Abstract
Prostaglandin E2 (PGE2) suppresses macrophage effector mechanisms; however, little is known about the function of PGD2 in infected alveolar macrophages (AMs). Using serum-opsonized Histoplasma capsulatum (Ops-H. capsulatum) in vitro, we demonstrated that AMs produced PGE2 and PGD2 in a time-dependent manner, with PGE2 levels exceeding those of PGD2 by 48 h postinfection. Comparison of the effects of both exogenous PGs on AMs revealed that PGD2 increased phagocytosis and killing through the chemoattractant receptor-homologous molecule expressed on Th2 lymphocytes receptor, whereas PGE2 had opposite effects, through E prostanoid (EP) receptor 2 (EP2)/EP4-dependent mechanisms. Moreover, PGD2 inhibited phospholipase C-γ (PLC-γ) phosphorylation, reduced IL-10 production, and increased leukotriene B4 receptor expression. In contrast, exogenous PGE2 treatment reduced PLC-γ phosphorylation, p38 and nuclear factor κB activation, TNF-α, H2O2, and leukotriene B4, but increased IL-1β production. Using specific compounds to inhibit the synthesis of each PG in vitro and in vivo, we found that endogenous PGD2 contributed to fungicidal mechanisms and controlled inflammation, whereas endogenous PGE2 decreased phagocytosis and killing of the fungus and induced inflammation. These findings demonstrate that, although PGD2 acts as an immunostimulatory mediator to control H. capsulatum infection, PGE2 has immunosuppressive effects, and the balance between these two PGs may limit collateral immune damage at the expense of microbial containment.
Keywords: histoplasmosis, phagocytosis, fungicidal activity
Histoplasmosis is a fungal disease caused by Histoplasma capsulatum, mainly affecting the respiratory tract (1). The incidence of histoplasmosis has increased worldwide, which is mostly associated with immunodeficiency, such as HIV (2–4). In the lung, the immune response begins after the uptake of the fungus by alveolar macrophages (AMs) and dendritic cells. AMs have a special importance in lung defense, where they are responsible for protecting the alveolar epithelium by clearing microorganisms by phagocytosis and intracellular killing (5). These innate immune sentinel cells are among the first and most essential participants in the successful elimination of the fungus. Furthermore, AM-derived cytokines and lipid mediators induce neutrophil and mononuclear cell recruitment (5) and activation of adaptive immune responses (6, 7), and regulate phagocytosis and antimicrobial activities of phagocytic cells (5, 7, 8).
In particular, the synthesis and signaling of lipid mediators known as leukotrienes (LTs) and prostaglandins (PGs) are increased during histoplasmosis and further regulate host defense (8, 9). Biosynthesis of PGs is coordinated by two distinct cyclooxygenase (COX) isoforms, the constitutive COX-1 and the inducible COX-2, which convert arachidonic acid (AA) to the unstable intermediate compound PGH2. Then, terminal synthase enzymes, including PGD and PGE synthases, generate PGD2 and PGE2, respectively, at sites of inflammation, resulting in either proinflammatory or anti-inflammatory effects, depending on the nature of the stimulus (10, 11). Recently, we demonstrated that, during lethal H. capsulatum infection, pharmacological inhibition of COX-2 by the compound celecoxib increased mouse survival and the phagocytic capacity of AMs, suggesting a contribution of PGs to pathogenesis of this infection (8). Additionally, during bacterial and other fungal infections, PGE2 and PGD2 production is enhanced in the lung (8, 12). However, the specific roles of PGD2 and PGE2 during infections, especially in histoplasmosis, remain incompletely defined.
Over time, PGE2 has emerged as a potent endogenous modulator of innate immunity and macrophage effector functions (10, 13). Generally, PGE2 promotes endothelial cell-mediated vasodilatation and recruitment of circulating leukocytes during inflammation to areas of infection, through mechanisms activated by PGE2 binding to E prostanoid (EP) receptors (EP1–4) coupled to G proteins present in the cell membrane (13). On the other hand, PGE2 inhibits macrophage effector mechanisms, such as phagocytosis, through EP2 receptor and bacterial killing through EP2–4 receptors, both coupled to Gαs proteins, leading to activation of adenylate cyclase, which increases cyclic adenosine monophosphate (cAMP) concentrations (14–16) and IL-1β production (16). PGD2, in turn, binds to the D prostanoid receptor 1 (DP1) and to the “chemoattractant receptor-homologous molecule expressed on Th2 lymphocytes” receptor (DP2 or CRTH2) (17). PGD2 binds to DP1, a transmembrane receptor coupled to the G-protein subunit Gαs. This prostanoid also induces elevation of cAMP, resulting in the inhibition of effector mechanisms of macrophages and other cells (18–20). However, when PGD2 binds to DP2, a Gαi protein subunit coupled receptor, it reduces cAMP concentrations (21). Consequently, the potentially contrasting immunoregulatory roles of PGD2 and PGE2 require further investigation, which is the focus of the present research.
Mammalian cells communicate with each other by exchanging signals that bind specifically to surface or intracellular receptors, followed by a cascade of events that amplify and transduce the incoming signal and eventually elicit a cellular response. The cAMP-dependent protein kinase A (PKA), mitogen-activated protein kinase (MAPK), and nuclear factor κB (NF-κB) cascades modulate common processes in the cell, and multiple levels of cross-talk between these signaling pathways have been described (22, 23). Because activation of PG receptors/cAMP axes is important to regulate macrophage effector functions (14, 15), the putative role of PGD2 and PGE2 in cell signaling activation and inflammatory mediator synthesis deserves detailed examination.
Despite the fact that PGE2 has been shown to inhibit phagocytosis and killing of pathogens by macrophages (14, 15), the role of PGD2 in modulating AM effector functions is not known. In the present study, we determined the contribution of endogenous and exogenous PGD2 and PGE2 on AM effector functions after immune serum (IS)-opsonized H. capsulatum (Ops-H. capsulatum) infection. Using rat AMs, we found that endogenous and exogenous PGD2 and PGE2 actions result in divergent effects on phagocytosis and fungicidal activities of these cells. Treatment of Ops-H. capsulatum-infected AMs with exogenous PGD2 increased phagocytosis and killing through the DP2 receptor; it also inhibited phosphorylation of phospholipase C-γ (PLC-γ) without affecting c-Jun N-terminal kinase 1/2 (JNK1/2), p38, and NF-κB. Furthermore, PGD2 inhibited IL-10 production by infected AMs, while increasing the expression of high-affinity receptors for LTB4 (BLT1). An opposite effect was observed for PGE2, which reduced phagocytosis and killing through an EP2/EP4-dependent manner. Additionally, it diminished phosphorylation of PLC-γ, p38, and NF-κB, while amplifying JNK1/2, and reducing TNF-α, H2O2, and LTB4 production by Ops-H. capsulatum-infected AMs. Finally, in vivo experiments demonstrated that inhibition of PGD2 synthesis increased susceptibility to infection in mice, as well as increased inflammatory cytokine production, while inhibition of PGE2 synthesis increased resistance against infection and diminished lung tissue damage. Also, endogenous PGD2 regulated only fungicidal mechanisms, whereas endogenous PGE2 coordinated phagocytosis and killing of the fungus by AMs. Given the opposite effects of PGD2 and PGE2 during AM infection, these lipids might be potential targets to treat fungal lung diseases, particularly histoplasmosis.
MATERIALS AND METHODS
Animals
Pathogen-free male Wistar rats (125–150 g) and male C57BL/6 mice (20–22 g) were obtained from the animal facilities of the Faculdade de Ciências Farmacêuticas de Ribeirão Preto, Universidade de São Paulo. All experiments were approved and conducted in accordance with the guidelines of the Animal Care Committee of the University of São Paulo (Protocols 09.1.375.53.5 and 013.2016-1). The in vitro experiments with H. capsulatum-infected AMs and infected animals were performed in level 3 biohazard facilities at Faculdade de Medicina de Ribeirão Preto, Universidade de São Paulo.
Culture of H. capsulatum
The H. capsulatum clinical isolate was obtained from a patient at the Hospital das Clínicas, Faculdade de Medicina de Ribeirão Preto, Universidade de São Paulo. The mycelia were obtained by culturing fungi at 25°C in Sabouraud dextrose agar tubes (Difco, Detroit, MI), and the live yeast fungus was subcultured at 37°C on glutamine-cysteine-sheep blood (5%) BHI (Detroit, MI) for 15 days. Yeast cells were used when their viability was ≥90% according to fluorescein diacetate (Sigma-Aldrich, St. Louis, MO) and ethidium bromide (Sigma-Aldrich) staining (8, 9).
IS and opsonization
Rats were intraperitoneally inoculated with 1 ml containing 108 yeast of H. capsulatum, and 10 days later were submitted to a second inoculation with an equal inoculum. After 7 days, the rats were decapitated, and blood was collected and centrifuged at 1,900 g for 10 min to obtain the IS. IS was heated at 56°C for 1 h to inactivate complement proteins and stored at −80°C (24). For fungus opsonization, 1 × 108 yeast in 1 ml of PBS was incubated with 10% IS for 30 min at 37°C on a rotating platform (7), and the opsonized fungus is referred to as Ops-H. capsulatum. Nonopsonized fungi (H. capsulatum) incubated only with PBS were used for comparison as described.
