Abstract
Conversion of diacylglycerol to phosphatidic acid is mediated by diacylglycerol kinases (DGKs), with DGKα specifically linked to adaptive immune responses. We determined the role of DGKα in obesity and inflammatory responses to a high-fat diet (HFD). DGKα KO and WT littermates were either a) chow-fed, b) HFD-fed for 12 weeks (Long-Term HFD), or c) HFD-fed for 3 days (Acute HFD). Body weight/composition, oxygen consumption, food intake, and glucose tolerance was unaltered between chow-fed DGKα KO and WT mice. Insulin concentration during the intraperitoneal glucose tolerance (IPGT) test was elevated in chow-fed DGKα KO mice, suggesting mild insulin resistance. Insulin concentration during the IPGT test was reduced in Long-Term HFD-fed DGKα KO mice, suggesting a mild enhancement in insulin sensitivity. Acute HFD increased hormone sensitive lipase protein abundance and altered expression of interleukin 1β mRNA, an inflammatory marker in perigonadal adipose tissue of DGKα KO mice. In conclusion, DGKα ablation is associated with mild alterations in insulin sensitivity. However, DGKα is dispensable for whole body insulin-mediated glucose uptake, hepatic glucose production, and energy homeostasis. Our results suggest DGKα aids in modulating the early immune response of adipose tissue following an acute exposure to HFD, possibly through modulation of acute T-cell action.
Keywords: cytokines, glucose, insulin
Diacylglycerol (DAG) is a precursor for triglycerides and phospholipids and acts as a second messenger. Infusion of free fatty acids in healthy humans increases DAG concentration and causes insulin resistance (1). As a second messenger, conversion of DAG to phosphatidic acid (PA) marks inactivation of DAG-sensitive targets; however, DAG to PA conversion can also mark activation of PA-sensitive targets. Diacylglycerol kinase (DGK) is responsible for the conversion of DAG to PA. DGKs constitute a family of 10 different enzymes that are classified into five subfamilies. Various DGK family members can be expressed in the same tissue (2, 3). Specific DGK isoforms have been linked to growth and metabolism. For example, DGKζ plays a role in skeletal muscle growth and differentiation (4, 5) and cardiac hypertrophy (6, 7). DGKδ has been associated with the development of insulin resistance and type 2 diabetes (8). Partial ablation of DGKδ in mice resulted in decreased DAG content in skeletal muscle, insulin resistance, and increased weight gain with age (8). Whether other DGKs influence growth and metabolic processes, including insulin sensitivity and obesity, remains to be determined.
DGKα is a member of the type I subfamily of DGKs (9). The type I DGK isoforms, including α, β, and γ, contain the DAG-binding C1 domains and catalytic domain, typical of all subfamilies, as well as calcium-binding EF hand motifs, that render these members more active in the presence of calcium (10). DGKα has been studied with respect to immunology and T-cell responses. DGKα is expressed in mouse spleen, skeletal muscle, lung, and testis (11). DGKα and DGKζ are the predominant DGK family members expressed in T-cells, and ablation of these DGK family members modulates T-cell responses (12, 13). Thus, T-cells lacking DGKα show impaired anergy in vitro, as demonstrated by interleukin (IL) 2 secretion and proliferation of T-cells following restimulation with antigen staphylococcal enterotoxin B (14). These findings highlight a prominent role for DGKα in modulating T-cell responses. Furthermore, DGKα overexpression protects cancer cells from TNF α-induced apoptosis (15). TNFα is a potent inducer of insulin resistance (16) and transcription of TNFα is increased in white adipose tissue (WAT) derived from obese and diabetic rodent models (17). This provides further evidence that DGKα modulates T-cell responses and TNFα-mediated signaling.
Excess caloric intake combined with unaltered energy expenditure increases WAT mass and leads to obesity. Obesity is a major prodrome of insulin resistance and type 2 diabetes. Increased adiposity is accompanied by a low-grade inflammatory state in WAT (18), which can further exacerbate metabolic abnormalities in obese individuals, including insulin resistance, type 2 diabetes, fatty liver disease, hypertension, dyslipidemia, atherosclerosis, and some cancers (19). The inflammatory state is visible by macrophages and T-cells that are recruited to WAT in obese individuals and ob/ob mice (20–22). The recruitment of these cells is facilitated by inflammatory cytokines that are secreted from WAT, including TNFα, IL6, and IL1β, which are associated with insulin resistance and obesity (23–25). Excessive intracellular lipid metabolites in peripheral tissues in obesity can also induce chronic inflammation (26). Thus, lipid metabolizing enzymes may also influence the immune tone of organs that control glucose and energy homeostasis.
As DGKα plays a role in inflammatory responses, we determined whether this lipid metabolizing enzyme is associated with obesity and insulin resistance. Specifically, we determined the role of DGKα in whole-body glucose and energy homeostasis as well as WAT inflammation. We report that DGKα deficiency is associated with reduced expression of IL1β in WAT in mice fed a high-fat diet (HFD) for 3 days. However, with prolonged (3 months) HFD, inflammatory responses and glucose homeostasis were unaltered. Our results suggest DGKα is involved in the early inflammatory responses to an Acute HFD but is dispensable for the Long-Term maintenance of glucose and energy homeostasis.
MATERIALS AND METHODS
Mouse models and experimental protocols
Animals were housed in a temperature- and light-controlled environment. Experiments were performed in female DGKα KO mice and WT littermates. Generation of the whole-body DGKα KO mouse model is described elsewhere (14). The expression of DGK δ, η, ε, and ζ isoforms known to be expressed in adipose tissue (27) was unaltered between DGKα KO and WT littermates (Data not shown). Thus, other DGK isoforms do not appear to compensate for the loss of DGKα in this model. Animals were maintained at a 12 h light-dark cycle and had ad libitum access to water and a standard rodent chow (Lantmännen, Stockholm, Sweden) or 55% adjusted fat HFD (TD.93075, Harlan Teklad, Indianapolis, IN). Three groups of mice were studied: a) 12-month-old mice fed a chow diet, b) 6-week-old mice fed HFD for 12 weeks (Long-Term HFD), and c) 10-week-old mice fed HFD for 3 days (Acute HFD). All animal experiments were approved by the Regional Ethical Committee on Animal Research, Stockholm North, Sweden.
Intraperitoneal glucose tolerance test
Glucose tolerance was assessed by an intraperitoneal injection of glucose (2 g/kg body weight). Mice were fasted 4 h prior to the intraperitoneal injection. Plasma glucose was determined directly after fasting and 15, 30, 60, and 120 min after injection with glucose. Blood samples were obtained prior to and 15 min after the glucose administration to assess plasma insulin levels using the ultra-sensitive insulin ELISA (Crystal Chem Inc. Downers Grove, IL).
Body composition
Whole-body composition was measured using an EchoMRI-100 Analyzer from EchoMRI LLC (Houston, TX). Fat and lean mass were measured.
