Abstract
Disregulation of fatty acid oxidation, one of the major mechanisms for maintaining hepatic lipid homeostasis under fasting conditions, leads to hepatic steatosis. Although obesity and type 2 diabetes-induced endoplasmic reticulum (ER) stress contribute to hepatic steatosis, it is largely unknown how ER stress regulates fatty acid oxidation. Here we show that fasting glucagon stimulates the dephosphorylation and nuclear translocation of histone deacetylase 5 (HDAC5), where it interacts with PPARα and promotes transcriptional activity of PPARα. As a result, overexpression of HDAC5 but not PPARα binding-deficient HDAC5 in liver improves lipid homeostasis, whereas RNAi-mediated knockdown of HDAC5 deteriorates hepatic steatosis. ER stress inhibits fatty acid oxidation gene expression via calcium/calmodulin-dependent protein kinase II-mediated phosphorylation of HDAC5. Most important, hepatic overexpression of a phosphorylation-deficient mutant HDAC5 2SA promotes hepatic fatty acid oxidation gene expression and protects against hepatic steatosis in mice fed a high-fat diet. We have identified HDAC5 as a novel mediator of hepatic fatty acid oxidation by fasting and ER stress signals, and strategies to promote HDAC5 dephosphorylation could serve as new tools for the treatment of obesity-associated hepatic steatosis.
Keywords: ER stress, HDAC5, PPARα, fatty acid oxidation, fasting signal
Fatty acid oxidation is an important mechanism for maintaining hepatic lipid homeostasis under fasted state. Impaired fatty acid oxidation leads to abnormal accumulation of triglycerides in the liver and results in hepatic steatosis (1, 2). Hepatic steatosis has become a major threat to human health worldwide, which could progress to nonalcoholic steatohepatitis, liver cirrhosis, and cancer (3, 4). Peroxisome proliferator–activated receptor α (PPARα) serves as a master transcriptional regulator of hepatic fatty acid oxidation (5–7) through regulating the transcription of key genes involved in fatty acid oxidation (8, 9). PPARα knockout mice exhibit decreased levels of fatty acid oxidation under fasted state and starvation (6). Fasting hormones such as glucagon regulate fatty acid oxidation in the liver through PPARα (10, 11). Glucagon stimulates PPARα activity and targets fatty acid oxidation gene expression, which is diminished in PPARα knockout mice (10). Despite the critical role of the fasting glucagon in the control of PPARα activity, the detailed mechanism still remains unclear and is currently under close investigation.
By binding to its receptor, glucagon stimulates the production of intracellular cAMP. Upon activation by intracellular cAMP, protein kinase A phosphorylates and inactivates salt-inducible kinases (SIKs; SIK1, 2, 3), which phosphorylate and suppress cAMP response element-binding (CREB)-regulated transcription coactivator (CRTC) (12) and CREB-binding protein/p300 (13). SIK2, a AMPK superfamily member, contributes to glucagon’s effect on PPARα activity through p300 (14). Glucagon also stimulates the efflux of cAMP, and the increase in extracellular cAMP promotes PPARα activity through activation of AMPK (15). Furthermore, SIKs also phosphorylate and suppress class II histone deacetylases (HDACs) (HDAC4, 5, 7), which deacetylate and inactivate Forkhead box O1 (FOXO1) (16, 17). However, it remains largely unknown whether class II HDACs affect hepatic fatty acid oxidation under fasted state.
Endoplasmic reticulum (ER) is responsible for protein folding, lipid and sterol biosynthesis, and calcium storage. Accumulation of unfolded proteins in ER leads to ER stress, and the unfolded protein response (UPR) through PKR-like endoplasmic reticulum kinase, inositol-requiring enzyme 1 (IRE1), and activating transcription factor 6 (ATF6) pathways serve as major mechanisms for restoring ER homeostasis under ER stress conditions (18). However, unresolved or prolonged ER stress influences cellular calcium metabolism (19), and the release of ER calcium stores into the cytosol activates calcium/calmodulin-dependent protein kinase II (CaMKII), which is critical for ER stress-induced apoptosis (20, 21). Hepatic ER stress is closely associated with obesity-induced steatosis (22–25). Obesity and type 2 diabetes directly induce hepatic ER stress (26, 27), which leads to steatosis (23). However, it is not completely understood how elevated ER stress in the liver contributes to steatosis.
In this study, we identify HDAC5, a major component of the fasting glucagon signaling pathway, as a key mediator of hepatic fatty acid oxidation gene expression. We demonstrate that fasting-induced dephosphorylation of HDAC5, which is suppressed by ER stress, promotes its binding to and activation of PPARα. As a result, ER stress-dependent hepatic steatosis is greatly attenuated in mice expressing a phosphorylation-defective HDAC5. Our data thus provide new evidence demonstrating the effect of HDAC5 on hepatic lipid homeostasis under physiological and pathological conditions. Furthermore, we demonstrate a potential, novel therapeutic strategy for treatment of obesity-associated hepatic steatosis.
MATERIALS AND METHODS
Cells, antibodies, and reagents
Primary hepatocytes were prepared as described (28, 29). Briefly, livers from fed mice were perfused with collagenase (type IV) (Sigma, St. Louis, MO, USA) dissolved in Hank’s balanced salt solution (Invitrogen, Waltham, MA) at a rate of 6 ml/min through the portal vein. Cells were seeded in medium M199 (Invitrogen, Waltham, MA), supplemented with 0.2% (weight/volume [w/v]) BSA and 2% (v/v) fetal bovine serum. After 2 h, medium was replaced with fresh M199. Cells were then infected with 1 plaque-forming unit (pfu) per cell of Ad-HDAC5, Ad-HDAC5 2SA, Ad-HDAC5Δ300-480, or Ad-green fluorescent protein (GFP) for 24 h for overexpression and Ad-HDAC5i or Ad-USi for 48 h for RNAi-mediated knockdown. Anti-pHDAC5 and anti-HDAC5 antibodies were purchased from Cell Signaling Technology (Danvers, MA). Anti-PPARα antibody was purchased from Abcam (Cambridge, UK). WY14643 (PPARα agonist) and thapsigargin (THA) were purchased from Sigma (St. Louis, MO). Forskolin (FSK) was purchased from Medchem Express (Monmouth Junction, NJ). All plasmids used in this study were from mouse origin. The Fgf21-luciferase reporter plasmid was described previously (30), and the −98/+5 promoter construct was used.
