Abstract
Rationale:
Primary cutaneous anaplastic large-cell lymphoma (C-ALCL) is a rare cancer belonging to the group of primary T-cell lymphoproliferative diseases. C-ALCL is characterized by the presence of single or multiple ulcerated lesions on the skin's surface.
Patient concerns:
This is the case of a 73-year-old man who reported to the Clinic of Cranio-Maxillofacial and Oral Surgery and Implantology, Medical University of Warsaw, owing to a skin tumor in the right parotideomasseteric region, initially diagnosed as discoid lupus erythematosus. During treatment for discoid lupus erythematosus, biopsy was repeated because of significant disease progression and dynamic tumor growth. Histopathological examination revealed the presence of pilomatrix carcinoma (trichilemmal carcinoma). Because of the discrepancy between clinical and histopathological findings, the tumor specimen was submitted to another facility, wherein lymphoma infiltration by anaplastic large cells was found in the dermis and subcutaneous tissue.
Diagnosis:
C-ALCL.
Interventions:
The patient was transferred to the Lymphoid Tumours Clinic of the Maria Skłodowska Curie Memorial Cancer Centre and Institute of Oncology in Warsaw, where chemotherapy was initiated.
Outcomes:
After 4 cycles of chemotherapy, a complete remission of skin lesions was achieved. During the 5-year follow-up, no recurrence occurred.
Lessons:
C-ALCL is a rare type of cancer. Misdiagnosis can lead to inappropriate therapy and result in disease progression or unnecessary harm to the patient.
Keywords: discoid lupus erythematosus, lymphomatoid papulosis, pilomatrix carcinoma, primary cutaneous anaplastic large-cell lymphoma
1. Introduction
The most common nonmelanoma skin cancer of the face is basal cell carcinoma, which accounts for 80% of all skin lesions, followed by squamous cell carcinoma.[1] The prevalence of these types of cancer in the general population is 10:1.[1] Meanwhile, primary skin lymphomas constitute a relatively rare occurrence among malignant neoplasms of the skin.
Primary skin lymphomas are a heterogeneous group of non-Hodgkin lymphomas, mainly derived from mature T-cells and B-lymphocytes (65% and 25% of primary skin lymphomas, respectively).[2] Other cancers of the skin are lymphomas derived from natural cytotoxic cells.[2] Skin tumors with at least 6 months of diagnosis and development are limited to the dermal layers and are not derived from systemic lymphomas recognized as primary skin lymphomas. According to the 2008 WHO-EORTC classification, primary cutaneous anaplastic large-cell lymphoma (C-ALCL)[3] is classified as primary skin lymphoma and constitutes about 10% of all skin lymphoma cases.[4]
Initially, C-ALCL, lymphomatoid papulosis (LyP), and borderline variants (where precise differentiation between C-ALCL and LyP is not possible) belong to a group of primary T-cell lymphoproliferative diseases, wherein C-ALCL represents 30% of all primary cutaneous T-cell lymphomas.[3,5]
Histopathologically, C-ALCL is characterized by loss of expression of one or more T-cell antigens (i.e., CD2, CD3, and CD5), high expression of CD30+ antigen, and usually absence of ALK- and EMA antigens.[2,3,6] CD30+ antigen expression in >75% of infiltration cells is a condition for C-ALCL diagnosis.[7] This type of lymphoma occurs mainly in adults and males, after the age of 60 years. C-ALCL cases in children have also been described.[8,9]
Clinically, C-ALCL is most prevalent on the skin of the face, trunk, buttocks, or limbs, in the form of single or multiple platelets or ulcerations of 1 to 10 cm in diameter, with reddish or violet color.[3,6,10,11] Establishing a proper diagnosis of C-ALCL is difficult and is often belated because of its nonspecific clinical picture, suggesting other disease entities related to primary or secondary CD30+.[7]
This article aimed to describe a case of C-ALCL in the right parotideomasseteric region, considering the rarity of this location and difficulties in the diagnostic process.