AM isolation, cell culture, and treatments
Resident AMs from naïve rats were obtained via ex vivo lung lavage (24) and suspended in incomplete RPMI 1640 at 2 × 106 cells per ml. Cells were allowed to adhere to tissue culture plates for 1 h (37°C, 5% CO2), followed by two washes with warm incomplete RPMI 1640, resulting in ± 99% of adherent cells identified as AMs by staining with Panoptic (Laborclin, Paraná, Brazil). Cells were cultured overnight in complete RPMI 1640 containing 10% FBS and 1% penicillin/streptomycin/amphotericin B (Gibco, Grand Island, NY). The following day, cells were washed twice with warm medium (incomplete RPMI 1640) to remove nonadherent cells. AMs were preincubated with indomethacin (10 μM) (COX1/2 inhibitor; Sigma-Aldrich), or celecoxib (10 μM) (COX-2 inhibitor; Celebra®, Pfizer, SP, Brazil), or HQL-79 (1 μM) (PGD2 synthase inhibitor); or CAY10526 (1 μM) (PGE2 synthase inhibitor); or BWA868c (1 μM) (DP1 antagonist); or Bay-u3405 (1 μM) (DP2 antagonist); or AH6809 (1 μM) (EP2 antagonist); or AH23848 (1 μM) (EP4 antagonist), preceding fungus infection. The enzyme inhibitors or antagonists were purchased from Cayman Chemical (Ann Arbor, MI), and incubated with AMs for 30 min before infection. When necessary, cells were incubated with PGD2 or PGE2 for 2 min (1 μM) (Cayman Chemical), before in vitro infection. Receptor antagonist and concentrations used were previously determined by our group (16, 25) or investigated in the literature (14, 15, 26, 27). Incomplete RPMI 1640 containing the same concentrations of DMSO and/or alcohol used to dissolve the compounds was used as control and identified as vehicle. Cells treated with compounds or vehicle were used for phagocytic and fungicidal assays.
Fluorometric phagocytosis assay with FITC-labeled H. capsulatum
A fluorometric phagocytosis assay was performed to test the capacity of AMs to phagocytize FITC-labeled H. capsulatum (or Ops-H. capsulatum) as published previously (7). Briefly, yeast cells were labeled with FITC (Amresco, OH) for 1 h at 37°C (7). FITC-labeled Ops-H. capsulatum or FITC-labeled H. capsulatum was added, and the number of yeast cells to be used by AMs was determined through multiplicity of infection (MOI) starting from 1:1; 1:5, or 1:10, respectively. The AMs were pretreated or not with the compounds as described above before the fungus was added and then incubated in the dark (37°C, 5% CO2). After 2 h, free yeast cells were removed by washing with warm sterile PBS, and the residual extracellular FITC was quenched with Trypan blue (250 mg/ml; Gibco) for 1 min. Fluorescence was determined by using a micro plate reader (485 nm excitation/535 nm emission, SPECTRAMax, Molecular Devices, Sunnyvale, CA). Phagocytosis was determined by the mean of relative fluorescence units (MFI) emitted from intracellular fungi.
Fungicidal activity assay
AMs were pretreated with IFN-γ (50 ng/ml) overnight to improve their effector mechanism as described by Peck (28), and submitted or not to the above treatments. Next, cells were incubated with H. capsulatum (opsonized or not) at MOI 1:10, and after 2 h, cells were washed twice with warm sterile PBS to remove the extracellular yeasts. Following another 48 h of incubation, the supernatants were collected and kept at −80°C until they were used for measurements, as described. Afterward, the cells were lysed by adding 200 µl of 0.05% saponin, and an aliquot was plated on BHI agar-blood (29). After 21 days of culture at 37°C, the colony-forming units (CFU) were counted and the fungicidal activity was calculated according to the formula: [100 - (100 × CFU experimental)/CFU control].
Quantitation of cytokines
AMs treated or not with the above compounds were incubated with Ops-H. capsulatum, and after 48 h the cell culture supernatants or lung homogenate of H. capsulatum-infected mice were obtained to measure TNF-α, IL-1β, and IL-10, by using commercially available ELISA kits (R&D Systems, Minneapolis, MN). For each sample, the cytokine concentrations were obtained from a standard curve established with the appropriate recombinant cytokine. The sensitivities were >10 pg/ml.
Hydrogen peroxide production
The release of H2O2 from AMs was determined by phenol red oxidation, as described previously (30), in supernatant from cells pretreated or not with PGD2 or PGE2, and incubated with Ops-H. capsulatum (MOI 1:10). After 48 h of incubation, the supernatants were replaced by supplemented assay medium (RPMI 1640-containing peroxidase and phenol red) and incubated for 2 h. Then, stop solution (NaOH 1N) was added, and optical density (OD) was determined at 620 nm.
Flow-cytometry analysis of BLT1 expression in AMs
The expression of BLT1 was determined by using the flow-cytometry immune staining protocol with antibodies conjugated with fluorochromes (BD Biosciences, Franklin Lakes, NJ). The AMs (2 × 105 cells) were pretreated with the compounds described above before the fungus was added (2 × 104 cells) and incubated at 37°C, 5% CO2. After 2 h, the supernatant was removed, and the cells were washed with 300 µl of PBS containing 2% FBS (Gibco). Cells were then suspended in polystyrene tubes and centrifuged at 400 g for 5 min. Cells were suspended in Fc block (200 µl), containing anti-CD16/CD32 antibodies at dilution 1:100 and incubated for 30 min at 4°C. Subsequently, the antibody against BLT1 was added to the AMs. After 40 min, cells were washed twice, and then fixed with PBS containing 1% (wt/vol) paraformaldehyde. A total of 20,000 events was acquired for each tube (FACSCantoTM; Becton Dickinson), using the FACSDiva software.
Measurements of PGE2, PGD2, and LTB4
Eicosanoids were purified from the supernatant of AMs cultures or lung homogenate (Mixer Homogenizer, IKA, Wilmington, NC) by using Sep-Pak C18 cartridges according to the manufacturer’s instructions (Waters Corp.. Milford, MA). Quantifications of PGE2 and LTB4 (Enzo Life Science, Farmingdale, NY) and PGD2 (Cayman Chemical) were performed by using specific enzyme immunoassay kits according to manufacturers’ instructions, and the results were expressed in picograms per milliliter, or, after the data were transformed, as percentages, and infected AM was set as 100%.
Phosphoprotein detection by cytometric bead array
Samples of AMs were prepared as previously described (31). After infection and treatments, cells were washed with ice-cold PBS and then lysed with buffer containing protease and phosphatase inhibitors according to the manufacturer’s protocol for adherent cells (Becton Dickinson, Heidelberg, Germany). The cell lysates were moved to Eppendorf tubes and immediately placed in a boiling water bath for 5 min. The total protein concentration was adjusted to 1 µg/µl, and the cell lysates were stored at −80°C until measurement of kinase phosphorylation. The total protein content of the lysates was determined by using the Bradford method (Sigma-Aldrich,). Quantitative determination of phospho-JNK1/2 (T183/Y185), phospho-p38 (T180/Y182), and phospho-PLC-γ (Y783) was performed by using antibodies from the multiplex Flex Set Cytometric Bead Array (Becton Dickinson). Flow-cytometric analysis was performed using FACSCantoTM, and FACSDiva was used for data acquisition and analysis (Becton Dickinson). A total of 900 events was acquired.
RAW-BlueTM cells experiment
To analyze the influence of PGE2 and PGD2 on NF-κB activation induced by the fungus, we used RAW-BlueTM cells (Invivogen). RAW-BlueTM is a cell line of macrophages that stably express the SEAP gene (secreted embryonic alkaline phosphatase), which is induced by NF-κB/AP-1 transcription factors and confers resistance to Zeocin™. These cells were grown in DMEM supplemented with 10% FBS, Normocin™ (50 μg/ml), and Zeocin™ (25 μg/ml). The cells were seeded in 96-well microculture plates at a density of 2 × 105 cells per well in DMEM supplemented with Normocin™ (50 mg/ml) and cultured at 37°C in a humidified 5% CO2 atmosphere for 18 h. After this period, the cells were stimulated with PGD2 or PGE2 for 2 min and then incubated with Ops-H. capsulatum (2 × 104 yeast per well) for 24 h. After this period, the medium was collected, and samples of 50 μl were mixed in 96-well plates at 37°C for 2 h, with 150 μl of QUANTI-Blue™ (Invivogen), which is a SEAP detection medium. The OD was then measured at 650 nm by using an ELISA reader (μQuant, Biotek Instruments Inc., Winooski, VT).
Infection and in vivo pharmacological treatments
Mice were anesthetized with ketamine and xylazine (10 and 20 mg/kg, respectively) and restrained on a small board. A 30-gauge needle attached to a tuberculin syringe was inserted into the trachea, and a lethal inoculum of H. capsulatum in PBS (1 × 106 yeast/100 µl per mouse) was intratracheally dispensed into the lungs. Infected mice were treated by gavage with inhibitor of PGD2 synthase (HQL-79, Cayman Chemical; 3 mg/kg/0.5 ml of water, dose chosen based on dose-response experiments ranging from 0.3 to 3 mg/kg) (32), or inhibitor of PGE2 synthase (CAY10526, Cayman Chemical; 5 mg/kg/0.5 ml of water) (33) or water (0.5 ml), 1 h before infection and daily for 30–40 days. As a control, mice were injected with PBS and treated daily with water.
