Abstract
Background:
In recent years, an increasing number of studies has been published analyzing the possible prognostic utility of glypican-3 (GPC3) in hepatocellular carcinoma (HCC), but the results are still controversial. The aim of this meta-analysis was to evaluate possible association between GPC3 expression and patients’ survival.
Methods:
Relevant publications which assessed GPC3 expression with survival outcome in HCC patients were searched from Pubmed, Embase, Web of Science, and the Cochrane library. Survival outcome (odds ratios or hazard ratios) was synthesized with a fixed or random effects meta-analysis. Publication bias and sensitivity analyses were also conducted. Statistical analysis was performed by STATA 12.0 and Review Manager software 5.3.
Results:
Fifteen studies including 2336 HCC cases were analyzed systematically in our meta-analysis. The main results showed that GPC3 high expression was significantly associated with later tumor stage, higher tumor grade, presence of vascular invasion, shortened overall survival, and disease-free survival. Subgroup analyses for GPC3 on HCC overall survival according to the studies categorized by sample size, follow-up period, and cut-offs were also conducted.
Conclusion:
Our findings suggested that GPC3 may play a role in cancer invasion and progression and may be related to poor prognosis of HCC. Further mechanical research or multicenter cohort studies are needed to confirm these findings.
Keywords: GPC3, HCC, meta-analysis, prognosis
1. Introduction
Based on the GLOBOCAN 2012 estimates, hepatocellular carcinoma (HCC) has become the most common primary liver cancer, ranking the 2nd-leading cause of cancer-related deaths worldwide.[1] Despite the progression in treatments for HCC, such as liver transplantation, surgical resection, liver directed therapy, and systemic therapy, the prognosis of HCC is still poor. The clinical treatment difficulties are mainly due to a lack of full understanding of HCC pathogenesis and effective biomarkers that predict HCC. Therefore, an in-depth exploration into new genes or molecules that are highly associated with HCC progression and prognosis is urgently needed for developing novel therapies and improving the survival outcome of HCC patients.
Glypican-3 (GPC3) is a type of glycosylphosphatidylinositol-anchored cell-surface heparin-sulfate proteoglycan[2] that has been repeatedly reported to be highly and selectively expressed in HCC patients.[3] An increasing number of studies have suggested that GPC3 plays crucial roles in cell proliferation and tumor suppression, contributing to the progression and metastasis of HCC patients.[4] It has been shown that GPC3 stimulated the growth of HCC cells and regulated migration, adhesion in tumor cells by activating autocrine/paracrine canonical Wnt signaling.[5,6] Recently, there is a growing interest in the possible prognostic utility of GPC3 in HCC, but the results of different studies with regard to disease-free survival (DFS) and/or overall survival (OS) are still controversial.
Meta-analysis is a quantitative statistical method that combine the data of different studies with the same theme, so as to give a more convincing conclusion.[7] Xiao et al[8] performed a meta-analysis previously to explore the association of GPC3 with DFS and overall survival (OS) in HCC patients. However, this meta-analysis included only 8 studies for estimating overall survival outcome. Studies published most recently which discussed the association between GPC3 and HCC prognosis in different populations have not been enrolled,[9–15] restricting the statistical power of meta-analyses. Therefore, we performed a meta-analysis that included the most updated data to gain a better insight about the direct relationship between expressions of GPC3 and patients’ survival statuses. Furthermore, we also tried to investigate the correlations of GPC3 expressions with patients’ clinical characteristics. To our knowledge, this is the most comprehensive meta-analysis regarding the association between GPC3 and HCC prognosis.
2. Materials and methods
2.1. Literature search
Pubmed database, Web of Science, the Cochrane library, and Embase database (updated to January 31, 2017) were searched systematically to identify studies concerning the association between GPC3 and HCC prognosis. The languages were limited to English. The search syntax was ((“glypican-3”[All Fields] OR “GPC3”[All Fields]) AND (“hepatocellular carcinoma” [All Fields] OR “HCC”[All Fields]) AND (“prognosis”[All Fields] OR “prognostic”[All Fields]) OR “survival”[All Fields])). The reference list of potential studies was searched manually for eligibility.
