Abstract
The objective of this study is to present the long-term results and to evaluate the safety and effectiveness of the Prestige-LP cervical disc replacement in treatment of patients with symptomatic 2-level cervical degenerative disc disease.
Twenty-four patients with 48 Prestige-LP disc were analyzed before surgery and at 1 week, 3 months, 6 months, 12 months, 24 months, and 60 months after surgery. Clinical assessments included 36-Short Form (SF-36), Japanese Orthopedic Assessment (JOA), visual analog scale (VAS), and Neck Disability Index (NDI) scores. Radiographic assessments included cervical lordosis (CL), disc height (DH), range of motion (ROM) of the total cervical spine, functional spinal unit (FSU) as well as upper and lower operated segment. Complications at the 5-year follow-up were collected as well.
Mean follow-up period was 64.22 months. There was clinical improvement in terms of SF-36, JOA, NDI, and VAS from the preoperative to the final follow-up (P < .05). Overall, ROM of the total cervical spine, FSU, and upper and lower operated segment were maintained during the follow-up. Statistically significant (P < .05) improvements in the trend of CL and DH were noted at the follow-up. Eight patients were observed an appearance of heterotopic ossification at the 5-year follow-up, with 6 patients appeared at Class II and 2 patients at Class III. Adjacent segment degeneration assessed by radiographic evidence was found in 2 patients.
Two-level cervical disc arthroplasty with Prestige-LP showed significant improvement in clinical outcomes at 5 years. It not only effectively preserves the motion of both total cervical spine and operated segments, but also restores normal CL and DH up to 5 years postoperation.
Keywords: 2-level disc disease, cervical arthroplasty, clinical outcome, Prestige-LP disc replacement, radiographic outcome
1. Introduction
Cervical degenerative disc disease (CDDD), a common inducement of severe cervical disease such as radiculopathy and myelopathy, frequently occurred in C4–5, C5–6, C6–7 and usually happened to be 2-level. For decades, anterior cervical discectomy and fusion (ACDF) has been widely performed and considered as the “gold-standard” technique for the treatment of CDDD, which showed an excellent clinical results.[1–3] However, the loss of motion at the operated level, which has been hypothesized to accelerated adjacent-level disc degeneration[4–9] as well as the common complication of this procedure including screw pull-out, dysphagia and plate fracture[10] still remains unignorable for both surgeons and patients. Goffin et al[11] have reported an significant rate of 92% of the adjacent segmental degeneration occurred after 8.6 years of fusion. When it comes to 2-level or multiple-level, the intradiscal pressure and likelihood of adjacent segment degeneration (ASD) can also substantially increase.[7] This may suggest a particular role for cervical total disc replacement (TDR) when 2- or multiple-level cervical reconstructions are required.
In recent years, a large number of articles have demonstrated the clinical efficacy of TDR and consider it an alternative treatment to fusion for CDDD. The US Food and Drug Administration has also approved it for the management of single-level cervical spondylosis. Theoretically, this type of nonfusion technology can effectively preserve the motion function of corresponding segments and restore the intervertebral disc space height, indirectly reduce the abnormal biomechanics of adjacent segments and avoid complications attributed to anterior cervical plating and cervical immobilization.[12] The Prestige-LP disc is the 5th-generation disc developed from the original Bristol–Cummins disc. It has a ball-in-trough articulation design consists of 2 components made of titanium ceramic composite materials. A set of rails is used for fixation and the porous titanium coating is beneficial for bone growth. The design of the Prestige-LP also allows for unrestricted multilevel implantation. At present, this prosthesis has been used to observe the biomechanical parameters for continuous 2-level TDR and gives positive feedback. Anup et al[13] have reported the biomechanical analysis of the contiguous 2-level TDR with Prestige-LP using cadaveric specimens, concluding it not only preserve the motion at the operated levels, but also maintain the normal motion at the adjacent levels. While to our knowledge, few studies have focused on the long-term treatment of the Prestige-LP disc for contiguous 2-level CDDD. In this study, our aim is to observe the safety and efficiency of TDR with Prestige-LP for the treatment of contiguous 2-level CDDD by comparing preoperative parameters, clinical and radiographic results, hope that this study will provide spine surgeons a viable alternative when facing contiguous 2-level CDDD.