Animals and adenovirus
Male C57BL/6J mice were purchased from Shanghai Laboratory Animal Center (Shanghai, China) and were adapted to colony cages with 12 h light/dark cycle in a temperature-controlled environment with free access to water and standard irradiated rodent diet (5% fat; Research Diet D12450, New Brunswick, NJ). For high-fat diet (HFD) studies, 6-week-old mice were maintained on HFD (60% fat; Research Diets D12492) for 12 weeks. For adenovirus injection, 1×108 pfu Ad-HDAC5, Ad-HDAC5 2SA, Ad-HDAC5Δ300-480, Ad-GFP, Ad-unspecific RNAi (USi), and Ad-HDAC5 RNAi (HDAC5i) were delivered by tail-vein injection. Six days after injection, mice were fasted for 24 h before sacrifice. All animal studies were approved by the animal experiment committee of Tongji University and in accordance with the guidelines of the School of Medicine, Tongji University.
In vitro analysis
Mouse tissues were frozen in liquid nitrogen and kept at –80°C until further use. Livers were homogenized by using tissue homogenizer at 4°C in lysis buffer (150 mM NaCl, 50 mM Tris-HCl pH 7.5, 5 mM EDTA, 30 mM sodium pyrophosphate, 30 mM sodium fluoride, 1% Triton-X 100, and protease inhibitor cocktail). Lysates were reserved for immunoblot and immunoprecipitation. Liver triglyceride levels were determined as previously reported (15).
Quantitative real-time PCR and immunoblot
Real-time PCR was performed as previously (31). Briefly, total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA), and reverse transcription was done using FastQuant RT kit (Tiangen, Shanghai, China). Real-time PCR was carried out using SuperReal SYBR Green kit (Tiangen, Shanghai, China) and Lightcycler 96 (Roche, Penzberg, Germany). All reactions were performed in duplicate. The amplification efficiency for each primer pair and the cycle threshold (Ct) were determined automatically by Lightcycler software (Roche, Penzberg, Germany). The fold-change was calculated by the comparative CT (2−ΔΔCT) method against β-actin (32). Immunoblot and immunoprecipitation were performed as described (33). Briefly, cells were washed with PBS and then resuspended in lysis buffer (150 mM NaCl, 1% Triton-X 100, 1 mM EDTA, 50 mM Tris pH7.5, and protease inhibitor cocktail). For immunoblot, protein content in the supernatant was determined by using the Micro BCA protein assay kit (Pierce, Rockford, IL) and suspended in sample buffer (100 mM Tris, PH 6.8, 4% SDS, 20% glycerol, 0.1% bromophenol blue). The samples were separated on SDS-PAGE gels, transferred, probed with antibodies, and visualized using ECL reagents. For immunoprecipitation, the supernatant was precleaned with protein A/G agarose for 30 min and then incubated overnight on a rocker with primary antibodies at 4°C, followed by incubation with Protein A/G agarose beads for another 2 h. The immunoprecipitates were extensively washed with lysis buffer and suspended in sample buffer for SDS-PAGE analysis.
Luciferase reporter assay
Human embryonic kidney (HEK) 293T cells were transfected with Fgf21-PPRE-luc and respiratory syncytial virus (RSV) β-gal, together with PPARα/retinoid X receptor α (RXRα) plasmid and indicated constructs for 24 h, and luciferase assays were performed by using the Promega GloMax96 system according to the manufacturer’s instructions (33). We used β-gal assay to normalize the expression levels.
Oil Red O staining
Livers embedded in optimal cutting temperature compound (Tissue-Tek, Laborimpex) were used for Oil Red O staining to assess hepatic steatosis.
Statistical analysis
All studies were performed on at least three independent occasions. Results are reported as mean ± SEM. Differences between two groups were assessed with unpaired Student’s t test. Data involving more than two groups were assessed by ANOVA with Bonferroni post hoc test. A P value of <.05 was considered statistically significant.
RESULTS
HDAC5 promotes hepatic fatty acid oxidation gene expression under fasted state
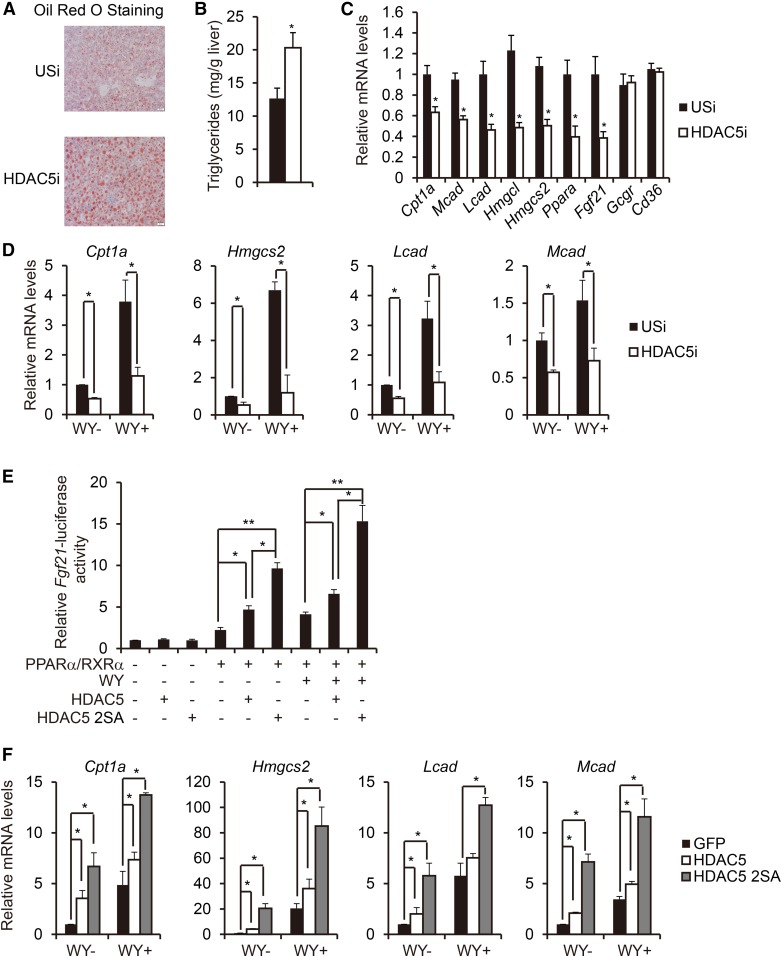