A study was included if it met the following inclusion criteria: full text publication evaluated the association between the expressions of GPC3 and overall survival (OS) and/or DFS in HCC; hazard ratios (HRs) and their 95% confidence intervals (95% CIs) for OS or DFS could be presented or calculated from the data; sample size had to be greater than 20; and GPC3 expression should be measured by immunohistochemistry. Reviews, conference abstracts, or comments with insufficient information from authors were excluded.
2.2. Data extraction and quality evaluation
All data were extracted independently by 2 reviewers according to the inclusion criteria. In case of disagreement, a 3rd author made a final decision for the discrepancy. The following information was extracted from each study: first author, year of publication, country, number of patients, numbers of different clinicopathological parameters, cut-off, follow-up period, and HR with 95% CI. If no HR and 95% CI were provided directly, it was digitized and extracted from the results of Kaplan–Meier curve using GetData Graph Digitizer 2.24 (http://getdata-graph-digitizer.com). No ethical approval and consent from patients are required as all analyses were based on previous published studies.
The Newcastle–Ottawa scale was used for evaluating the quality of included studies. The concrete content consists of the following items: patient selection, comparability, and ascertainment of outcome. Each item could be awarded 1 point except for the item on comparability, which is awarded 2 points. Studies were evaluated as low quality when scores were 0 to 4; those with scores of 5 to 9 were considered high quality.[16]
2.3. Statistical analysis
RevMan 5.3 software (Cochrane Collaboration, Oxford, UK) and STATA 12.0 (StataCorp LP, College Station, TX) were applied to conduct the meta-analysis. I2 statistic was used to show the heterogeneity between studies. When there was obvious heterogeneity between studies (I2 ≥ 50%), the random effect model was used; otherwise, the fixed effect model should be used. Odds ratios (ORs) with 95% CI were used to estimate the associations between GPC3 and clinicopathological parameters for HCC, including HBV/HCV status, Child–Pugh class, tumor size, tumor multifocality, histologic grade, stage, and tumor vascular invasion. The HR with its variance estimates (95% CI) was extracted or calculated to evaluate the correlations between GPC3 and HCC survival outcome. Sensitivity analysis was performed to assess the stability of the results by deleting 1 study each time to explore the influence of the individual data on the pooled HR.[17] Begg funnel plots and Egger linear regression method were used to test the publication bias.[18] All statistical tests were 2-sided and P < .05 was considered as statistically significant.
3. Results
3.1. Studies selection process and characteristics
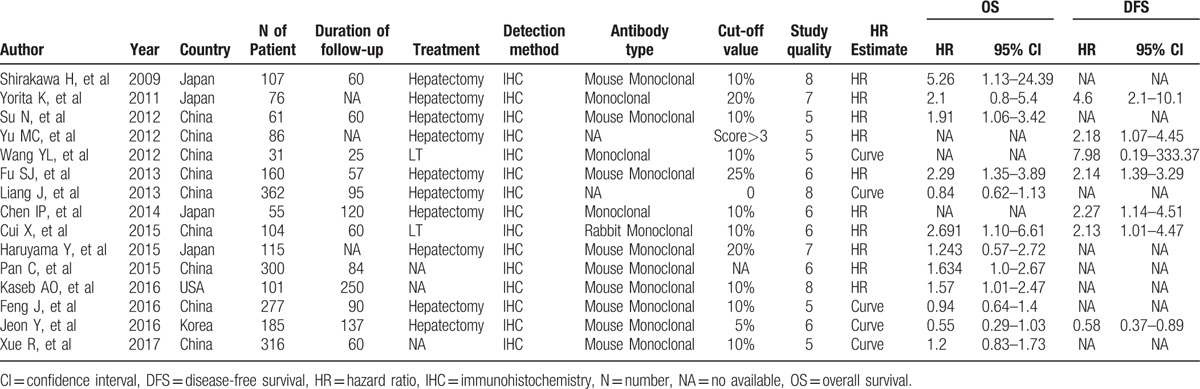
We identified 162 relevant citations with regard to the association between GPC3 and HCC after the initial literature search. Sixty-seven articles were excluded after the first screening based on titles or abstracts, since they were reviews, abstracts, letters to editor, animal/in vitro studies, and duplications. The remaining 95 studies were selected for further evaluation. After reading the full-text articles, 80 articles were excluded for not relevant to the current topic or lacking sufficient survival data. As a result, 15 eligible studies published between 2009 and 2017 met the inclusion criteria and were included in our meta-analysis. The 15 selected studies, which originated from 4 countries (USA, China, Japan, and Korea), included 2336 HCC cases. OS was presented in 12 studies, while DFS was reported in 7 studies. All the studies detected GPC3 by immunohistochemistry. The scores of study quality assessed by Newcastle–Ottawa quality assessment scale ranged from 5 to 8. The characteristics of studies enrolled were shown in Table 1.