2. Materials and methods
2.1. Patients
We reviewed the records of patients who accepted surgical treatment for 2-level contiguous CDDD by a single surgeon from January 2010 to March 2012 in our institution. Ethical approval was given by the medical ethics committee of West China Hospital of Sichuan University. All patients signed an informed consent and agreed to participate in the study. All patients underwent 2-level TDR with Prestige-LP disc. Inclusion criteria were as follows: aged between 18 and 65 years old, 2 contiguous level degenerative disc diseases between C3-T1, clinical symptoms of the spinal cord or nerve root compression following part of the symptoms and signs: upper limb numbness or pain, decreased muscle strength, fine activity disorders, Hoffmann positive sign, trunk banding feeling, computed tomography (CT), myelography or magnetic resonance imaging (MRI) confirmed spinal cord or nerve root compression, preoperative conservative treatment for 6 weeks without efficacy. Exclusion criteria were as follows: osteoporosis, cervical kyphosis or structural instability, ankylosing spondylitis, rheumatoid arthritis, ossification of posterior longitudinal ligament, type 1 diabetes, cervical infection, pregnancy, metal allergy, neuromuscular disease, and history of cervical spine surgery. According to these criteria, 24 patients were enrolled in this study, including 15 males and 9 females with a mean age of 45.38 years (range: 34–55).
2.2. Surgical procedure
All operations were performed by the same senior surgeon. Under general anesthesia and tracheal intubation, the patient took a supine position with a soft cushion under the neck, the cervical spine maintained the physiological forearm and was fixed in the neutral position. A standard Smith–Robinson anterior approach to the cervical spine was undertaken, took a C-arm fluoroscopy to confirm the operated segments before discectomy and decompression was performed under a protection to esophageal, touch the dural sac can feel the tension decreases, the swelling state returns, and visible pulsation. After decompression, moderately used a burr to dispose the osteophytes so that the endplates were flat and parallel, watching out not remove the unnecessary cortical bone. Then the prostheses were planted sequentially from the cephalad disc to caudal in accordance with the PRESTIGE LP artificial intervertebral disc implantation procedure and requirements. After a verification of the proper placement through anterior–posterior and lateral fluoroscopy, a drainage tube was inserted and the incision was closed in the standard fashion.
After surgery, conventional removed the drainage tube in 1 or 2 days. Patients were encouraged to ambulate as earlier as possible together with taking physical cervical activities under the guidance of the attending physician and gradually increase the amount of it. We suggested patients to take a neck collar while taking outdoor activities and carry out rehabilitation training to strengthen the neck and back muscle function after surgery.
2.3. Outcome assessment
The collected data included patient demographic data, clinical evaluations, and radiographic evaluations. All data were collected before surgery in inpatient ward and at 1 week, 3 months, 6 months, 12 months, 24 months, and 60 months after surgery through a routine follow-up. Before operation, patients were asked to take plain radiographs, CT scans, and MRI of the cervical spine.
Clinical evaluations included patient-reported assessments such as the Japanese Orthopedic Assessment (JOA) scoring system, used to evaluate the myelopathic status; the Neck Disability Index (NDI), used to assess the neck function; the visual analog scale (VAS), used to evaluate the neck and arm pain intensity; and 36-Item Short Form Healthy Survey (SF-36) mental and physical general health surveys (mental component summary and physical component summary), used to assess the quality of life.