Histology
At 7 days postinfection, the lungs were removed and immediately fixed in 10% formalin for conventional histological examination. The specimens were processed, embedded in paraffin, and cut into 5-µm sections. The sections were stained with hematoxylin and eosin (H&E) and analyzed in a blinded fashion at 100× magnification. ImageJ software (NIH, Bethesda, MD) was used to calculate the lung area damage in five random photomicrograph sections per slide, which was represented by percent lung area covered by infiltrating cells calculated for each mouse by dividing the sum of damage areas in these sections by the total area of the lung examined.
Determination of CFU
After 7 days of infection, the lungs were recovered to determine the fungal burden. H. capsulatum yeasts from the lung were examined as previously described (9). Two hundred microliter samples from each cell suspension were collected, and a 10-fold dilution was plated into BHI agar-blood. After incubation at 37°C for 21 days, the H. capsulatum CFU was counted and expressed as CFU/g lung (log 10).
Statistical analysis
Statistical analysis was performed by using GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA). Data are expressed as mean ± standard error of the mean (SEM). All experiments were conducted in triplicate unless otherwise noted, and repeated at least twice. Comparisons were performed by using ANOVA followed by Tukey’s multiple comparison test as described. In all comparisons, a significance level of P < 0.05 was considered to be significant. Differences in survival were analyzed by using the log-rank test. Significance level was set at 5%.
RESULTS
In vitro AMs phagocytized Ops-H. capsulatum more efficiently and released PGE2 and PGD2 differently
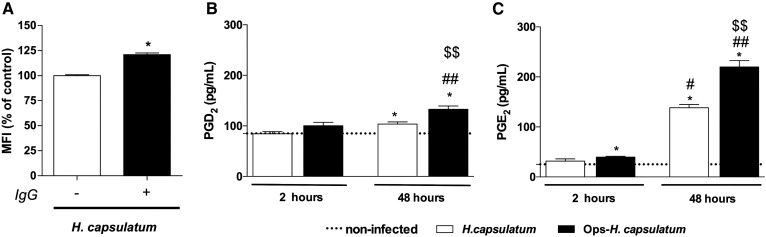
The usual immune response to H. capsulatum begins in the lung with the uptake of the fungus by resident AMs that are essential cells for clearing microorganisms by phagocytosis and intracellular killing (29). In addition, it has been demonstrated that H. capsulatum induces a humoral immune response and leads to production of circulating immune complexes (34). Since a humoral response is enhanced during the course of infection (34), it may be relevant in the setting of reinfection, particularly in endemic areas. Therefore, we first investigated whether resident AMs produce PGD2 and PGE2 after in vitro infection with nonopsonized (H. capsulatum) or with specific IS-opsonized H. capsulatum (Ops-H. capsulatum). As expected, after 2 h, Ops-H. capsulatum was more efficiently phagocytized than the nonopsonized fungus (Fig. 1A). The phagocytosis induced PGE2 production by AMs 2 and 48 h following infection, whereas PGD2 production was increased only at 48 h (Fig. 1B, C). The nonopsonized fungus induced lower amounts of PGs in comparison to the opsonized fungus. Based on these data, Ops-H. capsulatum was selected for the next experiments to assess the effects of PGD2 and PGE2 on AM effector functions.
Fig. 1.
Ops-H. capsulatum are more efficiently phagocytized by AMs and induced higher amounts of PGE2 and PGD2 than did H. capsulatum. A: AMs were incubated with H. capsulatum or Ops-H. capsulatum labeled with FITC (MOI 1:10), and phagocytosis was assessed 2 h later. Data are expressed as average of fluorescence intensity (MFI) from internalized yeast (n = 6). To determine PGD2 (B) and PGE2 (C) production, AMs were incubated with H. capsulatum or Ops-H. capsulatum (MOI 1:10) during 2 and 48 h, and the supernatants were collected for PG quantification by immunoassays as described in Materials and Methods (n = 3). * P < 0.05 [H. capsulatum vs. Ops-H. capsulatum (A); noninfected AMs vs. AMs + H. capsulatum or vs Ops-H. capsulatum (B, C)]; # P < 0.05 (48 h AMs + H. capsulatum vs. 2 h H. capsulatum); ## P < 0.05 (48 h AMs + Ops-H. capsulatum vs. 2 h Ops-H. capsulatum); $$ P < 0.05 (48 h AMs + H. capsulatum vs. 48 h Ops-H. capsulatum). One-way ANOVA and Tukey’s multiple comparison tests were used. Data are representative of two independent experiments (±SEM).
Inhibition of COX-1 and COX-2 increased phagocytosis and fungicidal activity of AMs and diminished PGD2 and PGE2 production
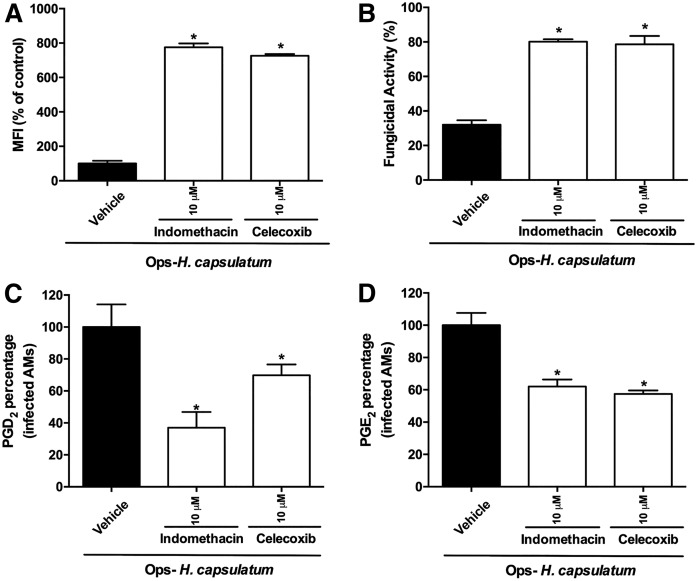
We next evaluated the extent to which endogenous PGs are required during FcR-mediated phagocytosis and killing of H. capsulatum by AMs. AMs were pretreated with indomethacin (10 μM), a dual COX-1/COX-2 inhibitor, or celecoxib (10 μM), a selective COX-2 inhibitor, and 30 min later were infected with IgG-H. capsulatum. Both inhibitors increased phagocytosis of Ops-H. capsulatum by AMs (Fig. 2A). Next, we evaluated the role of PGs in the fungicidal activity of AMs after 48 h of incubation with Ops-H. capsulatum. Nonspecific inhibition of COX enzymes or the specific inhibition of COX-2 augmented clearance of IgG opsonized fungi by AMs (Fig. 2B). As expected, both COX inhibitors suppressed the production of PGD2 and PGE2 (Fig. 2C, D). Our data demonstrate that inhibition of COX-1- or COX-2-mediated PG synthesis during fungal infection increased the phagocytic and fungicidal activity of AMs against Ops-H. capsulatum.
Fig. 2.
Inhibition of COX-1 and COX-2 decreased PGD2 and PGE2 and augmented phagocytosis and fungicidal activity of AMs. A: AMs were pretreated or not with indomethacin (10 μM) or celecoxib (10 μM) for 30 min prior to addition of Ops-H. capsulatum labeled with FITC (MOI 1:10). Phagocytosis was assessed 2 h later. Data are expressed as average of fluorescence intensity (MFI) from internalized yeast (n = 6). B: AMs were incubated with Ops-H. capsulatum at MOI 1:10 for 2 h to allow yeast internalization, and, after 48 h, AMs were lysed, and live yeast was determined by CFU number. Fungicidal activity was expressed as a percentage from total internalized yeast (100%) (n = 4). Measurement of PGD2 (C) and PGE2 (D) by immunoassays is described in Materials and Methods. The supernatants were collected 2 h after indomethacin or celecoxib treatment and fungal infection (n = 4). Production of PGD2 and PGE2 was expressed as a percentage, and infected and vehicle-incubated cells were set as 100% (AMs + Ops-H. capsulatum). * P < 0.05 [AMs + Ops-H. capsulatum (in vehicle) vs. other groups]. One-way ANOVA–Tukey’s multiple comparison tests were used. Data are representative of two independent experiments (±SEM).
