Supplemental Digital Content is available in the text
Keywords: database, hepatocellular carcinoma, survival, veterans
Abstract
Sorafenib is the only Food and Drug Administration (FDA)-approved first-line therapy shown to have survival benefit for patients with advanced hepatocellular carcinoma (HCC). Patients with advanced HCC are often but not exclusively transferred from non-oncologists to oncologists to initiate systemic therapy. The objective of this study was to assess whether sorafenib prescribing by non-oncologists has any impact on utilization, adverse effects, cost or outcome.
This was a retrospective cohort study utilizing data from patients prescribed sorafenib for HCC within Veterans Health Administration hospitals with 100% chart abstraction to confirm HCC diagnosis, identify prescribing provider specialty (oncology versus gastroenterology/hepatology), and obtain data required for cancer staging by the Barcelona Clinic Liver Cancer (BCLC) system. The primary outcome was overall survival from the time of sorafenib prescription.
A total of 4903 patients who prescribed sorafenib for HCC were identified, for whom 340 patients (6.9%) were prescribed drug by a non-oncologist (Onc). BCLC Stage, age, Child–Turcotte–Pugh score, and comorbidity indices were similar between patients prescribed sorafenib by oncologists and non-oncologists. Oncologists more often discontinued sorafenib due to progression, whereas non-oncologists were more likely to continue sorafenib until death resulting in greater pill utilization and cost. Overall survival in both unadjusted and multivariable models showed no significant impact of prescriber type on survival (222 vs 217 days, P = .96), confirmed with propensity-matched subcohorts.
Similar survival outcomes were observed for patients with HCC prescribed sorafenib by non-oncologists and oncologists, suggesting that non-oncologists with expertise in the management of HCC can safely and effectively administer sorafenib.
Key Points
In this large, retrospective, multi-institutional study of 4903 patients with HCC, we observed no inferiority of sorafenib administered by non-oncologists compared to oncologists.
Medication exposure and costs were slightly higher for sorafenib administered by non-oncologists.
Non-oncologists tended to utilize higher doses and continue drug despite progression more often than oncologists.
Non-Onc administration of sorafenib to patients with HCC has no negative impact on treatment outcomes.
1. Introduction
The incidence of hepatocellular carcinoma (HCC) has tripled in the United States over the past 30 years.[1,2] A minority of patients presenting with HCC are candidates for curative surgical therapy, either resection or transplantation.[3] Locoregional palliative ablative and embolic therapies may prolong overall survival (OS) in intermediate stage disease, but most patients with progressive or advanced disease are considered for treatment with sorafenib, a multikinase inhibitor[4,5] that currently is the only available FDA-approved first-line systemic therapy proven to provide consistent survival benefit.[6,7] However, sorafenib has frequent associated toxicities, including gastrointestinal upset, anorexia, hand–foot skin reactions, and fatigue with an overall 30% occurrence of grade 3–4 severity events requiring permanent discontinuation in approximately 28% of treated patients.[8]
Approximately 90% of patients developing HCC have underlying cirrhosis, which produces significant competing morbidity and mortality. Cirrhosis severity also impacts candidacy for surgical, locoregional, and systemic therapy.[5] Due to convention, formulary restriction, or drug familiarity, the initiation of systemic therapy often results in transfer of patient care to an oncologist (Onc). However, the ongoing needs for medical management for cirrhosis and continuity of care might prompt gastroenterologists or hepatologists to prescribe sorafenib without referral. Few data exist on the use of sorafenib by non-oncologists. In the preliminary reports of the Global Investigation of therapeutic DEcisions in HCC and of its treatment with sOrafeNib (GIDEON) registry, the plurality (49%) of prescribing physicians were surprisingly hepatologists or gastroenterologists, and only a minority were oncologists (39%).[9,10] In these abstracts, treatment duration appeared to be longer and at higher dose, but with more frequent dose reductions, when administered by hepatologists compared to oncologists. In addition to a specialist distribution likely not reflecting common practice, the GIDEON registry involves primarily tertiary, high volume centers and its findings may be limited by the selection biases inherent to academic registry studies.[11] The utilization of sorafenib and related outcomes in “real-world” practice remains unreported.
The objective of this study was to determine if sorafenib prescribing by gastroenterologists or hepatologists has any impact on survival, dosing, cost, or adverse events in a broad, unselected national cohort of patients receiving sorafenib for HCC.
