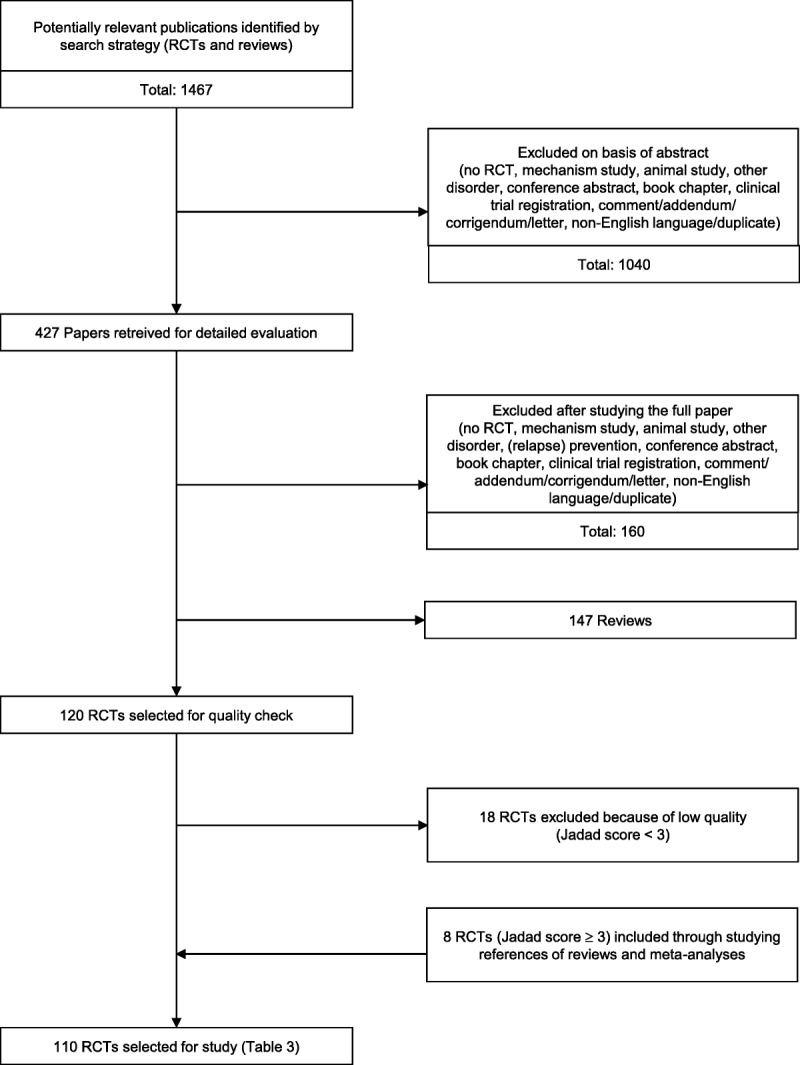
Flowchart of study selection.
TABLE 2.
Jadad Scale for Assessing the Quality of RCTs
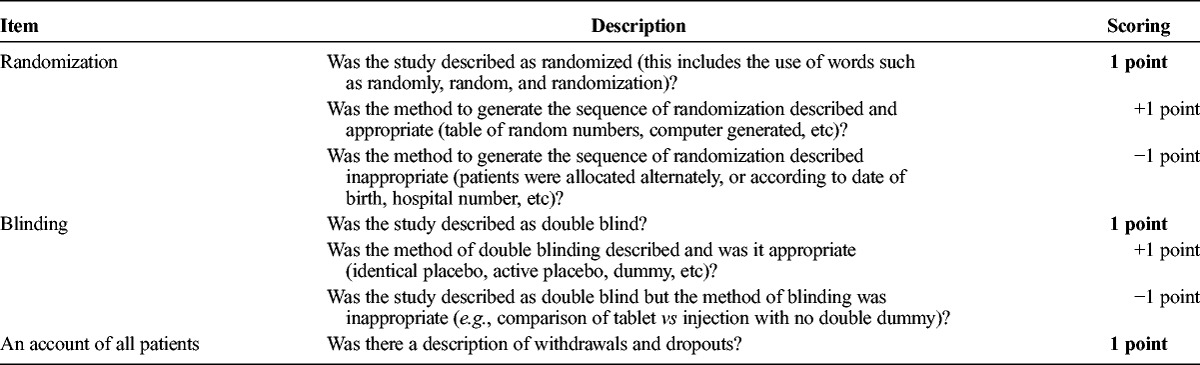
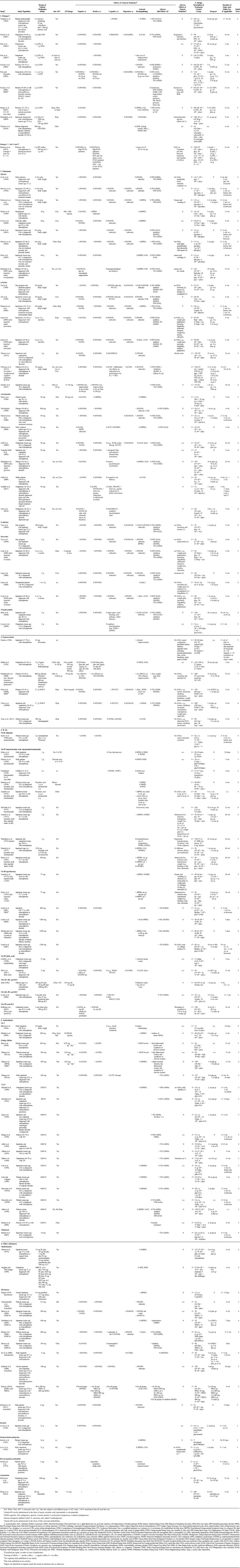
TABLE 3.
Overview of Effects of Natural Medicines for Psychotic Disorders
Classification of Agents
The RCTs included were divided into six groups based on supposed underlying mechanisms of action (Table 3). For a good grasp of the results, we briefly present the working mechanisms of the agents from five groups (not from the group “other substances”).
(i) Omega-3 fatty acids. Polyunsaturated fatty acids (PUFAs) are essential for brain functioning (Tsalamanios et al., 2006). They have multiple important biological roles, including membrane functioning, neurotransmission, signal transduction, and eicosanoid synthesis. Research suggests that PUFA level reduction is related to schizophrenia (Berger et al., 2006). Concordant with these findings, omega-3 PUFA may have positive effects in the treatment of schizophrenia (Emsley et al., 2002; Peet, 2008).
(ii) Glutamate. Besides dopamine, glutamate is thought to play a role in schizophrenia (Tsai and Lin, 2010). On the basis of the hypothesis that the glutamatergic system may be compromised in schizophrenia, the use of N-methyl-d-aspartate (NMDA) receptor modulators may compensate for alterations in the glutamate system (Singh and Singh, 2011). Agents with coagonistic properties to (glutaminergic) NMDA receptors are glycine (full, endogenous agonist), d-serine (full, endogenous agonist), d-cycloserine (partial, exogenous agonist), d-alanine (partial, endogenous agonist), and sarcosine (= methylglycine, acting as a reuptake inhibitor of glycine and source of glycine). The glycine transporter-1 (GlyT-1) plays a pivotal role in maintaining the glycine concentration within synapses at a subsaturating level. Sarcosine is a GlyT-1 inhibitor, meaning that its presence results in increased glycine concentrations. Lower cerebral glycine levels are suggested to be found in patients with schizophrenia. The administration of sarcosine is therefore proposed to relieve symptoms of schizophrenia when added to nonclozapine antipsychotics (Lane et al., 2006). Whereas the mechanisms of NAC are now beginning to be understood, NAC is probably exerting benefits beyond being a precursor to the antioxidant glutathione, also modulating glutamatergic, neurotropic, and inflammatory pathways (Dean et al., 2011).
(iii) Eastern (Chinese and ayurvedic) herbs. Eastern herbs are provided in the context of treatment with complete systems of medicine that evolved over thousands of years, such as TCM and Ayurveda. These treatments include prescription of herbal compounds, massage, diet, acupuncture, and the regulation of lifestyle (Clifford, 1994; Kaptchuck, 2000). Most clinical studies were performed on acupuncture (beyond the scope of this review) and on herbal compounds.
(iv) B vitamins. Nobel laureate Linus Pauling proposed a way of understanding and treating psychiatric disorders by correcting malfunctions in the body's chemistry, calling this approach “orthomolecular psychiatry” (Pauling, 1968). His idea was partly built on studies by Osmond and Hoffer (1962) and Hoffer and Osmond (1964), reporting good results when treating patients with schizophrenia with large doses of vitamins, especially vitamin B3. Hoffer (1971, 1972) published two more positive results with B vitamins. However, attempts to replicate his findings seem to have failed (Ban and Lehmann, 1975; Wittkopp and Abuzzahab, 1972). The contradicting findings may be explained because vitamine B is suggested to be effective in early psychosis but not in chronic schizophrenia (Hoffer and Osmond, 1964). One of the proposed mechanisms is abnormal one-carbon metabolism due to vitamin deficiencies (Hoffer, 2008). Variable levels of the components of one-carbon metabolism (folic acid [= vitamin B9] and vitamin B12) and consequently altered levels of homocysteine and phospholipid DHA have been reported both in medicated patients and in medication-naive first-episode psychotic patients (Kale et al., 2010). Folate status in patients with schizophrenia correlates inversely with negative symptoms (Goff et al., 2005).
(v) Antioxidants. Oxygen is essential in life but also generates reactive molecules (so-called free radicals) throughout the body. These free radicals are potentially harmful because they can damage essential molecules such as DNA and the enzymes necessary for proper cell functioning. Antioxidants may capture these reactive free radicals and convert them back to less reactive forms of the molecules (Singh et al., 2010). Research suggests that oxidative damage (maybe due to defective enzyme systems) may contribute to the course and outcome of schizophrenia (Fendri et al., 2006; Mahadik and Mukherjee, 1996; Mahadik et al., 2001) and is already present in patients with first-episode psychosis (Flatow et al., 2013).
Ascorbic acid (vitamin C), an antioxidant vitamin, plays an important role in protecting free radical-induced damage in the body. It is present in brain tissue and dopamine-dominant areas in higher concentrations compared with other organs (Harrison and May, 2009). Ginkgo biloba, an extract of the leaves of the ginkgo biloba tree, is also suggested to have antioxidant properties (Maclennan et al., 2002), improving brain circulation at the microvascular level (Kuboto et al., 2001; Sun et al., 2003; Yan et al., 2008) and, thus, improving outcome in psychosis.
