Abstract
Rationale:
Low-grade myofibroblastic sarcoma (LGMS) is a rare mesenchyme-derived tumor, which usually occurs in head, neck (especially tongue and mouth), and limbs. In this report, we described a case of gastric LGMS by 18F-fluoro-2-deoxy-d-glucose (FDG) positron emission tomography/computed tomography (PET/CT), which has not been reported previously.
Patient concerns:
A 51-year-old female patient was admitted to our hospital with upper abdominal discomfort for 1 year and gradually increased eating difficulties over the last 3 months. From gastroscopy, an ulcer of 1.0 cm × 1.2 cm at the entrance of cardia and stiffness of peripheral mucosa were found, leading to suspicion of cardia cancer. 18F-FDG PET/CT was performed for further diagnosis and staging.
Diagnoses:
According to pathological findings in combination with immunohistochemical features, diagnosis of gastric LGMS was made.
Interventions:
To relieve symptoms of upper gastrointestinal obstruction in the patient, proximal gastrectomy was carried out 1 week after the 18F-FDG PET/CT scan.
Outcomes:
The patient died due to advanced tumor.
Lessons:
18F-FDG PET/CT scan showed local thickening of the gastric wall, invasion of adjacent soft tissue, diaphragmatic and peritoneal metastasis at early stage, absence of regional lymph node metastasis, and increased 18F-FDG metabolism in primary tumor and metastatic tumor.
Keywords: 18F-FDG, gastric tumor, low-grade myofibroblastic sarcoma, PET/CT
1. Introduction
As an uncommon mesenchymal myofibroblastic tumor, low-grade myofibroblastic sarcoma (LGMS) has a low malignant potential. Local recurrences are common, while distant metastases are infrequently reported. LGMS predominantly occurs in adults, affecting slightly more men than women.[1] The most common LGMS-affected sites include head, neck (especially tongue and mouth), and limbs, but the gastric LGMS is extremely rare. The etiology and mechanism of LGMS remain largely unexplored, and the clinical symptoms are not typical. As an important imaging modality to assess MS, 18F-fluoro-2-deoxy-d-glucose (FDG) positron emission tomography/computed tomography (PET/CT) is able to detect metastases at unexpected sites through its whole-body screening, which is the major advantage of 18F-FDG PET/CT in the staging of MS patients over conventional imaging technologies, such as CT and magnetic resonance imaging (MRI). Furthermore, 18F-FDG PET seems promising in treatment monitoring.[2] To the best of our knowledge, we, for the first time, described the features of gastric LGMS using 18F-FDG PET/CT.
2. Case report
The study was approved by the Ethics Committee of our institute. The patient signed the informed consent form. The patient’ medical records were anonymous. A 51-year-old female patient was admitted to our hospital with upper abdominal discomfort for 1 year and gradually increased eating difficulties over the last 3 months. In addition, this patient had symptoms of nausea without vomiting, occasional palpitation, chest tightness, and weight loss of 5 kg in 6 months. In August 2012, the patient underwent x-ray of esophagram and abdominal ultrasound in our hospital, and no abnormalities were detected. Laboratory tests were carried out in May 2013, and results were shown as follows. There were no abnormalities in tumor markers (alpha-fetoprotein [AFP], carcinoembryonic antigen [CEA], carbohydrate antigen 19–9, and carbohydrate antigen 125), and her hemoglobin level was 96 g/L. From gastroscopy, an ulcer of 1.0 cm × 1.2 cm at the entrance of cardia and stiffness of peripheral mucosa were found, leading to suspicion of cardia cancer. 18F-FDG PET/CT scan was carried out for further diagnosis and staging. Results showed thickened gastric walls along with increased FDG metabolism. The wall thickness was approximately 1.5 cm, and the maximum standardized uptake value (SUVmax) was 5.7. The scan further revealed thickened left diaphragm, increased FDG metabolism, an SUVmax of 6.3, an indistinct interface between lesions and abdominal aorta, and local thickening of the left retroperitoneum with increased FDG metabolism and an SUVmax of 2.8 (Figs. 1 and 2). To relieve symptoms of obstruction in the patient, proximal gastrectomy was carried out 1 week after the scan. During the surgical operation, an ulcer type lesion with a diameter of about 1.0 cm was observed in the cardia, and narrowing of the cardia was caused by a solid soft-tissue compression at the posterior wall of the cardia. Pathology diagnosis showed low degree of malignant spindle cell tumor at the cardia, infiltration growth and invasion to the serosa, and no lymph node metastasis was observed in the small omental bursa. Immunohistochemistry data (Fig. 3) were as follows: vimentin (Vim) (+), smooth muscle actin (SMA) (+), cytokeratin (CK) (–), CEA (–), P53 (+), CD117 (–), CD34 lesion (+), Dog-1 (–), S-100 (–), desmin (–), fibronectin (FN) (+), β-catenin (–), and Ki67 (10%+). Therefore, the tumor was diagnosed as LGMS. The patient did not undergo radiotherapy or chemotherapy after surgery, and she died in July 2015 due to advanced tumor.