PGD2 and PGE2 have opposite effects on phagocytosis and fungicidal activity of AMs infected with Ops-H. capsulatum
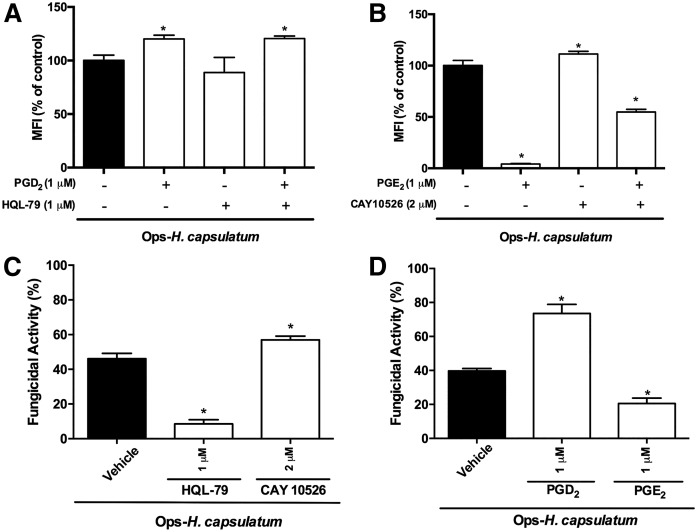
It has been demonstrated that PGE2 inhibits bacterial phagocytosis and killing by AMs (14, 15). Because we detected that both PGD2 and PGE2 are produced by AMs infected with H. capsulatum (Fig. 1), we subsequently investigated the biological effect of each PG in regulating phagocytosis and killing by AMs. First, we showed that the inhibition of endogenous PGD2 synthesis by HQL-79 at 1 μM (Fig. 3A) (and at other concentrations of 0.1 and 10 μM; data not shown) did not modify phagocytosis of Ops-H. capsulatum by AMs. In contrast, inhibition of endogenous PGE2 by CAY10526 at 2 μM increased phagocytosis (Fig. 3B) (and at other concentrations of 0.2 and 20 μM; data not shown). Next, we evaluated the effects of exogenous administration of each PG on phagocytosis. The addition of exogenous PGD2 (1 μM) increased (Fig. 3A), whereas exogenous PGE2 (1 μM) inhibited phagocytosis (Fig. 3B) of IgG-H. capsulatum. Inhibition of endogenous PGD2 or PGE2 did not distinctively alter the effects of the exogenous mediators (Fig. 3A, B).
Fig. 3.
PGD2 and PGE2 have opposite effects on phagocytosis and fungicidal activity of AMs. The effects of pretreating AMs with a specific inhibitor of PGD synthase (HQL-79) or PGE synthase (CAY10526), or soluble PGD2 or PGE2 on phagocytosis and fungicidal activity were assessed. AMs were pretreated with HQL-79 (1 μM) or/and PGD2 (1 μM) (A) or CAY10526 (2 μM) or/and PGE2 (1 μM) (B), respectively, 30 and 2 min before the addition of Ops-H. capsulatum (MOI 1:10) labeled with FITC. Phagocytosis was assessed 2 h later, and data are expressed as average of fluorescence intensity (MFI) from internalized yeast (n = 6). The fungicidal activity was also evaluated following treatment with HQL-79 or CAY10526 (C) or with PGD2 or PGE2 (D). After 48 h, AMs were lysed, and CFU number determined live yeast. Fungicidal activity was expressed as a percentage from total internalized yeast by AMs incubated with vehicle (100%) (n = 4). * P < 0.05 [AMs + Ops-H. capsulatum (in vehicle) vs. other groups]. One-way ANOVA and Tukey’s multiple comparison tests were used. Data are representative of two independent experiments (±SEM).
Interestingly, we also found that, after 48 h, inhibition of PGD synthase impaired the killing of Ops-H. capsulatum by AMs, whereas inhibition of PGE synthase increased this process (Fig. 3C). These findings indicate that the inhibition of endogenous PGD2 exerted no effect on the phagocytic ability of AMs. However, these treatments regulated the fungicidal activity of infected AMs differently. Adding exogenous PGD2 and PGE2, we confirmed that PGD2 increased the killing of IgG-H. capsulatum, whereas PGE2 reduced the ability of AMs to eliminate the fungus (Fig. 3D). Together, these data indicate the opposite effects of PGD2 and PGE2 on yeast killing by AMs and demonstrate, for the first time, that phagocytosis can be enhanced only by exogenous PGD2, which, in vivo, may be released by its own cells and other neighbors.
PGD2 and PGE2 receptors differently control phagocytosis and killing of Ops-H. capsulatum by AMs
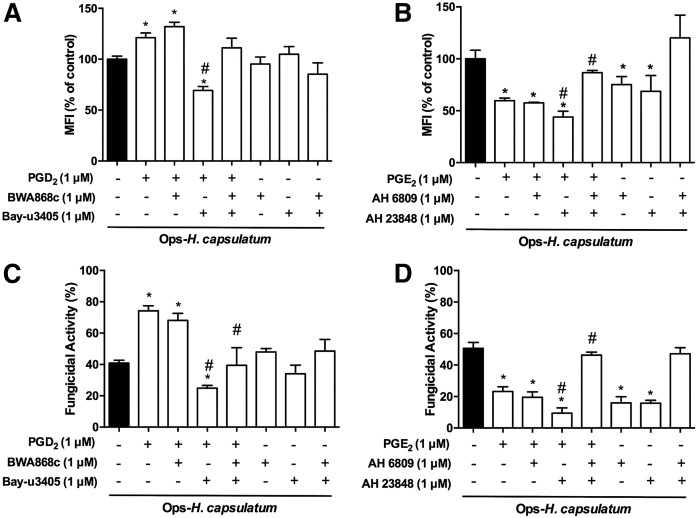
PGD2 and PGE2 signaling occurs through specific G-coupled receptors to mediate effector mechanisms in macrophages (14, 35). Therefore, we investigated which receptors are driving the effects of exogenous and endogenous PGD2 and PGE2 on phagocytosis and killing of Ops-H. capsulatum by AMs. Phagocytosis of Ops-H. capsulatum was increased by pretreatment of AMs with PGD2 alone or PGD2 in presence of a DP1 receptor antagonist (BWA868c), but was reduced after blocking DP2 receptors by the compound Bay-u3405. When used in combination, DP1/DP2 antagonists did not affect PGD2-increased phagocytosis. Interestingly, blocking DP1, DP2, or DP1/DP2 in the absence of exogenous PGD2 did not affect the phagocytosis of the fungus (Fig. 4A). On the other hand, in the presence of exogenous PGE2, an EP2 antagonist (compound AH6809) did not modify its inhibitory effects, whereas an EP4 antagonist (AH23848 compound) potentiated its inhibitory action. Unexpected simultaneous inhibition of EP2/EP4, in the presence of exogenous PGE2, almost reversed its inhibitory action. Nevertheless, when AMs were pretreated with EP1 or EP2 antagonists, in the absence of exogenous PGE2, significant and similar inhibition of phagocytosis was observed, but simultaneous EP2/EP4 blocking had no effect (Fig. 4B).
Fig. 4.
PGD2 and PGE2 receptors antagonists differently impacted phagocytosis and killing of Ops-H. capsulatum by AMs. AMs were pretreated or not for 20 min with DP1 antagonist (BWA868c; 1 μM) or/and DP2 antagonist (Bay-u3405; 1 μM) before addition of vehicle or PGD2 (1 μM) for 2 min. Subsequently, the cells were infected with Ops-H. capsulatum labeled with FITC (MOI 1:10), and phagocytosis (A) and fungicidal activity (C) were assessed 2 and 48 h later, respectively. In another set of experiments, AMs were pretreated or not with EP2 antagonist (AH 6809; 1 μM) and/or with EP4 antagonist (AH 23848; 1 μM) before the addition of vehicle or PGE2 (1 μM) for 2 min. Subsequently, the cells were infected with Ops- H. capsulatum labeled with FITC (MOI 1:10), and phagocytosis (B) and fungicidal activity (D) were assessed 2 and 48 h later, respectively. Phagocytosis is expressed as average of fluorescence intensity (MFI) from internalized yeast by AMs in vehicle (n = 6). For fungicidal activity, after 48 h, AMs were lysed, and the fungicidal activity was determined as described in Materials and Methods. Fungicidal activity was expressed as a percentage of live yeast recovered (CFU) from cells treated with the antagonists in comparison to total yeast recuperated from AMs incubated with vehicle (100%) (n = 4). * P < 0.05 (AMs + Ops-H. capsulatum vs. other groups); # P < 0.05 (AMs + Ops-H. capsulatum PGD2 or PGE2 vs. treatments). One-way ANOVA and Tukey’s multiple comparison tests were used. Data are representative of four independent experiments (±SEM).
When the fungicidal activity of AMs was evaluated in the presence of exogenous PGD2, and DP2 and DP1/DP2 antagonists, its fungicidal activity was reversed, unlike the DP1 antagonist. However, blocking DP1, DP2, or DP1/DP2 in the absence of exogenous PGD2 did not affect AM killing activity (Fig. 4C). However, only blocking the EP4 receptor potentiated exogenous PGE2-induced reduction of fungicidal activity of AMs infected with Ops-H. capsulatum. Simultaneous inhibition of EP2/EP4 completely blocked the inhibitory effects of exogenous PGE2. Similar results were observed by pretreating AMs with EP2 and/or EP4 antagonists in the absence of exogenous PGE2 (Fig. 4D). Together, these data further demonstrate that PGD2 increased phagocytosis and fungicidal activity through DP2 receptor activation, and PGE2-impaired phagocytosis and fungicidal activity are mediated by the cooperation of both receptors EP2 and EP4.