2. Materials and methods
2.1. Identification of patients
Data from patients treated at any of 128 United States Veterans Administration hospitals diagnosed with HCC prescribed sorafenib between July 1, 2007 and April 15, 2015 were identified from the Veterans Health Administration (VHA) Corporate Data Warehouse (CDW). HCC was identified by the presence of at least 2 outpatient or 1 inpatient encounter with International Classification of Diseases 9th Revision Clinical Modification (ICD9-CM) codes for malignant neoplasm of the liver (ICD9-CM 155.0 and 155.2).[12] Confirmation of HCC diagnosis was achieved by manual chart abstraction for 100% of identified patients. Patients were excluded for miscoding of their HCC ICD9-CM codes or if chart abstraction confirmed that prescriptions were either not picked up or not taken by the patient. This study was approved by the institutional review board (IRB) at VA Connecticut Healthcare System (West Haven, CT) and the Corporal Michael J. Crescenz VA Medical Center (Philadelphia, PA) with a waiver of informed consent.
2.2. Data collection
The VA utilizes the CDW, an integrated and centralized data repository that captures clinical, laboratory, radiology, procedural, and prescription data for all VHA patients. Baseline demographic data were collected from the CDW, including age, gender, race, ethnicity, concurrent comorbidities, and Alcohol Use Disorder Identification Test Consumption (AUDIT-C) scores.[13] Cirrhosis Comorbidity (CirCom) score was calculated based on relevant comorbidities,[14] but without including HCC in the malignancy subdomains. Active alcohol use was defined as patients with AUDIT-C score >4 in the 1 year prior to initiation of sorafenib. Laboratory data obtained within 90 days prior and closest to time of sorafenib start date were extracted from CDW for all patients. Model for End-Stage Liver Disease Sodium (MELD-Na) score was calculated based on component laboratory values.[15] Child–Turcotte–Pugh (CTP) score was determined using a previously validated algorithm.[16] The presence of concurrent liver-directed therapy, including transarterial chemoembolization (TACE), radiofrequency ablation, percutaneous ethanol injection, and Yttrium-90 transarterial radioembolization, was obtained by querying CDW radiology procedure tables using relevant search phrases. All pharmacy data were extracted from the VA CDW, including data on every sorafenib prescription filled at the VA. From this information, we calculated the time of first sorafenib prescription, daily prescribed sorafenib dose (mg/d) for each prescription filled, total cumulative number of sorafenib pills prescribed, total cumulative days on sorafenib, and total drug-related cost for each patient.
2.3. Chart abstraction
Manual chart abstraction was performed by 2 trained research assistants (RM and KD) for the remaining data not obtained from the CDW. Data were obtained from medical records within 90 days prior and closest to time of sorafenib initiation. Information extracted included date of HCC diagnosis, HCC tumor characteristics based on imaging closest to time of sorafenib initiation (including number and size[s] of HCC tumors, presence of macrovascular invasion, local invasion [e.g., into gallbladder or diaphragm], lymph node spread, and extrahepatic metastatic spread), presence and severity of ascites and hepatic encephalopathy (graded based on thresholds used in the CTP scoring system),[17] an estimate of Eastern Cooperative Oncology Group (ECOG) performance status classification, and specialty of the physician prescribing sorafenib. Date of HCC diagnosis were determined using the first date of imaging with contrast enhanced computed tomography or magnetic resonance imaging that met diagnostic criteria for HCC,[5] the date of pathology report if biopsies were performed, and date of tumor board discussion in cases where imaging was equivocal. Barcelona Clinic Liver Cancer (BCLC) stage was calculated based on collected component variables.[18]
2.4. Definition of outcomes
Our primary outcome of interest was OS, calculated as the time from initiation of sorafenib prescription to time of death, which was ascertained using the VA Vital Status Master File, an aggregate of all of the main federal mortality databases, previously shown to have >97% agreement with the gold standard state death certificate registries.[19] All survival data were censored as of December 31, 2015. Secondary outcomes included time to progression, total number of days on sorafenib, percentage of patients stopping sorafenib due to adverse events, and medication costs. Time to progression was calculated from initiation of sorafenib to time of radiologic progression, which was identified from radiology reports or clinical notes identifying progression. Total number of days on sorafenib estimates the total cumulative number of days that each patient took sorafenib, calculated as the sum of all prescription durations. Reason for sorafenib cessation was obtained from manual chart abstraction, as described above. If sorafenib was discontinued due to any adverse events, the adverse event(s) that led to sorafenib cessation was recorded.
2.5. Statistical analysis
Univariate analysis was performed using Fisher's exact test for dichotomous variables and Student t test or Wilcoxon rank-sum test for normal and non-normal continuous variables, respectively. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using the Cox-proportional-hazards model including clustering by clinical site. Separate Cox models were applied to each individual subgroup during subgroup analysis. Groups selected for subgroup analyses were selected a priori. Survival curves for time-to-event variables were estimated using the Kaplan–Meier method. Finally, a randomized control trial evaluating post-sorafenib survival was simulated using a nearest-neighbor propensity model to match patients treated by gastroenterology/hepatologists (GI/Hep) and oncologists for variables associated with treating specialist, concomitant liver-directed therapy, and outcome with adjustment for clustering by clinical site.