Fasting glucagon stimulates the gluconeogenesis via activation of CRTC2 (34) and HDAC5 pathways (16, 17). Glucagon also regulates fatty acid oxidation in the liver (10, 11), and CRTC2 has been reported to control hepatic lipid metabolism by regulating SREBP1 (35). However, whether or not HDAC5 affects hepatic fatty acid oxidation remains largely unknown. To investigate the function of HDAC5 on lipid homeostasis under fasted state, we injected regular diet (RD)-fed mice intravenously with adenovirus, encoding either unspecific RNAi (Ad-USi) or HDAC5 RNAi (Ad-HDAC5i) to specifically knockdown hepatic HDAC5 expression without affecting HDAC3 expression (supplemental Fig. S1A). After 24 h of fasting, while body weight, liver weight, and plasma glucagon levels remained unchanged, hepatic lipid accumulation and triglyceride levels as well as plasma NEFA levels were dramatically increased in Ad-HDAC5i-injected mice in comparison with Ad-Usi-injected mice, and plasma ketone bodies were decreased (Fig. 1A, B, and supplemental Fig. S1B, C). Consistently, the expression of PPARα target genes known to regulate fatty acid oxidation, including carnitine palmitoyltransferase 1A (Cpt1a), medium-chain acyl-CoA dehydrogenase (Mcad), long-chain acyl-CoA dehydrogenase (Lcad), 3-hydroxy-3-methylglutaryl-CoA lyase (Hmgcl), 3-hydroxy-3-methylglutaryl-CoA synthase 2 (Hmgcs2), Pparα, and Fgf21, were significantly decreased, whereas hepatic glucagon receptor Gcgr and fatty acid transporter Cd36 remained unchanged (Fig. 1C). Furthermore, exposure of primary hepatocytes to PPARα agonist WY14643 stimulated expression of PPARα target genes, including Cpt1a, Hmgcs2, Lcad, and Mcad; this effect was largely blocked when cells were infected with Ad-HDAC5i (Fig. 1D).
Fig. 1.
HDAC5 promotes hepatic fatty acid oxidation under fasted state. A: Representative Oil Red O staining of liver sections from RD-fed mice injected with either Ad-USi or Ad-HDAC5i (scale bar, 20 μm). B: Effect of Ad-USi or Ad-HDAC5i injection on hepatic triglyceride levels in RD-fed mice (n = 8). C: Effect of Ad-USi or Ad-HDAC5i injection on hepatic fatty acid oxidation gene expression in RD-fed mice (n = 8). D: Effect of Ad-USi or Ad-HDAC5i infection on mRNA amounts for WY (50 μM)–induced fatty acid oxidation genes including Cpt1a, Hmgcs2, Lcad, and Mcad in primary hepatocytes. Primary hepatocytes were infected with Ad-USi or Ad-HDAC5i for 48 h and followed by WY stimulation for 24 h (n = 8). E: Effect of HDAC5 or HDAC5 2SA on Fgf21-luc reporter activity. HEK293T cells were transfected with Fgf21-luc, RSV-β-gal, PPARα, and RXRα, together with HDAC5 or HDAC5 2SA for 24 h and followed by WY stimulation for 24 h (n = 4). F: Effect of Ad-HDAC5, Ad-HDAC5 2SA, or Ad-GFP on mRNA amounts for WY-induced fatty acid oxidation genes including Cpt1a, Hmgcs2, Lcad, and Mcad in primary hepatocytes. Primary hepatocytes were infected with Ad-GFP, Ad-HDAC5, or Ad-HDAC5 2SA for 24 h and followed by WY stimulation for 24 h (n = 3). All data are presented as means ± SEM. *P < 0.05. **P < 0.01.
Although HDAC5 is phosphorylated at consensus SIK recognition sites and sequestered in the cytoplasm under ad lib conditions, fasting triggered HDAC5 dephosphorylation at Ser256 and Ser498 and nuclear translocation (16). Changes in hepatic fatty acid oxidation in AD-HDAC5i-infected mice under fasted conditions prompt us to further investigate the effect of HDAC5 and the phosphorylation-defective HDAC5 mutant (HDAC5 S259/498A, HDAC5 2SA), which exhibits a permanent nuclear localization identical to wild-type HDAC5 localization upon glucagon or FSK treatment (16), on PPARα transcriptional activity. Although HDAC5 expression caused a significant increase of PPARα/RXRα-induced activation of the Fgf21-luciferase reporter in HEK293T cells, HDAC5 2SA expression further boosted the effect (Fig. 1E). This effect seemed to be PPARα ligand–independent, because PPARα activation function 2 (AF2) mutant (lacking the ligand-dependent activation) (36) and RXRα-induced activation of the Fgf21-luciferase reporter were still able to be promoted by HDAC5 (supplemental Fig. S2A). Consistently, Ad-HDAC5 expression promoted the expression of PPARα target genes known to regulate fatty acid oxidation, including Cpt1a, Hmgcs2, Lcad, and Mcad; this effect was further enhanced when primary hepatocytes were expressed with Ad-HDAC5 2SA (Fig. 1F). Together, these data indicate that HDAC5 plays an important role in regulating hepatic fatty acid oxidation gene expression under fasted state.