Whole-body energy homeostasis
Whole-body energy homeostasis was determined in DGKα KO and WT mice using the Comprehensive Lab Animal Monitoring System (Columbus Instruments, Columbus, OH). Mice were individually housed with ad libitum access to water and standard chow or HFD. After a 24-h acclimatization period, oxygen consumption was measured for 48 h. Thereafter, mice were fasted overnight and refed in the morning. Food intake was determined for the dark and light phase during 24 h before the fasting.
Euglycemic-hyperinsulinemic clamp
Whole body insulin-mediated glucose uptake and hepatic glucose production was determined in conscious DGKα KO and WT mice by means of the euglycemic-hyperinsulinemic clamp as described (8). A catheter was placed in the left jugular vein and mice were allowed to recover for at least 3 days. On the day of the experiment, mice were fasted for 5 h and placed in individual plastic containers and blood was sampled from the tail. Glucose turnover rate was measured in the basal state and during the euglycemic-hyperinsulinemic state, using a constant infusion of [3-3H]glucose (NET331C001MC, PerkinElmer, Waltham, MA). Basal glucose production and utilization were assessed at 65–75 min after the start of the tracer infusion. After 75 min, the euglycemic-hyperinsulinemic clamp was started. A priming dose of insulin (22 mU/kg; Actrapid, Novo Nordisk, Bagsvaerd, Denmark) was administered, followed by a constant infusion rate of 2.5 mU/kg/min. Glucose (30%) was infused to maintain euglycemia. When the glucose infusion rate reached a steady state, blood samples were drawn to determine whole-body glucose utilization and hepatic glucose production. Hepatic glucose production was determined by subtracting the average glucose infusion rate from whole-body glucose uptake.
DGK activity in WAT
Total DGK activity was measured via ATP dependent conversion of DAG to PA. Homogenates of perigonadal adipose tissue were prepared in ice-cold buffer containing 20 mM Tris.HCl (pH 7.5), 250 mM sucrose, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, protease inhibitor cocktail set 1 (Calbiochem, Darmstadt, Germany). Samples were subjected to centrifugation (800 g, 15 min, 4°C) and protein concentration was determined in supernatant. An octyl glucoside/phosphatidylserine mixed-micelle assay for DGK activity was performed using [γ-32P]ATP as described (28). Samples were subsequently separated by TLC and plates were developed. Regions of interest containing the phospholipid product were quantified by phospho-imaging and densitometry.
Lipolysis assay
Mice were fasted for 4 h, anesthetized with 2,2,2-tribromoethanol via intraperitoneal injection, and perigonadal WAT was collected. Tissue (∼20 mg) was incubated in the absence or presence of isoprenaline (10−5 M, 10−6 M) for 90 min at 37°C in D-PBS supplemented with 2% RIA-grade BSA. The tissue was removed and the glycerol concentration in the medium was determined. Glycerol release into the medium was measured as an index of lipolysis. Glycerol was measured using a Zenbio Glycerol Analysis Kit (Research Triangle Park, NC).
RNA extraction and gene expression analysis
RNA from perigonadal WAT was extracted using a Trizol reagent and RNeasy kit from Qiagen (Hilden, Germany). RNA was DNase treated (Qiagen) prior to the cDNA synthesis. cDNA was created by using the High Capacity Reverse Transcription Kit from Applied Biosystems (Foster City, CA). Quality and yield of RNA was assessed using a NanoDrop spectrophotometer from Thermo Fisher Scientific (Waltham, MA). Gene expression was determined using a StepOnePlus quantitative real time PCR machine from Applied Biosystems. Comparison of relative gene expression data was accomplished using the 2-ΔΔCt method. Gene expression data was normalized against peptidyl-prolyl isomerase A and hypoxanthine guanine phosphoribosyl transferase 1. Primers were from Applied Biosystems (Foster City, CA): TNFα (Mm00443260_g1), IFNγ (Mm01168134_m1), IL1β (Mm00434228_m1), IL2 (Mm00434256_m1), IL6 (Mm00446190_m1), F4/80 (Mm00802529_m1), hormone sensitive lipase (HSL) (Mm00495359_m1), peptidyl-prolyl isomerase A (Mm02342430_g1) and hypoxanthine guanine phosphoribosyl transferase 1 (Mm00446968_m1).
Protein extraction and Western blot analysis
Perigonadal WAT was homogenized in an ice-cold homogenization buffer (137 mM NaCl, 20 mM Tris.HCl pH 7.8, 2.7 mM KCl, 10% glycerol, 5 mM sodium pyrophosphate, 1% Triton X-100, 1 mM MgCl2, 10 mM NaF, 1 mM EDTA, 0.2 mM PMSF, 1 μg/ml aprotinin, 1 μg/ml leupeptin, 0.5 mM sodium orthovandate). From the resulting homogenate, the supernatant was collected and 15 μg protein was separated by SDS-PAGE and transferred to polyvinyl difluoride membranes. Membranes were incubated with primary antibodies, followed by incubation with an HRP tagged secondary antibody (Bio-Rad). Antibodies were visualized by enhanced chemiluminescence and quantified using ImageJ software. The antibodies against total HSL protein (#4107) and phosphorylated (p)-HSLSer565 (#4137) were from Cell Signaling (Danvers, MA). The IgG2c antibody (A90-136AP) was purchased from Bethyl Laboratories (Montgomery, TX). Equal loading was verified by Ponceau S staining of the polyvinyl difluoride membrane (see Fig. 6C) (29).