Radiographic evaluations included range of motion (ROM) of cervical spine, ROM of functional spinal unit (FSU) and each operated segment, cervical lordosis (CL), and disc height (DH). CL was defined as the Cobb angle in a neutral position. The ROM of cervical was measured using the Cobb angle between full flexion and extension in lateral radiographs. The ROM of FSU was defined as the sum of 2 angles formed by 2 lines drawn along the superior endplate of the cephalad vertebral body, and the inferior endplate of the caudal vertebral in full flexion and extension radiographs at the 2 operated segments. The ROM of each operated segment was defined as the angle formed preoperatively by the natural endplates and postoperatively by the shells of the prosthesis. DH was defined as the distance between the mid-point of upper and lower endplate in 1 disc space. ACDSee Canvas 14 software (ACD Systems, Victoria, Canada) was used to take all the measurements on radiographs. Other complications which could be observed through radiographs such as heterotopic ossification (HO), migration and subsidence would also be collected. HO was evaluated in accordance with the McAfee classification.[14] The assessment of ASD included the presence of any of the following radiographic parameters above or below the operated level: new anterior or enlarging osteophyte formation; increase or new narrowing of the disc space defined as ≤25% narrowing of the intervertebral disc space; and calcification of the anterior longitudinal ligament.[4,15]
Radiographic measurement data were collected from 2 trained observers, each of them measured 3 times. As for the evaluation of HO and ASD, we first tested the reliability of the assessing system in all 24 patients. Photographs were assigned to 2 observers in a random sequence at the interval of 2 weeks. Intraclass correlation (ICC) value was used to assess the intraobserver and interobserver reliability (excellent for the ICC value from 0.9 to 1, good for 0.7 to 0.89, fair for 0.5 to 0.69, low for 0.25 to 0.49, poor for 0 to 0.24). When 2 different grading results appeared in 1 patient, we chose the lower grade as the final result.
2.4. Statistical analysis
Statistical analysis was performed using SPSS software for Windows Version 19.0 (SPSS, Chicago, IL). A paired t test was used to assess the statistical significance of postoperative parameters change from the preoperative in CL, ROM, DH, VAS, JOA, NDI, and SF-36. P values of <.05 were considered statistically significant.
3. Results
3.1. Demographic and surgical data
A total of 24 patients completed the follow-up in this study, with an average follow-up period of 64.22 months (minimum 60 months). The mean blood loss was 77.73 mL. There were 15 males and 9 females in terms of gender issues. Among these patients, 15 (62.5%) presented with radiculopathy and 9 (37.5%) presented with myelopathy. The implanted levels included 16 cases of C4–C5–C6 (66.7%) and 8 cases of C5–C6–C7 (33.3%) (Table 1).
Table 1.
Patient's demographics.

3.2. Clinical evaluations
After surgery, for all of the following functional outcomes assessments, the last 5-year follow-up demonstrated statistical significant improvements compared with preoperative values and showed a trend that normal physiological function gradually improved as well as ill symptoms decreased with the time goes by.
Mean JOA improved significant over preoperative score at all postoperative follow-up time points (P < .05). Mean JOA score at 60 months was 15.4 ± 1.6 compared with preoperative score 10.3 ± 1.6. Mean NDI decreased significant over preoperative score at all postoperative follow-up (P < .05). Mean NDI score at 60 months was 9.6 ± 5.7 compared with preoperative score 26.3 ± 7.1. Mean VAS decreased significant over preoperative score at all postoperative follow-up (P < .05). Mean VAS score at 60 months was 1.9 ± 1.0 compared with preoperative score 5.3 ± 2.3. Mean SF-36 physical component summary (PCS) and mental component summary (MCS) scores improved significant over preoperative score at all postoperative follow-up (P < .05). Mean PCS and MCS at 60 months was 74.0 ± 13.5 and 81.6 ± 12.9 compared with preoperative scores 39.3 ± 13.2 and 50.8 ± 15.1, respectively. The variation tendency of above-mentioned assessments is shown in Fig. 1.