Long-term treatment with antipsychotics is associated with a variety of movement disorders, including tardive dyskinesia (TD). Both dopamine receptor supersensitivity and oxidative stress-induced neurotoxicity in the nigrostriatal system are suggested to be involved in its pathogenesis (Kulkarni and Naidu, 2003). The pineal hormone melatonin is a potent antioxidant and attenuates dopaminergic activity in the striatum and dopamine release from the hypothalamus (Shamir et al., 2001). Thus, treatment with antioxidative agents may have a beneficial effect for both treatment of psychotic symptoms and prevention of TD. Vitamin E has been suggested for TD because it is a lipid-soluble antioxidant that decreases free radical formation (Herrera and Barbas, 2001).
Risk of Bias Assessment
Two assessors (A.A.B.V. and N.K.V.) independently rated the methodological quality of the eligible RCTs using the Jadad scale (Jadad et al., 1996). Interrater agreement on the Jadad scores before consensus discussion amounted to 0.83. Besides, H.J.R.H. independently rated a random selection of 17 papers (15%) from the selected RCTs. Interrater agreement of all three assessors was 0.71. Any scoring disagreements between the assessors were resolved through consensus discussion between these three authors. The 110 RCTs with a Jadad score of 3 or higher were included in the current review, categorized into six groups (see the Classification of agents section).
For each of the 110 studies fulfilling the selection criteria, the following assessments were made: which natural agent was used; was this combined with antipsychotics, and if so, which antipsychotics and what dosage; the effect of the natural agent on negative, positive, cognitive, depressive, and general symptoms and on adverse effects of antipsychotics; possible adverse effects of the natural agent; number of participants in the study; control group characteristics; number of dropouts; study duration; and Jadad score. The results are shown in Table 3.
Results
In total, 110 RCTs that matched the inclusion criteria were identified. Detailed effects are given in Table 3. Most of the studies were performed in the United States, followed by (in decreasing order) Israel, Canada, Taiwan, China, India, United Kingdom, Australia, Iran, South Africa, Switzerland, the Netherlands, Austria, Ireland, Korea, and Norway.
(i) Omega-3 Fatty Acids
Eleven RCTs on omega-3 were included (Bentsen et al., 2013; Berger et al., 2007; Emsley et al., 2002, 2006, 2008; Fenton et al., 2001; Manteghiy et al., 2008; Peet et al., 2001; Peet and Horrobin, 2002; Toktam et al., 2010; Vaddadi et al., 1989), and one combined omega-3 with vitamins E and C (Bentsen et al., 2013). In studies combining antipsychotics with omega-3 PUFA, one (from five) study on negative symptoms in schizophrenia found some positive effect (in patients using clozapine; Peet and Horrobin, 2002), two (from four) found some positive effect on positive symptoms (Peet et al., 2001; Peet and Horrobin, 2002; one only in patients using clozapine [Peet and Horrobin, 2002]), one (from two) on cognitive symptoms (Vaddadi et al., 1989), none (from three) on depressive symptoms, and four (from eight) on general psychopathology (Emsley et al., 2002; Peet et al., 2001; Peet and Horrobin, 2002; Vaddadi et al., 1989; one only in patients using clozapine [Peet and Horrobin, 2002]). One (from one) study on omega-3 PUFA without antipsychotics reported a decrease of positive symptoms (Peet et al., 2001). Three (from six) reported less adverse effects of antipsychotics (EPS and/or dyskinesia) (Berger et al., 2007; Emsley et al., 2002; Vaddadi et al., 1989). Two studies reported less use of antipsychotics in the omega-3 PUFA group (Berger et al., 2007; Peet et al., 2001). One study reported an increase in positive symptoms by omega-3 (EPA), but only among those with low levels of red blood cell PUFA. This effect disappeared when EPA was combined with vitamin E and vitamin C (Bentsen et al., 2013). Some nonsevere adverse effects of omega-3 PUFA were reported, such as mild gastrointestinal problems and increased bleeding time.
(ii) Glutamate
Nine RCTs on glycine (Buchanan et al., 2007; Diaz et al., 2005; Evins et al., 2000; Heresco et al., 1996, 2004; Heresco-Levy et al., 1999; Javitt et al., 1994, 2001; Potkin et al., 1999), eight on d-serine (D'Souza et al., 2013; Ermilov et al., 2013; Heresco-Levy et al., 2005; Lane et al., 2005, 2010; Tsai et al., 1998, 1999; Weiser et al., 2012), ten on d-cycloserine (Buchanan et al., 2007; Cain et al., 2014; Duncan et al., 2004; Goff et al., 2005, 2008; Gottlieb et al., 2011; Heresco-Levy et al., 2002; Rosse et al., 1996; Van Berckel et al., 1999; Yurgelun-Todd et al., 2005), one on d-alanine (Tsai et al., 2006), five on sarcosine (Lane et al., 2005, 2006, 2008, 2010; Tsai et al., 2004), and two on NAC (Berk et al., 2008; Lavoie et al., 2008) were included.
Glycine improved negative symptoms when combined with antipsychotics in six (from seven) studies (Buchanan et al., 2007; Heresco et al., 1996, 2004; Heresco-Levy et al., 1999; Javitt et al., 1994, 2001), but not when combined with clozapine (two studies) (Potkin et al., 1999; Evins et al., 2000). Positive symptoms improved in one study (Heresco et al., 2004), worsened in another (with clozapine) (Potkin et al., 1999), and did not change in five (from seven) studies (Diaz et al., 2005; Evins et al., 2000; Heresco et al., 1996; Heresco-Levy et al., 1999; Javitt et al., 1994); cognitive improvement was shown in four (Heresco et al., 1996, 2004; Heresco-Levy et al., 1999; Heresco et al., 2004; Javitt et al., 2001) and no change in two (from seven) studies (Buchanan et al., 2007; Evins et al., 2000); depressive symptoms diminished in four (from four) studies (Heresco et al., 1996, 2004; Heresco-Levy et al., 1999; Javitt et al., 2001); and improvement of general psychopathology was shown in three (from eight) studies (Heresco et al., 1996, 2004; Heresco-Levy et al., 1999). No adverse effects of glycine were reported, except some mild gastrointestinal complaints.
d-Serine was shown to improve positive, negative, and cognitive symptoms and general psychopathology in two (from six) studies when added to antipsychotics (Heresco-Levy et al., 2005; Tsai et al., 1998). The three largest studies with the highest Jadad score did not show a significant effect of d-serine on any symptom (Lane et al., 2005; Lane et al., 2010; Weiser et al., 2012). In four (from six) studies, d-serine did not improve adverse effects of antipsychotics (Lane et al., 2005, 2010; Tsai et al., 1998, 1999). Insomnia, weight gain, palpitations, and other adverse effects of d-serine were reported. One study found improvement by d-serine without antipsychotics, but this was significantly less compared with the improvement in the olanzapine group; D-serine, however, caused less adverse effects (Ermilov et al., 2013).
d-Cycloserine showed an improvement of negative symptoms in three (from nine) studies when added to antipsychotics (Goff et al., 2008; Heresco-Levy et al., 2002; Yurgelun-Todd et al., 2005); some improvement of positive symptoms in one (Gottlieb et al., 2011) and worsening in another study (from seven) (Van Berckel et al., 1999); and little or no effect on cognitive and depressive symptoms or general psychopathology and no improvement of adverse effects of antipsychotics was shown. Five (from five) studies found no improvement of adverse effects of antipsychotics (Buchanan et al., 2007; Duncan et al., 2004; Goff et al., 2005; Heresco-Levy et al., 2002; Van Berckel et al., 1999). No studies were reported on d-cycloserine without antipsychotics. No adverse effects of d-cycloserine were reported.
The only study on d-alanine reported positive effects when added to antipsychotics on negative, positive, cognitive, and general symptoms, but no effect on depressive symptoms (Tsai et al., 2006). No effect on adverse effects of antipsychotics was found. Adverse effects of d-alanine (insomnia and nausea) were reported.
All three studies combining sarcosine with antipsychotics (not clozapine) found positive effects in almost all symptom domains (Lane et al., 2005, 2010; Tsai et al., 2004). When combined with clozapine (one study), no treatment effects were found (Lane et al., 2006). In addition, when given without antipsychotics (one study), sarcosine did not improve symptoms (Lane et al., 2008). Sarcosine did not improve adverse effects of antipsychotics in four (from four) studies (Lane et al., 2005, 2006, 2010; Tsai et al., 2004). Adverse effects of sarcosine included weight gain, insomnia, palpitations, dizziness, and sedation.
One large study on NAC added to antipsychotics reported improved positive symptoms but no improvement of negative, cognitive, or general symptoms and no improvement of adverse effects of antipsychotics (Berk et al., 2008), whereas one small study found some improvement of cognitive symptoms (Lavoie et al., 2008). The large study (Berk et al., 2008) reported that there were no adverse effects, and in the small study (Lavoie et al., 2008), occurrence of any adverse effect was not mentioned.
(iii) Eastern (Chinese and Ayurvedic) Herbs
Many studies on Eastern herbs were found, but only six had a Jadad score of three or higher (Chen et al., 2008a, 2008b, 2009; Mahal et al., 1976; Mundewadi et al., 2008; Naidoo, 1956). One old study on reserpine found “clinical improvement” after 11 weeks compared with placebo in 80 patients not treated with antipsychotics but with electroconvulsice therapy (Naidoo, 1956). Several adverse effects were reported: nasal congestion, periorbital edema, diarrhea, epigastric pain, salivating, pseudo-Parkinsonian state, severe headaches, and deep pains in limbs. Another old study (Mahal et al., 1976) found positive effects of brahmyadiyoga without antipsychotics compared with placebo and equal to chlorpromazin in 136 patients with schizophrenia (Mahal et al., 1976); no adverse effects were reported. Four (from six) more recent studies found significant effects on general psychopathology when adding ayurvedic herbs (reserpine: one study [Naidoo, 1956]; bacopa monnieri and nardostachys jatamansi: one study [Mundewadi et al., 2008]; a mixture of 13 Chinese herbs: two studies [Chen et al., 2008a, 2008b, 2009]) to antipsychotics.
The ayurvedic herbs were compared with 10 mg of olanzapine in a 76-week noninferiority study in 200 patients. No statistically significant differences were found between both groups examining improvement of positive and negative symptoms and general psychopathology. The ayurvedic group had less weight gain (Mundewadi et al., 2008). Two large studies by Chen et al. (2008a, 2008b, 2009) of a mixture of 13 Chinese herbs found an improvement on general psychopathology. When kidney yang was added to risperidone, an improvement on cognitive and depressive symptoms was found in one study (from two) (Chen et al., 2008a, 2009). One study found no effect of the Chinese herb sarsasapogenin compared with placebo when added to risperidone on positive, negative, and cognitive symptoms or general psychopathology in 90 patients during 8 weeks (Xiao et al., 2011). Many different nonsevere adverse effects were reported (e.g., gastrointestinal, drowsiness, and insomnia).