3. Results
3.1. Time trends, demographic data, and concomitant therapy
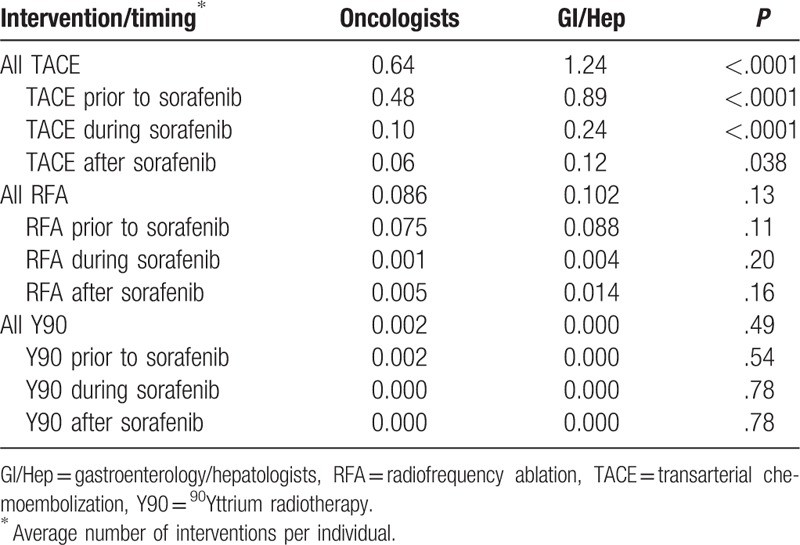
Initial case identification using ICD9-CM codes and pharmacy data identified 5172 patients with sorafenib prescriptions for HCC. Of these, 176 prescriptions were ordered but never picked up by the patient and in 92 patients the diagnosis of HCC could not be substantiated, yielding 4903 patients (94.7%) available for analysis. Sorafenib was prescribed by GI/Hep in 340 (6.9%) of cases, with the remainder prescribed by Oncologists (Onc). Prescribing by GI/Hep was infrequent but increased from 2007 to 2008, plateauing from 2009 to 2015 between 6% and 8.5% of sorafenib prescriptions, with a peak number of 55 cases in 2011 (Fig. 1A). As show in Figure 1B and Table 1, patients prescribed by Onc were slightly older (63.9 ± 8.0 vs 62.6 ± 7.3 years old, Wilcoxon P = .002), slightly less often of black race, and less often infected with either chronic hepatitis B or C infections but similar in terms of CirCom comorbidity, CTP Score and BCLC Stage as those managed by GI/Hep. The plurality of cases as expected were BCLC C (46.1%–47.4%) with the second largest fraction BCLC B (32.4%–33.9%) with similar fractions of early stage A and terminal stage D patients receiving sorafenib independent of provider type. Patients managed by GI/Hep were more likely to have undergone prior TACE procedures, averaging 0.89 TACE sessions compared with 0.48 for Onc patients and were twice as likely to receive additional TACE during and after sorafenib therapy (Table 2).
Figure 1.
