Abstract
The aim of the present study was to investigate the correlation of enhancer of Zeste homolog 2 (EZH2) and SET and MYND domain containing 3 (SMYD3) gene polymorphisms with breast cancer susceptibility and prognosis. A total of 712 patients with breast cancer and 783 healthy individuals were selected. Normal breast epithelial cells MCF-10A and breast cancer cells MCF-7, MDA-MB-231, T47D, and Bcap-37 were cultured. Polymerase chain reaction (PCR)-restriction fragment length polymorphism method was applied for genotyping. Reverse-transcription quantitative PCR (RT-qPCR) and Western blotting were used to examine EZH2 and SMYD3 expression in breast cancer tissues and cells. The risk factors and prognostic factors for breast cancer were estimated. The C allele of EZH2 rs12670401 (odds ratio (OR) =1.255, 95% confidence interval (95% CI): 1.085–1.452), T allele of EZH2 rs6464926 (OR =1.240, 95% CI: 1.071–1.435), and three alleles of SMYD3 variable number of tandem repeats (VNTRs) (OR =1.305, 95% CI: 1.097–1.552) could increase susceptibility to breast cancer. Combined genotypes of EZH2 rs12670401 (TC + CC) and EZH2 rs6464926 (CT + TT) were associated with breast cancer susceptibility. Breast cancer tissues had higher EZH2 and SMYD3 expression. EZH2 rs12670401, EZH2 rs6464926, age of menarche, and menopausal status were associated with breast cancer susceptibility. Patients with TT genotype of EZH2 rs12670401 or with CC genotype of EZH2 rs6464926 had higher overall survival (OS). EZH2 rs12670401, EZH2 rs6464926, and clinical staging were independent prognostic factors for breast cancer. SMYD3 VNTR polymorphism exhibited no association with susceptibility and prognosis. EZH2 rs12670401 and rs6464926 polymorphisms, EZH2 and SMYD3 expression, clinical staging, lymph node metastasis, human epidermal growth factor receptor-2 (HER2) status, and metastasis may be correlated with breast cancer susceptibility and prognosis.
Keywords: Breast cancer, EZH2, Polymorphism, Prognosis, SMYD3, Susceptibility
Introduction
Breast cancer, the most common malignant tumor in the world and second common malignant tumor in China, ranks sixth as the cause of female tumor deaths [1]. The hazard and developing trend of breast cancer is closely related to age, lifestyle, economic development, and environmental change, and the mortality of breast cancer in Chinese females shows a gradual upward trend in recent years [2]. Surgical therapy is currently the main treatment for breast cancer, including hormonal therapy, chemotherapy, radiotherapy, and molecular targetted therapy [3]. Many molecular targets have been identified in breast cancer: trastuzumab and lapatinib target the human epidermal growth factor receptor-2 (HER2) and are approved drugs for the treatment of metastatic breast cancer [4]. In addition, the expression abnormality of relative genes has been proved to be involved in the incidence of breast cancer and may cause the proliferation, invasion, recurrence, and metastasis of tumors [5]. Researches in genetic polymorphisms of breast cancer can provide a theoretical basis for breast cancer prevention, diagnosis, therapy, and prognosis [6–8].
Epigenetic histone modification, a kind of genetic modification, plays a role in the regulation of gene expression by affecting the affinity of histone, DNA duplexes, binding of transcription factors, and DNA structural gene promoters [9]. Histone methylation is a member of the histone modification with histone methyltransferase activity, wherein SET and MYND domain containing 3 (SMYD3) and enhancer of Zeste homolog 2 (EZH2) are reported to be involved in the development of multiple cancers and play important roles in transcriptional regulation [10]. It was found that EZH2 and SMYD3 could stimulate growth and increase invasion of multiple tumors [11,12]. In recent years, gene polymorphisms have been reported to play important roles in tumor development and progression [13]. EZH2 polymorphism has a significant influence on colorectal cancer (CRC) susceptibility in the Han Chinese population and plays an important role in the pathogenesis and prediction of CRC [14]. Polymorphisms (rs12670401 and rs6464926) of EZH2 were identified to be significantly associated with the risk of gastric cancer and C allele of EZH2 rs12670401 and T allele of EZH2 rs6464926 showed strong associations with increased gastric cancer susceptibility [15]. A variable number of tandem repeats (VNTRs) polymorphism in the SMYD3 promoter region is a risk factor for familial breast cancer and esophageal squamous cell carcinoma [16,17]. However, the correlation of EZH2 and SMYD3 polymorphisms with breast cancer susceptibility and prognosis has not yet been reported. Therefore, the present study aims to investigate the correlation of EZH2 and SMYD3 gene polymorphisms with breast cancer susceptibility and prognosis, in order to provide a certain theoretical basis for clinical application in the diagnosis and prognosis of breast cancer, as well as a reference for individualized therapy of breast cancer.
Materials and methods
Ethics statement
The experimental procedures were approved by the Human Ethics Committee of Shaanxi Provincial People’s Hospital and were performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Study subjects
From August 2010 to December 2012, 712 patients with breast cancer (all females, mean age: 49.88 ± 13.14 years) who were admitted in Shaanxi Provincial People’s Hospital and the co-operative hospital (First Hospital of Xi’an Jiao Tong University) were randomly selected as a case group. The inclusion criteria were as follows: patients received X-ray mammography and were confirmed with pathological examination as having breast cancer. A total of 783 cancer-free healthy people with no sibship with involved patients (mean age: 45.51 ± 11.21 years) who took physical examination in Shaanxi Provincial People’s Hospital in the same period were classified as the control group. The exclusion criteria were as follows: (i) patients with extensive metastasis; (ii) patients with tumor history in other organs; (iii) patients with systemic failure, systemic lupus erythematosus, or other autoimmune diseases; (iv) patients with recent trauma, surgeries, lymph nodes, or other malignant cancer; (v) patients who did not co-operate with surgery and investigation; (vi) patients without detailed clinical data or follow-up data. All 712 samples of breast cancer tissues not treated with radiotherapy or chemotherapy before operation were collected. All 783 normal adjacent tissues were extracted at least 4 cm from the cancer tissues. All tissues were stored at −80°C after cryopreservation.
Cell culture
Normal breast epithelial cells MCF-10A and breast cancer cells MCF-7, MDA-MB-231, T47D, and Bcap-37 were purchased from American Type Culture Collection (ATCC, Manassas, VA, U.S.A.). After resuscitation, MCF-10A was cultured in Dulbecco’s modified Eagle’s medium (DMEM)/F12 medium containing 5% horse serum, 10 g/ml insulin, 20 ng/ml epidermal growth factor (EGF), 100 ng/ml cholera toxin, and 0.5 g/ml hydrocortisone; MDA-MB-231 was cultured in RPMI1640 medium containing 10% FBS, 100 U/ml penicillin, 100 Ug/ml streptomycin, and 2 mol/l glutamine; MCF-7 was cultured in DMEM containing 10% FBS, 100 U/ml penicillin, 100 Ug/ml streptomycin, and 2 mol/l glutamine; T47D was cultured in DMEM containing 20% FBS, 100 U/ml penicillin, 100 Ug/ml streptomycin, and 2 mol/l glutamine; Bcap-37 was cultured in RPMI1640 medium containing 20% FBS, 100 U/ml penicillin, 100 Ug/ml streptomycin, and 2 mol/l glutamine. All five kinds of cells were cultured in 5% CO2 incubation at 37°C with saturated humidity. The culture medium was changed every 2–3 days. When the cells grew well and reached 80–90% confluence, they were collected.