HDAC5 promotes fatty acid oxidation gene expression via its interaction with PPARα
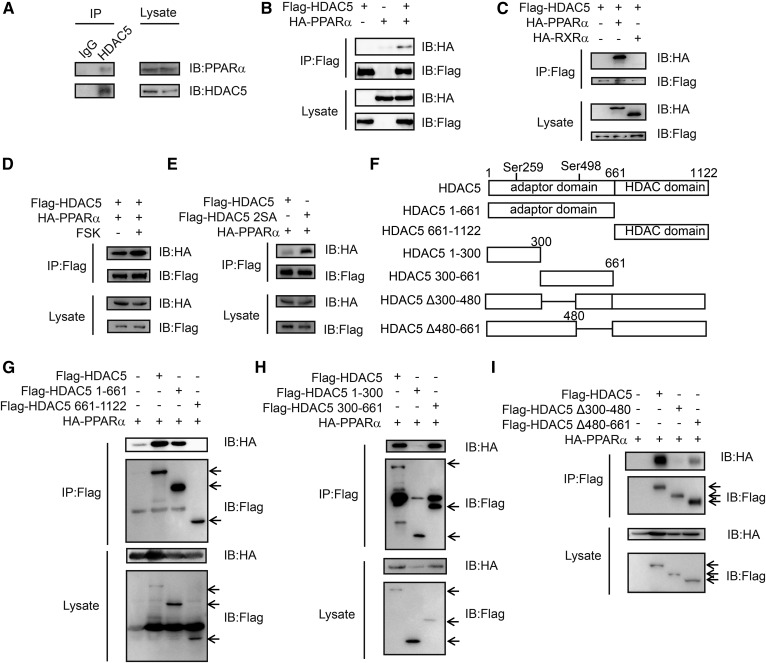
HDAC5 has been reported to interact with transcription factors, such as FOXO1 (16), MEF2 (37), and p65 (33), and modulate their transcriptional activity. On the basis of the effect of HDAC5 on PPARα activity, we tested whether HDAC5 associates with PPARα. Indeed, we recovered endogenous PPARα via immunoprecipitation with endogenous HDAC5 in primary hepatocytes (Fig. 2A). Consistent with this association, hemagglutinin (HA)–tagged PPARα but not HA-tagged RXRα could be pulled down by Flag-tagged HDAC5 in HEK293T cells (Fig. 2B, C). Interestingly, exposure to FSK greatly increased the interaction between HDAC5 and PPARα (Fig. 2D) and HDAC5 2SA showed a higher affinity to interacting with PPARα (Fig. 2E) in HEK293T cells. HDAC5 contains an adaptor domain in the N-terminal region and a conserved catalytic domain (HDAC domain) in the C-terminal region. To further establish the interaction domain of HDAC5 with PPARα, we tested various truncated forms of Flag-tagged HDAC5 for the ability to bind PPARα in HEK293T cells (Fig. 2F). HA-tagged PPARα was found to associate with Flag-tagged HDAC5 as well as HDAC5 1-661, HDAC5 300-661 mutants, and HDAC5 Δ480-661 mutant to a lesser extent; however, Δ300-480 truncated mutation of HDAC5 disrupted the HDAC5-PPARα interaction (Fig. 2G–I).
Fig. 2.
HDAC5 interacts with PPARα. A: Immunoblot showing amounts of endogenous PPARα recovered from immunoprecipitation of endogenous HDAC5 prepared from primary hepatocytes. B: Interaction between Flag-tagged HDAC5 and HA-tagged PPARα in HEK293T cells. C: Interaction between Flag-tagged HDAC5 and HA-tagged PPARα or HA-tagged RXRα in HEK293T cells. D: Interaction between Flag-tagged HDAC5 and HA-tagged PPARα in HEK293T cells exposed to FSK (10 μM, 1 h). E: Interaction between Flag-tagged HDAC5 or HDAC5 2SA and HA-tagged PPARα in HEK293T cells. F: Schematic diagram of the structure of HDAC5 and its truncation mutants. G-I: Immunoblots showing effects of mutants in HDAC5 on its association with PPARα in HEK293T cells. IB, immunoblot; IP, immunoprecipitation.
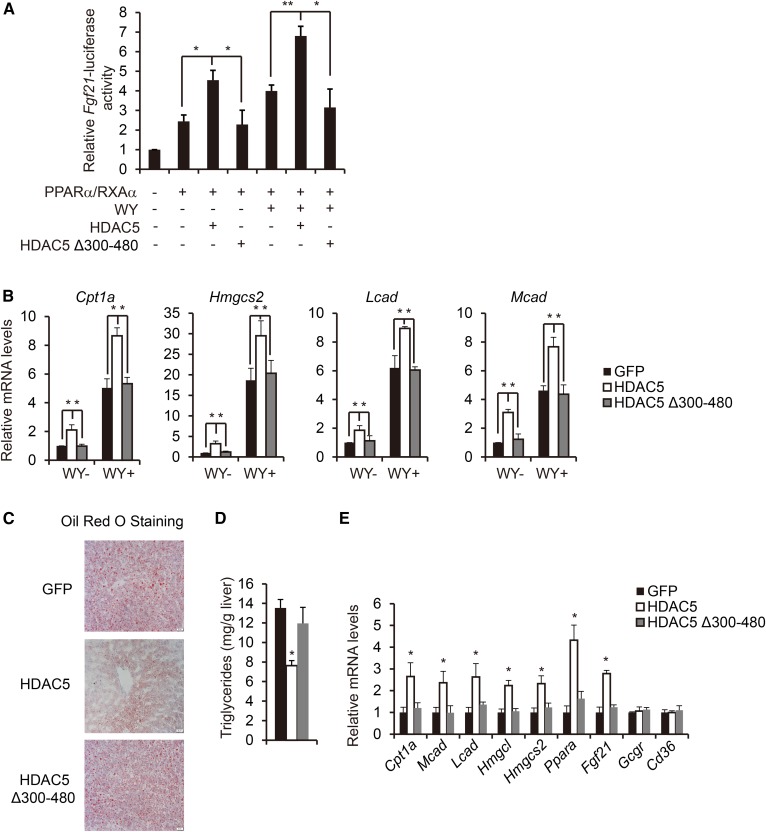
To explore whether HDAC5 interaction with PPARα directly modulates PPARα activity, we determined the effect of HDAC5 and HDAC5 Δ300-480 mutant on PPARα-induced activation of the Fgf21-luciferase reporter in HEK293T cells. In a manner consistent with the interaction data, HDAC5 but not HDAC5 Δ300-480 mutant significantly increased PPARα/RXRα-induced activation of the Fgf21-luciferase reporter (Fig. 3A). Furthermore, WY14643 stimulated expression of PPARα target genes (Cpt1a, Hmgcs2, Lcad, and Mcad) and was dramatically increased by Ad-HDAC5 but not Ad-HDAC5 Δ300-480 mutant infection in primary hepatocytes (Fig. 3B). We further tested the influence of HDAC5 Δ300-480 mutant on hepatic lipid homeostasis in vivo; mice were injected intravenously with adenovirus encoding Ad-GFP, Ad-HDAC5, or Ad-HDAC5 Δ300-480 mutant (supplemental Fig. S3A). After 24 h of fasting, while body weight, liver weight, and plasma glucagon levels remained unchanged, mice injected with Ad-HDAC5 but not Ad-HDAC5 Δ300-480 mutant showed decreased plasma NEFA levels, lipid accumulation, and triglyceride levels in liver and increased ketone bodies (Fig. 3C, D, and supplemental Fig. S3B, C). Consistently, expression of PPARα target genes known to regulate fatty acid oxidation (Cpt1a, Mcad, Lcad, Hmgcl, Hmgcs2, PPARa and Fgf21) was significantly increased in the livers of mice injected with Ad-HDAC5 but not Ad-HDAC5 Δ300-480 mutant (Fig. 3E). Taken together, these data suggest that HDAC5 promotes PPARα transcriptional activity through their interaction.