Figure 1.
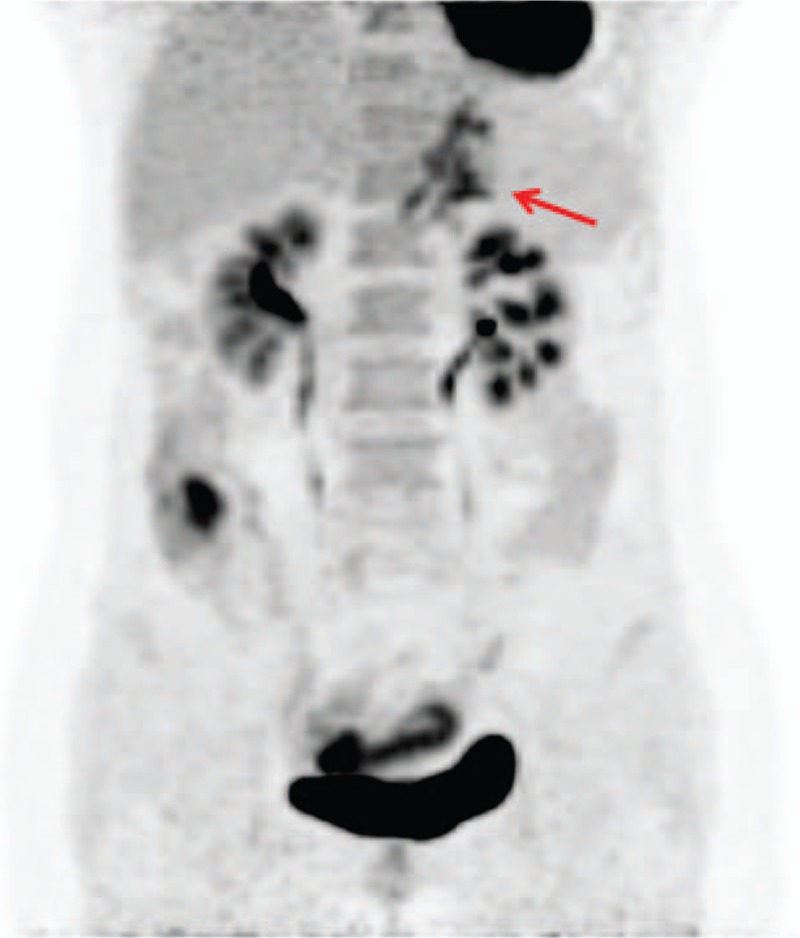
The maximum intensity projection image of PET showed intense FDG uptake in the left upper abdomen (arrow). FDG = fluoro-2-deoxy-d-glucose, PET = positron emission tomography.
Figure 2.
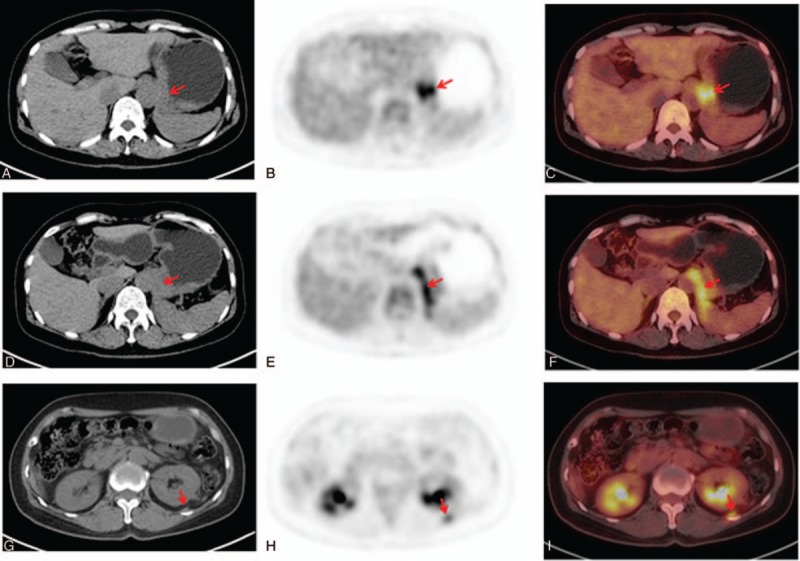
Axial low-dose CT (left), PET (center), and fused PET/CT (right) images showed that there was intense FDG uptake in both thickening gastric cardia with an SUVmax of 5.7 (A, B, C) and thickening left diaphragmatic crura with an SUVmax of 6.3 (D, E, F). Meanwhile, localized thickening of left retroperitoneala had mild 18F-FDG uptake with a SUVmax of 2.8 (G, H, I) (arrow). FDG = fluoro-2-deoxy-d-glucose, PET/CT = positron emission tomography/computed tomography, SUVmax = maximum standardized uptake value.