Peripheral venous blood collection and genomic DNA extraction
Fasting peripheral blood was collected in the case and control groups for EDTA anticoagulant and frozen preservation. Genomic DNA was extracted with Blood Genome DNA Extraction Kit (Takara Biotechnology Ltd., Dalian, China) after blood sampling. Under high-salt state, the DNA was specifically adsorbed by a silicone membrane; under the condition of low salt or aqueous solution, the DNA was eluted. The DNA concentration was adjusted to 100 ng/μl and preserved in a refrigerator at −20°C.
Polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP)
PCR-RFLP was used to detect and analyze polymorphic sites of genotypes and the prime 5.0 software was applied for primer design. All the primers were synthesized by Shanghai Sangon Biological Engineering Technology & Services Co., Ltd. (Shanghai, China) and the primer sequences are shown in Table 1 and Table 2.
Table 1. Primer sequences of SNP for EZH2 and SMYD3.
| SNP | Primer sequence | Temperature | Time | Circle |
|---|---|---|---|---|
| EZH2rs12670401 | F: 5′-ACGTTGGATGTACTTGGTTTAGATTGCCTG-3′ | 66°C | 45 s | 30 |
| R: 5′-ACGTTGGATGATGGACTAGGTGGTTCAAAC-3′ | ||||
| EZHrs6464926 | F: 5′-ACGTTGGATGAGAATTGCCAGGAGATCTCT-3′ | 58°C | 45 s | 40 |
| R: 5′-ACGTTGGATGTTCCTTCATTGCCTGTTGCC-3′ | ||||
| SMYD3VNTR | F: 5′-CGCCTGTCTTCTGCGCAGTCG-3′ | 56.5°C | 45 s | 40 |
| R: 5′-CACCTTCAGCGGCTCCATCCTC-3 |
Abbreviations: F, forward; R, reverse; SNP, single nucleotide polymerase.
Table 2. Primer sequences of EZH2 and SMYD3.
| Genes | Sequences (5′–3′) |
|---|---|
| EZH2 | F: 5′-AATCAGAGTACATGCGACTGAGA-3′ |
| R: 5′-GCTGTATCCTTCGCTGTTTCC-3′ | |
| SMYD3 | F: 5′-CGCGTCGCCAAATACTGTAGT-3′ |
| R: 5′-CAAGAAGTCGAACGGAGTCTG-3′ | |
| GAPDH | F: 5′-GGAGCGAGATCCCTCCAAAAT-3′ |
| R: 5′-GGCTGTTGTCATACTTCTCATGG-3′ |
Abbreviations: F, forward; GAPDH, glyceraldehyde phosphate dehydrogenase; R, reverse.
The PCR reaction system of EZH2 rs12670401: the total reaction system was 25 μl, including 12.5 μl of 2× Master Mix, 1 μl (10 mM) of upstream and downstream primers, respectively, 1 μg of DNA template, and 25 μl of ddH2O. PCR conditions: 2 min of predenaturation at 66°C, 30 cycles of 30 s of denaturation at 94°C, 45 s of annealing at 66°Cand 30 s of extension at 72°C, and finally 7 min of extension at 72°C.
The PCR reaction system of EZH2 rs6464926: the total reaction system was 50 μl, including 5 μl of 10× buffer, 4 μl (25 mM) of MgCl2, 1 μl (10 mM) of dNTPs, 1 μl (25 pmol/l) of upstream and downstream primers, respectively, 10 μl of DNA template, and 27 μl of ddH2O. PCR conditions: 2 min of predenaturation at 95°C, 40 cycles of 1 min of denaturation at 95°C, 45 s of annealing at 58°Cand 1 min of extension at 72°C, and finally 2 min of extension at 72°C.
The PCR reaction system of SMYD3 VNTR: the total reaction system was 50 μl, including 5 μl of 10× buffer, 4 μl (25 mM) of MgCl2, 1 μl (10 mM) of dNTPs, 1 μl (25 pmol/l) of upstream and downstream primers, respectively, 10 μl of DNA template, and 27 μl of ddH2O. PCR conditions: 2 min of predenaturation at 94°C, 40 cycles of 20 s of denaturation at 94°C, 45 s of annealing at 56.5°C, and 30 s of extension at 72°C, and finally 3 min of extension at 72°C.
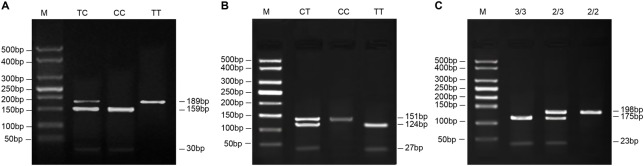
The PCR amplification product was digested with restricted enzyme. The total reaction system was 20 μl, including 17 μl of PCR amplification product, 2 μl of 10× reaction buffer, and 1 μl (10 U/μl) of matching buffer, followed by addition of incision enzyme (1 μl) for overnight digestion at 37°C. The enzyme digestion products were evaluated by 3.5% agarose gel electrophoresis (120 V, 40 min) and Ethidium Bromide staining. Moreover, the DNA bends (Figure 1) were observed and detected under a UV lamp. Ten percent samples randomly collected were sequenced from both directions by Shanghai Sangon Biological Engineering Technology & Services Co., Ltd. (Shanghai, China) to verify the results of PCR detection. Genotypes were determined according to the enzyme map.
Figure 1. Electrophoresis maps of EZH2 rs12670401, EZH2 rs6464926, and SMYD3VNTR gene polymorphisms.
(A) Electrophoresis maps of EZH2 rs12670401 gene polymorphisms. M, DNA marker; TC, TC heterozygotes digestion products, 189, 159, 30 bp; CC, CC homozygotes digestion products, 159 and 30 bp; TT, TT homozygotes digestion product, 189 bp. (B) Electrophoresis map of EZH2 rs6464926 gene polymorphisms. M, DNA marker; CC, CC homozygotes digestion products, 151 bp; TT, TT homozygotes digestion products, 124 and 27 bp; CT, CT heterozygotes digestion products, 124, 151, and 27 bp. (C) Electrophoresis map of SMYD3 VNTR gene polymorphisms. M, DNA marker; 3/3, 3/3 homozygote digestion products, 175 and 23 bp; 2/3, 2/3 heterozygotes digestion products, 198, 175, and 23 bp; 2/2, 2/2 homozygotes digestion products, 198 bp.
Reverse-transcription quantitative PCR
MiRNeasy Mini Kit (Qiagen Company, Hilden, Germany) was used to extract total RNA from tissues or cells. The absorbance and purity of RNA were determined by UV spectrophotometer at 260 and 280 nm. If the ratio of optical density (OD)260/OD280 was between 1.7 and 2.1, the purity was higher and could be used in subsequent experiments. The cDNA template was synthesized by reverse transcription in PCR amplification apparatus (ABI). ABI7500 quantitative PCR (ABI Company, Oyster Bay, NY, U.S.A.) was used to perform the reverse-transcription quantitative PCR (RT-qPCR) experiment, and the reaction conditions were as follows: 10 min of predenaturation at 95°C, 10 s of denaturation at 95°C, 20 s of annealing at 60°C, 34 s of extension at 72°C, totalling 40 cycles. The reaction system included SYBR Premix Ex Taq™ II 10 µl, PCR forward primer (10 µM) 0.8 µl, PCR reverse primer (10 µM) 0.8 µl, ROX Reference Dye 0.4 µl, cDNA template 2.0 µl, and sterilized distilled water 6.0 µl. The glyceraldehyde phosphate dehydrogenase (GAPDH) was used as a reference, and 2−ΔΔCt indicated the ratio of target gene expression between the experiment group and the control group. The formula was as follows: ΔΔCT = ΔCt experiment group – ΔCt control group, and ΔCt = Ct target gene – Ct GAPDH. Ct was the cycle numbers in which the fluorescence intensity reached the set threshold when the amplification was at logarithmic growth phase [18].