2. Case report
A 73-year-old man was admitted to the Clinic of Cranio-Maxillofacial and Oral Surgery and Implantology, Medical University of Warsaw, because of a skin tumor in the right parotideomasseteric region. According to medical history, the skin of the right parotideomasseteric region had shown a well-defined skin redness of 10 × 15 mm, 6 months earlier. His general health condition was good. The patient was seen by a dermatologist who performed the biopsy of the lesion and ordered blood tests. High levels of antimitochondrial and anti-nuclear antibodies were found in the serum. Immunofluorescence of the collected tissue revealed the presence of IgG and IgM deposits on the cutaneous epidermis, which led to the diagnosis of discoid lupus erythematosus and prescription of Arechin, 250 mg orally, twice a day. Four months after the onset of treatment, significant disease progression and dynamic tumor growth were observed; thus, the patient was referred to the Lymphoid Tumours Clinic of the Maria Skłodowska Curie Memorial Cancer Center and Institute of Oncology in Warsaw.
On admission, a well-demarcated exophytic skin tumor with irregular surface without ulceration, measuring 50 × 70 mm (Fig. 1), was observed in the right parotideomasseteric region. The patient reported no pain related to the tumor or impaired sensation on the skin of the face. There was no evidence of facial nerve palsy. Local anesthesia was induced for a histopathological examination that later indicated the presence of pilomatrix carcinoma (trichilemmal carcinoma) in the tumor specimen. Owing to the discrepancy between clinical features (appearance of the lesion and dynamics of the disease) and histopathological examination results, the biopsy material was submitted to another facility, wherein microscopic imaging clearly indicated the presence of anaplastic large cells in the dermis and subcutaneous tissue, characterized by the following immunohistochemistry: LCA+, CD30+, ALK-, CD3+ (weak membrane reaction), CD5-, CKAE1/3, MELAN A-, S100-, and Ki67+++ in about 90% of cells.
Figure 1.
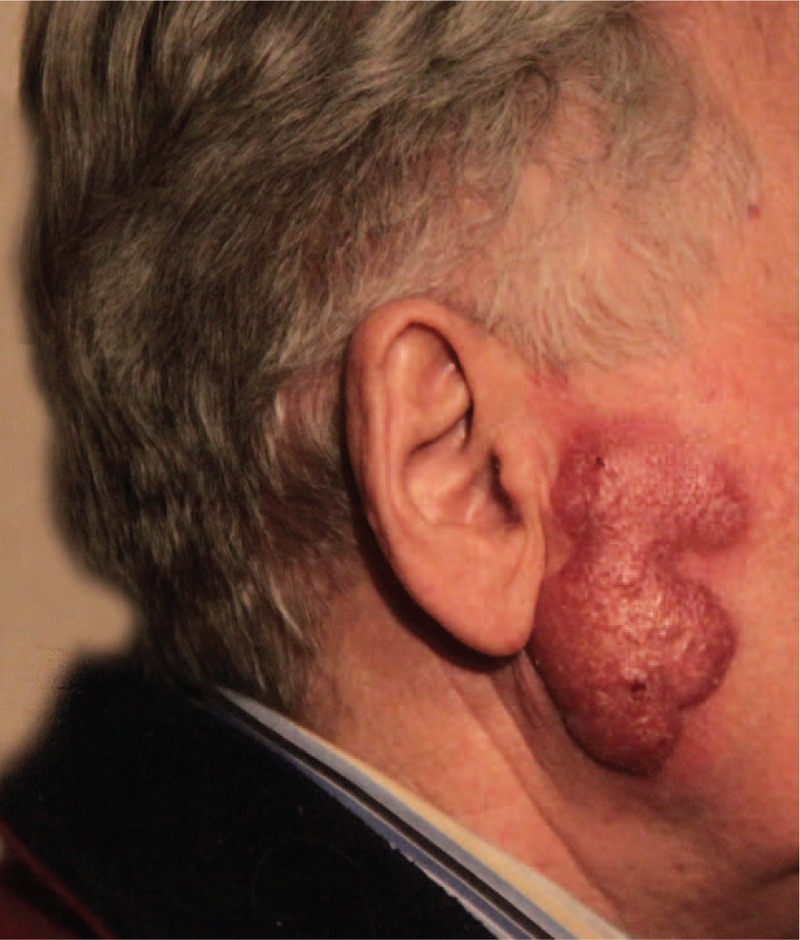
Well-demarcated exophytic skin tumor with irregular surface in the right parotideomasseteric region.