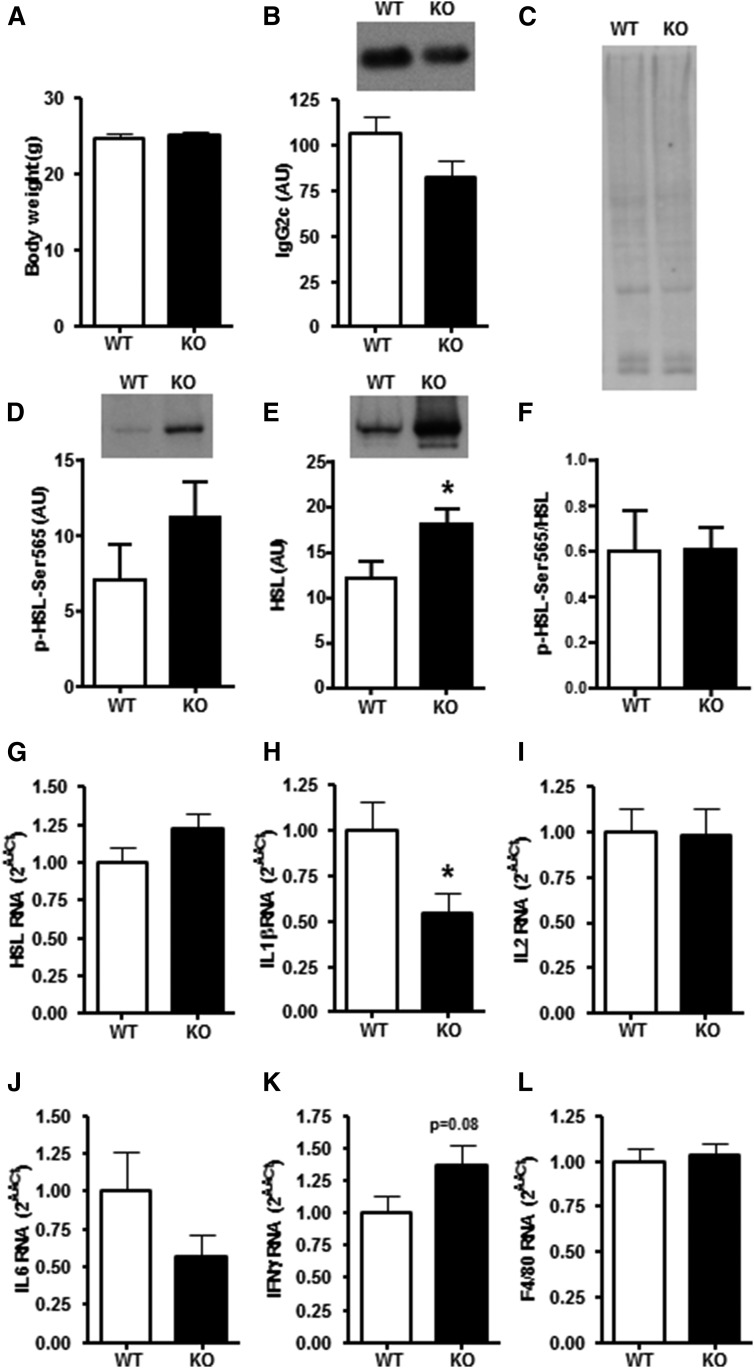
Fig. 6.
Body weight and expression of HSL and markers of inflammation in WAT of Acute HFD-fed DGKα KO and WT mice (group c). A: Body weight of DGKα KO (closed bar) and WT (open bar) mice after Acute HFD. Protein abundance of (B) IgG2c, (D) p-HSLSer565, and (E) HSL protein abundance in perigonadal WAT. (F) p-HSLSer565 to HSL abundance ratio and (C) Ponceau staining to verify equal loading. mRNA expression of (G) HSL and inflammatory markers including (H) IL1β, (I) IL2, (J) IL6, (K) IFNγ, and (L) F4/80 in perigonadal WAT. Results are mean ± SEM (n = 7–11 mice). *P < 0.05 versus WT mice.
Statistical analysis
Statistical differences were analyzed using a 2-way ANOVA. Student’s t-test was used for comparison of two parameters. Significance was accepted at P < 0.05.
RESULTS
Effect of DGKα ablation on glucose metabolism
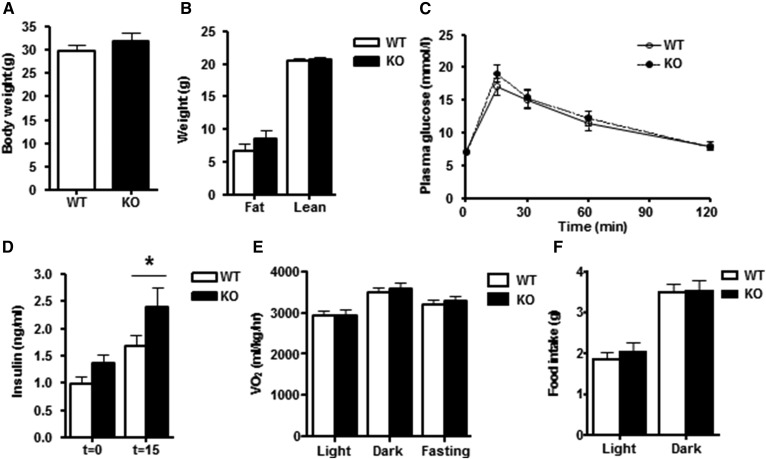
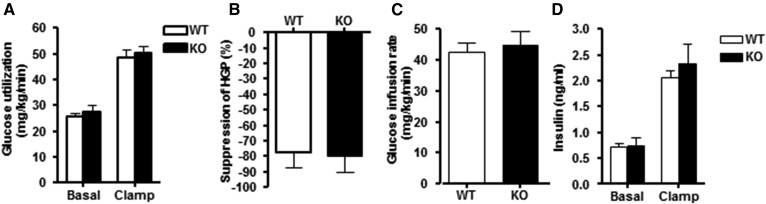
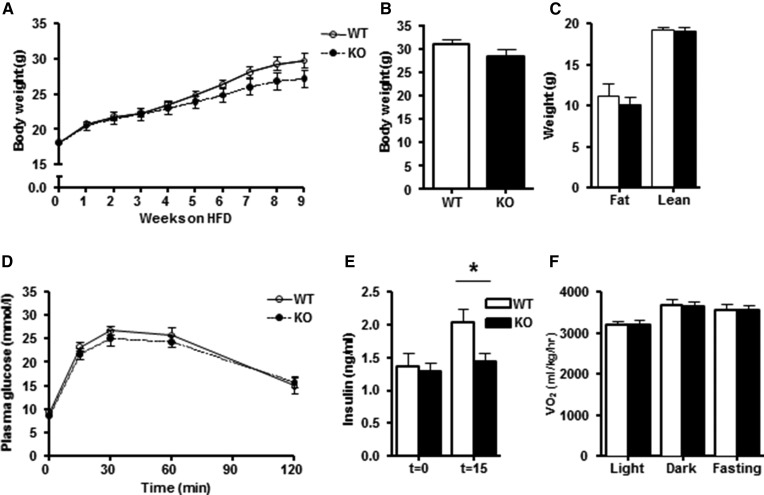
The role of DGKα on whole-body glucose metabolism and WAT was studied in chow-fed mice. Body weight and fat mass were unaltered in 12-month-old DGKα KO versus WT mice (Fig. 1A, B). Although glucose tolerance was unaltered between DGKα KO and WT mice (Fig. 1C), the insulin concentration measured at 15 min was elevated in DGKα KO mice (Fig. 1D), suggesting mild insulin resistance. Furthermore, the glucose-to-insulin ratio tended to be decreased in the DGKα KO mice (WT 8.7 ± 1.3 versus KO 5.5 ± 0.6; P = 0.07), suggesting mild insulin resistance. Whole-body energy expenditure was measured via indirect calorimetry. Changes in oxygen consumption (VO2) during the light/dark cycle, as well as with fasting, were similar between DGKα KO versus WT mice (Fig. 1E). In addition, food intake was not altered between WT and DGKα KO mice (Fig. 1F). To further study the effects of DGKα ablation on insulin sensitivity, we performed a euglycemic-hyperinsulinemic clamp in conscious mice. Whole body insulin-mediated glucose utilization and hepatic insulin sensitivity were similar between DGKα KO and WT mice (Fig. 2A–C). Insulin infusion was verified by measuring the insulin concentrations under basal- and insulin-stimulated (clamp state) conditions. We found that the insulin concentration during the euglycemic-hyperinsulinemic clamp was similar between DGKα KO and WT mice (Fig. 2D).