Western blotting
The cancer tissues and adjacent tissues were added with liquid nitrogen and ground until the tissues were homogeneous. Then, the tissues were added with protein lysate (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China), centrifuged at 12000 rev/min at 4°C for 20 min, and the supernatant was obtained and stored for further use. The cells were collected, lysed, and centrifuged, and the total protein was collected. The total protein concentration was measured by BCA Kit (Thermo Fisher Scientific, Carlsbad, California, U.S.A.). After taking the total protein of the cell and separating by SDS/PAGE (12% gel), the protein was transferred on to PVDF membrane and sealed by skimmed milk at room temperature for 1 h. The following primary antibodies were added – EZH2 (1:1000, ab186006), SMYD3 (1:2000, ab187149), and GAPDH (1:1000, ab8245) – and cultured at 4°C overnight. All antibodies were purchased from Abcam Inc. (Cambridge, MA, U.S.A.). The membrane was washed three times to remove the primary antibodies, and added with second antibody and cultured at room temperature for 1 h. The membrane was washed three times again and ECL reagent was applied to reveal Western blotting bands. Using GAPDH as a reference, the relative expression of protein was analyzed by Western blotting image (ImageJ2x software).
Follow-up
With approval of the patients and their families, follow-up was carried out on a regular basis by telephone, outpatient, letters, or clinical data consulting for data statistics. The follow-up duration was 60 months and the follow-up rate was 100%. The patients’ condition changes were informed regularly through follow-up feedback and the clinical pathological data and survival rate were collected for statistical analysis.
Statistical analysis
SPSS 19.0 statistical software (IBM Corp. Armonk, NY, U.S.A.) was applied for data processing. Hardy–Weinberg equilibrium was used to test the population representativeness. A P-value ≥0.05 indicated that the sample reached genetic equilibrium with good population representativeness. Univariate and multivariate logistic regression analyses were applied to calculate odds ratio (OR) and 95% confidence interval (95% CI), to estimate the strength of association between the mutation of each polymorphic locus and breast cancer. The enumeration data were represented as ratio or rate, and compared by χ2 test. The measurement data were represented as mean ± S.D., and compared by t test, with P<0.05 as statistically significant. P was a two-sided test, and P<0.05 was considered to be statistically significant.
Results
Baseline characteristics of study subjects
No significant difference in age (P>0.05), but obvious differences in age of menarche and menopausal status were found between the case and control groups (both P<0.05). In the case group, patients with age over 40 years accounted for 83.01%; 75.28% patients were positive in estrogen receptor (ER+) and 76.41% were positive in progesterone receptor (PR+); 91.85% patients were in clinical stage I or II, and 8.15% (58 cases) were in stage III or IV; 377 cases (52.94%) had lymph node metastasis (Table 3).
Table 3. Baseline characteristics of the study subjects between the case and control groups.
| Groups | Case group, n (%) | Control group, n (%) | P |
|---|---|---|---|
| Total | 712 | 783 | |
| Age (years) | |||
| ≥40 | 591 (83.01 ) | 673 (85.95) | 0.132 |
| <40 | 121 (16.99) | 110 (14.05) | |
| Age of menarche (years) | |||
| ≥13 | 495 (69.52) | 477 (60.92) | <0.001 |
| <13 | 217 (30.48) | 306 (39.08) | |
| Menopausal status | |||
| Premenopause | 81 (11.38) | 292 (37.29) | <0.001 |
| Postmenopause | 631 (88.62) | 491 (62.71) | |
| Tumor size (cm) | |||
| ≤2.0 | 323 ( 45.37) | ||
| 2.1–4 | 329 (46.21) | ||
| >4 | 60 (8.43) | ||
| Pathological type | |||
| IDC | 618 (86.80) | ||
| ILC | 31 (4.35) | ||
| Other | 63 (8.85) | ||
| Lymph node metastasis | |||
| Negative | 334 (46.91) | ||
| Positive | 378 (53.09) | ||
| Immunohistochemical indexes | |||
| ER (−) | 176 (24.72) | ||
| ER (+) | 536 (75.28) | ||
| PR (−) | 168 (23.60) | ||
| PR (+) | 544 (76.41) | ||
| HER2 status | |||
| Negative | 361 (50.70) | ||
| Positive | 351 (49.30) | ||
| FDRs with breast cancer | |||
| Yes | 251 (35.25) | ||
| No | 461 (64.75) | ||
| Clinical staging | 654 (91.85) | ||
| I/II | |||
| III/IV | 58 (8.15) | ||
| Subtypes of breast cancer | |||
| Triple negative | 133 (18.68) | ||
| Non-triple negative | 579 (81.32) | ||
| Metastasis situation | |||
| Distant metastasis | 154 (21.63) | ||
| Primary lesion | 558 (78.37) |
Abbreviations: FDR, first-degree relative; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma.
Electrophoresis map of EZH2 and SMYD3 gene polymorphisms
The amplified fragment length of EZH2 rs12670401 was 189 bp, wherein there were no fragments in wild-type (TT-type), two fragments (159 and 30 bp) in homozygous mutant (CC type), and three fragments (189, 159, and 30 bp) in heterozygous mutant (TC type) after HaeIII digestion (Figure 1A). The amplified fragment length of EZH2 rs6464926 was 151 bp, wherein there were two fragments (124 and 27 bp) in homozygous mutant (TT type), three fragments (124, 151, and 27 bp) in heterozygous mutant (CT type), and a fragment (151 bp) in wild-type (CC type) after NdeI digestion (Figure 1B). The amplified fragment length of SMYD3 VNTR was 198 bp, wherein there were two fragments (175 and 23 bp) in homozygous mutant (3/3 type), three fragments (198, 175, and 23 bp) in heterozygous mutation (2/3 type), and a fragment (198 bp) in wild genotype (2/2 type) after HinfI digestion (Figure 1C).
Frequency distribution of EZH2 and SMYD3 genotypes and alleles
The genotypes and alleles frequency distribution of EZH2 rs12670401, EZH2 rs6464926, and SMYD3 VNTR polymorphisms in the case and control groups conformed to Hardy–Weinberg equilibrium with population representativeness (P>0.05).
In the case and control groups, there were significant differences in the allele frequency (χ2 =9.356, P=0.002) and genotype frequency (χ2 =12.22, P<0.001) of EZH2 rs12670401, and C allele could increase the susceptibility to breast cancer (OR =1.255, 95% CI: 1.085–1.452, P=0.002). There was an obvious difference in allele frequency (χ2 =8.286, P=0.004) and genotype frequency (χ2 =8.972, P=0.003) of EZH2 rs6464926, and T allele could increase the susceptibility to breast cancer (OR =1.240, 95% CI: 1.071–1.435, P=0.004). There was a distinctive difference in allele frequency (χ2 =9.1, P=0.034) and genotype frequency (χ2 =39.971, P<0.001) of SMYD3 VNTR, and 3 allele could increase the susceptibility to breast cancer (OR =1.305, 95% CI: 1.097–1.552, P=0.003) (Table 4).