To rule out systemic anaplastic large-cell lymphoma, which involves locations other than the skin, trepanobiopsy of bone marrow was performed, and the image indicated no lymphoma infiltration. Diagnosis of potential lymphoma infiltration was further performed with computed tomography (CT) scans of the chest, abdominal cavity, and neck and detailed assessment of the nasopharynx (Fig. 2). CT confirmed the absence of lymphoma foci in other organs, which led to the definitive diagnosis of C-ALCL. The patient underwent 4 chemotherapy cycles consisting of intravenous vincristine 2 mg, doxorubicin 90 mg, endoxan 1400 mg, Dexaven 18 mg, and Zofran 8 mg. Blood parameters were monitored throughout the treatment period. During chemotherapy, gradual tumor regression was observed (Fig. 3A and B), and 1 year after the first cycle of chemotherapy, complete remission was achieved. At 5 years after the end of treatment, no recurrent lymphoma was evident (Fig. 4A and B).
Figure 2.
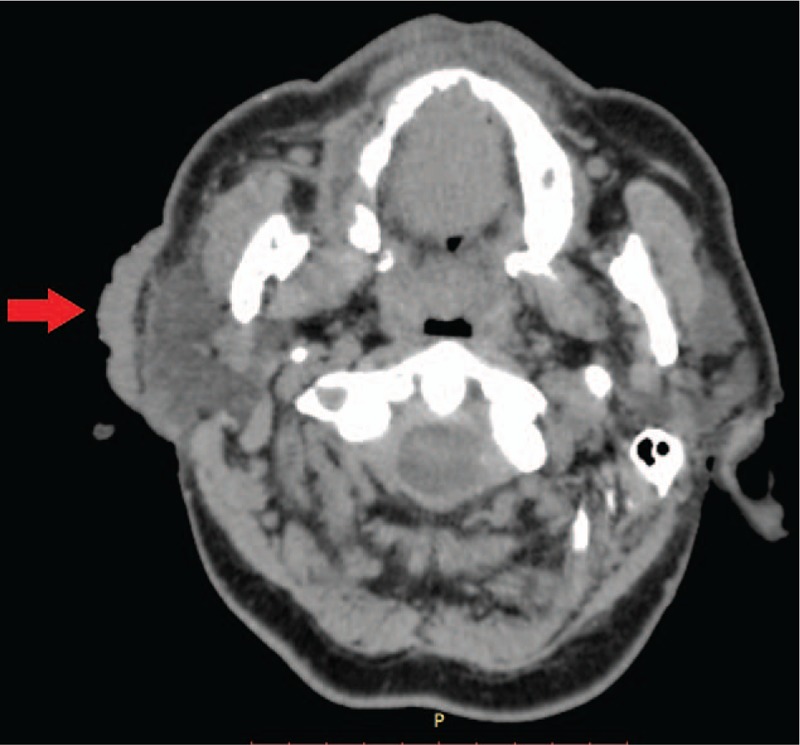
Computed tomography image showing a solid, obscure mass on the skin in the right parotideomasseteric region (arrowhead).
Figure 3.
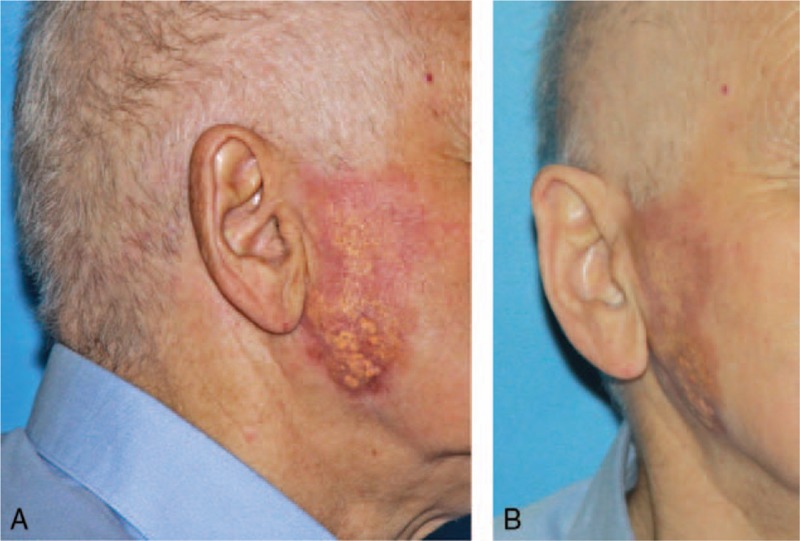
(A and B) Gradual tumor regression during chemotherapy.