Figure 1.
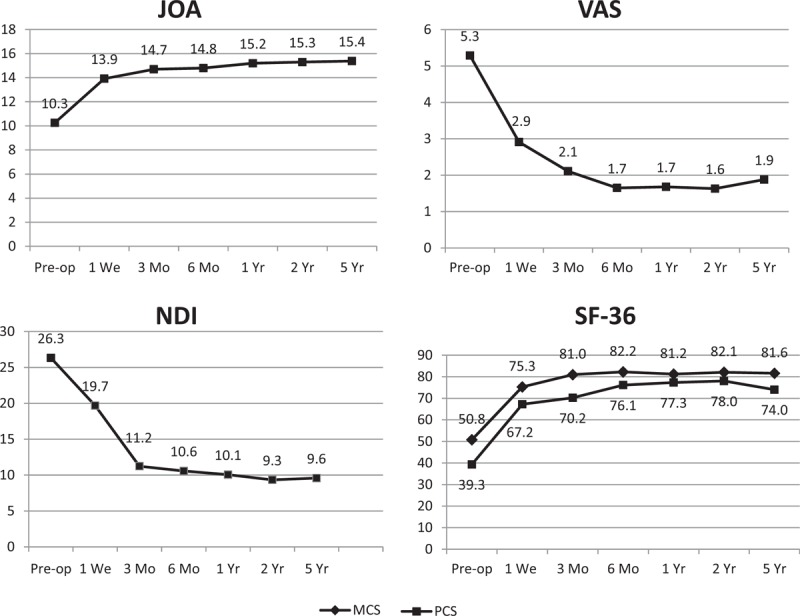
The variation tendency of Japanese Orthopedic Assessment (JOA), Neck Disability Index (NDI), visual analog scale (VAS), and 36-Short Form (SF-36: mental and physical component summary).
3.3. Radiographic evaluations
The mean CL at the each follow-up time point was 12.87° at 1 week, 11.88° at 3 months, 11.36° at 6 months, 12.46° at 12 months, 11.20° at 24 months, 10.73° at 60 months compared with 7.53° preoperatively (Table 2), with a significant increase before and after the surgery (P < .05).
Table 2.
Cervical alignment, range of motion (ROM), and disc height (DH) of the patients at each follow-up (mean ± SD).
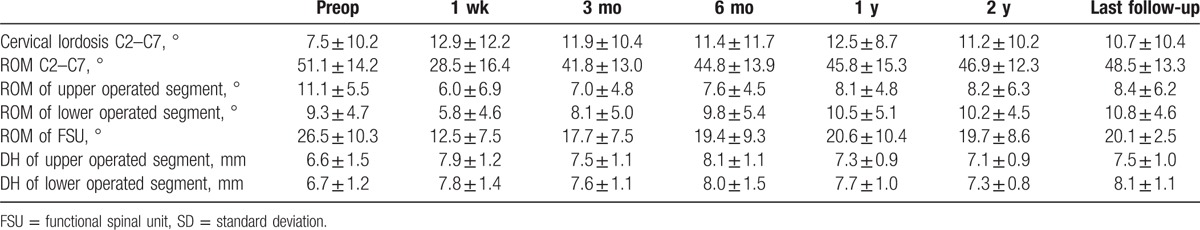
The ROM of the cervical spine was 28.49° at 1 week, 41.83° at 3 months, 44.84° at 6 months, 45.77° at 12 months, 46.91° at 24 months, 48.53° at 60 months compared with 51.12° preoperatively (Table 2). Data at 1 week and 3 months significantly decreased and after 6 months data showed no significant difference (P < .05).
The ROM of FSU was 26.50° preoperatively and 12.45° at 1 week, 17.66° at 3 months, 19.39° at 6 months, 20.58° at 12 months, 19.69° at 24 months, and 20.06° at 60 months (Table 2). Data at 1 week, 3 months, and 6 months were significantly decreased and other follow-up time points were not significantly different (P < .05).