Table 4. Genotype and allele frequency of EZH2 rs12670401, EZH2 rs6464926, and SMYD3 VNTR between the case and control groups.
| Genotype/allele | Case group, n (%) | Control group, n (%) | χ2 | P | OR (95% CI) |
|---|---|---|---|---|---|
| EZH2 rs12670401 | |||||
| TT | 232 (32.58) | 290 (37.04) | 1 | ||
| TC | 323 (45.37) | 372 (47.51) | 0.495 | 0.482 | 1.085 (0.864–1.363) |
| CC | 157 (22.05) | 121 (15.45) | 12.22 | <0.001 | 1.687 (1.257–2.265) |
| TC + CC | 480 (67.42) | 493 (62.96) | 3.254 | 0.071 | 1.217 (0.983–1.507) |
| T | 787 (55.27) | 952 (60.79) | 1 | ||
| C | 637 (44.73) | 614 (39.21) | 9.356 | 0.002 | 1.255 (1.085–1.452) |
| EZH2 rs6464926 | |||||
| CC | 243 (34.13) | 305 (38.95) | 1 | ||
| CT | 327 (45.93) | 365 (46.62) | 1.044 | 0.307 | 1.124 (0.898–1.408) |
| TT | 142 (19.94) | 113(14.43) | 8.972 | 0.003 | 1.577 (1.169–2.127) |
| CT + TT | 469 (65.87) | 478 (61.05) | 3.737 | 0.053 | 1.232 (0.997–1.521) |
| C | 813 (57.09) | 975 (62.26) | 1 | ||
| T | 611 (42.91) | 591 (37.74) | 8.286 | 0.004 | 1.240 (1.071–1.435) |
| SMYD3VNTR | |||||
| 2/2 | 427 (59.97) | 483 (61.69) | 1 | ||
| 2/3 | 222 (31.18) | 289 (36.91) | 1.596 | 0.207 | 0.869 (0.699–1.081) |
| 3/3 | 63 (8.85) | 11 (1.40) | 39.971 | <0.001 | 6.478 (3.369–12.456) |
| 2/3 + 3/3 | 285 (40.03) | 300 (38.31) | 0.459 | 0.498 | 1.075 (0.873–1.323) |
| 2 | 1076 (75.56) | 1255 (80.14) | 1 | ||
| 3 | 348 (24.44) | 311 (19.86) | 9.1 | 0.003 | 1.305 (1.097–1.552) |
Interaction of EZH2 and SMYD3 polymorphic loci with the susceptibility to breast cancer
As shown in Table 5, the combined genotype of EZH2 rs12670401 (TC + CC) and EZH2 rs6464926 (CT + TT) was significantly associated with the susceptibility to breast cancer (OR =1.465, 95% CI: 1.055–2.036, P=0.022). There was no statistical significance in the interaction of other genotypes with the susceptibility to breast cancer (all P>0.05).
Table 5. Correlation of interaction of EZH2 rs12670401, EZH2 rs6464926, and SMYD3 VNTR gene polymorphisms with breast cancer susceptibility in the case and control groups.
| Combined genotype | Case group, n (%) | Control group, n (%) | OR | 95% CI | P |
|---|---|---|---|---|---|
| EZH2 rs12670401 (TC + CC)/EZH2 rs6464926 (CT + TT) | 316 (44.41) | 303 (38.76) | 1.465 | 1.055–2.036 | 0.022 |
| EZH2 rs12670401 (TC + CC)/EZH2 rs6464926 (CC) | 164 (23.01) | 190 (24.20) | 1.213 | 0.849–1.732 | 0.288 |
| EZH2 rs12670401 (TT)/EZH2 rs6464926 (CT + TT) | 153 (21.46) | 179 (22.80) | 1.201 | 0.838–1.722 | 0.319 |
| EZH2 rs12670401 (TT)/EZH2 rs6464926 (CC) | 79 (11.12) | 111 (14.24) | Reference | ||
| EZH2 rs12670401 (TC + CC)/ SMYD3VNTR(2/3 + 3/3) | 192 (26.99) | 99 (12.69) | 1.312 | 0.918–1.874 | 0.136 |
| EZH2 rs12670401 (TC + CC)/ SMYD3VNTR (2/2) | 288 (40.43) | 160 (20.44) | 1.217 | 0.879–1.685 | 0.236 |
| EZH2 rs12670401 (TT)/ SMYD3VNTR(2/3 + 3/3) | 93 (13.04) | 58 (7.47) | 1.084 | 0.713–1.650 | 0.705 |
| EZH2 rs12670401 (TT)/ SMYD3VNTR (2/2) | 139 (19.54) | 94 (12.02) | Reference | ||
| SMYD3VNTR(2/3 + 3/3)/EZH2 rs6464926 (CT + TT) | 188 (26.37) | 185 (44.82) | 1.288 | 0.957–1.733 | 0.095 |
| SMYD3VNTR (2/3 + 3/3)/EZH2 rs6464926 (CC) | 97 (13.66) | 115 (27.99) | 1.069 | 0.756–1.512 | 0.707 |
| SMYD3VNTR (2/2)/EZH2 rs6464926 (CT + TT) | 281 (39.50) | 297 (72.17) | 1.195 | 0.911–1.567 | 0.198 |
| SMYD3VNTR (2/2)/EZH2 rs6464926 (CC) | 146 (20.47) | 186 (45.07) | Reference |
Reference, 95% CI is 1.
Expression of EZH2 and SMYD3 is higher in cancer tissues than in adjacent tissues
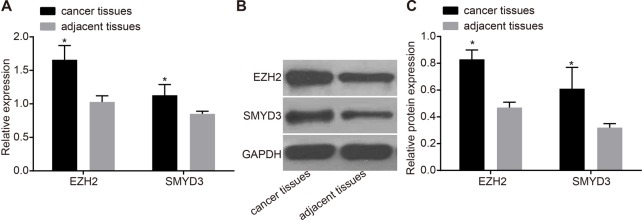
The mRNA and protein expression of EZH2 and SMYD3 in cancer tissues and adjacent tissues were detected by RT-qPCR and Western blotting. The results showed that the mRNA and protein expression of EZH2 and SMYD3 in cancer tissues were higher than those in adjacent tissues (P<0.05) (Figure 2). The results suggested that EZH2 and SMYD3 were in high expression in breast cancer tissues.
Figure 2. Expression of EZH2 and SMYD3 in cancer tissues and adjacent tissues.
(A) mRNA expression of EZH2 and SMYD3 in cancer tissues and adjacent tissues. (B) Western blotting. (C) Protein expression of EZH2 and SMYD3 in cancer tissues and adjacent tissues.
Expression of EZH2 and SMYD3 is higher in breast cancer cells
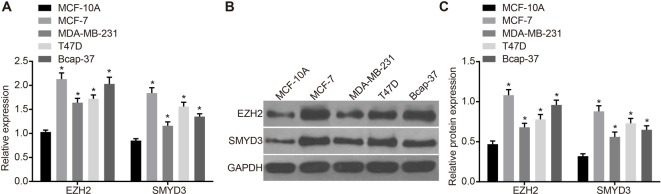
The mRNA and protein expression of EZH2 and SMYD3 in normal breast epithelial cells MCF-10A and breast cancer cells MCF-7, MDA-MB-231, T47D, and Bcap-37 were detected by RT-qPCR and Western blotting. The results showed that the mRNA and protein expression of EZH2 and SMYD3 in breast cancer cells MCF-7, MDA-MB-231, T47D, and Bcap-37 were higher than those in normal breast epithelial cells MCF-10A (P<0.05) (Figure 3). The results suggested that EZH2 and SMYD3 were in high expression in breast cancer cells.
Figure 3. mRNA and protein expression of EZH2 and SMYD3 in breast cancer cells.
(A) mRNA expression of EZH2 and SMYD3 in breast cancer cells. (B) Immunoblotting map. (C) Protein expression of EZH2 and SMYD3 in breast cancer cells; *P<0.05, compared with MCF-10A cells.