Figure 4.

(A and B) Five years after chemotherapy, no recurrence was observed.
3. Discussion
Pilomatrix carcinoma of the parotideomasseteric region is a rare, malignant cancer of the hair follicles, with only few cases reported in the literature.[12] In this study, the first results of histopathological examination indicating pilomatrix carcinoma did not appear to be clinically viable because of the clinical course of the disease and rarity in the skin.
In both C-ALCL and LyP, the expression of CD30+ antigen is a marker. Histologically, the difference between these 2 types of tumors is primarily based on medical history and in-depth clinical examination.[11,13] In adults, C-ALCL should also be distinguished from atopic dermatitis, gangrene, scleroderma, skin cancer, and other proliferative diseases.[13] In juvenile patients, C-ALCL should be differentiated from rhabdomyosarcoma.[14]
The diagnostic criteria for C-ALCL[5] include the expression of CD30+ antigen in >75% of infiltration cells; lack of clinical evidence of LyP, mycosis fungoides, or other cutaneous lymphomas; and no confirmed lymphoma in locations other than the skin layers.
To rule out systemic anaplastic large-cell lymphoma, it is advisable to perform CT scans of the chest, abdominal cavity, and neck in search for different localizations of the disease.[13] In this study, the definitive test was a histopathological examination with an immunohistochemical evaluation of the specimen section. Immunohistochemistry is needed to determine the subtype of skin lymphoma and to differentiate between primary and secondary disease.[4]
C-ALCL occurs mainly in males after the age of 60 years, particularly on the skin of the face, trunk, buttocks, or limbs. The age, clinical symptoms, and localization of the disease described in this study were consistent with the general description of C-ALCL. If the tumor affects the limbs, the prognosis is worse.[15] The literature also describes isolated cases of this disease in children.[8,9,11]
Depending on tumor size, tumor location, and the current course of the disease, several treatment regimens may be considered. Isolated small tumors can be excised or treated with 30 to 46 Gy (2 Gy/fraction) of radiotherapy with a 2 to 3 cm margin around the tumor.[2] Jepsen et al[11] obtained good results for C-ALCL at 16 to 20 Gy and photon energy of 40 to 50 kV. In about 10% of cases, C-ALCL can spread and affect regional lymph nodes or other organs. In such cases, treatment can involve suitable chemotherapy regimens as in the systemic form.[14] Chemotherapy is also reserved for patients diagnosed with multifocal C-ALCL or those with recurrent disease.[4] Moreover, methotrexate doses of up to 30 mg/week are used in patients with multifocal C-ALCL.[2] Other treatment regimens include the administration of interferon alfa, bexarotene, and anti-CD30 monoclonal antibodies (brentuximab vedotin).[16] In the case described here, because of the course of the disease and the tumor size, chemotherapy was administered using the CHOP scheme, and the patient achieved complete remission after 1 year.
Despite significant cell atypia in C-ALCL histopathology, the prognosis is good. The 10-year survival rate of patients is 90%.[10] Spontaneous or partial tumor remission was observed in 44% of cases.[17]
4. Conclusions
C-ALCL is a diagnostic challenge for both clinicians and histopathologists. Establishing a proper diagnosis is possible based on thorough medical history, clinical and imaging examinations, and histopathological evaluation of the biopsy sample from the lesion site. Misdiagnosis can lead to inappropriate therapy and result in disease progression or unnecessary harm to the patient.
Footnotes
Abbreviations: ALK = anaplastic lymphoma kinase (Ki-1), C-ALCL = cutaneous anaplastic large-cell lymphoma, CD2 = cluster of differentiation 2, CD3 = cluster of differentiation 3, CD30 = cluster of differentiation 30, CD5 = cluster of differentiation 5, CK = AE1/AE3 anti-cytokeratin monoclonal antibodies, AE1 and AE3, CT = computed tomography, EMA = epithelial membrane antigen, Ki 67 = antigen Ki67 (protein) cellular marker for proliferation, LCA = leukocyte common antigen, LyP = lymphomatoid papulosis, Melan A = melanocyte antigen, S100 = calcium-binding protein.