(iv) B Vitamins
Nineteen RCTs on B vitamins added to antipsychotics (Ananth et al., 1972, 1973; Deutsch et al., 1977; Godfrey et al., 1990; Hill et al., 2011; Joshi, 1982; Kline et al., 1967; Lerner et al., 2001, 2002, 2004, 2007; Levine et al., 2006; McGrath et al., 1972; Meltzer et al., 1969; Miodownik et al., 2006; Petrie et al., 1981; Ramsay et al., 1970; Roffman et al., 2013; Sacks et al., 1989; Wittenborn et al., 1973) and one on B3 without antipsychotics (Greenbaum 1970) were found. B1 showed some positive effect on general psychopathology (when combined with B6 and B12) in one study (Joshi, 1982) and on positive and negative symptoms in another (Sacks et al., 1989). B3 showed improved general psychopathology in three (from nine) studies (Ananth et al., 1972, 1973; Petrie et al., 1981). B6 improved general psychopathology in four (from five) studies (Ananth et al., 1973; Lerner et al., 2004; Miodownik et al., 2006; Petrie et al., 1981). In one study, general psychopathology improved after the administration of methylfolate (Godfrey et al., 1990). One study reported no effect of B9 (folic acid) (Hill et al., 2011). Another study showed a positive effect of combined B6, B9, and B12 on positive, negative, and cognitive symptoms (Levine et al., 2006). Yet, another study showed improved negative symptoms by adding B9 (folic acid) and B12 to antipsychotics, but only in those with a specific genotype (Roffman et al., 2013). B6 improved extrapyramidal adverse effects of antipsychotics (TD and neuroleptic induced akathisia) in four (from four) studies (Lerner et al., 2001, 2002, 2004, 2007; Miodownik et al., 2006). In one study on B3 in 57 children without antipsychotics, cognition and general psychopathology had not improved after 6 months (Greenbaum, 1970). Most B vitamins induced modest adverse effects, especially skin flushing and abnormal liver function induced by vitamin B3 and B6.
(v) Antioxidants
Two RCTs on vitamin C were found (Bhavani et al., 1962; Dakhale et al., 2005). One reported improved general psychopathology and reduced adverse effects (reduced serum malondialdehyde; a lipid peroxidation product) when added to olanzapine (10 mg), quetiapine (200 mg), or ziprasidone (40 mg) after 8 weeks (Dakhale et al., 2005). One study without antipsychotics found no effect on cognition or motor functioning after 10 days (Bhavani et al., 1962). Both studies reported no adverse effects of vitamin C.
Four studies on ginkgo biloba were found (Zhang et al., 2001a, 2001b, 2006, 2011b; Zhou et al., 1999). Three (from four) studies found improved positive symptoms (Zhang et al., 2001a, 2001b, 2006, 2011b; Zhou et al., 1999), two (from three) found improved general psychopathology (Zhang et al., 2001a; Zhou et al., 1999), and four (from four) reported no improvement of negative symptoms when added to antipsychotics (Zhang et al., 2001a, 2001b, 2006, 2011a; Zhou et al., 1999). In all four studies, adverse effects of antipsychotics improved (behavioral toxicity, symtoms of nervous system, and TD). No adverse effects of ginkgo were reported.
Thirteen studies of vitamin E were found (Adler et al., 1993, 1999; Akhtar et al., 1993; Dabiri et al., 1994; Dorevitch et al., 1997a, 1997b; Egan et al., 1992; Elkashef et al., 1990; Lam et al., 1994; Lohr and Caligiuri, 1996; Salmasi et al., 2009; Schmidt et al., 1991; Shriqui et al., 1992). Six (from 13) studies for reducing EPSs, while using antipsychotics, showed a decrease of TD (Adler et al., 1993; Akhtar et al., 1993; Dabiri et al., 1994; Egan et al., 1992; Elkashef et al., 1990; Lohr and Caligiuri 1996) and EPS (one study; Elkashef et al., 1990), and those with shorter duration of TD seemed to improve more; no adverse effects of vitamin E were reported, except mild diarrhea in two studies. Five (from five) reported no effect on general psychopathology (Adler et al., 1999; Dorevitch et al., 1997a; Elkashef et al., 1990; Lam et al., 1994; Lohr and Caligiuri, 1996). One study of melatonin for TD reported a decrease of TD and no adverse effects (Shamir et al., 2001).
(vi) Other Substances
Agents that did not fit in the five aforementioned categories were classified in this residual category. A total of 16 high-quality RCTs have been performed on multivitamins (Altman et al., 1973; Vaughan and McConaghy, 1999), hormones (DHEA; Nachshoni et al., 2005; Ritsner, 2010; Ritsner et al., 2006; Strous et al., 2003, 2007), pregnenolone (PREG; Ritsner, 2010), estradiol (Akhondzadeh et al., 2003; Kulkarni et al., 2008, 2011), protilerin (thyrotropin-releasing hormone) (Prange, 1979), testosterone (Ko et al., 2008), inositol (Levine et al., 1994), gamma-hydroxybutyrate (GHB; Levy et al., 1983; Schulz et al., 1981) and des-tyr-gamma-endorphin (Verhoeven et al., 1979).
Two (from five) studies on DHEA added to antipsychotics showed improvement of negative symptoms (Ritsner et al., 2006; Strous et al., 2003), two (from three) on positive symptoms (Ritsner, 2010; Ritsner et al., 2006), one (from three) on cognition (Ritsner et al., 2006), two (from two) on depression (Ritsner et al., 2006; Strous et al., 2003), and one (from four) on general functioning (Strous et al., 2003). Three (from four) improved adverse effects of drugs (Nachshoni et al., 2005; Ritsner, 2010; Strous et al., 2007). In one study of 30 patients with schizophrenia, using either 5 g of 1% testosterone gel or a placebo added to a fixed dosage of antipsychotic medication over a period of 4 weeks, negative symptoms improved without adverse effects (Ko et al., 2008). One (from one) small study (N = 12) on protilerin found improved general psychopathology (Prange, 1979). Three (from three) studies on estradiol showed improvement of general psychopathology (Akhondzadeh et al., 2003; Kulkarni et al., 2008, 2011), two (from three) of positive symptoms (Akhondzadeh et al., 2003; Kulkarni et al., 2008), one (from one) of improved cognition (Kulkarni et al., 2008), and none (from three) of negative symptoms.
One (from one) small study (N = 14) on inositol found no effect on positive or negative symptoms (Levine et al., 1994). Two (from two) studies on GHB found no improvement of general psychopathology (Levy et al., 1983; Schulz et al., 1981). One (from one) very small (N = 6) study on des-tyr-gamma-endorphin found improvement on general psychopathology and positive symptoms (Verhoeven et al., 1979). No serious adverse effects of these agents were reported.
One study (of two) on artemisinin (a natural medicine against malaria) found a significant effect on negative symptoms and clinical global impression, but no effect on positive or cognitive symptoms or on general psychopathology in first-episode treatment-naive patients that were treated with risperidone (Dickerson et al., 2011; Wang et al., 2014). The study of Dickerson et al. (2011) did not demonstrate clinical benefit of adjunctive artemisinin for schizophrenia symptoms.
DISCUSSION
This review describes the effects of natural agents in the treatment of psychotic disorders and of undesired effects of antipsychotics. Some studies suggest that glycine, sarcosine, NAC, several Chinese and ayurvedic herbs, ginkgo biloba, estradiol, and vitamin B6 may be effective for psychotic symptoms when added to antipsychotics (glycine not when added to clozapine). We found inconclusive or no evidence for omega-3 fatty acids, d-serine, d-alanine, d-cycloserine, other B vitamins, vitamin C, DHEA, PREG, inositol, GHB, and des-tyr-gamma-endorphin when added to antipsychotics. Reserpine without antipsychotics seemed effective in one old study but was poorly tolerated. Ayurvedic herbs seemed equally effective as olanzapine in only one study. Other agents as monotherapy (vitamin B3, vitamin C, sarcosine, glycine, and protilerin) were not effective or had only been tested in single or small trials. For alleviation of adverse effects, ginkgo and vitamin B6 seemed effective for TD and neuroleptic induced akathisia (NIA). The evidence for reducing some adverse effects of antipsychotics by omega-3 fatty acids, melatonin, and DHEA was inconclusive.
Apart from reserpine, all natural compounds studied caused no or mild undesired adverse effects. There is inconclusive evidence for improved outcome by combining omega-3 fatty acids with antipsychotics in schizophrenia. Earlier reviews reported similar conclusions (Boskovic et al., 2011; Irving et al., 2006; Tsalamanios et al., 2006). A meta-analysis of randomized placebo controlled trails showed a modest, nonsignificant, beneficial effect of fatty acids in schizophrenia (Fusar-Poli and Berger, 2012).
Glycine and sarcosine combined with antipsychotics may reduce negative symptoms, but not when combined with clozapine and neither as monotherapy. Inconclusive evidence was found for d-cycloserine and d-serine on clinical improvement. Our results concur with two reviews (Singh and Singh, 2011; Tsai and Lin, 2010) and are in line with a Cochrane review (Tiihonen and Wahlbeck, 2006). Conflicting results from studies on drugs targeting the glutamate/NMDA system may be explained by complicated dose-effect relationships, as recently found in studies with the GlyT-1 transporter antagonist bitopertin (Umbricht et al., 2013).
By adding Chinese or ayurvedic herbs to antipsychotics, general psychopathology may improve. One study (of two) on artemisinin (a natural medicine against malaria) found a significant effect on negative symptoms and clinical global impression, but no effect on positive or cognitive symptoms or on general psychopathology in first-episode treatment-naive patients who were treated with risperidone (Wang et al., 2014; Dickerson et al., 2011). The study of Dickerson et al. (2011) did not demonstrate clinical benefit of adjunctive artemisinin for schizophrenia symptoms. Rathbone et al. (2007) state that “the results suggest that combining Chinese herbal medicine with antipsychotics is beneficial.” Another Cochrane review (Agarwal et al., 2007) concludes that “ayurvedic medication may have some effects for treatment of schizophrenia, but has been evaluated only in a few small pioneering trials.” These results need further exploration and pharmacological differentation, as Chinese and ayurvedic herbs include hundreds of species combined in thousands of different combinations and are prescribed in a fundamentally different way than Western medicines (Clifford, 1994; Kaptchuck, 2000). The combined approach using knowledge from both conventional and Chinese medicine seems promising, as it may lead to innovation (Van der Greef, 2011) and possibly to improved outcomes (Zhang et al., 2011b).
Inconsistent beneficial outcomes of studies on B vitamins were identified, especially when given as a combination of B1, B3, B6, B9, and/or B12 with antipsychotics. One review concluded that no adequate support for the efficacy of B vitamins in schizophrenia can be identified (Kleijnen and Knipschild, 1991). Most studies with positive effects in our review, however, were published after the aforementioned review was published. Most convincing evidence was found for vitamin B6 added to antipsychotics, shown to be effective in diminishing general psychopathology and TD.