Relationship between mRNA expression of EZH2 and SMYD3 and clinicopathological features
The results showed that mRNA expression of EZH2 had no statistical significance with patients’ age, age of menarche, menopausal status, tumor size, pathological type, immunohistochemical indexes, and first-degree relatives (FDRs) with breast cancer (P>0.05), but had statistical significance with lymph node metastasis, HER2 status, clinical staging, subtypes of breast cancer, and metastasis situation. mRNA expression of SMYD3 had no statistical significance with patients’ age, age of menarche, menopausal status, tumor size, pathological type, immunohistochemical indexes, FDRs with breast cancer, and clinical staging (P>0.05), but had statistical significance with lymph node metastasis, HER2 status, subtypes of breast cancer, and metastasis situation (Table 6).
Table 6. The relationship between mRNA expression of EZH2 and SMYD3 and clinicopathological features.
| Pathological characteristics | Case | EZH2 mRNA expression | P | SMYD3 mRNA expression | P |
|---|---|---|---|---|---|
| Age (years) | 0.623 | 0.206 | |||
| ≥40 | 591 | 1.66 ± 0.20 | 1.13 ± 0.16 | ||
| <40 | 121 | 1.67 ± 0.22 | 1.15 ± 0.15 | ||
| Age of menarche (years) | 0.553 | 0.999 | |||
| ≥13 | 495 | 1.66 ± 0.21 | 1.13 ± 0.15 | ||
| <13 | 217 | 1.67 ± 0.20 | 1.13 ± 0.17 | ||
| Menopausal status | 0.677 | 0.113 | |||
| Premenopause | 81 | 1.67 ± 0.23 | 1.16 ± 0.16 | ||
| Postmenopause | 631 | 1.66 ± 0.20 | 1.13 ± 0.16 | ||
| Tumor size (cm) | 0.372 | 0.197 | |||
| ≤2.0 | 323 | 1.67 ± 0.19 | 1.14 ± 0.16 | ||
| 2.1–4 | 329 | 1.66 ± 0.22 | 1.13 ± 0.16 | ||
| >4 | 60 | 1.63 ± 0.20 | 1.10 ± 0.16 | ||
| Pathological type | 0.368 | 0.779 | |||
| IDC | 618 | 1.66 ± 0.20 | 1.13 ± 0.15 | ||
| ILC | 31 | 1.66 ± 0.20 | 1.15 ± 0.15 | ||
| Other | 63 | 1.66 ± 0.23 | 1.13 ± 0.19 | ||
| Lymph node metastasis | <0.001 | <0.001 | |||
| Negative | 335 | 1.61 ± 0.19 | 1.09 ± 0.14 | ||
| Positive | 377 | 1.71 ± 0.21 | 1.17 ± 0.17 | ||
| Immunohistochemical indexes | 0.392 | 0.433 | |||
| ER (−) | 176 | 1.66 ± 0.21 | 1.15 ± 0.15 | ||
| ER (+) | 536 | 1.66 ± 0.21 | 1.13 ± 0.16 | ||
| PR (−) | 168 | 1.66 ± 0.21 | 1.14 ± 0.15 | ||
| PR (+) | 544 | 1.66 ± 0.20 | 1.13 ± 0.16 | ||
| HER2 status | <0.001 | <0.001 | |||
| Negative | 361 | 1.62 ± 0.18 | 1.10 ± 0.14 | ||
| Positive | 351 | 1.71 ± 0.22 | 1.16 ± 0.17 | ||
| FDRs with breast cancer | 0.531 | 0.426 | |||
| Yes | 251 | 1.67 ± 0.21 | 1.14 ± 0.16 | ||
| No | 461 | 1.66 ± 0.20 | 1.13 ± 0.16 | ||
| Clinical staging | 0.004 | 0.154 | |||
| I/II | 654 | 1.66 ± 0.20 | 1.13 ± 0.15 | ||
| III/IV | 58 | 1.74 ± 0.25 | 1.16 ± 0.19 | ||
| Subtypes of breast cancer | <0.001 | <0.001 | |||
| Triple negative | 133 | 1.74 ± 0.25 | 1.18 ± 0.17 | ||
| Non-triple negative | 579 | 1.65 ± 0.19 | 1.12 ± 0.15 | ||
| Metastasis situation | <0.001 | <0.001 | |||
| Distant metastasis | 154 | 1.74 ± 0.21 | 1.19 ± 0.19 | ||
| Primary lesion | 558 | 1.64 ± 0.20 | 1.12 ± 0.14 |
Abbreviations: IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma.
mRNA expression of EZH2 and SMYD3 in patients with different genotypes
The results showed that mRNA expression of EZH2 in CC genotype carriers of EZH2 rs12670401 was higher than that in TT and TC genotype carriers (P<0.05); mRNA expression of EZH2 in C allele carriers of EZH2 rs12670401 was higher than that in T allele carriers (P<0.05); mRNA expression of EZH2 in TT genotype carriers of EZH2 rs6464926 was higher than that in CC and CT genotype carriers (P<0.05); mRNA expression of EZH2 in T allele carriers of EZH2 rs6464926 was higher than that in C allele carriers (P<0.05). mRNA expression of SMYD3 in 3/3 genotype carriers of SMYD3VNTR was higher than that in 2/3 and 2/2 genotype carriers, but the difference was not statistically significant (P>0.05). The difference in the mRNA expression of SMYD3 between the different allele carriers in SMYD3VNTR was not statistically significant (P>0.05) (Table 7).
Table 7. mRNA expression of EZH2 and SMYD3 in patients with different genotypes.
| Gene loci | Case | mRNA expression | P |
|---|---|---|---|
| EZH2 rs12670401 | |||
| TT | 232 | 1.63 ± 0.18 | 0.01 |
| TC | 323 | 1.67 ± 0.21 | |
| CC | 157 | 1.69 ± 0.22 | |
| T | 787 | 1.65 ± 0.20 | 0.007 |
| C | 637 | 1.68 ± 0.22 | |
| EZH2 rs6464926 | |||
| CC | 243 | 1.64 ± 0.18 | 0.02 |
| CT | 327 | 1.66 ± 0.22 | |
| TT | 142 | 1.70 ± 0.20 | |
| C | 813 | 1.65 ± 0.21 | 0.009 |
| T | 611 | 1.68 ± 0.22 | |
| SMYD3VNTR | |||
| 2/2 | 427 | 1.13 ± 0.16 | 0.631 |
| 2/3 | 222 | 1.13 ± 0.15 | |
| 3/3 | 63 | 1.15 ± 0.17 | |
| 2 | 1076 | 1.13 ± 0.16 | 0.304 |
| 3 | 348 | 1.14 ± 0.15 |
Univariate and multivariate logistic regression analyses of risk factors for breast cancer
Multivariate logistic regression analysis was carried out with breast cancer as a dependent variable, and different genotypes (EZH2 rs12670401, EZH2 rs6464926, and SMYD3 VNTR) in the case and control groups and baseline characteristics (age, age of menarche, and menopausal status) as independent variables. As shown in Table 8, EZH2 rs12670401 and EZH2 rs6464926 polymorphisms, age of menarche, and menopausal status were risk factors for breast cancer (all P<0.05), but age was not the risk factor of breast cancer (P>0.05).
Table 8. Multivariate logistic regression analysis of risk factors for breast cancer patients.
| B | S.E.M. | OR | 95% CI | P | |
|---|---|---|---|---|---|
| EZH2 rs12670401 | 0.697 | 0.095 | 2.007 | 1.666–2.418 | <0.001 |
| EZH2 rs6464926 | 0.236 | 0.116 | 1.266 | 1.008–1.590 | 0.042 |
| SMYD3VNTR | −0.173 | 0.118 | 1.223 | 0.667–1.060 | 0.143 |
| Age | −0.186 | 0.151 | 0.830 | 0.618–1.115 | 0.216 |
| Age of menarche | 0.444 | 0.117 | 1.558 | 1.238–1.962 | <0.001 |
| Menopausal status | 1.590 | 0.148 | 4.901 | 3.664–6.557 | <0.001 |
Abbreviation: B, regression coefficient.