Figure 3.
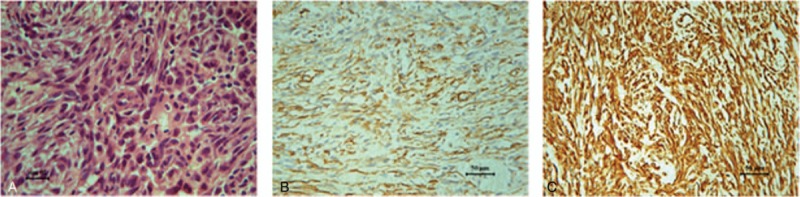
Micrographs revealing the histological appearance of the tumor. Hematoxylin and eosin stain revealed spindle cells arranged in long fascicles (magnification, ×400), and mitosis was found (A). The tumor cells were positive for SMA (B) and Vim (C) (magnification, ×200). SMA = smooth muscle actin, Vim = vimentin.
3. Discussion
As a rare mesenchyme-derived tumor, LGMS was first named by Mentzel et al.[3] In “Soft Tissue Tumor Classification (2002 edition)”, it clearly states that LGMS is an independent tumor type with intermediate or low malignant potential.[4,5] With a wide age range between 4 and 85 years old, LGMS predominantly occurs in the elderly, affecting slightly more men than women.[1] LGMS in head or neck is characterized with slow-growing and gradually increased painless mass, making it prone to missed diagnosis or misdiagnosis. In this case, LGMS primarily occurred in the stomach, which is extremely rare and has not been reported previously. The early symptoms, including nausea and eating difficulties, may be associated with the lesion in the cardia area and the cardia stenosis caused by left diaphragmatic oppression in the cardia.
LGMS imaging also lacks characteristics of the most common CT and MRI manifestation as follows: mostly single lumps, lobulated or irregular type, a small number of tumor have clear border, mostly invasive growth, easy to infiltrate adjacent fat, fibrous and muscle tissues, visible necrosis and calcification; CT scan: non-contrast enhanced scan reveals uneven or lower density, and contrast enhanced scan reveals the uniform or thick-walled ring enhancement and mostly delayed enhancement[6]; MRI characteristics: T1WI signal is mostly equal signal, T2WI signal is mostly slightly higher signal, the signal is not uniform, and there are cable-like signals.[7] Regarding to the 18F-FDG PET/CT scan of LGMS, only Morii et al[8] have reported a case of back (left split muscle) LGMS, in which abnormally increased FDG metabolism can be seen in the lesions with an SUVmax of 9.8. In our case, the PET/CT imaging of LGMS revealed the thickening of the stomach wall in the cardia area with increased FDG metabolism, and the SUVmax was 5.7. The high capacity of glucose utilization is a possible reflection of LGMS. The high SUVmax may be one of the informative biomarkers for the differential diagnosis between malignant and benign tumors.
The PET/CT imaging of gastric LGMS needs to be differentiated mainly from the following diseases: gastric cancer: manifested as irregular thickening of the stomach wall or formation of lumps in the cavity, usually with swollen lymph nodes around the stomach. 18F-FDG metabolic levels may vary depending on pathological type or differentiation level of the tumor. Though there was stomach wall thickening in the cardia area in this case, there were no abnormally swollen lymph nodes around the stomach. Instead, there was local thickening of the left diaphragm and peritoneum with increased FDG metabolism. Such PET/CT manifestation was different from that of gastric cancer. Inflammatory myofibroblastic tumor (IMT): similar to LGMS, IMT belongs to the myofibroblastoma, which is originated from mesenchymal tumors. IMT manifests as single rounds of circular masses with clear boundary, expansive and invasive growth, lower infiltration degree compared with LGMS, increased 18F-FDG metabolism[9] and low incidence. Gastric stromal tumors are characterized by circular mass with clear boundary, growth inside and outside of the cavity, which occurs preferably in the stomach body. However, malignant gastric stromal tumors can be expressed as irregular soft tissue mass, accompanied with cystic changes, necrosis, and invasion of surrounding tissue.[1] Lymphoma is mostly characterized with diffused or segmental thickening of the stomach wall and significantly increased 18F-FDG metabolism. In this case, PET/CT manifestations of the gastric LGMS included thickening of the stomach wall with increased FDG metabolism, indistinct border, and invasion to the adjacent surrounding soft tissues without regional lymph node metastasis, which were different from the characteristics of common gastric tumor. However, differentiation of LGMS from other types of mesenchymal tumors is relatively difficult, and the final diagnosis needs to be confirmed by pathological and immunohistochemical examinations.