The findings on the efficacy of vitamin C for schizophrenia in only two RCTs were inconsistent, hindering definite conclusions. The efficacy of vitamin E on TD remains inconclusive, as only half of the included studies found some positive results. Even so, a meta-analysis by Boskovic et al. (2011) claimed, “Vitamin E could potentially improve TD.” This may be due to the finding that those with a short history of TD tend to improve more than those with a longer history of TD. A Cochrane review in 2011 (Soares et al., 2011) came to a similar conclusion: “small trials of limited quality suggest that vitamin E may protect against deterioration of TD. There is no evidence that vitamin E improves symptoms of this problematic and disfiguring condition once established.”
Ginkgo biloba seems to benefit patients with schizophrenia in several ways when added to antipsychotics. Several studies suggested evidence for improving symptoms in various domains, especially an effect on positive symptoms and the reduction of adverse effects of antipsychotics.
On melatonin, one study provided preliminary evidence for diminishing TD (Shamir et al., 2001). As TD is difficult to investigate because of the fluctuating symptom severity, this study needs replication.
Some inconsistent evidence was found on improved outcomes by several hormones (DHEA, PREG, and testosterone) in schizophrenia, not allowing final conclusions. A Cochrane review on DHEA/testosterone drew a similar conclusion (Elias and Kumar, 2007). For estradiol, a Cochrane review reported no convincing evidence over placebo (Chua et al., 2005). Since then, two studies found that estradiol improves positive (but not negative) symptoms and general psychopathology in schizophrenia when added to antipsychotics (Kulkarni et al., 2008, 2011), however only in women of childbearing age. Therefore, using estradiol in schizophrenia warrants further study.
Limitations
There are several methodological limitations. First, the wide scope of this review allows only general descriptions of included studies in six domains. Second, it is unclear to which extent our findings are influenced by publication bias, in favor of publication of studies with positive results. Third, we used the Jadad score to select only RCTs of high quality (with a score of three or higher, as is in accordance with other reviews [e.g., see Thirthalli et al., 2016]). However, the Jadad score is not a perfect tool because it does not judge the selection of subjects, the sample size and power, and the quality of the data analyses. Therefore, RCTs with a Jadad score of 3 or higher might still have methodological weaknesses, which hamper drawing firm conclusions. Fourth, some studies (e.g., Bhavani et al., 1962; Greenbaum 1970; Naidoo, 1956) were done in the pre–Diagnostic and Statistical Manual of Mental Disorders, 3rd Edition (DSM-3) era, when standards of care and diagnostics may have been of lower quality than nowadays, which hampers interpretation of their results. Fifth, in most of the studies included, effect sizes were not provided nor was it possible to calculate them, which makes it difficult to compare the results or to estimate the clinical relevance of some of the findings. Sixth, it cannot be ruled out that some of the studies were underpowered, which might have hampered finding a significant effect.
Clinical Implications
Clinicians need to be aware that patients often use natural medicines without medical prescription, whereas some patients assume that natural is better than chemical and causes fewer adverse effects. Although beneficial effects may occur, this is certainly not always true. Some natural agents that may be suggested for treatment of psychotic disorders are toxic to humans (Topliss et al., 2002), and some herbal medicines can cause adverse effects or interact with medication (Ernst, 2003b). Only 3% of the user population is aware of the potential risks of interactions between herbs and prescription medication (Walter and Rey, 1999). From a medical perspective, it is therefore important to know what patients buy and try. Another concern are the media reports on contamination of Chinese herbs with heavy metals. However, after investigation of 334 samples, Harris et al. (2011) conclude that “the vast majority (95%) of medications in this study contained levels of heavy metals or pesticides that would be of negligible concern.” Because of these concerns, patients want their medical doctors to advise them on complementary (or natural) medicines (Gray et al., 1998; Hoenders et al., 2006). The World Health Organization (2013) has repeatedly advised its member states to “formulate national policy and regulation for the proper use of CAM and its integration into national health care systems; establish regulatory mechanisms to control the safety and quality of products and of CAM practice; create awareness about safe and effective CAM therapies among the public and consumers” and “promote therapeutically sound use of appropriate Traditional Medicine by practitioners and consumers.” Respecting patients' opinions and informing them may also improve the therapeutic relationship (Stevinson, 2001) and thus enhance treatment outcome (Gill, 2013; Koenig, 2000), which depends on the quality of the therapeutic alliance (Baldwin et al., 2007).
This review gives clinicians and patients an overview of the results of RCTs, which fit a minimal level of quality (minimum Jadad score of 3), on the efficacy and safety of natural medicines for psychotic disorders. However, many questions about clinical use (e.g., dosage, safety, interactions, and quality) remain unanswered.
ACKNOWLEDGMENTS
We thank Truus van Ittersum (University Medical Center Groningen) for expertly performing the literature searches, Sietse Dijk (University Medical Center Groningen) for his assistance in obtaining the papers for this review, Karen van der Ploeg, MSc (Center for Integrative Psychiatry, Lentis) for assisting in the selection procedure, and Edzard Geertsema, PhD (University Medical Center Groningen) for his detailed information about the chemical structure and characteristics of the agents.
DISCLOSURE
The authors declare no conflict of interest.
REFERENCES
- Adler LA, Peselow E, Rotrosen J, Duncan E, Lee M, Rosenthal M, Angrist B. (1993) Vitamin E treatment of tardive dyskinesia. Am J Psychiatry. 150:1405–1407. [DOI] [PubMed] [Google Scholar]
- Adler LA, Rotrosen J, Edson R, Lavori P, Lohr J, Hitzemann R, Raisch D, Caligiuri M, Tracy K. (1999) Vitamin E treatment for tardive dyskinesia. Veterans Affairs Cooperative Study #394 Study Group. Arch Gen Psychiatry. 56:836–841. [DOI] [PubMed] [Google Scholar]
- Agarwal V, Abhijnhan A, Raviraj P. (2007) Ayurvedic medicine for schizophrenia. Cochrane Database Syst Rev. DOI:10.1002/14651858. CD006867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhondzadeh S, Nejatisafa AA, Amini H, Mohammadi MR, Larijani B, Kashani L, Raisia F, Kamalipour A. (2003) Adjunctive estrogen treatment in women with chronic schizophrenia: A double-blind, randomized, and placebo-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry. 27:1007–1012. [DOI] [PubMed] [Google Scholar]
- Akhtar S, Jajor TR, Kumar S. (1993) Vitamin E in the treatment of tardive dyskinesia. J Postgrad Med. 39:124–126. [PubMed] [Google Scholar]
- Altman H, Mehta D, Evenson RC, Sletten IW. (1973) Behavioral effects of drug therapy on psychogeriatric inpatients. II. Multivitamin supplement. J Am Geriatr Soc. 21:249–252. [DOI] [PubMed] [Google Scholar]
- Ananth JV, Ban TA, Lehmann HE. (1973) Potentiation of therapeutic effects of nicotinic acid by pyridoxine in chronic schizophrenics. Can Psychiatr Assoc J. 18:377–383. [DOI] [PubMed] [Google Scholar]
- Ananth JV, Vacaflor L, Kekhwa G, Sterlin C, Ban TA. (1972) Nicotinic acid in the treatment of newly admitted schizophrenic patients: A placebo-controlled study. Int Z Klin Pharmakol Ther Toxikol. 5:406–410. [PubMed] [Google Scholar]
- Baldwin SA, Wampold BE, Imel ZE. (2007) Untangling the alliance-outcome correlation: Exploring the relative importance of therapist and patient variability in the alliance. J Consult Clin Psychol. 75:842–852. [DOI] [PubMed] [Google Scholar]
- Ban TA, Lehmann HE. (1975) Nicotinic acid in the treatment of schizophrenias: Canadian Mental Health Association Collaborative Study–Progress Report II. Can Psychiatr Assoc J. 20:103–112. [DOI] [PubMed] [Google Scholar]
- Bentsen H, Osnes K, Refsum H, Solberg DK, Bøhmer T. (2013) A randomized placebo-controlled trial of an omega-3 fatty acid and vitamins E + C in schizophrenia. Transl Psychiatry. 3:e335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger GE, Proffitt TM, McConchie M, Yuen H, Wood SJ, Amminger GP, Brewer W, McGorry PD. (2007) Ethyl-eicosapentaenoic acid in first-episode psychosis: A randomized, placebo-controlled trial. J Clin Psychiatry. 68:1867–1875. [DOI] [PubMed] [Google Scholar]
- Berger GE, Smesny S, Amminger GP. (2006) Bioactive lipids in schizophrenia. Int Rev Psychiatry. 18:85–98. [DOI] [PubMed] [Google Scholar]
- Berk M, Copolov D, Dean O, Lu K, Jeavons S, Schapkaitz I, Anderson-Hunt M, Judd F, Katz F, Katz P, Ording-Jespersen S, Little J, Conus P, Cuenod M, Do KQ, Bush AI. (2008) N-acetyl cysteine as a glutathione precursor for schizophrenia—A double-blind, randomized, placebo-controlled trial. Biol Psychiatry. 64:361–368. [DOI] [PubMed] [Google Scholar]