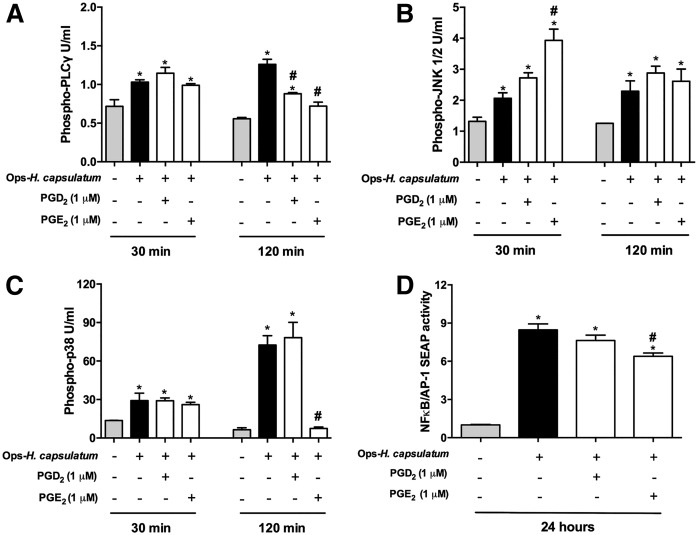
PGD2 and PGE2 modified PLC-γ, MAPK, and NF-κB/AP-1 signaling during Ops-H. capsulatum infection
In order to identify cell signaling pathways activated or inhibited by PGD2 and PGE2 during Ops-H. capsulatum AM infection, we investigated the phosphorylation of PLC-γ, JNK1/2, and p38 and the activation of NF-κB. As demonstrated by protein phosphorylation, PLC-γ, JNK1/2, p38, and NF-κB/AP-1 pathways were activated in AMs in response to Ops-H. capsulatum infection. Although Ops-H. capsulatum infection induced PLC-γ phosphorilation, the addition of exogenous PGD2 or PGE2 reduced phospho-PLC-γ after 2 h (Fig. 5A). In infected cells, exclusively, PGE2 potentiated phosphorylation of JNK1/2 at an earlier time point (Fig. 5B), abrogated phosphorylation of p38 (Fig. 5C), and reduced activation of NF-κB/AP-1. Together, these findings support the hypothesis that PGD2 and PGE2 have distinct effects on infected AMs, and that these effects can be mediated through distinct cell-signaling pathways.
Fig. 5.
MAPKs and NF-κB are involved in PG-induced macrophage activation. Quantification of phosphorylated PLC-γ (A), JNK1/2 (B), and p38 (C) were determined by cytometric bead array and expressed as units per milliliter. Phosphorylation was determined in AMs stimulated with PGD2 or PGE2 for 2 min. After the cells were infected with Ops-H. capsulatum infection (MOI 1:10) for 30 and 120 min, the cells were analyzed. Nontreated and noninfected cells were used as controls. D: Activation of NF-kB/AP-1 was measured in RAW-BlueTM cells incubated with either PGD2 or PGE2 for 2 min, before addition of Ops-H. capsulatum (MOI 1:10) for 24 h. * P < 0.05 (uninfected AMs vs. other groups); # P < 0.05 (AMs + Ops-H. capsulatum vs. other groups). One-way ANOVA and Tukey’s multiple comparison tests were used. Data are representative of one (A–C) and two (D) independent experiments (n = 4, ±SEM).
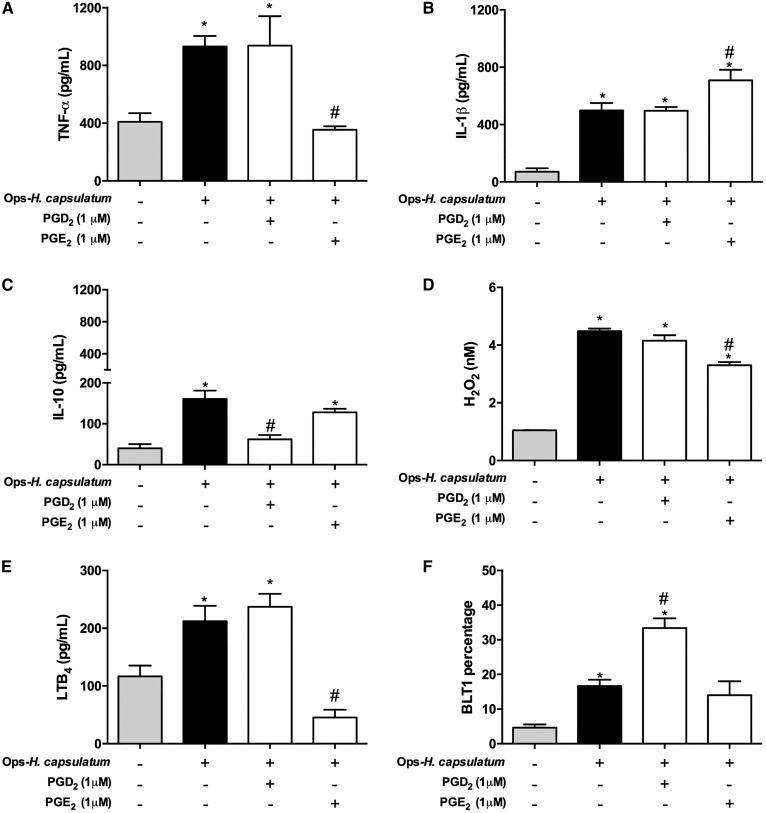
Distinct effects of PGD2 and PGE2 on production of inflammatory mediators and BLT1 receptor expression by AMs infected with Ops-H. capsulatum
In tissues and cells infected by H. capsulatum, production of different inflammatory mediators appears to influence the success or failure of the host to control the infection (9, 36–39). Therefore, we investigated the extent to which PGs could modulate TNF-α, IL-1β, IL-10, LTB4, and H2O2 production and BLT1 receptor expression by infected AMs. Ops-H. capsulatum infection induced production of all mediators listed above and increased the BLT1 when compared with uninfected cells. Interestingly, TNF-α, IL-10, and H2O2 were significantly reduced when AMs were preincubated with PGE2 (Fig. 6A, C, D), whereas IL-1β was increased (Fig. 6B). In contrast, administration of PGD2 did not modify TNF-α, IL-1β, and H2O2 (Fig. 6A, B, D), but inhibited IL-10 production (Fig. 6C). Specifically, regarding the cross-talk between lipid mediators, we observed that PGE2 negatively regulated LTB4 production, unlike PGD2, which exerted no effect (Fig. 6E). However, only PGD2 increased the expression of BLT1 induced by the infection (Fig. 6F). These findings reinforce the idea that PGE2 suppresses proinflammatory mediator production, which in fact might impair fungi elimination by AMs. Alternatively, PGD2 inhibited the production of IL-10, which represents a negative regulator of phagocytosis and killing, while simultaneously facilitating LTB4 beneficial effector functions.
Fig. 6.
TNF-α, IL-1β, IL-10, H2O2, and LTB4 production and BLT1 receptor expression by infected AMs induced by exogenous PGD2 and PGE2. AMs were pretreated with PGD2 (1 μM) or with PGE2 (1 μM) for 2 min before infection with Ops-H. capsulatum (MOI 1:10). The supernatants were collected after 48 h of incubation and TNF-α (A), IL-1β (B), IL-10 (C), H2O2 (D), and LTB4 (E) concentrations were determined by immunoassays. F: Expression of BLT1 receptor shown was analyzed by flow cytometry. * P < 0.05 (uninfected AMs vs. other groups); # P < 0.05 (AMs + Ops-H. capsulatum vs. other groups). One-way ANOVA and Tukey’s multiple comparison tests were used. Data are representative of two independent experiments (±SEM).