Fig. 1.
Body composition, energy metabolism and food intake in chow-fed DGKα KO and WT mice (group a). A: Body weight of DGKα KO (closed bar) and WT (open bar) mice. B: Fat mass and lean muscle mass of DGKα KO and WT mice as determined by MRI analysis. C: Intraperitoneal glucose tolerance (IPGT) in DGKα KO and WT mice. D: Plasma insulin concentration during IPGT test. E: VO2 assessed by indirect calorimetry. F: Food intake during dark and light phases. Results are mean ± SEM (n = 8–15 mice). *P < 0.05 versus WT mice.
Fig. 2.
Whole-body insulin-mediated glucose utilization and hepatic glucose production in chow-fed DGKα KO and WT mice (group a). Whole-body glucose utilization was assessed in conscious DGKα KO (closed bar) and WT (open bar) mice. A: Basal and insulin-stimulated whole-body glucose utilization. B: Suppression of hepatic glucose production (HGP). C: Glucose infusion rate during the euglycemic-hyperinsulinemic clamp. D: Plasma insulin concentration during basal and insulin-stimulated conditions of the euglycemic-hyperinsulinemic clamp. Results are mean ± SEM (n = 7–10 mice).
Effect of DGKα ablation on lipolysis and inflammatory markers in WAT of chow-fed mice
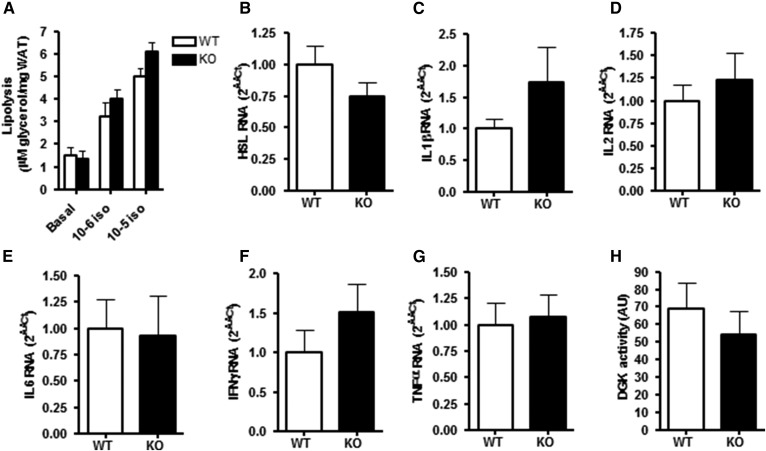
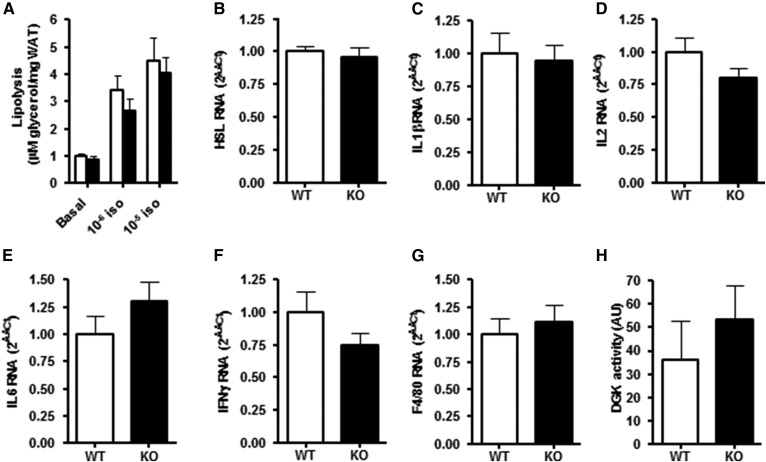
The role of DGKα was determined on lipolysis in perigonadal WAT ex vivo as measured by isoprenaline-stimulated glycerol release. Isoprenaline increased glycerol release in a concentration-dependent manner; however, glycerol release was unaltered between DGKα KO and WT mice (Fig. 3A), concomitant with unchanged HSL mRNA expression in adipose tissue (Fig. 3B). Expression of inflammatory markers IL1β, IL2, IL6, IFNγ, and TNFα were unaltered between DGKα KO and WT mice (Fig. 3C–G). Total DGK activity in perigonadal WAT was unchanged in WT and DGKα KO mice when determining concentrations of PA (Fig. 3H).
Fig. 3.
Lipolytic activity and inflammatory markers in WAT of 12-month-old chow-fed DGKα KO and WT mice (group a). A: Isoprenaline-mediated glycerol release was determined ex vivo in perigonadal WAT derived from DGKα KO (closed bar) and WT (open bar) mice. mRNA expression of (B) HSL, (C) IL1β, (D) IL2, (E) IL6, (F) IFNγ, and (G) TNFα was assessed in perigonadal WAT. H: Total DGK activity measured as DAG converted to PA in perigonadal WAT. Results are mean ± SEM (n = 8–10 mice).
Effects of DGKα ablation on body composition and glucose tolerance during Long-Term HFD
DGKα KO and WT mice were fed HFD for 12 weeks and measures of body weight, body composition, glucose tolerance and energy homeostasis were determined. Final body weight was unaltered between Long-Term HFD-fed DGKα KO and WT mice (Fig. 4A, B). Lean muscle mass and fat mass was unaltered between DGKα KO mice and WT mice (Fig. 4C). Although glucose tolerance was unaltered between Long-Term HFD-fed DGKα KO and WT mice (Fig. 4D), the insulin concentration measured at 15 min was reduced in DGKα KO mice (Fig. 4E), suggesting a modest enhancement of insulin sensitivity. The glucose to insulin ratio was however similar between WT and DGKα KO mice (WT 7.5 ± 1.1 versus KO 7.4 ± 0.9). VO2 was calculated in Long-Term HFD-fed DGKα KO and WT mice by indirect calorimetry. VO2 was similar between DGKα KO and WT mice during the light/dark cycle, as well with fasting (Fig. 4F), suggesting energy homeostasis is unaltered between genotypes.
Fig. 4.
Body composition and energy expenditure in Long-Term HFD-fed DGKα KO and WT mice (group b). A: Body weight of DGKα KO (closed circle) and WT (open circle) mice was assessed over the first 9 weeks of the 12-week Long-Term HFD. B: Body weight of DGKα KO (closed bar) and WT (open bar) mice after Long-Term HFD. C: Fat mass and lean muscle mass determined by MRI analysis. D: IPGT. E: Plasma insulin concentration during the IPGT test. F: VO2 assessed via indirect calorimetry. Results are mean ± SEM (n = 8–10 mice). *P < 0.05 versus WT mice.