Ethical Statement: I testify on behalf of all co-authors that our article submitted to Medicine.
Titled “Primary cutaneous anaplastic large-cell lymphoma (C-ALCL): A case report”.
The paper was coauthored by Zygmunt Stopa and Marta Siewert-Gutowska.
This manuscript has not been published or presented elsewhere in part or in entirety and is not under consideration by another journal. The subject of the study provided informed consent. We have read and understood your journal's policies, and we believe that neither the manuscript nor the study violates any of these.
The authors report no conflicts of interest.
All authors have been personally and actively involved in substantive work leading to the manuscript, and will hold themselves jointly and individually responsible for its content.
References
- [1].Kapka-Skrzypczak L, Dudra-Jastrzębska M, Czajka M, et al. Clinical characteristics and molecular basis of skin tumors (Charakterystyka kliniczna oraz molekularne podstawy nowotworów skóry). Hygeia Public Health 2014;49:39–45. [Google Scholar]
- [2].Sokołowska-Wojdyło M, Roszkiewicz J. Primary cutaneous lymphoma (Pierwotne chłoniaki skóry). Lublin: Wydawnictwo Czelej; 2008. [Google Scholar]
- [3].Lech-Marańda E. Therapy of primary cutaneous lymphoma (Leczenie pierwotnych chłoniaków skóry). Acta Haematol Pol 2010;41:343–55. [Google Scholar]
- [4].Uzuncakmak TK, Akdeniz N, Karadag AS, et al. Primary cutaneous CD 30 (+) ALK (-) anaplastic large cell lymphoma with dermoscopic findings: a case report. Dermatol Pract Concept 2017;7:59–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chao-Lo MP, King-Ismael D, Lopez RA. Primary cutaneous CD30+ anaplastic large cell lymphoma: report of a rare case. J Dermatol Case Rep 2008;2:31–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood 2005;105:3768–85. [DOI] [PubMed] [Google Scholar]
- [7].de Oliveira LSR, Nobrega MP, Monteiro MG, et al. Primary cutaneous anaplastic large-cell lymphoma—case report. An Bras Dermatol 2013;88(6 suppl 1):132–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Oschlies I, Lisfeld J, Lamant L, et al. ALK-positive anaplastic large cell lymphoma limited to the skin: clinical, histopathological and molecular analysis of 6 pediatric cases. A report from the ALCL99 study. Haematologica 2013;98:50–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hung TY, Lin YC, Sun HL, et al. Primary cutaneous anaplastic large cell lymphoma in a young child. Eur J Pediatr 2008;167:111–3. [DOI] [PubMed] [Google Scholar]
- [10].Ishida M, Hodohara K, Yoshii M, et al. Primary cutaneous anaplastic large cell lymphoma occurring in an atopic dermatitis patient: a case report with review of the literature with emphasis on their association. Int J Clin Exp Pathol 2014;7:1735–41. [PMC free article] [PubMed] [Google Scholar]
- [11].Jepsen ME, Gniadecki R. Treatment of primary cutaneous anaplastic large cell lymphoma with superficial X-rays. Dermatol Reports 2015;7:5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Liu JF, Li B, Fan ZX, et al. Pilomatrix carcinoma on the left side of the parotid region: a case report and review of the literature. Oncol Lett 2015;10:313–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rao SD, Ravi R, Govindarajan M. Primary cutaneous CD 30+ anaplastic large cell lymphoma. Indian J Surg 2010;72(Suppl 1):283–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Barbosa L, Brito MJ, Balaco I, et al. Anaplastic cutaneous lymphoma mimicking an infection. J Radiol Case Rep 2014;8:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sokołowska-Wojdyło M, Olek-Hrab K, Ruckemann-Dziurdzińska K. Primary cutaneous lymphomas: diagnosis and treatment. Postepy Derm Alergol 2015;32:368–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kaffenberger BH, Winardi FK, Frederickson J, et al. Periocular cutaneous anaplastic large cell lymphoma clearance with brentuximab vedotin. J Clin Aesthet Dermatol 2013;6:29–31. [PMC free article] [PubMed] [Google Scholar]
- [17].Kinney MC, Higgins RA, Medina EA. Anaplastic large cell lymphoma: twenty-five years of discovery. Arch Pathol Lab Med 2011;135:19–43. [DOI] [PubMed] [Google Scholar]