The preoperative ROM of the upper and lower operated segments were 11.12° and 9.26°, respectively, 6.01° and 5.76° at 1 week, 7.00° and 8.11° at 3 months, 7.61° and 9.75° at 6 months, 8.12° and 10.49° at 12 months, 8.17° and 10.21° at 24 months, 8.42° and 10.78° at 60 months (Table 2). Both segments showed a significant decrease at 1 week and 3 months, after 6 months data showed no significant difference.
The DH of the upper and lower operated segments significantly increased from 6.62 and 6.71 mm preoperatively to 7.88 and 7.81 mm at 1 week, 7.52 and 7.61 mm at 3 months, 8.05 and 8.01 mm at 6 months, 7.28 and 7.74 mm at 12 months, 7.12 and 7.28 mm at 24 months, and 7.49 and 8.14 mm at 60 months (Table 2). DH increased significantly over preoperative score at all postoperative follow-up (P < .05).
3.4. Complications
During the follow-up period, infection, local hematoma, migration, or subsidence of prosthesis were not identified, no reoperation was needed for the patients. At the last follow-up, no patient reported a complication of dysphagia. In the aspect of HO, Class II was observed in 6 patients and Class III was observed in 2 patients, Class I and Class IV HO was not observed. In the Class II group, 3 (50%) patients appeared at the upper operated segment and 3 (50%) patients appeared at the lower operated segment. In the Class III group, 1 (50%) patient appeared at the upper operated segment and another (50%) patient appeared at the lower operated segment. ASD assessed by the radiographic evidence developed in 2 patients underwent C4–C5–C6 TDR and both ASD happened at the lower C6–C7 segment.
4. Discussion
For over 50 years, ACDF remains the gold standard for managing symptomatic cervical disc degenerative disease. It has been proven to have a superb clinical efficacy.[1–3] However, despite the long-standing success, the problem of ASD has long been unsatisfied. It is partly because of this reason the advent of TDR came out. When it comes to multilevel CDDD, ASD, and other complications tend to become more serious.[7] There have been various studies demonstrating the excellent clinical outcome in 1-level TDR.[16,17] However, multilevel TDR seems to have those following problems: this might add difficulty to the technique and increase the possibility of disc prostheses complications with increasing implant levels, and the cost of multilevel replacement is quite high.[18] Even some authors considered TDR a contraindication in the multilevel disease treatment.[19] While majority authors still give a positive attitude for the application of multilevel TDR.
The results of the current study showed excellent long-term clinical outcomes. Compared with preoperative parameters, JOA, NDI, VAS, and SF-36 all showed a huge improvement from immediately after surgery to 3 or 6 months postoperation, then basically maintained at a favorable level. Radiographic evaluation demonstrated that either upper or lower implanted prosthesis maintained the normal motion at the last follow-up as well as the total cervical. In order to gain a more comprehensive evaluation of the results of surgery rather than divide it into 2 separate parts, we use the testing index ROM of FSU, considering those 2 operated segments as a whole to evaluate the ROM, the results also showed a maintenance of the motion function in those 2 operated segments compared with preoperative motion.
In our study, we observed a correction of the CL from the preoperative 7.53° changed to 10.73° at the last follow-up period, the difference is significant (P < .05). This is important since there have been many reports of segmental kyphosis after the use of Byran disc even the total cervical alignment is still fine, suggesting that the remaining nonoperative segments were compensated for the lordosis.[20,21] Chan et al[22] reported in their study that the lordotic segmental alignment was preserved after Prestige-LP insertion (14° lordotic at 6 months and 13° at 2 years postoperation). The postoperative segmental kyphosis would promote ASD in ACDF has been informed.[23] Although whether this kind of segmental kyphosis in TDR can lead to an ending of ASD or not is still unclear, we can consider that segmental lordosis would have a more positive influence than kyphosis, because longstanding cervical kyphosis can produce myelopathy with resultant permanent damage to the spinal cord.[24] So we can say Prestige-LP potentially plays a role in the prevention of ASD through preserve or correct the segmental lordosis.