Effect of DGKα ablation on lipolysis and inflammatory markers in WAT of Long-Term HFD mice
Lipolysis in perigonadal WAT was examined ex vivo as measured by isoprenaline-stimulated glycerol release. Isoprenaline increased glycerol release in a concentration dependent manner with similar effects noted between DGKα KO and WT mice (Fig. 5A). In addition, HSL mRNA expression in perigonadal WAT was unaltered between genotypes (Fig. 5B). Markers of inflammatory status were assessed in Long-Term HFD-fed DGKα KO and WT mice. mRNA expression of IL1β, IL2, IL6, IFNγ, and F4/80 was unaltered between genotypes (Fig. 5C–G). Moreover, conversion of DAG to PA was similar between DGKα KO and WT mice (Fig. 5H).
Fig. 5.
Lipolytic activity and inflammatory markers in WAT of Long-Term HFD-fed DGKα KO and WT mice (group b). A: Isoprenaline-mediated glycerol release determined ex vivo in perigonadal WAT of DGKα KO (closed bars) and WT (open bars) mice after 12 weeks of Long-Term HFD. mRNA expression of (B) HSL and inflammatory markers including (C) IL1β, (D) IL2, (E) IL6, (F) IFNγ, and (G) F4/80 in perigonadal WAT. H: Total DGK activity measured as DAG converted to PA in perigonadal WAT. Results are mean ± SEM (n = 8–10 mice).
Effect of DGKα ablation on inflammatory markers in WAT of Acute HFD mice
We next assessed the effects of Acute HFD on inflammatory markers in perigonadal WAT of DGKα KO and WT mice. This acute protocol affords the opportunity to assess the role of DGKα on inflammatory markers prior to the onset of weight gain that typically accompanies Long-Term HFD. Body weight was unaltered by Acute HFD in DGKα KO and WT mice (Fig. 6A). The inflammatory status of WAT was assessed by determining expression of markers associated with activation of T-cells (e.g., IL2, IFNγ), B-cells (e.g., IgG2c), macrophages (e.g., IL1β, F4/80), and secreted factors (i.e., IL6) from WAT. Acute HFD tended to decrease IgG2c protein abundance in perigonadal WAT of DGKα KO mice (P = 0.12; Fig. 6B). Acute HFD did not alter p-HSLSer565 (P = 0.22; Fig. 6D) whereas HSL protein abundance (Fig. 6E) was increased in perigonadal WAT from DGKα KO mice. HSL mRNA expression (P = 0.14; Fig. 6G) was unaltered in perigonadal WAT of DGKα KO mice. Conversely, Acute HFD decreased IL1β mRNA expression in perigonadal WAT of DGKα KO mice (Fig. 6H). Finally, Acute HFD tended to increase IFNγ mRNA expression in perigonadal WAT of DGKα KO mice (P = 0.08; Fig. 6K), whereas IL2, IL6, and F4/80 were unchanged between genotypes (Fig. 6I, J, L).
DISCUSSION
DGKs form a diverse family of enzymes that play a role in signal transduction and lipid homeostasis. DGKs modulate numerous biological processes by controlling the balance of DAG and PA at discrete cellular locations. The presence of multiple DGKs suggest isoform-specific physiological roles for individual DGK isoforms. For example, DGKδ deficiency is associated with peripheral insulin resistance and obesity (8), whereas DGKε ablation preserves glucose tolerance and modulates lipid metabolism (30). The opposing metabolic phenotypes observed between these KO models further reinforce that notion that isoform-specific actions of DGK may influence metabolism. Here, we report DGKα, a ubiquitously expressed isoform that modulates adaptive immune cell function (13, 14), is associated with alterations of insulin levels during a glucose tolerance test in chow-fed and Long-Term HFD fed mice. Moreover, DGKα appears to modulate the early immune response of perigonadal WAT following Acute HFD, possibly through modulation of T-cell action.
With obesity, T-cells infiltrate adipose tissue and thereby contribute to the development of adipose tissue inflammation (31). Ablation of DGKα increases DAG-dependent T cell receptor signaling and impairs anergy induction, leading to a state of immune unresponsiveness (14). The development of this T-cell unresponsive state is associated with increased IL2 production in response to anergy-producing stimulation (14). In a natural killer-like cell line, a feedback loop between DGKα and IL2 exists such that IL2 stimulation alters the subcellular localization of DGKα and DGKα inhibition reduces IL2-stimulated S phase entry of T-cells (32). Moreover, IL2 stimulation of T-cells reduces DGKα mRNA expression (33). Thus, we reasoned that ablation of DGKα may influence the immunological stress response to HFD. Although IL2 mRNA expression was unaltered in WAT of Acute HFD or 12-month-old chow-fed DGKα KO mice, we did note a modest decrease in IL2 mRNA in WAT of Long-Term HFD-fed DGKα KO mice (P = 0.16), indicating that T-cells may have reduced ability to transcribe more IL2 mRNA, which may alter IL2 production. This observation challenges the notion that T-cells lacking DGKα are hyperactive and secrete more IL2 (14). However, previous experiments demonstrating hyperactive T-cells in DGKα KO mice were based on two subsequent (in vivo and in vitro) exposures to staphylococcal enterotoxin B (14). Furthermore, the modest effect of DGKα ablation on immunological function may be related to activities of other DGK isoforms, namely DGKζ and DGKδ, which are also expressed in T-cells (14). Clearly DGKα works in concert with other isoforms to control immunological function. In addition, we have not sorted cells from the adipose tissue samples and therefore we cannot exclude the possibility that DGK activity may be altered in different cell types within adipose tissue. Total DGK activity in perigonadal adipose tissue samples was unaltered in DGKα KO mice. Due to the sensitivity of the DGK activity assay, we have not been able to analyze DGK activity in isolated cell types; thus, DGK activity was measured in the whole tissue. Furthermore, we determined total DGK activity, not the specific DGKα activity. Based on these caveats, we cannot exclude the possibility that total DGK activity, or more specifically DGKα activity, is altered in distinct cell types within adipose tissue.
Acutely inflamed WAT attracts immune cells and this may contribute to the development of insulin resistance (31, 34). IgG2c secreting B-cells accumulate in WAT after Acute HFD or aging in mice (35, 36). We found that DGKα KO mice expressed lower levels of pro-inflammatory cytokine IL1β in perigonadal WAT after Acute HFD compared with WT mice, which was further accompanied by a trend for decreased IgG2c protein abundance (P = 0.12) and increased IFNγ mRNA expression (P = 0.08). IgG2c-secreting B-cells further modulate the infiltration of other immune cells like T-cells and macrophages (36); consequently, a reduction of B-cells would suggest a decreased infiltration of inflammatory cells into WAT. Our finding of a trend for reduced IgG2c protein in perigonadal WAT of DGKα KO mice after Acute HFD indicates that the inflammatory response may be attenuated. An increase of IFNγ expression in WAT is associated with increased infiltration of other immune cells (35), which in the present scenario, could aid in battling a new stressor. Exogenous administration of IFNγ to 3T3-L1 adipocytes result in release of various chemokines (37), which could attract immune cells. T-cell inflammation in WAT has no direct effect on insulin sensitivity; however, IL6 infusion induces insulin resistance (38). Further involvement of IL6 is probable, as IL6 secretion from WAT is dependent on IL1β however, this was only observed in aged animals (39). IL6 mRNA expression tended to decrease after Acute HFD, which may also aid in reducing inflammation in WAT of DGKα KO mice (P = 0.18). Together, our findings suggest DGKα plays a role, in concert with WAT and immune cells, to acutely reduce inflammation in response to a short-term HFD.