Table 1.
Characteristics of studies enrolled.
3.2. Quantitative data synthesis
3.2.1. GPC3 and clinicopathological features of HCC patients
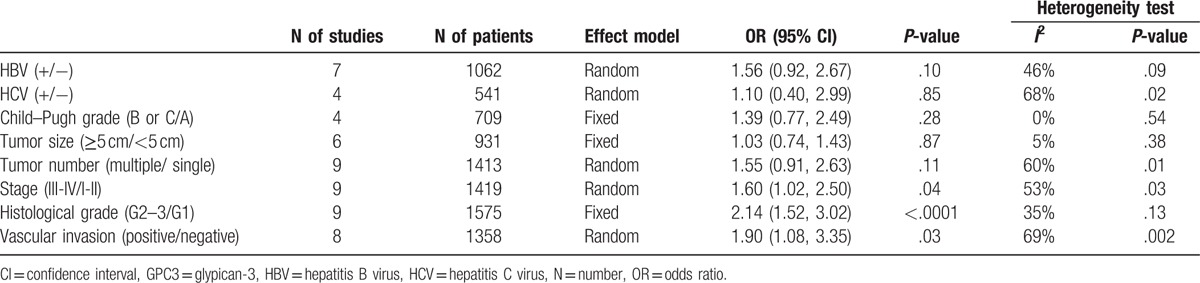
Several studies evaluated associations between GPC3 high expression and infection of HBV or HCV, Child–Pugh grade, tumor size, tumor number, stage, histological grade, and vascular invasion. Our analyses suggested that GPC3 high expression was significantly associated with later tumor stage (OR = 1.60, 95% CI: 1.02–2.50, P = .04), higher tumor grade (OR = 2.14, 95% CI: 1.52–3.02, P < .001), and presence of vascular invasion (OR = 1.90, 95% CI: 1.08–3.35, P = 3.35) (Table 2).
Table 2.
Association between GPC3 high expression and clinicopathological features.
3.2.2. GPC3 and OS in HCC
OS was reported in 12 studies with a total of 2164 HCC patients. Since heterogeneity is obvious in the study (I2 = 65.3%, P = .001), pooled HR was calculated by the random effect model. As a result, it was demonstrated that a significant association existed between high GPC3 expression and lower OS with a pooled HR of 1.38 (95% CI: 1.05–1.80, P = .02) (Fig. 1).
Figure 1.
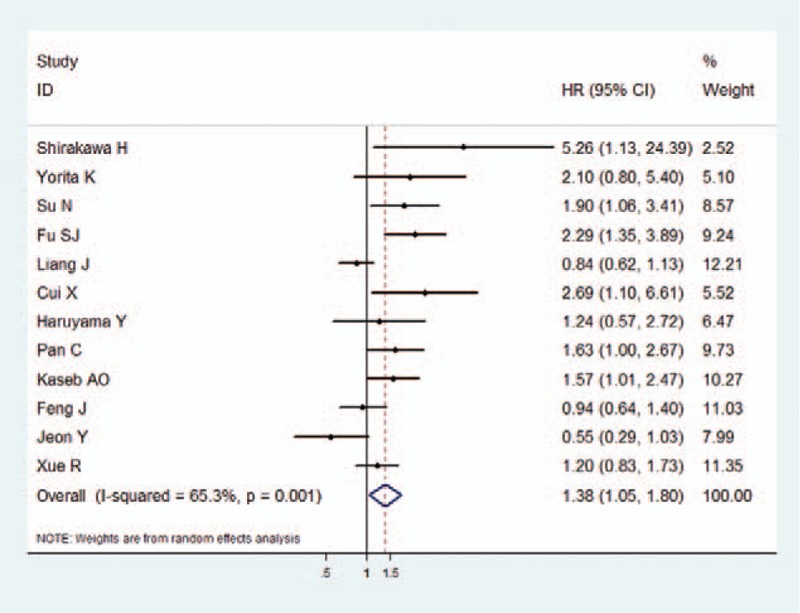
The association between GPC3 overexpression and overall survival of HCC. The summary HR and 95% CIs were shown. CI = confidence interval, GPC3 = glypican-3, HCC = hepatocellular carcinoma, HR = hazard ratio.