In the current study, there was no evidence of device-related complications such as implant failures, migration/dislocations, or subsidence. Chan et al conducted a prospective study of a sample of 40 patients with 59 prostheses demonstrated a zero happening of adverse event and concluded that this method of rail fixation was feasible and has the advantage of eliminating the anterior profile of the device as well as allowing for multiple implantation.[22] At the last follow-up, no dysphagia was observed among our patients; however, according to a former study, 2-level surgery was associated with a higher incidence of dysphagia, while TDR with Prestige-LP can significantly reduce both transient and persistent postoperative dysphagia compared with ACDF.[25]
The DH also showed a significant increase after operation till the last follow-up. The postoperative DH may have a influence in the ROM, the main purpose of TDR is to restore the height of the intervertebral space and to maintain operated segments mobility, if the postoperative intervertebral DH repair is insufficient, it will limit the postoperative operated segments activity; while if the postoperative DH is too large, it will otherwise lead to a longstanding high tension of the facet joint capsule of the corresponding segments and other soft tissue, even facet joint subluxation may occur, and that can also result in a decrease of the ROM.[26] Peng et al[27] observed the relationship between postoperative DH and ROM with the ProDisc-C prosthesis and draw a conclusion that the optimal postoperative DH range to maximize ROM is between 5 and 7 mm.
Occurrence of HO is an evitable postoperative complication after cervical TDR, which is contrary to the fundamental goal of TDR.[27,28] In our study, we observed 8 occurrences with 6 grade II examples and 2 grade III examples. A total occurrence rate was 33.3%. Yi et al[29] reported that a total occurrence of HO was 40.5% in TDR, occurrence varied from different prosthesis types with 21.0% in Byran disc, 52.5% in Mobi-C, and 71.4% in ProDisc-C. Most of the patients (78.3%) were classified as grade I and II. This result was similar to our observation as an occurrence of 75.0% of the HO in grade I and II. While we find all ossification occurred at the posterior location which were different from a former study.[30] We think probably using different type prosthesis and the intervention of the anterior vertebral in the surgery can explain this phenomenon.
In our study, we found 2 cases of ASD with an appearance of new anterior osteophyte formation in radiographs at the last follow-up. The total ASD occurrence is 8.33%, and no surgical intervention was needed in these 2 patients due to symptomatic ASD. Long-term outcome data suggest that in patients who underwent ACDF, 25.6% of them would have new disease at an adjacent segment with 10 years after the operation.[7] More recently, Goffin et al[11] demonstrated a 6.11% reoperation rate due to symptomatic ASD after at least 5-year follow-up. Comparing all the previous data of the ASD after ACDF, we may draw a conclusion that TDR may provide the benefits of neural decompression without the drawback of placing adjacent segments at risk for accelerated degeneration.[24]
The current study is limited by its retrospective nature, lack of a control group and relatively small sample size. In the future, a large-sample, prospective randomized controlled study will be required to ascertain the cervical disc arthroplasty with Prestige-LP as an optimal surgical choice for contiguous 2-level CDDD.
5. Conclusion
The use of Prestige-LP implantation in the treatment of contiguous 2-level cervical disc degeneration disease is safe and effective. It maintains that the physiologic motion at 5 years after operation with satisfactory clinical outcomes and a relatively low occurrence and degree of adjacent degeneration comparing to ACDF. Hence, for the patients of contiguous 2-level cervical disc degeneration disease, we may consider TDR with Prestige-LP as a feasible alternative procedure.