Fig. 3.
HDAC5 promotes fatty acid oxidation via its binding to and activation of PPARα. A: Effect of HDAC5 or HDAC5 Δ300-480 on Fgf21-luc reporter activity. HEK293T cells were transfected with Fgf21-luc, RSV-β-gal, PPARα, and RXRα, together with HDAC5 or HDAC5Δ300-480 for 24 h and followed by WY stimulation for 24 h (n = 4). B: Effect of Ad-HDAC5, Ad-HDAC5 Δ300-480, or Ad-GFP on mRNA amounts for WY-induced fatty acid oxidation genes, including Cpt1a, Hmgcs2, Lcad, and Mcad in primary hepatocytes. Primary hepatocytes were infected with Ad-GFP, Ad-HDAC5, or Ad-HDAC5 Δ300-480 for 24 h and followed by WY stimulation for 24 h (n = 8). C: Representative Oil Red O staining of liver sections from Ad-GFP, Ad-HDAC5, or Ad-HDAC5 Δ300-480-injected RD-fed mice (scale bar, 20 μm). D: Effect of Ad-GFP, Ad-HDAC5, or Ad-HDAC5 Δ300-480 injection on hepatic triglyceride levels in RD-fed mice (n = 8). E: Effect of Ad-GFP, Ad-HDAC5, or Ad-HDAC5 Δ300-480 injection on hepatic fatty acid oxidation gene expression in RD-fed mice (n = 8). All data are presented as means ± SEM. *P < 0.05. **P < 0.01.
Obesity-induced ER stress suppresses fatty acid oxidation gene expression through phosphorylation of HDAC5
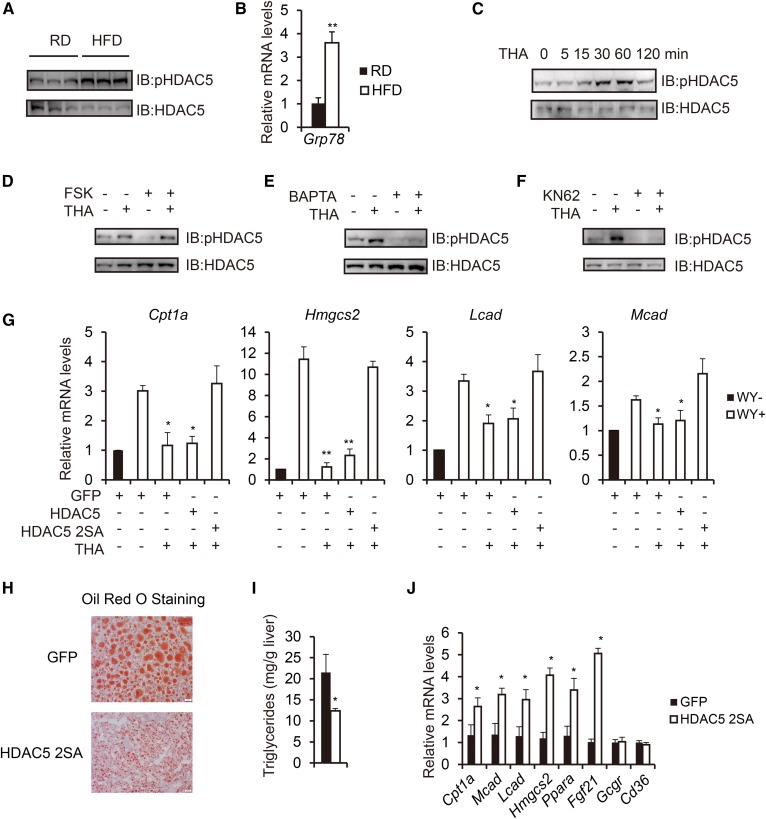
Considering that obesity is characterized by hyperglucagonemia (38) and that HDAC5 undergoes glucagon-stimulated dephosphorylation, we tested whether HDAC5 phosphorylation is altered in this setting. Surprisingly, contrary to what we expected, HFD-fed mice exhibited increased hepatic amounts of phosphorylated HDAC5 in relation to RD controls under fasted state (Fig. 4A), suggesting that another mechanism besides glucagon might contribute to its phosphorylation. It is well known that obesity-induced ER stress contributes to hepatic steatosis (27), and we further confirmed the induction of ER stress in HFD-fed mouse livers by showing that hepatic mRNA level of GRP78, an ER chaperone (27), was elevated in HFD-fed mice in comparison with controls (Fig. 4B). ER stress stimulates the release of ER calcium stores into the cytosol, which activates CaMKII (20, 21). On the basis of the fact that HDAC5 has been implicated as a substrate of CaMKII (39–41), we tested whether obesity-induced ER stress accounted for the increased HDAC5 phosphorylation through calcium-CaMKII pathway. Indeed, HDAC5 phosphorylation was significantly upregulated in a time-dependent manner by the ER stress inducer, THA treatment in primary hepatocytes (Fig. 4C). Interestingly, FSK-stimulated dephsphorylation of HDAC5 was also inhibited by THA treatment in primary hepatocytes (Fig. 4D). Pretreatment of primary hepatocytes with the intracellular calcium chelator 1,2-bis(2-aminophenoxy)ethane-N,N,N’,N’-tetraacetate or CaMKII inhibitor KN62 blocked THA-induced HDAC5 phosphorylation (Fig. 4E, F). We next directly tested the requirement of HDAC5 phosphorylation for the effect of ER stress on fatty acid oxidation gene expression. While exposure of primary hepatocytes to THA strongly inhibited fatty acid oxidation gene expression, expression of HDAC5 2SA, but not HDAC5, fully restored fatty acid oxidation gene expression (Cpt1a, Hmgcs2, Lcad, and Mcad) (Fig. 4G). These data suggest that ER stress inhibits PPARα activity via CaMKII-induced HDAC5 phosphorylation.