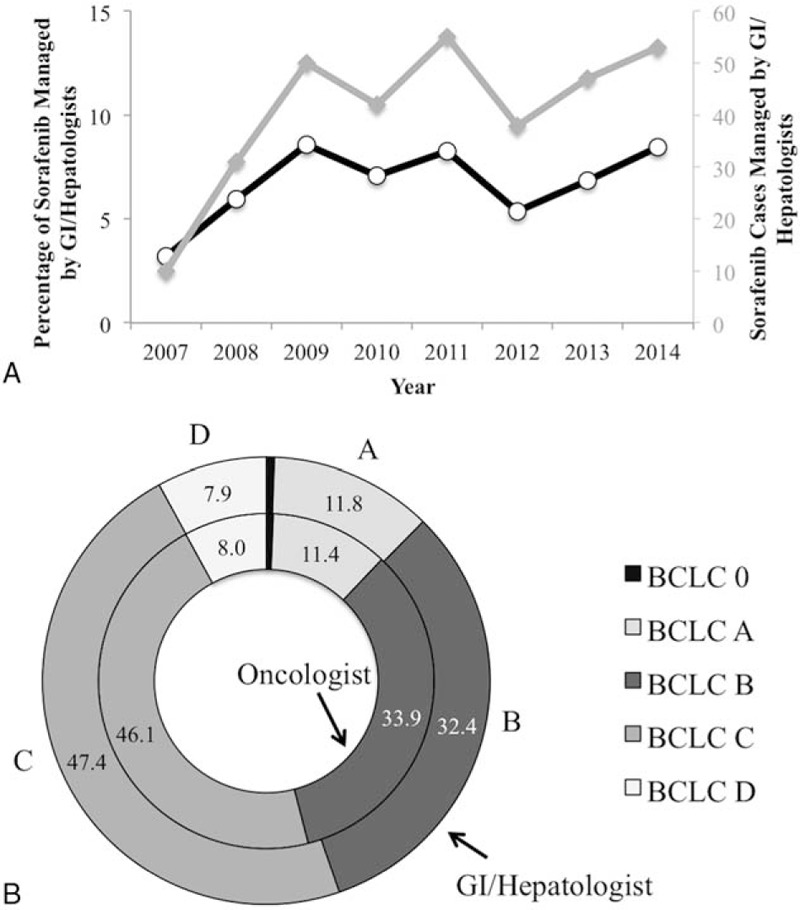
Evolution and characteristics of non-oncologist-prescribed sorafenib-receiving patients. (A) Percentage (black line) and absolute numbers (grey line) of patients receiving sorafenib prescriptions by GI/Hep by year. (B) Distribution of BCLC Stage of patients receiving sorafenib prescribed by oncologists or GI/Hep. No significant differences were obtained by Wilcoxon rank-sum tests. BCLC = Barcelona Clinic Liver Cancer, GI/Hep = gastroenterology/hepatologists.
Table 1.
Baseline characteristics.
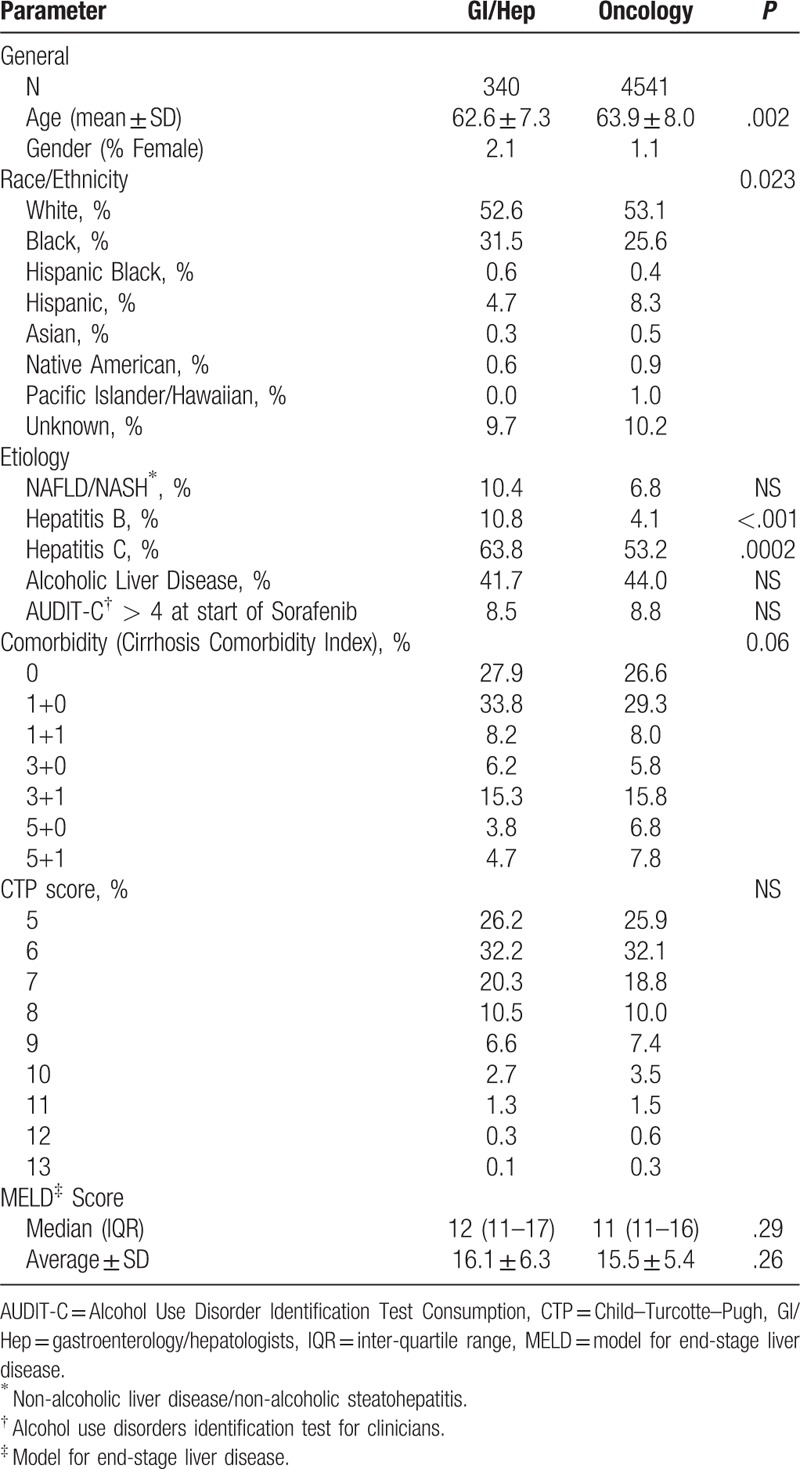
Table 2.