3.2.3. GPC3 and DFS in HCC
The data for DFS were reported in 7 studies. A random effect model was used in the meta-analysis, as there was significant heterogeneity in the data (I2 = 81.4%, P < .001). The pooled analyses for DFS were similar to that for OS, showing that high GPC3 expression was associated with poor DFS (HR = 1.98, 95% CI: 1.08–3.62, P = .027) (Fig. 2).
Figure 2.
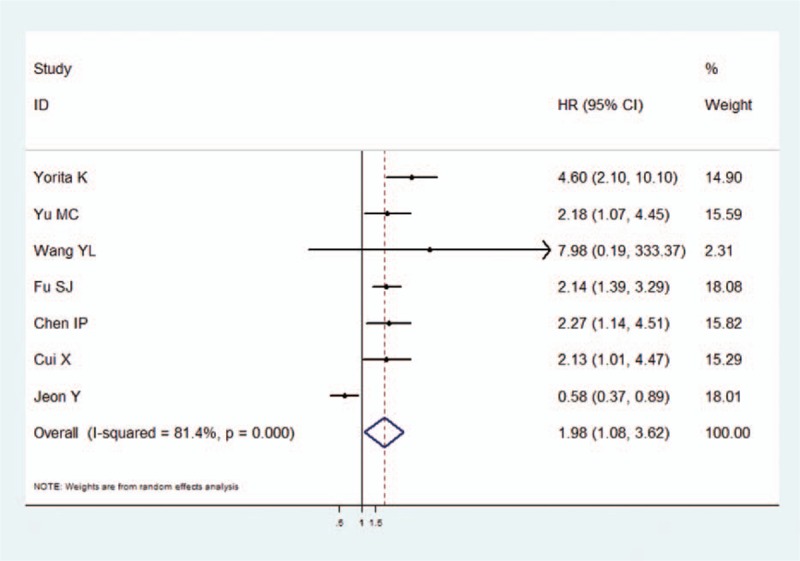
The association between GPC3 overexpression and disease-free survival of HCC. The summary HR and 95% CIs were shown. CI = confidence interval, GPC3 = glypican-3, HCC = hepatocellular carcinoma, HR = hazard ratio.
3.3. Subgroup analysis
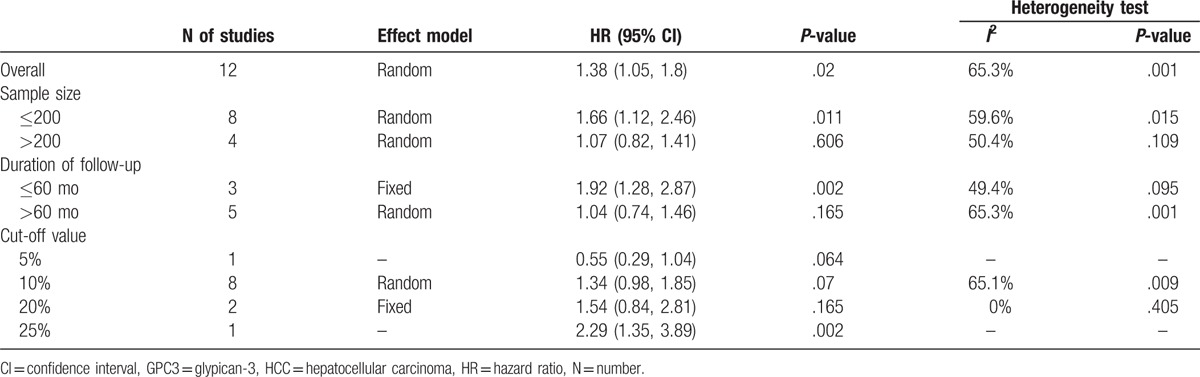
Subgroup analyses for the association between GPC3 and OS, based on sample size, follow-up period, and cut-offs, were conducted (Table 3). In the subgroup analysis according to sample size, the combined HR of the studies with 200 or fewer cases was 1.66 (95% CI: 1.12–2.46, P = .015). However, the combined HR was 1.07 (95% CI: 0.82–1.41, P = .109) based on studies with more than 200 cases. When aggregating the studies separately according to the follow-up period, the association between GPC3 and poor OS was found only for studies of shorter follow-up period (≤60 months) (HR = 1.92, 95% CI: 1.28–2.87, P = .002). Furthermore, subgroup analysis was also performed to evaluate whether the pooled estimate of OS was different according to the GPC3 cut-off values reported in the included studies.