DGK isoforms have been implicated in the pathogenesis of several diseases, including type 2 diabetes and obesity (40, 41). Leptin-deficiency in mice, with severe obesity and insulin resistance, influences DGK isoform mRNA expression in skeletal muscle, adipose tissue, and liver (27). We have previously reported that DGKδ deficiency increases DAG content in skeletal muscle and WAT and causes peripheral insulin resistance and obesity (8). DGKδ protein abundance is reduced in skeletal muscle of type 2 diabetic patients (8) with expression regulated by glucose and free fatty acid levels (8, 42). We have also determined the role of DGKε on glucose and energy homeostasis (30). In contrast with the insulin resistant phenotype observed in the DGKδ deficient mice (30), DGKε KO led to increased glucose tolerance, reduced whole-body respiratory exchange ratio, and increased abundance of mitochondrial markers in skeletal muscle, indicating a greater reliance on fat oxidation and intracellular lipid metabolism (30). In cultured MIN6 cells, DGKα siRNA attenuates insulin secretion (43), implicating a role for this isoenzyme in type 2 diabetes. PPARγ agonists increase DGKα mRNA and protein as well as DGK activity, thereby suppressing DAG-PKC signaling (44). In Long-Term HFD animals, we found DGKα ablation was associated with reduced insulin levels during a glucose tolerance test, suggesting enhanced insulin sensitivity or altered insulin secretion. However, our findings of only modest changes in insulin sensitivity and insulin levels in DGKα KO mice indicate that expression of other DGK isoforms may play a more fundamental role in the regulation of insulin sensitivity. Indeed, whole body insulin-mediated glucose utilization and hepatic insulin sensitivity were similar between chow-fed DGKα KO and WT mice, indicating DGKα is dispensable for whole body glucose homeostasis.
We used two different HFD paradigms to separate the effects of lipid overload due to an Acute HFD from the effects of chronic low-grade inflammation, obesity, and subsequent insulin resistance of a Long-Term HFD. DGKα activity is increased by phosphatidylethanolamine and cholesterol (45). DGKα activity is stimulated by increased levels of phosphatidylinositol 3-kinase lipid products by a calcium-independent mechanism (46). Thus, an increase in lipid products may directly stimulate DGKα activity. Alternatively, the reduction in IL1β in WAT, an inflammation marker, may contribute to the modest alteration in insulin sensitivity in DGKα KO mice. Aside from T-cell regulation, DGKα plays a role in female sex hormone estradiol-mediated proliferation of cancer cells (47) and regulation of vascular endothelial growth factor action (48). Thus, DGKα may directly influence proliferation and angiogenesis of WAT independent of immune cells. Curiously, insulin levels were decreased in Long-Term HFD DGKα KO mice during the glucose tolerance test. Although the mechanism for the differences in the insulin response in chow- versus Long-Term HFD-fed mice is unclear, DGKα deficiency does not appear to have a severe effect on glucose and energy homeostasis. Our results suggest that DGK isoforms have different roles in maintaining metabolic homeostasis.
In contrast to other DGK isoform-specific KO mouse models including DGKδ, DGKε, and DGKζ (8, 30, 49), the metabolic phenotype of DGKα KO mice is subtle. Several of the assays utilized in this study have also been applied to other DGK isoform-specific KO mouse models. We studied younger and older DGKα deficient mice in order to ascertain whether DGKα deficiency modifies glucose homeostasis. Metabolic derangements in DGKδ haploinsufficient mice are more profound with ageing. Therefore, we studied both young and old mice and utilized HFD as an environmental stressor to promote insulin resistance. In many cases, subjecting rodents to HFD can more rapidly reveal a genotype-phenotype interaction that might not be as obvious in an unstressed chow-fed animal. Despite these extensive paradigms, DGKα deficiency does not appear to influence whole body glucose homeostasis.
In conclusion, DGKα is dispensable for the regulation of whole body insulin-mediated glucose utilization, hepatic glucose production, and energy homeostasis. Our results suggest that DGKα aids in modulating the early immune response of perigonadal WAT following an acute exposure to HFD, possibly through modulation of acute T-cell action. Therapeutic approaches targeting DGKα may aid in reducing recruitment and activation of immune cells to WAT, which, left untreated, may trigger obesity and insulin resistance.
Acknowledgments
The authors are grateful to Dr. Matthew K. Topham (University of Utah) for providing the DGKα KO mouse model and to Drs. Marc Gilbert and Robby Z. Tom for valuable input during the initial stages of this study.
Footnotes
Abbreviations:
- Acute HFD
- HFD-fed for 3 days
- DAG
- diacylglycerol
- DGK
- diacylglycerol kinase
- HFD
- high fat diet
- HSL
- hormone sensitive lipase
- IL
- interleukin
- IPGT
- intraperitoneal glucose tolerance
- Long-Term HFD
- HFD-fed for 12 weeks
- PA
- phosphatidic acid
- p-HSL
- phosphorylated HSL
- VO2
- oxygen consumption
- WAT
- white adipose tissue
This work was funded by the Novo Nordisk Foundation, Strategic Research Program in Diabetes at Karolinska Institutet, European Research Council Ideas Program (ICEBERG, ERC-2009-AdG233285), Swedish Research Council (2011-3550), Swedish Diabetes Foundation (DIA2012-082), and Swedish Foundation for Strategic Research (SRL10-0027). The authors have no relevant conflicts of interest to disclose.