- Bhavani AD, Sen NN, Punekar BD. (1962) Impact of ascorbic acid administration on memory, attention and motor functions in schizophrenia. In Transactions of All-India Institute of Mental Health (pp 80–88). Bangalore, India: All-India Institute of Mental Health. [Google Scholar]
- Boskovic M, Vovk T, Plesnicar BK, Grabnar I. (2011) Oxidative stress in schizophrenia. Curr Neuropharmacol. 9:301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan RW, Javitt DC, Marder SR, Schooler NR, Gold JM, McMahon RP, Heresco-Levy U, Carpenter WT. (2007) The Cognitive and Negative Symptoms in Schizophrenia Trial (CONSIST): The efficacy of glutamatergic agents for negative symptoms and cognitive impairments. Am J Psychiatry. 164:1593–1602. [DOI] [PubMed] [Google Scholar]
- Buckley PF, Stahl SM. (2007) Pharmacological treatment of negative symptoms of schizophrenia: Therapeutic opportunity or Cul-de-sac? Acta Psychiatr Scand. 115:93–100. [DOI] [PubMed] [Google Scholar]
- Cain CK, McCue M, Bello I, Creedon T, Tang DI, Laska E, Goff DC. (2014) d-Cycloserine augmentation of cognitive remediation in schizophrenia. Schizophr Res. 153:177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZH, Wang GH, Wang XP, Chen RY, Wang HL, Yang MH, Huo YX, Mei HB. (2008a) Effectiveness and tolerability of warm-supplementing kidney yang added to risperidone in improving cognitive impairment in patients with schizophrenia: An 8-week, multicenter, randomized, double-blind, placebo-controlled clinical trial. Curr Ther Res Clin Exp. 69:104–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZH, Wang GH, Wang XP, Chen RY, Wang HL, Yang MH, Huo YX, Mei HB. (2008b) Effects of warm-supplementing kidney yang (WSKY) capsule added on risperidone on cognition in chronic schizophrenic patients: A randomized, double-blind, placebo-controlled, multi-center clinical trial. Hum Psychopharmacol. 23:465–470. [DOI] [PubMed] [Google Scholar]
- Chen ZH, Wang GH, Wang XP, Huo YX, Yang MH, Li L, Mei H. (2009) Effect of Warm-Supplementing Kidney Yang (WSKY) added to risperidone on quality of life in patients with schizophrenia: A randomized controlled trial. Clin Rehabil. 23:963–972. [DOI] [PubMed] [Google Scholar]
- Chua WL, de Izquierdo SA, Kulkarni J, Mortimer A. (2005) Estrogen for schizophrenia. Cochrane Database Syst Rev. DOI: 10.1002/14651858 CD004719. [DOI] [PubMed] [Google Scholar]
- Clifford T. (1994) Tibetan Buddhist medicine and psychiatry: The diamond healing. Delhi, India: Motilal Banarsidass. [Google Scholar]
- Cooper C, Bebbington P, King M, Brugha T, Meltzer H, Bhugra D, Jenkins R. (2007) Why people do not take their psychotropic drugs as prescribed: Results of the 2000 National Psychiatric Morbidity Survey. Acta Psychiatr Scand. 116:47–53. [DOI] [PubMed] [Google Scholar]
- Dabiri LM, Pasta D, Darby JK, Mosbacher D. (1994) Effectiveness of vitamin-E for treatment of long-term tardive-dyskinesia. Am J Psychiatry. 151:925–926. [DOI] [PubMed] [Google Scholar]
- Dakhale GN, Khanzode SD, Khanzode SS, Saoji A. (2005) Supplementation of vitamin C with atypical antipsychotics reduces oxidative stress and improves the outcome of schizophrenia. Psychopharmacology (Berl). 182:494–498. [DOI] [PubMed] [Google Scholar]
- Dean O, Giorlando F, Berk M. (2011) N-acetylcysteine in psychiatry: Current therapeutic evidence and potential mechanisms of action. J Psychiatry Neurosci. 36:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch M, Ananth JV, Ban TA. (1977) Nicotinic acid in the treatment of chronic hospitalized schizophrenic patients: A placebo-controlled clinical study. Psychopharmacol Bull. 13:21–23. [PubMed] [Google Scholar]
- Diaz P, Bhaskara S, Dursun SM, Deakin B. (2005) Double-blind, placebo-controlled, crossover trial of clozapine plus glycine in refractory schizophrenia negative results. J Clin Psychopharmacol. 25:277–278. [DOI] [PubMed] [Google Scholar]
- Dickerson F, Stallings C, Vaughan C, Origoni A, Goga J, Khushalani S, Yolken R. (2011) Artemisinin reduces the level of antibodies to gliadin in schizophrenia. Schizophr Res. 129:196–200. [DOI] [PubMed] [Google Scholar]
- Dorevitch A, Kalian M, Shlafman M, Lerner V. (1997a) Treatment of long-term tardive dyskinesia with vitamin E. Biol Psychiatry. 41:114–116. [DOI] [PubMed] [Google Scholar]
- Dorevitch A, Lerner V, Shalfman M, Kalian M. (1997b) Lack of effect of vitamin E on serum creatine phosphokinase in patients with long-term tardive dyskinesia. Int Clin Psychopharmacol. 12:171–173. [DOI] [PubMed] [Google Scholar]
- D'Souza DC, Radhakrishnan R, Perry E, Bhakta S, Singh NM, Yadav R, Abi-Saab D, Pittman B, Chaturvedi SK, Sharma MP, Bell M, Andrade C. (2013) Feasibility, safety, and efficacy of the combination of d-serine and computerized cognitive retraining in schizophrenia: An international collaborative pilot study. Neuropsychopharmacology. 38:492–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan EJ, Szilagyi S, Schwartz MP, Bugarski-Kirola D, Kunzova A, Negi S, Stephanides M, Efferen TR, Angrist B, Peselow E, Corwin J, Gonzenbach S, Rotrosen JP. (2004) Effects of d-cycloserine on negative symptoms in schizophrenia. Schizophr Res. 71:239–248. [DOI] [PubMed] [Google Scholar]
- Egan MF, Hyde TM, Albers GW, Elkashef A, Alexander RC, Reeve A, Blum A, Saenz RE, Wyatt RJ. (1992) Treatment of tardive dyskinesia with vitamin E. Am J Psychiatry. 149:773–777. [DOI] [PubMed] [Google Scholar]
- Elias A, Kumar A. (2007) Testosterone for schizophrenia. Cochrane Database Syst Rev. DOI: 10.1002/14651858 CD006197. [DOI] [PubMed] [Google Scholar]
- Elkashef AM, Ruskin PE, Bacher N, Barrett D. (1990) Vitamin E in the treatment of tardive dyskinesia. Am J Psychiatry. 147:505–506. [DOI] [PubMed] [Google Scholar]
- Elwyn G, Edwards A, Kinnersley P, Grol R. (2000) Shared decision making and the concept of equipoise: The competences of involving patients in healthcare choices. Br J Gen Pract. 50:892–899. [PMC free article] [PubMed] [Google Scholar]
- Emsley R, Myburgh C, Oosthuizen P, van Rensburg SJ. (2002) Randomized, placebo-controlled study of ethyl-eicosapentaenoic acid as supplemental treatment in schizophrenia. Am J Psychiatry. 159:1596–1598. [DOI] [PubMed] [Google Scholar]
- Emsley R, Niehaus DJ, Koen L, Oosthuizen PP, Turner HJ, Carey P, Janse van Rensburg S, Maritz JS, Murck H. (2006) The effects of eicosapentaenoic acid in tardive dyskinesia: A randomized, placebo-controlled trial. Schizophr Res. 84:112–120. [DOI] [PubMed] [Google Scholar]
- Emsley R, Niehaus DJ, Oosthuizen PP, Koen L, Ascott-Evans B, Chiliza B, van Rensburg SJ, Smit RM. (2008) Safety of the omega-3 fatty acid, eicosapentaenoic acid (EPA) in psychiatric patients: Results from a randomized, placebo-controlled trial. Psychiatry Res. 161:284–291. [DOI] [PubMed] [Google Scholar]
- Ermilov M, Gelfin E, Levin R, Lichtenberg P, Hashimoto K, Javitt DC, Heresco-Levy U. (2013) A pilot double-blind comparison of d-serine and high-dose olanzapine in treatment-resistant patients with schizophrenia. Schizophr Res. 150:604–605. [DOI] [PubMed] [Google Scholar]
- Ernst E. (2003a) UK government funds CAM research. FACT. 397–401. [Google Scholar]
- Ernst E. (2003b) Serious psychiatric and neurological adverse effects of herbal medicines—A systematic review. Acta Psychiatr Scand. 108:83–91. [DOI] [PubMed] [Google Scholar]
- Evins AE, Fitzgerald SM, Wine L, Rosselli R, Goff DC. (2000) Placebo-controlled trial of glycine added to clozapine in schizophrenia. Am J Psychiatry. 157:826–828. [DOI] [PubMed] [Google Scholar]
- Fendri C, Mechri A, Khiari G, Othman A, Kerkeni A, Gaha L. (2006) Oxidative stress involvement in schizophrenia pathophysiology: A review. Encéphale. 32:244–252. [DOI] [PubMed] [Google Scholar]
- Fenton WS, Dickerson F, Boronow J, Hibbeln JR, Knable M. (2001) A placebo-controlled trial of omega-3 fatty acid (ethyl eicosapentaenoic acid) supplementation for residual symptoms and cognitive impairment in schizophrenia. Am J Psychiatry. 158:2071–2074. [DOI] [PubMed] [Google Scholar]
- Flatow J, Buckley P, Miller BJ. (2013) Meta-analysis of oxidative stress in schizophrenia. Biol Psychiatry. 74:400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Berger G. (2012) Eicosapentaenoic acid interventions in schizophrenia: Meta-analysis of randomized, placebo-controlled studies. J Clin Psychopharmacol. 32:179–185. [DOI] [PubMed] [Google Scholar]
- Gill PS. (2013) Patient engagement: An investigation at a primary care clinic. Int J Gen Med. 6:85–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey PS, Toone BK, Carney MW, Flynn TG, Bottiglieri T, Laundy M, Chanarin I, Reynolds EH. (1990) Enhancement of recovery from psychiatric illness by methylfolate. Lancet. 336:392–395. [DOI] [PubMed] [Google Scholar]
- Goff DC, Cather C, Gottlieb JD, Evins AE, Walsh J, Raeke L, Otto MW, Schoenfeld D, Green MF. (2008) Once-weekly d-cycloserine effects on negative symptoms and cognition in schizophrenia: An exploratory study. Schizophr Res. 106:320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff DC, Herz L, Posever T, Shih V, Tsai G, Henderson DC, Freudenreich O, Evins AE, Yovel I, Zhang H, Schoenfeld D. (2005) A six-month, placebo-controlled trial of d-cycloserine co-administered with conventional antipsychotics in schizophrenia patients. Psychopharmacology (Berl). 179:144–150. [DOI] [PubMed] [Google Scholar]