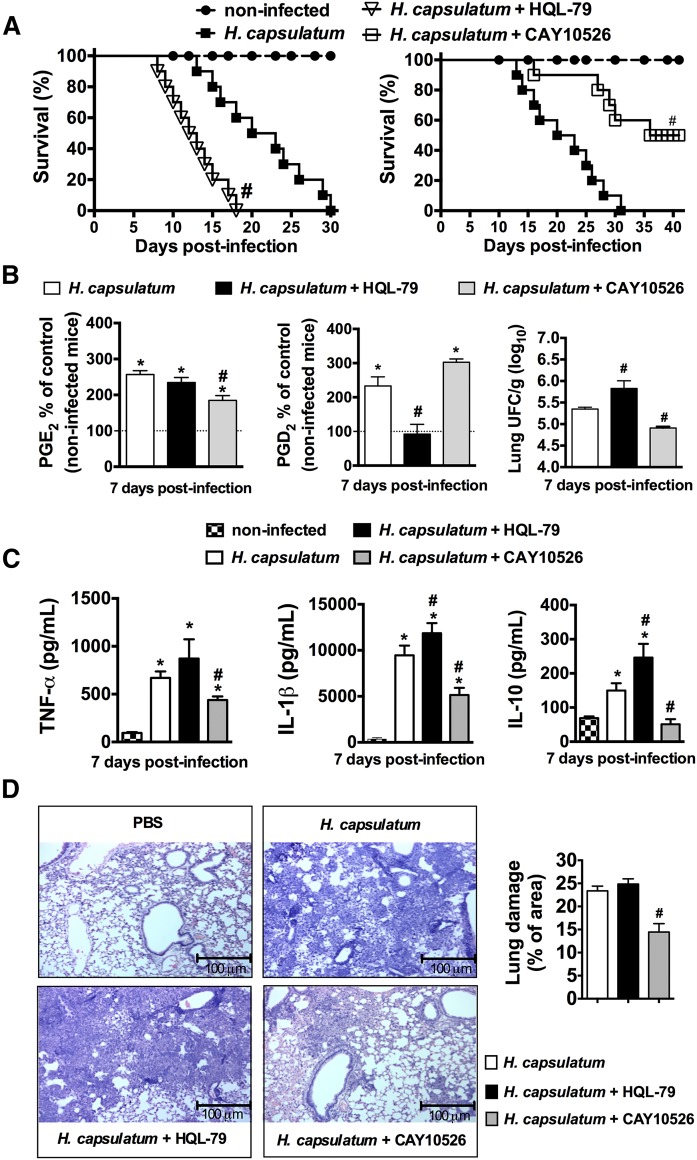
Differential effects of inhibition of PGD2 and PGE2 during H. capsulatum infection in vivo
Previous experiments in our laboratory showed that PGs are important mediators of the pathogenesis of pulmonary histoplasmosis (8). In this study, we showed that the two PGs had different effects on activating AMs for phagocytosis and killing. To examine the physiological differences between PGD2 and PGE2, mice were infected with a lethal H. capsulatum inoculum and treated daily with selective PGD2 or PGE2 synthesis inhibitors. Infected mice began to die 14 days postinfection; mice treated with the selective inhibitor of PGD synthase HQL-79 showed a higher death rate, whereas mice treated with CAY10526, an inhibitor of microsomal PGE synthase activity, showed increased survival (Fig. 7A). The specificity effect of HQL-79 and CAY10526 was observed 7 days postinfection based on inhibition of PGD2 and PGE2 production (Fig. 7B). Also, inhibition of PGD2 synthesis increased the fungal burden (Fig. 7B). H. capsulatum-infected mice treated with CAY10526 decreased TNF-α, IL-1β, and IL-10 production (Fig. 7C). However, HQL-79 treatment significantly increased IL-1β and IL-10 production 7 days after H. capsulatum infection (Fig. 7C). The lung inflammation was observed in Fig. 7D, where the inhibition of PGE2 synthesis decreased inflammatory cell infiltrate and lung damage. These data demonstrate that inhibition of PGD2 increases the susceptibility of mice to H. capsulatum infection, whereas inhibition of PGE2 increases resistance, but reduces tissue damage.
Fig. 7.
PG synthesis inhibition differently affects the survival of mice infected with lethal inoculum of H. capsulatum and inflammatory parameters. A: Mice were treated daily orally with water or HQL-79 (inhibitor of PGD2 synthesis: 3 mg/kg/0.5 ml) for 30 days (n = 10) or treated daily with CAY10526 (inhibitor of PGE2 synthesis: 5 mg/kg/0.5 ml) for 40 days (n = 10). A group of noninfected mice was used as controls (n = 10). B: Lipid mediators PGD2 and PGE2 in the lung parenchyma were measured by enzyme immunoassay and fungal burden in the lungs. C: Lung cytokine concentrations (TNF-α, IL-1β, and IL-10) were determined at 7 days following infection by ELISA. D: Lung tissues were also processed and stained with H&E to detect leukocyte infiltration after 7 days. Percent of cell infiltrated area corresponding to extent of lung damage. # P < 0.05 (H. capsulatum + H2O vs. H. capsulatum + HQL-79 or H. capsulatum + CAY10526 treatment); * P < 0.05 [PBS (non-infected mice, dashed line) vs. H. capsulatum + H2O or H. capsulatum + HQL-79 or H. capsulatum + CAY10526 treatment]. A: Log rank test for survival analysis was used. B–D: One-way ANOVA and Tukey’s multiple comparison test were used. Data are representative of two independent experiments (±SEM).
DISCUSSION
Histoplasmosis is a fungal disease that affects the lungs, especially in immunocompromised patients worldwide (1). Previously, we demonstrated that the host control of H. capsulatum infection was dependent on the production of cytokines and lipid mediators (8, 9). The specific importance of endogenous PGs in this lung fungal infection was highlighted by the treatment of H. capsulatum-infected mice with a selective inhibitor of COX-2, which primarily reduced PG synthesis while increasing LTB4 production (presumably through shunting of AA metabolism), consequently reducing inflammation and increasing the resolution of the infection (8, 9). Nevertheless, a well-orchestrated pulmonary host response against infection requires activation of AMs, which themselves produce and respond to mediators that regulate critical functions such as phagocytosis and killing of microorganisms (7, 14, 15, 40, 41). The investigation of lipid mediator effects on AMs is extremely relevant (42, 43), since these cells contribute to innate and adaptive immune response triggered by microorganisms (5). It has been suggested that PGE2 and PGD2 can induce opposite, antagonistic actions (10), but nothing has been described about the effects of these two PGs on AM functions during H. capsulatum infection. Therefore, in the present study, we explored distinctive roles of PGE2 and PGD2 in AM effector functions after in vitro Ops-H. capsulatum infection.
Our results demonstrate that PGD2 has a protective role, by enhancing the phagocytosis and killing of Ops-H. capsulatum, whereas PGE2 contributes to susceptibility, by impairing phagocytosis and fungicidal capacity of AMs. Investigation of the receptors involved in PGD2 actions revealed that DP2 is a key receptor in exogenous PGD2, boosting AM phagocytosis and killing. DP2 is a receptor coupled to subunit Gαi of G protein (21), which is a molecule involved in elevation of intracellular calcium and reduction of cAMP production (21). The role of cAMP in macrophage effector mechanisms is well documented; there is a strong inverse correlation between the production of this second messenger and the ability of macrophages to engage in phagocytosis and killing (15, 44, 45). Thus, we suggest that PGD2-induced improvements in AM phagocytosis and killing are likely the result of decreased cAMP levels via DP2 receptors (17, 21). Conversely, we demonstrated that exogenous PGD2, signaling via DP2, increased the phagocytosis and killing of Ops-H. capsulatum. Treatment with DP2 antagonist, in the presence of exogenous PGD2, may impair the control on cAMP levels and activation of PKA, resulting in the inhibition of effector mechanisms of macrophages and other cells (18–20). Furthermore, we investigated the action of DP1 or DP2 antagonists on endogenous PGD2 produced by infected AMs, and we did not observe any effects on phagocytosis and killing activity. Therefore, we suggest that DP1 and DP2 are differentially expressed in AMs, resulting in distinct and opposite cell signaling, as previously described (18, 42).
As opposed to PGD2, we found that PGE2 inhibited effector AM mechanisms, and its actions were mediated by both EP2 and EP4 receptors. Engagement of PGE2 on EP2 and EP4 inhibits FcγR-mediated phagocytosis and bacterial killing, by mechanisms driven by elevation of cAMP (14, 15). Also, we investigated the action of EP2 or EP4 antagonists alone on endogenous PGE2 produced by infected AMs and reduced on phagocytosis and fungicidal activity were observed. We speculated that blocking only one of these receptors might have resulted in compensatory increases in the endogenous signaling of the other receptor pathway. The involvement of cAMP as a key element in the actions of PGE2 and PGD2 on AM effector function is also supported by the impact of these two lipids on cytokine production (20, 42, 46). Other work demonstrated that cAMP upregulates IL-10 and inhibits TNF-α synthesis (47). Also, increased release of cAMP prevented the generation of reactive oxygen and nitrogen intermediates, phagosome acidification and lysosomal enzyme release, and consequently microorganism killing (48). In histoplasmosis, effective host defense requires the production of cytokines, such IL-12, IFN-γ, TNF-α, IL-1β (20, 36, 37, 39), and LTB4 (9), which, acting via BLT1 receptors, contribute to the elimination of the fungus (49). In contrast, IL-10 is a negative regulator that inhibits phagosome maturation, leading to profound intracellular proliferation of microorganisms (50). Production of these cytokines is under the control of the transcription factor NF-κB, which translocates to the nucleus after activation of MAPK pathway in response to pathogens (51). Infection of AMs with Ops-H. capsulatum induced phosphorylation of p38 MAPK, JNK1/2, and PLC-γ. Interestingly, PGE2 treatment in AMs within 30 min of infection increased the phosphorylation of JNK1/2, with subsequent inhibition of phosphorylation of p38 after 120 min of infection and reduced NF-κB/AP-1 activity and TNF-α production after 24 h. In this regard, it was previously demonstrated that p38 signaling is important for TNF-α production and macrophage activation (52), whereas PKA activation is necessary for downregulation of TNF-α by lipopolysaccharide (53). Activation of JNK can be negatively regulated by NF-κB (54). Based on these findings, we hypothesized that activation of the MAPK, PLC-γ, and NF-κB/AP-1 pathways by PGE2 and PGD2 in Ops-H. capsulatum-infected AMs contributed differently to phagocytosis and fungicidal activity. Further studies are needed to investigate the pivotal role of these pathways in AM effector functions and the cross-talk between them. Moreover, we demonstrated that PGD2 reduced IL-10 production by AMs, and inhibition of PGD2 production in vivo enhanced IL-10 release in the H. capsulatum infected lung, which correlated with increased phagocytosis and killing of H. capsulatum. In contrast, PGE2 treatment inhibited the production of TNF-α and H2O2, which are important mediators of macrophage activation and killing (55, 56). Finally, we demonstrated the effects of PGD2 that were opposite to those of PGE2 during H. capsulatum infection in mice. While PGD2 showed protective effects in infected mice, PGE2 showed suppressive action in the lung during infection. On this model, inhibition of PGE2 production diminished inflammatory cell infiltrate and lung damage, accompanied by reduced production of TNF-α and IL-1β.