Fig. 4.
Obesity-induced ER stress suppresses fatty acid oxidation through phosphorylation of HDAC5. A: Immunoblot analysis of hepatic phosphorylated HDAC5 levels in RD-fed and HFD-fed mice under fasted state. B: Real-time PCR analysis of hepatic Grp78 mRNA amounts in RD-fed and HFD-fed mice (n = 3). C: Immunoblot showing effects of THA (500 nM) treatment on HDAC5 phosphorylation in primary hepatocytes at indicated times. D: Immunoblot showing effect of THA (500 nM, 1 h) treatment on FSK (10 μM)–induced HDAC5 dephosphorylation in primary hepatocytes. E: Immunoblot showing effect of BAPTA (1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate, 10 μM, 1 h) treatment on THA-induced HDAC5 phosphorylation in primary hepatocytes. F: Immunoblot showing effect of KN62 (10 μM, 1 h) treatment on THA-induced HDAC5 phosphorylation in primary hepatocytes. G: Effect of THA on mRNA amounts for WY-induced fatty acid oxidation genes, including Cpt1a, Hmgcs2, Lcad, and Mcad in primary hepatocytes reconstituted with HDAC5 or HDAC5 2SA (n = 3). H: Representative Oil Red O staining of liver sections from HFD-fed mice injected with either Ad-GFP or Ad-HDAC5 2SA (scale bar, 20 μm). I: Effect of Ad-GFP or Ad-HDAC5 2SA injection on hepatic triglyceride levels in HFD-fed mice (n = 8). J: Effect of Ad-GFP or Ad-HDAC5 2SA injection on hepatic fatty acid oxidation gene expression in HFD-fed mice (n = 8). All data are presented as means ± SEM. *P < 0.05. **P < 0.01.
Aberrant increase in ER stress-induced HDAC5 phosphorylation levels in HFD-fed mice indicate that strategies to promote HDAC5 dephosphorylation could serve as potential new tools to ameliorate obesity-associated hepatic steatosis. Indeed, Ad-HDAC5 2SA injection in HFD-fed mice (supplemental Fig. S4A) greatly decreased plasma NEFA levels, hepatic lipid accumulation, triglyceride levels in liver, and increased ketone bodies compared with controls, whereas body weight, liver weight, and plasma glucagon levels remained unchanged (Fig. 4H, I, and supplemental S4B, C). Consistently, expression of PPARα target genes known to regulate fatty acid oxidation (Cpt1a, Mcad, Lcad, Hmgcs2, Ppara, and Fgf21) were significantly increased in the livers of mice injected with Ad-HDAC5 2SA (Fig. 4J).
DISCUSSION
The liver is a major organ that controls glucose and lipid metabolism in response to hormonal signals. In the past decade, the ER stress-induced UPR pathway has emerged as an important modulator of hepatic glucose and lipid metabolism. ATF6 reduces hepatic glucose output by disrupting the CREB-CRTC2 interaction (42) and increases fatty acid oxidation to attenuate hepatic steatosis through PPARα (43). IRE1α promotes glucagon-stimulated gluconeogenesis (44) and prevents hepatic steatosis through repressing expression of key metabolic transcriptional regulators such as PPARγ (25). XBP1s inhibits hepatic gluconeogenesis by targeting FOXO1 for proteasomal degradation (45) and meanwhile promotes lipogenesis (22). Beside the UPR pathways, ER stress also leads to the release of Ca2+ from the ER lumen to the cytosol to activate CaMKII (20, 21). Here, we report that the calcium-CaMKII-HDAC5 pathway mediates ER stress-induced suppression of fatty acid oxidation gene expression. Meanwhile, it has been reported that HDAC5 could also interact with LXRα to impact lipogenesis (46), and we were also able to detect the interaction of HDAC5 with LXRα when expressed in HEK293T cells (supplemental Fig. S5A). Thus, it is tempting to speculate that this pathway may contribute to broader hepatic metabolic pathways, which will need further investigation.
Glucagon levels are elevated in subjects with type 2 diabetes and contribute to the development of excessive hepatic glucose production and hyperglycemia (47). Although glucagon is known to induce hepatic fatty acid oxidation and suppress lipogenesis in liver, excessive triacylglycerol deposits cause steatosis in subjects with type 2 diabetes. The detailed mechanism for this paradox still remains unsolved and greatly limits the use of glucagon antagonism as a potential strategy for type 2 diabetes in human. Our previous work showed that impaired glucagon-stimulated cAMP efflux from liver by obesity accounts for the excessive triacylglycerol deposits in the pathophysiology of type 2 diabetes (15). Here, we provide a new insight into the mechanism by showing that ER stress induced by obesity and type 2 diabetes suppresses glucagon-stimulated HDAC5 dephosphorylation and HDAC5-mediated PPARα activity, which lead to hepatic steatosis. Hence, hyperglucagonemia with defective glucagon signaling defines a new glucagon resistance status in obesity and type 2 diabetes, and ER stress functions as an important inducer of glucagon resistance together with insulin resistance (48).
Taken together, we show that regulation of HDAC5 phosphorylation status by fasting glucagon and ER stress serves as an important mechanism for modulating PPARα activity and hepatic fatty acid oxidation. This mechanism has an important role in the development of hepatic steatosis under both physiological and pathological conditions. Thus, a systematic investigation into the role of the glucagon signal pathway and the ER stress pathway in fatty acid oxidation would likely lead to novel therapeutic strategies for manipulating obesity-associated hepatic steatosis.