Footnotes
Abbreviations: ACDF = anterior cervical discectomy and fusion, ASD = adjacent segment degeneration, CDDD = cervical degenerative disc disease, CL = cervical lordosis, CT = computed tomography, DH = disc height, FSU = functional spinal unit, HO = heterotopic ossification, JOA = Japanese Orthopedic Assessment, MRI = magnetic resonance imaging, NDI = Neck Disability Index, ROM = range of motion, TDR = total disc replacement, VAS = visual analog scale.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Yue WM, Brodner W, Highland TR. Long-term results after anterior cervical discectomy and fusion with allograft and planting: a 5 to 11-year radiologic and clinical follow-up study. Spine 2005;30:2138–44. [DOI] [PubMed] [Google Scholar]
- [2].Epstein NE. Anterior cervical diskectomy and fusion without plate instrumentation in 178 patients. J Spinal Disord 2000;13:1–8. [DOI] [PubMed] [Google Scholar]
- [3].Thorell W, Cooper J, Hellbusch L, et al. The long-term clinical outcomes of patients undergoing anterior cervical discectomy with and without intervertebral bone graft placement. Neurosurgery 1998;43:268–73. [DOI] [PubMed] [Google Scholar]
- [4].Robertson JT, Papadopoulos SM, Traynelis VC. Assessment of adjacent-segment disease in patients treated with cervical fusion or arthroplasty: a prospective 2-year study. J Neurosurg Spine 2005;3:417–23. [DOI] [PubMed] [Google Scholar]
- [5].Eck JC, Humphreys SC, Lim TH, et al. Biomechanical study on the effect of cervical spine fusion on adjacent-level intradiscal pressure and segmental motion. Spine 2002;27:2431–4. [DOI] [PubMed] [Google Scholar]
- [6].Matsunaga S, Kabayama S, Yamamoto T, et al. Strain on intervertebral discs after anterior cervical decompression and fusion. Spine 1999;24:670–5. [DOI] [PubMed] [Google Scholar]
- [7].Hilibrand AS, Carlson GD, Palumbo MA, et al. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg Am 1999;81:519–28. [DOI] [PubMed] [Google Scholar]
- [8].Wigfield C, Gill S, Nelson R, et al. Influence of an artificial cervical joint compared with fusion on adjacent-level motion in the treatment of degenerative cervical disc disease. J Neurosug (Spine 1) 2002;96:17–21. [DOI] [PubMed] [Google Scholar]
- [9].Baba H, Furusawa N, Imura S, et al. Late radiographic findings after anterior cervical fusion for spondylotic myeloradiculopathy. Spine 1993;18:2167–73. [DOI] [PubMed] [Google Scholar]
- [10].Kaiser MG, Haid RW, Jr, Subach BR, et al. Anterior cervical plating enhances arthrodesis after discectomy and fusion with cortical allograft. Neurosurgery 2002;50:229–36. [DOI] [PubMed] [Google Scholar]
- [11].Goffin J, Geusens E, Vantomme N, et al. Long-term follow-up after interbody fusion of the cervical spine. Paper presentation at the 28th Annual Meeting of the Cervical Spine Research Society in Charleston, SC, 2000. [Google Scholar]
- [12].Hisey MS, Bae HW, Davis RJ, et al. Prospective, randomized comparison of cervical total disc replacement vs. anterior cervical fusion: results at 48 months follow-up. J Spinal Disord Tech 2015;28:E237–43. [DOI] [PubMed] [Google Scholar]
- [13].Anup AG, Swathi K, Nicole A, et al. Biomechanical analysis of cervical disc replacement and fusion using single level, two level, and hybrid constructs. Spine 2015;40:1578–85. [DOI] [PubMed] [Google Scholar]
- [14].McAfee PC, Cunningham BW, Devine J, et al. Classification of heterotopic ossification (HO) in artificial disk replacement. J Spinal Disord Tech 2003;16:384–9. [DOI] [PubMed] [Google Scholar]