To our knowledge, the present study describes, for the first time, the role of PGE2 and PGD2 in AM effector mechanisms in the context of H. capsulatum infection. We demonstrated a new role for PGD2 as a key mediator for effective phagocytosis and fungicidal functions of AMs. On the other hand, these studies revealed that PGE2 impairs phagocytosis and killing of the fungus. Notwithstanding, these findings shed light on novel perspectives for the treatment of fungal diseases using PGD2 as a potential pharmacological immunomodulatory agent to control the infection.
Acknowledgments
We would like to thank Dr. Huy Ong at the University of Montréal (Canada) for kindly donating the RAW-BlueTM cells used in this study; Elaine Floreano Peixoto for helping with the histology techniques; and Fabiana Rosseto de Morais for helping with Flow Cytometer analysis.
Footnotes
Abbreviations:
- AM
- alveolar macrophage
- BLT1
- leukotriene B4 receptor
- CFU
- colony-forming unit
- COX
- cyclooxygenase
- DP1
- D prostanoid receptor 1
- DP2
- chemoattractant receptor-homologous molecule expressed on Th2 lymphocytes
- EP
- E prostanoid
- IS
- immune serum
- JNK1/2
- c-Jun N-terminal kinase 1/2
- LT
- leukotriene
- MFI
- mean of relative fluorescence units
- MOI
- multiplicity of infection
- NF-κB
- nuclear factor κB
- ops-H. capsulatum
- opsonized Histoplasma capsulatum
- PG
- prostaglandin
- PKA
- protein kinase A
- PLC-γ
- phospholipase C-γ
This study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo Grants Process 2009/07169-5 and 2014/07125-6; and the Conselho Nacional de Desenvolvimento Científico e Tecnológico. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the paper. The authors declare that there are no conflicts of interest.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Kauffman C. A. 2009. Histoplasmosis. Clin. Chest Med. 30: 217–225. [DOI] [PubMed] [Google Scholar]
- 2.Kasuga T., White T. J., Koenig G., McEwen J., Restrepo A., Castaneda E., Lacaz C., Heins-Vaccari E. M., De Freitas R. S., Zancope-Oliveira R. M., et al. . 2003. Phylogeography of the fungal pathogen Histoplasma capsulatum. Mol. Ecol. 12: 3383–3401. [DOI] [PubMed] [Google Scholar]
- 3.Colombo A. L., Tobon A., Restrepo A., Queiroz-Telles F., and Nucci M.. 2011. Epidemiology of endemic systemic fungal infections in Latin America. Med. Mycol. 49: 785–798. [DOI] [PubMed] [Google Scholar]
- 4.Antinori S. 2014. Histoplasma capsulatum: more widespread than previously thought. Am. J. Trop. Med. Hyg. 90: 982–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lohmann-Matthes M. L., Steinmuller C., and Franke-Ullmann G.. 1994. Pulmonary macrophages. Eur. Respir. J. 7: 1678–1689. [PubMed] [Google Scholar]
- 6.Medeiros A. I., Sá-Nunes A., Turato W. M., Secatto A., Frantz F. G., Sorgi C. A., Serezani C. H., Deepe G. S. Jr., and Faccioli L. H.. 2008. Leukotrienes are potent adjuvant during fungal infection: effects on memory T cells. J. Immunol. 181: 8544–8551. [DOI] [PubMed] [Google Scholar]
- 7.Secatto A., Rodrigues L. C., Serezani C. H., Ramos S. G., Dias-Baruffi M., Faccioli L. H., and Medeiros A. I.. 2012. 5-Lipoxygenase deficiency impairs innate and adaptive immune responses during fungal infection. PLoS One. 7: e31701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pereira P. A., Trindade B. C., Secatto A., Nicolete R., Peres-Buzalaf C., Ramos S. G., Sadikot R., Bitencourt Cda S., and Faccioli L. H.. 2013. Celecoxib improves host defense through prostaglandin inhibition during Histoplasma capsulatum infection. Mediators Inflamm. 2013: 950981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Medeiros A. I., Sa-Nunes A., Soares E. G., Peres C. M., Silva C. L., and Faccioli L. H.. 2004. Blockade of endogenous leukotrienes exacerbates pulmonary histoplasmosis. Infect. Immun. 72: 1637–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris S. G., Padilla J., Koumas L., Ray D., and Phipps R. P.. 2002. Prostaglandins as modulators of immunity. Trends Immunol. 23: 144–150. [DOI] [PubMed] [Google Scholar]
- 11.Joo M., and Sadikot R. T.. 2012. PGD synthase and PGD2 in immune response. Mediators Inflamm. 2012: 503128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joo M., Kwon M., Sadikot R. T., Kingsley P. J., Marnett L. J., Blackwell T. S., Peebles R. S., Urade Y. Jr., and Christman J. W.. 2007. Induction and function of lipocalin prostaglandin D synthase in host immunity. J. Immunol. 179: 2565–2575. [DOI] [PubMed] [Google Scholar]
- 13.Nakanishi M., and Rosenberg D. W.. 2013. Multifaceted roles of PGE2 in inflammation and cancer. Semin. Immunopathol. 35: 123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serezani C. H., Chung J., Ballinger M. N., Moore B. B., Aronoff D. M., and Peters-Golden M.. 2007. Prostaglandin E2 suppresses bacterial killing in alveolar macrophages by inhibiting NADPH oxidase. Am. J. Respir. Cell Mol. Biol. 37: 562–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aronoff D. M., Canetti C., and Peters-Golden M.. 2004. Prostaglandin E2 inhibits alveolar macrophage phagocytosis through an E-prostanoid 2 receptor-mediated increase in intracellular cyclic AMP. J. Immunol. 173: 559–565. [DOI] [PubMed] [Google Scholar]
- 16.Zoccal K. F., Sorgi C. A., Hori J. I., Paula-Silva F. W. G., Arantes E. C., Serezani C. H., Zamboni D. S., and Faccioli L. H.. 2016. Opposing roles of LTB4 and PGE2 in regulating the inflammasome-dependent scorpion venom-induced mortality. Nat. Commun. 7: 10760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirai H., Tanaka K., Yoshie O., Ogawa K., Kenmotsu K., Takamori Y., Ichimasa M., Sugamura K., Nakamura M., Takano S., et al. . 2001. Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J. Exp. Med. 193: 255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jandl K., Stacher E., Balint Z., Sturm E. M., Maric J., Peinhaupt M., Luschnig P., Aringer I., Fauland A., Konya V., et al. . 2016. Activated prostaglandin D2 receptors on macrophages enhance neutrophil recruitment into the lung. J. Allergy Clin. Immunol. 137: 833–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y., Perussia B., and Campbell K. S.. 2007. Prostaglandin D2 suppresses human NK cell function via signaling through D prostanoid receptor. J. Immunol. 179: 2766–2773. [DOI] [PubMed] [Google Scholar]
- 20.Faveeuw C., Gosset P., Bureau F., Angeli V., Hirai H., Maruyama T., Narumiya S., Capron M., and Trottein F.. 2003. Prostaglandin D2 inhibits the production of interleukin-12 in murine dendritic cells through multiple signaling pathways. Eur. J. Immunol. 33: 889–898. [DOI] [PubMed] [Google Scholar]
- 21.Sawyer N., Cauchon E., Chateauneuf A., Cruz R. P., Nicholson D. W., Metters K. M., O’Neill G. P., and Gervais F. G.. 2002. Molecular pharmacology of the human prostaglandin D2 receptor, CRTH2. Br. J. Pharmacol. 137: 1163–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ubersax J. A., and Ferrell J. E. Jr. 2007. Mechanisms of specificity in protein phosphorylation. Nat. Rev. Mol. Cell Biol. 8: 530–541. [DOI] [PubMed] [Google Scholar]
- 23.Han J., and Ulevitch R. J.. 2005. Limiting inflammatory responses during activation of innate immunity. Nat. Immunol. 6: 1198–1205. [DOI] [PubMed] [Google Scholar]
- 24.Mancuso P., and Peters-Golden M.. 2000. Modulation of alveolar macrophage phagocytosis by leukotrienes is Fc receptor-mediated and protein kinase C-dependent. Am. J. Respir. Cell Mol. Biol. 23: 727–733. [DOI] [PubMed] [Google Scholar]
- 25.Assis P. A., Espindola M. S., Paula-Silva F. W., Rios W. M., Pereira P. A., Leao S. C., Silva C. L., and Faccioli L. H.. 2014. Mycobacterium tuberculosis expressing phospholipase C subverts PGE2 synthesis and induces necrosis in alveolar macrophages. BMC Microbiol. 14: 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J. W., Woodward D. F., Martos J. L., Cornell C. L., Carling R. W., Kingsley P. J., and Marnett L. J.. 2016. Multitargeting of selected prostanoid receptors provides agents with enhanced anti-inflammatory activity in macrophages. FASEB J. 30: 394–404. [DOI] [PubMed] [Google Scholar]