Prior therapy.
3.2. Utilization
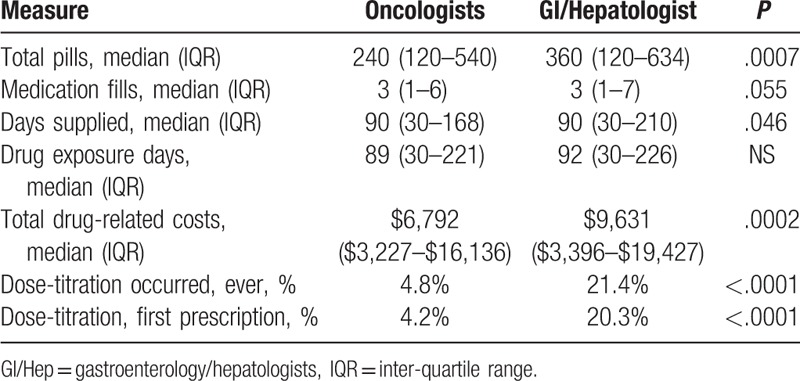
Patients managed by GI/Hep providers received more total pills, a median 360 compared to 240 from Onc (P = .0007) with a trend towards a greater number of medication fills and days of medication supplied (Table 3). Due to higher pill delivery, sorafenib pill costs were significantly greater for GI/Hep than Onc with a median cost of $9631 compared to $6792 (P = .0002). GI/Hep were not statistically significantly more likely to start patients at full dose 800 mg/d but were much more likely to increase doses if the initial prescribed dose was lower than 800 mg/d (21.4% vs 4.8%, P < .0001). These differences in prescribing behavior likely explain the higher pill utilization and cost by GI/Hep-managed patients.
Table 3.
Pharmacy utilization.
3.3. Outcomes
In univariate Cox proportional hazard models, there was no difference in OS for patients managed by GI/Hep or Onc patients, with a median OS 222.5 (Inter-quartile range [IQR] 187–275) days for GI/Hep compared to 217 (206–227) days for Onc (Fig. 2). The absence of a statistical contribution of provider type on survival outcomes persisted in multivariable models including BCLC Stage, CTP status, receipt of prior TACE, multidisciplinary care, initial dosing (full dose or <800 mg/d), and treating center (data not shown). GI/Hep were more likely to continue sorafenib until death (24% vs 17%) and less likely to discontinue sorafenib for radiological progression (20% vs 29%) than Onc with similar discontinuation rates for adverse effects, functional decline, or other causes (Fig. 3A). Adverse events prompting discontinuation did not differ by provider type (Fig. 3B).
Figure 2.
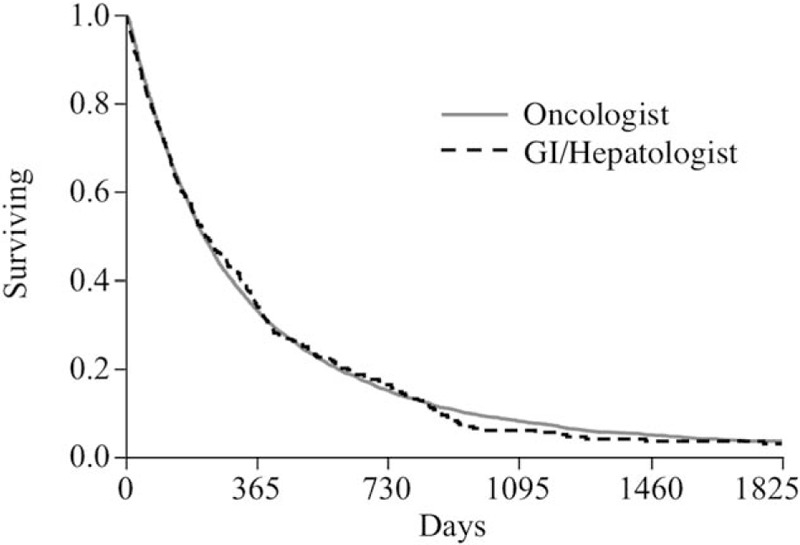
Kaplan–Meier survival curves for patients receiving sorafenib prescribed by oncologists or GI/Hep. No significant differences were identified by Kaplan–Meier or Cox proportional hazard methodologies. GI/Hep = gastroenterology/hepatologists.