Table 3.
Subgroup analyses for GPC3 on HCC overall survival.
3.4. Publication bias and sensitivity analyses
Test of publication bias in our meta-analysis was performed using Begg funnel plot and Egger regression method. In all enrolled studies, there was no asymmetry observed in funnel plot, with P = .05 in the Egger test (Fig. 3), indicating no evidence of significant publication bias. For sensitivity analyses, we omitted 1 study per time to check if individual study affected the final results. All the results were not materially altered.
Figure 3.
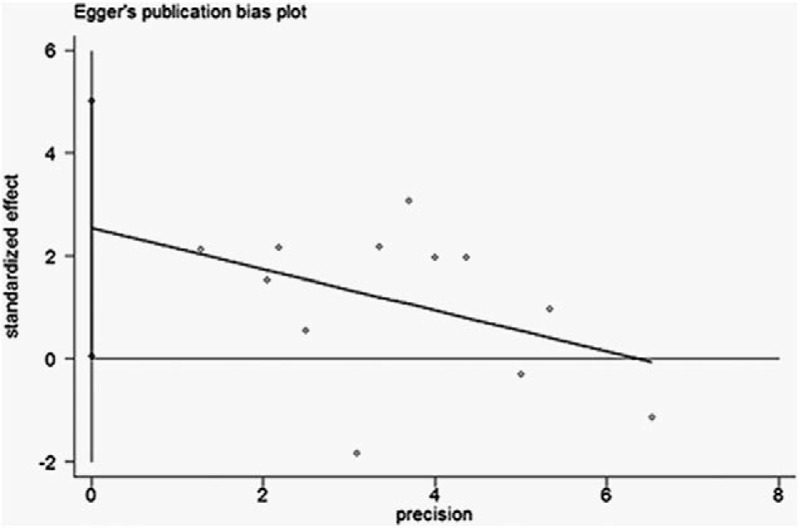
Funnel plots of Egger to detect publication bias on overall estimate.
4. Discussion
Current studies have indicated that GPC3 expression was closely related to proliferation, invasion, and progression of HCC.[19–21] Nevertheless, the results from different published studies were inconsistent, so the prognostic value of GPC3 in HCC is still unclear. Thus, the present meta-analysis was performed to comprehensively analyze all of the available researches which compared the survival outcomes of HCC patients according to expression status of GPC3. To date, as we know, this study is the most comprehensive meta-analysis exploring the prognostic value of GPC3 in HCC.
Compared with the previous meta-analysis by Xiao, we analyzed 15 studies with a total of 2336 patients, doubling the cases compared to Xiao's study. With respect to the correlations between GPC3 and clinicopathological features, the present study showed that high expression of GPC3 was significantly associated with higher tumor grade, later tumor stage, and presence of vascular invasion, while ORs for infection of HBV/HCV, Child–Pugh grade, tumor size, or tumor number were not significant, indicating that GPC3 high expression may be a marker of invasiveness in HCC. Our results are in line with that of basic studies, which have reported that overexpression of GPC3 in HCC could promote the in vivo and in vitro growth of HCC by stimulating canonical Wnt signaling, FGF activity, and insulin growth factor signaling pathway.[21–24] Whereas, suppression of GPC3 in HCC cells overexpressing GPC3 inhibited cell proliferation associated with an increase in phosphorylation of SMAD2/3 and also arrested cell cycle progression at the G1 phase.[21] Furthermore, the main results showed promising prognostic value of GPC3 detected in tumor samples for both OS and DFS of HCC. Patients with elevated GPC3 expression had 1.38 times higher risk of poor overall survival and 1.98 times higher risk of poor DFS, compared with those with low GPC3 expression. However, the pooled HR for OS was lower than that in Xiao's study. Several reasons may explain this discrepancy. First of all, our meta-analysis enrolled more studies than Xiao's, which may be more powerful statistically and more reliable to draw conclusions. Second, our meta-analysis included populations from more regions with diverse backgrounds than previous meta-analysis, leading to more conclusive results. Therefore, although elevated GPC3 expression showed positive association with poorer HCC survival in our study, the exact prognostic value of GPC3 in clinical applications needed to be discussed.