Supplementary Material
Footnotes
Abbreviations:
- ATF6
- activating transcription factor 6
- CaMKII
- Calcium/calmodulin-dependent protein kinase II
- CREB
- cAMP-response element binding protein
- CRTC
- CREB-regulated transcription coactivator
- CPT1a
- carnitine palmitoyltransferase 1A
- ER
- endoplasmic reticulum
- FOXO1
- Forkhead box O1
- HDAC
- histone deacetylase
- HMGCL
- 3-hydroxy-3-methylglutaryl-CoA lyase
- HMGCS2
- 3-hydroxy-3-methylglutaryl-CoA synthase 2
- IRE1
- inositol-requiring enzyme 1
- Lcad
- long-chain acyl-CoA dehydrogenase
- Mcad
- medium-chain acyl-CoA dehydrogenase
- SIK
- salt-inducible kinase
- UPR
- unfolded protein response
This research was supported by grants from 1000 Talents Program for Young Scholars of China (B.L., C.Z.); the National Science Foundation of China Grants 81671110, 81570760, and 31771283 and Shanghai Rising-Star Program Grants 17QA1402900 and 15QA1403600 (Z-N. Z., C.Z.); the National Key Research and Development Program of China (Grants 2017YFA0103900, 2017YFA0103902, 2017YFA0106500, and 2016YFA0102200); the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning (A11323) (C.Z.), and the Fundamental Research Funds for the Central Universities of Tongji University. The authors declare no competing financial interests.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Marchesini G., Bugianesi E., Forlani G., Cerrelli F., Lenzi M., Manini R., Natale S., Vanni E., Villanova N., Melchionda N., et al. . 2003. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 37: 917–923. [DOI] [PubMed] [Google Scholar]
- 2.Hooper A. J., Adams L. A., and Burnett J. R.. 2011. Genetic determinants of hepatic steatosis in man. J. Lipid Res. 52: 593–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGarry J. D., and Foster D. W.. 1980. Regulation of hepatic fatty acid oxidation and ketone body production. Annu. Rev. Biochem. 49: 395–420. [DOI] [PubMed] [Google Scholar]
- 4.Cohen J. C., Horton J. D., and Hobbs H. H.. 2011. Human fatty liver disease: old questions and new insights. Science. 332: 1519–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chawla A., Repa J. J., Evans R. M., and Mangelsdorf D. J.. 2001. Nuclear receptors and lipid physiology: opening the X-files. Science. 294: 1866–1870. [DOI] [PubMed] [Google Scholar]
- 6.Kersten S., Seydoux J., Peters J. M., Gonzalez F. J., Desvergne B., and Wahli W.. 1999. Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. J. Clin. Invest. 103: 1489–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans R. M., Barish G. D., and Wang Y. X.. 2004. PPARs and the complex journey to obesity. Nat. Med. 10: 355–361. [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto T., Cook W. S., Qi C., Yeldandi A. V., Reddy J. K., and Rao M. S.. 2000. Defect in peroxisome proliferator-activated receptor alpha-inducible fatty acid oxidation determines the severity of hepatic steatosis in response to fasting. J. Biol. Chem. 275: 28918–28928. [DOI] [PubMed] [Google Scholar]
- 9.Rakhshandehroo M., Knoch B., Muller M., and Kersten S.. 2010. Peroxisome proliferator-activated receptor alpha target genes. PPAR Res. Epub ahead of print. September 26, 2010; doi:10.1155/2010/612089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Longuet C., Sinclair E. M., Maida A., Baggio L. L., Maziarz M., Charron M. J., and Drucker D. J.. 2008. The glucagon receptor is required for the adaptive metabolic response to fasting. Cell Metab. 8: 359–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Meyenn F., Porstmann T., Gasser E., Selevsek N., Schmidt A., Aebersold R., and Stoffel M.. 2013. Glucagon-induced acetylation of Foxa2 regulates hepatic lipid metabolism. Cell Metab. 17: 436–447. [DOI] [PubMed] [Google Scholar]
- 12.Dentin R., Liu Y., Koo S. H., Hedrick S., Vargas T., Heredia J., Yates J. 3rd, and Montminy M.. 2007. Insulin modulates gluconeogenesis by inhibition of the coactivator TORC2. Nature. 449: 366–369. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y., Dentin R., Chen D., Hedrick S., Ravnskjaer K., Schenk S., Milne J., Meyers D. J., Cole P., Yates J. 3rd, et al. . 2008. A fasting inducible switch modulates gluconeogenesis via activator/coactivator exchange. Nature. 456: 269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Z. N., Gong L., Lv S., Li J., Tai X., Cao W., Peng B., Qu S., Li W., Zhang C., et al. . 2016. SIK2 regulates fasting-induced PPARalpha activity and ketogenesis through p300. Sci. Rep. 6: 23317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lv S., Qiu X., Li J., Liang J., Li W., Zhang C., Zhang Z., and Luan B.. 2017. Glucagon-induced extracellular cAMP regulates hepatic lipid metabolism. J. Endocrinol. 234: 73–87. [DOI] [PubMed] [Google Scholar]
- 16.Mihaylova M. M., Vasquez D. S., Ravnskjaer K., Denechaud P. D., Yu R. T., Alvarez J. G., Downes M., Evans R. M., Montminy M., and Shaw R. J.. 2011. Class IIa histone deacetylases are hormone-activated regulators of FOXO and mammalian glucose homeostasis. Cell. 145: 607–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang B., Moya N., Niessen S., Hoover H., Mihaylova M. M., Shaw R. J., Yates J. R. 3rd, Fischer W. H., Thomas J. B., and Montminy M.. 2011. A hormone-dependent module regulating energy balance. Cell. 145: 596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hetz C., Chevet E., and Harding H. P.. 2013. Targeting the unfolded protein response in disease. Nat. Rev. Drug Discov. 12: 703–719. [DOI] [PubMed] [Google Scholar]
- 19.Michalak M., Robert Parker J. M., and Opas M.. 2002. Ca2+ signaling and calcium binding chaperones of the endoplasmic reticulum. Cell Calcium. 32: 269–278. [DOI] [PubMed] [Google Scholar]