Figure 3.
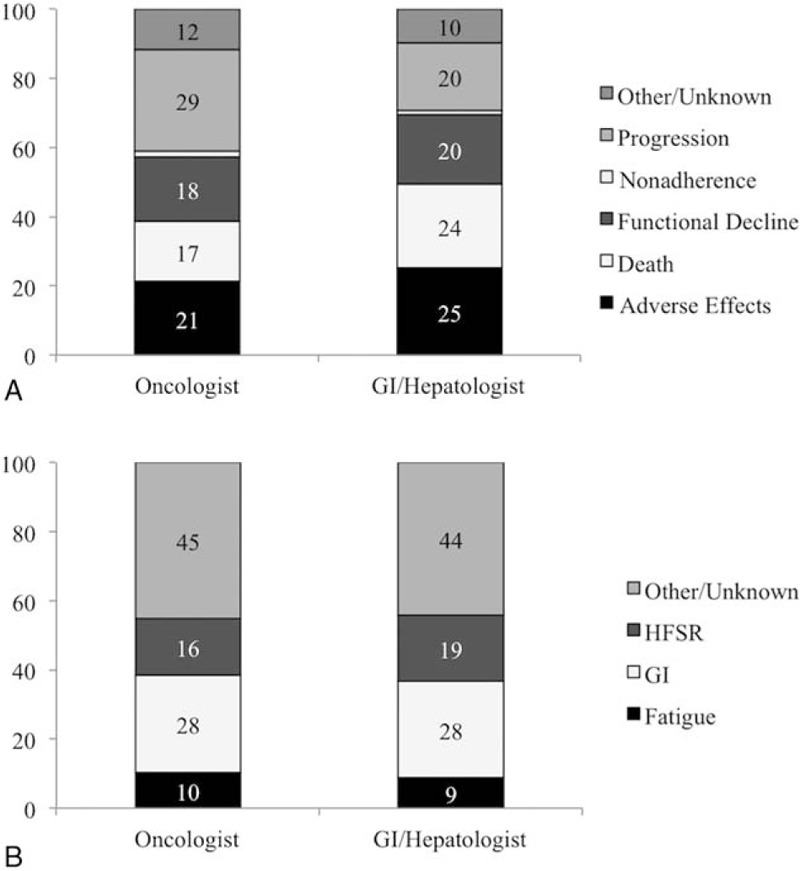
Treatment discontinuation and adverse effects by provider type. (A) Reasons for treatment discontinuation by provider type. (B) Adverse effects leading to treatment discontinuation by provider type. Data only includes cases in which adverse effects were primary reason for treatment cessation.
3.4. Propensity score-matched survival outcomes
To simulate a clinical trial in which patients might be randomly assigned to a GI/Hep or Onc, we performed a propensity-matched analysis in which matched 1:2 patients managed by GI/Hep to patients managed by Onc based on age, gender, comorbidities, BCLC Stage, CTP status, MELD score, receipt of prior TACE, initial dosing (full dose or <800 mg/d), and treating center. We were able to match 202 GI/Hep managed patients to 404 Onc patients with complete balancing of covariates except for receipt of prior TACE and post-sorafenib TACE (Supplemental Table 1). Among this highly matched subcohort, there was no significant difference in mortality with an HR 0.98 (95% CI 0.89–1.25) for patients managed by GI/Hep. The Kaplan–Meier survival curves are presented in Supplemental Figure 1.
4. Discussion
The Sorafenib for Hepatocellular carcinomA in VEterans (SHAVE) cohort of 4903 patients represents the largest North American cohort of patients prescribed sorafenib for HCC reported to date. Compared with the multinational GIDEON registry cohort,[20] the SHAVE cohort shares a similar age range but includes a greater frequency of CTP B and C patients (36% vs 21%) and higher frequency of BCLC B rather than C stage (B/C 34/46% vs 21/56%). Although not reported in manuscript form, initial abstracts from the GIDEON group suggested very high prescription rates by non-oncologists,[9] which do not reflect actual utilization patterns in the United States,[21] where by convention and/or formulary restriction, sorafenib most commonly is prescribed by oncologists. Assuming that gastroenterologists or hepatologists offer better management of cirrhosis-related complications such as ascites, encephalopathy, and variceal hemorrhage, it might be hypothesized that sorafenib prescription by gastroenterologist/hepatologist providers could be associated with improved survival outcomes for HCC.