REFERENCES
- 1.Itani S. I., Ruderman N. B., Schmieder F., and Boden G.. 2002. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes. 51: 2005–2011. [DOI] [PubMed] [Google Scholar]
- 2.Topham M. K., and Epand R. M.. 2009. Mammalian diacylglycerol kinases: molecular interactions and biological functions of selected isoforms. Biochim. Biophys. Acta. 1790: 416–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Topham M. K., and Prescott S. M.. 1999. Mammalian diacylglycerol kinases, a family of lipid kinases with signaling functions. J. Biol. Chem. 274: 11447–11450. [DOI] [PubMed] [Google Scholar]
- 4.Evangelisti C., Riccio M., Faenza I., Zini N., Hozumi Y., Goto K., Cocco L., and Martelli A. M.. 2006. Subnuclear localization and differentiation-dependent increased expression of DGK-zeta in C2C12 mouse myoblasts. J. Cell. Physiol. 209: 370–378. [DOI] [PubMed] [Google Scholar]
- 5.Evangelisti C., Tazzari P. L., Riccio M., Fiume R., Hozumi Y., Fala F., Goto K., Manzoli L., Cocco L., and Martelli A. M.. 2007. Nuclear diacylglycerol kinase-zeta is a negative regulator of cell cycle progression in C2C12 mouse myoblasts. FASEB J. 21: 3297–3307. [DOI] [PubMed] [Google Scholar]
- 6.Bilim O., Takeishi Y., Kitahara T., Arimoto T., Niizeki T., Sasaki T., Goto K., and Kubota I.. 2008. Diacylglycerol kinase zeta inhibits myocardial atrophy and restores cardiac dysfunction in streptozotocin-induced diabetes mellitus. Cardiovasc. Diabetol. 7: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harada M., Takeishi Y., Arimoto T., Niizeki T., Kitahara T., Goto K., Walsh R. A., and Kubota I.. 2007. Diacylglycerol kinase zeta attenuates pressure overload-induced cardiac hypertrophy. Circ. J. 71: 276–282. [DOI] [PubMed] [Google Scholar]
- 8.Chibalin A. V., Leng Y., Vieira E., Krook A., Bjornholm M., Long Y. C., Kotova O., Zhong Z., Sakane F., Steiler T., et al. . 2008. Downregulation of diacylglycerol kinase delta contributes to hyperglycemia-induced insulin resistance. Cell. 132: 375–386. [DOI] [PubMed] [Google Scholar]
- 9.Raben D. M., and Wattenberg B. W.. 2009. Signaling at the membrane interface by the DGK/SK enzyme family. J. Lipid Res. 50(Suppl): S35–S39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamada K., Sakane F., Matsushima N., and Kanoh H.. 1997. EF-hand motifs of alpha, beta and gamma isoforms of diacylglycerol kinase bind calcium with different affinities and conformational changes. Biochem. J. 321: 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanjuán M. A., Pradet-Balade B., Jones D. R., Martinez A. C., Stone J. C., Garcia-Sanz J. A., and Merida I.. 2003. T cell activation in vivo targets diacylglycerol kinase alpha to the membrane: a novel mechanism for Ras attenuation. J. Immunol. 170: 2877–2883. [DOI] [PubMed] [Google Scholar]
- 12.Gorentla B. K., Wan C. K., and Zhong X. P.. 2011. Negative regulation of mTOR activation by diacylglycerol kinases. Blood. 117: 4022–4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo R., Wan C. K., Carpenter J. H., Mousallem T., Boustany R. M., Kuan C. T., Burks A. W., and Zhong X. P.. 2008. Synergistic control of T cell development and tumor suppression by diacylglycerol kinase alpha and zeta. Proc. Natl. Acad. Sci. USA. 105: 11909–11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olenchock B. A., Guo R., Carpenter J. H., Jordan M., Topham M. K., Koretzky G. A., and Zhong X. P.. 2006. Disruption of diacylglycerol metabolism impairs the induction of T cell anergy. Nat. Immunol. 7: 1174–1181. [DOI] [PubMed] [Google Scholar]
- 15.Yanagisawa K., Yasuda S., Kai M., Imai S., Yamada K., Yamashita T., Jimbow K., Kanoh H., and Sakane F.. 2007. Diacylglycerol kinase alpha suppresses tumor necrosis factor-alpha-induced apoptosis of human melanoma cells through NF-kappaB activation. Biochim. Biophys. Acta. 1771: 462–474. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen S. T., Lehrskov-Schmidt L., Krogh-Madsen R., Solomon T. P., Lehrskov-Schmidt L., Holst J. J., and Moller K.. 2013. Tumour necrosis factor-alpha infusion produced insulin resistance but no change in the incretin effect in healthy volunteers. Diabetes Metab. Res. Rev. 29: 655–663. [DOI] [PubMed] [Google Scholar]
- 17.Hotamisligil G. S., Shargill N. S., and Spiegelman B. M.. 1993. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 259: 87–91. [DOI] [PubMed] [Google Scholar]
- 18.Weisberg S. P., McCann D., Desai M., Rosenbaum M., Leibel R. L., and Ferrante A. W. Jr. 2003. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112: 1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blüher M. 2009. Adipose tissue dysfunction in obesity. Exp. Clin. Endocrinol. Diabetes. 117: 241–250. [DOI] [PubMed] [Google Scholar]
- 20.Kintscher U., Hartge M., Hess K., Foryst-Ludwig A., Clemenz M., Wabitsch M., Fischer-Posovszky P., Barth T. F., Dragun D., Skurk T., et al. . 2008. T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler. Thromb. Vasc. Biol. 28: 1304–1310. [DOI] [PubMed] [Google Scholar]
- 21.Pettersson U. S., Walden T. B., Carlsson P. O., Jansson L., and Phillipson M.. 2012. Female mice are protected against high-fat diet induced metabolic syndrome and increase the regulatory T cell population in adipose tissue. PLoS One. 7: e46057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rausch M. E., Weisberg S., Vardhana P., and Tortoriello D. V.. 2008. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int. J. Obes. (Lond.) 32: 451–463. [DOI] [PubMed] [Google Scholar]
- 23.Steinberg G. R., Michell B. J., van Denderen B. J., Watt M. J., Carey A. L., Fam B. C., Andrikopoulos S., Proietto J., Gorgun C. Z., Carling D., et al. . 2006. Tumor necrosis factor alpha-induced skeletal muscle insulin resistance involves suppression of AMP-kinase signaling. Cell Metab. 4: 465–474. [DOI] [PubMed] [Google Scholar]
- 24.Deiuliis J., Shah Z., Shah N., Needleman B., Mikami D., Narula V., Perry K., Hazey J., Kampfrath T., Kollengode M., et al. . 2011. Visceral adipose inflammation in obesity is associated with critical alterations in tregulatory cell numbers. PLoS One. 6: e16376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ehses J. A., Lacraz G., Giroix M. H., Schmidlin F., Coulaud J., Kassis N., Irminger J. C., Kergoat M., Portha B., Homo-Delarche F., et al. . 2009. IL-1 antagonism reduces hyperglycemia and tissue inflammation in the type 2 diabetic GK rat. Proc. Natl. Acad. Sci. USA. 106: 13998–14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zierath J. R. 2007. The path to insulin resistance: paved with ceramides? Cell Metab. 5: 161–163. [DOI] [PubMed] [Google Scholar]