Association of gene polymorphisms and mRNA expression of EZH2 and SMYD3 with breast cancer prognosis
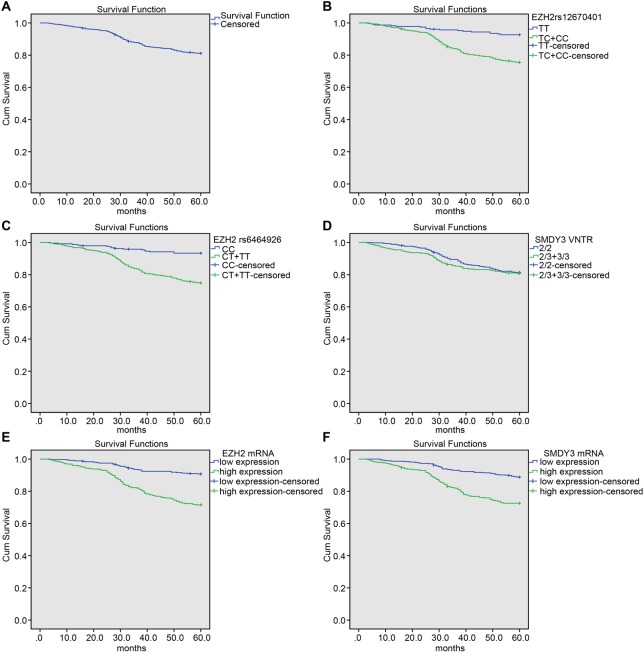
The overall survival (OS) after 24, 36, 48, and 60 months of the patients were 95.23, 87.76, 84.13, and 81.18%, respectively (Figure 4). The association between polymorphisms and breast cancer prognosis was analyzed by Kaplan–Meier survival, and the result showed that the OS of patients with TT genotype was higher than those with TC + CC genotype in EZH2 rs12670401 locus, and the OS of the patients with CC genotype was higher than those with CT + TT genotype in EZH2 rs6464926 locus (both P<0.05); the OS of the patients with 2/2 genotype was higher than those with 2/3 + 3/3 genotype in SMYD3 VNTR locus, but the difference was not statistically significant (P>0.05) (Figure 4). Patients were allocated into EZH2 high expression and EZH2 low expression, SMYD3 high expression, and SMYD3 low expression with the median of EZH2 and SMYD3mRNA expression as boundary. The Kaplan–Meier survival analysis showed that the OS of patients with EZH2 high expression was lower than those with EZH2 low expression (P<0.05), and the OS of patients with SMYD3 high expression was lower than those with SMYD3 low expression (P<0.05).
Figure 4. Correlation of EZH2 rs12670401, EZH2 rs6464926, and SMYD3VNTR gene polymorphisms with the OS of patients with breast cancer.
(A) OS curve for patients with breast cancer. (B) Survival curve for patients with EZH2 rs12670401. (C) Survival curve for patients with EZH2 rs6464926. (D) Survival curve for patients with SMYD3 VNTR genotypes. (E) Survival curve for mRNA expression of EZH2. (F) Survival curve for mRNA expression of SMYD3.
Result of Cox multivariate analysis declared that EZH2 rs12670401 and EZH2 rs6464926 genotypes, mRNA and protein expression of EZH2 and SMYD3, clinical staging, lymph node metastasis, HER2 status, and metastasis situation were independent prognostic factors for the survival rate of breast cancer (all P<0.05), but other factors had no significant difference with the survival rate (Table 9).
Table 9. Multivariate survival analysis of OS using Cox’s regression model for breast cancer patients.
| Genotypes | B | S.E.M. | OR | 95% CI | P |
|---|---|---|---|---|---|
| EZH2 rs12670401 | 1.004 | 0.277 | 2.729 | 1.586–4.695 | <0.001 |
| EZH2 rs6464926 | 0.928 | 0.275 | 2.529 | 1.474–4.339 | 0.001 |
| SMYD3VNTR | 0.358 | 0.184 | 1.431 | 0.998–2.051 | 0.052 |
| EZH2 mRNA | 0.463 | 0.219 | 1.589 | 1.034–2.440 | 0.035 |
| SMYD3mRNA | 0.614 | 0.198 | 1.848 | 1.253–2.726 | 0.002 |
| EZH2 protein | 0.574 | 0.198 | 1.776 | 1.205–2.617 | 0.004 |
| SMYD3 protein | 0.495 | 0.191 | 1.640 | 1.128–2.385 | 0.010 |
| Age | −0.172 | 0.528 | 0.842 | 0.299–2.372 | 0.745 |
| Age of menarche | 0.158 | 0.205 | 1.171 | 0.783–1.750 | 0.443 |
| Menopausal status | 0.594 | 0.471 | 1.810 | 0.720–4.553 | 0.207 |
| Tumor size | 0.012 | 0.142 | 1.012 | 0.767–1.336 | 0.931 |
| ER (+) | −0.247 | 0.766 | 0.781 | 0.174–3.504 | 0.747 |
| PR (+) | 0.319 | 0.832 | 1.376 | 0.269–7.034 | 0.701 |
| Histology | −0.049 | 0.151 | 0.952 | 0.708–1.281 | 0.746 |
| Clinical staging | 1.193 | 0.237 | 3.298 | 2.072–5.249 | <0.001 |
| Lymph node metastasis | 0.742 | 0.223 | 2.101 | 1.358–3.251 | 0.001 |
| HER2 status | 1.204 | 0.255 | 3.335 | 2.024–5.494 | <0.001 |
| FDRs with breast cancer | −0.125 | 0.190 | 0.883 | 0.608–1.282 | 0.513 |
| Subtypes of breast cancer | −0.088 | 0.287 | 0.915 | 0.522–1.606 | 0.758 |
| Metastasis situation | 0.668 | 0.189 | 1.950 | 1.345–2.826 | <0.001 |
Abbreviation: B, regression coefficient.
Discussion
The present study aims to explore the correlations of EZH2 and SMYD3 gene polymorphisms with breast cancer susceptibility and prognosis. Our findings suggest that EZH2 rs12670401 and EZH2 rs6464926 polymorphisms may be significantly correlated with breast cancer susceptibility and prognosis.
Our study found that C allele of EZH2 rs12670401, T allele of EZH2 rs6464926, and 3 allele of SMYD3 VNTR could increase the susceptibility to breast cancer. EZH2 gene, a histone methyltransferase gene and an important member of polycomb group (PcG), has been reported to be closely related to the formation and development of a variety of primary tumors (breast cancer included) and can play a role in the regulation of PcG through construction of polycomb-repressive complex 2 (PRC2) and in the modification of epigenetic genes [11,19]. EZH2 rs12670401 polymorphism is located in the intron region of EID combined with EED, and EZH2 rs6464926 is located in intron region of D2 combined with SUZ12 (PcG protein). PRC2 was formed by the combination of EZH2 with EED and SUZ12 that could activate histone methyltransferase enzyme [20–23]. Therefore, the polymorphism of the above loci may indirectly affect the combination of EZH2 with EED and SUZ12, which may influence the histone methyltransferase function of EZH2 so as to affect the function of histone in chromosomal methylation of EZH2 [24–26]. EZH2 as the subunit of the PRC2 complex catalyzes trimethylation of histone H3 lysine 27 (H3K27) [27]. It is reported that the methylation level of H3K27 is closely related to the occurrence and prognosis of tumors [28]. Therefore, we concluded that the polymorphisms of EZH2 may influence H3K27 methylation so as to function in the occurrence and prognosis of breast cancer. Besides, our results revealed that EZH2 and SMYD3 were in high expression in breast cancer tissues and cells. EZH2, an inhibitor of gene transcription, was related to biological malignancy in various cancers [29]. A previous study also found that high expression of EZH2 was associated with poor outcome in breast cancer [30]. SMYD3, a histone methyltransferase, played an important role in transcriptional regulation in human carcinogenesis [31]. High SMYD3 expression was critical for the development of breast cancer cells [32].