Significant heterogeneity was detected in this meta-analysis for OS and DFS, so stratified subgroup analyses for GPC3 on OS were also performed to increase the homogeneity. When follow-up period was taken into account, we found that the association was significant for studies with follow-up period ≤60 months, while no significant association was observed for studies with longer follow-up period (>60 months), indicating that the GPC3 expression status might be more valuable on predicting short-term outcome of HCC. Nevertheless, significant association between high GPC3 and poor prognosis was only found in the studies with 200 or fewer cases, implying that the prognostic significance of GPC3 in HCC was mainly based on the results of 8 small-sample studies. Therefore, more large-sample studies are needed to verify the exact value of GPC3 for predicting HCC overall survival.
There are limitations in our meta-analysis that should be acknowledged. First of all, an important limitation was publication bias. In this analysis, although publication bias was absent statistically, the possibility of publication bias may still exist since only fully published studies and studies in English were included. Besides, potential source of bias may be related to the method of extrapolation of HR. Some survival outcomes calculated from survival curves may have introduced some imprecision. Second, there was no consistent standard for cut-off values in our eligible studies, so subgroup analysis was performed according to the GPC3 cut-off values. However, due to a lack of sufficient data in some subgroups, we were unable to determine the optimized cut-off value for predicting OS. Third, because of the limited number of included studies, we did not perform subgroup analyses based on treatments, types of primary antibody, or laboratory infrastructure. All above factors might result in confounding bias. Therefore, large sample and multicenter RCTs are still needed.
In conclusion, despite the above limitations, our meta-analysis found that GPC3 was significantly associated with poor prognosis of HCC based on currently obtained data. Particularly, GPC3 may play a role in cancer invasion and progression. However, our results need to be confirmed by adequately designed prospective studies which further evaluate the relationship between GPC3 and the prognosis of patients with HCC.
Acknowledgments
The authors thank the Natural Science Foundation of China (81672882 and 81502441) and the Science and Technology Support Program of Sichuan Province (2017SZ0003, 2014SZ0039, 2014SZ0002-4, and 2017SZ0151) for the support.
Footnotes
Abbreviations: DFS = disease-free survival, GPC3 = glypican-3, HCC = hepatocellular carcinoma, HR = hazard ratio, OR = odds ratio.
Funding/support: This work was supported by grants from the Natural Science Foundation of China (81672882 and 81502441) and the Science and Technology Support Program of Sichuan Province (2017SZ0003, 2014SZ0039, 2014SZ0002-4, and 2017SZ0151).
The authors have no conflicts of interest to disclose.