- Gottlieb JD, Cather C, Shanahan M, Creedon T, Macklin EA, Goff DC. (2011) d-Cycloserine facilitation of cognitive behavioral therapy for delusions in schizophrenia. Schizophr Res. 131:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray RE, Fitch M, Greenberg M. (1998) A comparison of physician and patient perspectives on unconventional cancer therapies. Psychooncology. 7:445–452. [DOI] [PubMed] [Google Scholar]
- Greenbaum GH. (1970) An evaluation of niacinamide in the treatment of childhood schizophrenia. Am J Psychiatry. 127:89–92. [DOI] [PubMed] [Google Scholar]
- Harris ES, Cao S, Littlefield BA, Craycroft JA, Scholten R, Kaptchuk T, Fu Y, Wang W, Liu Y, Chen H, Zhao Z, Clardy J, Woolf AD, Eisenberg DM. (2011) Heavy metal and pesticide content in commonly prescribed individual raw Chinese herbal medicines. Sci Total Environ. 409:4297–4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison FE, May JM. (2009) Vitamin C function in the brain: Vital role of the ascorbate transporter SVCT2. Free Radic Biol Med. 46:719–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazra M, Noh S, Boon H, Taylor A, Moss K, Mamo D. (2010) Complementary and alternative medicine in psychotic disorders. J Altern Complement Med. 7:1–15. [Google Scholar]
- Heresco LU, Ermilov M, Lichtenberg P, Bar G, Javitt DC. (2004) High-dose glycine added to olanzapine and risperidone for the treatment of schizophrenia. Biol Psychiatry. 55:165–171. [DOI] [PubMed] [Google Scholar]
- Heresco LU, Javitt DC, Ermilov M, Mordel C, Horowitz A, Kelly D. (1996) Double-blind, placebo-controlled, crossover trial of glycine adjuvant therapy for treatment-resistant schizophrenia. Br J Psychiatry. 169:610–617. [DOI] [PubMed] [Google Scholar]
- Heresco-Levy U, Ermilov M, Shimoni J, Shapira B, Silipo G, Javitt DC. (2002) Placebo-controlled trial of d-cycloserine added to conventional neuroleptics, olanzapine, or risperidone in schizophrenia. Am J Psychiatry. 159:480–482. [DOI] [PubMed] [Google Scholar]
- Heresco-Levy U, Javitt DC, Ebstein R, Vass A, Lichtenberg P, Bar G, Catinari S, Ermilov M. (2005) d-Serine efficacy as add-on pharmacotherapy to risperidone and olanzapine for treatment-refractory schizophrenia. Biol Psychiatry. 57:577–585. [DOI] [PubMed] [Google Scholar]
- Heresco-Levy U, Javitt DC, Ermilov M, Mordel C, Silipo G, Lichtenstein M. (1999) Efficacy of high-dose glycine in the treatment of enduring negative symptoms of schizophrenia. Arch Gen Psychiatry. 56:29–36. [DOI] [PubMed] [Google Scholar]
- Herrera E, Barbas C. (2001) Vitamin E: Action, metabolism and perspectives. J Physiol Biochem. 57:43–56. [PubMed] [Google Scholar]
- Hill M, Shannahan K, Jasinski S, Macklin EA, Raeke L, Roffman JL, Goff DC. (2011) Folate supplementation in schizophrenia: A possible role for MTHFR genotype. Schizophr Res. 127:41–45. [DOI] [PubMed] [Google Scholar]
- Hoenders HJ, Appelo MT, Milders CF. (2006) Complementary and alternative medicine and psychiatry: Opinions of patients and psychiatrists. Tijdschr Psychiatr. 48:733–737. [PubMed] [Google Scholar]
- Hoenders HJ, Appelo MT, van den Brink EH, Hartogs BM, de Jong JT. (2011) The Dutch Complementary and Alternative Medicine (CAM) protocol: To ensure the safe and effective use of complementary and alternative medicine within Dutch mental health care. J Altern Complement Med. 17:1197–1201. [DOI] [PubMed] [Google Scholar]
- Hoenders HJR. (2013) Integrative psychiatry: Conceptual foundation, implementation and effectiveness. PhD thesis Groningen: University of Groningen. [Google Scholar]
- Hoffer A. (1971) Megavitamin B-3 therapy for schizophrenia. Can Psychiatr Assoc J. 16:499–504. [DOI] [PubMed] [Google Scholar]
- Hoffer A. (1972) LSD-induced psychosis and vitamin B3. Am J Psychiatry. 128:1155. [DOI] [PubMed] [Google Scholar]
- Hoffer A, Osmond H. (1964) Treatment of schizophrenia with nicotinic acid. A ten year follow-up. Acta Psychiatr Scand. 40:171–189. [DOI] [PubMed] [Google Scholar]
- Hoffer LJ. (2008) Vitamin therapy in schizophrenia. Isr J Psychiatry Relat Sci. 45:3–10. [PubMed] [Google Scholar]
- Irving CB, Mumby-Croft R, Joy LA. (2006) Polyunsaturated fatty acid supplementation for schizophrenia. Cochrane Database Syst Rev. DOI: 10.1002/14651858. CD001257. [DOI] [PubMed] [Google Scholar]
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. (1996) Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials. 17:1–12. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Silipo G, Cienfuegos A, Shelley AM, Bark N, Park M, Lindenmayer JP, Suckow R, Zukin SR. (2001) Adjunctive high-dose glycine in the treatment of schizophrenia. Int J Neuropsychopharmacol. 4:385–391. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zylberman I, Zukin SR, Heresco-Levy U, Lindenmayer JP. (1994) Amelioration of negative symptoms in schizophrenia by glycine. Am J Psychiatry. 151:1234–1236. [DOI] [PubMed] [Google Scholar]
- Joshi VG. (1982) Vitamins B1, B6, and B12 in the adjunctive treatment of schizophrenia: Further studies to examine the effect of reduction of chlorpromazine dosage. J Orthomol Psychiatry. 11:45–49. [Google Scholar]
- Kale A, Naphade N, Sapkale S, Kamaraju M, Pillai A, Joshi S, Mahadik S. (2010) Reduced folic acid, vitamin B12 and docosahexaenoic acid and increased homocysteine and cortisol in never-medicated schizophrenia patients: Implications for altered one-carbon metabolism. Psychiatry Res. 175:47–53. [DOI] [PubMed] [Google Scholar]
- Kane JM, Correll CU. (2010) Pharmacologic treatment of schizophrenia. Dialogues Clin Neurosci. 12:345–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaptchuck T. (2000) The Web that has no weaver. Chicago, IL: Contemporary, McGraw-Hill. [Google Scholar]
- Kleijnen J, Knipschild P. (1991) Niacin and vitamin B6 in mental functioning: A review of controlled trials in humans. Biol Psychiatry. 29:931–941. [DOI] [PubMed] [Google Scholar]
- Kline NS, Barclay GL, Cole JO, Esser AH, Lehmann H, Wittenborn JR. (1967) Controlled evaluation of nicotinamide adenine dinucleotide in the treatment of chronic schizophrenic patients. Br J Psychiatry. 113:731–742. [DOI] [PubMed] [Google Scholar]
- Ko YH, Lew YM, Jung SW, Joe SH, Lee CH, Jung HG, Lee MS. (2008) Short-term testosterone augmentation in male schizophrenics: A randomized, double-blind, placebo-controlled trial. J Clin Psychopharmacol. 28:375–383. [DOI] [PubMed] [Google Scholar]
- Koenig HG. (2000) MSJAMA: Religion, spirituality, and medicine: Application to clinical practice. JAMA. 284:1708. [PubMed] [Google Scholar]
- Kuboto Y, Tanaka N, Umegaki K, Takenaka H, Mizuno H, Nakamura K, Shinozuka K, Kunitomo M. (2001) Ginkgo biloba extract-induced relaxation of rat aorta is associated with increase in endothelial intracellular calcium level. Life Sci. 69:2327–2336. [DOI] [PubMed] [Google Scholar]
- Kulkarni J, de Castella A, Fitzgerald PB, Gurvich CT, Bailey M, Bartholomeusz C, Burger H. (2008) Estrogen in severe mental illness—A potential new treatment approach. Arch Gen Psychiatry. 65:955–960. [DOI] [PubMed] [Google Scholar]
- Kulkarni J, de Castella A, Headey B, Marston N, Sinclair K, Lee S, Gurvich C, Fitzgerald PB, Burger H. (2011) Estrogens and men with schizophrenia: Is there a case for adjunctive therapy? Schizophr Res. 125:278–283. [DOI] [PubMed] [Google Scholar]
- Kulkarni SK, Naidu PS. (2003) Pathophysiology and drug therapy of tardive dyskinesia: Current concepts and future perspectives. Drugs Today (Barc). 39:19–49. [DOI] [PubMed] [Google Scholar]
- Lake JH. (2007) Textbook of integrative mental health care. New York: Thieme Medical Publishers. [Google Scholar]
- Lam LC, Chiu HF, Hung SF. (1994) Vitamin E in the treatment of tardive dyskinesia: A replication study. J Nerv Ment Dis. 182:113–114. [DOI] [PubMed] [Google Scholar]
- Lane HY, Chang YC, Liu YC, Chiu CC, Tsai GE. (2005) Sarcosine or d-serine add-on treatment for acute exacerbation of schizophrenia: A randomized, double-blind, placebo-controlled study. Arch Gen Psychiatry. 62:1196–1204. [DOI] [PubMed] [Google Scholar]
- Lane HY, Huang CL, Wu PL, Liu YC, Chang YC, Lin PY, Chen PW, Tsai G. (2006) Glycine transporter I inhibitor, N-methylglycine (sarcosine), added to clozapine for the treatment of schizophrenia. Biol Psychiatry. 60:645–649. [DOI] [PubMed] [Google Scholar]
- Lane HY, Lin CH, Huang YJ, Liao CH, Chang YC, Tsai GE. (2010) A randomized, double-blind, placebo-controlled comparison study of sarcosine (N-methylglycine) and d-serine add-on treatment for schizophrenia. Int J Neuropsychopharmacol. 13:451–460. [DOI] [PubMed] [Google Scholar]
- Lane HY, Liu YC, Huang CL, Chang YC, Liau CH, Perng CH, Tsai GE. (2008) Sarcosine (N-methylglycine) treatment for acute schizophrenia: A randomized, double-blind study. Biol Psychiatry. 63:9–12. [DOI] [PubMed] [Google Scholar]
- Lavoie S, Murray MM, Deppen P, Knyazeva MG, Berk M, Boulat O, Bovet P, Bush AI, Conus P, Copolov D, Fornari E, Meuli R, Solida A, Vianin P, Cuénod M, Buclin T, Do KQ. (2008) Glutathione precursor, N-acetyl-cysteine, improves mismatch negativity in schizophrenia patients. Neuropsychopharmacology. 33:2187–2199. [DOI] [PubMed] [Google Scholar]
- Lerner V, Bergman J, Statsenko N, Miodownik C. (2004) Vitamin B6 treatment in acute neuroleptic-induced akathisia: A randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 65:1550–1554. [DOI] [PubMed] [Google Scholar]
- Lerner V, Miodownik C, Kaptsan A, Bersudsky Y, Libov I, Sela BA, Witztum E. (2007) Vitamin B6 treatment for tardive dyskinesia: A randomized, double-blind, placebo-controlled, crossover study. J Clin Psychiatry. 68:1648–1654. [DOI] [PubMed] [Google Scholar]