Unfortunately, we failed to reveal any correlations between SMYD3 VNTR polymorphism and breast cancer susceptibility and prognosis. SMYD3, a protein with histone methylation function that could accelerate the methylation of chromosomes histone, is associated with the transcriptional cell regulation and presents high expression in a variety of tumors (breast cancer included) [32]. It has been reported that there exist differences in polymorphism of VNTR in the 5′ regulatory region of SMYD3, which might be closely related to the individual differences in the occurrence of tumorigenesis [16,17]. The 5′ regulatory region of SMYD3 is a binding site of transcription factor E2F-1 and plays an important role in cell cycle regulation and the occurrence of tumors. The increase in E2F in copy number of the region could enhance the affinity of SMYD3 and E2F-1 and increase the possibility in occurrence of breast cancer and poor prognosis [16]. Thus, we hypothesized that SMYD3 gene polymorphisms may be involved in the development and progression of breast cancer through combining with other genes and environmental factors.
Our study also found that the combined genotype of EZH2 rs12670401 (TC + CC) and EZH2 rs6464926 (CT + TT) could result in an obvious increase in the susceptibility to breast cancer, indicating that there may be interactions between the two loci. EZH2 rs12670401 and EZH2 rs6464926 polymorphisms have been proved to be in significant correlation with breast cancer susceptibility and prognosis [33]. The interaction mechanism of EZH2 rs12670401 and EZH2 rs6464926 polymorphisms in the development and progression of breast cancer is needed to be further studied in the future. Besides, we found that the age of menarche and menopause status had a certain relationship with breast cancer susceptibility and prognosis as well, which was consistent with a former study [34].
Another significant finding was that OS of patients with TT genotype was higher than those with TC + CC genotype of EZH2 rs12670401 and OS of patients with CC genotype was higher than those with CT + TT genotype of EZH2 rs6464926. The OS of patients with EZH2 high expression and SMYD3 high expression was lower than those with EZH2 low expression and SMYD3 low expression. Result of Cox multivariate analysis showed that EZH2 rs12670401, EZH2 rs6464926 and clinical staging, mRNA and protein expression of EZH2 and SMYD3, lymph node metastasis, HER2 status, and metastasis situation were independent prognostic factors for survival rate of breast cancer patients. EZH2 plays varied roles in cancer development relying on the type of cancers [35]. EZH2 expression is related to the OS of cancer patients and high EZH2 expression as a prognostic factor shows shorter OS for patients with breast cancer [36]. The T allele of EZH2 148505302 gene is associated with a longer OS in cholangiocarcinoma patients [37].
To summarize, our study provided strong evidence that EZH2 rs12670401 and rs6464926 polymorphisms may be correlated with breast cancer susceptibility and prognosis. However, SMYD3 VNTR polymorphism exhibited no association with breast cancer susceptibility and prognosis. Our findings provide a theoretical basis for breast cancer susceptibility assessment, breast cancer therapy, and clinical reference for personalized therapy of breast cancer. But the mechanism in the susceptibility to and survival of breast cancer remained unclear and therefore constant follow-up research is required.
Acknowledgments
We thank the reviewers for their helpful comments on this article.
Abbreviations
- 95% CI
95% confidence interval
- CRC
colorectal cancer
- DMEM
Dulbecco’s modified Eagle’s medium
- ER+
estrogen receptor
- EZH2
enhancer of Zeste homolog 2
- FDRs
first-degree relatives
- GAPDH
glyceraldehyde phosphate dehydrogenase
- H3K27
histone H3 lysine 27
- HER2
human epidermal growth factor receptor-2
- IDC
invasive ductal carcinoma
- ILC
invasive lobular carcinoma
- OD
optical density
- OR
odds ratio
- OS
overall survival
- PcG
polycomb group
- PR+
progesterone receptor
- PRC2
polycomb-repressive complex 2
- RT-qPCR
reverse-transcription quantitative polymerase chain reaction
- SMYD3
SET and MYND domain containing 3
Funding
This work was supported in part by the Science and Technology Project of Shaanxi Social Development [grant number 2016SF-168].
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
Author contribution
S.-J.M. and Y.-M.L. wrote the paper and conceived and designed the experiments. Y.-L.Z. analyzed the data. M.-W.C. and W.C. collected and provided the sample for the present study.
References
- 1.Liu H.J., Yan H., Yan J., Li H., Chen L., Han L.R. et al. (2016) Substance P promotes the proliferation, but inhibits differentiation and mineralization of osteoblasts from rats with spinal cord injury via RANKL/OPG system. PLoS ONE 11, e0165063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao Y., Huang Y., Song F., Dai H., Wang P., Li H. et al. (2016) Urban-rural disparity of overweight/obesity distribution and its potential trend with breast cancer among Chinese women. Oncotarget 7, 56608–56618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamada A., Narui K., Sugae S., Shimizu D., Takabe K., Ichikawa Y. et al. (2016) Operation with less adjuvant therapy for elderly breast cancer. J. Surg. Res. 204, 410–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orphanos G. and Kountourakis P. (2012) Targeting the HER2 receptor in metastatic breast cancer. Hematol. Oncol. Stem Cell Ther. 5, 127–137 [DOI] [PubMed] [Google Scholar]
- 5.Naushad S.M., Divya C., Janaki Ramaiah M., Hussain T., Alrokayan S.A. and Kutala V.K. (2016) Population-level diversity in the association of genetic polymorphisms of one-carbon metabolism with breast cancer risk. J. Community Genet. 7, 279–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sergentanis T.N. and Economopoulos K.P. (2010) Four polymorphisms in cytochrome P450 1A1 (CYP1A1) gene and breast cancer risk: a meta-analysis. Breast Cancer Res. Treat. 122, 459–469 [DOI] [PubMed] [Google Scholar]
- 7.Yu K.D., Chen A.X., Yang C., Qiu L.X., Fan L., Xu W.H. et al. (2010) Current evidence on the relationship between polymorphisms in the COX-2 gene and breast cancer risk: a meta-analysis. Breast Cancer Res. Treat. 122, 251–257 [DOI] [PubMed] [Google Scholar]
- 8.Beeghly-Fadiel A., Shu X.O., Long J., Li C., Cai Q., Cai H. et al. (2009) Genetic polymorphisms in the MMP-7 gene and breast cancer survival. Int. J. Cancer 124, 208–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erdel F. and Greene E.C. (2016) Generalized nucleation and looping model for epigenetic memory of histone modifications. Proc. Natl. Acad. Sci. U.S.A. 113, E4180–E4189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sponziello M., Durante C., Boichard A., Dima M., Puppin C., Verrienti A. et al. (2014) Epigenetic-related gene expression profile in medullary thyroid cancer revealed the overexpression of the histone methyltransferases EZH2 and SMYD3 in aggressive tumours. Mol. Cell. Endocrinol. 392, 8–13 [DOI] [PubMed] [Google Scholar]