References
- [1].Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- [2].Filmus J. The contribution of in vivo manipulation of gene expression to the understanding of the function of glypicans. Glycoconj J 2002;19:319–23. [DOI] [PubMed] [Google Scholar]
- [3].Filmus J, Capurro M. Glypican-3: a marker and a therapeutic target in hepatocellular carcinoma. FEBS J 2013;280:2471–6. [DOI] [PubMed] [Google Scholar]
- [4].Roncalli M, Borzio M, Di Tommaso L. Hepatocellular dysplastic nodules. Hepatol Res 2007;37(Suppl 2):S125–34. [DOI] [PubMed] [Google Scholar]
- [5].Pan Z, Chen C, Long H, et al. Overexpression of GPC3 inhibits hepatocellular carcinoma cell proliferation and invasion through induction of apoptosis. Mol Med Rep 2013;7:969–74. [DOI] [PubMed] [Google Scholar]
- [6].Gao W, Kim H, Feng MQ, et al. Inactivation of Wnt signaling by a human antibody that recognizes the heparan sulfate chains of glypican-3 for liver cancer therapy. Hepatology 2014;60:576–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhang Y, Zhang J, Zeng L, et al. The -2518A/G polymorphism in the MCP-1 gene and tuberculosis risk: a meta-analysis. PloS One 2012;7:e38918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Xiao WK, Qi CY, Chen D, et al. Prognostic significance of glypican-3 in hepatocellular carcinoma: a meta-analysis. BMC Cancer 2014;14:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cui X, Li Z, Gao P-J, et al. Prognostic value of glypican-3 in patients with HBV-associated hepatocellular carcinoma after liver transplantation. Hepatobiliary Pancreat Dis Int 2015;14:157–63. [DOI] [PubMed] [Google Scholar]
- [10].Feng J, Zhu R, Chang C, et al. CK19 and glypican 3 expression profiling in the prognostic indication for patients with HCC after surgical resection. PloS One 2016;11:e0151501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Haruyama Y, Yorita K, Yamaguchi T, et al. High preoperative levels of serum glypican-3 containing N-terminal subunit are associated with poor prognosis in patients with hepatocellular carcinoma after partial hepatectomy. Int J Cancer 2015;137:1643–51. [DOI] [PubMed] [Google Scholar]
- [12].Jeon Y, Kim H, Jang ES, et al. Expression profile and prognostic value of glypican-3 in post-operative South Korean hepatocellular carcinoma patients. APMIS 2016;124:208–15. [DOI] [PubMed] [Google Scholar]
- [13].Pan C, Wang X, Chen W, et al. Reevaluation of glypican-3 as a prognostic marker in HCC using X-tile software. Med Oncol 2015;32:359. [DOI] [PubMed] [Google Scholar]
- [14].Xue R, Feng J, Meng Q, et al. The significance of glypican-3 expression profiling in the tumor cellular origin theoretical system for hepatocellular carcinoma progression. J Gastroenterol Hepatol 2017;32:1503–11. [DOI] [PubMed] [Google Scholar]
- [15].Kaseb AO, Hassan M, Lacin S, et al. Evaluating clinical and prognostic implications of Glypican-3 in hepatocellular carcinoma. Oncotarget 2016;7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fugang W, Zhaopeng Y, Meng Z, et al. Long-term outcomes of laparoscopy vs. open surgery for colorectal cancer in elderly patients: a meta-analysis. Mol Clin Oncol 2017;7:771–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med 1997;127:820–6. [DOI] [PubMed] [Google Scholar]
- [18].Begg CB. A comparison of methods to detect publication bias in meta-analysis by P. Macaskill, S. D. Walter and L. Irwig. Statistics in Medicine 2001;20:641–54. Stat Med. 2002;21(12):1803. [DOI] [PubMed] [Google Scholar]
- [19].Capurro MI, Xiang YY, Lobe C, et al. Glypican-3 promotes the growth of hepatocellular carcinoma by stimulating canonical Wnt signaling. Cancer Res 2005;65:6245–54. [DOI] [PubMed] [Google Scholar]
- [20].Zittermann SI, Capurro MI, Shi W, et al. Soluble glypican 3 inhibits the growth of hepatocellular carcinoma in vitro and in vivo. Int J Cancer 2010;126:1291–301. [DOI] [PubMed] [Google Scholar]
- [21].Sun CK, Chua MS, He J, et al. Suppression of glypican 3 inhibits growth of hepatocellular carcinoma cells through up-regulation of TGF-beta2. Neoplasia 2011;13:735–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gauglhofer C, Sagmeister S, Schrottmaier W, et al. Up-regulation of the fibroblast growth factor 8 subfamily in human hepatocellular carcinoma for cell survival and neoangiogenesis. Hepatology 2011;53:854–64. [DOI] [PubMed] [Google Scholar]
- [23].Galli A, Roure A, Zeller R, et al. Glypican 4 modulates FGF signalling and regulates dorsoventral forebrain patterning in Xenopus embryos. Development 2003;130:4919–29. [DOI] [PubMed] [Google Scholar]
- [24].Cheng W, Tseng CJ, Lin TT, et al. Glypican-3-mediated oncogenesis involves the insulin-like growth factor-signaling pathway. Carcinogenesis 2008;29:1319–26. [DOI] [PMC free article] [PubMed] [Google Scholar]