- 27.Mannerås-Holm L., Kirchner H., Bjornholm M., Chibalin A. V., and Zierath J. R.. 2015. mRNA expression of diacylglycerol kinase isoforms in insulin-sensitive tissues: effects of obesity and insulin resistance. Physiol. Rep. 3: pii: e12372. doi: 10.14814/phy2.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee I. K., Koya D., Ishi H., Kanoh H., and King G. L.. 1999. d-Alpha-tocopherol prevents the hyperglycemia induced activation of diacylglycerol (DAG)-protein kinase C (PKC) pathway in vascular smooth muscle cell by an increase of DAG kinase activity. Diabetes Res. Clin. Pract. 45: 183–190. [DOI] [PubMed] [Google Scholar]
- 29.Romero-Calvo I., Ocon B., Martinez-Moya P., Suarez M. D., Zarzuelo A., Martinez-Augustin O., and de Medina F. S.. 2010. Reversible Ponceau staining as a loading control alternative to actin in Western blots. Anal. Biochem. 401: 318–320. [DOI] [PubMed] [Google Scholar]
- 30.Mannerås-Holm L., Schonke M., Brozinick J. T., Vetterli L., Bui H. H., Sanders P., Nascimento E. B. M., Bjornholm M., Chibalin A. V., and Zierath J. R.. 2017. Diacylglycerol kinase epsilon deficiency preserves glucose tolerance and modulates lipid metabolism in obese mice. J. Lipid Res. 58: 907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boutens L., and Stienstra R.. 2016. Adipose tissue macrophages: going off track during obesity. Diabetologia. 59: 879–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flores I., Casaseca T., Martinez A. C., Kanoh H., and Merida I.. 1996. Phosphatidic acid generation through interleukin 2 (IL-2)-induced alpha-diacylglycerol kinase activation is an essential step in IL-2-mediated lymphocyte proliferation. J. Biol. Chem. 271: 10334–10340. [DOI] [PubMed] [Google Scholar]
- 33.Martínez-Moreno M., Garcia-Lievana J., Soutar D., Torres-Ayuso P., Andrada E., Zhong X. P., Koretzky G. A., Merida I., and Avila-Flores A.. 2012. FoxO-dependent regulation of diacylglycerol kinase alpha gene expression. Mol. Cell. Biol. 32: 4168–4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahn C. S., Han J. A., Lee H. S., Lee S., and Pai H. S.. 2011. The PP2A regulatory subunit Tap46, a component of the TOR signaling pathway, modulates growth and metabolism in plants. Plant Cell. 23: 185–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frasca D., Diaz A., Romero M., Vazquez T., and Blomberg B. B.. 2017. Obesity induces pro-inflammatory B cells and impairs B cell function in old mice. Mech. Ageing Dev. 162: 91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winer D. A., Winer S., Shen L., Wadia P. P., Yantha J., Paltser G., Tsui H., Wu P., Davidson M. G., Alonso M. N., et al. . 2011. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat. Med. 17: 610–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bastos L. F., Angusti A., Vilaca M. C., Merlo L. A., Nascimento E. B. Jr., Rocha L. T., Godin A. M., Solano A. G., Jarussophon S., Nunan E. A., et al. . 2008. A novel non-antibacterial, non-chelating hydroxypyrazoline derivative of minocycline inhibits nociception and oedema in mice. Br. J. Pharmacol. 155: 714–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sultan A., Strodthoff D., Robertson A. K., Paulsson-Berne G., Fauconnier J., Parini P., Ryden M., Thierry-Mieg N., Johansson M. E., Chibalin A. V., et al. . 2009. T cell-mediated inflammation in adipose tissue does not cause insulin resistance in hyperlipidemic mice. Circ. Res. 104: 961–968. [DOI] [PubMed] [Google Scholar]
- 39.Starr M. E., Saito M., Evers B. M., and Saito H.. 2015. Age-associated increase in cytokine production during systemic inflammation-ii: the role of il-1beta in age-dependent Il-6 upregulation in adipose tissue. J. Gerontol. A Biol. Sci. Med. Sci. 70: 1508–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakane F., Imai S., Kai M., Yasuda S., and Kanoh H.. 2008. Diacylglycerol kinases as emerging potential drug targets for a variety of diseases. Curr. Drug Targets. 9: 626–640. [DOI] [PubMed] [Google Scholar]
- 41.Sakane F., Mizuno S., and Komenoi S.. 2016. Diacylglycerol kinases as emerging potential drug targets for a variety of diseases: an update. Front. Cell Dev. Biol. 4: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sakiyama S., Usuki T., Sakai H., and Sakane F.. 2014. Regulation of diacylglycerol kinase delta2 expression in C2C12 skeletal muscle cells by free fatty acids. Lipids. 49: 633–640. [DOI] [PubMed] [Google Scholar]
- 43.Kurohane Kaneko Y., Kobayashi Y., Motoki K., Nakata K., Miyagawa S., Yamamoto M., Hayashi D., Shirai Y., Sakane F., and Ishikawa T.. 2013. Depression of type I diacylglycerol kinases in pancreatic beta-cells from male mice results in impaired insulin secretion. Endocrinology. 154: 4089–4098. [DOI] [PubMed] [Google Scholar]
- 44.Verrier E., Wang L., Wadham C., Albanese N., Hahn C., Gamble J. R., Chatterjee V. K., Vadas M. A., and Xia P.. 2004. PPARgamma agonists ameliorate endothelial cell activation via inhibition of diacylglycerol-protein kinase C signaling pathway: role of diacylglycerol kinase. Circ. Res. 94: 1515–1522. [DOI] [PubMed] [Google Scholar]
- 45.Fanani M. L., Topham M. K., Walsh J. P., and Epand R. M.. 2004. Lipid modulation of the activity of diacylglycerol kinase alpha- and zeta-isoforms: activation by phosphatidylethanolamine and cholesterol. Biochemistry. 43: 14767–14777. [DOI] [PubMed] [Google Scholar]
- 46.Ciprés A., Carrasco S., Merino E., Diaz E., Krishna U. M., Falck J. R., Martinez A. C., and Merida I.. 2003. Regulation of diacylglycerol kinase alpha by phosphoinositide 3-kinase lipid products. J. Biol. Chem. 278: 35629–35635. [DOI] [PubMed] [Google Scholar]
- 47.Filigheddu N., Sampietro S., Chianale F., Porporato P. E., Gaggianesi M., Gregnanin I., Rainero E., Ferrara M., Perego B., Riboni F., et al. . 2011. Diacylglycerol kinase alpha mediates 17-beta-estradiol-induced proliferation, motility, and anchorage-independent growth of Hec-1A endometrial cancer cell line through the G protein-coupled estrogen receptor GPR30. Cell. Signal. 23: 1988–1996. [DOI] [PubMed] [Google Scholar]
- 48.Baldanzi G., Mitola S., Cutrupi S., Filigheddu N., van Blitterswijk W. J., Sinigaglia F., Bussolino F., and Graziani A.. 2004. Activation of diacylglycerol kinase alpha is required for VEGF-induced angiogenic signaling in vitro. Oncogene. 23: 4828–4838. [DOI] [PubMed] [Google Scholar]
- 49.Benziane B., Borg M. L., Tom R. Z., Riedl I., Massart J., Bjornholm M., Gilbert M., Chibalin A. V., and Zierath J. R.. 2017. DGKzeta deficiency protects against peripheral insulin resistance and improves energy metabolism. J. Lipid Res. 58: 2324–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]