In this large retrospective cohort, we confirmed that a minority of patients received sorafenib prescriptions (6.9%) from gastroenterologist/hepatologists, but there was no significant survival improvement or reduction associated with provider type. BCLC stage, CTP status, ECOG performance status, and age did not differ across provider types; moreover, no differences in provider-related outcome could be unmasked in multivariable models suggesting that the absence of difference is highly robust. Hepatologists were more likely to up-titrate from lower initial doses if utilized, resulting in a higher number of pills provided. Oncologists were more likely to discontinue sorafenib due to radiological progression, whereas hepatologists tended to continue sorafenib until death. These prescribing behaviors did not translate into statistically longer drug exposure periods or survival, but did result in modestly increased costs.
The rate of prescription by non-oncologists was initially low but rapidly plateaued, suggesting stability of prescriber distributions over time. There was significant clustering of non-Onc utilization with prescription by GI/Hep in more than 25% of cases occurring at only 7/128 hospitals (data not shown) suggesting that very specific local factors and/or providers drove this prescribing practice. These factors could have included the degree of interest of the non-Oncologist provider in liver cancer, local access to oncologists with interest and/or expertise in HCC, and institutional norms. It is therefore likely that GI/Hep prescribing sorafenib were highly selected, and that similar findings might or might not be reproduced were sorafenib prescription more broadly adopted by gastroenterologists or hepatologists.
Our study has several strengths, including the large size of our nationally based cohort, which allowed us to power our study for survival and adequately evaluate several secondary outcomes. Second, this multicenter data from 128 VHA centers affords us high external validity, as it reflects recent practice patterns and patient characteristics across the United States in both academic and community centers. Finally, experienced research assistants performed 100% chart confirmation of diagnosis, tumor characteristics, and patient outcomes to provide robust staging and characterization of events leading to drug discontinuation.
We noted a similar, but relatively high, off-label use of sorafenib by GI/Hep and Onc providers (BCLC A patients, 11.7%; BCLC D, 8%). We speculate that use in BCLC D patients, whereas not expected to have a significant impact on patient outcome, was compassionate-use related to patient requests for therapy. Higher than expected utilization in BCLC A patients could reflect patient preferences, reduced access to standard therapies, bridging therapy for patients on transplant waitlists, or other practices and merits further study.
Our study has several notable limitations. As with any observational cohort study, the potential for unmeasured confounding must be acknowledged. This was a study of mostly male U.S. Veterans who are older, may be more affected by medical comorbidities, and have worse outcomes than the general U.S. population. However, these data serve to fill important knowledge gaps not addressed in clinical trials that typically enroll younger patients with no advanced liver disease. Although much of the tumor staging and receipt of therapy data were abstracted from chart review, ICD-9-CM diagnosis codes were used to determine comorbidity and underlying liver disease, possibly introducing misclassification bias. No information was collected on veterans who did not primarily receive VA care for HCC; they may have had more commercial insurance and different access to specialty care and transplantation. Though geographic and provider differences in care patterns and survival were noted, precise reasons for these differences (e.g., provider expertise, patient access and adherence to treatment recommendations) were not elucidated in this study and should be explored further. The imbalanced sample size with 93% oncology prescribing and 7% non-Onc may contribute to a type II error with relation to the absence of survival differences between provider types. The sample size of 340 cases prescribed by hepatologists only provided 80% power to detect a 17% difference in survival (HR 0.83). Therefore, we cannot exclude that small but statistically significant differences in survival between provider types could exist. It is unlikely however that such differences would be large enough to be clinically important. Due to the inability to capture patient-reported outcomes during sorafenib treatment, we cannot assess the impact that specific prescriber behaviors, for example, up-titration or continuing sorafenib after radiological progression, could have had in patient quality of life.
5. Conclusion
A minority of sorafenib prescriptions for HCC originate from non-oncologists. Although modest differences in prior HCC treatment, concomitant therapy, dosing and utilization exist between non-Onc and Onc prescribers, case mix and survival rates are remarkably similar. These data suggest that hepatologists with expertise in the management of HCC can safely and effectively administer sorafenib and potentially other systemic therapies for HCC.