- 11.Ferraro A., Boni T. and Pintzas A. (2014) EZH2 regulates cofilin activity and colon cancer cell migration by targeting ITGA2 gene. PLoS ONE 9, e115276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo N., Chen R., Li Z., Liu Y., Cheng D., Zhou Q. et al. (2011) Hepatitis C virus core upregulates the methylation status of the RASSF1A promoter through regulation of SMYD3 in hilar cholangiocarcinoma cells. Acta. Biochim. Biophys. Sin. (Shanghai) 43, 354–361 [DOI] [PubMed] [Google Scholar]
- 13.Su C.H., Lane H.Y., Hsiao C.L., Liu L.C., Ji H.X., Li H.T. et al. (2016) Matrix metalloproteinase-1 genetic polymorphism in breast cancer in Taiwanese. Anticancer Res. 36, 3341–3345 [PubMed] [Google Scholar]
- 14.Wang J., Ma Z.B., Li K. and Guo G.H. (2014) Association between EZH2 polymorphisms and colorectal cancer risk in Han Chinese population. Med. Oncol. 31, 874. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Y., Du W.D., Wu Q., Liu Y., Chen G., Ruan J. et al. (2014) EZH2 genetic variants affect risk of gastric cancer in the Chinese Han population. Mol. Carcinog. 53, 589–597 [DOI] [PubMed] [Google Scholar]
- 16.Tsuge M., Hamamoto R., Silva F.P., Ohnishi Y., Chayama K., Kamatani N. et al. (2005) A variable number of tandem repeats polymorphism in an E2F-1 binding element in the 5′ flanking region of SMYD3 is a risk factor for human cancers. Nat. Genet. 37, 1104–1107 [DOI] [PubMed] [Google Scholar]
- 17.Wang H., Liu Y., Tan W., Zhang Y., Zhao N., Jiang Y. et al. (2008) Association of the variable number of tandem repeats polymorphism in the promoter region of the SMYD3 gene with risk of esophageal squamous cell carcinoma in relation to tobacco smoking. Cancer Sci. 99, 787–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ayuk S.M., Abrahamse H. and Houreld N.N. (2016) The role of photobiomodulation on gene expression of cell adhesion molecules in diabetic wounded fibroblasts in vitro. J. Photochem. Photobiol. B. 161, 368–374 [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez M.E., Moore H.M., Li X., Toy K.A., Huang W., Sabel M.S. et al. (2014) EZH2 expands breast stem cells through activation of NOTCH1 signaling. Proc. Natl. Acad. Sci. U.S.A. 111, 3098–3103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kong X., Chen L., Jiao L., Jiang X., Lian F., Lu J. et al. (2014) Astemizole arrests the proliferation of cancer cells by disrupting the EZH2-EED interaction of polycomb repressive complex 2. J. Med. Chem. 57, 9512–9521 [DOI] [PubMed] [Google Scholar]
- 21.Cho K.S., Oh H.Y., Lee E.J. and Hong S.J. (2007) Identification of enhancer of zeste homolog 2 expression in peripheral circulating tumor cells in metastatic prostate cancer patients: a preliminary study. Yonsei Med. J. 48, 1009–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi J.H., Song Y.S., Yoon J.S., Song K.W. and Lee Y.Y. (2010) Enhancer of zeste homolog 2 expression is associated with tumor cell proliferation and metastasis in gastric cancer. APMIS 118, 196–202 [DOI] [PubMed] [Google Scholar]
- 23.Fiskus W., Pranpat M., Balasis M., Herger B., Rao R., Chinnaiyan A. et al. (2006) Histone deacetylase inhibitors deplete enhancer of zeste 2 and associated polycomb repressive complex 2 proteins in human acute leukemia cells. Mol. Cancer Ther. 5, 3096–3104 [DOI] [PubMed] [Google Scholar]
- 24.Simon J.A. and Lange C.A. (2008) Roles of the EZH2 histone methyltransferase in cancer epigenetics. Mutat. Res. 647, 21–29 [DOI] [PubMed] [Google Scholar]
- 25.Martinez-Garcia E. and Licht J.D. (2010) Deregulation of H3K27 methylation in cancer. Nat. Genet. 42, 100–101 [DOI] [PubMed] [Google Scholar]
- 26.Nichol J.N., Dupere-Richer D., Ezponda T., Licht J.D. and Miller W.H. Jr (2016) H3K27 methylation: a focal point of epigenetic deregulation in cancer. Adv. Cancer Res. 131, 59–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCabe M.T., Graves A.P., Ganji G., Diaz E., Halsey W.S., Jiang Y. et al. (2012) Mutation of A677 in histone methyltransferase EZH2 in human B-cell lymphoma promotes hypertrimethylation of histone H3 on lysine 27 (H3K27). Proc. Natl. Acad. Sci. U.S.A. 109, 2989–2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Avila-Moreno F., Armas-Lopez L., Alvarez-Moran A.M., Lopez-Bujanda Z., Ortiz-Quintero B., Hidalgo-Miranda A. et al. (2014) Overexpression of MEOX2 and TWIST1 is associated with H3K27me3 levels and determines lung cancer chemoresistance and prognosis. PLoS ONE 9, e114104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He L.R., Liu M.Z., Li B.K., Jia W.H., Zhang Y., Liao Y.J. et al. (2010) High expression of EZH2 is associated with tumor aggressiveness and poor prognosis in patients with esophageal squamous cell carcinoma treated with definitive chemoradiotherapy. Int. J. Cancer 127, 138–147 [DOI] [PubMed] [Google Scholar]
- 30.Reijm E.A., Timmermans A.M., Look M.P., Meijer-van Gelder M.E., Stobbe C.K., van Deurzen C.H. et al. (2014) High protein expression of EZH2 is related to unfavorable outcome to tamoxifen in metastatic breast cancer. Ann. Oncol. 25, 2185–2190 [DOI] [PubMed] [Google Scholar]
- 31.Chen D., Liu L., Luo X., Mu A., Yan L., Chen X. et al. (2017) Effect of SMYD3 on the microRNA expression profile of MCF-7 breast cancer cells. Oncol. Lett. 14, 1831–1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamamoto R., Silva F.P., Tsuge M., Nishidate T., Katagiri T., Nakamura Y. and Furukawa Y. (2006) Enhanced SMYD3 expression is essential for the growth of breast cancer cells. Cancer Sci. 97, 113–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tao R., Chen Z., Wu P., Liu C., Peng Y., Zhao W. et al. (2015) The possible role of EZH2 and DNMT1 polymorphisms in sporadic triple-negative breast carcinoma in southern Chinese females. Tumour Biol. 36, 9849–9855 [DOI] [PubMed] [Google Scholar]
- 34.Zong H., Xu H., Geng Z., Ma C., Ming X., Shang M. et al. (2014) Reproductive factors in relation to risk of brain tumors in women: an updated meta-analysis of 27 independent studies. Tumour Biol. 35, 11579–11586 [DOI] [PubMed] [Google Scholar]
- 35.Ernst T., Chase A.J., Score J., Hidalgo-Curtis C.E., Bryant C., Jones A.V. et al. (2010) Inactivating mutations of the histone methyltransferase gene EZH2 in myeloid disorders. Nat. Genet. 42, 722–726 [DOI] [PubMed] [Google Scholar]
- 36.Jang S.H., Lee J.E., Oh M.H., Lee J.H., Cho H.D., Kim K.J. et al. (2016) High EZH2 protein expression is associated with poor overall survival in patients with luminal A breast cancer. J. Breast Cancer 19, 53–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paolicchi E., Pacetti P., Giovannetti E., Mambrini A., Orlandi M., Crea F. et al. (2013) A single nucleotide polymorphism in EZH2 predicts overall survival rate in patients with cholangiocarcinoma. Oncol. Lett. 6, 1487–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]