- Lerner V, Miodownik C, Kaptsan A, Cohen H, Loewenthal U, Kotler M. (2002) Vitamin B6 as add-on treatment in chronic schizophrenic and schizoaffective patients: A double-blind, placebo-controlled study. J Clin Psychiatry. 63:54–58. [DOI] [PubMed] [Google Scholar]
- Lerner V, Miodownik C, Kaptsan A, Cohen H, Matar M, Loewenthal U, Kotler M. (2001) Vitamin B(6) in the treatment of tardive dyskinesia: A double-blind, placebo-controlled, crossover study. Am J Psychiatry. 158:1511–1514. [DOI] [PubMed] [Google Scholar]
- Leucht S, Hierl S, Kissling W, Dold M, Davis JM. (2012) Putting the efficacy of psychiatric and general medicine medication into perspective: Review of meta-analyses. Br J Psychiatry. 200:97–106. [DOI] [PubMed] [Google Scholar]
- Levine J, Goldberger I, Rapaport A, Schwartz M. (1994) CSF inositol in schizophrenia and high-dose inositol treatment of schizophrenia. Eur Neuropsychopharmacol. 4:487–490. [DOI] [PubMed] [Google Scholar]
- Levine J, Stahl Z, Sela BA, Ruderman V, Shumaico O, Babushkin I, Osher Y, Bersudsky Y, Belmaker RH. (2006) Homocysteine-reducing strategies improve symptoms in chronic schizophrenic patients with hyperhomocysteinemia. Biol Psychiatry. 60:265–269. [DOI] [PubMed] [Google Scholar]
- Levy MI, Davis BM, Mohs RC, Trigos GC, Mathé AA, Davis KL. (1983) Gamma-hydroxybutyrate in the treatment of schizophrenia. Psychiatry Res. 9:1–8. [DOI] [PubMed] [Google Scholar]
- Lohr JB, Caligiuri MP. (1996) A double-blind placebo-controlled study of vitamin E treatment of tardive dyskinesia. J Clin Psychiatry. 57:167–173. [PubMed] [Google Scholar]
- Maclennan KM, Darlington CL, Smith PE. (2002) The CNS effects of Ginkgo biloba extracts and ginkgolide B. Prog Neurobiol. 67:235–257. [DOI] [PubMed] [Google Scholar]
- Mahadik SP, Evans D, Lal H. (2001) Oxidative stress and role of antioxidant and omega-3 essential fatty acid supplementation in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 25:463–493. [DOI] [PubMed] [Google Scholar]
- Mahadik SP, Mukherjee S. (1996) Free radical pathology and antioxidant defense in schizophrenia: A review. Schizophr Res. 19:1–17. [DOI] [PubMed] [Google Scholar]
- Mahal AS, Ramu NG, Chaturvedi DD, Thomas KM, Senapati HM, Narasimha Murthy NS. (1976) Double blind controlled study of brahmyadiyoga and tagara in the management of various types of unmada (schizophrenia). Indian J Psychiatry. 18:283–292. [Google Scholar]
- Manteghiy A, Shakeri MT, Koohestani L, Salari E. (2008) Beneficial antipsychotic effects of omega-3 fatty acids add-on therapy for the pharmacological management of patients with schizophrenia. Iran J Psychiatry Behav Sci. 2:35–40. [Google Scholar]
- McGrath SD, O'Brien PF, Power PJ, Shea JR. (1972) Short report: Nicotinamide treatment of schizophrenia. Schizophr Bull. 1:74–76. [Google Scholar]
- Meltzer H, Shader R, Grinspoon L. (1969) The behavioral effects of nicotinamide adenine dinucleotide in chronic schizophrenia. Psychopharmacologia. 15:144–152. [DOI] [PubMed] [Google Scholar]
- Miodownik C, Lerner V, Statsenko N, Dwolatzky T, Nemets B, Berzak E, Bergman J. (2006) Vitamin B6 versus mianserin and placebo in acute neuroleptic-induced akathisia: A randomized, double-blind, controlled study. Clin Neuropharmacol. 29:68–72. [DOI] [PubMed] [Google Scholar]
- Morrison AP, Hutton P, Shiers D, Turkington D. (2012) Antipsychotics: Is it time to introduce patient choice? Br J Psychiatry. 201:83–84. [DOI] [PubMed] [Google Scholar]
- Mundewadi AA, Joshi DD, Arekar AS, Bakre GB, Mundewadi RA. (2008) A randomized, controlled, clinical trial of a herbal combination of aqueous extracts of bacopa monnieri and nardostachys jatamansi in schizophrenia, compared to standard anti-psychotic drug, olanzapine as an active control. Bombay Hospital J. 50:456–465. [Google Scholar]
- Nachshoni T, Ebert T, Abramovitch Y, Assael-Amir M, Kotler M, Maayan R, Weizman A, Strous RD. (2005) Improvement of extrapyramidal symptoms following dehydroepiandrosterone (DHEA) administration in antipsychotic treated schizophrenia patients: A randomized, double-blind placebo controlled trial. Schizophr Res. 79:251–256. [DOI] [PubMed] [Google Scholar]
- Naidoo D. (1956) The effects of reserpine (serpasil) on the chronic disturbed schizophrenic: A comparative study of rauwolfia alkaloids and electroconvulsive therapy. J Nerv Ment Dis. 123:1–13. [DOI] [PubMed] [Google Scholar]
- Osmond H, Hoffer A. (1962) Massive niacin treatment in schizophrenia. Review of a nine-year study. Lancet. 1:316–319. [DOI] [PubMed] [Google Scholar]
- Pai NB, Vella SC. (2012) Reason for clozapine cessation. Acta Psychiatr Scand. 125:39–44. [DOI] [PubMed] [Google Scholar]
- Pauling L. (1968) Orthomolecular psychiatry. Varying the concentrations of substances normally present in the human body may control mental disease. Science. 160:265–271. [DOI] [PubMed] [Google Scholar]
- Peet M. (2008) Omega-3 polyunsaturated fatty acids in the treatment of schizophrenia. Isr J Psychiatry Relat Sci. 45:19–25. [PubMed] [Google Scholar]
- Peet M, Brind J, Ramchand CN, Shah S, Vankar GK. (2001) Two double-blind placebo-controlled pilot studies of eicosapentaenoic acid in the treatment of schizophrenia. Schizophr Res. 49:243–251. [DOI] [PubMed] [Google Scholar]
- Peet M, Horrobin DF, E-E Multicentre Study Group (2002) A dose-ranging exploratory study of the effects of ethyl-eicosapentaenoate in patients with persistent schizophrenic symptoms. J Psychiatr Res. 36:7–18. [DOI] [PubMed] [Google Scholar]
- Petrie WM, Ban TA, Ananth JV. (1981) The use of nicotinic acid and pyridoxine in the treatment of schizophrenia. Int Pharmacopsychiatry. 16:245–250. [DOI] [PubMed] [Google Scholar]
- Porter N. (1998) Webster's revised unabridged dictionary. Springfield, MA: C. & G. Merriam Co. [Google Scholar]
- Potkin SG, Jin Y, Bunney BG, Costa J, Gulasekaram B. (1999) Effect of clozapine and adjunctive high-dose glycine in treatment-resistant schizophrenia. Am J Psychiatry. 156:145–147. [DOI] [PubMed] [Google Scholar]
- Prange AJ. (1979) Behavioral and endocrine responses of schizophrenic patients to TRH (protirelin). Arch Gen Psychiatry. 36:1086–1093. [DOI] [PubMed] [Google Scholar]
- Ramsay RA, Ban TA, Lehmann HE, Saxena BM, Bennett J. (1970) Nicotinic acid as adjuvant therapy in newly admitted schizophrenic patients. Can Med Assoc J. 102:939–942. [PMC free article] [PubMed] [Google Scholar]
- Rathbone J, Zhang L, Zhang M, Xia J, Liu X, Yang Y, Adams CE. (2007) Chinese herbal medicine for schizophrenia: Cochrane systematic review of randomised trials. Br J Psychiatry. 190:379–384. [DOI] [PubMed] [Google Scholar]
- Ritsner MS. (2010) Pregnenolone, dehydroepiandrosterone, and schizophrenia: Alterations and clinical trials. CNS Neurosci Ther. 16:32–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritsner MS, Gibel A, Ratner Y, Tsinovoy G, Strous RD. (2006) Improvement of sustained attention and visual and movement skills, but not clinical symptoms, after dehydroepiandrosterone augmentation in schizophrenia: A randomized, double-blind, placebo-controlled, crossover trial. J Clin Psychopharmacol. 26:495–499. [DOI] [PubMed] [Google Scholar]
- Roffman JL, Lamberti JS, Achtyes E, Macklin EA, Galendez GC, Raeke LH, Silverstein NJ, Smoller JW, Hill M, Goff DC. (2013) Randomized multicenter investigation of folate plus vitamin B12 supplementation in schizophrenia. JAMA Psychiat. 70:481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosse RB, Fay-McCarthy M, Kendrick K, Davis RE, Deutsch SI. (1996) d-Cycloserine adjuvant therapy to molindone in the treatment of schizophrenia. Clin Neuropharmacol. 19:444–450. [DOI] [PubMed] [Google Scholar]
- Rummel-Kluge C, Komossa K, Schwarz S, Hunger H, Schmid F, Asenjo Lobos C, Kissling W, Davis JM, Leucht S. (2010) Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: A systematic review and meta-analysis. Schizophr Res. 123:225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks W, Esser AH, Feitel B, Abbott K. (1989) Acetazolamide and thiamine: An ancillary therapy for chronic mental illness. Psychiatry Res. 28:279–288. [DOI] [PubMed] [Google Scholar]
- Salmasi FB, Jazayeri M, Ghaeli P, Hashemian F, Akhondzadeh S, Raisi F, Hosseini SH, Setareh MJ, Khavidaki SD. (2009) Comparing the effects of high-dose vitamin E with those of placebo on insulin resistance in patients with schizophrenia treated with olanzapine. J Clin Psychopharmacol. 29:182–183. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Meister P, Baumann P. (1991) Treatment of tardive dyskinesias with vitamin E. Eur Psychiatry. 6:201–207. [Google Scholar]
- Schulz SC, van Kammen DP, Buchsbaum MS, Roth RH, Alexander P, Bunney WE., Jr (1981) Gamma-hydroxybutyrate treatment of schizophrenia: A pilot study. Pharmacopsychiatria. 14:129–134. [DOI] [PubMed] [Google Scholar]
- Shamir E, Barak Y, Shalman I, Laudon M, Zisapel N, Tarrasch R, Elizur A, Weizman R. (2001) Melatonin treatment for tardive dyskinesia: A double-blind, placebo-controlled, crossover study. Arch Gen Psychiatry. 58:1049–1052. [DOI] [PubMed] [Google Scholar]
- Shriqui CL, Bradwejn J, Annable L, Jones BD. (1992) Vitamin E in the treatment of tardive dyskinesia: A double-blind placebo-controlled study. Am J Psychiatry. 149:391–393. [DOI] [PubMed] [Google Scholar]
- Singh SP, Singh V. (2011) Meta-analysis of the efficacy of adjunctive NMDA receptor modulators in chronic schizophrenia. CNS Drugs. 25:859–885. [DOI] [PubMed] [Google Scholar]