- [15].Kim SW, Limson MA, Kim SB, et al. Comparison of radiographic changes after ACDF versus Bryan disc arthroplasty in single and bi-level cases. Eur Spine J 2009;18:218–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Rabin D, Bertagnoli R, Wharton N, et al. Sagittal balance influences range of motion: an in vivo study with the ProDisc-C. Spine J 2009;9:128–33. [DOI] [PubMed] [Google Scholar]
- [17].Ahn PG, Kim KN, Moon SW, et al. Changes in cervical range of motion and sagittal alignment in early and late phases after total disc replacement: radiographic follow-up exceeding 2 years. J Neurosurg Spine 2009;11:688–95. [DOI] [PubMed] [Google Scholar]
- [18].Mao NF, Wu JH, Zhang Y, et al. A comparison of anterior cervical corpectomy and fusion combined with artificial disc replacement and cage fusion in patients with multilevel cervical spondylotic myelopathy. Spine 2015;40:1277–83. [DOI] [PubMed] [Google Scholar]
- [19].Auerbach JD, Jones KJ, Fras CI, et al. The prevalence of indications and contraindications to cervical total disc replacement. Spine J 2008;8:711–6. [DOI] [PubMed] [Google Scholar]
- [20].Shim CS, Lee SH, Park HJ, et al. Early clinical and radiologic outcomes of cervical arthroplasty with Bryan cervical disc prosthesis. J Spinal Disord Tech 2006;19:465–70. [DOI] [PubMed] [Google Scholar]
- [21].Johnson JP, Lauryssen C, Cambron HO, et al. Sagittal alignment and the Bryan cervical artificial disc. Neurosurg Focus 2004;17:E14. [DOI] [PubMed] [Google Scholar]
- [22].Chan WB, Wai MY, Abdul B, et al. Intermediate results of the Prestige LP cervical disc replacement. Spine 2011;36:105–11. [DOI] [PubMed] [Google Scholar]
- [23].Katsuura A, Hukuda S, Saruhashi Y, et al. Kyphotic malalignment after anterior cervical fusion is one of the factors promoting the degenerative process in adjacent intervertebral levels. Eur Spine J 2001;10:320–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Frech RD, Shad A, Cadoux-Hudson TA, et al. Anterior correction of cervical kyphosis deformity: effect on myelopathy, neck pain, and sagittal alignment. J Neurosurg 2004;100:13–9. [DOI] [PubMed] [Google Scholar]
- [25].Yang Y, Ma L, Liu H, et al. Comparison of the incidence of patient-reported post-operative dysphagia between ACDF with a traditional anterior plate and artificial cervical disc replacement. Clin Neurol Neurosurg 2016;148:72–8. [DOI] [PubMed] [Google Scholar]
- [26].Liu JY, Ebraheim NA, Haman SP, et al. How the increase of the cervical disc space height affects the facet joint: an anatomy study. Spine 2006;31:E350–4. [DOI] [PubMed] [Google Scholar]
- [27].Peng CW, Qurino M, Bendo JA, et al. Effect of intervertebral disc height on postoperative motion and clinical outcomes after ProDisc-C cervical disc replacement. Spine J 2009;9:551–5. [DOI] [PubMed] [Google Scholar]
- [28].Leung C, Casey AT, Goffin J, et al. Clinical significance of heterotopic ossification in cervical disc replacement: a prospective multicenter clinical trail. Neurosurgery 2005;57:759–63. [DOI] [PubMed] [Google Scholar]
- [29].Yi S, Kim NY, Yang MS, et al. Difference in occurrence of heterotopic ossification according to prosthesis type in the cervical artificial disc replacement. Spine 2010;35:1556–61. [DOI] [PubMed] [Google Scholar]
- [30].Yi S, Ahn PG, Kim DH, et al. Cervical artificial disc replacement: part 2: clinical experience with the cervical artificial disc. Neurosurg Quart 2008;18:96–103. [Google Scholar]