- 27.Aritake K., Kado Y., Inoue T., Miyano M., and Urade Y.. 2006. Structural and functional characterization of HQL-79, an orally selective inhibitor of human hematopoietic prostaglandin D synthase. J. Biol. Chem. 281: 15277–15286. [DOI] [PubMed] [Google Scholar]
- 28.Peck R. 1985. A one-plate assay for macrophage bactericidal activity. J. Immunol. Methods. 82: 131–140. [DOI] [PubMed] [Google Scholar]
- 29.Brummer E., and Stevens D. A.. 1995. Antifungal mechanisms of activated murine bronchoalveolar or peritoneal macrophages for Histoplasma capsulatum. Clin. Exp. Immunol. 102: 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pick E., Charon J., and Mizel D.. 1981. A rapid densitometric microassay for nitroblue tetrazolium reduction and application of the microassay to macrophages. J. Reticuloendothel. Soc. 30: 581–593. [PubMed] [Google Scholar]
- 31.Zoccal K. F., Bitencourt Cda S., Paula-Silva F. W., Sorgi C. A., de Castro Figueiredo Bordon K., Arantes E. C., and Faccioli L. H.. 2014. TLR2, TLR4 and CD14 recognize venom-associated molecular patterns from Tityus serrulatus to induce macrophage-derived inflammatory mediators. PLoS One. 9: e88174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsushita N., Hizue M., Aritake K., Hayashi K., Takada A., Mitsui K., Hayashi M., Hirotsu I., Kimura Y., Tani T., et al. . 1998. Pharmacological studies on the novel antiallergic drug HQL-79: I. Antiallergic and antiasthmatic effects in various experimental models. Jpn. J. Pharmacol. 78: 1–10. [DOI] [PubMed] [Google Scholar]
- 33.Coulombe F., Jaworska J., Verway M., Tzelepis F., Massoud A., Gillard J., Wong G., Kobinger G., Xing Z., Couture C., et al. . 2014. Targeted prostaglandin E2 inhibition enhances antiviral immunity through induction of type I interferon and apoptosis in macrophages. Immunity. 40: 554–568. [DOI] [PubMed] [Google Scholar]
- 34.Tristão F. S., Leonello P. C., Nagashima L. A., Sano A., Ono M. A., and Itano E. N.. 2012. Carbohydrate-rich high-molecular-mass antigens are strongly recognized during experimental Histoplasma capsulatum infection. Rev. Soc. Bras. Med. Trop. 45: 232–237. [DOI] [PubMed] [Google Scholar]
- 35.Schröder R., Merten N., Mathiesen J. M., Martini L., Kruljac-Letunic A., Krop F., Blaukat A., Fang Y., Tran E., Ulven T., et al. . 2009. The C-terminal tail of CRTH2 is a key molecular determinant that constrains Galphai and downstream signaling cascade activation. J. Biol. Chem. 284: 1324–1336. [DOI] [PubMed] [Google Scholar]
- 36.Deepe G. S. Jr., and Gibbons R. S.. 2006. T cells require tumor necrosis factor-alpha to provide protective immunity in mice infected with Histoplasma capsulatum. J. Infect. Dis. 193: 322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allendoerfer R., and Deepe G. S. Jr. 1998. Blockade of endogenous TNF-alpha exacerbates primary and secondary pulmonary histoplasmosis by differential mechanisms. J. Immunol. 160: 6072–6082. [PubMed] [Google Scholar]
- 38.Peng J. K., Lin J. S., Kung J. T., Finkelman F. D., and Wu-Hsieh B. A.. 2005. The combined effect of IL-4 and IL-10 suppresses the generation of, but does not change the polarity of, type-1 T cells in Histoplasma infection. Int. Immunol. 17: 193–205. [DOI] [PubMed] [Google Scholar]
- 39.Deepe G. S. Jr., and McGuinness M.. 2006. Interleukin-1 and host control of pulmonary histoplasmosis. J. Infect. Dis. 194: 855–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soares E. M., Mason K. L., Rogers L. M., Serezani C. H., Faccioli L. H., and Aronoff D. M.. 2013. Leukotriene B4 enhances innate immune defense against the puerperal sepsis agent Streptococcus pyogenes. J. Immunol. 190: 1614–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Serezani C. H., Kane S., Medeiros A. I., Cornett A. M., Kim S. H., Marques M. M., Lee S. P., Lewis C., Bourdonnay E., Ballinger M. N., et al. . 2012. PTEN directly activates the actin depolymerization factor cofilin-1 during PGE2-mediated inhibition of phagocytosis of fungi. Sci. Signal. 5: ra12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tajima T., Murata T., Aritake K., Urade Y., Hirai H., Nakamura M., Ozaki H., and Hori M.. 2008. Lipopolysaccharide induces macrophage migration via prostaglandin D(2) and prostaglandin E(2). J. Pharmacol. Exp. Ther. 326: 493–501. [DOI] [PubMed] [Google Scholar]
- 43.Pereira P. A., Bitencourt Cda S., dos Santos D. F., Nicolete R., Gelfuso G. M., and Faccioli L. H.. 2015. Prostaglandin D2-loaded microspheres effectively activate macrophage effector functions. Eur. J. Pharm. Sci. 78: 132–139. [DOI] [PubMed] [Google Scholar]
- 44.Rogers L. M., Thelen T., Fordyce K., Bourdonnay E., Lewis C., Yu H., Zhang J., Xie J., Serezani C. H., Peters-Golden M., et al. . 2014. EP4 and EP2 receptor activation of protein kinase A by prostaglandin E2 impairs macrophage phagocytosis of Clostridium sordellii. Am. J. Reprod. Immunol. 71: 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peres C. M., Aronoff D. M., Serezani C. H., Flamand N., Faccioli L. H., and Peters-Golden M.. 2007. Specific leukotriene receptors couple to distinct G proteins to effect stimulation of alveolar macrophage host defense functions. J. Immunol. 179: 5454–5461. [DOI] [PubMed] [Google Scholar]
- 46.Hilkens C. M., Snijders A., Snijdewint F. G., Wierenga E. A., and Kapsenberg M. L.. 1996. Modulation of T-cell cytokine secretion by accessory cell-derived products. Eur. Respir. J. Suppl. 22: 90s–94s. [PubMed] [Google Scholar]
- 47.Kim S. H., Serezani C. H., Okunishi K., Zaslona Z., Aronoff D. M., and Peters-Golden M.. 2011. Distinct protein kinase A anchoring proteins direct prostaglandin E2 modulation of Toll-like receptor signaling in alveolar macrophages. J. Biol. Chem. 286: 8875–8883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Serezani C. H., Ballinger M. N., Aronoff D. M., and Peters-Golden M.. 2008. Cyclic AMP: master regulator of innate immune cell function. Am. J. Respir. Cell Mol. Biol. 39: 127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Secatto A., Soares E. M., Locachevic G. A., Assis P. A., Paula-Silva F. W., Serezani C. H., de Medeiros A. I., and Faccioli L. H.. 2014. The leukotriene B(4)/BLT(1) axis is a key determinant in susceptibility and resistance to histoplasmosis. PLoS One. 9: e85083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nguyen T., Robinson N., Allison S. E., Coombes B. K., Sad S., and Krishnan L.. 2013. IL-10 produced by trophoblast cells inhibits phagosome maturation leading to profound intracellular proliferation of Salmonella enterica Typhimurium. Placenta. 34: 765–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lawrence T. 2009. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 1: a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kang Y. J., Chen J., Otsuka M., Mols J., Ren S., Wang Y., and Han J.. 2008. Macrophage deletion of p38alpha partially impairs lipopolysaccharide-induced cellular activation. J. Immunol. 180: 5075–5082. [DOI] [PubMed] [Google Scholar]
- 53.Zhu W., Downey J. S., Gu J., Di Padova F., Gram H., and Han J.. 2000. Regulation of TNF expression by multiple mitogen-activated protein kinase pathways. J. Immunol. 164: 6349–6358. [DOI] [PubMed] [Google Scholar]
- 54.Tang G., Minemoto Y., Dibling B., Purcell N. H., Li Z., Karin M., and Lin A.. 2001. Inhibition of JNK activation through NF-kappaB target genes. Nature. 414: 313–317. [DOI] [PubMed] [Google Scholar]
- 55.Kunkel S. L., Spengler M., May M. A., Spengler R., Larrick J., and Remick D.. 1988. Prostaglandin E2 regulates macrophage-derived tumor necrosis factor gene expression. J. Biol. Chem. 263: 5380–5384. [PubMed] [Google Scholar]
- 56.Sporn P. H., Peters-Golden M., and Simon R. H.. 1988. Hydrogen-peroxide-induced arachidonic acid metabolism in the rat alveolar macrophage. Am. Rev. Respir. Dis. 137: 49–56. [DOI] [PubMed] [Google Scholar]