The biological behavior of LGMS is benign with occasional local recurrence and very rare distant metastasis.[4] A retrospective study has shown that the 5-year survival rate of LGMS is 71.6%.[10] In this case, the survival length was only 26 months, which might be attributed to local tumor infiltration and multiple metastases at early stage. Regarding to treatment of LGMS, it is generally believed that complete resection of the tumor is of great significance to reduce the recurrence of LGMS. However, the outcome of radiotherapy and chemotherapy remains unclear. Meanwhile, radioactive particle therapy could be used as a tentative adjuvant therapy.
In summary, we, for the first time, reported the characteristics of 18F-FDG PET/CT imaging of gastric LGMS, showing local thickening of the gastric wall, invasion of adjacent soft tissue, diaphragmatic, and peritoneal metastases at early stage, absence of regional lymph node metastasis, and increased 18F-FDG metabolism in primary tumor and metastatic tumor. Integrated 18F-FDG PET/CT, which can evaluate tumor function and perform a morphological assessment at the same time, may therefore have an advantage for differentiating a malignant tumor from a benign soft tissue tumor. In this case, the size, location, infiltration area, and metastasis of the tumor were confirmed by PET/CT scan. These findings provided a reliable evidence for the selection of treatment regimen. Additionally, PET/CT imaging displayed superior efficacy compared with other imaging technologies, with a better correlation between the early and significant decline in metabolic activity and response to therapy in MS.
Footnotes
Abbreviations: LGMS = low-grade myofibroblastic sarcoma, MRI = magnetic resonance imaging, PET/CT = positron emission tomography/computed tomography, SUVmax = maximum standardized uptake value.
This work was supported by Key Development Foundation of Jiangsu Province, China (No. BE2015635) and National Natural Science Foundation of China-Youth Fund (NO. 81701737, 81701734).
The authors declare that they have no conflict of interest.
References
- [1].Qiu JY, Liu P, Shi C, et al. Low-grade myofibroblastic sarcomas of the maxilla. Oncol Lett 2015;9:619–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Springer, Cham, Ceyssens S, Stroobants S. Vanhoenacker F, Parizel P, Gielen J. PET and PET-CT in Soft Tissue Sarcoma. Imaging of Soft Tissue Tumors 2017;59–69. [Google Scholar]
- [3].Mentzel T, Dry S, Katenkamp D, et al. Low-grade myofibroblastic sarcoma: analysis of 18 cases in the spectrum of myofibroblastic tumors. Am J Surg Pathol 1998;22:1228–38. [DOI] [PubMed] [Google Scholar]
- [4].Fletcher CDM, Unni KK, Mertens F. Fibroblastic/myofibroblastic tumours. World Health Organization Classification of Tumors. Pathology and Genetics. Tumors of Soft Tissue and Bone. 2002;Lyon: IARCP Press, 94–95. [Google Scholar]
- [5].Yu Y, Xiao J, Wang L, et al. Low-grade myofibroblastic sarcoma in the mandibular canal: a case report. J Oral Maxillofac Surg 2016;74:1505.e1–5. [DOI] [PubMed] [Google Scholar]
- [6].Han SR, Yee GT. Low grade myofibroblastic sarcoma occurred in the scalp. J Korean Neurosurg Soc 2015;58:385–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Niedzielska I, Janic T, Mrowiec B. Low-grade myofibroblastic sarcoma of the mandible: a case report. J Med Case Rep 2009;3:8458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Morii T, Mochizuki K, Sano H, et al. Occult myofibroblastic sarcoma detected on FDG-PET performed for cancer screening. Ann Nucl Med 2008;22:811–5. [DOI] [PubMed] [Google Scholar]
- [9].Chong A, Ha JM, Hong R, et al. Inflammatory myofibroblastic tumor mimicking gastric gastrointestinal stromal tumor on 18F-FDG PET/CT. Clin Nucl Med 2014;39:725–7. [DOI] [PubMed] [Google Scholar]
- [10].Chan JY, Gooi Z, Wong EW, et al. Low-grade myofibroblastic sarcoma: a population-based study. Laryngoscope 2017;127:116–21. [DOI] [PubMed] [Google Scholar]