- 20.Timmins J. M., Ozcan L., Seimon T. A., Li G., Malagelada C., Backs J., Backs T., Bassel-Duby R., Olson E. N., Anderson M. E., et al. . 2009. Calcium/calmodulin-dependent protein kinase II links ER stress with Fas and mitochondrial apoptosis pathways. J. Clin. Invest. 119: 2925–2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozcan L., and Tabas I.. 2010. Pivotal role of calcium/calmodulin-dependent protein kinase II in ER stress-induced apoptosis. Cell Cycle. 9: 223–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee A. H., Scapa E. F., Cohen D. E., and Glimcher L. H.. 2008. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 320: 1492–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malhi H., and Kaufman R. J.. 2011. Endoplasmic reticulum stress in liver disease. J. Hepatol. 54: 795–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rutkowski D. T., Wu J., Back S. H., Callaghan M. U., Ferris S. P., Iqbal J., Clark R., Miao H., Hassler J. R., Fornek J., et al. . 2008. UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Dev. Cell. 15: 829–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang K., Wang S., Malhotra J., Hassler J. R., Back S. H., Wang G., Chang L., Xu W., Miao H., Leonardi R., et al. . 2011. The unfolded protein response transducer IRE1alpha prevents ER stress-induced hepatic steatosis. EMBO J. 30: 1357–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozcan U., Yilmaz E., Ozcan L., Furuhashi M., Vaillancourt E., Smith R. O., Gorgun C. Z., and Hotamisligil G. S.. 2006. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 313: 1137–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ozcan U., Cao Q., Yilmaz E., Lee A. H., Iwakoshi N. N., Ozdelen E., Tuncman G., Gorgun C., Glimcher L. H., and Hotamisligil G. S.. 2004. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 306: 457–461. [DOI] [PubMed] [Google Scholar]
- 28.Dentin R., Pegorier J. P., Benhamed F., Foufelle F., Ferre P., Fauveau V., Magnuson M. A., Girard J., and Postic C.. 2004. Hepatic glucokinase is required for the synergistic action of ChREBP and SREBP-1c on glycolytic and lipogenic gene expression. J. Biol. Chem. 279: 20314–20326. [DOI] [PubMed] [Google Scholar]
- 29.Lv S., Qiu X., Li J., Li W., Zhang C., Zhang Z. N., and Luan B.. 2016. Suppression of CRTC2-mediated hepatic gluconeogenesis by TRAF6 contributes to hypoglycemia in septic shock. Cell Discov. 2: 16046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inagaki T., Dutchak P., Zhao G., Ding X., Gautron L., Parameswara V., Li Y., Goetz R., Mohammadi M., Esser V., et al. . 2007. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 5: 415–425. [DOI] [PubMed] [Google Scholar]
- 31.Lv S., Li J., Qiu X., Li W., Zhang C., Zhang Z. N., and Luan B.. 2017. A negative feedback loop of ICER and NF-kappaB regulates TLR signaling in innate immune responses. Cell Death Differ. 24: 492–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmittgen T. D., and Livak K. J.. 2008. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3: 1101–1108. [DOI] [PubMed] [Google Scholar]
- 33.Luan B., Goodarzi M. O., Phillips N. G., Guo X., Chen Y. D., Yao J., Allison M., Rotter J. I., Shaw R., and Montminy M.. 2014. Leptin-mediated increases in catecholamine signaling reduce adipose tissue inflammation via activation of macrophage HDAC4. Cell Metab. 19: 1058–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Altarejos J. Y., and Montminy M.. 2011. CREB and the CRTC co-activators: sensors for hormonal and metabolic signals. Nat. Rev. Mol. Cell Biol. 12: 141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han J., Li E., Chen L., Zhang Y., Wei F., Liu J., Deng H., and Wang Y.. 2015. The CREB coactivator CRTC2 controls hepatic lipid metabolism by regulating SREBP1. Nature. 524: 243–246. [DOI] [PubMed] [Google Scholar]
- 36.Pyper S. R., Viswakarma N., Yu S., and Reddy J. K.. 2010. PPARalpha: energy combustion, hypolipidemia, inflammation and cancer. Nucl. Recept. Signal. 8: e002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berdeaux R., Goebel N., Banaszynski L., Takemori H., Wandless T., Shelton G. D., and Montminy M.. 2007. SIK1 is a class II HDAC kinase that promotes survival of skeletal myocytes. Nat. Med. 13: 597–603. [DOI] [PubMed] [Google Scholar]
- 38.Unger R. H., Aguilar-Parada E., Muller W. A., and Eisentraut A. M.. 1970. Studies of pancreatic alpha cell function in normal and diabetic subjects. J. Clin. Invest. 49: 837–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haberland M., Montgomery R. L., and Olson E. N.. 2009. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat. Rev. Genet. 10: 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Backs J., Backs T., Bezprozvannaya S., McKinsey T. A., and Olson E. N.. 2008. Histone deacetylase 5 acquires calcium/calmodulin-dependent kinase II responsiveness by oligomerization with histone deacetylase 4. Mol. Cell. Biol. 28: 3437–3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McKinsey T. A., Zhang C. L., and Olson E. N.. 2000. Activation of the myocyte enhancer factor-2 transcription factor by calcium/calmodulin-dependent protein kinase-stimulated binding of 14–3-3 to histone deacetylase 5. Proc. Natl. Acad. Sci. USA. 97: 14400–14405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y., Vera L., Fischer W. H., and Montminy M.. 2009. The CREB coactivator CRTC2 links hepatic ER stress and fasting gluconeogenesis. Nature. 460: 534–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen X., Zhang F., Gong Q., Cui A., Zhuo S., Hu Z., Han Y., Gao J., Sun Y., Liu Z., et al. . 2016. Hepatic ATF6 increases fatty acid oxidation to attenuate hepatic steatosis in mice through peroxisome proliferator-activated receptor alpha. Diabetes. 65: 1904–1915. [DOI] [PubMed] [Google Scholar]
- 44.Mao T., Shao M., Qiu Y., Huang J., Zhang Y., Song B., Wang Q., Jiang L., Liu Y., Han J. D., et al. . 2011. PKA phosphorylation couples hepatic inositol-requiring enzyme 1alpha to glucagon signaling in glucose metabolism. Proc. Natl. Acad. Sci. USA. 108: 15852–15857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou Y., Lee J., Reno C. M., Sun C., Park S. W., Chung J., Lee J., Fisher S. J., White M. F., Biddinger S. B., et al. . 2011. Regulation of glucose homeostasis through a XBP-1-FoxO1 interaction. Nat. Med. 17: 356–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jia H. Y., Li Q. Z., and Lv L. F.. 2016. HDAC5 inhibits hepatic lipogenic genes expression by attenuating the transcriptional activity of liver X receptor. Cell Physiol Biochem. 39: 1561–1567. [DOI] [PubMed] [Google Scholar]
- 47.Maharaj A., Zhu L., Huang F., Qiu H., Li H., Zhang C. Y., Jin T., and Wang Q.. 2012. Ectopic expression of glucagon receptor in skeletal muscles improves glucose homeostasis in a mouse model of diabetes. Diabetologia. 55: 1458–1468. [DOI] [PubMed] [Google Scholar]
- 48.Flamment M., Hajduch E., Ferre P., and Foufelle F.. 2012. New insights into ER stress-induced insulin resistance. Trends Endocrinol Metab. 23: 381–390. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.