- Singh V, Singh SP, Chan K. (2010) Review and meta-analysis of usage of ginkgo as an adjunct therapy in chronic schizophrenia. Int J Neuropsychopharmacol. 13:257–271. [DOI] [PubMed] [Google Scholar]
- Soares WK, Maayan N, McGrath J. (2011) Vitamin E for neuroleptic-induced tardive dyskinesia. Cochrane Database Syst Rev. DOI: 10.1002/14651858. CD000209. [DOI] [PubMed] [Google Scholar]
- Stahl SM. (2008) Stahl's essential psychopharmacology: Neuroscientific basis and practical applications. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Stevinson C. (2001) Why patients use complementary and alternative medicine. In Ernst E. (Ed), The Desktop Guide to Complementary and Alternative Medicine, an Evidence-based Approach. Edinburgh, UK: Mosby, by Hartcourt Publishers Limited. [Google Scholar]
- Strous RD, Maayan R, Lapidus R, Stryjer R, Lustig M, Kotler M, Weizman A. (2003) Dehydroepiandrosterone augmentation in the management of negative, depressive, and anxiety symptoms in schizophrenia. Arch Gen Psychiatry. 60:133–141. [DOI] [PubMed] [Google Scholar]
- Strous RD, Stryjer R, Maayan R, Gal G, Viglin D, Katz E, Eisner D, Weizman A. (2007) Analysis of clinical symptomatology, extrapyramidal symptoms and neurocognitive dysfunction following dehydroepiandrosterone (DHEA) administration in olanzapine treated schizophrenia patients: A randomized, double-blind placebo controlled trial. Psychoneuroendocrinology. 32:96–105. [DOI] [PubMed] [Google Scholar]
- Sun BL, Zhang J, Wang XC, Xia ZL, Yang MF, Zhang SM, Ye WJ, Yuan H. (2003) Effects of extract of Ginkgo biloba on spasms of the basilar artery and cerebral microcirculatory perfusion in rats with subarachnoid hemorrhage. Clin Hemorheol Microcirc. 29:231–238. [PubMed] [Google Scholar]
- Thirthalli J, Zhou L, Kumar K, Gao J, Vaid H, Liu H, Hankey A, Wang G, Gangadhar BN, Nie JB, Nichter M. (2016) Traditional, complementary, and alternative medicine approaches to mental health care and psychological wellbeing in India and China. Lancet Psychiatry. 3:660–672. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Wahlbeck K. (2006) Glutamatergic drugs for schizophrenia. Cochrane Database Syst Rev. DOI: 10.1002/14651858. CD003730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toktam F, Padideh G, Adel J, Javad MG, Vandad S, Shahin A. (2010) Effect of early intervention with omega-3 on insulin resistance in patients initiated on olanzapine with either sodium valproate or lithium: A randomized, double-blind, placebo-controlled trial. Iran J Psychiatry. 5:18–22. [PMC free article] [PubMed] [Google Scholar]
- Topliss JG, Clark AM, Ernst E, Hufford CD, Johnston GA, Rimoldi JM, Weimann BJ. (2002) Natural and synthetic substances related to human health–(IUPAC Technical Report). Pure Appl Chem. 74:1957–1985. [Google Scholar]
- Tsai G, Lane HY, Yang P, Chong MY, Lange N. (2004) Glycine transporter I inhibitor, N-methylglycine (sarcosine), added to antipsychotics for the treatment of schizophrenia. Biol Psychiatry. 55:452–456. [DOI] [PubMed] [Google Scholar]
- Tsai G, Yang P, Chung LC, Lange N, Coyle JT. (1998) d-Serine added to antipsychotics for the treatment of schizophrenia. Biol Psychiatry. 44:1081–1089. [DOI] [PubMed] [Google Scholar]
- Tsai GE, Lin PY. (2010) Strategies to enhance N-methyl-d-aspartate receptor–mediated neurotransmission in schizophrenia, a critical review and meta-analysis. Curr Pharm Des. 16:522–537. [DOI] [PubMed] [Google Scholar]
- Tsai GE, Yang P, Chang YC, Chong MY. (2006) d-Alanine added to antipsychotics for the treatment of schizophrenia. Biol Psychiatry. 59:230–234. [DOI] [PubMed] [Google Scholar]
- Tsai GE, Yang P, Chung LC, Tsai IC, Tsai CW, Coyle JT. (1999) d-Serine added to clozapine for the treatment of schizophrenia. Am J Psychiatry. 156:1822–1825. [DOI] [PubMed] [Google Scholar]
- Tsalamanios E, Yanni AE, Koutsari C. (2006) Omega-3 fatty acids: Role in the prevention and treatment of psychiatric disorders. Curr Psychiatry Rev. 2:215–234. [Google Scholar]
- Umbricht D, Lentz E, Lalonde J, Martin-Facklam M, Santarelli L. (2013) The effect of bitopertin, a glycine reuptake inhibitor, on key negative symptom dimensions and association with estimated GlyT1 occupancy. Schizophr Bull. 39:s355–s356. [Google Scholar]
- Vaddadi KS, Courtney P, Gilleard CJ, Manku MS, Horrobin DF. (1989) A double-blind trial of essential fatty acid supplementation in patients with tardive dyskinesia. Psychiatry Res. 27:313–323. [DOI] [PubMed] [Google Scholar]
- Van Berckel BN, Evenblij CN, Loon BJ, Maas MF, Geld MA, Wynne HJ, van Ree JM, Kahn RS. (1999) D-Cycloserine increases positive symptoms in chronic schizophrenic patients when administered in addition to antipsychotics: A double-blind, parallel, placebo-controlled study. Neuropsychopharmacology. 21:203–210. [DOI] [PubMed] [Google Scholar]
- Van der Greef J. (2011) Perspective: All systems go. Nature. 480:S87. [DOI] [PubMed] [Google Scholar]
- Van Os J, Kapur S. (2009) Schizophrenia. Lancet. 374:635–645. [DOI] [PubMed] [Google Scholar]
- Vaughan K, McConaghy N. (1999) Megavitamin and dietary treatment in schizophrenia: A randomised, controlled trial. Aust N Z J Psychiatry. 33:84–88. [DOI] [PubMed] [Google Scholar]
- Verhoeven WM, van Praag HM, van Ree JM, de Wied D. (1979) Improvement of schizophrenic patients treated with [des-Tyr1]-gamma-endorphin (DTgammaE). Arch Gen Psychiatry. 36:294–298. [DOI] [PubMed] [Google Scholar]
- Walter G, Rey JM. (1999) The relevance of herbal treatments for psychiatric practice. Aust N Z J Psychiatry. 33:482–489. [DOI] [PubMed] [Google Scholar]
- Wang HL, Xiang YT, Li QY, Wang XP, Liu ZC, Hao SS, Liu X, Liu LL, Wang GH, Wang DG, Zhang PA, Bao AY, Chiu HF, Ungvari GS, Lai KY, Buchanan RW. (2014) The effect of artemether on psychotic symptoms and cognitive impairment in first-episode, antipsychotic drug-naive persons with schizophrenia seropositive to Toxoplasma gondii. J Psychiatr Res. 53:119–124. [DOI] [PubMed] [Google Scholar]
- Weiser M, Heresco-Levy U, Davidson M, Javitt DC, Werbeloff N, Gershon AA, Abramovich Y, Amital D, Doron A, Konas S, Levkovitz Y, Liba D, Teitelbaum A, Mashiach M, Zimmerman Y. (2012) A multicenter, add-on randomized controlled trial of low-dose d-serine for negative and cognitive symptoms of schizophrenia. J Clin Psychiatry. 73:e728–e734. [DOI] [PubMed] [Google Scholar]
- Wittenborn JR, Weber ES, Brown M. (1973) Niacin in the long-term treatment of schizophrenia. Arch Gen Psychiatry. 28:308–315. [DOI] [PubMed] [Google Scholar]
- Wittkopp TA, Abuzzahab FS., Sr (1972) Nicotinic acid and nicotinamide adenine dinucleotide (NAD) therapy in schizophrenia: A review. Behav Neuropsychiatry. 4:6–12. [PubMed] [Google Scholar]
- World Health Organization (2013) Traditional Medicine Strategy 2014–2023. Available at: http://www.who.int/medicines/publications/traditional/trm_strategy14_23/en/.
- Xiao SF, Xue HB, Li X, Chen C, Li GJ, Yuan CM, Zhang MY. (2011) A double-blind, placebo-controlled study of traditional Chinese medicine sarsasapogenin added to risperidone in patients with negative symptoms dominated schizophrenia. Neurosci Bull. 27:258–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan F, Zheng Y, Zhao F. (2008) Effects of Ginkgo biloba extract EGb761 on expression of RAGE and LRP-1 in cerebral microvascular endothelial cells under chronic hypoxia and hypoglycemia. Acta Neuropathol. 116:529–535. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd DA, Coyle JT, Gruber SA, Renshaw PF, Silveri MM, Amico E, Cohen B, Goff DC. (2005) Functional magnetic resonance imaging studies of schizophrenic patients during word production: Effects of d-cycloserine. Psychiatry Res. 138:23–31. [DOI] [PubMed] [Google Scholar]
- Zhang WF, Tan YL, Zhang XY, Chan RC, Wu HR, Zhou DF. (2011a) Extract of Ginkgo biloba treatment for tardive dyskinesia in schizophrenia: A randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 72:615–621. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Zhou DF, Cao LY, Wu GY. (2006) The effects of Ginkgo biloba extract added to haloperidol on peripheral T cell subsets in drug-free schizophrenia: A double-blind, placebo-controlled trial. Psychopharmacology (Berl). 188:12–17. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Zhou DF, Su JM, Zhang PY. (2001a) The effect of extract of ginkgo biloba added to haloperidol on superoxide dismutase in inpatients with chronic schizophrenia. J Clin Psychopharmacol. 21:85–88. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Zhou DF, Zhang PY, Wu GY, Su JM, Cao LY. (2001b) A double-blind, placebo-controlled trial of extract of Ginkgo biloba added to haloperidol in treatment-resistant patients with schizophrenia. J Clin Psychiatry. 62:878–883. [DOI] [PubMed] [Google Scholar]
- Zhang ZJ, Tan QR, Tong Y, Wang XY, Wang HH, Ho LM, Wong HK, Feng YB, Wang D, Ng R, McAlonan GM, Wang CY, Wong VT. (2011b) An epidemiological study of concomitant use of Chinese medicine and antipsychotics in schizophrenic patients: Implication for herb-drug interaction. PLoS One. 6:e17239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Zhang X, Su J, Nan Z, Cui Y, Liu J, Guan Z, Zhang P, Shen Y. (1999) The effects of classic antipsychotic haloperidol plus the extract of ginkgo biloba on superoxide dismutase in patients with chronic refractory schizophrenia. Chin Med J (Engl). 112:1093–1096. [PubMed] [Google Scholar]