Supplementary Material
Footnotes
Abbreviations: AUDIT-C = Alcohol Use Disorder Identification Test Consumption, BCLC = Barcelona Clinic Liver Cancer, CDW = Corporate Data Warehouse, CI = confidence interval, CirCom = Cirrhosis Comorbidity, CTP = Child–Turcotte–Pugh, ECOG = Eastern Cooperative Oncology Group, FDA = Food and Drug Administration, GI/Hep = gastroenterology/hepatologists, GIDEON = Global Investigation of therapeutic DEcisions in HCC and of its treatment with sOrafeNib, HCC = hepatocellular carcinoma, HR = hazard ratio, ICD9-CM = International Classification of Diseases 9th Revision Clinical Modification, MELD-Na = Model for End-Stage Liver Disease Sodium, Onc = oncologist, OS = overall survival, RFA = radiofrequency ablation, SHAVE = Sorafenib for Hepatocellular carcinomA in VEterans, TACE = transarterial chemoembolization, VHA = Veterans Health Administration.
This work was supported by unrestricted research funds from Bayer Healthcare Pharmaceuticals and the VA HIV, Hepatitis and Related Conditions Programs in the Office of Specialty Care Services. The funding sources had no role in data acquisition, analysis or interpretation of data.
The views expressed in this article are those of the authors and do not necessarily represent the views of the U.S. Department of Veterans Affairs of the U.S. Government. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Departments of Veterans Affairs and Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
None of the authors have any relevant conflicts of interest to disclose with regard to content of this work.
Supplemental Digital Content is available for this article.
Contributor Information
Collaborators: for the Sorafenib for Hepatocellular carcinomA in VEterans (SHAVE) Study Investigators
References
- [1].El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med 1999;340:745–50. [DOI] [PubMed] [Google Scholar]
- [2].Kaczynski J, Oden A. The rising incidence of hepatocellular carcinoma. N Engl J Med 1999;341:451.author reply 452. [DOI] [PubMed] [Google Scholar]
- [3].Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology 1999;30:1434–40. [DOI] [PubMed] [Google Scholar]
- [4].Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet 2012;379:1245–55. [DOI] [PubMed] [Google Scholar]
- [5].Bruix J, Sherman M. American Association for the Study of Liver D. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378–90. [DOI] [PubMed] [Google Scholar]
- [7].Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25–34. [DOI] [PubMed] [Google Scholar]
- [8].Lencioni R, Kudo M, Ye SL, et al. GIDEON (Global Investigation of therapeutic DEcisions in hepatocellular carcinoma and Of its treatment with sorafeNib): second interim analysis. Int J Clin Pract 2014;68:609–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Venook AP, Lencioni R, Marrero JA, et al. First interim results of the global investigation of therapeutic decisions in hepatocellular carcinoma (HCC) and of its treatment with sorafenib (GIDEON) study: use of sorafenib (Sor) by oncologists and nononcologists in the management of HCC. J Clin Oncol 2011;29:157.21135274 [Google Scholar]
- [10].Venook AP, Lencioni R, Marrero JA, et al. Second interim analysis of the Global Investigation of Therapeutic Decisions in Unresectable HCC (uHCC) and of Its Treatment with Sorafenib (GIDEON): differences in AE reporting across physician specialties. J Clin Oncol 2012;30:286. [Google Scholar]
- [11].Krumholz HM. Registries and selection bias: the need for accountability. Circ Cardiovasc Qual Outcomes 2009;2:517–8. [DOI] [PubMed] [Google Scholar]
- [12].Lo Re V, 3rd, Lim JK, Goetz MB, et al. Validity of diagnostic codes and liver-related laboratory abnormalities to identify hepatic decompensation events in the Veterans Aging Cohort Study. Pharmacoepidemiol Drug Saf 2011;20:689–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bush K, Kivlahan DR, McDonell MB, et al. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med 1998;158:1789–95. [DOI] [PubMed] [Google Scholar]
- [14].Jepsen P, Vilstrup H, Lash TL. Development and validation of a comorbidity scoring system for patients with cirrhosis. Gastroenterology 2014;146:147–56. quiz e115–146. [DOI] [PubMed] [Google Scholar]
- [15].Kim WR, Biggins SW, Kremers WK, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med 2008;359:1018–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kaplan DE, Dai F, Aytaman A, et al. Development and performance of an algorithm to estimate the Child-Turcotte-Pugh score from a National Electronic Healthcare Database. Clin Gastroenterol Hepatol 2015;13:2333-41.e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973;60:646–9. [DOI] [PubMed] [Google Scholar]
- [18].Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999;19:329–38. [DOI] [PubMed] [Google Scholar]
- [19].Sohn MW, Arnold N, Maynard C, et al. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr 2006;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Marrero JA, Kudo M, Venook AP, et al. Observational registry of sorafenib use in clinical practice across Child-Pugh subgroups: the GIDEON study. J Hepatol 2016;65:1140–7. [DOI] [PubMed] [Google Scholar]
- [21].Sanyal A, Poklepovic A, Moyneur E, et al. Population-based risk factors and resource utilization for HCC: US perspective. Curr Med Res Opin 2010;26:2183–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


